 Open Access Article
Open Access ArticleSustainable seawater electrolysis: evaluating environmental impacts and technological development opportunities
Monireh
Khosravi
 a,
Haylee
Thomas
b,
Luk
Peeters
c,
Kate
Holland
c,
Tara
Hosseini
d,
Lachlan
Carter
e,
Bita
Bayatsarmadi
a,
Haylee
Thomas
b,
Luk
Peeters
c,
Kate
Holland
c,
Tara
Hosseini
d,
Lachlan
Carter
e,
Bita
Bayatsarmadi
 *a and
Yuyang
Hou
*a and
Yuyang
Hou
 *a
*a
aCSIRO Mineral Resources, Clayton, VIC 3168, Australia. E-mail: bita.bayatsarmadi@csiro.au; yuyang.hou@csiro.au
bCSIRO Environment, Waterford, WA 6152, Australia
cCSIRO Environment, Urrbrae, SA 5064, Australia
dCSIRO Energy, Clayton, VIC 3168, Australia
eCSIRO Energy, Pullenvale, QLD 4069, Australia
First published on 10th July 2025
Abstract
As the global energy landscape shifts towards sustainable development, seawater electrolysis is increasingly recognised as a promising technology for green hydrogen production. This approach utilises the abundant availability of seawater, presenting a more environmentally friendly alternative to conventional hydrogen generation techniques. Despite its promise, seawater electrolysis faces significant challenges in energy efficiency, catalyst performance, and the management of undesirable by-products. This review provides a comprehensive analysis of recent advancements in seawater electrolysis, with particular emphasis on its environmental impacts and the technological opportunities it presents. The challenges of seawater electrolysis are discussed, with issues related to electrodes, electrolytes and membranes being highlighted. Furthermore, recent solutions and technological developments aimed at addressing these challenges are outlined. Finally, the current state of seawater electrolysis is summarised, and its prospects are discussed, highlighting promising research directions that may enhance its viability in supporting the worldwide shift towards clean energy solutions.
1. Introduction
The transition towards a sustainable energy economy is heavily dependent on the integration of renewable energy sources. However, the intermittent nature of resources such as wind and solar presents a major challenge to ensuring a reliable and continuous energy supply. To overcome this issue and meet the ever-increasing energy demands of society, efficient and scalable energy storage systems are required.1 Electrochemical water splitting, specifically water electrolysis, offers a promising pathway for converting excess electricity produced by renewable energy into chemical energy in the form of hydrogen, which can be stored and utilised during periods of low electricity generation. Hydrogen production via water electrolysis serves as efficient energy storage while also being adaptable for use in diverse sectors, including households, transportation, and industry for the synthesis of valuable chemicals. Moreover, hydrogen holds the potential to play a critical role in combatting climate change and achieving carbon neutrality, as its generation, consumption, and regeneration create a closed-loop cycle with minimal environmental impact. However, the associated costs, including investment, maintenance, and transportation, impose significant economic burdens and affect the viability and widespread adoption of hydrogen as a sustainable energy carrier.2Renewable hydrogen, generated via water electrolysis driven by renewable energy, will establish novel interconnections between the energy and water sectors,3 and securing a sustainable water supply is fundamental to its success in transitioning the energy sector to meet global net-zero targets. Global demand for renewable hydrogen is expected to grow significantly over the coming decades. Utilising freshwater for electrolysis reduces the treatment requirements to reach the high quality needed by conventional electrolysers; however, less than 1% of the water on earth exists as non-frozen freshwater.4 In countries with inland freshwater resources (e.g., surface water and groundwater), such as Australia, hydrogen production through water electrolysis is feasible. However, the uneven distribution of water resources across the land presents a challenge for hydrogen production in inland regions.5 Additionally, many regions experiencing rapid growth in renewable energy are located along extensive coastlines, where the development of hydrogen production facilities may be challenged by limited access to freshwater resources.6
Freshwater availability is undergoing significant changes worldwide, with a general declining trend observed between 2001 and 2020, particularly in the southern hemisphere.7,8 Among the key drivers of this variability, precipitation has been identified as the dominant factor.8 Climate change is expected to intensify the global hydrological cycle, altering the spatial and temporal distribution of water resources and further constraining freshwater supplies.9 Projections indicate that river flows in regions such as South America and Australia will continue to decline under ongoing warming trends.8 In parallel, the past five decades have seen a dramatic rise in global groundwater extraction, largely driven by population growth, which heightens the risk of aquifer depletion and long-term water insecurity.10 The combination of multiple stressors on water availability such as population growth, industry, and climate change are highly challenging for water resource management. In this context, seawater electrolysis can potentially mitigate pressures on freshwater resources by utilising abundant ocean water, positioning it as a promising option for hydrogen production in regions with limited freshwater availability.
Commercially available water electrolysis systems commonly rely on freshwater resources for their water feedstocks. However, global freshwater systems increasingly threatened by the growing human population, rising water consumption, industrial development pressures, and anthropogenic climate change.11 Recognising the need for alternative water sources, seawater as a limitless resource can be a viable approach to hydrogen production. Seawater electrolysis is still in its infancy compared to pure water electrolysis due to limitations raised by seawater characteristics and the complexity of electrochemical reactions. As a result, multiple methods, such as purification, desalination, and deionisation, are required to treat seawater. There are two approaches for seawater electrolysis (Fig. 1): direct seawater electrolysis and conventional electrolysis coupled with desalination. Each presenting several compelling arguments in its favour. Conventional electrolysis integrates external mature desalination technologies and pre-treatment plants with commercial water-electrolysers. On the other hand, direct seawater electrolysis involves treating seawater inside the electrolysers or consuming seawater with no pre-treatment.
 | ||
| Fig. 1 Schematic of direct seawater electrolysis and conventional electrolysis coupled with desalination.4 Reproduced from ref. 4 with permission from the Royal Society of Chemistry, copyright 2021. | ||
Some studies suggest that conventional electrolysis is more practical because the cost of purifying seawater upfront is much lower than that of electrolysis. The initial stage of seawater treatment involves the removal of microorganisms and sediments through straightforward filtration methods.12–14 However, further research has shown that the water quality purified by seawater desalination technologies may not be adequate for direct use, and the soluble ion species remain within the seawater, entering the electrolysis system, which can harm the electrolysers over time.15 Thus, it becomes imperative to consider the presence of these ion species during seawater electrolysis. Additionally, the chemical compounds and contaminants present in seawater progressively diminish the efficiency of the electrolyser.15 Therefore, additional purification steps are required to ensure high purity water feeds, significantly increasing the overall cost.13,14,16 Consequently, especially in large-scale offshore hydrogen production, bypassing the pre-treatment systems through direct seawater electrolysis offers tremendous potential to enhance economic viability regarding system design, reduced capital costs, minimised maintenance, and smaller physical footprints.
In summary, seawater electrolysis stands as a promising solution at the intersection of sustainable energy and water resource management. As global efforts intensify to address environmental challenges, further research, development, and implementation of seawater electrolysis technologies become imperative for a resilient and environmentally conscious future. This review mainly explores environmental impact, technical challenges and the state-of-the-art in direct seawater electrolysis in depth from various perspectives.
2. Environmental impact of seawater electrolysis
Physical and ecological features of the environment can be impacted during the construction, operation, and decommissioning of desalination and electrolysis plants through various causal pathways. The intensity of impacts depends on the degree of disturbance and the ecosystem's inherent sensitivity, varying based on the unique characteristics of the local habitat and communities.17 A high-level causal pathway for two drivers: conventional seawater electrolysis (combined desalination–electrolysis) and direct seawater electrolysis, has been developed for this review (Fig. 2). | ||
| Fig. 2 Causal network of hydrogen production pathways for direct seawater electrolysis and conventional seawater electrolysis. | ||
The planned activities triggered by seawater electrolysis include several essential developments including electricity generation, earthworks/construction, desalination, electrolysis, and waste generation. These can lead to stressors, which are negative impacts on the environment resulting from activities. Stressors, in turn, cause a change in processes, which are natural mechanisms occurring in the environment, and these can affect endpoints, the marine and terrestrial ecosystems. In this review, potential impacts on the marine environment have been investigated.
2.1 Vegetation removal
Direct effects on coastal and marine systems may arise from habitat loss caused by vegetation clearance and seafloor disruption associated with constructing and operating seawater intake and outfall pipelines. In addition to marine ecosystems, terrestrial habitats are also affected, as vegetation is often cleared to accommodate desalination plants, electrolyser systems, pipeline corridors, and related infrastructure. These activities contribute to habitat fragmentation and loss, which can in turn lead to biodiversity decline.18 While the severity of these impacts varies depending on local ecological conditions, the degradation of natural habitats has been widely recognised as a key driver of biodiversity loss.18 The extent of such environmental impacts is closely linked to the physical footprint of the infrastructure involved.17 For example, the construction of the Alkimos seawater desalination plant in Western Australia is estimated to have caused the loss of approximately 8.3 hectares of reef, seagrass, and microalgal habitats,19 with largely irreversible effects on benthic communities.17 In this context, direct seawater electrolysis offers potential environmental advantages. By eliminating the need for large-scale desalination plants, it can substantially reduce land and marine area requirements, thereby minimising vegetation clearance and habitat disruption. This reduced physical footprint may help mitigate ecosystem fragmentation and the associated biodiversity impacts, particularly in sensitive coastal zones.2.2 Atmospheric emissions
Emissions to the atmosphere from seawater desalination and electrolysis can include greenhouse gases (GHG), particularly CO2, as well as chlorine (Cl2) or oxygen (O2), depending on the type of electrolysis applied.20 Among these, CO2 emissions are largely determined by the electricity source used to power various systems, including seawater intake and pre-treatment, reverse osmosis (RO), product water distribution, electrolysers, and other balance-of-plant components.21,22 Electricity for these systems can come from onshore/offshore wind, photovoltaic (PV) solar, or the local electricity grid, each carrying different emission intensities.23 While seawater reverse osmosis (SWRO) is less energy-intensive than conventional water electrolysis (Table 1), direct seawater electrolysis offers a potential benefit in that it does not require additional energy input compared to current methods such as alkaline and PEM electrolysis.24| Process | Functional unit | Energy consumption | Reference |
|---|---|---|---|
| SWRO | kWh per m3 desalinated water | 2–7 | 4, 6, 14, 21, 23 and 25–28 |
| SWRO | kWh per m3 water | 3.5–5 | 29 |
| Alkaline | kWh pe kg H2 | 47–66 | 30 and 31 |
| PEM | kWh per kg H2 | 47–63 | 24, 27, 28, 30 and 31 |
| SOEC | kWh per kg H2 | 42.3 | 30 |
| Seawater electrolysis | kWh per kg H2 | 50–56.2 | 31 and 32 |
| Electrolysis | kWh per kg H2 | 40–60 | 29 |
Renewable energy such as wind or PV solar generates no CO2 emissions compared to the Australian electricity grid mix in 2021–22, in which fossil fuels contributed 71% resulting in a total emission of 0.66 kg CO2-eq. kWh. A comparison of different electricity mixes for seawater desalination22 found that emissions during the operation phase were 692 CO2-eq. if using the Australian electricity mix and 26 CO2-eq. if using 100% renewable energy over a 26-year period. Emissions for water electrolysis under two scenarios: COP26 (mostly solar, nuclear, and hydro energy) and EU27 (mostly coal, natural gas, nuclear, wind, and hydro) electricity mixes,33 were estimated at 2.329 kg CO2-eq. kg H2 (COP26) and 22.964 kg CO2-eq. kg H2 (EU27) respectively. A separate study indicated CO2-eq. emissions from grid-connected water electrolysis could be between 1.5–41.1 kg CO2-eq. kg H2.23 The global warming potential of electrolysis powered by electricity from wind was estimated at 0.85 kg CO2-eq. kg H2 for alkaline electrolyser and 0.95 kg CO2-eq. kg H2 for PEM electrolyser.34
The release of CO2 into the atmosphere, if unmanaged, leads to the enhancement of the greenhouse effect and hastens climate warming. As more CO2 dissolves in the ocean, seawater pH decreases (becomes more acidic) due to the formation of carbonic acid and the release of hydrogen ions (H+).35 Certain marine taxonomic groups are more vulnerable to acidification than others, as increased energy is required to form and sustain the exoskeletons of phytoplankton, crustaceans, mollusks, and corals under low pH conditions.35 Under future acidification scenarios, the growth and survival of approximately half of benthic species could be reduced. Deoxygenation and hypoxia are also closely linked to ocean acidification. The effects of global warming, however, may differ between ocean basins due to differences in circulation patterns.
2.3 Seawater extraction
Stoichiometric calculations indicate that the production of 1 kg of hydrogen through electrolysis requires a minimum of 8.9 litres of water;30 however, additional volumes are needed to meet the high purity requirement for the electrolyser, cooling of the system, and to account for system inefficiencies.36 The recovery of water using reverse osmosis for desalination is around 40–50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 and electro-deionisation is around 90%.38 This means that the required seawater intake volume to produce the 1 kg of hydrogen could be more than double the stoichiometric requirement. In addition, volumes for electrolyser cooling have been estimated at 88.1 L per kg H2 for alkaline and PEM electrolysis, 152 L per kg H2 for PEM electrolysis using evaporative cooling, and 645 L per kg H2 for SOEC electrolysis.30
37 and electro-deionisation is around 90%.38 This means that the required seawater intake volume to produce the 1 kg of hydrogen could be more than double the stoichiometric requirement. In addition, volumes for electrolyser cooling have been estimated at 88.1 L per kg H2 for alkaline and PEM electrolysis, 152 L per kg H2 for PEM electrolysis using evaporative cooling, and 645 L per kg H2 for SOEC electrolysis.30
Impacts on the marine environment associated with seawater intake depend on the nature of the local environment at the intake area, the intake capacity, type, and structure.25 Seawater is extracted from the ocean using either surface (open) intake or subsurface intake structures.26 Surface intakes can be classified into three categories: offshore submerged intakes, nearshore submerged intakes, or nearshore surface intakes. In contrast, subsurface intakes can be categorised as onshore, utilising vertical wells, or offshore, employing wells or seabed infiltration galleries.26
The extraction of large quantities of seawater may disrupt natural circulation of water, especially in regions with weak currents and waves.17 The method of extraction can result in the entrainment, entrapment, and/or impingement of marine organisms, thus reducing species population numbers which may impact on ecosystem productivity.17,26
Entrainment occurs when small organisms with limited or no swimming capabilities are drawn into intake pipes, thereby being removed from the natural environment.17 Open seawater intake usually results in the entrainment and loss of fish eggs and larvae and benthic invertebrate species, algae and seagrass spores, phytoplankton and zooplankton, and other smaller marine organisms.17 The effects of entrainment can be substantial depending on local conditions. Impingement involves the physical confinement of marine organisms against intake screens due to high-flow conditions and the associated hydraulic forces. This typically results in injury, weakening, or even mortality, leading to a decrease in the local abundance and diversity of marine life.25 Meanwhile, entrapment refers to the process where organisms are captured within the intake system without the ability to return to their natural environment, which may affect the overall diversity of the local marine ecosystem.25
2.4 Waste and by-product disposal
The main waste and by-products from seawater electrolysis are (i) brines, (ii) chemicals added (additives) during pre-treatment of seawater and cleaning of RO membranes and (iii) inert solid waste including RO membranes.Disposal of brine effluent represents a significant challenge due to both its environmental impact and the high costs involved.38 While direct ocean discharge remains the most common practice,39 alternative onshore disposal methods, including deep well injection or evaporation ponds, are hindered by factors such as substantial capital investment, limited land availability, and stringent regulatory controls, as well as the inherent risks of soil and groundwater contamination. Offshore disposal via diffusers or coastal release attempts to mitigate some environmental risks, yet the long-term ecological consequences remain a concern. Emerging strategies emphasize transforming brine from a waste product into a resource, notably through ‘zero liquid discharge’ (ZLD) approaches.40 These focus on recovering valuable salts and metals, offering economic incentives that could offset disposal costs. Additionally, innovative applications such as fish farming and halophyte cultivation leverage the unique chemical composition of brine to create sustainable bioproduct systems.38 Despite these promising avenues, comprehensive assessments of environmental trade-offs and economic feasibility are crucial before widespread implementation can be achieved. Thus, managing brine not only poses a technical challenge but also an opportunity to rethink waste valorisation within seawater electrolysis frameworks.
The total dissolved solid (TDS) concentration of seawater is generally between 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–40
000–40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1.37 Seawater desalination plants typically produce brine which is 1.5 to 2 times more concentrated than ambient seawater (60
000 mg L−1.37 Seawater desalination plants typically produce brine which is 1.5 to 2 times more concentrated than ambient seawater (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–80
000–80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg per L TDS).37,41 When brine is discharged into the ocean, it creates a high-density hypersaline plume that usually follows the gradient of the seabed.42 The dispersion and dilution of the brine plume are shaped by the discharge method (coastal or submarine outfall), the hydrodynamic characteristics of the environment (e.g., currents, waves, and tides), and the bathymetry of the seabed, which makes the dispersal and impact extent location-specific.42,43 Variability in the flow can result in long-term chronic exposure at the plume centre as well as episodic short-term exposure of marine organisms to the waste brine at the perimeters of the plume.40 Based on the aforementioned factors, a typical coastal desalination plant and its associated brine disposal system are illustrated in Fig. 3.44
000 mg per L TDS).37,41 When brine is discharged into the ocean, it creates a high-density hypersaline plume that usually follows the gradient of the seabed.42 The dispersion and dilution of the brine plume are shaped by the discharge method (coastal or submarine outfall), the hydrodynamic characteristics of the environment (e.g., currents, waves, and tides), and the bathymetry of the seabed, which makes the dispersal and impact extent location-specific.42,43 Variability in the flow can result in long-term chronic exposure at the plume centre as well as episodic short-term exposure of marine organisms to the waste brine at the perimeters of the plume.40 Based on the aforementioned factors, a typical coastal desalination plant and its associated brine disposal system are illustrated in Fig. 3.44
 | ||
| Fig. 3 Schematic illustrating a coastal desalination plant and its brine disposal system.44 Reproduced from ref. 44 with permission from Elsevier, copyright 2018. | ||
Marine organisms exist in an osmotic balance with their environment, and an increase in the concentration of salts can cause osmotic stress on a wide range of marine organisms.40,45,46 Osmotic imbalances from salinity increases may lead to irreversible cell dehydration (‘lethal osmotic shock’), reduced turgor pressure, and even death.14,47,48 However, different species show varying levels of sensitivity to increased salinity.43 The scale and severity of impacts depends on factors such as species' vulnerability and conservation status, the prevailing environmental conditions (e.g., oceanography, climate, and bathymetry), and the chemical composition and disposal mechanism of the brine discharge.49 Effects tend to worsen where water circulation is limited and for sessile organisms such as seagrass and benthic fauna. Seagrass, for instance, is known to be particularly sensitive to changes in salinity and may serve as a bioindicator for habitat degradation.50 Numerous studies have investigated the effect of brine on seagrass species such as Cymodocea nodosa, Posidonia oceanica, and Posidonia australis.14,50 Laboratory experiments have demonstrated that salinity levels exceeding 39.1 g L−1 reduce the vitality of P. oceanica, evidenced by decreased leaf growth, necrotic spots, and premature leaf senescence. The species C. nodosa showed negative effects from increased salinity in both laboratory and in situ conditions. P. australis exhibited a broader tolerance range for salinity, from 27 to 60 g L−1.14 Consequently, extensive studies advise that brine discharge should avoid seagrass ecosystems altogether or remain below a critical salinity threshold of 37.7 g L−1.50 Similarly, the abundance of Echinoderms has been used as an early warning of osmotic stress near the Javea facility in Spain.50 Another study presents a seven-year evaluation of the impact of discharge from the Sydney Desalination Plant on fish communities near rocky reefs, showing a significant rise in fish assemblages at the outlet in comparison to reference locations.51
The discharge of the highly saline SWRO brine can also alter the abiotic marine environment by increasing the ambient ocean temperature, decreasing the dissolved oxygen content, altering the pH, and/or causing eutrophication.47 Membrane-based desalination plants produce brine at a temperature close to that of the intake seawater, with reported temperature differences ranging from 1 to 2.5 °C.14 In the vicinity of the outlet of Australia's first desalination plant in Cockburn Sound, Western Australia, a notable reduction in dissolved oxygen (DO) levels was observed.52 Reduced dissolved oxygen availability may lead to changes in photosynthesis, metabolic, and growth rates.25 Thermal stress and deoxygenation of receiving waters can occur in the case of thermal distillation. Rejected brine from saline electrolysis, containing precipitated Mg(OH)2 and Ca(OH)2, has a pH of >14 and, therefore a strong alkalising effect on the receiving marine environment.20
Sodium metabisulphite, used as a disinfectant in membrane-based desalination methods, can react with dissolved oxygen releasing sulphur dioxide (SO2). This results in acidification and de-oxygenation (hypoxia) of the effluent.49 Such conditions negatively impact the growth and survival of benthic marine organisms and may lead to changes in the composition and spatial arrangement of benthic habitats and communities. Anti-scaling agents containing organic phosphorus can enrich the effluent with nutrients stimulating plant growth. Coagulants are known to enhance water turbidity and reduce light penetration and/or lead to the burial of sessile benthic organisms.54 Thermal desalination systems also employ anti-foaming agents and higher concentration of anti-biofouling additives, compared to membrane-based systems, which may pose risks to the life cycles of marine species.55 Desalination, especially through thermal systems that experience higher corrosion rates, can lead to the release of heavy metals into effluent streams, contingent on the specific metallic alloys and their characteristics within the system.56
In seawater electrolysis, chlorine Cl2 (g) (0.23 kg per kg H2) is produced at the anode as a by-product and released to the atmosphere if no special measures are undertaken.20 Chlorine is over twice as dense as air and tends to accumulate near its release point unless dispersed by air currents. Additionally, chlorine gas is highly soluble in water and exhibits significant reactivity.57 Residual chlorine at low concentrations can be detrimental to aquatic life when the environment is heavily chlorinated. Research has demonstrated that marine invertebrates, like shrimps, hermit crabs, and amphipods, show particular sensitivity to chlorine exposure.58 Chlorine undergoes hydrolysis to produce hydrochloric acid (HCl) and hypochlorous acid (HOCl), the latter being a powerful oxidising agent with sanitising properties. Chlorine also combines with organic compounds in seawater, leading to the creation of chlorinated and halogenated organic by-products, some of which could be harmful to aquatic life or even carcinogenic.59
It's noteworthy that alkaline seawater electrolysis generates alkaline brine containing calcium and magnesium that can react with dissolved CO2 in the ocean, providing a pathway for ocean CO2 mineralisation. This approach not only reduces some brine waste but also aids in removing carbon dioxide from the ocean, which has positive environmental implications.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours for proton exchange membrane (PEM), less than 40
000 hours for proton exchange membrane (PEM), less than 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours for solid oxide electrolyser cells (SOEC), and up to 90
000 hours for solid oxide electrolyser cells (SOEC), and up to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours for alkaline electrolysers—while overall system lifespans range from 10–20 years for PEM to 20–30 years for alkaline types.61 However, unlike RO modules, electrolysers do not require frequent large-scale physical replacements of polymer-based units.
000 hours for alkaline electrolysers—while overall system lifespans range from 10–20 years for PEM to 20–30 years for alkaline types.61 However, unlike RO modules, electrolysers do not require frequent large-scale physical replacements of polymer-based units.
From a life-cycle waste perspective, direct seawater electrolysis may offer advantages by avoiding the substantial volume of waste associated with RO membrane disposal. This distinction highlights another potential environmental benefit of direct electrolysis pathways, particularly in long-term sustainability assessments.
3. Challenges of seawater electrolysis
Seawater electrolysis presents a significant challenge due to the complex chemical composition of seawater. Seawater contains both inorganic and organic compounds, including dissolved salt ions (Cl−, Na+, K+, Mg2+, Ca2+), sediments containing heavy metals (Sr2+, Cd2+, Pb2+, Cu2+ and Fe2+/3+), sulphates (SO42−), carbonates (CO32− and HCO3−) in deep seawater, CO2 in surface seawater, dissolved F−, N− and B− anions,62,63 as well as micro-organism species including bacteria, fungi and microalgae.64 Moreover, the characteristics of seawater, such as composition, salinity, and pH, vary depending on the depth, geographical locations, and seasonal fluctuations.62,63 These property fluctuations necessitate adapted approaches for seawater electrolysis, as the electrolytic parameters must be constantly adjusted to accommodate these variations. The complex relationship of these factors underscores the complexity of seawater electrolysis, making it imperative for researchers and engineers to develop innovative solutions to harness the potential of this abundant resource for sustainable energy production.65 Herein, the key challenges of direct seawater electrolysis are presented.3.1 Conductivity
Although seawater contains various ionic compounds, its salinity and ionic conductivity are low, which results in low electrical conductivity for seawater. This low electrical conductivity decreases the electrolyser efficiency by increasing the cell voltage input and energy consumption.66,673.2 pH
While the pH of seawater changes depending on the geographical locations and seasonal changes, another seawater splitting challenge is the variation of local pH at the cathode and anode during electrolysis. By increasing the potential, the local pH increases at the cathode during HER and reduces at the anode during OER. Generally, the bulk pH of seawater is close to neutral, about 8.62 However, the local pH variation at electrodes can be between 5 to 9 pH units, which causes electrode degradation and reduces their stability and efficiency. Although the carbonates in seawater can act as buffers during the electrolysis, their performance is not sufficient to prevent fluctuations in the local pH at electrodes.68 The local pH increases at the cathode results in precipitation of metal hydroxides such as Mg(OH)2 (at pH 10.7–11) and Ca(OH)2 as well as Ca(CO)3 on the cathode surface and block the cathode active sites,6,69,70 consequently reducing the HER efficiency of the cathode. Moreover, at the specific potentials, the soluble metal cations, including Cu, Pb and Cd, will electrodeposit on the cathode surface and block active sites for HER. These metal depositions not only compete with HER but also reduce the efficiency and durability of the cathode.3.3 Chloride
The presence of electrochemical active Cl− anions in seawater is another key challenge in seawater electrolysis. Chloride electro-oxidation chemistry in aqueous solutions is a complex mechanism that tends to compete with oxygen evolution reaction (OER) and is influenced by several variables, including pH, applied potentials, and temperature. At a temperature of 25 °C and a fixed total 0.5 M concentration of chlorine species, which is representative of seawater conditions, a Pourbaix diagram was constructed to depict the prevailing reactions under different pH conditions (Fig. 4).71 When the pH drops below 3.0, under acidic conditions, the free chlorine evolution reaction (ClER) becomes the dominant chloride oxidation process. As the pH increases to the range of 3 to 7.5, hypochlorous acid (HClO) formation becomes the major reaction, while hypochlorite (ClO−) forms at pH values exceeding 7.5, corresponding to the pKa of hypochlorous acid.71 The presence of chlorine dissolved in water and the disproportionation of hypochlorite ions under higher temperatures add further complexity to the chemistry of chlorine species. | ||
| Fig. 4 The Pourbaix diagram displays (a) chloride chemistry and (b) the oxygen evolution reaction (OER) in an aqueous saline electrolyte. The red line corresponds to the acidic oxidation of chloride to Cl2 gas, while the green line represents the thermodynamic equilibrium between H2O and O2. The black and purple lines indicate the onset of chloride oxidation to HOCl or ClO−.71,72 Reproduced from ref. 71 with permission from Wiley-VCH, copyright 2016 and from ref. 72 with permission from Elsevier, copyright 2022. | ||
Thermodynamically, the chloride oxidation reactions are less favourable than the OER. The potential difference between these reactions and the OER grows with increasing pH, eventually reaching a maximum value near 480 mV when hypochlorite formation starts, at which point it stabilises.71 This implies that under alkaline conditions, by maximising the potential difference between the OER and the ClER, the formation of toxic chlorine compounds can be effectively suppressed, which is known as the “alkaline design criterion” in saline water electrolysis. Furthermore, the chloride reactions involve two electron transfers, whereas the OER involves four electrons. This discrepancy in the number of electrons participating in the OER reaction mechanisms contributes to the commonly observed higher overpotential for the OER compared to chloride oxidation, making the OER kinetically unfavourable. Consequently, the development of highly selective anode catalysts is crucial to prevent the evolution of chlorine gas during seawater electrolysis.73–75
Moreover, the presence of Cl− anions, along with the production of harmful Cl2 gas and hypochlorite, degrades catalysts and electrode materials while also corroding other components of the electrolyser through the formation of metal chloride and hydroxide compounds. This results in reduced efficiency and stability of the electrolysis process, ultimately shortening the lifespan of the electrolyser.
3.4 Organic and biological impurities
Organic and biological impurities in seawater significantly impact the efficiency and longevity of water electrolysers. During electrolysis, they can lead to various issues such as the production of undesired compounds, increased gas impurity, reduced performance, membrane fouling, and even the normal operation of electrolysers.15 Organic compounds, for instance, might not contribute to conductivity but can disrupt the electrolysis process. Biological impurities introduce complexities in electrochemical water splitting and can cause corrosion and affect the overall functionality of electrolysers. Therefore, considering the impact of these impurities, it is essential to design electrolyser components for the efficient functioning of seawater electrolysis, ensuring consistent and sustainable hydrogen production.4. Potential solutions and recent advancements
In addressing the challenges faced in direct seawater electrolysis, researchers have adopted a multitude of strategies aimed at overcoming these obstacles. A pivotal focus of these efforts has been the examination and modification of key components of conventional water electrolysis systems for their applicability in seawater electrolysis. The primary components under study are electrodes, electrolytes, and membranes, each of which plays a crucial role in the electrolytic process. In addition to these fundamental components, researchers have explored innovative solutions and developed hybrid systems that combine different electrolysis technologies to optimise the efficiency and stability of seawater electrolysis. Fig. 5 represents a summary of these key concepts and innovations. The following sections examine the progress made in addressing the issues associated with seawater electrolysis. | ||
| Fig. 5 The key concepts in seawater electrolysis research and development include three main areas: electrode design, electrolyte improvement, and membrane innovation. | ||
4.1 Electrodes
Researchers have investigated the enhancement of electrode materials and designs to improve their catalytic activity and stability in seawater conditions. Noble metal-based electrodes have been a common choice due to their high catalytic activity and corrosion resistivity, but efforts have been made to replace them with transition metal-based counterparts, which are more cost-effective.76 Approaches such as improving electrical conductivity, increasing the number of active sites, reducing the reaction energy barrier, and developing protective layers have been investigated to enable efficient electrolysis in the challenging seawater environment.65 Some representative methods of electrode design are highlighted in Fig. 5.One of the investigated approaches to enhance the electronic properties of electrocatalysts is applying conductive supports/substrates. The structure, conductivity and wettability of substrates can significantly affect the electrocatalytic performance of catalysts. Studies showed that substrates with 3D structures (e.g. Ni/Co/Cu foams and carbon cloth/papers) have demonstrated better performance compared to flat surfaces, such as glassy carbon electrodes. It was shown that in situ synthesis of catalysts on substrates with 3D structures facilitates the electron and mass transfer, increase electrochemical active surface area, and provides better gas diffusion. It is noteworthy to say that the dispersion of catalysts on the substrate can affect its electrochemical performance. Uniform and mono dispersion of catalytic materials on substrates can increase the active sites, surface area and electrochemical performance while it results in less materials being used. For instance, nano-dispersion of noble metals on carbon-based substrates has been introduced as an efficient electrode for seawater electrolysis. These electrodes provide a large surface area and exposure to active sites due to the presence of noble metals. Furthermore, the carbon-based substrates protect the noble metals from corrosion in seawater electrolysis.79,80 This type of catalyst not only enhances the conductivity and performance of OER/HER but also provides good durability in seawater due to its excellent mechanical stability and structural diversity. Moreover, other carbon-based materials and metal-based materials have been studied as suitable conductive supports for HER catalysts to enhance electron transfer.66,81–85 It should be noted that conductive supports do not alter the intrinsic catalytic activity but rather improve the electron transfer and increase catalytic surface area.86
Another approach is to develop highly active electrocatalysts to enhance the electrical conductivity and increase seawater electrolysis performance. Researchers implement different methods to design electrocatalysts with excellent activity. One of the studied methods to enhance electrical conductivity involves the strategic design of heterostructures composed of two or more active components, which enable rapid charge transfer due to their optimised interfacial structure.87–89 Heteroatom doping of catalytic materials serves as an alternative way to adjust the electronic structure and enhance conductivity.90 Furthermore, rational defect engineering is another way to optimise electrical conductivity.91
Overall, the high electrical conductivity is crucial for accelerating electron, facilitating gas production, and improving electrocatalytic activity. Over the last decades, many electrocatalysts have been developed with high faradaic efficiency for use in pure water electrolysers that can be investigated for seawater electrolysis.
Another parameter that can improve the electrochemical performance is improving the reaction energy barriers. For both HER and OER, the electrocatalytic performance is determined by the free energy of adsorption and desorption of hydrogen and oxygen intermediates on the catalyst surface. The reaction energy barriers of the catalysts can be optimised by improving the electronic structure of the catalyst's surface. Researchers have studied various methods to reduce the energy barriers, such as interface and defect engineering phase transition and atom doping.88,90,99,104–108
The creation of active sites is a crucial factor in developing highly efficient electrocatalysts, which can be achieved through two main approaches: (a) increasing the number of active sites and (b) enhancing their intrinsic activity to optimise reaction energy barriers. While most research in this field focuses on designing electrocatalysts with a greater number of active sites, the effects of impurities or hybrid materials on their deactivation have received little attention. Therefore, identifying effective strategies or pre-treatment methods to mitigate the impact of seawater impurities on electrocatalytic active sites is essential.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88), cationic selective polyanion (e.g., NiFe/NiSx,121 NimConSx122 and MoS2
88), cationic selective polyanion (e.g., NiFe/NiSx,121 NimConSx122 and MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 88), small organic molecules (SOMs)123 and carbon coating (e.g., graphene oxide82) that can provide superior resistance to chloride corrosion and increase the stability of electrocatalysts.124 Moreover, researchers have employed structural buffer engineering to develop a catalyst that exhibits high stability and selectivity for OER. The Cl− within the lattice structure of these catalysts acts as a structural buffer, and its gradual leaching during the OER process creates vacancies that allow seawater Cl− to enter, preventing catalyst deactivation.125 Recently, the intercalation of anions, sulphates, phosphate, carbonate, and selenide ions, within the structure of catalysts has been drawing attention due to their high chloride rippling ability.126,127
88), small organic molecules (SOMs)123 and carbon coating (e.g., graphene oxide82) that can provide superior resistance to chloride corrosion and increase the stability of electrocatalysts.124 Moreover, researchers have employed structural buffer engineering to develop a catalyst that exhibits high stability and selectivity for OER. The Cl− within the lattice structure of these catalysts acts as a structural buffer, and its gradual leaching during the OER process creates vacancies that allow seawater Cl− to enter, preventing catalyst deactivation.125 Recently, the intercalation of anions, sulphates, phosphate, carbonate, and selenide ions, within the structure of catalysts has been drawing attention due to their high chloride rippling ability.126,127
4.2 Electrolytes
The performance, hydrogen purity and longevity of commercial water electrolysis systems are widely recognised to be influenced by the quality of the water feed. Impurities in the electrolyte feed can detrimentally impact electrolyser efficiency and the overall quality of produced hydrogen, as well as contribute to device degradation over time. Two different sources of impurities in water electrolysis processes are identified: exogenous and endogenous.15 Exogenous impurities are those introduced by the feed water, including ionic and organic impurities as well as dissolved gases (e.g. nitrogen, oxygen, argon and carbon dioxide). Endogenous impurities are generated in situ by degradation processes within the electrolyser and total plant itself. Impurities may also be introduced by the electrolyser due to leaching from its components and contamination during manufacturing and maintenance. Like freshwater electrolysis, seawater electrolysis struggles with both types of impurities. However, the complex composition of seawater makes the scenario more complicated. This section focuses specifically on exogenous impurities in the electrolyte, and some representative methods are listed, as shown in Fig. 5. | ||
| Fig. 6 Schematic representations of (a) the creation of an alkaline microenvironment on anode with Lewis acid modification, promoting OER and suppressing chlorine chemistry, and (b) the formation of alkaline microenvironment on cathode with Lewis acid modification, aiding HER and preventing precipitation. EDL = electrical double layer, LA = Lewis acid site, E = external electric field.135 Reproduced from ref. 135 with permission from Springer Nature, copyright 2023. | ||
In order to overcome the low conductivity of seawater and prevent local pH gradient in seawater electrolysis, a possible effective strategy can be applying additives to the electrolyte. Several research have used buffered seawater to get improved results. For instance, 1 M KOH + 1 M phosphate (KPi) + 1 M borate (KBi)136 or NaH2PO4 + Na2HPO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 137 were added to natural seawater to control the pH during seawater electrolysis. While the utilisation of buffering seawater electrolytes may result in reduced pH gradients across the electrolytic cell and improved catalyst performance, the process of adding salts to seawater and then filtering them presents two minor challenges. Firstly, the process of direct seawater splitting has been modified to an indirect approach involving pre-treatment of seawater to reduce the concentration of ions. Secondly, regular addition of salts is necessary to maintain a steady reaction. These concerns are more intensified in the case of alkaline seawater, as the process of alkalising seawater necessitates the use of KOH prior to electrolysis. Although several methods involve the use of more affordable salts such as Na2CO3 to eliminate Ca2+/Mg2+ cations, followed by the addition of NaOH/KOH to induce alkalinity, these pre-treatment steps effectively covert the process from direct electrolysis of natural seawater to indirect electrolysis of artificially alkalised seawater.138
137 were added to natural seawater to control the pH during seawater electrolysis. While the utilisation of buffering seawater electrolytes may result in reduced pH gradients across the electrolytic cell and improved catalyst performance, the process of adding salts to seawater and then filtering them presents two minor challenges. Firstly, the process of direct seawater splitting has been modified to an indirect approach involving pre-treatment of seawater to reduce the concentration of ions. Secondly, regular addition of salts is necessary to maintain a steady reaction. These concerns are more intensified in the case of alkaline seawater, as the process of alkalising seawater necessitates the use of KOH prior to electrolysis. Although several methods involve the use of more affordable salts such as Na2CO3 to eliminate Ca2+/Mg2+ cations, followed by the addition of NaOH/KOH to induce alkalinity, these pre-treatment steps effectively covert the process from direct electrolysis of natural seawater to indirect electrolysis of artificially alkalised seawater.138
An alternative approach is the process of electrolysis in acidic seawater that offers certain advantages, such as reduced precipitation of cations on electrodes and enhanced efficiency in HER. However, this method also introduces challenges, including unlimited chloride oxidation, acid etching and intensive corrosion. Currently, there is few research investigating acidic seawater electrolysis.79,135 Although direct electrolysis in acidic electrolytes offers a simpler process, there are now more severe criteria for both electrodes and membranes. In a PEM electrolyser system, cationic impurities, substitute protons within the membrane, diminishing ionic conductivity and hindering electrochemical reactions. Organics often adsorb on the catalyst, impeding reactions. For example, those with –OH and –CN functional groups cause metal leaching, and some, like methanol, oxidise at the anode, but gaseous products can poison the cathode.15 It is evident that the cost-effectiveness of direct electrolysis compared to conventional electrolysis, using an acid electrolyte, is possible only when both electrode and membrane exhibit sufficient durability. Therefore, more comprehensive research is needed to evaluate the impact of these impurities on direct seawater electrolysis under acidic conditions.
 | ||
| Fig. 7 Flowsheets illustrating the (a) suggested process for chlorine-free seawater electrolysis to produce H2 and O2, and (b) laboratory setup used for continuous electrolysis experiments.141 Reproduced from ref. 141 with permission from Elsevier, copyright 2018. | ||
In this regard, another approach is developing electrolysers with asymmetric electrolyte feed instead of using a symmetric electrolyte feed that removes the limitation of dependency on additives. A novel electrolyser system was tested with asymmetric electrolyte feed using synthetic seawater (0.5 M NaCl) as electrolyte for the cathode compartment and KOH solution (0.5 M KOH) for the anode compartment (Fig. 8).142 It was reported that this electrolysis system reduces the cost of electrolyte by removing the need for additives to the electrolyte, alleviates anode corrosion by improving OER selectivity and operates at high current density, triggering ClOR, consequently increasing the efficiency.
 | ||
| Fig. 8 Schemes for anion exchange membrane (AEM) electrolysers utilising separate electrolyte feeds with varying compositions: (a) symmetric alkaline feeds, (b) asymmetric alkaline catholyte, (c) asymmetric KOH catholyte and NaCl anolyte, (d) symmetric KOH + NaCl feed, (e) asymmetric NaCl catholyte, (f) asymmetric NaCl catholyte and KOH anolyte.142 Reproduced from ref. 142 with permission from the Royal Society of Chemistry, copyright 2020. | ||
 | ||
| Fig. 9 (a) The Pourbaix diagram for OER, HER, HzOR, and ClOR in synthetic seawater containing 0.5 M Cl− at pH values ranging from 7 to 14. (b) Schematic illustration of hybrid seawater electrolysis design (HSE) (top) vs. alkaline seawater electrolysis design (ASE) (bottom).81 Reproduced from ref. 81 with permission from Springer Nature, copyright 2021. | ||
In another study, a combination of water electrolysis and sulphion degradation was reported (Fig. 10).151 In comparison to OER, the cell voltage for sulphion oxidation is decreased by a range of 1.3 to 1.4 V. This reduction effectively mitigates the issues of corrosion and competition of ClER in the process of seawater electrolysis. The hydrogen production rate in this study was reported as 5.34 mol h−1 g−1 (of catalyst) at a current density of 300 mA cm−2, and the energy consumption exhibits a reduction of 60% in comparison to the alkaline water electrolysers. Since urea oxidation is thermodynamically more favourable than OER, urea sources, such as wastewater streams containing high concentrations of urea, can be potential electrolyte feed for hydrogen production from seawater.152,153
 | ||
| Fig. 10 (a) Pourbaix diagram of SOR, HER, OER, and ClOR under alkaline conditions. Below pH 13, the polysulfides in the aqueous solution are not stable. (b) Schematic illustration of hydrogen production at cell voltages below 1.0 V by coupling seawater reduction with SOR on Co-based electrocatalysts.151 Reproduced from ref. 151 with permission from Wiley-VCH, copyright 2021. | ||
The performance of Ru/P–NiMoO4 catalyst with Ni foam support was evaluated as a bifunctional electrocatalyst for HER in alkaline seawater electrolyte and urea oxidation reaction (Fig. 11).154 This system required 1.73 V cell voltage at a current density of 500 mA cm−2 for overall urea splitting, and H2 production was stable for 145 h operating at a current density of 100 mA cm−2.
4.3 Membrane
As discussed earlier, during water electrolysis, fluctuations in local pH levels can results in an acidic environment at the anode and an alkaline environment at the cathode, which directly impacts the electrode potential and reaction rate. At the cathode, in alkaline conditions, insoluble deposits like Mg(OH)2, Ca(OH)2, and MgO are more likely to form on the electrode surface. Notably, these blockages are not limited to the cathode but also affect the anode, as well as the porous diaphragm or membrane. In PEM electrolysers, metal ions may occupy the proton exchange sites of the membrane, increasing the resistance to charge transfer and slowing down the reaction. In a similar manner, Cl− ions can hinder the ion exchange efficiency within the anion exchange membrane of AEM electrolysers. Moreover, solid impurities and microbial contaminations, including bacteria and microbes, lead to precipitation and adhesion on the electrode and membrane surfaces. These factors further impede the transfer of charge and diffusion of water during the electrolysis process and affect the efficiency of seawater electrolysis. Consequently, in designing electrolysers for direct seawater splitting, it is crucial to develop membranes that effectively prevent impurities from reaching the electrodes and also minimise membrane fouling caused by these impurities. Some innovative works on membrane technology are listed as shown in Fig. 5.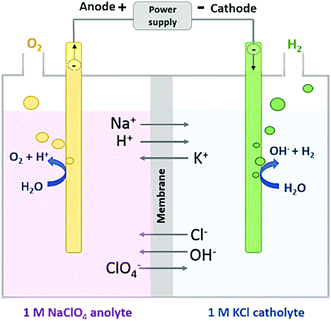 | ||
| Fig. 12 Schematic illustrating ion transfer under a constant current, with 1 M NaClO4 for the anolyte and 1 M KCl for the catholyte instead of NaCl to demonstrate cation transport under varying conditions.155 Reproduced from ref. 155 with permission from the Royal Society of Chemistry, copyright 2020. | ||
 | ||
| Fig. 13 Schematics illustrating (a) a forward-osmosis electrolyser that addresses the challenge associated with precipitation of Ca(OH)2/Mg(OH)2, and (b) the issue of operating an unprotected cathode and Ca(OH)2/Mg(OH)2 precipitation challenge in a conventional cell design for seawater electrolysis.156 Reproduced from ref. 156 with permission from American Chemical Society, copyright 2022. | ||
 | ||
| Fig. 14 Schematics showing the placement of ion-exchange membranes and catalyst/ionomer-coated porous transport layers (PTL), along with the feed circulation used for (a) zero-gap BPMWE and (b) zero-gap PEMWE electrolyser design. (c) Schematic illustrating the primary ion-transport mechanisms: Donnan exclusion (curved arrows), diffusion (solid arrows), migration effects (dashed arrows), and that govern the ion transport across ion-exchange membranes for PEMWE (left), BPMWE (middle), and AEMWE (right) electrolyser systems.163 Reproduced from ref. 163 with permission from Elsevier, copyright 2023. | ||
Based on a comprehensive evaluation encompassing current density, faradaic efficiency, operational stability, energy demand, and overall cost, self-driven purification technology emerges as the most promising option. This approach demonstrates superior electrochemical characteristics, combined with relatively low operational costs and satisfactory durability. Although the performance of forward osmosis is not as high, it still shows considerable potential as an alternative method. On the other hand, both the vapour-fed system and the bipolar membrane approach are hindered by limited current output and stability issues, alongside elevated energy requirements and high implementation costs, which collectively constrain their feasibility for practical deployment.
5. Summary and outlook
In this review, the environmental impact of seawater electrolysis, the technical barriers posed by the complexity of seawater composition, and various strategies to address the challenges of direct seawater electrolysis are extensively discussed. These strategies encompassed diverse approaches, from exploring innovative electrodes to adjusting the electrolyte composition and membrane designs, aiming to achieve efficient, cost-effective, and sustainable seawater electrolysis. Some recent breakthroughs in the development of novel seawater electrolysis systems were also highlighted, shedding light on their potential but also acknowledging their existing limitations. Building upon these discussions, the potential future research directions are identified below, which could significantly shape the field of direct seawater electrolysis (Fig. 15).(1) Although efforts have been carried out to design electrodes for direct seawater electrolysis by multi-scale design, the performance and stability of developed electrodes are still far from the industrial target values. Therefore, it is recommended to extend research on industrialising electrodes using multiple strategies.
(2) Efforts should focus on manipulating electrocatalysts' electronic structure for optimal adsorption energy and improving selectivity, particularly for OER. In this regard, both morphology and electronic engineering have demonstrated their efficacy in enhancing catalytic performance. Exploring specialised hydrophilic nanostructure catalysts, especially porous or multilayered ones, that provide abundant active sites and prevent bubble formations, enhancing overall efficiency and stability.
(3) Achieving high stability for electrodes requires enhancing chloride corrosion resistance. Recommended further investigations in this area include: (a) exploring suitable anti-corrosion coatings, considering diverse pH conditions without compromising catalytic performance, is crucial for industrial seawater splitting. For example, developing core–shell structures by integrating a catalytically active core with specific protective shells (manganese oxides, transition metal phosphides, or graphene) that enhance corrosion resistance and stability; (b) evaluating the performance of high-entropy materials with stable structure resilient to corrosion, for seawater electrolysis.
(4) Limited research has been conducted on electrode poisoning by insoluble and organic impurities in seawater. For example, the precipitation of magnesium/calcium hydroxides during electrolysis clogs catalyst channels and covers active sites, severely impairing catalytic performance and reducing catalyst lifespan. Exploring the impact of these impurities on electrode performance, preventing strategies, and electrode regenerating techniques are crucial for direct seawater electrolysis.
(5) Since the suitable choice of substrate composition and structure enhances the mass transfer, catalytic activity, and corrosion resistance, further research on substrates/supports for electrocatalysts is suggested. Particularly, extending research on developing self-supported electrocatalysts with controllability of morphology and structure is highly recommended.
(6) Transition metal-based electrocatalysts hold promise as replacements for precious metal-based catalysts, but their large-scale production remains a significant concern. Current synthesis methods involve high-temperature hydrothermal and solvothermal processes, leading to low yield, energy inefficiency, and scaling issues to meet industrial demand. There's a crucial need for cost effective, sustainable, and simplified production techniques to enable the broad application of these electrocatalysts in seawater splitting.
(7) Owing to the intricate chemical makeup of seawater, the electrochemical processes occurring during its electrolysis are considerably more complex than those in freshwater systems. As such, comprehensive investigations into the underlying reaction mechanisms are crucial for advancing knowledge of electrode structural dynamics, active site behaviour, electronic characteristics, and electrocatalytic pathways in seawater electrolysis. In this regard, combining electrochemical and in situ characterisation techniques with computational chemistry methods (including density functional theory calculations (DFT) and Molecular Dynamics (MD) simulations) would provide a clear understanding of electrochemical reactions and structural alterations during seawater electrolysis.
(8) Despite significant investment in the development of seawater-based electrocatalysts, the actual hydrogen yields, power costs, and electrolyser testing for realistic applications have been disregarded, which are necessary to the commercialisation of extended hydrogen generation by seawater electrolysis. As a result, increasing hydrogen production experiments, appropriate power consumption studies, and the design of an efficient electrolytic cell were critical.
(9) The impact of organic and biological/microbial impurities is underexplored. Therefore, further comprehensive research is essential to evaluate the influence of these impurities on electrocatalysts, membranes, electrochemical reactions over a range of pHs and overall electrolyser performance.
(10) Future research could target the creation of membranes that possess high robustness in harsh seawater electrolytes and high selectivity for specific ions, ensuring that impurities do not hinder the electrolysis process. Additionally, the development of self-cleaning or fouling-resistant membranes could contribute to prolonged system operation without significant efficiency losses.
(11) The efficiency of seawater electrolysis is not solely dependent on individual components but also on the holistic system design. Research in this area could explore innovative reactor designs and flow patterns that enhance mass transfer and minimise energy losses, making the technology more scalable and adaptable to various applications.
(12) Innovative and efficient integration strategies are essential for direct seawater electrolysis. Technologies allowing simultaneous self-purification and electrolysis are desirable, such as solid oxide electrolysis cells (SOEC) dissociating water into hydrogen and oxygen at high temperatures, coupling interfacial-solar stream and water electrolysis or integrating flow-electrode capacitive deionisation devices with electrolysers.
(13) Further research into the environmental impacts of direct seawater electrolysis is critical as it could provide a more sustainable pathway for hydrogen production. To advance knowledge in this area, suggesting research directions include: investigate marine ecosystem impacts of brine discharge, develop mitigation strategies for chlorine emissions, assess the impact of alkaline brine on ocean acidification, investigate “zero liquid discharge” (ZLD) strategies and methods for resource recovery from brine, particularly valuable salts and minerals like magnesium or lithium, assess cumulative ecological impact of electrolysis plants along coastlines (particularly in regions with high biodiversity or sensitive ecosystems), develop predictive models and monitoring systems that track the long-term ecological impacts of direct seawater electrolysis and inform adaptive management.
(14) Comprehensive life cycle assessment (LCA) is essential to quantify and compare the environmental impacts of direct seawater electrolysis versus conventional electrolysis (using desalinated water) across their full life cycles.
Data availability
This article is a review and does not involve the creation or analysis of new data. All data supporting this work are derived from publicly available sources, as cited within the text.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by CSIRO's Hydrogen Energy Future Science Platform. The authors thank Dr Nikolai Kinaev for many helpful discussions.References
- N. S. Lewis and D. G. Nocera, Powering the planet: Chemical challenges in solar energy utilization, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- S. A. Gorji, Challenges and opportunities in green hydrogen supply chain through metaheuristic optimization, J. Comput. Des. Eng., 2023, 10, 1143–1157 Search PubMed.
- P. Woods, H. Bustamante and K.-F. Aguey-Zinsou, The hydrogen economy – Where is the water?, Energy Nexus, 2022, 7, 100123 CrossRef.
- J. N. Hausmann, R. Schlögl, P. W. Menezes and M. Driess, Is direct seawater splitting economically meaningful?, Energy Environ. Sci., 2021, 14, 3679–3685 RSC.
- A. Feitz, E. Tenthorey and R. Coghlan, Prospective Hydrogen Production Regions of Australia, 2019 Search PubMed.
- H. Yu, J. Wan, M. Goodsite and H. Jin, Advancing direct seawater electrocatalysis for green and affordable hydrogen, One Earth, 2023, 6, 267–277 CrossRef.
- M. Rodell, J. S. Famiglietti, D. N. Wiese, J. T. Reager, H. K. Beaudoing, F. W. Landerer and M.-H. Lo, Emerging trends in global freshwater availability, Nature, 2018, 557, 651–659 CrossRef CAS PubMed.
- Y. Zhang, C. Li, F. H. S. Chiew, D. A. Post, X. Zhang, N. Ma, J. Tian, D. Kong, L. R. Leung, Q. Yu, J. Shi and C. Liu, Southern Hemisphere dominates recent decline in global water availability, Science, 2023, 382, 579–584 CrossRef CAS PubMed.
- W. Liu, W. H. Lim, F. Sun, D. Mitchell, H. Wang, D. Chen, I. Bethke, H. Shiogama and E. Fischer, Global Freshwater Availability Below Normal Conditions and Population Impact Under 1.5 and 2 °C Stabilization Scenarios, Geophys. Res. Lett., 2018, 45, 9803–9813 CrossRef.
- P. G. Cook, M. Shanafield, M. S. Andersen, S. Bourke, I. Cartwright, J. Cleverly, M. Currell, T. M. Doody, H. Hofmann, R. Hugmann, D. J. Irvine, A. Jakeman, J. McKay, R. Nelson and A. D. Werner, Sustainable management of groundwater extraction: An Australian perspective on current challenges, J. Hydrol.: Reg. Stud., 2022, 44, 101262 Search PubMed.
- C. J. Vörösmarty, P. B. McIntyre, M. O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S. Glidden, S. E. Bunn, C. A. Sullivan, C. R. Liermann and P. M. Davies, Global threats to human water security and river biodiversity, Nature, 2010, 467, 555–561 CrossRef PubMed.
- S. Liyanaarachchi, L. Shu, S. Muthukumaran, V. Jegatheesan and K. Baskaran, Problems in seawater industrial desalination processes and potential sustainable solutions: a review, Rev. Environ. Sci. Bio/Technol., 2014, 13, 203–214 CrossRef CAS.
- K. M. Shah, I. H. Billinge, X. Chen, H. Fan, Y. Huang, R. K. Winton and N. Y. Yip, Drivers, challenges, and emerging technologies for desalination of high-salinity brines: A critical review, Desalination, 2022, 538, 115827 CrossRef CAS.
- A. Panagopoulos and K.-J. Haralambous, Environmental impacts of desalination and brine treatment - Challenges and mitigation measures, Mar. Pollut. Bull., 2020, 161, 111773 CrossRef CAS PubMed.
- H. Becker, J. Murawski, D. V. Shinde, I. E. L. Stephens, G. Hinds and G. Smith, Impact of impurities on water electrolysis: a review, Sustainable Energy Fuels, 2023, 7, 1565–1603 RSC.
- Y. Li, Q. Zhang, Y. Li, Y. Xu, L. Li and D. Tian, Improving the efficiency of seawater desalination and hydrogen production: Challenges, strategies, and the future of seawater electrolysis, Desalination, 2025, 609, 118882 CrossRef CAS.
- UNEP and WHO, Desalination Resource and Guidance Manual for Environmental Impact Assessments, Manama, Bahrain, 2008.
- L. A. Yeager, J. Estrada, K. Holt, S. R. Keyser and T. A. Oke, Are Habitat Fragmentation Effects Stronger in Marine Systems? A Review and Meta-analysis, Curr. Landscape Ecol. Rep., 2020, 5, 58–67 CrossRef.
- Water Corporation, Alkimos Seawater Desalination Plant Environmental Review Document, 2019.
- R. d'Amore-Domenech and T. J. Leo, Sustainable Hydrogen Production from Offshore Marine Renewable Farms: Techno-Energetic Insight on Seawater Electrolysis Technologies, ACS Sustain. Chem. Eng., 2019, 7, 8006–8022 CrossRef.
- N. Voutchkov, Energy use for membrane seawater desalination – current status and trends, Desalination, 2018, 431, 2–14 CrossRef CAS.
- M. Heihsel, M. Lenzen and F. Behrendt, Desalination and sustainability: a triple bottom line study of Australia, Environ. Res. Lett., 2020, 15, 114044 CrossRef CAS.
- T. Terlouw, C. Bauer, R. McKenna and M. Mazzotti, Large-scale hydrogen production via water electrolysis: a techno-economic and environmental assessment, Energy Environ. Sci., 2022, 15, 3583–3602 RSC.
- Y. Zheng and S.-Z. Qiao, Direct seawater splitting to hydrogen by a membrane electrolyzer, Joule, 2023, 7, 20–22 CrossRef.
- K. Elsaid, M. Kamil, E. T. Sayed, M. A. Abdelkareem, T. Wilberforce and A. Olabi, Environmental impact of desalination technologies: A review, Sci. Total Environ., 2020, 748, 141528 CrossRef CAS PubMed.
- Intakes and Outfalls for Seawater Reverse-Osmosis Desalination Facilities, ed. T. M. Missimer, B. Jones and R. G. Maliva, Springer International Publishing, Cham, 2015 Search PubMed.
- Y. Morales, P. Samanta, F. Tantish, H. Horn and F. Saravia, Water management for Power-to-X offshore platforms: an underestimated item, Sci. Rep., 2023, 13, 12286 CrossRef CAS PubMed.
- M. A. Khan, T. Al-Attas, S. Roy, M. M. Rahman, N. Ghaffour, V. Thangadurai, S. Larter, J. Hu, P. M. Ajayan and M. G. Kibria, Seawater electrolysis for hydrogen production: a solution looking for a problem?, Energy Environ. Sci., 2021, 14, 4831–4839 RSC.
- A. Baldinelli, G. Cinti, L. Barelli and G. Bidini, Hydrogen production from low-quality water: challenges and perspectives, J. Phys.: Conf. Ser., 2022, 2385, 012048 CrossRef CAS.
- N. Gerloff, Comparative Life-Cycle-Assessment analysis of three major water electrolysis technologies while applying various energy scenarios for a greener hydrogen production, J. Energy Storage, 2021, 43, 102759 CrossRef.
- S. Dresp, F. Dionigi, M. Klingenhof and P. Strasser, Direct Electrolytic Splitting of Seawater: Opportunities and Challenges, ACS Energy Lett., 2019, 4, 933–942 CrossRef CAS.
- H. Xie, Z. Zhao, T. Liu, Y. Wu, C. Lan, W. Jiang, L. Zhu, Y. Wang, D. Yang and Z. Shao, A membrane-based seawater electrolyser for hydrogen generation, Nature, 2022, 612, 673–678 CrossRef CAS PubMed.
- R. Hren, A. Vujanović, Y. Van Fan, J. J. Klemeš, D. Krajnc and L. Čuček, Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment, Renewable Sustainable Energy Rev., 2023, 173, 113113 CrossRef CAS.
- N. Tenhumberg and K. Büker, Ecological and economic evaluation of hydrogen production by different water electrolysis technologies, Chem. Ing. Tech., 2020, 92, 1586–1595 CrossRef CAS.
- P. A. K, M. M, S. Rajamanickam, S. Sivarethinamohan, M. K. R. Gaddam, P. Velusamy, G. R, G. Ravindiran, T. R. Gurugubelli and S. K. Muniasamy, Impact of climate change and anthropogenic activities on aquatic ecosystem – A review, Environ. Res., 2023, 238, 117233 CrossRef PubMed.
- R. E. Lester, D. Gunasekera, W. Timms and D. Downie, Water Requirements for Use in Hydrogen Production in Australia, Potential Public Policy and Industry-Related Issues, Geelong, 2022.
- P. Xu, T. Y. Cath, A. P. Robertson, M. Reinhard, J. O. Leckie and J. E. Drewes, Critical review of desalination concentrate management, Treatment and Beneficial Use, Environ. Eng. Sci., 2013, 30, 502–514 CrossRef CAS.
- E. Jones, M. Qadir, M. T. H. van Vliet, V. Smakhtin and S. Kang, The state of desalination and brine production: A global outlook, Sci. Total Environ., 2019, 657, 1343–1356 CrossRef CAS PubMed.
- O. Barron, E. Campos-Pozuelo and C. Fernandez, in Urban Water Reuse Handbook, ed. S. Eslamian, CRC Press, 1st edn, 2016 Search PubMed.
- K. L. Petersen, H. Frank, A. Paytan and E. Bar-Zeev, in Sustainable Desalination Handbook, Elsevier, 2018, pp. 437–463 Search PubMed.
- N. Voutchkov, Overview of seawater concentrate disposal alternatives, Desalination, 2011, 273, 205–219 CrossRef CAS.
- R. Navarro, J. L. Sánchez Lizaso and I. Sola, Assessment of Energy Consumption of Brine Discharge from SWRO Plants, Water, 2023, 15, 786 CrossRef.
- R. Einav, K. Harussi and D. Perry, The footprint of the desalination processes on the environment, Desalination, 2003, 152, 141–154 CrossRef CAS.
- O. Abessi, in Sustainable Desalination Handbook, Elsevier, 2018, pp. 259–303 Search PubMed.
- P. H. Gomes, S. P. Pereira, T. C. L. Tavares, T. M. Garcia and M. O. Soares, Impacts of desalination discharges on phytoplankton and zooplankton: Perspectives on current knowledge, Sci. Total Environ., 2023, 863, 160671 CrossRef CAS PubMed.
- D. A. Roberts, E. L. Johnston and N. A. Knott, Impacts of desalination plant discharges on the marine environment: A critical review of published studies, Water Res., 2010, 44, 5117–5128 CrossRef CAS PubMed.
- A. Gautam, T. Dave and S. Krishnan, Can solar energy help ZLD technologies to reduce their environmental footprint? - A Review, Sol. Energy Mater. Sol. Cells, 2023, 256, 112334 CrossRef CAS.
- M. L. Cambridge, A. Zavala-Perez, G. R. Cawthray, J. Statton, J. Mondon and G. A. Kendrick, Effects of desalination brine and seawater with the same elevated salinity on growth, physiology and seedling development of the seagrass Posidonia australis, Mar. Pollut. Bull., 2019, 140, 462–471 CrossRef CAS PubMed.
- E. Portillo, M. Ruiz de la Rosa, G. Louzara, J. M. Ruiz, L. Marín-Guirao, J. Quesada, J. C. González, F. Roque, N. González and H. Mendoza, Assessment of the abiotic and biotic effects of sodium metabisulphite pulses discharged from desalination plant chemical treatments on seagrass (Cymodocea nodosa) habitats in the Canary Islands, Mar. Pollut. Bull., 2014, 80, 222–233 CrossRef CAS PubMed.
- I. Sola, D. Zarzo, A. Carratalá, Y. Fernández-Torquemada, J. A. de-la-Ossa-Carretero, Y. Del-Pilar-Ruso and J. L. Sánchez-Lizaso, Review of the management of brine discharges in Spain, Ocean Coastal Manage., 2020, 196, 105301 CrossRef.
- B. P. Kelaher, G. F. Clark, E. L. Johnston and M. A. Coleman, Effect of Desalination Discharge on the Abundance and Diversity of Reef Fishes, Environ. Sci. Technol., 2020, 54, 735–744 CrossRef CAS PubMed.
- J. Kämpf and B. Clarke, How robust is the environmental impact assessment process in South Australia? Behind the scenes of the Adelaide seawater desalination project, Mar. Policy, 2013, 38, 500–506 CrossRef.
- Victoria Department of Sustainability and Environment, GHD (Firm) and Maunsell Australia, Victorian Desalination Project Environment Effects Statement : Environmental effects of marine structures, Victorian Government Department of Sustainability and Environment, Melbourne, 2088, vol. 2.
- S. Lattemann and T. Höpner, Environmental impact and impact assessment of seawater desalination, Desalination, 2008, 220, 1–15 CrossRef CAS.
- A. Shokri and M. Sanavi Fard, A comprehensive overview of environmental footprints of water desalination and alleviation strategies, Int. J. Environ. Sci. Technol., 2023, 20, 2347–2374 CrossRef.
- M. Sharifinia, M. Keshavarzifard, P. Hosseinkhezri, M. H. Khanjani, C. K. Yap, W. O. Smith, M. Daliri and A. Haghshenas, The impact assessment of desalination plant discharges on heavy metal pollution in the coastal sediments of the Persian Gulf, Mar. Pollut. Bull., 2022, 178, 113599 CrossRef CAS PubMed.
- Australian & New Zealand Guidelines for fresh and marine water quality, Chlorine in freshwater and marine water, https://www.waterquality.gov.au/anz-guidelines/guideline-values/default/water-quality-toxicants/toxicants/chlorine-2000, accessed 28 May 2025.
- T. M. Manning, S. P. Wilson and J. C. Chapman, Toxicity of Chlorine and Other Chlorinated Compounds to Some Australian Aquatic Organisms, Bull. Environ. Contam. Toxicol., 1996, 56, 971–976 CrossRef CAS PubMed.
- G. A. Tularam and M. Ilahee, Environmental concerns of desalinating seawater using reverse osmosis, J. Environ. Monit., 2007, 9, 805 RSC.
- W. Lawler, J. Alvarez-Gaitan, G. Leslie and P. Le-Clech, Comparative life cycle assessment of end-of-life options for reverse osmosis membranes, Desalination, 2015, 357, 45–54 CrossRef CAS.
- R. Bhandari, C. A. Trudewind and P. Zapp, Life cycle assessment of hydrogen production via electrolysis – a review, J. Cleaner Prod., 2014, 85, 151–163 CrossRef CAS.
- F. J. Millero, R. Feistel, D. G. Wright and T. J. McDougall, The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale, Deep Sea Res. Part I, 2008, 55, 50–72 CrossRef.
- H. D. Holland, Sea level, sediments and the composition of seawater, Am. J. Sci., 2005, 305, 220–239 CrossRef CAS.
- P. Dewapriya and S. Kim, Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods, Food Res. Int., 2014, 56, 115–125 CrossRef CAS.
- Y. Wang, H. Zhu, T. Ou, T. Xing, Y. Liang, R. Ren, Y. Gu, L. Liu, Y. Su and J. Zhang, Recent Advances in Efficient Seawater Hydrogen Evolution Electrocatalysts, Eur. J. Inorg. Chem., 2025, 28, e202400831 CrossRef CAS.
- L. Yu, L. Wu, S. Song, B. McElhenny, F. Zhang, S. Chen and Z. Ren, Hydrogen Generation from Seawater Electrolysis over a Sandwich-like NiCoN|NixP|NiCoN Microsheet Array Catalyst, ACS Energy Lett., 2020, 5, 2681–2689 CrossRef CAS.
- X. Lu, J. Pan, E. Lovell, T. H. Tan, Y. H. Ng and R. Amal, A sea-change: manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater, Energy Environ. Sci., 2018, 11, 1898–1910 RSC.
- R. L. Pindar, C. Batchelor-McAuley, M. Yang and R. G. Compton, Towards Direct Electroanalysis in Seawater: Understanding the Role of the Buffer Capacity of Seawater in Proton-Coupled Electron Transfer Reactions, J. Phys. Chem. C, 2021, 125, 27949–27958 CrossRef CAS.
- D. Kirk and A. Ledas, Precipitate formation during sea water electrolysis, Int. J. Hydrogen Energy, 1982, 7, 925–932 CrossRef CAS.
- E. M. Kapp, The Precipitation of Calcium and Magnesium from Sea water by Sodium hydroxide, Biol. Bull., 1928, 55, 453–458 CrossRef CAS.
- F. Dionigi, T. Reier, Z. Pawolek, M. Gliech and P. Strasser, Design Criteria, Operating Conditions, and Nickel–Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis, ChemSusChem, 2016, 9, 962–972 CrossRef CAS PubMed.
- F.-Y. Gao, P.-C. Yu and M.-R. Gao, Seawater electrolysis technologies for green hydrogen production: challenges and opportunities, Curr. Opin. Chem. Eng., 2022, 36, 100827 CrossRef.
- L. Zhuang, S. Li, J. Li, K. Wang, Z. Guan, C. Liang and Z. Xu, Recent Advances on Hydrogen Evolution and Oxygen Evolution Catalysts for Direct Seawater Splitting, Coatings, 2022, 12, 659 CrossRef CAS.
- H.-Y. Wang, C.-C. Weng, J.-T. Ren and Z.-Y. Yuan, An overview and recent advances in electrocatalysts for direct seawater splitting, Front. Chem. Sci. Eng., 2021, 15, 1408–1426 CrossRef.
- L. Yu, Q. Zhu, S. Song, B. McElhenny, D. Wang, C. Wu, Z. Qin, J. Bao, Y. Yu, S. Chen and Z. Ren, Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis, Nat. Commun., 2019, 10, 5106 CrossRef PubMed.
- M. G. Lee, S. Kim and Y. Lee, Direct seawater electrolysis: Breaking the dependency on noble metal-based electrocatalysts, Int. J. Hydrogen Energy, 2025, 130, 89–107 CrossRef CAS.
- F. Zhou, Y. Zhou, G.-G. Liu, C.-T. Wang and J. Wang, Recent advances in nanostructured electrocatalysts for hydrogen evolution reaction, Rare Met., 2021, 40, 3375–3405 CrossRef CAS.
- R. Mudoi, U. Bora, D. Lahon, S. Jyoti Kalita and L. Saikia, Insights into role of advanced 2D materials for sustainable hydrogen production through seawater electrolysis, Int. J. Hydrogen Energy, 2025, 133, 520–545 CrossRef CAS.
- N. Nie, D. Zhang, Z. Wang, S. Ge, Y. Gu, B. Yang, J. Lai and L. Wang, Stable p-block metals electronic perturbation in PtM@CNT (M=Ga, In, Pb and Bi) for acidic seawater hydrogen production at commercial current densities, Appl. Catal., B, 2023, 322, 122100 CrossRef CAS.
- S. Gao, G.-D. Li, Y. Liu, H. Chen, L.-L. Feng, Y. Wang, M. Yang, D. Wang, S. Wang and X. Zou, Electrocatalytic H2 production from seawater over Co, N-codoped nanocarbons, Nanoscale, 2015, 7, 2306–2316 RSC.
- F. Sun, J. Qin, Z. Wang, M. Yu, X. Wu, X. Sun and J. Qiu, Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation, Nat. Commun., 2021, 12, 4182 CrossRef CAS PubMed.
- A. R. Jadhav, A. Kumar, J. Lee, T. Yang, S. Na, J. Lee, Y. Luo, X. Liu, Y. Hwang, Y. Liu and H. Lee, Stable complete seawater electrolysis by using interfacial chloride ion blocking layer on catalyst surface, J. Mater. Chem. A, 2020, 8, 24501–24514 RSC.
- M. Sarno, E. Ponticorvo and D. Scarpa, Active and stable graphene supporting trimetallic alloy-based electrocatalyst for hydrogen evolution by seawater splitting, Electrochem. Commun., 2020, 111, 106647 CrossRef CAS.
- T. Liu, H. Liu, X. Wu, Y. Niu, B. Feng, W. Li, W. Hu and C. M. Li, Molybdenum carbide/phosphide hybrid nanoparticles embedded P, N co-doped carbon nanofibers for highly efficient hydrogen production in acidic, alkaline solution and seawater, Electrochim. Acta, 2018, 281, 710–716 CrossRef CAS.
- X. Zhai, Q. Yu, G. Liu, J. Bi, Y. Zhang, J. Chi, J. Lai, B. Yang and L. Wang, Hierarchical microsphere MOF arrays with ultralow Ir doping for efficient hydrogen evolution coupled with hydrazine oxidation in seawater, J. Mater. Chem. A, 2021, 9, 27424–27433 RSC.
- Q. Fu, J. Han, X. Wang, P. Xu, T. Yao, J. Zhong, W. Zhong, S. Liu, T. Gao, Z. Zhang, L. Xu and B. Song, 2D Transition Metal Dichalcogenides: Design, Modulation, and Challenges in Electrocatalysis, Adv. Mater., 2021, 33, 1907818 CrossRef CAS PubMed.
- Z. Wang, W. Xu, K. Yu, Y. Feng and Z. Zhu, 2D heterogeneous vanadium compound interfacial modulation enhanced synergistic catalytic hydrogen evolution for full pH range seawater splitting, Nanoscale, 2020, 12, 6176–6187 RSC.
- J. A. Nasir, Z. ur Rehman, S. N. A. Shah, A. Khan, I. S. Butler and C. R. A. Catlow, Recent developments and perspectives in CdS-based photocatalysts for water splitting, J. Mater. Chem. A, 2020, 8, 20752–20780 RSC.
- J. Guo, K. Lang, X. Yang, R. Yang, M. Ru, X. Xiao, H. Meng and B. Jiang, Efficient and stable La2O3/MoS2 hetero-nanosheet architecture as bifunctional catalysts for seawater electrolysis, Int. J. Hydrogen Energy, 2025, 130, 373–380 CrossRef CAS.
- J. Yang, Y. Wang, J. Yang, Y. Pang, X. Zhu, Y. Lu, Y. Wu, J. Wang, H. Chen, Z. Kou, Z. Shen, Z. Pan and J. Wang, Quench-Induced Surface Engineering Boosts Alkaline Freshwater and Seawater Oxygen Evolution Reaction of Porous NiCo2O4 Nanowires, Small, 2022, 18, 2106187 CrossRef CAS PubMed.
- X. H. Wang, Y. Ling, B. Wu, B. L. Li, X. L. Li, J. L. Lei, N. B. Li and H. Q. Luo, Doping modification, defects construction, and surface engineering: Design of cost-effective high-performance electrocatalysts and their application in alkaline seawater splitting, Nano Energy, 2021, 87, 106160 CrossRef CAS.
- D. Wu, D. Chen, J. Zhu and S. Mu, Ultralow Ru Incorporated Amorphous Cobalt-Based Oxides for High-Current-Density Overall Water Splitting in Alkaline and Seawater Media, Small, 2021, 17, 2102777 CrossRef CAS PubMed.
- L. Wu, L. Yu, Q. Zhu, B. McElhenny, F. Zhang, C. Wu, X. Xing, J. Bao, S. Chen and Z. Ren, Boron-modified cobalt iron layered double hydroxides for high efficiency seawater oxidation, Nano Energy, 2021, 83, 105838 CrossRef CAS.
- W.-L. Ding, Y.-H. Cao, H. Liu, A.-X. Wang, C.-J. Zhang and X.-R. Zheng, In situ growth of NiSe@Co0.85Se heterointerface structure with electronic modulation on nickel foam for overall water splitting, Rare Met., 2021, 40, 1373–1382 CrossRef CAS.
- C.-H. An, W. Kang, Q.-B. Deng and N. Hu, Pt and Te codoped ultrathin MoS2 nanosheets for enhanced hydrogen evolution reaction with wide pH range, Rare Met., 2022, 41, 378–384 CrossRef CAS.
- L. Yu, L. Wu, B. McElhenny, S. Song, D. Luo, F. Zhang, Y. Yu, S. Chen and Z. Ren, Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting, Energy Environ. Sci., 2020, 13, 3439–3446 RSC.
- Y. Surendranath, M. Dincǎ and D. G. Nocera, Electrolyte-Dependent Electrosynthesis and Activity of Cobalt-Based Water Oxidation Catalysts, J. Am. Chem. Soc., 2009, 131, 2615–2620 CrossRef CAS PubMed.
- F. Cheng, X. Feng, X. Chen, W. Lin, J. Rong and W. Yang, Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH, Electrochim. Acta, 2017, 251, 336–343 CrossRef CAS.
- Y. Zhao, B. Jin, Y. Zheng, H. Jin, Y. Jiao and S. Qiao, Charge State Manipulation of Cobalt Selenide Catalyst for Overall Seawater Electrolysis, Adv. Energy Mater., 2018, 8, 1801926 CrossRef.
- K. S. Exner, J. Anton, T. Jacob and H. Over, Chlorine Evolution Reaction on RuO2(110): Ab initio Atomistic Thermodynamics Study - Pourbaix Diagrams, Electrochim. Acta, 2014, 120, 460–466 CrossRef CAS.
- V. Petrykin, K. Macounova, O. A. Shlyakhtin and P. Krtil, Tailoring the Selectivity for Electrocatalytic Oxygen Evolution on Ruthenium Oxides by Zinc Substitution, Angew. Chem., Int. Ed., 2010, 49, 4813–4815 CrossRef CAS PubMed.
- C. Zhao, Z. Ding, K. Zhang, Z. Du, H. Fang, L. Chen, H. Jiang, M. Wang and M. Wu, Comprehensive Chlorine Suppression: Advances in Materials and System Technologies for Direct Seawater Electrolysis, Nano-Micro Lett., 2025, 17, 113 CrossRef CAS PubMed.
- K. Jiang, W. Liu, W. Lai, M. Wang, Q. Li, Z. Wang, J. Yuan, Y. Deng, J. Bao and H. Ji, NiFe Layered Double Hydroxide/FeOOH Heterostructure Nanosheets as an Efficient and Durable Bifunctional Electrocatalyst for Overall Seawater Splitting, Inorg. Chem., 2021, 60, 17371–17378 CrossRef CAS PubMed.
- W. Zang, T. Sun, T. Yang, S. Xi, M. Waqar, Z. Kou, Z. Lyu, Y. P. Feng, J. Wang and S. J. Pennycook, Efficient Hydrogen Evolution of Oxidized Ni-N3 Defective Sites for Alkaline Freshwater and Seawater Electrolysis, Adv. Mater., 2021, 33, 2003846 CrossRef CAS PubMed.
- L. Xiu, W. Pei, S. Zhou, Z. Wang, P. Yang, J. Zhao and J. Qiu, Multilevel Hollow MXene Tailored Low-Pt Catalyst for Efficient Hydrogen Evolution in Full-pH Range and Seawater, Adv. Funct. Mater., 2020, 30, 1910028 CrossRef CAS.
- Y. Huang, L. Hu, R. Liu, Y. Hu, T. Xiong, W. Qiu, M.-S. (Jie Tang) Balogun, A. Pan and Y. Tong, Nitrogen treatment generates tunable nanohybridization of Ni5P4 nanosheets with nickel hydr(oxy)oxides for efficient hydrogen production in alkaline, seawater and acidic media, Appl. Catal., B, 2019, 251, 181–194 CrossRef CAS.
- Y. Lin, K. Sun, S. Liu, X. Chen, Y. Cheng, W. Cheong, Z. Chen, L. Zheng, J. Zhang, X. Li, Y. Pan and C. Chen, Construction of CoP/NiCoP Nanotadpoles Heterojunction Interface for Wide pH Hydrogen Evolution Electrocatalysis and Supercapacitor, Adv. Energy Mater., 2019, 9, 1901213 CrossRef.
- G. Zhou, Z. Guo, Y. Shan, S. Wu, J. Zhang, K. Yan, L. Liu, P. K. Chu and X. Wu, High-efficiency hydrogen evolution from seawater using hetero-structured T/Td phase ReS2 nanosheets with cationic vacancies, Nano Energy, 2019, 55, 42–48 CrossRef CAS.
- T. ul Haq and Y. Haik, S doped Cu2O-CuO nanoneedles array: Free standing oxygen evolution electrode with high efficiency and corrosion resistance for seawater splitting, Catal. Today, 2022, 400–401, 14–25 CrossRef.
- L. Wu, L. Yu, B. McElhenny, X. Xing, D. Luo, F. Zhang, J. Bao, S. Chen and Z. Ren, Rational design of core-shell-structured CoP@FeOOH for efficient seawater electrolysis, Appl. Catal., B, 2021, 294, 120256 CrossRef CAS.
- Q. Tu, W. Liu, M. Jiang, W. Wang, Q. Kang, P. Wang, W. Zhou and F. Zhou, Preferential Adsorption of Hydroxide Ions onto Partially Crystalline NiFe-Layered Double Hydroxides Leads to Efficient and Selective OER in Alkaline Seawater, ACS Appl. Energy Mater., 2021, 4, 4630–4637 CrossRef CAS.
- J. G. Vos, T. A. Wezendonk, A. W. Jeremiasse and M. T. M. Koper, MnOx/IrOx as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution, J. Am. Chem. Soc., 2018, 140, 10270–10281 CrossRef CAS PubMed.
- A. A. Bhardwaj, J. G. Vos, M. E. S. Beatty, A. F. Baxter, M. T. M. Koper, N. Y. Yip and D. V. Esposito, Ultrathin Silicon Oxide Overlayers Enable Selective Oxygen Evolution from Acidic and Unbuffered pH-Neutral Seawater, ACS Catal., 2021, 11, 1316–1330 CrossRef CAS.
- Z.-D. Huang, C. Feng, J.-P. Sun, B. Xu, T.-X. Huang, X.-K. Wang, F.-N. Dai and D.-F. Sun, Ultrathin Metal–Organic Framework Nanosheets-Derived Yolk–Shell Ni0.85Se@NC with Rich Se-Vacancies for Enhanced Water Electrolysis, CCS Chem., 2021, 3, 2696–2711 CrossRef CAS.
- L. Wu, L. Yu, F. Zhang, B. McElhenny, D. Luo, A. Karim, S. Chen and Z. Ren, Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting, Adv. Funct. Mater., 2021, 31, 2006484 CrossRef CAS.
- H. Saada, G. Benoit, J. Dabboussi, C. Lagrost, B. Fabre and G. Loget, Direct Seawater Electrolysis with Stabilized High-Performance NiCr Anodes, Adv. Mater. Interfaces, 2025, 2500305 CrossRef.
- D. He, P. Yang, K. Yang, J. Qiu and Z. Wang, Durable seawater electrolysis enabled by chloride rejection on hydroxide trapping anode, J. Energy Chem., 2025, 107, 407–415 CrossRef CAS.
- Y. Lin, K. Sun, X. Chen, C. Chen, Y. Pan, X. Li and J. Zhang, High-precision regulation synthesis of Fe-doped Co2P nanorod bundles as efficient electrocatalysts for hydrogen evolution in all-pH range and seawater, J. Energy Chem., 2021, 55, 92–101 CrossRef CAS.
- J. Li, Y. Liu, H. Chen, Z. Zhang and X. Zou, Design of a Multilayered Oxygen-Evolution Electrode with High Catalytic Activity and Corrosion Resistance for Saline Water Splitting, Adv. Funct. Mater., 2021, 31, 2101820 CrossRef CAS.
- K. Obata and K. Takanabe, A Permselective CeOx Coating to Improve the Stability of Oxygen Evolution Electrocatalysts, Angew. Chem., Int. Ed., 2018, 57, 1616–1620 CrossRef CAS PubMed.
- Y. Kuang, M. J. Kenney, Y. Meng, W.-H. Hung, Y. Liu, J. E. Huang, R. Prasanna, P. Li, Y. Li, L. Wang, M.-C. Lin, M. D. McGehee, X. Sun and H. Dai, Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 6624–6629 CrossRef CAS PubMed.
- C. Wang, M. Zhu, Z. Cao, P. Zhu, Y. Cao, X. Xu, C. Xu and Z. Yin, Heterogeneous bimetallic sulfides based seawater electrolysis towards stable industrial-level large current density, Appl. Catal., B, 2021, 291, 120071 CrossRef CAS.
- Q. Niu, F. Gao, X. Sun, Y. Zheng and S. Qiao, Chloride-Mediated Electron Buffering on Ni-Fe Anodes for Ampere-Level Alkaline Seawater Electrolysis, Adv. Funct. Mater., 2025, 2504872 CrossRef.
- H. J. Song, H. Yoon, B. Ju, D.-Y. Lee and D.-W. Kim, Electrocatalytic Selective Oxygen Evolution of Carbon-Coated Na2Co1–xFexP2O7 Nanoparticles for Alkaline Seawater Electrolysis, ACS Catal., 2020, 10, 702–709 CrossRef CAS.
- L. Zhuang, J. Li, K. Wang, Z. Li, M. Zhu and Z. Xu, Structural Buffer Engineering on Metal Oxide for Long-Term Stable Seawater Splitting, Adv. Funct. Mater., 2022, 32, 2201127 CrossRef CAS.
- W.-H. Hung, B.-Y. Xue, T.-M. Lin, S.-Y. Lu and I.-Y. Tsao, A highly active selenized nickel–iron electrode with layered double hydroxide for electrocatalytic water splitting in saline electrolyte, Mater. Today Energy, 2021, 19, 100575 CrossRef CAS.
- B. Zhang, S. Liu, S. Zhang, Y. Cao, H. Wang, C. Han and J. Sun, High Corrosion Resistance of NiFe-Layered Double Hydroxide Catalyst for Stable Seawater Electrolysis Promoted by Phosphate Intercalation, Small, 2022, 18, 2203852 CrossRef CAS PubMed.
- S. Dresp and P. Strasser, Non-Noble Metal Oxides and their Application as Bifunctional Catalyst in Reversible Fuel Cells and Rechargeable Air Batteries, ChemCatChem, 2018, 10, 4162–4171 CrossRef CAS.
- S. Dresp, F. Dionigi, S. Loos, J. Ferreira de Araujo, C. Spöri, M. Gliech, H. Dau and P. Strasser, Direct Electrolytic Splitting of Seawater: Activity, Selectivity, Degradation, and Recovery Studied from the Molecular Catalyst Structure to the Electrolyzer Cell Level, Adv. Energy Mater., 2018, 8, 1800338 CrossRef.
- C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, An Advanced Ni–Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS PubMed.
- B. C. M. Martindale and E. Reisner, Bi-Functional Iron-Only Electrodes for Efficient Water Splitting with Enhanced Stability through In Situ Electrochemical Regeneration, Adv. Energy Mater., 2016, 6, 1502095 CrossRef.
- W.-H. Huang and C.-Y. Lin, Iron phosphate modified calcium iron oxide as an efficient and robust catalyst in electrocatalyzing oxygen evolution from seawater, Faraday Discuss., 2019, 215, 205–215 RSC.
- J. Juodkazytė, B. Šebeka, I. Savickaja, M. Petrulevičienė, S. Butkutė, V. Jasulaitienė, A. Selskis and R. Ramanauskas, Electrolytic splitting of saline water: Durable nickel oxide anode for selective oxygen evolution, Int. J. Hydrogen Energy, 2019, 44, 5929–5939 CrossRef.
- J. Guo, Y. Zheng, Z. Hu, C. Zheng, J. Mao, K. Du, M. Jaroniec, S.-Z. Qiao and T. Ling, Direct seawater electrolysis by adjusting the local reaction environment of a catalyst, Nat. Energy, 2023, 8, 264–272 CAS.
- A. J. Esswein, Y. Surendranath, S. Y. Reece and D. G. Nocera, Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutral and natural waters, Energy Environ. Sci., 2011, 4, 499–504 RSC.
- M. Li, H.-J. Niu, Y. Li, J. Liu, X. Yang, Y. Lv, K. Chen and W. Zhou, Synergetic regulation of CeO2 modification and (W2O7)2- intercalation on NiFe-LDH for high-performance large-current seawater electrooxidation, Appl. Catal., B, 2023, 330, 122612 CrossRef CAS.
- S. Jiang, Y. Liu, H. Qiu, C. Su and Z. Shao, High Selectivity Electrocatalysts for Oxygen Evolution Reaction and Anti-Chlorine Corrosion Strategies in Seawater Splitting, Catalysts, 2022, 12, 261 CrossRef CAS.
- W. Tong, M. Forster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan and P. Farràs, Electrolysis of low-grade and saline surface water, Nat. Energy, 2020, 5, 367–377 CrossRef CAS.
- I. Sohrabnejad-Eskan, A. Goryachev, K. S. Exner, L. A. Kibler, E. J. M. Hensen, J. P. Hofmann and H. Over, Temperature-Dependent Kinetic Studies of the Chlorine Evolution Reaction over RuO2 (110) Model Electrodes, ACS Catal., 2017, 7, 2403–2411 CrossRef CAS.
- G. Amikam, P. Nativ and Y. Gendel, Chlorine-free alkaline seawater electrolysis for hydrogen production, Int. J. Hydrogen Energy, 2018, 43, 6504–6514 CrossRef CAS.
- S. Dresp, T. Ngo Thanh, M. Klingenhof, S. Brückner, P. Hauke and P. Strasser, Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds, Energy Environ. Sci., 2020, 13, 1725–1729 RSC.
- M. Cai, Q. Zhu, X. Wang, Z. Shao, L. Yao, H. Zeng, X. Wu, J. Chen, K. Huang and S. Feng, Formation and Stabilization of NiOOH by Introducing α-FeOOH in LDH: Composite Electrocatalyst for Oxygen Evolution and Urea Oxidation Reactions, Adv. Mater., 2023, 35, 2209338 CrossRef CAS PubMed.
- Q. Qian, J. Zhang, J. Li, Y. Li, X. Jin, Y. Zhu, Y. Liu, Z. Li, A. El-Harairy, C. Xiao, G. Zhang and Y. Xie, Artificial Heterointerfaces Achieve Delicate Reaction Kinetics towards Hydrogen Evolution and Hydrazine Oxidation Catalysis, Angew. Chem., Int. Ed., 2021, 60, 5984–5993 CrossRef CAS PubMed.
- Y. Zhu, J. Zhang, Q. Qian, Y. Li, Z. Li, Y. Liu, C. Xiao, G. Zhang and Y. Xie, Dual Nanoislands on Ni/C Hybrid Nanosheet Activate Superior Hydrazine Oxidation-Assisted High-Efficiency H2 Production, Angew. Chem., Int. Ed., 2022, 61, e202113082 CrossRef CAS PubMed.
- Y. Liu, J. Zhang, Y. Li, Q. Qian, Z. Li, Y. Zhu and G. Zhang, Manipulating dehydrogenation kinetics through dual-doping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production, Nat. Commun., 2020, 11, 1853 CrossRef CAS PubMed.
- W. Zhou, S. Chen, X. Meng, J. Li and J. Gao, Energy-saving cathodic H2 production enabled by non-oxygen evolution anodic reactions: A critical review on fundamental principles and applications, Int. J. Hydrogen Energy, 2023, 48, 15748–15770 CrossRef CAS.
- L. Chen and J. Shi, Chemical-assisted hydrogen electrocatalytic evolution reaction (CAHER), J. Mater. Chem. A, 2018, 6, 13538–13548 RSC.
- X. Liu, J. He, S. Zhao, Y. Liu, Z. Zhao, J. Luo, G. Hu, X. Sun and Y. Ding, Self-powered H2 production with bifunctional hydrazine as sole consumable, Nat. Commun., 2018, 9, 4365 CrossRef PubMed.
- Y. Li, X. Wei, L. Chen, J. Shi and M. He, Nickel-molybdenum nitride nanoplate electrocatalysts for concurrent electrolytic hydrogen and formate productions, Nat. Commun., 2019, 10, 5335 CrossRef PubMed.
- L. Zhang, Z. Wang and J. Qiu, Energy-Saving Hydrogen Production by Seawater Electrolysis Coupling Sulfion Degradation, Adv. Mater., 2022, 34, 2109321 CrossRef CAS.
- Y. Tong, P. Chen, M. Zhang, T. Zhou, L. Zhang, W. Chu, C. Wu and Y. Xie, Oxygen Vacancies Confined in Nickel Molybdenum Oxide Porous Nanosheets for Promoted Electrocatalytic Urea Oxidation, ACS Catal., 2018, 8, 1–7 CrossRef.
- Z.-Y. Yu, C.-C. Lang, M.-R. Gao, Y. Chen, Q.-Q. Fu, Y. Duan and S.-H. Yu, Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis, Energy Environ. Sci., 2018, 11, 1890–1897 RSC.
- L. Guo, J. Chi, J. Zhu, T. Cui, J. Lai and L. Wang, Dual-doping NiMoO4 with multi-channel structure enable urea-assisted energy-saving H2 production at large current density in alkaline seawater, Appl. Catal., B, 2023, 320, 121977 CrossRef CAS.
- L. Shi, R. Rossi, M. Son, D. M. Hall, M. A. Hickner, C. A. Gorski and B. E. Logan, Using reverse osmosis membranes to control ion transport during water electrolysis, Energy Environ. Sci., 2020, 13, 3138–3148 RSC.
- S. S. Veroneau, A. C. Hartnett, A. E. Thorarinsdottir and D. G. Nocera, Direct Seawater Splitting by Forward Osmosis Coupled to Water Electrolysis, ACS Appl. Energy Mater., 2022, 5, 1403–1408 CrossRef CAS.
- S. Z. Oener, M. J. Foster and S. W. Boettcher, Accelerating water dissociation in bipolar membranes and for electrocatalysis, Science, 2020, 369, 1099–1103 CrossRef CAS PubMed.
- Z. Yan and T. E. Mallouk, Bipolar Membranes for Ion Management in (Photo)Electrochemical Energy Conversion, Acc. Mater. Res., 2021, 2, 1156–1166 CrossRef CAS.
- L. Chen, Q. Xu, S. Z. Oener, K. Fabrizio and S. W. Boettcher, Design principles for water dissociation catalysts in high-performance bipolar membranes, Nat. Commun., 2022, 13, 3846 CrossRef CAS PubMed.
- M. A. Shehzad, A. Yasmin, X. Ge, Z. Ge, K. Zhang, X. Liang, J. Zhang, G. Li, X. Xiao, B. Jiang, L. Wu and T. Xu, Shielded goethite catalyst that enables fast water dissociation in bipolar membranes, Nat. Commun., 2021, 12, 9 CrossRef CAS PubMed.
- D. A. Vermaas and W. A. Smith, Synergistic Electrochemical CO2 Reduction and Water Oxidation with a Bipolar Membrane, ACS Energy Lett., 2016, 1, 1143–1148 CrossRef CAS.
- M. A. Blommaert, D. Aili, R. A. Tufa, Q. Li, W. A. Smith and D. A. Vermaas, Insights and Challenges for Applying Bipolar Membranes in Advanced Electrochemical Energy Systems, ACS Energy Lett., 2021, 6, 2539–2548 CrossRef CAS PubMed.
- D. H. Marin, J. T. Perryman, M. A. Hubert, G. A. Lindquist, L. Chen, A. M. Aleman, G. A. Kamat, V. A. Niemann, M. B. Stevens, Y. N. Regmi, S. W. Boettcher, A. C. Nielander and T. F. Jaramillo, Hydrogen production with seawater-resilient bipolar membrane electrolyzers, Joule, 2023, 7, 765–781 CrossRef CAS.
- J. Han, Exploring the Interface of Porous Cathode/Bipolar Membrane for Mitigation of Inorganic Precipitates in Direct Seawater Electrolysis, ChemSusChem, 2022, 15, e202200372 CrossRef CAS PubMed.
- J.-H. Han, E. Jwa, H. Lee, E. J. Kim, J.-Y. Nam, K. S. Hwang, N. Jeong, J. Choi, H. Kim, Y.-C. Jeung and T. D. Chung, Direct seawater electrolysis via synergistic acidification by inorganic precipitation and proton flux from bipolar membrane, Chem. Eng. J., 2022, 429, 132383 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |





