Multifunctional hybrid poly(ester-urethane)urea/resveratrol electrospun nanofibers for a potential vascularizing matrix†
Chen
Liang‡
a,
Yanan
Wang‡
b,
Renliang
Zhao‡
c,
Juan
Du
a,
Jin
Yao
a,
Atta ur Rehman
Khan
a,
Youwei
Zhu
*fgh,
Huitang
Xia
*de and
Tonghe
Zhu
 *a
*a
aMultidisciplinary Centre for Advanced Materials, Institute for Frontier Medical Technology, School of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, 333 Longteng Rd., Shanghai 201620, P. R. China. E-mail: zhutonghe89@163.com
bDepartment of Minimally Invasive Spine Surgery, Shandong Wendeng Orthopedic Hospital, 1 Fengshan Rd., Weihai 264400, Shandong, P. R. China
cOrthopedics Research Institute, Trauma Medical Center, Department of Orthopedics, West China Hospital, Sichuan University, 37 Guoxue Ln., Chengdu 610041, Sichuan, P. R. China
dDepartment of Plastic Surgery, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, 16766 Jingshi Rd., Jinan 250014, Shandong, P. R. China. E-mail: xiahuitang@163.com
eJinan Clinical Research Center for Tissue Engineering Skin Regeneration and Wound Repair, 16766 Jingshi Rd., Jinan 250014, Shangdong, P. R. China
fDepartment of General Surgery, Pancreatic Disease Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, 197 Ruijin 2nd Rd., Shanghai 200025, P. R. China. E-mail: ywzhu555@163.com
gShanghai Key Laboratory of Pancreatic Neoplasms Translational Medicine, 197 Ruijin 2nd Rd., Shanghai 200025, P. R. China
hResearch Institute of Pancreatic Diseases, Shanghai Jiao Tong University School of Medicine, 197 Ruijin 2nd Rd., Shanghai 200025, P. R. China
First published on 26th November 2024
Abstract
The challenges for clinical application of small-diameter vascular graft are mainly acute/chronic thrombosis, inadequate endothelialization, intimal hyperplasia caused by inflammation, oxidative stress, and the mismatch of mechanical compliance after transplantation. How to construct an effective regenerative microenvironment through a material with uniform dispersion of active components is the premise of maintaining patency of a vascular graft. In this study, we have compounded poly(ester-urethane)urea (PEUU) with various optimized concentrations of resveratrol (Res) by homogeneous emulsion blending, followed by electrospinning into the hybrid PEUU/Res nanofibers (P/R-0, P/R-0.5, P/R-1.0, and P/R-1.5). Then the microstructure, surface wettability, mechanical properties, degradation, Res sustained release properties, hemocompatibility, and cytocompatibility of P/R were evaluated comprehensively. The results indicate that Res can be gradually released from the P/R, and both the hydrophilicity and antioxidant ability of the nanofiber gradually increase with the increase of Res content. Moreover, with the increase of Res, the viability and proliferation behavior of HUVECs were significantly improved. Meanwhile, tube formation and migration experiments showed that Res promoted the formation of a neovascularization network. In brief, it is concluded that P/R-1.0 is the optimal candidate with a uniform microstructure, moderate wettability, optimized mechanical properties, reliable hemocompatibility and cytocompatibility, and strongest ability to promote endothelial growth for the vascularizing matrix.
1. Introduction
Cardiovascular disease (CVD) remains a threat to human life and health and is one of the leading causes of death, accounting for 30% of the population.1–3 For vessels that lose normal function, the most effective treatment currently focuses on bypass transplantation. Autologous vascular transplantation is regarded as the gold standard of bypass transplantation, but it cannot meet the needs of most surgeries due to factors such as lack of donors, trauma, anatomical abnormalities, and size mismatch.4–6 Therefore, the use of artificial blood vessels as an alternative to autologous blood vessels for tissue repair has been widely studied.7The existing large-diameter artificial blood vessels, including polytetrafluoroethylene (ePTFE), poly(ethylene-terephthalate) (PET), and other materials of artificial blood vessels, have achieved unprecedented success in clinical application.8–10 However, due to both the slower blood flow rate and mismatch in the mechanical properties of small-diameter artificial blood vessels (<6 mm), early thrombosis, lumen stenosis, and intimal hyperplasia occurred frequently after vascular transplantation.11–13 For this, there have been many studies in this aspect, such as pre-inoculation of cells and growth factors in the inner layer of small-diameter vascular grafts, or modification of the lumen surface with anticoagulant drugs. But these methods still need to be improved due to the complex production process, insufficient stability, and poor continuity.14–16 Therefore, after decades of research, scientists have not found a suitable biomaterial to replace the matrix of small blood vessels.
Previous studies have shown that the rapid formation of complete endothelial cell layers after transplantation is still the primary consideration in the design of small-diameter artificial blood vessels.17–20 The extracellular matrix plays a decisive role in cell induction and thus in vascular endothelial remodeling in vivo. Therefore, artificial blood vessel materials should have the same properties as a natural extracellular matrix, including mechanical properties, blood compatibility, topographical cues, biological signal transduction, etc., to guide vascular endothelial formation.21–23
Resveratrol (Res) is a small molecule of natural polyphenols extracted from the traditional Chinese medicinal plant polygonum cuspidatum.24 Its vascularization, anti-inflammatory, and antioxidant properties have been extensively studied.25 Many studies have shown that Res plays a positive role in the growth of vascular endothelial cells and the generation of vascular networks at appropriate concentrations.26 However, due to the low water solubility of Res (<1 mg mL−1), and only 2% oral bioavailability of Res, the low bioaccumulation of Res in the cardiovascular system (such as in heart and aortic tissues) is another problem.27 The concentration of Res achieved in the target organ is significantly lower than the effective dose applied in cell and animal studies.28 Before reaching target cells, Res may degrade rapidly in the gastrointestinal tract, be over-metabolized by intestinal flora, or be over-consumed by other non-target cells, so a more efficient delivery system in vivo is needed.29,30
Electrospinning is currently a relatively mature and simple method for preparing nanostructured materials. The nanofibers made by electrospinning have a three-dimensional porous structure similar to that of a natural tissue extracellular matrix, which is suitable for cell adhesion and growth, and can also encapsulate different types of drug molecules, as well as have been widely used in the preparation of tissue engineering scaffolds.31
Poly(ester-urethane)urea (PEUU) has biomechanical properties matching those of vascularized tissues, excellent processing properties, and stable biocompatibility, and has been increasingly used for tissue regeneration.32,33 In this work, a double solvent method was introduced to prepare PEUU and Res co-blended electrospun solutions with different proportions for electrospinning nanofibers to overcome the limitation of uneven dispersion of Res in vascular material. With this, Res molecules could be efficiently combined into the nanofibers to achieve continuous drug delivery in targeted organs hence solving the low bioavailability of Res. The nanofibers were extensively characterized and the effect of nanofibers with different Res contents on the cell behavior of vascular endothelial cells was studied in detail.
2. Materials and methods
2.1 Materials
The materials used in the experiments are shown in the ESI† in Section S1.1.2.2 Synthesis of the PEUU elastomer and preparation of hybrid P/R fibers
We prepared P/R nanofibers by using HFIP and DMF to dissolve PEUU and Res, respectively. The synthesis of PEUU elastomer and the preparation of P/R hybrid nanofibers were carried out according to the ESI† in Section S1.2.2.3 Characterization and testing
The detailed tests and parameters for micromorphology, infrared spectroscopy, hydrophilicity, porosity and water absorption can be found in the ESI† in Section S1.3.2.4 Degradation and Res release in vitro
The ethanol solution of Res was prepared by removing 5 mL of anhydrous ethanol in the centrifuge tube and adding a trace amount of Res. Anhydrous ethanol was used as the control group. The UV spectrum of Res was scanned by a UV-VIS spectrophotometer to determine the maximum UV absorption wavelength of Res.The standard concentration curve of Res was drawn: a Res solution of 100 μg mL−1 was prepared by accurately weighing 0.01 g Res powder and dissolving it in 10 mL ethanol, and then diluting the volume to 100 mL in a 100 mL volumetric bottle. After that, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8 mL solutions were accurately removed using a pipette through the volume bottle to 5 mL Res solutions of 2, 4, 6, 8, 10, 12, 14, and 16 μg mL−1 were prepared. The absorbance of Res at different concentrations was tested by a photometer, the standard concentration curve of Res was drawn and the standard concentration formula was calculated by Origin software.
The dry weight of four groups of nanofibers was recorded, and each group of nanofibers was immersed in 4 mL of protease K solution (2 U mL−1 in PBS). It was then placed in a temperature vibrator at 37 °C (100 rpm). In order to maintain appropriate protease K activity throughout the process, the protease K solution was changed every 72 hours. The nanofibers were removed at the set time node, repeatedly cleaned with deionized (DI) water, frozen overnight in a −80 °C refrigerator, and lyophilized. The weight of the lyophilized nanofibers was recorded and three samples were taken in parallel from each set of nanofibers at each time point for testing. The weight of the nanofibers for four weeks was recorded and the residual mass ratio was calculated using the following eqn (1):
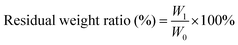 | (1) |
The 10 mg nanofiber sample was weighed and placed into a 15 mL centrifuge tube containing 10 mL of PBS solution. The centrifuge tubes were then incubated in a vibrator at 37 °C. At the designated time point, 4 mL of slow-release solution was withdrawn from each centrifuge tube, and promptly replaced with 4 mL of fresh PBS solution to maintain a total volume of 10 mL. Three parallel samples were included in each P/R group. The absorbance of Res was measured at 315 nm, and the cumulative release (mc) and cumulative release rate (Rc) of Res were calculated by eqn (2) and (3):
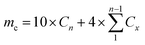 | (2) |
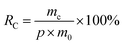 | (3) |
2.5 Antioxidant activity test in vitro
The antioxidant activity of P/R nanofibers was evaluated by the free radical scavenging efficiency of 1,1-diphenyl-2-picrohydrazine (DPPH). A purple DPPH solution was first prepared by dissolving 100 μg of DPPH in 50 mL of absolute ethanol. 4 mL DPPH solution was removed from a 10 mL centrifuge tube, and 5 mg nanofiber was added, respectively. DPPH solution without P/R nanofiber was used as a blank control group. It was incubated in a dark environment for 30 min. After incubation, absorbance (OD value) at 517 nm was measured by a UV-VIS spectrophotometer. Finally, the antioxidant efficiency of the P/R nanofibers was calculated using the following eqn (4):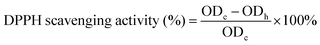 | (4) |
2.6 Hemocompatibility test
All animal experimental protocols are in accordance with the policy of the Institutional Animal Care and Use Committee (IACUC) of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and approved by the IACUC. The ethical principles were followed throughout all experimental procedures. All animal experiments were performed according to the Animal Management Regulations of China (1988 and revised in 2001, Ministry of Science and Technology) and all animals were purchased from Shanghai Slaccas Experimental Animal Co., Ltd (Shanghai, China).The hemocompatibility of the fibers was analyzed through hemolysis and platelet adhesion tests. Rabbit blood taken from healthy New Zealand white rabbit ear veins was diluted in normal saline at a ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) for use. P/R nanofibers were cut into 14 mm diameter fiber plates and put into a centrifuge tube containing 10 mL saline. For the positive control group, 10 mL of deionized water was used, and for the negative control group, 10 mL of PBS phosphate buffer was used. All centrifuge tubes were placed in a constant temperature shaker at 37 °C for 30 min, followed by the addition of 0.2 mL of diluted rabbit blood and incubation for 1 h. After incubation, the centrifugal tube in a low-speed centrifuge was centrifuged for 5 min at a speed of 1200 rpm. Finally, the supernatant was removed and the absorbance (OD value) at 545 nm was measured by a UV-visible spectrophotometer. The following eqn (5) was used to calculate the hemolysis rate:
10 (v/v) for use. P/R nanofibers were cut into 14 mm diameter fiber plates and put into a centrifuge tube containing 10 mL saline. For the positive control group, 10 mL of deionized water was used, and for the negative control group, 10 mL of PBS phosphate buffer was used. All centrifuge tubes were placed in a constant temperature shaker at 37 °C for 30 min, followed by the addition of 0.2 mL of diluted rabbit blood and incubation for 1 h. After incubation, the centrifugal tube in a low-speed centrifuge was centrifuged for 5 min at a speed of 1200 rpm. Finally, the supernatant was removed and the absorbance (OD value) at 545 nm was measured by a UV-visible spectrophotometer. The following eqn (5) was used to calculate the hemolysis rate:
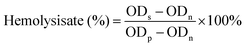 | (5) |
For the platelet adhesion experiment, platelet-rich plasma (PRP) and platelet-poor plasma (PPP) from fresh rabbit blood were separated by centrifugation at 1200 rpm. The P/R nanofibers were cut into 14 mm round sheets, placed on 24-well plates after alcohol fumigation and ultraviolet sterilization, added to 500 μL PRP, and incubated in a constant temperature shaking table at 37 °C for 2 h. Repeated rinsing with PBS phosphate buffer several times after incubation ensured that the platelets not adhering to the surface of the material were successfully washed away. 4% paraformaldehyde solution was added to fix for 4 h, and finally dehydrated (10%, 30%, 50%, 60%, 75%, 80%, 90%, and 100% after gradient alcohol dehydration treatment, respectively), soaked for 10 min each time, and the platelet adhesion on the surface of the three groups of materials was observed by scanning electron microscopy (SEM, Hitachi SU8010). Then, the area of platelets was measured in ImageJ software. Eqn (6) shows the calculation method of platelets coverage:
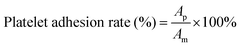 | (6) |
2.7 Cytocompatibility test
2.8 Statistical analysis
Statistical analysis of the data was performed utilizing IBM SPSS statistics software. The results were articulated as the mean value accompanied by the standard deviation (SD) for each set of data. One-way ANOVA was performed on the results using Microsoft Office Excel. The obtained P-values were considered to be statistically significant. ns: not significant, *p < 0.05 statistical difference; **p < 0.01 significant statistical difference; ***p < 0.001 very significant statistical difference.3. Results and discussion
The PEUU/HFIP solution was mixed with Res/DMF solution to obtain the solution and prepare the nanofibers by electrospinning. The electrospinning process in this method was stable and controllable, and the nanofibers P/R-0, P/R-0.5, P/R-1.0, and P/R-1.5 were prepared by this method. Electrospinning is a facile nanofiber fabricating method driven by electricity. Given a positive charge by an applied electric field, the polymer is stretched into ultra-fine fibers. With the volatilization of solvent, the tissue engineering scaffold obtained by electrospinning has the characteristics of large surface area, high porosity, and adjustable mechanical properties, which can simulate the three-dimensional mechanical environment of the extracellular matrix to a large extent. It is beneficial to cell adhesion, invasion, and growth, and promotes tissue regeneration.35 Electrospinning has become one of the most common methods to manufacture artificial blood vessels.36–38Polyurethane elastomers are suitable as scaffolds for implantation in vivo because of their adjustable mechanical properties and excellent biocompatibility.39 But certain limitations are also associated such as low cell adhesion, lack of anticoagulation and antiplatelet deposition ability, and easy thrombosis in the body.33 Therefore, the rapid formation of endodermis after implantation has become the focus of current research.40
3.1 Chemical properties, microstructure and surface wettability of nanofibers
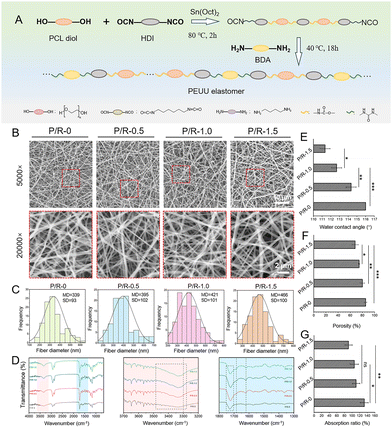
PEUU was successfully synthesized by the two-step method (Fig. 1A). The synthesis of PEUU was accomplished through the utilization of PCL diol as the flexible chain segment, HDI as the rigid chain segment, and putrescine serving as the agent for chain extension. To observe the morphological characteristics of the fibrous scaffold surface, SEM observation showed that the fibers of the four groups of nanofibers were uniformly distributed and arranged in a random cross-network structure, and no similar drug recrystallization was found, which proved that the composite spinning of the two solutions was feasible (Fig. 1B). Electrospinning nanofiber diameter is affected by many processing parameters, including applied voltage, flow rate, concentration of spinning solution, conductivity, and distance from syringe to collector. The process parameters of electrospinning are also mentioned in the ESI.† They are all carried out under relatively the same conditions, and the obtained nanofiber membrane is directly used after waiting for the solvent to completely volatilize, without any post-treatment process. The clipping carried out in the course of various experiments will not affect the overall loading rate of Res, so the loading rate of Res is 100% in theory. Therefore, we believe that the load rate of resveratrol and the consistency of resveratrol load between different samples can be guaranteed. According to the SEM images, the average diameters of P/R-0, P/R-0.5, P/R-1.0, and P/R-1.5 nanofibers were 339.48 ± 93.01 nm, 395.25 ± 102.25 nm, 420.98 ± 110.83 nm, and 465.51 ± 99.90 nm, respectively (Fig. 1C). The nanofiber diameter increased slightly with the increase in Res molecule concentration. It has been noted that higher drug concentrations have led to an increase in viscosity resistance in the mixed solution resulting in an inability to maintain flow at the tip of the Taylor cone.41 In turn, large-diameter fibers were produced. The possible reason is that with the increase in the mass of Res, the viscosity of the solution system slightly rises due to the increase in the solid content. Under the same electrospinning process conditions, the fiber is difficult to be fully stretched under the condition of high viscosity, so the fiber diameter increases.The incorporation of Res into the nanofiber was determined by FTIR analysis (Fig. 1D). The FTIR spectra of P/R nanofibers showed that the wide absorption peaks at 3500–3200 cm−1, 1680 cm−1, and 1610 cm−1 corresponded to O–H stretching of Res, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– stretching, and –C
C– stretching, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– stretching (aromatic), respectively. Moreover, an obvious absorption peak of the carbamate bond was observed at 1800–1700 cm−1, and a –N–H absorption peak appeared at 3300 cm−1 in the spectrum of the P/R-0 nanofiber, which proved the successful synthesis of PEUU.
C– stretching (aromatic), respectively. Moreover, an obvious absorption peak of the carbamate bond was observed at 1800–1700 cm−1, and a –N–H absorption peak appeared at 3300 cm−1 in the spectrum of the P/R-0 nanofiber, which proved the successful synthesis of PEUU.
The general rule is that the hydrophilic scaffold material is more conducive to cell adhesion, and the hydrophilic surface will make the cell form more stretched, thus healthier and faster growing.42,43 The measurement of dynamic water contact angle showed that the hydrophilicity of nanofibers changed to hydrophobicity with more Res added (Fig. 1E). Despite Res being classified as a hydrophobic substance, pertinent research has indicated that in scenarios where the hydrophilicity of the material remains relatively consistent, a decrease in the material's surface roughness correlates with a reduction in its contact angle.44,45 As mentioned above, with the increase in Res load, the fiber diameter increases, the grooves between the fibers become shallow and the roughness decreases, so the contact angle of the nanofibers decreases.
Porosity is also an important consideration for the effect of nanofiber scaffolds on tissue regeneration, because appropriate space is necessary for the invasive growth and proliferation of cells. In general, porosity should not be too low for invasive cell growth.46 Utilizing the solvent displacement method to ascertain porosity (Fig. 1F), it was observed that as the loading of Res incrementally increased, there was a corresponding decrease in the porosity of the nanofibers. The values of P/R-0, P/R-0.5, P/R-1.0, and P/R-1.5 are 85.30 ± 1.98%, 79.96 ± 3.60%, 74.55 ± 0.78%, and 67.96 ± 5.26%, respectively, which are also consistent with the results shown by SEM images and fiber diameter statistics above. The damaged part of the vascularized tissue is often infiltrated by a large amount of blood and exudate, which is not conducive to the long term repair of the tissue.47 It can be seen from the water contact angle that although the four groups of nanofibers are not hydrophilic when the nanofibers are fully infiltrated by water, the water absorption rates of P/R-0, P/R-0.5, P/R-1.0, and P/R-1.5 could still reach 132.09 ± 10.38%, 110.52 ± 9.87%, 105.83 ± 10.86%, and 90.43 ± 10.03%, respectively (Fig. 1G). These results indicate that the P/R nanofiber can absorb the exudate of the damaged tissue and create a suitable environment for tissue regeneration.
3.2 Mechanical properties
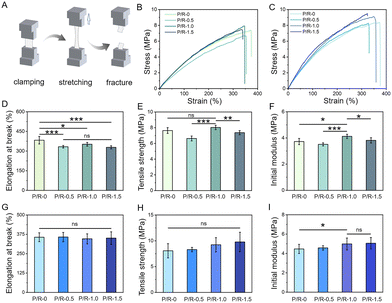
The mechanical properties of tissue engineering scaffolds are one of the important guarantees to ensure that fiber scaffolds can have their expected effects in the body. Without an appropriate mechanical support, scaffolds may collapse, tear, and degrade in the body, thus losing their biological role, leading to repair failure, and even triggering the body's inflammatory response and rejection reaction.48–51Fig. 2A shows the testing scheme of the mechanical properties of P/R nanofiber. After the fixtures equipped with mechanical terminals fix the two ends of the sample, they stretch in the opposite direction at a constant speed until they break. The optimum mechanical property of polyurethane elastomer is one of the reasons for choosing as scaffolds implanted materials. Studies report that rabbit carotid artery of the initial modulus range in 2.8–3.0 MPa.52Fig. 2B shows the representative tensile stress–strain curve of P/R-0, P/R-0.5, P/R-1.0, and P/R-1.5. As the concentration of Res mixed with PEUU increased, Res small molecules produced a certain hydrogen bond crosslinking network after mixing with PEUU, and its structure contained two benzene rings with a certain rigidity. Therefore, the relative displacement of the elongation at break of the scaffold is somewhat constrained due to the enhanced intermolecular connectivity facilitated by hydrogen bonding (Fig. 2D), resulting in an observed increment in both initial modulus and tensile strength (Fig. 2E and F).In addition, to simulate the environment in which the nanofibers fully infiltrated in the body, the nanofibers were soaked in PBS buffer and then taken out for mechanical testing in the wet state. As shown in Fig. 2C, the representative stress–strain curves of the four groups of nanofibers in a wet state are shown. Fig. 2G–I show the corresponding elongation at break, tensile strength, and initial modulus in the wet state, respectively. After water absorption, the expansion volume of the fibers increased, resulting in an increase in the contact area between single fibers and thus an increase in friction. Therefore, the tensile strength and initial modulus of the four groups of nanofibers after water absorption are higher than their dry state, indicating that the P/R nanofiber can still meet the needs of use in the in vivo environment.
3.3 Degradation and resveratrol sustained release properties in vitro
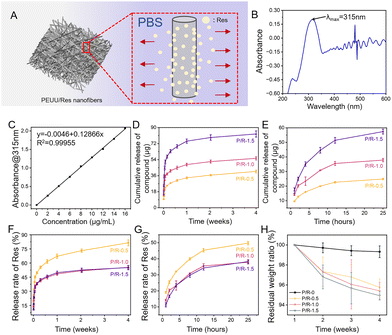
Fig. 3A shows the release diagram of Res from P/R nanofibers in PBS solution. According to UV-visible absorption spectrum analysis, the maximum absorption wavelength of Res is 315 nm (Fig. 3B). Then, the standard concentration curve of Res is drawn at 315 nm (Fig. 3C). The formula of the standard concentration curve obtained by the test is y = 0.12866x − 0.0046. R2 = 0.99955, and the linear relationship is highly satisfactory. Fig. 3D and E show the drug release of the three groups of P/R nanofibers loaded with Res. The effect of different Res loading content on its release from the nanofibers is obvious. The cumulative release rates of P/R-0.5, P/R-1.0, and P/R-1.5 in the fourth week were 81.43 ± 3.10%, 55.44 ± 1.83%, and 55.19 ± 2.31%, respectively (Fig. 3F). It can be seen that Res was released in large quantities within the first day, followed by slow release. The cumulative release rates of P/R-0.5, P/R-1.0, and P/R-1.5 in 25 h were 49.76 ± 1.07%, 37.87 ± 1.15%, and 38.26 ± 1.12%, respectively (Fig. 3G). Due to their rapid short-term release, it is assumed that P/R nanofiber can effectively inhibit short-term acute coagulation after implantation. The higher cumulative release rate of Res in P/R-0.5 than in the other two groups may be attributed to the fact that Res is mainly distributed on the fiber surface or in shallow layers and the fiber structure has a larger porosity allowing easier penetration of PBS. The P.R-1.0 and P/R-1.5 nanofibers, on the other hand, tended to have similar release rates and lower overall release rates due to the deep embedding of Res molecules, reduced porosity, and possible molecular interactions. Resveratrol release is closely related to fiber degradation, which in turn is influenced by synergistic effects of hydrophilicity, porosity, matrix material properties and three-dimensional structure. As the resveratrol content increased, the fiber diameter also increased. This may be due to the fact that resveratrol plays a certain plasticizing or viscosity-enhancing role in the spinning process, which makes the fibers more difficult to stretch during the forming process, resulting in the formation of thicker fibers. The increase in fiber diameter usually leads to a decrease in the void space between the fibers, which may be the reason for the decrease in porosity. The tensile strength of P/R nanofibers decreased with the increase in Res content, and the elongation at break increased slowly, which also proved that the increase in Res content may play a plasticizing role for the intermolecular PEUU, and reduce the intermolecular force of PEUU, thus reducing the water resistance and stability of the P/R nanofibers. Secondly, with the increase in resveratrol content, the contact angle of the fiber membrane gradually decreases, indicating that the hydrophilicity of the material surface increases. This may be due to the fact that the hydrophobicity of resveratrol is relatively low compared with that of PEUU, and the doping will reduce the hydrophobicity of the whole fiber membrane, which makes it easier to adsorb water, so it is more likely to be released from the material, and with the release of resveratrol in this process, micropores or cracks may appear on the surface of the material, which will make the material unstable and accelerate the degradation, and further enhance the release of resveratrol. The above reasons may have contributed to the result that the mass residual rate of P/R nanofibers shown in Fig. 3H decreased with the increase of Res addition.3.4 Antioxidation and hemocompatibility in vitro
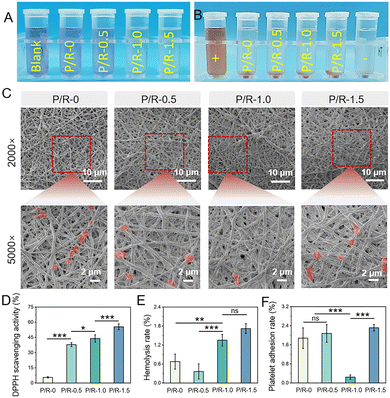
Overproduction of reactive oxygen species (ROS) is the main cause of intimal hyperplasia and eventually vascular restenosis.53,54 Therefore, it is necessary to evaluate the oxidation resistance of the materials. As a phenolic compound, Res has been widely studied and proved to have antioxidant effects such as scavenging or inhibiting the generation of free radicals, inhibiting lipid peroxidation,55 and regulating the activity of oxidation-related enzymes.56DPPH is a stable free radical with unpaired valence electrons on one atom of the nitrogen bridge.57 DPPH free radical scavenging is a common method for determining antioxidant activity. The antioxidant activity of four groups of nanofibers were evaluated by the DPPH clearance test. As shown in Fig. 4A, it was found that the antioxidant capacity of the nanofibers increased with the addition of Res, and the purple color of DPPH could hardly be observed at P/R-1.0 and P/R-1.5. The clearance rates of P/R-0 and P/R-0.5 were 43.98 ± 3.61% and 55.51 ± 2.87%, respectively, which showed obvious antioxidant activity increased from 5.58 ± 0.58% to 37.90 ± 1.95% (Fig. 4D).
In addition, as a blood contact material, it should not cause symptoms such as hemolysis, organ ischemia or hypoxia, nor should it activate platelets to make them gather in large numbers to form thrombosis, so the evaluation of blood compatibility is crucial.58 We performed a series of blood compatibility assessments on four groups of P/R. Fig. 4B shows the hemolysis test digital photograph of the material and the SEM image of platelets attached to the P/R nanofibers as shown in Fig. 4C. The hemolysis rates of P/R in the four groups were 0.36 ± 0.24% for P/R-0, 0.68 ± 0.24% for P/R-0.5, 1.35 ± 0.18% for P/R-1.0 and 1.72 ± 0.16% for P/R-1.5, respectively. By comparing with the ISO10993-4 standard, observations indicate that the hemolytic percentage associated with the P/R in each of the four groups significantly falls below the 5% threshold as per the established criterion (Fig. 4E). The platelet coverage on the nanofiber surface of each group is quantitatively analyzed in Fig. 4F. Analysis reveals that the platelet adhesion rate of P/R-1.0 was 0.25 ± 0.09%, which was far less than the rates of 1.88 ± 0.43%, 2.08 ± 0.37%, and 2.32 ± 0.13% for P/R-0, P/R-0.5 and P/R-1.5. Compared with the other three groups of nanofibers, P/R-1.0 has a good antithrombin production effect, greatly reducing the adhesion and activation of platelets, and has obvious anticoagulant potential. A large number of studies have shown that the inhibition of platelet adhesion becomes stronger with the increase in resveratrol concentration.59,60 However, the results of this study are ambiguous, and P/R-1.5 does not seem to show the inhibition of platelet adhesion. The possible reason is that during the platelet adhesion experiment, the co-culture time of platelets and P/R nanofibers was 2 h. Combined with the release results of Res in Fig. 3E, it can be seen that the release amounts of P/R-1.0 and P/R-1.5 Res in the first two hours were not very different and both were greater than P/R-0.5. Compared with P/R-1.5, the nanofiber diameter of P/R-1.0 nanofibers is smaller, and the P/R-1.0 nanofiber membrane has larger porosity, so it has a larger specific surface area. Under short-term conditions, more Res molecules will migrate from the pores to the surface of the nanofiber membrane and act on platelets, resulting in a local concentration of more than P/R-1.5 on the surface of the nanofiber membrane, so P/R-1.0 showed a better ability to inhibit platelet activation and adhesion.
3.5 Cytocompatibility
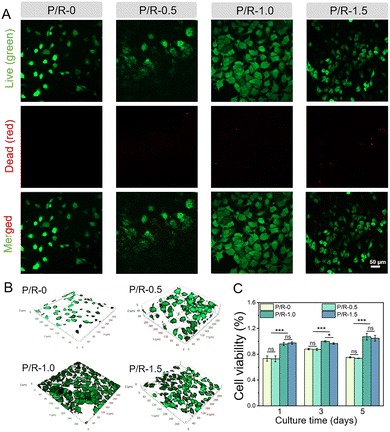
As a temporary substitute for damaged tissues, the nanofibers’ mechanical properties are pre-requisite. Apart from this, they are also very important for the adhesion and growth of cells. As a vascular graft, firstly, they do not have obvious cytotoxicity toward cells. Secondly, if the cells can be well attached to the surface of the graft for proliferation and growth, it plays a crucial role in the rapid in situ repair of damaged tissues.61–63Studies have shown that Res has a biphasic effect on angiogenesis, and plays an active role in promoting angiogenesis at lower concentrations, while excessive Res accumulation causes damage to vascular cells.26 HUVECs were co-cultured with the nanofibers to evaluate its cytocompatibility. As shown in Fig. 5A, after co-culture with a P/R nanofiber membrane for 5 days, the cell viability of HUVECs was evaluated by live/dead staining. It is evident from the data that the proliferation effect of cells on the nanofiber membrane P/R-1.0 and P/R-1.5 is better than that of P/R-0 and P/R-0.5, and it can be seen that P/R-0, P/R-0.5 and P/R-1.0 have no obvious toxicity toward cells, while a few dead cells (red) are observed on the nanofiber membrane P/R-1.5. At the same time, combining with the three-dimensional reconstruction images of laser confocal microscopy (Fig. 5B), it can be seen that the HUVECs coverage area on the P/R-1.0 nanofiber membrane is the largest, and monolayer endothelial structures have been formed, and the endothelization degree is the best. Meanwhile, the results of CCK-8 were analyzed (Fig. 5C). There was no difference between P/R-0 and P/R-0.5 in promoting the proliferation of HUVECs. HUVECs co-cultured in P/R-1.0 and P/R-1.5 groups all prolificated gradually with time, and there was no significant difference on the 1st and 5th day, while the survival rate of P/R-1.0 cells was higher than that of P/R-1.5 cells at 3 d. The cell viability of the two P/R culture groups with high Res content was considerably higher than that of the two P/R culture groups with low Res content. Although the P/R-1.0 and P/R-1.5 groups did not exhibit a notable difference in terms of cell survival rates, the growth of HUVECs on different nanofibers obtained by laser confocal microscopy layer scanning on the 5th day of co-culture showed the most extended HUVEC morphology on the P/R-1.0 nanofibers. However, the cells on the P/R-1.5 nanofiber membrane tended to curl up and aggregate. These results suggest that, as in many previous studies, increasing the concentration of Res may not always be beneficial for the proliferation and growth of HUVECs and the degree of endothelialization.
3.6 Cell migration assay
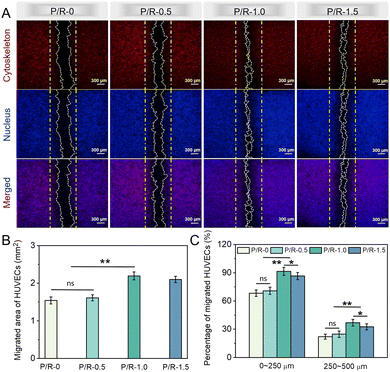
Cell migration, a fundamental cellular process, is integral to a spectrum of physiological functions, including tissue regeneration, embryonic development, repair of wounds, immune system reactions, and the metastatic spread of cancer, with particular significance in the context of vascular tissue regeneration.64–67 To a large extent, the enduring patency rate of synthetic conduits with a small caliber, designed to emulate blood vessels, remains a significant concern. It depends on whether the endothelial cells on the side of the autologous blood vessel can quickly migrate to the suture site of the artificial blood vessel and form the inner cortex in the middle of the artificial blood vessel.68,69Fluorescent staining images of HUVECs cultured on P/R-0, P/R-0.5, P/R-1.0 and P/R-1.5 for 48 h are presented in Fig. 6A and Fig. S1 (ESI†). The experimental findings indicated that, under the conditions associated with the P/R-1.0 formulation, the migration of HUVECs towards the central migration zone from opposing sides was superior to that observed in the remaining trio of groups. Furthermore, within the migration zone, the morphology of HUVECs exhibited distinct pseudopodia and a spindle-like form. In contrast, a majority of HUVECs in the non-migration zone maintained their initial round configuration, characteristic of the early developmental phase of cellular growth. We also performed quantitative analysis on the migratory ability of HUVECs across the aforementioned four samples. In assessing cellular migration capacity, we focused our quantitative analysis on two parameters: the cell area within the migration zone, as illustrated in Fig. 6B, and the proportion of cells exhibiting varying migration distances relative to the entire population of migratory cells, as depicted in Fig. 6C. As delineated by the quantitative data presented in Fig. 6C, the P/R-1.0 group demonstrated the highest prevalence within the migration distance categories of 0–250 μm and 250–500 μm. This finding corroborates the superior migratory capacity of the HUVECs in this group, specifically highlighting the chemotactic allure of the P/R-1.0 in attracting cells. In conclusion, cellular growth and expansion are orchestrated processes that encompass cell adhesion, migration, and proliferation. These processes are directed towards establishing a rapid in situ cellular presence on the material surface, thereby facilitating effective tissue regeneration and structural formation.
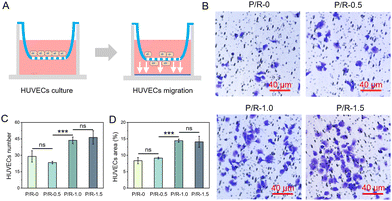
The transwell cell migration experiment seeded HUVECs into the upper chamber of the transwell. The migration effect of the cells from the upper chamber to the lower chamber was observed by using fibrous membrane soaking medium for 30 min (Fig. 7A). The transwell cell migration experiment was conducted to evaluate the induced migration effect of P/R nanofibers on HUVECs, which was used to show whether P/R nanofibers can promote the rapid migration of HUVECs to form the inner cortex and the bridging of the vascular suture connecting artificial blood vessels and autologous blood vessels during vascular regeneration. We can observe representative images of cells migrating from the upper chamber of the transwell to the lower surface of the transwell through light microscopy (Fig. 7B). Three fields were randomly selected in each group, and the number of HUVECs in the P/R-1.0 and P/R-1.5 nanofibers were 43.7 ± 3.1 and 46.3 ± 6.1, respectively, which showed no significant difference but were much higher than the 29 ± 5.2 and 23.3 ± 1.2 cells in P/R-0 and P/R-0.5 (Fig. 7C). The cell coverage of HUVECs was also calculated as 8.36 ± 0.96% for P/R-0, 9.16 ± 0.28% for P/R-0.5, 14.45 ± 0.48% for P/R-1.0, and 14.11 ± 1.69% for P/R-1.5, respectively (Fig. 7D). The results showed that P/R nanofiber with Res loading of 1 wt% and 1.5 wt% had a positive effect on cell migration.
Combined with the results of Fig. 3E, it can be seen that when the P/R nanofibers release Res for 1 hour, there is little difference in the release amount of P/R-1.0 and P/R-1.5 Res, and both are greater than P/R-0.5. The possible reason is that compared with P/R-1.5, P/R-1.0 has a finer nanofiber diameter and larger porosity and specific surface area. Coupled with the hydrophobic property of the PEUU material itself, P/R-1.5 is not fully infiltrated by the medium in a short period of time, and Res molecules with the same mass as P/R-1.0 are released. There was no significant difference in the ability of P/R-1.0 and P/R-1.5 to induce vertical cell migration. When HUVECs are co-cultured with P/R nanofibers as shown in the horizontal migration results mentioned above, HUVECs on P/R-1.0 perform best. Different from the transwell migration experiment, HUVECs are in direct contact with P/R nanofibers in the horizontal migration experiment, and Res on the surface of P/R nanofibers can directly affect HUVECs. It can be inferred that P/R-1.0 nanofibers may be more favorable to the migration of HUVECs.
3.7 In vitro tube formation of HUVECs
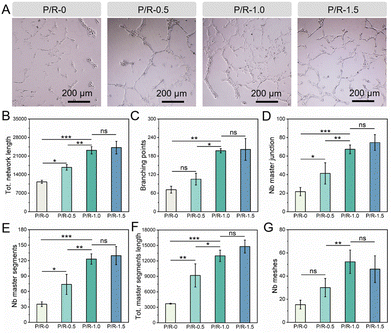
The formation of the capillary-like structure of endothelial cells in vitro involves the steps of cell adhesion, migration, arrangement, protease secretion, and tube formation, which is of great reference value for evaluating the vascularization ability of active molecules.70–72 The activity of Res on angiogenesis was measured by an in vitro tube formation experiment, and the total network length, branching points, number of meshes, master segments, and master junctions and the total master segment length were counted. The longer the total network length, the stronger the angiogenesis activity, and branching points refer to the nodes where new branches of the vascular network form. The number of branching points can reflect the complexity and maturity of the vascular network. In the process of angiogenesis, the formation of branching points is a key step in the expansion of the vascular network. The number of meshes refers to the number of individual grids, or loops, formed in the network of blood vessels. This index can reflect the connectivity and stability of the vascular network. Master segments refer to the vascular segments that form the backbone of the vascular network. This index can reflect the main structural and functional parts of the vascular network. Master junctions refer to branching points that play major roles in the blood vessel network; these points play a key role in the formation and expansion of the network. Total master segment length refers to the total length of the vascular segments that form the backbone of the vascular network. This index can reflect the development degree of the main blood vessels and the main blood vessel supply capacity of the network. As shown in Fig. 8A, the influence of four groups of nanofibers on the formation of tubes in HUVECs can be directly seen. It can be seen that under the condition of P/R-0 culture, HUVECs are almost all spherical, scattered and locally clustered, and can hardly form a continuous grid connected to each other. HUVECs are connected on P/R-0.5, forming a small number of dendritic structures. In contrast, P/R-1.0 and P/R-1.5 obviously observed that HUVECs were in a good growth state, and the cell morphology was extended in long strips with obvious pseudopods, and the cells formed tubes connected to each other, and finally formed an impressive grid-like structure. Then, the image was further quantitatively analyzed, as shown in Fig. 8B–G. There was no significant difference between P/R-1.0 and P/R-1.5 in the total network length, branching points, number of meshes, master segments, master junctions and the total master segment length, but both were much higher than the other two groups. The master segments, master junctions and the total master segment length of P/R-0.5 are higher than those of P/R-0. Therefore, it can be preliminarily concluded that a small amount of Res loading is beneficial for tube formation, and P/R-1.0 and P/R-1.5 may be the most beneficial for tube formation in HUVECs.4. Conclusions
In this study, a series of P/R nanofibers loaded with resveratrol (Res) were prepared by dual-solution electrospinning for enhancing the bioactivity of vascular endothelial cells and improving blood compatibility. The mechanical properties of P/R nanofiber in both dry and wet conditions were matched with the implantation requirements. The anticoagulation and anti-oxidation effects of P/R nanofibers increase with the increase of Res content. Moreover, P/R nanofibers with 1 wt% loading capacity had the most significant effect on promoting endothelial cell activity, and endothelial cells could form a continuous single layer of endodermal structure on the surface of the nanofibers. Combined with the results of the platelet adhesion experiment, it indicated that the P/R nanofiber presented a continuous and effective anticoagulation effect. In conclusion, these multifunctional hybrid poly(ester-urethane)urea/resveratrol electrospun nanofibers (P/R-1.0) may be a potential candidate for potential vascularizing matrices.Author contributions
Cheng Liang: writing – original draft; data curation; validation; visualization; formal analysis. Yanan Wang: visualization; investigation; visualization. Renliang Zhao: visualization; investigation; visualization. Juan Du: resources; investigation. Jin Yao: visualization; formal analysis. Atta ur Rehman Khan: writing – review & editing. Tonghe Zhu: conceptualization; supervision; project administration; methodology; writing – review & editing; formal analysis. Huitang Xia: resources; investigation. Youwei Zhu: funding acquisition.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
All authors declare no conflict of interest.Acknowledgements
This work was financially supported by the Science and Technology Commission of Shanghai Municipality (22S31904700), the National Natural Science Foundation of China (82303265), Shanghai Sailing Program (22YF1426200), and Shanghai College Students Innovation Training Project (202410856019).Notes and references
- L. Liberale, L. Badimon, F. Montecucco, T. F. Lüscher, P. Libby and G. G. Camici, Inflammation, aging, and cardiovascular disease: JACC review topic of the week, J. Am. Coll. Cardiol., 2022, 79, 837–847 CrossRef CAS.
- H. O. Ventura, A. Elagizi and C. J. Lavie, Optimal prevention of cardiovascular diseases: The earlier the better, J. Am. Coll. Cardiol., 2023, 81, 1162–1164 CrossRef PubMed.
- W. C. Poller, M. Nahrendorf and F. K. Swirski, Hematopoiesis and cardiovascular disease, Circ. Res., 2020, 126, 1061–1085 CrossRef CAS.
- K. Park, S. An, J. Kim, S. Yoon, J. Song, D. Jung, J. Park, Y. Lee, D. Son and J. Seo, Resealable antithrombotic artificial vascular graft integrated with a self-healing blood flow sensor, ACS Nano, 2023, 17, 7296–7310 CrossRef CAS.
- T. Schweizer, S. M. Shambat, V. D. Haunreiter, C. Mestres, A. Weber, F. Maisano, A. Zinkernagel and B. Hasse, Polyester vascular graft material and risk for intracavitary thoracic vascular graft infection, Emerging Infect. Dis., 2020, 26, 2448 CrossRef CAS.
- Z. Xing, S. T. Wu, C. Zhao, Y. T. Bai, D. W. Jin, M. Yin, H. F. Liu and Y. B. Fan, Vascular transplantation with dual-biofunctional ePTFE vascular grafts in a porcine model, J. Mater. Chem. B, 2021, 9, 7409–7422 RSC.
- H. X. Su, W. C. Liu, X. F. Li, G. X. Li, S. Q. Guo, C. Liu, T. Yang, C. B. Ou, J. H. Liu, Y. Z. Li, C. C. Wei, Q. Huang, T. Xu and C. Z. Duan, Cellular energy supply for promoting vascular remodeling of small-diameter vascular grafts: a preliminary study of a new strategy for vascular graft development, Biomater. Sci., 2023, 11, 3197–3213 RSC.
- K. R. Adhikari, J. Zimmerman, P. S. Dimble, B. S. Tucker and V. Thomas, Kink-free electrospun PET/PU-based vascular grafts with 3D-printed additive manufacturing reinforcement, J. Mater. Res., 2021, 3, 4013–4023 CrossRef.
- P. Zhao, Q. Fang, D. S. Gao, Q. Wang, Y. B. Cheng, Q. Ao, X. H. Wang, X. H. Tian, Y. H. Zhang, H. Tong, N. Yan, X. K. Hu and J. Fan, Klotho functionalization on vascular graft for improved patency and endothelialization, Biomater. Adv., 2022, 133, 112630 CrossRef CAS.
- L. Yu, E. R. Newton, D. C. Gillis, K. Sun, B. C. Cooley, A. N. Keith, S. S. Sheiko, N. D. Tsihlis and M. R. Kibbe, Coating small-diameter ePTFE vascular grafts with tunable poly(diol-co-citrate-co-ascorbate) elastomers to reduce neointimal hyperplasia, Biomater. Sci., 2021, 9, 5160–5174 RSC.
- W. Y. Jin, H. B. Liu, Z. H. Li, P. Nie, G. X. Zhao, X. Cheng, G. M. Zheng and X. H. Yang, Effect of hydrogel contact angle on wall thickness of artificial blood vessel, Int. J. Mol. Sci., 2022, 23, 11114 CrossRef CAS PubMed.
- B. C. Yi, Y. B. Shen, H. Tang, X. L. Wang, B. Li and Y. H. Zhang, Stiffness of aligned fibers regulates the phenotypic expression of vascular smooth muscle cells, ACS Appl. Mater. Interfaces, 2019, 11, 6867–6880 CrossRef CAS.
- S. Zia, A. Djalali-Cuevas, M. Pflaum, J. Hegermann, D. Dipresa, P. Kalozoumis, A. Kouvaka, K. Burgwitz, S. Andriopoulou, A. Repanas, F. Will, K. Grote, C. Schrimpf, S. Toumpaniari, M. Mueller, B. Glasmacher, A. Haverich, L. Morticelli and S. Korossis, Development of a dual-component infection-resistant arterial replacement for small-caliber reconstructions: A proof-of-concept study, Front. Bioeng. Biotechnol., 2023, 11, 957458 CrossRef PubMed.
- Z. P. Fang, Y. H. Xiao, X. Geng, L. J. Jia, Y. H. Xing, L. Ye, Y. Q. Gu, A. Y. Zhang and Z. G. Feng, Fabrication of heparinized small diameter TPU/PCL bi-layered artificial blood vessels and in vivo assessment in a rabbit carotid artery replacement model, Biomater. Adv., 2022, 133, 112628 CrossRef CAS.
- S. Masumoto, A. Ono, A. Ito, Y. Kawabe and M. Kamihira, Hypoxia-responsive expression of vascular endothelial growth factor for induction of angiogenesis in artificial three-dimensional tissues, J. Biosci. Bioeng., 2021, 132, 399–407 CrossRef CAS.
- Y. M. Li, Y. Zhou, W. H. Qiao, J. W. Shi, X. F. Qiu and N. G. Dong, Application of decellularized vascular matrix in small-diameter vascular grafts, Front. Bioeng. Biotechnol., 2023, 10, 1081233 CrossRef PubMed.
- Q. W. Zhang, S. Duncan, D. A. Szulc, C. Mestral and M. J. Kutryk, Development of a universal, oriented antibody immobilization method to functionalize vascular prostheses for enhanced endothelialization for potential clinical application, J. Biol. Eng., 2023, 17, 37 CrossRef CAS PubMed.
- L. J. Wang, C. E. Miao, F. B. Liang, Y. S. Shang, Y. Sun, J. Zhang, J. Shen, M. Yin and J. Yuan, Hydrogen sulfide releasing and carboxybetaine modified vascular graft with enhanced anticoagulant, anticalcification, and pro-endothelialization properties, Appl. Mater. Today, 2023, 35, 101976 CrossRef.
- I. Łopianiak, W. Rzempołuch, M. Civelek, I. Cicha, T. Ciach and B. A. Butruk-Raszeja, Multilayered blow-spun vascular prostheses with luminal surfaces in Nano/Micro range: the influence on endothelial cell and platelet adhesion, J. Biol. Eng., 2023, 17, 20 CrossRef.
- M. Hu, F. Jia, W. P. Huang, X. Li, D. F. Hu, J. Wang, K. F. Ren, G. S. Fu, Y. B. Wang and J. Ji, Substrate stiffness differentially impacts autophagy of endothelial cells and smooth muscle cells, Bioact. Mater., 2021, 6, 1413–1422 CAS.
- H. Alkazemi, T. Huang, M. Mail, Z. Lokmic-Tomkins, D. E. Heath and A. J. O’Connor, Spontaneous orthogonal alignment of smooth muscle cells and endothelial cells captures native blood vessel morphology in tissue-engineered vascular grafts, ACS Appl. Mater. Interfaces, 2023, 15, 34631–34641 CrossRef CAS.
- L. Pocivavsek, S. H. Ye, J. Pugar, E. Tzeng, E. Cerda, S. Velankar and W. R. Wagner, Active wrinkles to drive self-cleaning: A strategy for anti-thrombotic surfaces for vascular grafts, Biomaterials, 2019, 192, 226–234 CrossRef CAS.
- X. H. Xie, Q. Y. Wu, Y. H. Liu, C. Y. Chen, Z. G. Chen, C. Xie, M. Z. Song, Z. L. Jiang, X. K. Qi, S. X. Liu, Z. J. Tang and Z. S. Wu, Vascular endothelial growth factor attenuates neointimal hyperplasia of decellularized small-diameter vascular grafts by modulating the local inflammatory response, Front. Bioeng. Biotechnol., 2022, 10, 1066266 CrossRef PubMed.
- B. Sun, Y. L. Zheng, S. K. Yang, J. R. Zhang, X. Y. Cheng, R. Ghiladi, Z. Ma, J. Wang and W. W. Deng, One-pot method based on deep eutectic solvent for extraction and conversion of polydatin to resveratrol from polygonum cuspidatum, Food Chem., 2021, 343, 128498 CrossRef CAS PubMed.
- Z. H. Wang, Y. F. Wu, J. N. Wang, C. N. Zhang, H. Y. Yan, M. F. Zhu, K. Wang, C. Li, Q. B. Xu and D. L. Kong, Effect of resveratrol on modulation of endothelial cells and macrophages for rapid vascular regeneration from electrospun poly(ε-caprolactone) scaffolds, ACS Appl. Mater. Interfaces, 2017, 9, 19541–19551 CrossRef CAS.
- H. Wang, H. B. Zhou, Y. X. Zou, Q. Liu, C. H. Guo, G. M. Gao, C. S. Shao and Y. Q. Gong, Resveratrol modulates angiogenesis through the GSK3β/β-catenin/TCF-dependent pathway in human endothelial cells, Biochem. Pharmacol., 2010, 8, 1386–1395 CrossRef PubMed.
- F. D. Oliveira Júnior and R. L. Cunha, Soy protein-based delivery systems as carriers of trans-resveratrol: Bioaccessibility using different in vitro digestion models, Food Res. Int., 2022, 161, 111837 CrossRef.
- Y. Zhu, B. Feng, S. M. He, Z. Q. Su and G. Zheng, Resveratrol combined with total flavones of hawthorn alleviate the endothelial cells injury after coronary bypass graft surgery, Phytomedicine, 2018, 40, 20–26 CrossRef CAS.
- R. Karimi-Soflou, E. Mohseni-Vadeghani and A. Karkhaneh, Controlled release of resveratrol from a composite nanofibrous scaffold: Effect of resveratrol on antioxidant activity and osteogenic differentiation, J. Biomed. Mater. Res., Part A, 2022, 110, 21–30 CrossRef CAS PubMed.
- N. Zhang, C. B. Zhang, J. M. Liu, C. Z. Fan, J. J. Yin and T. Wu, An oral hydrogel carrier for delivering resveratrol into intestine-specific target released with high encapsulation efficiency and loading capacity based on structure-selected alginate and pectin, Food Funct., 2022, 13, 12051–12066 RSC.
- M. Tahir, S. Vicini and A. Sionkowska, Electrospun Materials Based on Polymer and Biopolymer Blends – A Review, Polymers, 2023, 15, 1654 CrossRef CAS PubMed.
- J. J. Zhu, D. Chen, J. Du, X. X. Chen, J. H. Wang, H. M. Zhang, S. H. Chen, J. L. Wu, T. H. Zhu and X. M. Mo, Mechanical matching nanofibrous vascular scaffold with effective anticoagulation for vascular tissue engineering, Composites, Part B, 2020, 186, 107788 CrossRef CAS.
- T. H. Zhu, H. B. Gu, H. M. Zhang, H. S. Wang, H. T. Xia, X. M. Mo and J. L. Wu, Covalent grafting of PEG and heparin improves biological performance of electrospun vascular grafts for carotid artery replacement, Acta Biomater., 2021, 119, 211–224 CrossRef CAS.
- T. H. Zhu, K. Yu, M. A. Bhutto, X. R. Guo, W. Shen, J. Wang, W. M. Chen, H. El-Hamshary, S. S. Al-Deyab and X. M. Mo, Synthesis of RGD-peptide modified poly(ester-urethane) urea electrospun nanofibers as a potential application for vascular tissue engineering, Chem. Eng. J., 2017, 315, 177–190 CrossRef CAS.
- D. F. Wang, Y. Y. Xu, Q. Li and L. S. Turng, Artificial small-diameter blood vessels: materials, fabrication, surface modification, mechanical properties, and bioactive functionalities, J. Mater. Chem. B, 2020, 8, 1801–1822 RSC.
- X. Y. Xu, Y. F. Si, Y. Zhao, Q. F. Ke and J. L. Hu, Electrospun textile strategies in tendon to bone junction reconstruction, Adv. Fiber Mater., 2023, 5, 764–790 CrossRef.
- W. Pi, Y. L. Zhang, L. F. Li, C. Li, M. Zhang, W. Zhang, Q. Cai and P. X. Zhang, Polydopamine-coated polycaprolactone/carbon nanotube fibrous scaffolds loaded with brain-derived neurotrophic factor for peripheral nerve regeneration, Biofabrication, 2022, 14, 035006 CrossRef CAS PubMed.
- M. B. Stie, K. Kalouta, C. F. B. Cunha, H. M. Feroze, V. Vetri and V. Foderà, Sustainable strategies for waterborne electrospinning of biocompatible nanofibers based on soy protein isolate, Sustainable Mater. Technol., 2022, 34, e00519 CrossRef CAS.
- M. Kitsara, P. Joanne, S. E. Boitard, I. B. Dhiab, B. Poinard, P. Menasché, C. Gagnieu, P. Forest, O. Agbulut and Y. Chen, Fabrication of cardiac patch by using electrospun collagen fibers, Microelectron. Eng., 2015, 144, 46–50 CrossRef CAS.
- J. Y. Zhou, M. Y. Wang, T. T. Wei, L. C. Bai, J. Zhao, K. Wang and Y. K. Feng, Endothelial cell-mediated gene delivery for in situ accelerated endothelialization of a vascular graft, ACS Appl. Mater. Interfaces, 2021, 13, 16097–16105 CrossRef CAS.
- M. A. Wsoo, S. I. A. Razak, S. P. M. Bohari, S. Shahir, R. Salihu, M. R. A. Kadir and N. H. M. Nayan, Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies, Int. J. Biol. Macromol., 2021, 181, 82–98 CrossRef CAS.
- Y. C. Jiang, Y. Y. Guo, H. N. Wang, X. F. Wang and Q. Li, Hydrogel coating based on dopamine-modified hyaluronic acid and gelatin with spatiotemporal drug release capacity for quick endothelialization and long-term anticoagulation, Int. J. Biol. Macromol., 2023, 230, 123113 CrossRef CAS PubMed.
- G. Q. Hu, G. L. Li, L. Chen and F. F. Hong, Production of novel elastic bacterial nanocellulose/polyvinyl alcohol conduits via mercerization and phase separation for small-caliber vascular grafts application, Int. J. Biol. Macromol., 2023, 239, 124221 CrossRef CAS PubMed.
- V. Edachery, R. Shashank and S. V. Kailas, Influence of surface texture directionality and roughness on wettability, sliding angle, contact angle hysteresis, and lubricant entrapment capability, Tribol. Int., 2021, 158, 106932 CrossRef.
- M. M. Amrei, M. Davoudi, G. G. Chase and H. V. Tafreshi, Effects of roughness on droplet apparent contact angles on a fiber, Sep. Purif. Technol., 2017, 180, 107–113 CrossRef CAS.
- Q. C. Zhang, S. He, X. B. Zhu, H. L. Luo, M. Gama, M. X. Peng, X. Y. Deng and Y. Z. Wan, Heparinization and hybridization of electrospun tubular graft for improved endothelialization and anticoagulation, Mater. Sci. Eng., C, 2021, 122, 111861 CrossRef CAS PubMed.
- J. Shi, X. Y. Zhang, L. Jiang, L. Zhang, Y. S. Dong, A. C. Midgley, D. L. Kong and S. F. Wang, Regulation of the inflammatory response by vascular grafts modified with Aspirin-Triggered Resolvin D1 promotes blood vessel regeneration, Acta Biomater., 2019, 97, 360–373 CrossRef CAS.
- H. L. Xu, K. J. Liu, Y. M. Su, Z. J. Liu, Y. Wei, Y. C. Hu, L. Q. Zhao, L. F. Chen, X. J. Lian and D. Huang, Rapid formation of ultrahigh strength vascular graft: Prolonging clotting time micro-dimension hollow vessels with interpenetrating polymer networks, Composites, Part B, 2023, 250, 110456 CrossRef CAS.
- C. X. Liu, J. Y. Dai, X. Q. Wang and X. Y. Hu, The Influence of Textile Structure Characteristics on the Performance of Artificial Blood Vessels, Polymers, 2023, 15, 3003 CrossRef CAS PubMed.
- Y. Yao, G. Pohan, M. F. A. Cutiongco, Y. Jeong, J. Kunihiro, A. M. Zaw, D. David, H. Shangguan, A. C. H. Yu and E. K. F. Yim, In vivo evaluation of compliance mismatch on intimal hyperplasia formation in small diameter vascular grafts, Biomater. Sci., 2023, 11, 3297–3307 RSC.
- A. Weekes, G. Wehr, N. Pinto, J. Jenkins, Z. Y. Li, C. Meinert and T. J. Klein, Highly compliant biomimetic scaffolds for small diameter tissue-engineered vascular grafts (TEVGs) produced via melt electrowriting (MEW), Biofabrication, 2023, 16, 015017 CrossRef.
- D. W. Jin, J. F. Hu, D. K. Xia, Al Liu, H. Z. Kuang, J. Du, X. M. Mo and M. Yin, Evaluation of a simple off-the-shelf bi-layered vascular scaffold based on poly(L-lactide-co-ε-caprolactone)/silk fibroin in vitro and in vivo, Int. J. Nanomed., 2019, 14, 4261–4276 CrossRef CAS PubMed.
- L. Wang, T. Yu, H. Lee, D. K. O'Brien, H. Sesaki and Y. Yoon, Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia, Cardiovasc. Res., 2015, 106, 272–283 CrossRef CAS.
- F. Yang, G. Guo and Y. Wang, Inflammation-directed nanozyme-eluting hydrogel coating promotes vascular tissue repair by restoring reactive oxygen species homeostasis, Chem. Eng. J., 2023, 454, 140556 CrossRef CAS.
- M. D. Turner, B. Nedjai, T. Hurst and D. J. Pennington, Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease, Biochim. Biophys. Acta, 2014, 184, 2563–2582 CrossRef.
- N. Xia, A. Daiber, U. Förstermann and H. Li, Antioxidant effects of resveratrol in the cardiovascular system, Br. J. Pharmacol., 2017, 17, 1633–1646 CrossRef PubMed.
- İ. Gulcin and S. H. Alwasel, DPPH Radical Scavenging Assay, Processes, 2023, 11, 2248 CrossRef.
- M. Marumo, K. Ekawa and I. Wakabayashi, Resveratrol inhibits Ca2+ signals and aggregation of platelets, Environ. Health Prev. Med., 2020, 25, 70 CrossRef CAS PubMed.
- L. L. Katie, A. R. Majed, K. T. Sara, N. M. Craig, B. Neil, P. P. Richard and L. S. Sherry, Resveratrol preserves the function of human platelets stored for transfusion, Br. J. Haematol., 2015, 172, 794–806 Search PubMed.
- J. Zhao, L. C. Bai, X. K. Ren, J. T. Guo, S. H. Xia, W. C. Zhang and Y. K. Feng, Co-immobilization of ACH11 antithrombotic peptide and CAG cell-adhesive peptide onto vascular grafts for improved hemocompatibility and endothelialization, Acta Biomater., 2019, 97, 344–359 CrossRef CAS PubMed.
- Z. W. Hao, T. H. Chen, Y. Wang, Q. Y. Feng, J. Y. Chen, H. K. Li, J. W. Wang, Z. P. Wang, Z. Y. Zhang, R. X. Chen, G. Shi, Z. W. Zou, L. Cai, T. H. Zhu and J. F. Li, Self-Assembling Peptide Nanofibers Anchored Parathyroid Hormone Derivative for Bone Tissue Engineering, Adv. Fiber Mater., 2024, 6, 583–606 CrossRef CAS.
- L. V. Antonova, V. N. Silnikov, V. V. Sevostyanova, A. E. Yuzhalin, L. S. Koroleva, E. A. Velikanova, A. V. Mironov, T. S. Godovikova, A. G. Kutikhin, T. V. Glushkova, I. Y. Serpokrylova, E. A. Senokosova, V. G. Matveeva, M. Y. Khanova, T. N. Akentyeva, E. O. Krivkina, Y. A. Kudryavtseva and L. S. Barbarash, Biocompatibility of small-diameter vascular grafts in different modes of RGD modification, Polymers, 2019, 11, 174 CrossRef PubMed.
- Z. T. Li, J. Giarto, J. Zhang, N. Kulkarni, E. Mahalingam, W. Klipstine and L. S. Turng, Anti-thrombotic poly(AAm-co-NaAMPS)-xanthan hydrogel-expanded polytetrafluoroethylene (ePTFE) vascular grafts with enhanced endothelialization and hemocompatibility properties, Biomater. Adv., 2023, 154, 213625 CrossRef CAS PubMed.
- S. J. Atkinson, T. S. Ellison, V. Steri, E. Gould and S. D. Robinson, Redefining the role(s) of endothelial αvβ3-integrin in angiogenesis, Biochem. Soc. Trans., 2014, 42, 1590–1595 CrossRef CAS PubMed.
- X. Zhao and J. L. Guan, Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis, Adv. Drug Delivery Rev., 2011, 63, 610–615 CrossRef CAS PubMed.
- A. Arora, A. M. Kivelä, L. Wang, R. Minkeviciene, J. H. Taskinen, B. Zhang, A. Koponen, J. Sun, M. Shirane, Y. Zhou, P. Hotulainen, C. Raiborg and V. M. Olkkonen, Protrudin regulates FAK activation, endothelial cell migration and angiogenesis, Cell. Mol. Life Sci., 2022, 79, 220 CrossRef CAS PubMed.
- X. Liu, Q. Wei, Z. J. Sun, S. N. Cui, X. Z. Wan, Z. Q. Chu, Y. K. Zhang, W. C. Zhong, L. Lu, L. X. Shi, X. B. Fu, C. P. Zhang and S. T. Wang, Small extracellular vesicles: Yields, functionalization and applications in diabetic wound management, Interdiscip. Med., 2023, 1, e20230019 CrossRef.
- Y. H. Liu, L. R. Wang, Z. Liu, Y. H. Kang, T. H. Chen, C. Xu and T. H. Zhu, Durable immunomodulatory nanofiber niche for the functional remodeling of cardiovascular tissue, ACS Nano, 2024, 18, 951–971 CrossRef CAS PubMed.
- W. X. Ma, Z. Liu, T. H. Zhu, L. M. Wang, J. Du, K. Wang and C. Xu, Fabric-enhanced vascular graft with hierarchical structure for promoting the regeneration of vascular tissue, Adv. Healthcare Mater., 2024, 2302676 CrossRef CAS.
- D. Wa, L. Ren, M. H. Hamblin and Y. Fan, Endothelial cell glucose metabolism and angiogenesis, Biomedicines, 2021, 9, 147 CrossRef.
- M. D. Palma, D. Biziato and T. V. Petrova, Microenvironmental regulation of tumour angiogenesis, Nat. Rev. Cancer, 2017, 17, 457–474 CrossRef PubMed.
- X. Li, X. D. Sun and P. Carmeliet, Hallmarks of endothelial cell metabolism in health and disease, Cell Metab., 2019, 30, 414–433 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm00937a |
| ‡ Co-first authors. |
| This journal is © The Royal Society of Chemistry 2025 |