 Open Access Article
Open Access ArticleEmpowering catalysis and separation: morphology control of MFI zeolites using organic additives
Jun
Zhao
ab,
Haijun
Yu
ab,
Haimei
Xu
b,
Zhiyu
He
 a,
Feng
Shao
a,
Feng
Shao
 *a,
Peng
Lu
*b and
Valentin
Valtchev
*a,
Peng
Lu
*b and
Valentin
Valtchev
 *bc
*bc
aKey Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China. E-mail: feng.shao@ouc.edu.cn
bThe ZeoMat Group, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Laoshan District, Qingdao 266101, China. E-mail: lupeng@qibebt.ac.cn
cNormandie University, ENSICAEN, UNICAEN, CNRS, Laboratoire Catalyse et Spectrochimie, F-14000 Caen, France. E-mail: valentin.valtchev@ensicaen.fr
First published on 10th December 2024
Abstract
As one of the most successful inorganic materials, MFI zeolite has been widely used in petrochemical and fine chemical industries. However, the presence of only micropores in MFI zeolite creates diffusion barriers and thus precludes its usage in processes involving large substrates. It is highly desirable to mitigate the diffusion pathways in MFI zeolites. One of the efficient methods is the morphology control strategy, which has become a hot topic in the past few decades. In this review, we summarize the progress of MFI zeolite morphology control using specific organic additives as morphology modifiers to enhance the catalytic and separation performance. Organic additives, including urea, amino acids, small organic molecules, and polymers, were categorized based on the MFI zeolites induced by them. The morphologies generated can be classified as nanocrystals, aggregated nanoparticles, nanosheets, intergrown nanosheets, plates, intergrown plates, needles, and bulky prismatic crystals, depending on the specific additives. The formation mechanisms of different morphological MFI zeolites and their properties are also discussed. This review is of great importance for the controllable synthesis of zeolites and rational design of zeolite catalysts.
1. Introduction
Zeolites, one of the most important inorganic microporous materials with a plethora of applications such as ion-exchangers, adsorbents, and catalysts,1–7 are constructed from tetrahedral atoms (T-atoms, such as Si, Al, and P) bridged by oxygen atoms to form three-dimensional frameworks. Uniform-sized micropores and high thermal and hydrothermal stability provide zeolites with excellent properties, especially as molecular sieves for size-related processes. MFI zeolite, a “star zeolite”, has been used extensively in petrochemical and fine chemical industries. The structure of MFI zeolite comprises two-channel systems, i.e., a sinusoidal channel (5.1 × 5.5 Å) along the a–c direction and a straight channel (5.4 × 5.6 Å) along the b-axis.8–10 According to its framework compositions, MFI zeolite subtypes mainly include ZSM-5 (aluminosilicate),11 silicalite-1 (silicate),12 TS-1 (titanosilicate),13etc. ZSM-5 was the first zeolite with MFI topology to be reported in the 1970s by Mobil.14 Based on their compositions, ZSM-5, silicalite-1, and TS-1 are widely used as acidic catalysts, adsorbents/membranes, and oxidation catalysts, respectively.Due to their microporous nature, MFI zeolites showed limitations in processes involving large substrates, thus hindering their potential applications. Strategies for mitigating the diffusion limitations have focused on decreasing sizes to make nanocrystals (variation of gel/sol concentration and composition and modifiers)15–19 and two-dimensional sheets/plates (organic structure-directing agents, OSDAs),20,21 and generating mesopores through templates (surfactants)22–25 or post-treatments (alkaline and acid etching).26–28 A prominent breakthrough among those approaches was achieved by Ryoo et al., who synthesized hierarchical MFI zeolites composed of unit-cell nanosheets with mesopores using pre-designed surfactant-based OSDAs.29 Fluoride-mediated synthesis has been proven effective for obtaining two-dimensional MFI zeolite plates.30 Although the surfactant and fluoride strategies are very promising from the academic point of view, their practical implication might be hindered considering the high cost and non-environmental friendliness. Thus, economic and eco-friendly methods are still needed for obtaining MFI zeolites with desired morphologies.
In this review, instead of providing a comprehensive overview of morphology manipulation methods, we focused on summarizing morphology control approaches of MFI zeolites using different organic modifiers, including urea, amino acids, small organic molecules, and polymers (Scheme 1). These modifiers have demonstrated capability to induce different morphologies, such as nanocrystals, nanosheets, plates, prisms, house-of-cards and intergrown sheets/plates. Importantly, these organic modifiers are cost-effective, considering industrial applications. The possible formation mechanisms of MFI zeolites with certain morphological features together with their advantageous applications are briefly summarized using specific additives. The perspectives and future challenges are also presented. A summary of MFI zeolites with various morphologies is given in Table 1, where the categorization is made based on the additive types, OSDA types, phases or products obtained, morphologies of the crystals and preferred orientation together with dimensions along the specific crystallographic axis.
| Additive type | OSDA type | Phase/product | Morphology | Preferred orientation/thickness | Ref. |
|---|---|---|---|---|---|
| Urea only | TPAOH | ZSM-5 | Sheet | b-Axis/90–110 nm | 31 |
| b-Axis/80–100 nm | 32 | ||||
| b-Axis/0.12–0.33 μm | 33 | ||||
| TPABr | ZSM-5 | Sheet | c-Axis/2.1 μm | 34 | |
| TPAOH | TS-1 | Sheet | b-Axis/50 nm | 35 | |
| TPAOH | H-ZSM-5 | Sheet | b-Axis/720–245 nm | 36 | |
| TPAOH | Silicalite-1 | Plate or sheet | b-Axis/50 nm | 37 | |
| TPAOH | MFI | Sheet | b-Axis/50–100 nm | 38 | |
| TPAOH | ZSM-5 | Plate | c-Axis/— | 39 | |
| TPAOH | TS-1 | Flat-plate or sheet | b-Axis/100 nm | 40 | |
| Urea & isopropyl alcohol (IPA) | TPAOH | TS-1 | Sheet | b-Axis/80 nm | 41 |
| b-Axis/95 nm | 42 | ||||
| TPAOH | ZSM-5 | Sheet | b-Axis/100 nm | 43 | |
| TPAOH | H-ZSM-5 | Plate | b-Axis/60 nm | 44 | |
| TPAOH | H-ZSM-5 | Coffin | b-Axis/60–250 nm | 45 | |
| Urea as alkalinity regulator | TPAOH | ZSM-5 | Aggregated nanocrystals | b-Axis/∼160 nm | 46 |
| L-Carnitine & ethanol | TPAOH | TS-1 | Elongated platelet | b-Axis/∼300 nm | 47 |
| D-Arginine | TPAOH | MFI | Elongated plate | b-Axis/— | 48 |
| L-Lysine | TPAOH | MFI | Hexagonal prism | b-Axis/∼56 nm, ∼37 nm | 49 |
| L-Glutamic acid | TPAOH | Silicalite-1 | Nanocrystals | —/av. 60 nm | 50 |
| TS-1 | —/av. 100 nm | ||||
| L-Lysine | TPAOH | ZSM-5 | Nanocrystals | —/∼100 nm | 51 |
| L-Lysine | TPAOH | Silicalite-1 | Nanocrystals | —/50–450 nm | 52 |
| Diols (EG, DEG, TEG, and tEG) | TPAOH | Silicalite-1 | Plate (hexagonal prism) | b-Axis/0.11–0.25 μm | 53 |
| Pyrocatechol | TPA+ | H-ZSM-5 | Sheet | c-Axis/∼548–1530 nm | 54 |
| Ethanol | TPAOH | ZSM-5 | Sheet | b-Axis/90 nm | 55 |
| Spermine | TPAOH | Silicalite-1 | Platelet | b-Axis/<300 nm | 56 |
| Tributylphosphine oxide (TBPO) | TPAOH | b-Axis/<150 nm | |||
| Triethylenetetramine (TETA) | TPAOH | Coffin | —/— | ||
| Triethylamine | C18−6−6Br2 | ZSM-5 | Intertwined nanosheets; multilamellar nanosheets with house-of-cards | b-Axis/8–10 nm | 57 |
| N-Methyl-2-pyrrolidone | — | ZSM-5 | House-of-cards | b-Axis/100 nm | 58 |
| Allophanamide | TPABr | ZSM-5 | Sheet | c-Axis/— | 34 |
| Tetramethylguanidine (TMG) | TPAOH | ZSM-5 | Plate | b-Axis/85 nm | 59 |
| Guanidine | TPAOH | ZSM-5, TS-1 | Plate | b-Axis/75–110 nm | 60 |
| Imidazolium salts | TPABr | Silicalite-1 | Coffin | c-Axis/5.8–22.9 μm | 61 |
| Glucose | TPAOH | ZSM-5 | Large prismatic | —/— | 62 |
| L c = 3.5–5.0 μm | |||||
| TPAOH | H-ZSM-5 | Plate | b-Axis/300–500 nm | 63 | |
| Propylene diamine | TPABr | ZSM-5 | Plate | —/— | 64 |
| Piperidine | |||||
| Catechol | |||||
| Pyrrolidine | TPABr | ZSM-5 | Sheet | b-Axis/90 nm | |
| Tetrahydrofuran | |||||
| Hexamethylenetetramine (H-amine) | TPAOH | ZSM-5 | Noodle-like | c-Axis/— | 39 |
| L c/Lb = 35; La/Lb = 3 | |||||
| Tributylphosphine oxide (TBPO) | TPAOH | MFI | Coffin (membrane) | b-Axis/1.1 ± 0.1 μm | 65 |
| Poly(hexamethylene biguanide) hydrochloride | TPAOH | MFI | Coffin (membrane) | b-Axis/∼400 nm | 66 |
| PEG2000 | TPAOH | H-ZSM-5 | Hexagonal prism | b-Axis/300–500 nm | 67 |
| Gelatin | TPABr | ZSM-5 | Sheet | b-Axis/— | 68 |
| TPAOH | TS-1 | Plate | b-Axis/40–300 nm | 69 | |
| Polyacrylamide | TPAOH | H-ZSM-5 | Hexagonal prism | c-Axis/30 μm | 70 |
| Elongated prism | c-Axis/40 μm | ||||
| Polyethylene oxide–polypropylene oxide–polyethylene oxide (P123) | TPAOH | TS-1 | Stacked nanoplate | b-Axis/20–25 nm (unilamellar) | 71 |
2. Morphology control of MFI zeolites using organic additives
In recent years, great efforts have been made in enhancing the catalytic and separation properties based on the zeolite morphology control strategy. The current morphology control strategy mainly includes additive-assisted synthesis. Urea and amino acids are both classical morphology modifiers in the controllable synthesis of zeolites; thus in this work, we separate them from small organic molecules (Section 3), and review them as distinct categories on their own, respectively.2.1 Urea
 | ||
| Fig. 1 (A) SEM images of (a) s-ZSM-5 and (b) n-ZSM-5. (B) Performance of catalysts, using different mixing methods, in the LT-FTO. Reprinted from ref. 31 with permission from Springer Nature Ltd, copyright 2022. | ||
Liu et al. synthesized sheet-like ZSM-5 zeolites with controllable c-axis length (called U-ZSM-5-x, where x = urea/silica) using tetrapropylammonium bromide (TPABr) as the OSDA and urea as the modifier by a one-pot solvent-free method.34 SEM inspection revealed that compared with a urea-free zeolite (named SH-ZSM-5), the c-axis of the nanosheet synthesized using U-ZSM-5-0.03 is increased by about twice (from 1.2 μm to 2.1 μm), and the b-axis is reduced by about half (from 0.9 μm to 0.4 μm). The performance of methanol to gasoline (MTG) was evaluated. The methanol conversion rate was 100%, which was higher than that of ZSM-5 with conventional morphology, and the lifetime was up to 900 h.
Bao's group drew inspiration from previous urea-assisted methods for the preparation of sheet-like ZSM-5 crystals, aiming to improve their catalytic activity in syngas to aromatics (STA) reaction.33 They investigated the optimal synthesis conditions for ZSM-5 nanosheets by adding different amounts of urea. The synthesis was performed using the gel molar composition of SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3
Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPAOH
TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea = 1
urea = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0025
0.0025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x, where x = 0–2.8, and the mixture was crystallized at 180 °C for 4 days. The XRD pattern shows that the zeolite sample with a urea/silica ratio of 2.8 exhibits a preferred orientation at 2θ = 8.9°, indicating that the sample has a sheet-like structure. When the urea/silica ratio is 0.5, the crystal has sheet-like morphology (Lc = 1.58 μm and Lb = 0.12 μm). Then, they continued to increase the amount of urea and found that the crystal size on each axis became larger. They performed the STA reaction over ZnCrOx–ZSM-5, and found that a lower b/a axis length ratio of zeolites led to formation of more aromatics, but the selectivity of CH4 and C2–C4 was not influenced.
x, where x = 0–2.8, and the mixture was crystallized at 180 °C for 4 days. The XRD pattern shows that the zeolite sample with a urea/silica ratio of 2.8 exhibits a preferred orientation at 2θ = 8.9°, indicating that the sample has a sheet-like structure. When the urea/silica ratio is 0.5, the crystal has sheet-like morphology (Lc = 1.58 μm and Lb = 0.12 μm). Then, they continued to increase the amount of urea and found that the crystal size on each axis became larger. They performed the STA reaction over ZnCrOx–ZSM-5, and found that a lower b/a axis length ratio of zeolites led to formation of more aromatics, but the selectivity of CH4 and C2–C4 was not influenced.
Zhang et al. synthesized sheet-like TS-1 zeolite with a short b-axis at 170 °C by introducing urea as a growth inhibitor in the initial synthesis and adding seeds.35 The b-axis thickness of the TS-1 zeolite prepared by pre-crystallization at the aging solution stage is as small as 50 nm. From SEM images, the TS-1 particles without urea and seed crystals are ellipsoidal (particle size over 500 nm). The morphology of the TS-1 synthesized by adding urea changed from an elliptical shape to a thin plate shape (ca. 250 nm), while the TS-1 synthesized by adding urea and seed crystals had a shorter b-axis (ca. 190 nm). Meanwhile, based on the synergistic synthesis of sheet-like TS-1 zeolite with urea and seed crystals, they also found that pre-crystallization at 80 °C can produce 50–60 nm thick sheet-like TS-1 zeolite. The performance of epoxidation of 1-hexene was evaluated between sheet-like TS-1 and conventional TS-1. The conversion rates of 1-hexene and the selectivity of 1,2-epoxyhexane were 48.2% vs. 50.7% and 80.4% vs. 33.5%, respectively.
Zhu et al. investigated the effects of adding urea and different aluminum source types in the initial gel on MFI-type zeolites and prepared HZSM-5 zeolites with different b-axis thicknesses and Si/Al ratios.36 Herein, the initial aluminosilicate gel with molar composition of 1SiO2/mAl2O3/0.12Na2O/0.25TPAOH/100H2O/nurea (m = 0.004–0.01, n = 0.6, 1.0, 2.0) was crystallized at 150 °C. ZSM-5 zeolite with a b-axis thickness of 340 nm was synthesized when urea content was equal to 1.0. At the same time the crystallization mechanism of ZSM-5 zeolite after the addition of urea was studied. They found that during the crystallization process, the –NH2 of urea interacts with the Si–OH on the surface of the crystal [010], significantly inhibiting the growth of ZSM-5 crystals in the b-axis direction (Fig. 2). The zeolite was then used for hexane cracking. The selectivity of light olefins was the highest (46.5%), which was 12.2% higher than that of the control sample under the same conditions due to lower CB/CL synergism and better mass transfer and diffusion properties.
 | ||
| Fig. 2 Mode of action of urea in the b-axis direction. Reprinted from ref. 36 with permission from Elsevier Inc., copyright 2023. | ||
Lv et al. synthesized Co@MFI zeolites with a controllable short b-axis and sheet thickness ranging from 50 nm to 130 nm with the assistance of urea.37 The cobalt metal introduced in situ existed in the form of subnanometer CoO clusters with an average particle size of 0.88 nm. The in situ incorporation of cobalt did not affect the synthesis of the short b-axis morphology. The limited Co clusters in MFI zeolites have three kinds of pore placement, namely, in straight pore channels, sinusoidal pore channels and cross pore channels. The lowering of thickness increases the proportion of CoO subnano-clusters in straight pore channels by nearly two times. In addition, the acidic properties of the catalyst are also modulated, and the thinner the thickness, the stronger the Lewis acidity, and the stronger the ability to complex with NO probe molecules. When the three kinds of Co active sites catalyze propane dehydrogenation, Co in the straight pore channel has the lowest first step dehydrogenation heat. Therefore, we hypothesized that CoO subnano-clusters located in the straight pore channel are the best active sites for Co-based catalysts to catalyze propane dehydrogenation. Experimental results show that the catalyst with the thinnest b-axis thickness (denoted as Co@MFI-P50) has excellent propane activation ability. The initial conversion rate of propane was 41.8%, the highest propylene generation rate was 15 mmol C3H6 per gCo per min, the propylene selectivity was 92%, and propane remained active after 9 regeneration cycles.
Recently, Mintova and co-workers reported the synthesis of plate-like MFI zeolites (silicalite-1 and ZSM-5) with controllable b-axis thickness ranging from 50–100 nm using urea as an additive.38 They used TPAOH as the OSDA, the concentration of which, together with parameters like water and urea content, determined the growth behavior of MFI zeolites. The mechanistic study performed on pure silica MFI showed that urea could control the anisotropic growth at the early nucleation stage, generating rectangular crystals; then the growth along the b-axis can be suppressed to obtain plate-like morphology with synergistic effects of optimized synthesis parameters (Fig. 3). The introduction of Al in the synthesis of plate-like MFI showed that the increase in Al increased the crystal size. Besides, the choice of Si and Al sources could affect the morphology, e.g., plate-like single crystals were obtained when TEOS and Al(OH)3 were employed, whereas agglomerated or intergrown crystals formed when other silica sources were used.
 | ||
| Fig. 3 Schematic representation of the MFI crystallization in the absence or presence of urea as an additive. Hexagonal prism crystals were obtained in the absence of urea (A). When the urea additive was added, rectangular crystallites were observed after about 1 hour (B), followed by the growth of nanosheet crystals with unfinished two ends (C), and finally coffin-shaped MFI nanosheets were formed (D). Reprinted from ref. 38 with permission from Springer Nature Ltd, copyright 2023. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3
Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPAOH
TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaO
NaO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea
urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol = 0.053
isopropanol = 0.053![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.016
0.016![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0025
0.0025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03
0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.0016 (x = 0.0044–0.00089) and found that the plate-like H-ZSM-5 zeolite (Si/Al = 75) with a b-axis thickness of about 60 nm achieved a high glycerol conversion (98%) and acrolein selectivity (87%) at a relatively high WHSV (4 h−1). Later, they also synthesized b-oriented H-ZSM-5 zeolites (60, 130, 180 and 250 nm, Si/Al = 200) via the similar aluminosilicate gel composition and evaluated their performance on GDTA.45 The results showed that H-ZSM-5 with a thickness of 60 nm had the highest acrolein selectivity up to 88%, ascribed to the higher microporous active site accessibility and shorter mass transfer channels in short b-axis MFI zeolites.
0.0016 (x = 0.0044–0.00089) and found that the plate-like H-ZSM-5 zeolite (Si/Al = 75) with a b-axis thickness of about 60 nm achieved a high glycerol conversion (98%) and acrolein selectivity (87%) at a relatively high WHSV (4 h−1). Later, they also synthesized b-oriented H-ZSM-5 zeolites (60, 130, 180 and 250 nm, Si/Al = 200) via the similar aluminosilicate gel composition and evaluated their performance on GDTA.45 The results showed that H-ZSM-5 with a thickness of 60 nm had the highest acrolein selectivity up to 88%, ascribed to the higher microporous active site accessibility and shorter mass transfer channels in short b-axis MFI zeolites.
 | ||
| Fig. 4 SEM images of (A) TS-1-S1, (B) TS-1-S2, (C) TS-1-C1, and (D) TS-1-C2 samples. Reprinted from ref. 41 with permission from the Royal Society of Chemistry, copyright 2011. | ||
Xie's group aimed to investigate the effect of a few specific additives on the morphology of ZSM-5 zeolites by adding them to aluminosilicate gel and studied the mechanism of additive adsorption preferences on the anisotropic growth of ZSM-5.39 ZSM-5 zeolites with different morphologies, such as sphere-like (butanone), plate-like (urea and pyrocatechol), and noodle-like (hexamethylenetetramine) were synthesized. Using TPAOH as the OSDA, ZSM-5 with added urea has a length-to-height ratio between 5 and 20 and an average width-to-height ratio of ∼3, showing a two-dimensional plate-like structure, but its b-axis thickness is slightly greater. To understand the mechanism of the effect of additives on the anisotropic growth of ZSM-5 crystals, the authors conducted parallel controlled experiments and selected 8 typical additives to regulate the crystal morphology of ZSM-5 zeolites. The authors believe that there are two main reasons for the anisotropic growth of ZSM-5 zeolite: one is the adsorption preference of additives, and the other is the different crystallization kinetics.
Ping et al. added urea into the synthesis system to study the effect of urea addition on the morphology of MFI-type titanosilicate zeolite (TS-1), and further investigated its performance in propylene gas phase epoxidation to propylene oxide (PO).42 They used commercial TPAOH as the OSDA, urea as a crystal growth regulator, and a small amount of isopropyl alcohol as an auxiliary to synthesize sheet-like titanium silicalite-1 zeolites (abbreviated as TS-1-S-x, x representing urea/SiO2) at 170 °C. With the increase in urea content from 0.15 to 0.35, TS-1-S-0.35 (Lb = 95 nm) with a thinner b-axis thickness was obtained, indicating that urea significantly inhibited the growth of crystals along the b-axis. Finally, TS-1 loaded with Au (marked as Au/TS-1) was used to catalyze PO. Compared with TS-1 and TS-1-S-0.35, TS-1-S-0.15 has the highest PO formation rates of 85 gPO h−1 kgcat.−1 over 80 gPO h−1 kgcat.−1 and 57 gPO h−1 kgcat.−1. Although the short b-axis TS-1-S is favorable for mass transfer and diffusion, excessive urea is unfavorable for Ti incorporation into the zeolite framework, resulting in poorer performance of PO compared to TS-1 zeolite.
Later, Xu et al. also studied the catalytic performance of propylene epoxidation with H2 and O2 (HOPO process).40 TS-1 zeolites with different amounts of urea were synthesized as supports. SEM analysis showed that when x was 0.15, the TS-1 morphology was elliptic. When x is increased to 0.30, TS-1 zeolite has a flat morphology with a b-axis length of about 100 nm. Then, they loaded Au onto the modified TS-1 zeolite (Au/TS-1) for HOPO to propylene oxide (PO). Compared with common ellipsoidal TS-1 zeolites with 200 nm and 500 nm crystal sizes, plate-like TS-1 zeolites have the highest PO formation rate (ca. 82 gPO h−1 kgTS−1).
Interestingly, unlike the previous work on urea-assisted preparation of nanosheets, using urea as an additive, Ma et al. obtained a hierarchical mesoporous MFI-type ZSM-5 zeolite with an aggregated nanocrystalline structure through one-pot hydrothermal synthesis.46 Compared with urea as a modifier to control the anisotropic growth of zeolites, urea here provides a mild, stable and homogeneous alkaline medium in the synthesis of hierarchical mesoporous zeolites. The alkalinity is provided by ammonia, which is the product of hydrolysis reaction of urea solution under controlled conditions. In addition, the crystallinity of zeolites treated with urea solution is perfectly preserved and mesoporosity can be successfully developed.
2.2 Amino acids
Watanabe et al. synthesized zeolites by adding an amino acid called L-glutamate (L-Glu) to the gel system.50 Nano-sized all-silica MFI zeolite (silicalite-1) with an average particle size of 60 nm was synthesized with a gel molar ratio of 1.0TEOS/0.25TPAOH/0.016 L-Glu/11H2O (Fig. 5A). Among them, L-Glu acts as a growth inhibitor to reduce the size of the crystal nucleus for obtaining nano-zeolite crystals. They also successfully synthesized titanosilicate zeolite (TS-1, av. 100 nm) by adding L-Glu (Fig. 5B). The epoxidation of cyclohexene with different sizes of TS-1 (100 nm, 190 nm and 305 nm) was carried out. The initial reaction rates of cyclohexene conversion were 3.0 × 10−3. 1.2 × 10−3 and 9.8 × 10−4 mmol min−1, respectively. | ||
Fig. 5 (A) Synthesis of nanosized silicalite-1 with an av. size of 62 nm. (a) XRD pattern, (b) FE-SEM image, (c) DLS curve, and (d) HR-TEM image. (B) FE-SEM images of the TS-1 samples synthesized with the gel molar composition of 1.0 TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.033 TBOT 0.033 TBOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.23 TPAOH 0.23 TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.016 L-Glu 0.016 L-Glu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x H2O. (a) x =12, (b) x =18, and (c) x =32. Reprinted from ref. 50 with permission from Elsevier Inc., copyright 2011. x H2O. (a) x =12, (b) x =18, and (c) x =32. Reprinted from ref. 50 with permission from Elsevier Inc., copyright 2011. | ||
Deng et al. added L-lysine to the parent gel system to adjust the morphology of silicalite-1.52 A series of silicalite-1 catalysts with a particle size between 50 and 450 nm were prepared by gel hydrothermal synthesis with a molar composition of 1.0TEOS/0.3TPAOH/xL-lysine/yH2O. It was observed by SEM that crystal grains have smooth surfaces and uniform size. It is worth mentioning that by adding L-lysine, they significantly reduced the size of the silicalite-1 grains, and the nanoscale nucleus is favorable for the subsequent crystallization of thinner nanoparticles or nanoparticle zeolites. Herein, the synthesized nano-silicalite-1 and its NaOH-modified derivatives catalyzed the gas-phase Beckmann rearrangement of cyclohexanone oxime to caprolactam at WHSV = 6 h−1, 370 °C. The results show that nano-silicalite-1 with the smallest particle size over cyclohexanone oxime conversion is nearly 100%, while the selectivity to ε-caprolactam reaches a maximum of 96.4%.
Yu's group obtained high-quality single-crystalline MFI-type nanozeolites (silicalite-1) with hexagonal prism morphology under the synergistic action of amino acids and multi-step crystallization.49 Nanosized silicalite-1 (10–50 nm) was synthesized by two-step crystallization at 80 °C and 170 °C using a gel molar ratio of 1.0SiO2/0.45TPAOH/xL-lysine/9H2O (x = 0 and 0.1). The first step at low temperature (80 °C) is favorable for forming metastable irregular nanoparticles. Next, the temperature was raised to 170 °C, resulting in high-quality nanosized crystals with regular morphology. During the crystallization process, the growth inhibitor L-lysine causes metastable irregular nanoparticles to rearrange into morphology toward an equilibrium crystal shape (Fig. 6). They used a similar method to synthesize ZSM-5 zeolites to evaluate the performance in methanol to propylene (MTP) conversion, and found that the propylene selectivity was as high as 49% and the catalyst life was extended by 54 h. Subsequently, they used a L-carnitine-assisted one-step hydrothermal route to synthesize plate-like TS-1 zeolites with different coordinated Ti species, which were respectively denoted as TS-1#A_15Et:0.4LC (hexagonal plate, TiO4) and TS-1#B_15Et:0.6LC (elongated platelet, TiO6).47 XRD shows that the TS-1#B_15Et:0.6LC and TS-1#C_0Et:0.6LC samples show a preferred orientation on the [020] crystal surface, which suggests the growth of plate-like zeolites. Meanwhile, SEM showed that L-carnitine/SiO2 = 0.6 was the best addition amount (Fig. 7). Finally, they carried out the epoxidation of alkene (EOA) with synthetic TS-1 zeolite samples. TS-1#B_15Et:0.6LC has excellent catalytic activity with a turnover frequency of 131 h−1, twice that of conventional TS-1 zeolites.
 | ||
| Fig. 6 Scheme for the proposed formation process of nanosized zeolites via synergetic use of the L-lysine-assisted approach and two-step crystallization in a concentrated gel system. Reprinted from ref. 49 with permission from American Chemical Society, copyright 2018. | ||
 | ||
| Fig. 7 SEM images of TS-1 zeolites assisted by L-carnitine. (A) TS-1#con, (B) TS-1#A_15Et:0.4LC, (C) TS-1#B_15Et:0.6LC, and (D) TS-1#C_0Et:0.6LC. Reprinted from ref. 47 with permission from American Chemical Society, copyright 2020. | ||
Peng et al. also synthesized ZSM-5 nano-zeolites with L-lysine to support MnOx species and investigated the catalytic performance of the MnOx/ZSM-5 catalyst in the oxidation of toluene.51 The molar composition of gel is 1.0SiO2/0.54TPAOH/0.017Al2O3/0.0017Na2O/xL-lysine/9H2O (x = 0 and 0.2), in which L-lysine is used as a growth modifier, and small particles of ZSM-5 zeolite (named Z5-L0.2) of 100 nm size are obtained. Z5-L0.2 is more favorable for separating supported MnOx than large-sized zeolites with a size of 500 nm. Meanwhile, due to the abundant oxygen vacancies and surface adsorbed oxygen of Z5-L0.2, the temperatures at which the conversion of toluene reaches 90% (T90) is 315 °C for MnOx/Z5-L0, while the T90 for MnOx/Z5-L0.2 is 276 °C.
Rimer and co-workers found that introducing D-arginine (D-Arg) into the gel could effectively control the anisotropic growth of silicalite-1 zeolites.56 The addition of D-Arg results in a significant increase in the thickness of the b-axis of platelet-like silicalite-1 and a decrease in its length in the direction of the c-axis. This is due to the inhibitory effect of D-Arg on the growth of its [302] facet. This group investigated in depth the effect of D-Arg addition on the morphology of silicalite-1 zeolite48 (Fig. 8). The addition of 0.3 wt% D-Arg to the gel increased the b-axis thickness of zeolites, so they concluded that D-Arg was more likely to bind to the [101] surface of silicalite-1 zeolites. Furthermore, they synthesized a series of hexagonal platelike zeolites by adding 0–7 wt% D-Arg. However, through crystal analysis and SEM, the crystal morphology was found to exhibit an unexpected changing trend. The b-axis thickness of the crystal increases with the increase in D-Arg concentration when the addition amount is 0–1 wt% D-Arg. In the range of 1–7 wt% D-Arg, the b-axis thickness of zeolite decreases monotonically with the increase in D-Arg concentration. Finally, they also proposed a crystallization mechanism for D-Arg, whereby D-Arg is decomposed in situ into ornithine lactam, which acts as a growth regulator to induce zeolite crystal size reduction, not through the traditional mechanism of preferentially binding crystal faces, but through its ability to hinder nanoparticle addition.
 | ||
Fig. 8 Effect of the modifier on the morphology of silicalite-1 crystals synthesized from growth solution S(I). (A) Crystal thickness along the b-direction in the presence of D-Arg (red squares) or R/S-ornithine-lactam (orange circles) relative to the crystal thickness of the control, thickness0, as a function of the modifier content. Each symbol is the average of more than 50 measurements from a single batch. Dashed lines are interpolated to help guide the eye, and error bars represent two standard deviations. (B–E) Scanning electron micrographs of silicalite-1 crystals prepared with the following synthesis conditions: (B) control (absence of the modifier), (C) 1, (D) 3, and (E) 7 wt% D-Arg. Solution S(I)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40SiO2 40SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40TPAOH 40TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9420H2O 9420H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160EtOH. Reprinted from ref. 48 with permission from American Chemical Society, copyright 2019. 160EtOH. Reprinted from ref. 48 with permission from American Chemical Society, copyright 2019. | ||
2.3 Small organic molecules
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.357TPAOH
0.357TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4EtOH
4EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x diols
x diols![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) yH2O, where x = 6–9, y = 21.55–45.55, the hexagonal prismatic plate-like silicalite-1 with c-axis orientation was synthesized. In addition, the type and concentration of diols also affect the c-axis length of silicalite-1 crystals. Finally, they proposed the kinetic mechanism of crystal growth of silicalite-1 zeolite under the action of diols as co-solvents. They suggested that this is related to adsorption theory, where the –O– groups of diols can form hydrogen bonds with local multi-Si–OH groups compared to monols. Under this strong force, the adjacent –CH2–CH2– groups come in close contact with the silicon species on the framework, thus further reducing the surface energy of the silicalite-1 crystals [010] and [100].
yH2O, where x = 6–9, y = 21.55–45.55, the hexagonal prismatic plate-like silicalite-1 with c-axis orientation was synthesized. In addition, the type and concentration of diols also affect the c-axis length of silicalite-1 crystals. Finally, they proposed the kinetic mechanism of crystal growth of silicalite-1 zeolite under the action of diols as co-solvents. They suggested that this is related to adsorption theory, where the –O– groups of diols can form hydrogen bonds with local multi-Si–OH groups compared to monols. Under this strong force, the adjacent –CH2–CH2– groups come in close contact with the silicon species on the framework, thus further reducing the surface energy of the silicalite-1 crystals [010] and [100].
Xie's group synthesized H-ZSM-5 zeolites with short b-axis direct channels but different c-axis lengths by regulating zeolite morphology with the aid of pyrocatechol.54 Herein, under alkaline conditions, H-ZSM-5 zeolites were prepared using the initial gel molar composition of 6TPA+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20SiO2
20SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5Na2O
2.5Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.025Al2O3
0.025Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x pyrocatechol
x pyrocatechol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80EtOH
80EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800H2O, where x = 0–1. The SEM images showed that the a-axis and b-axis lengths of the synthesized H-ZSM-5 samples were about 250 nm and 100 nm, respectively, but only the c-axis length varied with the amount of pyrocatechol added (Fig. 9).
800H2O, where x = 0–1. The SEM images showed that the a-axis and b-axis lengths of the synthesized H-ZSM-5 samples were about 250 nm and 100 nm, respectively, but only the c-axis length varied with the amount of pyrocatechol added (Fig. 9).
 | ||
| Fig. 9 The morphology of the as-synthesized H-ZSM-5 zeolites assisted by pyrocatechol. (A and B) SEM results of H-ZSM-5 zeolites with similar thicknesses of a- and b-axis but different lengths of c-axis. Left: Z-cS; middle: Z-cM; right: Z-cL. (C) TEM image of Z-cL sample with exposed facets of [010] plane. (D) The corresponding SAED patterns of [010] plane. (E) Aberration-corrected STEM image of Z-cL sample with exposed facets of [010] plane, with the inset on the top right displaying the STEM image with high resolution and the framework structure of H-ZSM-5 projected along [010] plane. (F–H) TEM image, the corresponding SAED patterns, and the aberration-corrected STEM image of Z-cL sample with exposed facets of [100] plane. Reprinted from ref. 54 with permission from Springer Nature Ltd, copyright 2021. | ||
Zhu et al. constructed sheet-like ZSM-5 (marked as S-ZSM-5) zeolites with a controllable aspect ratio only by using an ethanol (EtOH) assisted strategy.55 Herein, they systematically investigated various parameters (the kind of alcohol and the content of ethanol and H2O) to screen out the optimal synthesis conditions for two-dimensional S-ZSM-5 zeolites, and synthesized S-ZSM-5 zeolites with a b-axis thickness of 90 nm and different aspect ratios. They also proposed the mechanism by which EtOH assisted in regulating the morphology of ZSM-5 zeolites. They suppose that EtOH containing –OH groups may preferentially interact with the exposed Si–OH group on the crystal [010] facets, inhibiting the growth along the b-axis and easily forming zeolites with a sheet-like morphology (Fig. 10). Because of its short straight channel and optimized acidity, S-ZSM-5 exhibits higher benzene conversion (ca. 8.5%) compared to conventional benzene-ring-like ZSM-5 (C-ZSM-5) (ca. 3.8%), and slightly higher ethylbenzene selectivity (ca. 95% vs. ca. 93%) in the alkylation of benzene with ethanol.
 | ||
| Fig. 10 Illustration of the formation of the sheet-like ZSM-5 zeolite with ethanol as an additive. Reprinted from ref. 55 with permission from Wiley-VCH GmbH, copyright 2023. | ||
 | ||
| Fig. 11 (A) Native crystal habit (top) can be modified (bottom) by using molecules that bind to specific surfaces and alter anisotropic growth. (B) The amorphous silica exoskeletons of diatoms are formed through interactions with amine-rich proteins, which we mimic in silicalite-1 synthesis by using synthetic ZGM analogues. Reprinted from ref. 56 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, copyright 2012. | ||
Wang et al. synthesized nanosheet-stacked hierarchical ZSM-5 zeolite with the assistance of triethylamine (TEA) as a modifier combined with crystal seeds.57 Herein, they used the di-quaternary ammonium cation [C18H37–N+(CH3)2C6H12–N+(CH3)2–C6H12]Br2 (abbreviated as C18−6−6Br2) as the OSDA, and studied the influence of TEA on the formation and morphology of ZSM-5 zeolites according to the mother gel molar composition of 7.5Na2O/1Al2O3/125SiO2/2.5C18−6−6Br2/4000H2O/xTEA (where, x = 0–40). When the molar addition of TEA increased from 5 to 40, the SEM images showed that the morphologies of ZSM-5 zeolites gradually transformed from monolayer platelet-like ZSM-5 zeolites with a small aspect ratio to a multilayer ZSM-5 stacked with nanosheets (Fig. 12). It is worth noting that the thickness of nanosheets is very thin, about 8–10 nm. Due to the easy access to acid sites and the hierarchical pore structure of nanosheet-stacked hierarchical ZSM-5 zeolites, they exhibit excellent catalytic activity and stability in catalyzing n-octane to light olefins, with a selectivity of 70.7%, representing an increase of 6.6% compared to conventional spherical ZSM-5.
 | ||
| Fig. 12 (A) Graphical illustration of the formation of hierarchical ZSM-5 nanosheets during hydrothermal crystallization with different amounts of triethylamine addition. (B) SEM and TEM images of the ZSM-5 zeolite samples upon adding triethylamine. (a1 and a2) HZ5-0/0, (b1 and b2) HZ5-2.5/0, (c1 and c2) HZ5-2.5/5, (d1 and d2) HZ5-2.5/10, (e1 and e2) HZ5-2.5/20, and (f1 and f2) HZ5-2.5/40. Reprinted from ref. 57 with permission from MDPI, copyright 2022. | ||
Liu et al. constructed a new pathway for synthesizing house-of-cards-like ZSM-5 (abbreviated as HCL-ZSM-5) in the zeolite synthesis system without the addition of the OSDA, using a N-methyl-2-pyrrolidone (NMP) assisted strategy.58 Based on the parent gel molar composition of 0.2Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.025Al2O3
0.025Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2
SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17.8H2O
17.8H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.808NMP, the ZSM-5 obtained after crystallization at 140 °C for 48 h has higher crystallinity and is the pure MFI phase. Meanwhile, the SEM images show that the ZSM-5 zeolite is composed of intergrown sheets with a single sheet thickness of 100 nm (Fig. 13). HCL-ZSM-5 synthesized with the assistance of NMP has two advantages: firstly, it has a larger outer surface, and secondly, it has stronger acidic sites. Compared to conventional zeolite catalysts, HCL-ZSM-5 has the highest 1,3,5-triisopropylbenzene (TIPB) conversion of 90.5%, compared to 15.7% for ZSM-5, 21.3% for Y and 41.8% for Al-MCM-41.
0.808NMP, the ZSM-5 obtained after crystallization at 140 °C for 48 h has higher crystallinity and is the pure MFI phase. Meanwhile, the SEM images show that the ZSM-5 zeolite is composed of intergrown sheets with a single sheet thickness of 100 nm (Fig. 13). HCL-ZSM-5 synthesized with the assistance of NMP has two advantages: firstly, it has a larger outer surface, and secondly, it has stronger acidic sites. Compared to conventional zeolite catalysts, HCL-ZSM-5 has the highest 1,3,5-triisopropylbenzene (TIPB) conversion of 90.5%, compared to 15.7% for ZSM-5, 21.3% for Y and 41.8% for Al-MCM-41.
 | ||
| Fig. 13 SEM images of the products synthesized by introducing N-methyl-2-pyrrolidone at 140 °C for (A) 6 h, (B) 18 h, (C) 24 h and (D) 48 h. (E and F) TEM images of the products prepared by introducing N-methyl-2-pyrrolidone at 140 °C for 18 h and 24 h, respectively. Reprinted from ref. 58 with permission from RSC Publishing Group, copyright 2014. | ||
Liu et al. synthesized c-axis oriented ZSM-5 zeolites with the assistance of allophanamide, an organic compound similar to urea, by a one-pot solvent-free method.34 They used TPABr as the OSDA and added an organic amine (allophanamide) to the initial gel to obtain c-axis oriented sheet-like ZSM-5 zeolites. Allophanamide has a similar action mechanism to urea, and can be decomposed into NH3 adsorbed on certain crystal facets at experimental temperatures. The sheet-like ZSM-5 catalyst for methanol to gasoline (MTG) achieves a methanol conversion of 100%, a high C5+ selectivity of 63.8%, and an extremely long lifetime of up to 900 h.
Shi's group reported the use of nitrogen-rich tetramethylguanidine (TMG) to assist the synthesis of short b-axis oriented ZSM-5 zeolites.59 With an initial gel molar composition of 1.0SiO2/0.01Al2O3/0.45TPAOH/18H2O/xTMG (x = 0.2, 0.3, 0.5, 0.7 and 0.9), plate-like ZSM-5 samples (abbreviated as P-ZSM-5-x) were hydrothermally synthesized by two-stage crystallization at 80 °C and 170 °C (Fig. 14). The SEM images show that P-ZSM-5-0.3 has crystal sizes of 290 × 85 × 840 nm (a × b × c). Finally, they put forward the mechanism of TMG, and believed that the existence of TMG transformed the initial gel into a colloid, and the colloidal particles attached to the crystal surface mainly along the a- and c-axis through the solid phase transition pathway. Later, this group selected more guanidine compounds, including tetramethylguanidine (TMG), dodecylguanidine hydrochloride (DGH), and polyhexamethylene biguanide hydrochloride (PHMB).60 Plate-like MFI zeolites (silicalite-1, ZSM-5 and TS-1) with b-axis thicknesses of 75–110 nm were also obtained via a similar gel composition. Among the three guanidine compounds, the addition of DGH and PHMB appeared to effectively control the crystal growth compared to TMG. During the synthesis of ZSM-5 or TS-1 zeolites, more heteroatoms (Al or Ti) can enter the framework due to the action of organic guanidine modifiers.
 | ||
| Fig. 14 Formation mechanism of single-crystalline hierarchical plate-like ZSM-5 crystals for zeolite syntheses in one step (at 170 °C) or in two steps (at 80 °C and then at 170 °C). Reprinted from ref. 59 with permission from the Royal Society of Chemistry, copyright 2022. | ||
Rong et al.61 investigated the effect of imidazole based ionic liquids on the morphology control of MFI zeolites by changing the type and dosage of imidazole based ionic liquids, which include 1-butyl-3-methylimidazole bromide ([C4MIm]Br), 1-octyl-3-methylimidazole bromide ([C8MIm]Br), 1-dodecyl-3-methylimidazole bromide ([C12MIm]Br), and 1-hexadecyl-3-methylimidazole bromide ([C16MIm]Br). The SEM images show that increasing the alkyl chain length or concentration (3, 5, 7.5 and 10 mmol) of imidazolium ionic liquids can result in small sized c-axis-oriented coffin-like silicalite-1 zeolites, indicating that the anisotropic growth of zeolites is affected by spatial steric hindrance.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50SiO2
50SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0Na2O
2.0Na2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8TPAOH
8TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000H2O
3000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j glucose (where j = 0, 10, 15, 20, 25). SEM images show that ZSM-5-T3, with the optimal addition of glucose, displayed a large prismatic morphology (Lc = 3.5–5 μm). The methanol to propylene (MTP) reaction was carried out at 450 °C and a methanol WHSV of 3.8 h−1, and ZSM-5-T3 exhibited the highest propylene selectivity up to 45.2%, a propylene/ethylene ratio of 8.4 and a C=2–C=4 selectivity of 63.9%. Later, Feng and co-workers again synthesized b-axis oriented nanosized H-ZSM-5 zeolites (Lb = 300–500 nm) via a similar gel molar composition of 50SiO2
j glucose (where j = 0, 10, 15, 20, 25). SEM images show that ZSM-5-T3, with the optimal addition of glucose, displayed a large prismatic morphology (Lc = 3.5–5 μm). The methanol to propylene (MTP) reaction was carried out at 450 °C and a methanol WHSV of 3.8 h−1, and ZSM-5-T3 exhibited the highest propylene selectivity up to 45.2%, a propylene/ethylene ratio of 8.4 and a C=2–C=4 selectivity of 63.9%. Later, Feng and co-workers again synthesized b-axis oriented nanosized H-ZSM-5 zeolites (Lb = 300–500 nm) via a similar gel molar composition of 50SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0Al2O3
1.0Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8TPAOH
8TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :12 glucose
:12 glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000H2O
3000H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nNH4OH (n = 10, 20, 30, 50, 75, 100, and 125), with enhanced performance in the MTP reaction.63 The obtained samples were abbreviated as Z5-G(1–7) corresponding to the values of n. In particular, the Z5-G3 catalyst presents the best catalytic performance (a high propylene selectivity of 50.98%, the highest light olefin selectivity of 79.46%, and the longest catalytic lifetime of 24 h).
nNH4OH (n = 10, 20, 30, 50, 75, 100, and 125), with enhanced performance in the MTP reaction.63 The obtained samples were abbreviated as Z5-G(1–7) corresponding to the values of n. In particular, the Z5-G3 catalyst presents the best catalytic performance (a high propylene selectivity of 50.98%, the highest light olefin selectivity of 79.46%, and the longest catalytic lifetime of 24 h).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.025TiO2
0.025TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.36TPAOH
0.36TPAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1KOH
0.1KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 citric acid/nitrilotriacetic acid
0.15 citric acid/nitrilotriacetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35H2O, TS-1 zeolites were obtained by crystallization at 170 °C. Among them, TS-1 was marked as TS-1-CA for citric acid addition, with a crystal size of 1.5 × 0.2 × 2.0 μm (a × b × c) and TS-1-NTA (0.3 × 0.06 × 0.6 μm) for nitilotriotropic acid addition, respectively (Fig. 15). The XRD patterns show that TS-1 zeolites synthesized with the aid of polycarboxylic acid are pure MFI phase; the peak strength of TS-1 zeolite regulated by citric acid at the [020] crystal surface is higher than that at the [101] crystal surface, indicating a preferred orientation, which implies that TS-1-CA is a lamellar zeolite. TS-1-CA and TS-1-NTA both have large aspect ratios (Lc/Lb) greater than 10, which is one of the largest aspect ratios reported for TS-1 zeolites. The plate-like TS-1 modified with organic weak acids was used for 1-hexene epoxidation, showing higher conversion rates of 20.9% and 20.2%, and turnover numbers of 104 and 100 compared to conventional nano-sized TS-1, respectively.
35H2O, TS-1 zeolites were obtained by crystallization at 170 °C. Among them, TS-1 was marked as TS-1-CA for citric acid addition, with a crystal size of 1.5 × 0.2 × 2.0 μm (a × b × c) and TS-1-NTA (0.3 × 0.06 × 0.6 μm) for nitilotriotropic acid addition, respectively (Fig. 15). The XRD patterns show that TS-1 zeolites synthesized with the aid of polycarboxylic acid are pure MFI phase; the peak strength of TS-1 zeolite regulated by citric acid at the [020] crystal surface is higher than that at the [101] crystal surface, indicating a preferred orientation, which implies that TS-1-CA is a lamellar zeolite. TS-1-CA and TS-1-NTA both have large aspect ratios (Lc/Lb) greater than 10, which is one of the largest aspect ratios reported for TS-1 zeolites. The plate-like TS-1 modified with organic weak acids was used for 1-hexene epoxidation, showing higher conversion rates of 20.9% and 20.2%, and turnover numbers of 104 and 100 compared to conventional nano-sized TS-1, respectively.
 | ||
| Fig. 15 SEM and TEM images of the calcined plate-like TS-1 product with the assistance of additives. (A and B) TS-1-CA and (C and D) TS-1-NTA. Reprinted from ref. 72 with permission from Elsevier B.V., copyright 2022. | ||
Wang et al. proposed to recover Xe from exhaled gas (CO2/Xe) using a high-flux b-oriented MFI zeolite membrane of silica-coated alumina supports65 (Fig. 16). They used commercial TPAOH as the OSDA and added 0–0.5 wt% tributylphosphine oxide (TBPO) as a morphology modifier. Under the action of TBPO, the b-face dimension of MFI zeolite membranes significantly increased, while promoting the secondary membrane-forming of coffin-like crystal seeds. The b-axis length of the monolayer of the MFI zeolite membrane remained around 350 nm when prepared with the assistance of TBPO. The prepared b-oriented MFI zeolite membranes exhibited a CO2 permeance of 1213, which is several orders of magnitude higher than that of carbon molecular sieving membranes and polymeric membranes.
 | ||
| Fig. 16 Illustration of the role of TBPO in MFI crystal nucleation and growth. Synthesis condition: 1TEOS/0.2TPAOH/110H2O at 150 °C. Reprinted from ref. 65 with permission from American Chemical Society, copyright 2018. | ||
2.4 Polymers
Lv et al. used polymer-assisted synthesis for hierarchical TS-1 zeolite stacked 2D-nanoplates with constrained mesopores earlier.71 They used commercial TPAOH as the OSDA and utilized polyethylene oxides–polypropylene oxides–polyethylene oxide (P123) lamellar micelles through a self-assembly process combined with multi-step crystallization to obtain lamellar nanoplate-like TS-1 zeolite. Among them, P123 plays two roles in the system: first, as a template for mesoporous TS-1 zeolite and the second as a morphology modifier for platelike TS-1 zeolites. The SEM images show that the total thickness of TS-1 zeolite stacked nanoplates is 100–120 nm, while the thickness of single-layer nanoplates is 20–25 nm (Fig. 17). The oxidative desulfurization (ODS) performance of TS-1 zeolites with hierarchical pores was evaluated. TS-1 zeolite with hierarchically stacked nanoplates significantly shortened the time of a total oxidation of dibenzothiophene (DBT) within 40 min compared to the oxidation of DBT is 99.8% for 2 h of oxidation when using hierarchical TS-1 zeolite as the catalyst.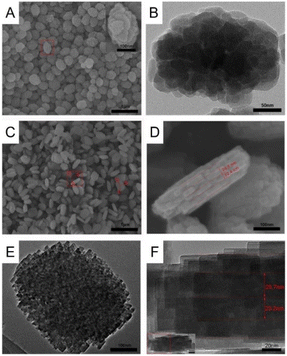 | ||
| Fig. 17 SEM images and high-magnification SEM image of hierarchical TS-1 particles in the inset (A), TEM image of hierarchical TS-1 zeolite (B), SEM image of hierarchical TS-1 nanoplates (C), high magnification SEM image of hierarchical TS-1 nanoplates (D), and TEM image of hierarchical TS-1 nanoplates (E and F). Reprinted from ref. 71 with permission from Elsevier B.V., copyright 2018. | ||
Chen et al. synthesized well-dispersed ZSM-5 zeolite with the aid of natural polymer gelatin.68 Gelatin–H2O mixture solutions with different mass ratios (gelatin/H2O = 0–0.04) were prepared first, and then reagents such as OSDA, silicon source and aluminum source were dissolved in the solution, and the initial gel with a molar composition of 100SiO2/Al2O3/8.5Na2O/12TPABr/2500H2O was obtained by hydrolysis overnight. After static crystallization at 180 °C for 48 h, ZSM-5 zeolites were obtained. When gelatin/H2O is equal to 2 wt%, the morphology of ZSM-5 zeolite exhibits hexagonal sheets with the thinnest b-axis thickness (Fig. 18). Using the zeolite for catalyzing the conversion of methanol to hydrocarbons (MTH), the catalyst shows the longest lifetime of 95 h, with a selectivity of 28.5% for propylene, which is significantly better than that of ZSM-5 zeolite without the gelatin modifier. Later, Guo's group also used gelatin (GE) as a modifier to obtain TS-1 nanoplates with a b-axis thickness of 40–300 nm.69 The SEM images show that when m(GE/SiO2) increases to 1.5, the obtained zeolites changed from nano aggregates to smooth sheet-like crystals (Lb = 40 nm). They also analyzed the working mechanism of gelatin on TS-1 nanoplates, and concluded that the synergistic action of amino and carboxyl groups in gelatin led to the morphological changes of TS-1 zeolites.
 | ||
Fig. 18 (A) Schematic representation of the synthesis of ZSM-5 zeolite by using gelatin hydrogels. (B) SEM images of the ZSM-5 crystals obtained in the reaction systems with different mass ratios of gelatin/H2O. (a and e) Gelatin/H2O = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100; (b and f) gelatin/H2O = 1 100; (b and f) gelatin/H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100; (c and g) gelatin/H2O = 2 100; (c and g) gelatin/H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100; (d and h) gelatin/H2O = 4 100; (d and h) gelatin/H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. Reprinted from ref. 68 with permission from Taylor & Francis, copyright 2019. 100. Reprinted from ref. 68 with permission from Taylor & Francis, copyright 2019. | ||
Guo et al. prepared c-axis-oriented HZSM-5 zeolites by the crosslinked polyacrylamide (C-PAM) hydrogel-assisted method.70 By adding in situ synthesized C-PAM (0–2%) to the aluminosilicate gel, ZSM-5 zeolite grows directionally along the c-axis. SEM images show that the hexagonal prism-like ZSM-5 (called Z-2) containing 2% C-PAM gel has a large aspect ratio (Lc/Lb) (Fig. 19). The NH3-TPD characterization results indicate that Z-2 has the strongest acidity, with a strong acid content of 0.163 mmol g−1. Z-2 was used for the methanol to olefin (MTO) reaction, and it demonstrated complete methanol conversion, higher light olefin selectivity (81.2%), and the longest lifetime of over 23 h.
 | ||
| Fig. 19 Schematic representation of HZSM-5 zeolites directly synthesized in crosslinked polyacrylamide (C-PAM) hydrogels. Reprinted from ref. 70 with permission from Springer Science Business Media, LLC part of Springer Nature, copyright 2020. | ||
Zhou et al. synthesized hierarchical lamellar H-ZSM-5 zeolite (marked as HLHZ) through the one-step hydrothermal synthesis strategy assisted by water-soluble polymer polyethylene glycol (PEG2000).67 A series of short b-axis HLHZ with hexagonal prism shapes were obtained using the gel molar ratio of 50SiO2/1.0Al2O3/10TPAOH/1500H2O/1.0PEG2000/x urea (x = 20, 40, 60 and 80). The NH4+ from urea decomposition could balance the negative charge of the zeolite framework. PEG2000 serves as the template for ZSM-5 zeolite with a hierarchical pore structure, and also acts as a modifier to inhibit growth along the b-axis, shortening the b-axis length from 1 μm to about 300–500 nm.
Yang et al. introduced the modifier poly(hexamethylene biguanide) hydrochloride to fabricate highly b-oriented MFI zeolite (abbreviated as BOMZ) membranes on porous Al2O3 support during the secondary growth of crystal seeds.66 When the ratio of initial gel is 1.0TEOS/0.2TPAOH/200H2O/0.008PHMB, the effect of inhibiting twin growth is the best. In addition, they also conducted in-depth research on the growth mechanism of BOMZ membranes in the presence of PHMB, and concluded that the introduction of PHMB regulates the crystallization kinetics by controlling the pH value of the synthesis system and preferential adsorption of the amino groups on the [010] surface of MFI crystals. The 650 nm thick BOMZ membrane prepared on a porous Al2O3 carrier exhibited excellent separation performance in the pervaporation of a 5 wt% EtOH/H2O solution at 60 °C, achieving a separation coefficient of 71 and a flux of 2.8 kg m−2 h−1.
3. Effect of zeolite morphology on the catalytic performance
Two-dimensional zeolites (nanosheets, plates, intergrown nanosheets, intergrown plates, etc.) are characterized by their open framework structure, large external surface area, optimized surface acidity, easy access to active sites, and short diffusion path lengths, which endow it with excellent catalytic performance in reactions such as conversion of methanol, cracking, carbonylation, isomerization, alkylation, acylation and oxidation. Except for the spatial confinement effect, the metal dispersion and stability of the catalysts were also significantly affected by the interaction between the metal species and zeolite supports. Two-dimensional zeolites can effectively fulfill the requirements of an optimal support ascribed to their large external surface area and the presence of rich silanol defects. In particular, the abundant silanol groups in two-dimensional zeolites can effectively anchor the metal species, which can enhance the interaction between the metal and the support via the formation of strong covalent metal–oxygen bonds, and significantly improve the confinement effect and dispersion of the metal species in the interlayers and pores, thus forming and stabilizing the ultrasmall metal species, and thus improving the metal utilization. In addition, two-dimensional MFI zeolites possess a short b-axis direction, which is favorable for the rapid diffusion of molecules, avoiding deep reactions.While the composition and pore structure of zeolites are essential, the particle size also significantly influences their properties and thus the performance in many chemical processes such as catalysis and separation. Low-dimensional zeolites such as “nanozeolites” (<100 nm) can improve reactivity and accelerate kinetics due to the much larger external surface area and shorter diffusion pathways. For instance, the gas-phase Beckmann rearrangement of cyclohexanone oxime to caprolactam52 and methanol to propylene (MTP)49 reactions were significantly improved by reducing the particle size of zeolites from the micro- to the nano-scale.
Therefore, based on a comprehensive review, it is not difficult to find that zeolite morphology has a significant effect on catalytic and separation performance upon comparison.
Finally, there are still important aspects to consider when choosing additives for the target morphology of zeolites, such as:
(1) First, the catalytic reaction itself should be considered (reactants/products and the reaction process) so that the ideal support morphology can be identified and then the morphology control can be performed purposefully.
(2) The principle of controlled synthesis of two-dimensional zeolites is to utilize the adsorption preference of additives to the crystal surface to influence the growth of a certain crystal surface. Consideration can be given to the choice of organic molecules containing –NH2 and –OH groups as well as inorganic compounds (carbonates and nitrates).
(3) For the synthesis of low-dimensional zeolites such as nanocrystals, L-lysine is an ideal option.
4. Conclusions
The morphology of zeolite is one of the main factors that determine its catalytic, adsorption and separation performance. Therefore, the preparation of zeolite crystals with ideal morphology has always been the goal of researchers' efforts, and promising progress has been achieved. It has been shown that synthesis conditions have a great influence on the morphology of zeolite crystals, including the synthesis formula and its ratios, the crystallization temperature and time, and the synthesis method, because these factors significantly affect the nucleation and crystal growth rate of zeolites by altering or modifying the gelation and crystallization processes. To control the anisotropic growth of zeolites, the addition of morphology modifiers to the synthesized gel is one of the effective methods to obtain the desired zeolite crystal shape.The strategies for manipulating MFI zeolite morphologies using organic modifiers are categorized and presented in this review. The use of urea solely or with a second additive, amino acids, small organic molecules (alcohols, N-compound, sugar, organic acids, and others) and polymers, was reviewed in detail. Various morphologies, including nanocrystals, aggregated nanoparticles, nanosheets, intergrown nanosheets, plates, intergrown plates, needles and bulky prismatic crystals were obtained using different additive types. Most of the studies were dedicated to preparing short-b-axis MFI type zeolites. The proposed potential role of additives in forming nanocrystals or nanosheets/plates is the adsorption of additive molecules on specific crystal facets, thus hindering further growth along that direction during the crystallization process. Applications using the different morphological MFI zeolites for catalytic reactions and separations were presented following each zeolite, showing superiors performance compared to that of the conventional MFI zeolite crystals, mainly due to the mitigation of diffusion limitation along the specific crystallographic axis, of which the dimensions were prominently reduced.
Despite the successful synthesis of MFI zeolites with various morphologies, the role of most additives in directing certain morphologies remains elusive. Hence, rationale explanations based on sophisticated characterization (isotope-labelled experiments and operando spectroscopy) and simulations are necessary to fundamentally elucidate the growth mechanisms to provide guidelines for producing specific morphological MFI zeolites tailored to specific needs. Although the limitations of current characterization techniques and computation capacity do not allow us to directly investigate and explicitly illustrate such a complex system, we have reasons to believe that with scientific and technological advancements, the rational design of zeolite crystal morphology can be achieved in the future. Furthermore, cost efficiency should be considered to screen out practically viable additives to ensure the economical and scalable industrial production and use of the materials. Lastly, the potential pollution caused by organic additives during and after zeolite synthesis should also be considered since more stringent legislation is introduced on controlled carbon emissions and environmental protection.
Data availability
This article is a review article without any research data. All analysis results have been presented in the manuscript.Author contributions
Jun Zhao: writing – review & editing – original draft, validation, investigation, formal analysis, and data curation. Haijun Yu: visualization, validation, conceptualization. Haimei Xu: software, methodology. Zhiyu He: discussion. Feng Shao: co-supervision. Peng Lu: writing – review & editing, supervision, project administration, funding acquisition. Valentin Valtchev: writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The ZeoMat Group acknowledges the starting grant provided by QIBEBT (Qingdao Institute of Bioenergy and Bioprocess Technology) and the support provided by the Shandong Energy Institute (SEI S202107). P. L. acknowledges the support by QIBEBT International Collaboration Program (202305) and by the Key Laboratory of Photoelectric Conversion and Utilization of Solar Energy (Innovation Fund Project No. KLPCU2024DP01).Notes and references
- C. J. Rhodes, Sci. Prog., 2010, 93, 223–284 CrossRef PubMed.
- S. Montalvo, C. Huilinir, R. Borja, E. Sanchez and C. Herrmann, Bioresour. Technol., 2020, 301, 122808 CrossRef PubMed.
- X. Chi, M. Li, J. Di, P. Bai, L. Song, X. Wang, F. Li, S. Liang, J. Xu and J. Yu, Nature, 2021, 592, 551–557 CrossRef PubMed.
- R. Ryoo, J. Kim, C. Jo, S. W. Han, J.-C. Kim, H. Park, J. Han, H. S. Shin and J. W. Shin, Nature, 2020, 585, 221–224 CrossRef PubMed.
- J. Shi, Y. Wang, W. Yang, Y. Tang and Z. Xie, Chem. Soc. Rev., 2015, 44, 8877–8903 RSC.
- B. M. Weckhuysen and J. Yu, Chem. Soc. Rev., 2015, 44, 7022–7024 RSC.
- B. Min, A. Korde, S. Yang, Y. Kim, C. W. Jones and S. Nair, AIChE J., 2021, 67, e17048 CrossRef CAS.
- C. S. Cundy and P. A. Cox, Chem. Rev., 2003, 103, 663–701 CrossRef CAS PubMed.
- G. T. Kokotailo, S. L. Lawton, D. H. Olson and W. M. Meier, Nature, 1978, 272, 437–438 CrossRef.
- T. C. T. Pham, T. H. Nguyen and K. B. Yoon, Angew. Chem., Int. Ed., 2013, 52, 8693–8698 CrossRef.
- G. T. M. Kadja, M. D. Rukmana, R. R. Mukti, M. H. Mahyuddin, A. G. Saputro and T. D. K. Wungu, Mater. Lett., 2021, 290, 129501 CrossRef.
- C. Zhang, S. Chu, J. Jiang, J. Zhao, S. Wen, B. Sun and W. Xu, Front. Chem., 2022, 10, 860795 CrossRef PubMed.
- M. Liu, Z. Huang, W. Wei, X. Wang and Y. Wen, Front. Chem., 2021, 9, 682404 CrossRef PubMed.
- B. Bensafi, N. Chouat and F. Djafri, Coord. Chem. Rev., 2023, 496, 215397 CrossRef.
- R. Aiello, F. Crea, E. Nigro, F. Testa, R. Mostowicz, A. Fonseca and J. B. Nagy, Microporous Mesoporous Mater., 1999, 28, 241–259 CrossRef.
- L. Zhang, Y. Song, G. Li, Q. Zhang, S. Zhang, J. Xu, F. Deng and Y. Gong, RSC Adv., 2015, 5, 61354–61363 RSC.
- J. Dedecek, V. Balgova, V. Pashkova, P. Klein and B. Wichterlova, Chem. Mater., 2012, 24, 3231–3239 CrossRef.
- K. A. Sashkina, Z. Qi, W. Wu, A. B. Ayupov, A. I. Lysikov and E. V. Parkhomchuk, Microporous Mesoporous Mater., 2017, 244, 93–100 CrossRef.
- H. Li, X. Liu, S. Qi, L. Xu, G. Shi, Y. Ding, X. Yan, Y. Huang and J. Geng, Angew. Chem., Int. Ed., 2017, 56, 14090–14095 CrossRef PubMed.
- M. Y. Jeon, D. Kim, P. Kumar, P. S. Lee, N. Rangnekar, P. Bai, M. Shete, B. Elyassi, H. S. Lee, K. Narasimharao, S. N. Basahel, S. Al-Thabaiti, W. Xu, H. J. Cho, E. O. Fetisov, R. Thyagarajan, R. F. DeJaco, W. Fan, K. A. Mkhoyan, J. I. Siepmann and M. Tsapatsis, Nature, 2017, 543, 690–694 CrossRef CAS PubMed.
- G. Gwak, J.-h. Park and D. Kim, Microporous Mesoporous Mater., 2023, 349, 112424 CrossRef CAS.
- Y. Seo, S. Lee, C. Jo and R. Ryoo, J. Am. Chem. Soc., 2013, 135, 8806–8809 CrossRef CAS.
- K. Na, C. Jo, J. Kim, K. Cho, J. Jung, Y. Seo, R. J. Messinger, B. F. Chmelka and R. Ryoo, Science, 2011, 333, 328–332 CrossRef CAS.
- L. Emdadi, Y. Wu, G. Zhu, C.-C. Chang, W. Fan, P. Trong, R. F. Lobo and D. Liu, Chem. Mater., 2014, 26, 1345–1355 CrossRef CAS.
- P. Rani, R. Srivastava and B. Satpati, Cryst. Growth Des., 2016, 16, 3323–3333 CrossRef CAS.
- Y. Liu, W. Qiang, T. Ji, M. Zhang, M. Li and J. Lu, Sci. Adv., 2020, 6, eaay5993 CrossRef.
- Z. Qin, L. Pinard, M. A. Benghalem, T. J. Daou, G. Melinte, O. Ersen, S. Asahina, J.-P. Gilson and V. Valtchev, Chem. Mater., 2019, 31, 4639–4648 CrossRef.
- J. Prech, K. N. Bozhilov, J. El Fallah, N. Barrier and V. Valtchev, Microporous Mesoporous Mater., 2019, 280, 297–305 CrossRef.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246–249 CrossRef.
- W. Dai, C. Kouvatas, W. Tai, G. Wu, N. Guan, L. Li and V. Valtchev, J. Am. Chem. Soc., 2021, 143, 1993–2004 CrossRef PubMed.
- C. Wang, W. Fang, Z. Liu, L. Wang, Z. Liao, Y. Yang, H. Li, L. Liu, H. Zhou, X. Qin, S. Xu, X. Chu, Y. Wang, A. Zheng and F.-S. Xiao, Nat. Nanotechnol., 2022, 17, 714–720 CrossRef PubMed.
- J. Duan, W. Chen, C. Wang, L. Wang, Z. Liu, X. Yi, W. Fang, H. Wang, H. Wei, S. Xu, Y. Yang, Q. Yang, Z. Bao, Z. Zhang, Q. Ren, H. Zhou, X. Qin, A. Zheng and F.-S. Xiao, J. Am. Chem. Soc., 2022, 144, 14269–14277 CrossRef PubMed.
- J. Yang, K. Gong, D. Miao, F. Jiao, X. Pan, X. Meng, F. Xiao and X. Bao, J. Energy Chem., 2019, 35, 44–48 CrossRef.
- Z. Liu, D. Wu, S. Ren, X. Chen, M. Qiu, X. Wu, C. Yang, G. Zeng and Y. Sun, ChemCatChem, 2016, 8, 3317–3322 CrossRef.
- M. Zhang, S. Ren, Q. Guo and B. Shen, ChemistrySelect, 2023, 8, e202203687 CrossRef.
- J. Zhu, S. Yan, G. Xu, X. Zhu and F. Yang, J. Solid State Chem., 2023, 318, 123772 CrossRef.
- X. Lv, M. Yang, S. Song, M. Xia, J. Li, Y. Wei, C. Xu, W. Song and J. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 14250–14260 CAS.
- Q. Yue, C. Liu, H. Zhao, H. Liu, P. Ruterana, J. Zhao, Z. Qin and S. Mintova, Nano Res., 2023, 16, 12196–12206 CrossRef CAS.
- J. Shi, Y. Du, W. He, G. Zhao, Y. Qin, L. Song, J. Hu, Y. Guan, J. Zhu, C. Wang, J. Teng and Z. Xie, Chem.–Eur. J., 2022, 28, e202201781 CrossRef CAS.
- J. Xu, Z. Zhang, D. Yu, W. Du, N. Song, X. Duan and X. Zhou, Nano Res., 2023, 16, 6278–6289 CrossRef CAS.
- Z. Shan, H. Wang, X. Meng, S. Liu, L. Wang, C. Wang, F. Li, J. P. Lewis and F.-S. Xiao, Chem. Commun., 2011, 47, 1048–1050 RSC.
- C. Ping, Q. Zhu, W. Ma, C. Hu and Y. Zhang, J. Porous Mater., 2022, 29, 1919–1928 CrossRef CAS.
- Y. Liu, X. Zhou, X. Pang, Y. Jin, X. Meng, X. Zheng, X. Gao and F.-S. Xiao, ChemCatChem, 2013, 5, 1517–1523 CrossRef CAS.
- B. A. Qureshi, X. Lan, M. T. Arslan and T. Wang, Ind. Eng. Chem. Res., 2019, 58, 12611–12622 CrossRef CAS.
- B. Ali, X. Lan, M. T. Arslan, S. Z. A. Gilani, H. Wang and T. Wang, J. Ind. Eng. Chem., 2020, 88, 127–136 CrossRef.
- Y. Ma, Z. Li, N. Zhao, F. Han and Q. Kan, Nano, 2020, 15, 2050026 CrossRef.
- X. Song, X. Yang, T. Zhang, H. Zhang, Q. Zhang, D. Hu, X. Chang, Y. Li, Z. Chen, M. Jia, P. Zhang and J. Yu, Inorg. Chem., 2020, 59, 13201–13210 CrossRef PubMed.
- W. Qin, A. Agarwal, M. K. Choudhary, J. C. Palmer and J. D. Rimer, Chem. Mater., 2019, 31, 3228–3238 CrossRef.
- Q. Zhang, G. Chen, Y. Wang, M. Chen, G. Guo, J. Shi, J. Luo and J. Yu, Chem. Mater., 2018, 30, 2750–2758 CrossRef.
- R. Watanabe, T. Yokoi and T. Tatsumi, J. Colloid Interface Sci., 2011, 356, 434–441 CrossRef PubMed.
- S. Peng, G. Yang and J. Zhang, Inorg. Chem. Commun., 2022, 143, 109759 CrossRef.
- Y.-Q. Deng, S.-F. Yin and C.-T. Au, Ind. Eng. Chem. Res., 2012, 51, 9492–9499 CrossRef.
- X. Chen, W. Yan, X. Cao, J. Yu and R. Xu, Microporous Mesoporous Mater., 2009, 119, 217–222 CrossRef.
- X. Liu, J. Shi, G. Yang, J. Zhou, C. Wang, J. Teng, Y. Wang and Z. Xie, Commun. Chem., 2021, 4, 107 CrossRef PubMed.
- P. Zhu, J. Wang, F. Xia, W. Zhang, H. Liu and X. Zhang, Eur. J. Inorg. Chem., 2023, 26, e202200664 CrossRef.
- A. I. Lupulescu and J. D. Rimer, Angew. Chem., Int. Ed., 2012, 51, 3345–3349 CrossRef PubMed.
- P. Wang, X. Xiao, Y. Pan, Z. Zhao, G. Jiang, Z. Zhang, F. Meng, Y. Li, X. Fan, L. Kong and Z. Xie, Catalysts, 2022, 12, 351 CrossRef.
- L. Liu, H. Wang, R. Wang, C. Sun, S. Zeng, S. Jiang, D. Zhang, L. Zhu and Z. Zhang, RSC Adv., 2014, 4, 21301–21305 RSC.
- Z. Shang, Y. Chen, L. Zhang, X. Zhu, X. Wang and C. Shi, Inorg. Chem. Front., 2022, 9, 1456–1466 RSC.
- Z. Shang, Y. Chen, L. Zhang, X. Zhu, X. Wang and C. Shi, Inorg. Chem. Front., 2022, 9, 2097–2103 RSC.
- Y. Rong, X. Zhang, H. Wang, D. Tan, H. Wang and T. Zhang, Microporous Mesoporous Mater., 2022, 341, 112094 CrossRef.
- R. Feng, X. Wang, J. Lin, Z. Li, K. Hou, X. Yan, X. Hu, Z. Yan and M. J. Rood, Microporous Mesoporous Mater., 2018, 270, 57–66 CrossRef.
- R. Feng, X. Yan, X. Hu, J. Wu and Z. Yan, Microporous Mesoporous Mater., 2020, 302, 110246 CrossRef.
- J. Shi, G. Zhao, J. Teng, Y. Wang and Z. Xie, Inorg. Chem. Front., 2018, 5, 2734–2738 RSC.
- X. Wang, P. Karakiliç, X. Liu, M. Shan, A. Nijmeijer, L. Winnubst, J. Gascon and F. Kapteijn, ACS Appl. Mater. Interfaces, 2018, 10, 33574–33580 CrossRef CAS.
- Y. Yang, X. Lu, H. Zhang, X. Yu, Q. Zheng and Z. Wang, Chem.–Asian J., 2023, 18, e202300218 CrossRef PubMed.
- Z. Zhou, R. Jiang, X. Chen, X. Wang and H. Hou, J. Solid State Chem., 2021, 298, 122132 CrossRef.
- X. Chen, R. Jiang, Z. Zhou and X. Wang, J. Dispersion Sci. Technol., 2021, 42, 561–568 CrossRef.
- Y. Ji, Y. Zuo, M. Liu, F. Wang, C. Song and X. Guo, Microporous Mesoporous Mater., 2021, 321, 111100 CrossRef.
- L. Guo, Z. Wang, J. Wang, Z. Wang, S. Xue, X. Jiang, T. Lu, J. Xu, Y. Zhan and L. Han, J. Sol-Gel Sci. Technol., 2020, 96, 256–263 CrossRef.
- G. Lv, S. Deng, Y. Zhai, Y. Zhu, H. Li, F. Wang and X. Zhang, Appl. Catal., A, 2018, 567, 28–35 CrossRef.
- B. Zhang, Y. Shao, T. Tatsumi and J. Wang, Mater. Lett., 2022, 317, 132076 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |

