Unlocking efficient overall water splitting reactions using sulphur-doped carbon dot electrocatalysts†
Rohini A.
Kale
a,
Pratiksha D.
Tanwade
a,
Balaji B.
Mulik
ab and
Bhaskar R.
Sathe
 *ac
*ac
aDepartment of Chemistry, Dr Babasaheb Ambedkar Marathwada University, Chhatrapati Sambhajinagar, MH 431004, India. E-mail: bsathe.chemistry@bamu.ac.in; bhaskarsathe@gmail.com
bMGM University, Chhatrapati Sambhajinagar, MH 431004, India
cDepartment of Nanoscience and Technology, Dr Babasaheb Ambedkar Marathwada University, Chhatrapati Sambhajinagar, MH 431004, India
First published on 2nd April 2025
Abstract
Herein, the synthesis of bifunctional, metal-free, and highly efficient sulphur-doped carbon dots (S-CDs) through a facile chemical method is demonstrated for electrocatalytic water splitting reactions, specifically targeting both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). S-CDs were characterized using thermogravimetric analysis (TGA), which demonstrated remarkable thermal stability up to 800 °C in Ar, and transmission electron microscopy (TEM) revealed a well-defined crystalline structure with a d-spacing of approximately 0.375 nm. The elemental composition confirmed using energy-dispersive analysis of X-ray (EDAX) showed the presence of carbon (15.91%), oxygen (62.49%), and sulphur (21.60%). X-ray diffraction (XRD) analysis exhibited a broad peak at 2θ = 24°, indicating the graphitic nature of the sulphur doping. Brunauer–Emmett–Teller (BET) surface area analysis revealed a large surface area of 160.49 m2 g−1 and a pore volume of 0.2209 cc g−1 for S-CDs. Fourier-transform infrared (FTIR) spectroscopy identified three distinct peaks corresponding to the functional groups associated with S-CDs. The electrocatalytic performance for the HER was characterized by a low overpotential of −0.64 V vs. RHE to achieve a current density of 10 mA cm−2, with a Tafel slope of 108 mV dec−1 in 1 M H2SO4. For the OER, S-CDs exhibited an overpotential of 1.94 V vs. RHE, with a Tafel slope of 21 mV dec−1 in 1 M KOH. These results demonstrate the potential of S-CDs as cost-effective and sustainable electrocatalysts for overall water splitting, and they hold promise as leading candidates for futuristic renewable energy applications.
1. Introduction
Currently, the economy of most of the countries largely depends on transportation, communication, and industrialization, and hence, effective planning for energy management and consumption is a crucial aspect of their development. Despite the progress made by many countries, the intensified development in these sectors has come with the cost of environmental degradation owing to the substantial increase in fossil fuel consumption and subsequent emission of CO2, NO2 and SO2 gases.1 The growing global energy demand and the environmental impact of fossil fuel usage have spurred intense research into clean energy alternatives, with the electrochemical production of hydrogen (H2) via water splitting emerging as a highly promising approach.2 In the crucial electrochemical process of H2 evolution, the use of advanced electrocatalysts is essential to reduce the overpotential required to drive the reaction, thereby achieving fast kinetics and making it viable for practical applications.3,4Consequently, noble metals, such as Pt, Ag, Au, Ir, and Rh, are frequently employed as catalysts in water splitting reactions owing to their exceptionally high stability (current/potential).5,6 Pt-based nano catalysts are well known for their high efficiency in the hydrogen evolution reaction (HER), whereas Ru and Ir are more extensively studied for their superior performance in the oxygen evolution reaction (OER). Nevertheless, their high expense and scarcity hinder their extensive applications as electrode materials.7 For the generation of H2 energy there is strong need to replace noble metal electrocatalysts with earth-abundant and cost-effective alternatives has become a great challenge in ongoing researches.8,9 Another challenge is the optimization of electrocatalytic systems by minimizing their use while maximizing their performance by fabricating materials at their nanoscale level. The development of cost-effective, high-performance nanostructured bifunctional electrocatalysts for both the HER and the OER is crucial for bridging the gap between precious and non-precious materials, ultimately enabling their commercial viability.
For instance, a wide range of efficient electrocatalysts, including earth-abundant metals, their alloys, sulphides, carbides, and nanocomposites composed of metal and carbon, have been reported by many groups. For example, Ni/NiO@rGO,10 Co3O4-rGO,11 CoNRCNTs,12 Cu2ZnSnS4,13 and Ni- and Fe-doped Cu2ZnSnS4.14 The commercialization of renewable energy technology is hindered by the high cost and limited lifespan of expensive metal-based electrocatalysts, which pose significant obstacles to widespread adoption.15 Research efforts are converging on the development of electrocatalysts that are efficient, abundant, non-toxic and free from precious metals, as this is a critical need in the pursuit of renewable energy and green technology advancement.13
Indeed, materials such as graphene and carbon nanotubes exhibit remarkable properties, and have combined features of metals and semiconductors. Their unique electronic, thermal, and mechanical characteristics make them promising for diverse applications from electronics to advanced material engineering.16,17 Carbon dots (CDs) indeed have garnered significant attention from scientists for their remarkable properties such as favourable biocompatibility and low toxicity. Additionally, their large surface-to-volume ratio is advantageous for adsorption during the process of catalysis. Moreover, the tunable optical and electrical properties of CDs open up opportunities for diverse applications in many fields.18 Heteroatom doping, which involves introducing non-carbon atoms (such as N, B, P or S) into carbon nanomaterials, has indeed been reported to improve their optical and electronic properties. For example, Dong et al. demonstrated a hydrothermal method for synthesizing N-doped carbon dots (N-CDs) using citric acid and EDTA as precursors.19 Furthermore, Ito et al. used a CVD method to synthesize CDs co-doped with N and S to achieve a high quantum yield.20 Herein, CDs and S-doped carbon dots (S-CDs) were synthesized for electrocatalytic overall water splitting reactions (Scheme 1). As compared to CDs, S-CDs electrocatalysts exhibit an excellent response for both the HER and the OER. Sulphur atoms can introduce more active sites with a suitable binding energy for H2 and oxygen (O2) adsorption due to their moderate electronegativity, facilitating the reaction kinetics. Sulphur doping can create synergistic effects with carbon, further promoting the catalytic activity through charge transfer and modification of the electronic structure.
 | ||
| Scheme 1 Schematic of the overall water splitting reactions using a sulphur-doped carbon dot (S-CD) electrocatalytic system. | ||
The growing demand for clean and sustainable energy solutions has driven significant research efforts toward developing efficient and environmentally friendly electrocatalysts. Among various nanomaterials, carbon dots (CDs) and quantum carbon dots (QCDs) have emerged as promising candidates due to their unique properties including high surface area, tunable optical and electronic characteristics, and favorable biocompatibility. These features make them highly versatile for applications ranging from bioimaging and sensing to catalysis and energy conversion.
Recent studies have explored doping strategies to enhance the electrocatalytic activity of CDs and QCDs by modifying their electronic structure and creating more active sites for specific reactions. For instance, nitrogen and sulphur co-doped CDs exhibit superior performance in photocatalysis applications due to their improved charge separation and light absorption capabilities. Similarly, sulphur doping in CDs has demonstrated significant enhancement in electrocatalytic performance, particularly for hydrogen and oxygen evolution reactions, by altering the charge transfer dynamics and optimizing adsorption energies for reaction intermediates. Despite these advances, the design and synthesis of bifunctional, metal-free CDs for overall water splitting remain challenges. The current approach often suffers from issues such as low conductivity, limited stability, or high energy barriers for electrocatalytic reactions. To address these challenges, innovative strategies are required for fine-tuning the structure and composition of CDs, enabling improved catalytic efficiency and durability. In this context, the present study demonstrates the synthesis of S-CDs as bifunctional electrocatalysts for overall water splitting reactions. S-CDs exhibit excellent activity for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), characterized by low overpotentials, high current densities, and superior stability under acidic and alkaline conditions. This work not only highlights the potential of S-CDs as cost-effective and sustainable catalysts but also advances the understanding of doping effects on the electrocatalytic properties of carbon-based materials.21–24
Sulphur doping enhances the crystallinity of carbon materials through multiple mechanisms. First, sulphur incorporation facilitates the rearrangement of carbon atoms in the lattice, leading to a more ordered structure. Experimental results such as X-ray photoelectron spectroscopy (XPS) indicate that sulphur doping effectively reduces the oxygen content, which otherwise contributes to structural disorder. The removal of these oxygen-related defects allows for better lattice organization and improves the overall crystallinity of the material. Additionally, sulphur atoms play a critical role in stabilizing graphitic domains. The formation of C–S bonds within the carbon framework enhances the stability of the sp2 hybridized structure. This stabilization prevents the collapse of graphitic layers and reinforces the integrity of the material. X-ray diffraction (XRD) analysis further supports this, showing sharper diffraction peaks in sulphur-doped materials, indicating an increased degree of crystallinity. The elimination of amorphous carbon phases and the improved alignment of carbon layers contribute to enhanced electrical conductivity and catalytic efficiency. Another crucial factor in crystallinity enhancement is the effect of sulphur on the electronic structure of carbon materials. Sulphur doping modifies the electronic density and charge distribution, reinforcing π-conjugation within the carbon lattice. This stronger conjugation improves structural order and increases the stability of graphitic layers. As a result, sulphur-doped materials exhibit higher durability and better electrochemical performance. The combined effects of oxygen removal, defect healing, graphitic domain stabilization, and π-conjugation enhancement make sulphur doping an effective strategy for improving the crystallinity and functionality of carbon-based catalysts.25,26
2. Experimental
2.1. Chemicals
Citric acid (C6H8O7), sodium thiosulphate pentahydrate (Na2S2O3·5H2O), potassium hydroxide (KOH), sulphuric acid (99% H2SO4), sodium hydroxide (NaOH), and ethanol (99.99% C2H6OH) were purchased from Loba Chemicals Pvt Ltd, India. Deionized water (DI) (18 MΩ cm−1) obtained from a Milli-Q system was used for electrochemical and electrocatalytic experiments.2.2. Synthesis of carbon dots (CDs)
CDs were prepared from citric acid by a simple chemical method, as shown in Scheme 2. In brief, 11.11 mL of conc. H2SO4 (4 M) was taken into a 100 mL beaker and heated at 100 °C for 30 min. Then, 10 g of citric acid was added to the beaker containing H2SO4 under vigorous stirring. Citric acid was dissolved in H2SO4 to form a colourless solution. The colour of the solution rapidly turned to brown, and finally became black after heating at 100 °C for 4 h. Then heating was stopped, and the resulting black solution was filtered by vacuum filtration through a 0.5 μm PTFE membrane. Additional purification steps are crucial to ensure the removal of unreacted precursors and potential impurities from the carbon dots [Materials Today Advances, 2023, 18, 100376]. Accordingly, we performed vacuum filtration using a 0.5 μm PTFE membrane. The collected carbon dot mass was then redispersed in deionised water, followed by the same filtration process and repeated washing with ethanol five times. After that, the black suspension was dried at 70 °C in a vacuum oven for 12 h and denoted as CDs and used for further analysis, doping and electrochemical studies. | ||
| Scheme 2 Schematic of the synthetic methodology for CDs and S-CDs using citric acid and sodium thiosulfate. | ||
2.3. Synthesis of sulphur-doped carbon dots (S-CDs)
The synthesis of S-CDs involves mixing 10 gm of as-synthesized CDs with 10 gm of sodium thiosulphate pentahydrate (Na2S2O3·5H2O) as a sulphur source. The mixture was heated at 100 °C for 3 h, with continuous agitation at 1200 rpm, and then filtered using vacuum filtration. Additional purification steps and repeated washing with deionized water followed by ethanol were carried out before drying the reaction mixture. The resulting black suspension was dried in a vacuum oven at 75 °C for 10 h to yield the final product, denoted as S-CDs, as shown in Scheme 2.The synthesis of S-CDs employs a straightforward chemical method using readily available precursors such as citric acid and sodium thiosulfate pentahydrate. These materials are cost-effective and widely accessible, making the process amenable to scale-up. The reactor development was optimized for large-scale production without compromising the uniformity and quality of S-CDs. This synthesized material was used for the overall water splitting reactions.
2.4. Characterization
The XRD patterns of the materials were obtained using an Ultima IV fully automatic high-resolution X-ray diffractometer equipped with an X-ray generator operating at 40 kV and 40 Ma, with Cu Kα radiation (λ = 1.5406 Å). Scans were performed over a 2θ range of 10° to 80° with a step size of 0.02° at room temperature. The crystalline grain size was calculated using the Scherrer equation, and the lattice distortion was analysed for gaining insights into the structural properties of S-CDs. Elemental composition and mapping were performed using an energy dispersive spectra (EDS) system (JEOL JEM 2100) integrated into a field-emission scanning electron microscope (FESEM). The electron beam energy was set to 20 keV, and the take-off angle was fixed at 35°. Elemental analysis was performed to confirm the presence of sulphur (S), oxygen (O), and carbon (C). Quantitative data were calibrated using a ZAF correction method. XPS analysis was performed using a Thermo-Fischer Scientific ESCALAB Xi+ instrument equipped with a monochromatic Al Kα source (hν = 1486.6 eV). The X-ray source was operated at a power of 150 W (15 kV and 10 mA). Spectra were recorded at a pass energy of 50 eV for high-resolution scans and 200 eV for survey scans, with a step size of 0.1 eV. Calibration was performed using the C 1s peak at 284.8 eV as the standard. Peak fitting was conducted using Gaussian–Lorentzian functions with a Shirley background subtraction. High-resolution TEM images and SAED patterns were obtained using a JEOL JEM-2100 instrument operating at an accelerating voltage of 200 kV. The interplanar spacings and particle sizes were determined from the images, and crystalline features were matched to the XRD data. The FTIR spectra were recorded using a Jasco V750 spectrometer in the range of 4000–400 cm−1. Samples were prepared as KBr pellets, and spectral peaks were assigned based on standard databases to identify functional groups associated with the sulphur-doped carbon dots. The thermal stability of the synthesized CDs and S-CDs was evaluated using a PerkinElmer STA 8000 instrument. Measurements were conducted under an argon atmosphere at a heating rate of 10 °C min−1 over a temperature range of 30–800 °C. Weight loss data were analysed to identify thermal degradation steps and assess the relative stability of S-CDs compared to CDs. The BET surface area and pore volume measurements were conducted using a Micromeritics ASAP 2020 analyser. The analysis involved nitrogen adsorption–desorption isotherms at 77 K. The pore size distribution was derived from the adsorption branch using the Barrett–Joyner–Halenda (BJH) method. The electrochemical studies were conducted using a conventional a three-electrode setup of CHI660E potentiostat (CH-instrument, USA), and a saturated calomel electrode, a Pt wire, and glassy carbon were used as the reference, counter and working electrodes, respectively.3. Results and discussion
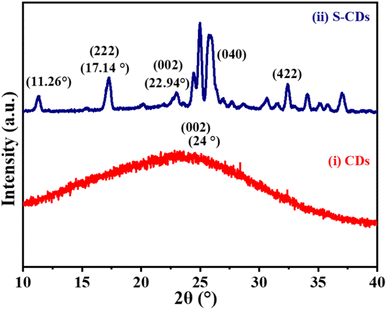
The graphitic structure of the CDs was further confirmed by the XRD patterns, which showed a broad 002 peak at approximately 2θ = 24°. As shown in Fig. 1(i).27 The sharp peaks between 24 and 28° in S-CDs correspond to the graphite plane, indicating some level of crystallinity.28 The XRD analysis revealed a crystallite grain size of ∼17.16 nm for S-CDs. The XRD results indicate that the S-CDs sample exhibited stronger intensities than those of CDs. The XRD pattern of S-CDs is shown in Fig. 1(ii). A sharp peak at 2θ = 11.26° was observed, which could correspond to the presence of specific ordered regions associated with sulphur-containing functional groups or small crystalline domains introduced during the doping process. Broad peaks at 2θ = 17.14° and 22.94° suggest a highly disordered structure, typical of amorphous carbon. These broad features are indicative of reduced long-range order and the presence of localized graphitic structures interspersed with amorphous regions. This combination indicates a structure containing both crystalline and amorphous phases in the synthesized S-CDs.29 The presence of S atoms can distort the carbon lattice, introducing strain and defect sites. Such modifications can lead to a combination of amorphous and semi-crystalline phases within the material. The XRD findings align with the amorphous nature and the disordered carbon network observed in S-CDs, rather than suggesting a fully crystalline structure. The interpretation is consistent with the reported effects of heteroatom doping in carbon materials, where doping induces localized graphitic ordering but disrupts overall crystalline coherence, leading to a mixed-phase structure. This combination of amorphous and ordered regions is crucial for the enhanced catalytic activity of the material. Reference for the graphitic structure is taken from ICDD PDF Card No. 41-1487.The widening of the diffraction peak can be attributed to two possible mechanisms: grain refinement and lattice distortion. However, in this case, lattice distortion is a more reasonable explanation for the doping of sulphur.28
Sulphur doping has a profound impact on the structural characteristics of carbon-based materials. The XRD patterns (Fig. 1(ii)) of S-CDs reveal sharper and more defined peaks than those of undoped CDs. This indicates an enhancement in crystallinity. The increase in crystallinity can be attributed to the following factors: sulphur atoms introduce specific interactions within the carbon matrix, such as π–π stacking and covalent bonding between sulphur and carbon atoms (e.g., C–S and C–SOx bonds). These interactions promote a more ordered arrangement of carbon atoms, leading to the formation of graphitic domains, as evidenced by the sharper diffraction peaks around 2θ = 24°. By considering the grain refinement mechanism, sulphur doping results in a reduction in the crystallite size reported in the XRD analysis (grain size of ∼17.16 nm). This refinement may arise due to lattice distortions caused by sulphur atoms, which create nucleation sites and facilitate the growth of smaller but more crystalline domains.
In addition to this, the synergistic effects of sulphur and carbon were observed, and they altered the electronic structure with bonding dynamics of the carbon matrix. The moderate electronegativity of the sulphur atom had an influence on the charge distribution and enabled better alignment of carbon planes, contributing to an increase in crystalline character.
Additionally, sulphur doping partially converts the disordered (amorphous) regions of carbon dots into more ordered graphitic domains. This transition is consistent with the XRD data, which show both sharp crystalline peaks and residual broad peaks indicative of amorphous regions. This results in an amorphous-to-crystalline transition of the synthesized materials under experimental conditions.
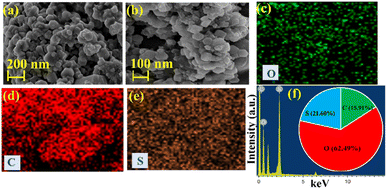
S-CDs can indeed exhibit a hexagonal structure when viewed under a scanning electron microscope, as shown in Fig. 2. The hexagonal structure arises due to the arrangements of carbon atoms in the carbon dots, which can be influenced by the presence of sulphur dopants. This arrangement often manifests as hexagonally shaped clusters or patterns when observed at high magnifications using a FESEM. Moreover, the elemental mapping along with its distribution shown in Fig. 2(c–e) confirms the formation of S-CDs. Furthermore, the ED spectrum of S-CDs shows the presence of the elements C, O, and S (Fig. 2(f)). The weight percentage of synthesized S-CDs catalysts has been shown in Fig. 2(f) (inset) and were found to be C (15.91%), O (62.49%) and S (21.60%).
S-CDs exhibit a hexagonal morphology in high-resolution transmission electron microscopy (HR-TEM), as shown in Fig. 3, and have a well-defined crystalline structure, potentially indicating the formation of S-containing carbon nanomaterials with ordered atomic arrangements. The TEM analysis revealed a d-spacing of approximately 0.375 nm, corresponding to the (002) plane of graphite matching with the XRD results. The selected area electron diffraction (SAED) pattern shown in Fig. 3(d) reveals distinct diffraction spots corresponding to the crystalline structure of S-CDs. These patterns indicate that while S-CDs are primarily amorphous, the incorporation of sulphur dopants introduces regions of localized crystallinity, aligning with the XRD findings of mixed crystalline and amorphous phases. This localized crystallinity enhances electron transfer and contributes to the superior electrocatalytic performance of S-CDs in the HER and OER.
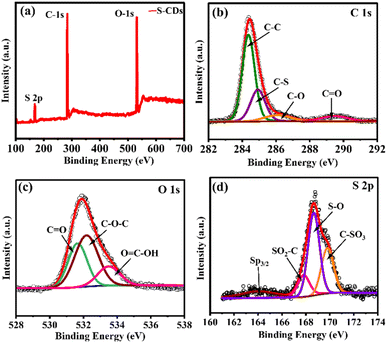
The chemical bonding, oxidation states, and elemental composition of S-CDs are well-characterized by X-ray photoelectron spectroscopy (XPS). Fig. 4 illustrates the high-resolution XP spectra of C 1s, O 1s, and S 2p, which correspond to C, O and S, respectively, at specific binding energies. These spectra provide critical insights into the chemical composition and oxidation states of S-CDs. In particular, the S 2p spectrum reveals detailed information about sulphur species present in the material. The C 1s spectrum offers insights into the carbon bonding environments, while the O 1s spectrum reflects the presence of oxygen-containing functional groups. Fig. 4(a) shows the full XPS survey spectrum of S-CDs, highlighting the elemental composition with distinct peaks corresponding to S, O and C. The intensity and position of these peaks give valuable information about the relative abundance of each element within the sample. The deconvoluted C 1s spectrum, shown in Fig. 4(b), presents four distinct peaks, indicating the presence of various carbon environments and functional groups. These peaks are attributed to different bonding configurations as follows: C–C at 284.3 eV, C–S at 284.9 eV, and C–O at 286.2 eV. The peak observed at 289.5 eV is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, consistent with the results reported in the literature. Similarly, the O 1s spectrum, as illustrated in Fig. 4(c), shows peaks at binding energies of 531.6 eV, 532.1 eV, and 533.5 eV, corresponding to C
O bonds, consistent with the results reported in the literature. Similarly, the O 1s spectrum, as illustrated in Fig. 4(c), shows peaks at binding energies of 531.6 eV, 532.1 eV, and 533.5 eV, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–C, and O
O, C–O–C, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH groups, respectively. These peaks confirm the presence of oxygen-based functional groups. Fig. 4(d) depicts the S 2p spectrum, with four peaks identified. The peak at 163.09 eV is attributed to the S p3/2 binding energy, which corresponds to the presence of sulphur atoms bonded to carbon atoms. This peak suggests that the S-atom is covalently bonded to the carbon lattice, forming C–S bonds.30 While the peaks at 167.8 eV, 168.8 eV, and 169.8 eV correspond to S(O2)–C bonding, S–O, and C–SO3 bonding, respectively. These spectral features collectively provide a comprehensive understanding of the chemical structure and composition of S-CDs. Moreover, the XPS spectrum of the C-dots is showed in Fig. S3(i),† which display two distinct peaks at 283.8 and 531.5 eV, corresponding to C 1s (49.4 at%) and O 1s (50.6 at%), respectively, whereas the C 1s spectrum (Fig. S3b†) reveals three peaks at 283.8, 284.8, and 286.6 eV, which are assigned to C–C, C–O, and C
C–OH groups, respectively. These peaks confirm the presence of oxygen-based functional groups. Fig. 4(d) depicts the S 2p spectrum, with four peaks identified. The peak at 163.09 eV is attributed to the S p3/2 binding energy, which corresponds to the presence of sulphur atoms bonded to carbon atoms. This peak suggests that the S-atom is covalently bonded to the carbon lattice, forming C–S bonds.30 While the peaks at 167.8 eV, 168.8 eV, and 169.8 eV correspond to S(O2)–C bonding, S–O, and C–SO3 bonding, respectively. These spectral features collectively provide a comprehensive understanding of the chemical structure and composition of S-CDs. Moreover, the XPS spectrum of the C-dots is showed in Fig. S3(i),† which display two distinct peaks at 283.8 and 531.5 eV, corresponding to C 1s (49.4 at%) and O 1s (50.6 at%), respectively, whereas the C 1s spectrum (Fig. S3b†) reveals three peaks at 283.8, 284.8, and 286.6 eV, which are assigned to C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, correspondingly. The spectrum O1s (Fig. S3c†) revolved those two well-defined peaks at 531.6 and 532.8 eV, which are attributed to oxygen in C
O bonds, correspondingly. The spectrum O1s (Fig. S3c†) revolved those two well-defined peaks at 531.6 and 532.8 eV, which are attributed to oxygen in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, indicative of carbonyl functionalities, and C–O–C groups, representing hydroxyl or ether functionalities, respectively, highlighting the diverse O-containing functional groups present on the C-dot surface.
O groups, indicative of carbonyl functionalities, and C–O–C groups, representing hydroxyl or ether functionalities, respectively, highlighting the diverse O-containing functional groups present on the C-dot surface.
In addition to this, the BET-surface area and pore volume of CDs and S-CDs were determined by N2 adsorption–desorption analysis, and are shown in Fig. S1.† These materials exhibited characteristic type-IV in the Langmuir model. Accordingly, detailed observation demonstrated in Fig. S1(b) and (d).† Indicated that S-CDs featured a higher BET surface area value of 160.489 m2 g−1 and a pore volume of 2.209 × 10−1 cc g−1. The observed values for S-CDs are high as those of CDs, as shown in Fig. S1(a) and (c)† (104.181 m2 g−1 and 1.278 × 10−1 cc g−1). The presence of sulphur can modify the electronic structure of the CDs, i.e. S-CDs lead to improved adsorption capabilities and surface reactivity compared to CDs material.
For confirming the functional group present in the fabricated CD and S-CD electrocatalysts, FTIR spectroscopy was carried out, and the spectra are displayed in Fig. S2.† It was found that the citric acid infrared spectrum exhibits two notable absorption bands with a strong stretch at 3430 cm−1, indicative of the hydroxy group presence, and a distinct C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at 1680 cm−1 characteristic of the carbonyl group vibration. The C–OH stretching at 1180 cm−1 peak is indicative of the –OH group bonded to carbon, as shown in Fig. S2(a.i).†31 As shown, three peaks i.e., C–O, C
O stretch at 1680 cm−1 characteristic of the carbonyl group vibration. The C–OH stretching at 1180 cm−1 peak is indicative of the –OH group bonded to carbon, as shown in Fig. S2(a.i).†31 As shown, three peaks i.e., C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O–H of S-CDs, are similar to those of CDs.32 The three peaks observed in Fig. S2(a.ii)† at 785 cm−1, 1034 cm−1, and 1120 cm−1 correspond to C–S–C, SO4, and C–S bonding.29 These absorption bands indicated the successful synthesis of S-CDs.
O, and O–H of S-CDs, are similar to those of CDs.32 The three peaks observed in Fig. S2(a.ii)† at 785 cm−1, 1034 cm−1, and 1120 cm−1 correspond to C–S–C, SO4, and C–S bonding.29 These absorption bands indicated the successful synthesis of S-CDs.
Thermal degradation studies of the CDs and S-CDs were carried out under an inert (Ar) atmosphere. As shown in Fig. S2(c),† the weight loss observed at 120 °C (7%) in pure CDs could be attributed to the loss of water molecules associated with the CDs. Meanwhile, the weight loss at 300 °C (13.00%) might indicate the gradual loss of oxygen-bearing moieties, potentially including –C–H groups from the CDs, which is supported by the literature.33 The weight loss of 3–5% at a temperature of 25–150 °C is due to the associated water molecules with S-CDs. A steady weight loss was observed between 200 and 540 °C, which was attributed to the loss of oxygen functional groups, and finally, complete decomposition occurs at 800 °C.34 The result indicates the high thermal stability of S-CDs as compared to the CDs electrocatalytic material.
4. Electrochemical investigations
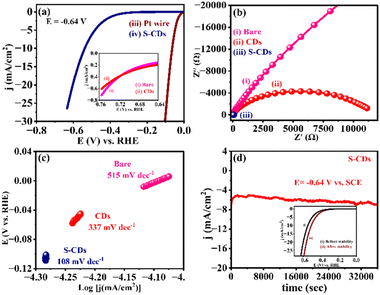
The electrocatalytic activities of the as-synthesized CDs and S-CDs was evaluated for the HER. The linear sweep voltammetry (LSV) profile of S-CDs in 1 M H2SO4 solution (Fig. 5(a)) demonstrates an increase in current density at a potential as low as −0.64 V vs. RHE. Hence, it has been confirmed that S-CDs show superior electrocatalytic activity towards the HER compared to the bare GCE and CDs.Compared to CDs, sulphur dopants can further lower the conversion barriers for the transformation of H+ to H2 during the HER, making the process more efficient. This is because of sulphur's electronic structure and its ability to facilitate favourable interactions with H-atoms and other intermediate species during the HER processes. Additionally, the presence of sulphur enhances the electrocatalytic activity by altering the surface chemistry and electronic properties of the CDs, promoting faster reaction kinetics and ultimately enhancing the catalytic activity of the materials.
According to Xia et al., the electrochemical surface area (ECSA) analysis showed that SA-Came had a significantly larger number of reaction-active sites compared to undoped carbon (C-AC). Moreover, the double-layer capacitance (Cdl) of SA-Came (47.44 mF cm−2) was much higher than that of S-Came (23.05 mF cm−2) and C-AC (5.63 mF cm−2), confirming more electrochemically active sites. SA-Came required a lower overpotential (154 mV at 10 mA cm−2) than that of S-Came (166 mV) and C-AC (228 mV), confirming its superior catalytic efficiency. SA-Came exhibited a lower Tafel slope (89.92 mV dec−1), indicating faster reaction kinetics for the HER. SA-Came remained stable for over 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s under HER conditions, proving its long-term durability. A-Came exhibited a low overpotential (340 mV at 10 mA cm−2), significantly better than C-AC. The Tafel slope of SA-Came for the OER was 86.01 mV dec−1, suggesting efficient oxygen evolution kinetics. The manuscript discusses the formation of a built-in electric field at the interface of sulphur-doped reduced graphene oxide (S-rGO) and amorphous carbon. This field attracts OH− and H+ ions more efficiently, which enhances the HER and OER activities by facilitating intermediate species adsorption and desorption. 35
000 s under HER conditions, proving its long-term durability. A-Came exhibited a low overpotential (340 mV at 10 mA cm−2), significantly better than C-AC. The Tafel slope of SA-Came for the OER was 86.01 mV dec−1, suggesting efficient oxygen evolution kinetics. The manuscript discusses the formation of a built-in electric field at the interface of sulphur-doped reduced graphene oxide (S-rGO) and amorphous carbon. This field attracts OH− and H+ ions more efficiently, which enhances the HER and OER activities by facilitating intermediate species adsorption and desorption. 35
Impedance measurements at an applied potential of −0.64 V vs. RHE were conducted for further evaluation of the electrochemical and electrocatalytic properties of all the electrocatalytic systems, and the results are presented in Fig. 5(b). The result observed that S-CDs shows lower impedance (Zf) (i.e., faradaic and charge transfer impedance) (2853 Ω), as shown in Fig. S4(d).† The result revealed that reduced Zf for S-CDs suggests that CDs showed markedly faster HER kinetics in electrocatalysis after sulphur atoms are doped in it. This is observed due to the synergistic effect that sulphur atoms significantly play at the interface between the material and the electrode.
The linear proportions of Tafel plots as shown in Fig. 5(c) were fit to the Tafel equation (η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a, where “j” is the current density and “b” is the Tafel slope), yielding Tafel slopes of ∼515 mV dec−1, ∼337 mV dec−1 and ∼108 mV dec−1 for bare, CDs and S-CDs, respectively. A small Tafel value for S-CDs compared to bare and CDs suggests superior electrocatalytic activity for HER. According to Fig. 5(d), the durability tests based on chronoamperometric measurements revealed that the electrocatalyst is very stable towards the HER up to 12
j + a, where “j” is the current density and “b” is the Tafel slope), yielding Tafel slopes of ∼515 mV dec−1, ∼337 mV dec−1 and ∼108 mV dec−1 for bare, CDs and S-CDs, respectively. A small Tafel value for S-CDs compared to bare and CDs suggests superior electrocatalytic activity for HER. According to Fig. 5(d), the durability tests based on chronoamperometric measurements revealed that the electrocatalyst is very stable towards the HER up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s at −0.64 V vs. RHE. This result also indicated that S-CD electrocatalysts had a longer stability. In addition to this, the LSV studies have been revealed before and after stability (i–t) for S-CDs, and are shown in the inset of Fig. 5(d). Accordingly, the peak current density slightly decreases after i–t stability (12
000 s at −0.64 V vs. RHE. This result also indicated that S-CD electrocatalysts had a longer stability. In addition to this, the LSV studies have been revealed before and after stability (i–t) for S-CDs, and are shown in the inset of Fig. 5(d). Accordingly, the peak current density slightly decreases after i–t stability (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s).
000 s).
Furthermore, the electrolyte concentration-dependent studies were performed using LSV by varying the concentration of sulphuric acid, as shown in Fig. S4(a).† Accordingly, with the concentration of sulphuric acid increasing from 1 M to 5 M, the concentration of proton also increases proportionality and hence increases the rate of reduction of protons to produce H2. The rate of this reaction is dependent on the availability of protons at the electrode surface. Higher will be the proton concentrations, leading to a greater number of collisions between protons and electrons at the electrode surface, resulting in an increased rate of HER. On the other hand, a higher concentration of sulphuric acid also increases the ionic strength and, hence, the conductivity of the electrolyte. This promotes the movement of ions, including protons, towards the electrode surface, facilitating a more efficient HER.
The LSV results for S-CDs demonstrate a superior response for the HER in sulphuric acid to pH-4, pH-7, pH-9, and KOH solutions, respectively, as shown in Fig. S4(b).† This is likely due to the enhanced catalytic activity of S-CDs in acidic environments, where the presence of sulphur facilitates proton adsorption and promotes efficient HER kinetics. Furthermore, Fig. S4(c)† indicates the stability of all materials for 1000 s. S-CDs show better stability than CDs. Sulphur doping can improve the electrical conductivity of CDs, which is beneficial for maintaining stable current over time during chronoamperometric measurements.
In order to further investigate the HER activity of CDs and S-CD catalysts, cyclic voltammetry curves were measured at different scan rates in the non-faradaic region. The double layer capacitance (Cdl) was determined from a CV using the equation: Cdl = Δj(ja − jc)/2ν, where ja and jc are the anodic and cathodic current densities at ΔE and ν is the scan rate in mV s−1. The non-faradic current density-based electrochemically active surface area (ECSA) was estimated according to the equation: ECSA = Cdl/Cs, where Cs is the specific capacitance of the electrode. The fitting slope (Cdl) can provide information about the size of the electrochemical active area (ECSA).36 In this study, the scan rate vs. current density difference was plotted, and the fitting slopes were obtained, as shown in Fig. S5.† The Cdl values for CDs and S-CDs are 42.73 and 46.29 mF cm−2, respectively with the calculated roughness factors of 601.83 and 651.97, respectively. The highest value for S-CDs indicated that a higher number of active sites were available for interactions followed by electrocatalytic transformation.
The LSV curves presented in Fig. S6† provide compelling evidence of the electrocatalyst's stability and durability toward the HER. These curves, corresponding to the 1st and 1000th cycles, allow a direct comparison of the electrocatalyst's initial and long-term performance under cyclic stability measurements. Such an analysis is crucial for evaluating the material's suitability for real-world applications in sustainable energy technologies. The LSV curve for the 1st cycle represents the initial electrochemical behavior of the electrocatalyst. It provides key information, such as the onset potential, where the HER begins, and the peak potential, which corresponds to the voltage at maximum catalytic efficiency. Additionally, the current density reflects the rate of hydrogen production, serving as a marker for the catalyst's activity. The sharpness and position of the peak potential during this initial cycle suggest the electrocatalyst's high catalytic efficiency. The LSV curve for the electrocatalyst after 1000 cycles is shown in Fig. S6,† which indicates minimal deviation from the 1st cycle. While slight changes in the peak potential are observed, these are negligible, indicating exceptional stability. The nearly unchanged current density further highlights that the catalyst retains its activity even after prolonged operation. This behaviour implies that the active sites responsible for the HER on the catalyst's surface remain intact, demonstrating its robustness. The small changes observed in the peak potential after 1000 cycles might be attributed to minor surface modifications, such as oxidation or adsorption of intermediates. However, these changes are insufficient to compromise the electrocatalyst's performance. Such durability under extended cycling underscores the material's ability to maintain its structural and functional integrity, which is a critical factor for practical applications. In conclusion, the LSV curves confirm that the electrocatalyst exhibits excellent stability and durability toward the HER (Table 1), maintaining its performance over 1000 cycles. This long-term reliability is a significant advantage, making the electrocatalyst a strong candidate for use in renewable hydrogen production processes. The findings highlight its potential for real-world applications, ensuring efficient and sustainable energy conversion over extended periods.
| Sr. no. | Materials | Onset potential (V) | Tafel slope (mV dec−1) | Ref. |
|---|---|---|---|---|
| 1 | P-Doped carbons | −0.27 V | −112 to −214 | 37 |
| 2 | B-SuG | −0.20 V | 99 | 38 |
| 3 | N,S-CN | −0.29 V | 76.9 | 39 |
| 4 | ONPPGC/OCC | −0.4 V | 84 | 40 |
| 5 | N,P-graphene nanosheet | −0.42 V | 91 | 41 |
| 6 | FPGC | −0.30 | 212 | 42 |
| 7 | Activated carbon nanotubes | −0.10 | 71.3 | 43 |
| 8 | S-CDs | −0.48 | 108 | Present work |
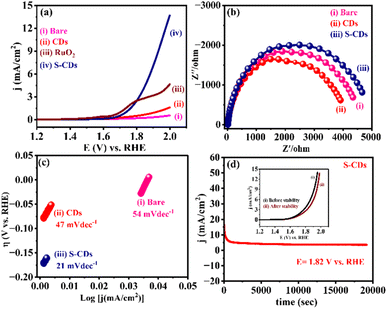
Furthermore, the electrocatalytic performance of S-CDs against the OER evaluated using LSV in 1 M KOH solution (as an electrolyte) and represented in Fig. 6(a). The onset potential is a crucial parameter when evaluating the performance of an OER electrocatalyst. However, the potential at which a current of 10 mA cm−2 is achieved is often used as a practical benchmark. In the context of OER, the overpotential, often denoted as “η” (eta), is the potential difference between the potential recorded at a specific current density during the reaction and the thermodynamic equilibrium potential.44 S-CDs typically exhibit more electrocatalytic activity than bare and CDs, as shown in Fig. 6(a).
The kinetics of the oxygen evolution reaction at the interface of electrode/electrocatalysts was studied via. electrochemical impedance spectroscopy (EIS), The EIS spectra were measured at 1.82 V vs. RHE frequency range from 10 KHz, as shown in Fig. 6(b), focusing on bare, CDs and S-CDs. Accordingly, S-CDs exhibited a lower Rct (986.4 Ω) value, as shown in Fig. S7(d),† indicating a higher charge transport efficiency.
According to Fig. 6(c), a lower Tafel slope leads to a faster increase in OER rate with the increase in overpotential, making it the main index for evaluating OER performances. The Tafel slope for S-CDs exhibits the lowest value of 21 mV dec−1, corresponding to faster OER kinetics. This indicates that the highest OER activity by S-CDs (iii) (21 mV dec−1) compared with the bare (i) (54 mV dec−1) and CDs (ii) (47 mV dec−1). Next, the stability of S-CDs was investigated further by chronoamperometric measurements at an applied potential of 1.82 V vs. RHE for 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, as shown in Fig. 6(d). These results signify an excellent stability of S-CD metal-free electrocatalysts towards the OER. This promoted activity could be due to sulphur, which supports enhanced electron transfer activity. Furthermore, the LSV studies have been revealed before and after stability (i–t) studies for S-CDs and have been shown in the inset of Fig. 6(d). Accordingly, the peak current density slightly decreases after i–t stability (11
000 s, as shown in Fig. 6(d). These results signify an excellent stability of S-CD metal-free electrocatalysts towards the OER. This promoted activity could be due to sulphur, which supports enhanced electron transfer activity. Furthermore, the LSV studies have been revealed before and after stability (i–t) studies for S-CDs and have been shown in the inset of Fig. 6(d). Accordingly, the peak current density slightly decreases after i–t stability (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s). Furthermore, Fig. S7(a)† demonstrates the electrolyte concentration-dependent LSV of S-CDs from 0.5 M to 2 M KOH. The increase in current density with concentration suggests enhanced electrocatalytic activity, possibly due to improved mass transport or availability of active sites. The slight shift in potential for oxygen evolution reactions could indicate changes in reaction kinetics or surface properties induced by different concentrations of KOH.
000 s). Furthermore, Fig. S7(a)† demonstrates the electrolyte concentration-dependent LSV of S-CDs from 0.5 M to 2 M KOH. The increase in current density with concentration suggests enhanced electrocatalytic activity, possibly due to improved mass transport or availability of active sites. The slight shift in potential for oxygen evolution reactions could indicate changes in reaction kinetics or surface properties induced by different concentrations of KOH.
Moreover, the role of solution pH on the LSV profile for the OER is shown in Fig. S7(b).† The notable response observed in 1 M KOH suggests that alkaline conditions favour the OER activity, likely due to the presence of OH- ions, facilitating the reaction kinetics. Comparatively, acidic conditions may hinder the OER due to proton competition at the active sites or changes in surface chemistry. As shown in Fig. S7(c),† for better understanding the stability of S-CDs compared to CDs, the chronoamperometric stability was studied for 1000 s. As compared to CDs, S-CDs show good stability towards the OER. However, Fig. S8† shows the Cdl values for CDs and S-CDs as 23.02 and 26.12 mF cm−2, respectively, with roughness factors as 324.22 and 367.88, respectively. The highest value observed for S-CDs indicated that a higher number of sites were available for interactions.
The Linear Sweep Voltammetry (LSV) curves shown in Fig. S9† also provide critical insights into the electrocatalyst's performance toward the oxygen evolution reaction (OER) (Table 2). These curves, representing the 1st and 1000th cycles, demonstrate the stability and durability of the electrocatalyst when subjected to extended operational cycles, which is essential for practical applications in renewable energy systems like water splitting. The LSV curve for the 1st cycle captures the initial electrochemical activity of the electrocatalyst during the OER. Key metrics such as the onset potential, which marks the beginning of the oxygen evolution process, and the peak potential, indicating the voltage for maximum catalytic efficiency, are observed. The high current density achieved reflects the material's excellent ability to facilitate oxygen evolution. The sharpness of the peak and its favourable position highlight the electrocatalyst's strong catalytic performance in the initial stage. After 1000 cycles, the LSV curve reveals minimal changes compared to the 1st cycle, showcasing the electrocatalyst's remarkable stability under prolonged use. The small change in peak potential indicates that the energy required for oxygen evolution does not significantly increase over time. Similarly, the current density remains stable, signifying that the active sites on the catalyst surface continue to function effectively even after extended cycling. This stability is a testament to the material's resistance to the degradation or loss of activity. The small changes observed in the peak potential over 1000 cycles are likely due to minor surface alterations, such as oxidation or the accumulation of reaction intermediates. However, these changes are negligible, highlighting the robustness of the electrocatalyst. Its ability to maintain consistent performance over numerous cycles indicates that the structural integrity of the active sites remains intact, which is a critical requirement for OER electrocatalysts in practical applications. In conclusion, the LSV curves confirm the electrocatalyst's exceptional stability and durability for the OER, retaining its efficiency after 1000 cycles. This robustness makes it a promising candidate for renewable energy technologies such as water splitting, where the stability and reliability of the oxygen evolution process are vital for long-term operation. These findings reinforce the material's potential for efficient and sustainable energy conversion, ensuring consistent oxygen production over extended periods.
Sulphur doping introduces electronic modifications by incorporating sulphur atoms into the carbon dot structure. This substitution creates new active sites due to sulphur's moderate electronegativity, facilitating better adsorption of reaction intermediates.30 The resulting charge redistribution enhances electron mobility, thereby promoting efficient charge transfer (Scheme 3). This improves reaction kinetics by lowering the activation energy required for both hydrogen and oxygen evolution reactions. Sulphur atoms form C–S and C–SO3 bonds, which provide favorable binding energies for reaction intermediates, further accelerating the electrocatalytic activity.30
 | ||
| Scheme 3 Mechanism for the electrochemical water splitting reaction on active sites of S-CDs, with probable electron transfer pathways for the HER and OER in 1 M H2SO4 and KOH, respectively. | ||
Conclusion
S-CDs composites were synthesized by a simple chemical method and characterized by morphological and structural characterization techniques such as SEM, HRTEM, XPS, XRD, FTIR spectroscopy, TGA and BET surface area measurements. Furthermore, CDs and S-CDs were evaluated for overall water splitting reactions (both the HER and the OER). Significantly, for the HER superior cathodic current density has been observed at an low overpotential −0.53 V vs. RHE and smaller Tafel slope ∼108 mV dec−1 in acidic medium also for OER high anodic current density of 10 mA cm−2 at an low overpotential 1.94 V vs. RHE and smaller Tafel slope 21 mV dec−1 in basic medium compared to bare and CDs. Moreover, their chronoamperometric current stability is good up to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s for the HER and 11
000 s for the HER and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s for the OER at their respective onset potentials, which make them efficient bifunctional materials for overall water splitting reactions. Furthermore, the concentration-dependent LSV studies of S-CDs were conducted in acidic and basic media. The increase in current density with the increase in concentration suggests enhanced electrocatalytic activity, possibly due to improved mass transport or active site availability. The slight shift in potential could indicate changes in reaction kinetics or surface properties induced by the varying concentrations of H2SO4 and KOH. The pH dependence of LSV and repeatability have been studied and the best result has been observed in acidic and basic media for the HER and OER, respectively. Sulphur doping increases the electrical conductivity of carbon dots, facilitating faster electron transfer and improved reaction kinetics. The combination of S and CDs exhibited excellent electrocatalytic activity, stability and durability, outperforming the conventional metal-free catalysts.
000 s for the OER at their respective onset potentials, which make them efficient bifunctional materials for overall water splitting reactions. Furthermore, the concentration-dependent LSV studies of S-CDs were conducted in acidic and basic media. The increase in current density with the increase in concentration suggests enhanced electrocatalytic activity, possibly due to improved mass transport or active site availability. The slight shift in potential could indicate changes in reaction kinetics or surface properties induced by the varying concentrations of H2SO4 and KOH. The pH dependence of LSV and repeatability have been studied and the best result has been observed in acidic and basic media for the HER and OER, respectively. Sulphur doping increases the electrical conductivity of carbon dots, facilitating faster electron transfer and improved reaction kinetics. The combination of S and CDs exhibited excellent electrocatalytic activity, stability and durability, outperforming the conventional metal-free catalysts.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to HRDG-CSIR (Ref. 01(2922)18/EMR-II) for the financial support and the Department of Chemistry, Dr Babasaheb Ambedkar Marathwada University, Chhatrapati Sambhajinagar for laboratory facilities.Notes and references
- A. V. Munde, B. B. Mulik, R. P. Dighole and B. R. Sathe, New J. Chem., 2020, 44, 15776–15784 RSC.
- L. Yu, B. Y. Xia, X. Wang and X. W. Lou, Adv. Mater., 2016, 28, 92–97 CrossRef CAS PubMed.
- Z. Ge, B. Fu, J. Zhao, X. Li, B. Ma and Y. Chen, J. Mater. Sci., 2020, 55, 14081–14104 CrossRef CAS.
- T. F. Jaramillo, K. P. Jørgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100–102 CrossRef CAS PubMed.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nat. Mater., 2012, 11, 963–969 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- L. Tian, Z. Li, X. Xu and C. Zang, J. Mater. Chem. A, 2021, 9, 13459–13470 RSC.
- S. Roy, S. Karmarkar, I. Mondal, K. Naskar, W. Sarkar and I. Deb, Chem. Commun., 2023, 59, 9074–9077 RSC.
- D. Wang and D. Astruc, Chem. Soc. Rev., 2017, 46, 816–854 RSC.
- S. S. Narwade and R. V. Digraskar, Int. J. Hydrogen Energy, 2019, 44, 1–10 CrossRef.
- A. V. Munde, B. B. Mulik, R. P. Dighole and B. R. Sathe, New J. Chem., 2020, 44, 15776–15784 RSC.
- X. Zou, X. Huang, A. Goswami, R. Silva, B. Sathe and T. Asefa, Angew. Chem., Int. Ed., 2014, 53, 4372–4376 CrossRef CAS PubMed.
- R. V. Digraskar, V. S. Sapner, S. M. Mali, S. S. Narwade, A. V. Ghule and B. R. Sathe, ACS Omega, 2019, 4, 7650–7657 CrossRef CAS PubMed.
- R. V. Digraskar, S. M. Mali, S. B. Tayade, A. V. Ghule and B. R. Sathe, Int. J. Hydrogen Energy, 2019, 44, 8144–8155 CrossRef CAS.
- S. S. Narwade, S. M. Mali and B. R. Sathe, New J. Chem., 2021, 45, 3932–3939 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- V. S. Sapner, B. B. Mulik, R. V. Digraskar, S. S. Narwade and B. R. Sathe, RSC Adv., 2019, 9, 6444–6451 RSC.
- M. Hatimuria, P. Phukan, S. Bag, J. Ghosh, K. Gavvala, A. Pabbathi and J. Das, Catalysts, 2023, 13, 1–19 CrossRef.
- Y. Dong, H. Pang, H. Yang, J. Jiang, Y. Chi and T. Yu, RSC Adv., 2014, 4, 32791–32795 RSC.
- Y. Ito, W. Cong, T. Fujita, Z. Tang and M. Chen, Angew. Chem., Int. Ed., 2015, 54, 2131–2136 CrossRef CAS PubMed.
- X. Wang, Y. Feng, P. Dong and J. Huang, Front Chem, 2019, 7, 1–9 CrossRef PubMed.
- V. C. Hoang, K. Dave and V. G. Gomes, Nano Energy, 2019, 66, 104093 CrossRef CAS.
- Z. Liu, C. Shan, G. Wei, L. Jiang, G. Hu, Z. Fang, T. Tang and M. Li, Molecules, 2023, 28, 1–12 Search PubMed.
- G. Wei, T. Tang, R. Xu, Z. Xie, S. Diao, J. Wen, L. Jilang, G. Hu and M. Li, Molecules, 2024, 29, 1–11 Search PubMed.
- X. Li, X. Duan, C. Han, X. Fan, Y. Li, F. Zhang, G. Zhang, W. Peng and S. Wang, Carbon, 2019, 148, 540–549 CrossRef CAS.
- A. M. El-Sawy, I. M. Mosa, D. Su, C. J. Guild, S. Khalid, R. Joesten, J. F. Rusling and S. L. Suib, Adv. Energy Mater., 2016, 6, 1–12 Search PubMed.
- P. Zuo, X. Lu, Z. Sun, Y. Guo and H. He, Microchim. Acta, 2016, 183, 519–542 CrossRef CAS.
- X. Qiao, M. A. Gondal, K. Shen and Q. Xu, J. Nanosci. Nanotechnol., 2017, 17, 1–13 CrossRef.
- S. R. Kamali, C. N. Chen, D. C. Agrawal and T. H. Wei, J. Anal. Sci. Technol., 2021, 12, 1–11 CrossRef.
- A. Kagkoura, R. Arenal and N. Tagmatarchis, Nanomaterials, 2020, 10, 1–11 Search PubMed.
- P. Pimpang, R. Sumang and S. Choopun, J. Sci., 2018, 45, 2005–2014 CAS.
- P. Pimpang, R. Sumang and S. Choopun, Chiang Mai J. Sci., 2018, 45, 2005–2014 CAS.
- S. Pandey, A. Mewada, M. Thakur, A. Tank and M. Sharon, RSC Adv., 2013, 48, 1–7 Search PubMed.
- H. Sadhanala, S. Senapati and K. Nanda, Carbon, 2018, 2, 1–24 Search PubMed.
- C. Xia, S. Surendran, S. Ji, D. Kim, Y. Chae, J. Kim, M. Je, M. Han, W. Choe, C. Choi, H. Choi, J. Kim and U. Sim, Carbon Energy, 2022, 4, 491–505 CrossRef CAS.
- S. Sun, H. Li and Z. J. Xu, Joule, 2018, 2, 1024–1027 CrossRef.
- S. García-Dalí, J. Quílez-Bermejo, J. Castro-Gutiérrez, N. Baccile, M. T. Izquierdo, A. Celzard and V. Fierro, Carbon, 2023, 1–34 Search PubMed.
- B. R. Sathe, X. Zou and T. Asefa, Catal. Sci. Technol., 2013, 4, 1–8 Search PubMed.
- K. Qu, Y. Zheng, X. Zang, K. Davey, S. Dai and S. Qiao, ACS Nano, 2017, 11, 7293–7300 CrossRef CAS PubMed.
- J. Lai, S. Li, F. Wu, M. Saqib, R. Luque and G. Xu, Energy Environ. Sci., 2012, 1–6 Search PubMed.
- Y. Zheng, Y. Jiao, L. Li, T. Xing, Y. Chen, M. Jaroniec and S. Qiao, ACS Nano, 2014, 8, 5290–5296 CrossRef CAS PubMed.
- X. Yue, S. Huang, J. Cai, Y. Jin and P. Shen, J. Mater. Chem. A, 2017, 1–44 Search PubMed.
- W. Cui, Q. Liu, N. Cheng, A. M. Asiri and X. Sun, Chem. Commun., 2014, 50, 9340–9342 RSC.
- J. Song, C. Wei, Z. F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC.
- T. Y. Ma, S. Dai, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed. Engl., 2014, 53, 1–6 CrossRef.
- G. L. Tian, M. Q. Zhao, D. Yu, X. Y. Kong, J. Q. Huang, Q. Zhang and F. Wei, Small, 2014, 10, 2251–2259 CrossRef CAS PubMed.
- H. Bin Yang, J. Miao, S. F. Hung, J. Chen, H. B. Tao, X. Wang, L. Zhang, R. Chen, J. Gao, H. M. Chen, L. Dai and B. Liu, Sci. Adv., 2016, 2, 1–11 Search PubMed.
- S. Dresp, F. Luo, R. Schmack, S. Kühl, M. Gliech and P. Strasser, Energy Environ. Sci., 2016, 9, 2020–2024 RSC.
- S. Liu, H. Zhang, Q. Zhao, X. Zhang, R. Liu, X. Ge, G. Wang, H. Zhao and W. Cai, Carbon, 2016, 106, 74–83 CrossRef CAS.
- S. Narwade, S. Mali and B. R. Sathe, New J. Chem., 2021, 45, 3932–3939 RSC.
- Z. Li, Y. Yao, Y. Niu, W. Zhang, B. Chen, X. Zeng and J. Zou, Chem. Eng. J., 2021, 418, 129–321 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: BET surface area measurement studies, concentration, and pH dependent studies, i–t measurements for HER, ECSA studies for HER, concentration, and pH dependent studies, i–t measurements for OER, ECSA studies for OER. See DOI: https://doi.org/10.1039/d5se00315f |
| This journal is © The Royal Society of Chemistry 2025 |