Open Access Article
Open Access ArticleAdvancing hydrothermal liquefaction of Canadian forestry biomass for sustainable biocrude production: co-solvent integration, co-liquefaction, and process optimization†
Sreenavya
Awadakkam
a,
Vasu
Chaudhary
a,
Ramesh
Kalagnanam
a,
Venu Babu
Borugadda
ab and
Ajay K.
Dalai
 *a
*a
aCatalysis and Chemical Reaction Engineering Laboratories, Department of Chemical and Biological Engineering, University of Saskatchewan, Saskatoon, S7N5A9, Canada. E-mail: ajay.dalai@usask.ca
bTidewater Renewables Ltd, 900, 222 3rd Avenue SW, Calgary, AB T2P 0B4, Canada
First published on 28th January 2025
Abstract
Canadian hardwood and softwood species were screened for hydrothermal liquefaction to produce sustainable biocrude. Based on the availability of the feedstock, their biocrude yield, and oxygen content, spruce (softwood) and poplar (hardwood) species were found to be promising and selected for the optimization of process parameters to maximize biocrude yield while minimizing the oxygen content. Solvent (ethanol) assisted hydrothermal liquefaction was performed to evaluate the effect of process parameters such as temperature, retention time, catalyst loading, and different ethanol concentrations. The highest yield of biocrude obtained from spruce and poplar was ∼36 wt% with an HHV of ∼27 MJ kg−1 under the optimized HTL conditions. HTL experiments were conducted to study the effect of recycling the hydrothermal liquefaction aqueous phase and co-liquefaction of hardwood and softwood species. The HTL aqueous phase recycling improved the quantity (47 wt%) and quality (HHV of 29.9 MJ kg−1) of the biocrude obtained from spruce liquefaction. The co-liquefaction of spruce and poplar (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt%) showed a potential synergistic effect on biocrude yield and quality at a lower reaction temperature (260 °C). The GC-MS analysis of spruce and poplar wood biocrude indicated that the majority of the compounds were phenolic in nature. BET results confirmed the high surface area of spruce and poplar wood-derived hydrochar. The gaseous products formed during HTL were mainly composed of CO2, CO, H2, O2, CH4, and C2H2.
50 wt%) showed a potential synergistic effect on biocrude yield and quality at a lower reaction temperature (260 °C). The GC-MS analysis of spruce and poplar wood biocrude indicated that the majority of the compounds were phenolic in nature. BET results confirmed the high surface area of spruce and poplar wood-derived hydrochar. The gaseous products formed during HTL were mainly composed of CO2, CO, H2, O2, CH4, and C2H2.
1 Introduction
The global push for net-zero emissions by 2050 has accelerated efforts to adopt lower-carbon, sustainable transportation fuels from renewable sources.1–4 Canada, in line with this goal, has enacted clean fuel regulations requiring liquid fuel producers and importers to blend renewable fuels into gasoline (5 vol%) and diesel (2 vol%).5,6 Biomass, a renewable carbonaceous resource, shows great promise for producing such fuels. Research worldwide focuses on sustainable fuel production from second-generation biomass sources like forestry residues, woody biomass, agricultural byproducts, animal waste, sewage, municipal solid waste, and industrial residues.7,8Canada, covering 362 million hectares of forest land (9% of the world's total), is home to 140 native tree species. Key species include spruce, pine, poplar, birch, aspen, hemlock, fir, cedar, maple, and oak.9 According to the National Forest Inventory, spruce accounts for 44% of forest volume and poplar 13%, with the remaining volume comprising other species.9
Woody biomass is classified as softwood and hardwood species based on lignin content and it is considered as a potential resource for biofuel production. Softwoods like spruce, pine, and fir have high lignin content (25–35 wt%), predominantly G units (guacyl, lignin monomer coniferyl alcohol), while hardwoods like maple, oak, and poplar have lower lignin content (18–25 wt%) with variable S (syringyl, lignin monomer sinapyl alcohol) and G unit ratios, affecting their reactivity and solubility.10,11 Key Canadian forestry biomass sources include timber processing residues (wood shavings, sawdust, bark), beetle-killed and fire-damaged trees, and logging residues. Saskatchewan alone has 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 oven-dry tonnes of forestry residues, half from timber processing.12–15 Often underutilized in low-value applications like landfilling, these residues hold promise for biocrude production via thermochemical conversions.16 Biocrude, a renewable fossil fuel alternative, contains valuable chemicals like ketones, esters, acids, and phenolics. It can be used as a fuel or upgraded to biofuels like biodiesel, renewable gasoline and sustainable aviation fuel, making woody biomass an economical, non-food-competing option for large-scale renewable energy projects.17
000 oven-dry tonnes of forestry residues, half from timber processing.12–15 Often underutilized in low-value applications like landfilling, these residues hold promise for biocrude production via thermochemical conversions.16 Biocrude, a renewable fossil fuel alternative, contains valuable chemicals like ketones, esters, acids, and phenolics. It can be used as a fuel or upgraded to biofuels like biodiesel, renewable gasoline and sustainable aviation fuel, making woody biomass an economical, non-food-competing option for large-scale renewable energy projects.17
Hydrothermal liquefaction (HTL) and pyrolysis are key methods for producing biocrudes and bio-oils from renewable lignocellulosic biomass, followed by catalytic upgrading to transportation fuels.18 HTL is particularly promising for converting high-moisture biomass like woody feedstock into biocrude with low oxygen content, along with by-products like gas, aqueous phase, and hydrochar.18 This process uses water as the liquefying medium under sub- and super-critical conditions (250–375 °C, 4–25 MPa), with or without catalysts.19 Hot compressed water breaks down biomass polymers (cellulose, hemicellulose, lignin), yielding a viscous, dark biocrude that requires significant upgrading for fuel specifications.20
Catalysts play a crucial role in enhancing biocrude yield and quality during HTL by inhibiting side reactions, increasing reaction rates, reducing char formation, and improving biocrude properties.21 Both homogeneous and heterogeneous catalysts are used, with alkali catalysts like K2CO3, KOH, Na2CO3, and NaOH being widely effective for lignocellulosic biomass liquefaction.22,23 Studies show that K2CO3 and KOH significantly boost biocrude yields, often doubling outputs compared to non-catalytic processes.24 For example, K2CO3 increased biocrude yield from 18 wt% to 35 wt% in barley straw HTL,25 and KOH achieved a 40% yield in woody biomass, reducing solid residues from 33% to 12%.26 Potassium salts are particularly effective, promoting repolymerization, enhancing biocrude separation, and avoiding corrosion issues associated with metal hydroxides.23 Based on insights from the literature, K2CO3 was chosen as the catalyst for this study, due to its effectiveness in enhancing reaction rates, facilitating the breakdown of biomass, and improving biocrude properties.
Solvent-assisted HTL enhances lignocellulosic biomass depolymerization, with solvent choice significantly impacting reaction rates, pathways, product yields, and oxygen content.27 Polar solvents, especially water, are commonly used for their ability to dissolve biomass components and polar biocrude compounds.27 However, water alone often results in high char yields.28 Alcohols, such as ethanol, are effective co-solvents for hydrothermal liquefaction of various biomass due to their hydrogen-donating properties, improving biocrude quality by reducing oxygen content viscosity, density and enhancing energy content.29 Ethanol, a green solvent, also facilitates hydrodeoxygenation and dissolves heavy molecular fractions while being less corrosive than water.30,31 Studies report the synergistic effects of ethanol–water mixtures in HTL, yielding higher biocrude output and biomass conversion rates.32–34 For example, Cheng et al. demonstrated the synergistic effects of alcohol (methanol or ethanol) and water on the direct liquefaction of pine sawdust.35 Ethanol-assisted HTL of poplar with a bimetal catalyst improved biocrude yield, energy recovery, and hydrodeoxygenation.36 Similarly, ethanol–water co-solvents with alkaline catalysts enhanced biocrude yield, carbon content, and HHV, demonstrating their efficiency in sustainable biomass conversion.37 In the current research investigation, an ethanol–water co-solvent mixture is used as the reaction medium for the HTL of soft and hardwoods to explore the potential of this solvent system in optimizing biocrude yield and quality. The dual role of ethanol as a solvent and an in situ hydrogen donor simplifies the operation by eliminating the need for an external hydrogen supply, thereby reducing process complexity and costs. Additionally, the study highlights the impact of aqueous phase recycling during HTL, which has been shown to significantly improve biocrude yield and reduce oxygen content—critical for optimizing biocrude fuel properties and minimizing the need for costly upgrading processes.
Extensive research exists on the co-hydrothermal liquefaction (co-HTL) of diverse biomass feedstocks, such as lignocellulosic biomass with sewage sludge, swine manure, or microalgae.38–46 Studies indicate that co-HTL can yield synergistic effects, improving biocrude quality, yield, and reducing feedstock collection and transportation costs.41 while co-HTL of diverse biomass types in subcritical water is well-documented, reports on similar biomass combinations are limited. Sharma et al. studied co-HTL of similar lignocellulosic biomasses (wheat straw, eucalyptus, pinewood) in supercritical water with K2CO3 at 400 °C affording lower biocrude and higher solid yields, indicating antagonistic effects.47 However, no studies have addressed co-HTL of hardwoods and softwoods. By leveraging the unique chemical and structural properties of both softwoods and hardwoods, co-HTL enables the efficient conversion of mixed biomass into valuable biocrude. This approach maximizes resource utilization and provides a pathway to address variability in feedstock availability and composition, ensuring a more robust and flexible biocrude production system. Also, it provides a stable and consistent supply throughout the year, unlike some biomass types that may depend on seasonal or environmental conditions. Such innovations are critical for advancing the sustainable energy sector and enhancing the economic viability of biomass-based energy solutions in Canada. This study explores the co-hydrothermal liquefaction of spruce and poplar wood, and identifies a potential synergistic effect that significantly enhances the biocrude yield at a relatively low reaction temperature of 260 °C. This reduction in HTL reaction temperature, while maintaining high yield and acceptable quality, represents a significant advancement in the HTL process, as it can potentially lower operational costs and energy requirements in biofuel production.
The present research aimed to produce sustainable biocrude from Canadian woody biomass and residues via hydrothermal liquefaction. The study investigated the effects of process parameters such as pre-treatment, co-solvent, temperature, catalyst loading, retention time, extraction solvent, aqueous phase recycling, and co-liquefaction on biocrude yield and quality from high- and low-lignin woody biomass. Additionally, the study analysed the properties of biocrude and by-products from softwood and hardwood species to identify their potential applications.
2 Materials and methods
2.1 Materials
The softwood and hardwood species used in this work to conduct the HTL process were collected from the Saskatoon region of Saskatchewan province in Canada. The softwood species were tamarack, pine, spruce, fir, and cedar. The hardwood species were aspen, red birch, white birch, maple, oak, and poplar. Spruce and poplar wood biomass in the form of sawdust was supplied by a local lumber mill (Saskatoon, Canada). All the biomass feedstocks were ground and sieved to particle size ≤1 mm. All the softwood and hardwood feedstock's proximate, ultimate, HHV, and fiber analyses were performed. Ethanol, acetone, potassium carbonate, sodium hydroxide, and urea were purchased from Sigma Aldrich (analytical grade) and used as received.2.2 Biocrude production via hydrothermal liquefaction
Hydrothermal liquefaction experiments were conducted in a 1 L high-pressure/high-temperature Parr batch reactor (model 4577 with a 4848 model reactor controller) having an upper limit pressure and temperature range of up to 5000 psi and 500 °C, respectively. For each experiment, 70 g of woody biomass, 3.5 g of K2CO3 catalyst (5 wt%), and 700 mL (biomass to solvent ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) of ethanol-–deionized water mixture (ratio of ethanol and deionized water is 2.33) were loaded into the reactor. The reactor was sealed, purged with N2 for leak test, and pressurized to an initial pressure of 100 psi and the reaction was carried out at 800 rpm. Performing HTL in a nitrogen atmosphere avoids oxidative reactions, altered product pathways, increased char formation, and significant safety risks, all of which lower biocrude yields and complicate processing when performed in an air atmosphere. The total pressure inside the reactor varied throughout the reaction to reach a final pressure in the range of 1000 to 2000 psi depending on the initial pressure (constant), temperature, amount of solvent (constant), ratio of ethanol and water, reaction time, and gaseous product formation. The reactor was heated to a target temperature of 260, 270, 280, and 300 °C at a retention time of 0, 15, 30, and 45 minutes; where 0 minutes corresponds to the immediate start of cooling by an electric fan after removing the heating jacket when the temperature reaches a set temperature value.
10) of ethanol-–deionized water mixture (ratio of ethanol and deionized water is 2.33) were loaded into the reactor. The reactor was sealed, purged with N2 for leak test, and pressurized to an initial pressure of 100 psi and the reaction was carried out at 800 rpm. Performing HTL in a nitrogen atmosphere avoids oxidative reactions, altered product pathways, increased char formation, and significant safety risks, all of which lower biocrude yields and complicate processing when performed in an air atmosphere. The total pressure inside the reactor varied throughout the reaction to reach a final pressure in the range of 1000 to 2000 psi depending on the initial pressure (constant), temperature, amount of solvent (constant), ratio of ethanol and water, reaction time, and gaseous product formation. The reactor was heated to a target temperature of 260, 270, 280, and 300 °C at a retention time of 0, 15, 30, and 45 minutes; where 0 minutes corresponds to the immediate start of cooling by an electric fan after removing the heating jacket when the temperature reaches a set temperature value.
The liquefaction yields and energy recovered in biocrude or hydrochar were calculated using the following eqn (1)–(5):
| Biocrude yield (wt%) = (mass of biocrude/mass of dried feedstock) × 100 | (1) |
| Hydrochar yield (wt%) = (mass of hydrochar/mass of dried feedstock) × 100 | (2) |
| Aqueous phase products (wt%) + gas phase products (wt%) = 100 − (biocrude (wt%) + hydrochar (wt%)) | (3) |
| Energy recovery (%)biocrude = [(mass of biocrude × HHV of biocrude)/(mass of dried feedstock) × (HHV of dried feedstock)] × 100 | (4) |
| Energy recovery (%)hydrochar = [(mass of hydrochar × HHV of hydrochar)/(mass of dried feedstock) × (HHV of dried feedstock)] × 100 | (5) |
2.3 Biocrude recovery and separation of the aqueous phase and hydrochar
Upon completion of the HTL reaction, the reactor was cooled to room temperature, and the non-condensable gas samples were collected in a tedlar bag for analysis. The remaining HTL slurry inside the reactor consists of an aqueous phase, hydrochar, and biocrude. The aqueous phase was separated by vacuum filtration. The remaining solid residue was subjected to solvent extraction using a suitable solvent (ethanol/acetone) followed by vacuum filtration to separate hydrochar from biocrude. Rota evaporation of the resulting biocrude and solvent mixture separates the solvent from the biocrude, which is a dark-colored highly viscous semi-solid. A schematic representation of the overall outline of the HTL process is shown in Fig. 1.2.4 Physicochemical characterization of woody biomass and the HTL products
Softwood and hardwood feedstocks were tested for their ultimate and proximate analysis using standard operating procedures. Moisture, ash, and volatile matter content of the feedstock were analyzed at 105 °C, 575 °C, and 950 °C based on ASTM 3173-87, ASTM 3174-04, and ASTM D3175-89, respectively. The fixed carbon percentage was calculated by deducting the moisture, ash, and volatile matter content from the total weight of the tested feedstock. The fiber analysis was conducted by a modified Van Soest method applying an Ankom 200 Fiber Analyzer (ANKOM Technology, Macedon, NY), determining cellulose, hemicellulose, and lignin proportion in the biomass.Elemental composition (CHNS) of the woody biomass feedstocks, biocrude, and hydrochar was performed on a Vario EL III CHNS Elemental Analyzer (Elementar Americas, Inc., Ronkonkoma, NY, USA). Oxygen was calculated based on the material balance as follows:
| Oxygen = 100 − (C + H + N + S + Ash) |
An oxygen bomb calorimeter (Parr 6400 calorimeter, IL, USA) was used for the measurement of the higher heating value of biomass feedstocks and their corresponding optimized biocrudes and hydrochars based on ASTM D5865. The mineral contents of raw biomass, optimal biocrudes, including K, Na, Ca, Mg, Fe, and Al were measured by inductively coupled plasma-optical emission spectrometry (ICP-OES; Optima 83000) and TOC analyzer (Lotix combustion TOC analyzer, Teledyne Tekmar, USA), respectively. The moisture content of the optimal biocrude was measured using a Karl Fischer coulometer (Mettler Toledo DL32: ASTM D6304-20 procedure B). The chemical composition of the biocrude was determined using gas chromatography-mass spectrometry (JEOL JMS-T100 GCy AccuTOF-GCv4G mass spectrometer with a 28 m DB5 capillary column having 0.25 μm film thickness). The textural properties of the calcined hydrochar (calcination at 550 °C for 3 hours in an inert atmosphere) were studied by N2 adsorption–desorption at 77 K using a Micromeritics ASAP 3500 Porosity Analyzer (degassing at 90 °C for 1 h followed by 350 °C for 4 h). Analysis of the gaseous products was performed using an Agilent 7890 GC having a Flame Ionization Detector (FID) and a Thermal Conductivity Detector (TCD). Analysis of the permanent gases was performed in the TCD detector using the HaysepQ column in series with a molecular sieve column. The gaseous hydrocarbons were analyzed using a Varian capillary column (25 m, 0.53 mm O. D, 10 μm thickness of Al2O3/KCl). During the gas analysis, the oven was programmed from 30 °C to 150 °C with an initial hold at 30 °C for 17 min and a ramp rate of 20 °C to 150 °C. The total acid number (TAN) of biocrude was determined by titration on a pH meter (Titroline 7000) using 0.01 N KOH and phenolphthalein as the titration solution and indicator, respectively. TAN was calculated in milligrams of KOH per gram of the biocrude sample as follows:
| TAN = [(A − B)N × 56.1]/W. | (6) |
3 Results and discussion
3.1 Proximate, ultimate, and fiber analysis of the Canadian-grown soft and hardwoods
A set of softwood species was characterized by proximate, ultimate, and fiber analysis (Table S1†) and the lignin content of the species varies in the range of 23–32 wt%. The species were subjected to HTL experiments under identical conditions to select a suitable feedstock for further process optimization studies (Table S2†). All the softwood species achieved a biocrude yield in the range of 23–31 wt%. The oxygen content in their biocrude ranged from 20 to 23 wt% (Table S2†). In a biorefinery process, the selection of feedstocks for large-scale processes depends mainly on their availability, the biocrude yield, and their oxygen content. Among the selected hardwood feedstock, spruce is the major species used in the Canadian lumber industry and its residue is abundantly available. The spruce wood has a lignin content of 26 wt% and yielded ∼25 wt% biocrude with 20.5 wt% oxygen content under the tested reaction conditions. Based on these factors, spruce wood is chosen from high-lignin woody biomass to optimize the process parameters to obtain a high quantity and quality of the sustainable biocrude.Similarly, a set of hardwood species (aspen, red birch, white birch, maple, oak, and poplar) were selected and characterized by proximate, ultimate, and fiber analysis (Table S3†). The lignin content of the species varies in the range of 9–19 wt%. The species were subjected to HTL experiments under identical conditions to select a suitable feedstock for further process optimization studies (Table S4†). All the hardwood species achieved a biocrude yield in the range of 23–31 wt% with oxygen content in the range of 17–23 wt%. Among the hardwood species poplar is the one majorly occupying Canadian forest land (13%) and its residue is abundantly available in the Canadian lumber industry. The lignin content in poplar wood is 9.9 wt% and yielded a biocrude in the range of ∼24 wt% with an oxygen content of 21 wt%. So, for further process optimization studies to obtain a better biocrude yield from a low-lignin woody biomass, poplar is selected from the screened feedstocks.
3.2 Optimization of process parameters for biocrude production from high lignin woody biomass (spruce wood)
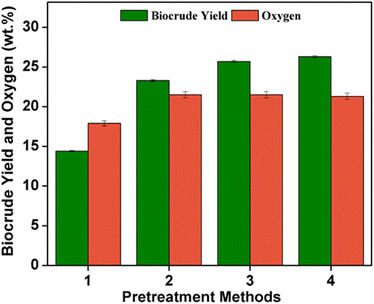
Pre-treatment methods often help to swell/open the fiber structure of wood fibers, which could increase the effectiveness of the HTL process. One of the pre-treatment methods employed in the pulp and paper industry is the dissolution of wood pulp in an aqueous NaOH/urea solution for inducing swelling or opening of the fiber structure and the direct dissolution of lignin.49,50 In this study, the spruce wood is pretreated in an aqueous NaOH/urea solution (1) followed by HTL yielding ∼15 wt% of biocrude with 18 wt% oxygen. Among the different pre-treatment methods employed, spruce wood treated under alkaline pre-treatment using NaOH (2) & (4) and K2CO3 (3) yielded higher biocrude yield (26.3 and 25 wt% respectively) during HTL under identical conditions. In a bio-refinery concept, the pre-treatment of lignocellulosic biomass is a highly energy-intensive and expensive process. Instead of pre-treating the spruce wood biomass under alkaline conditions, the effect of NaOH and K2CO3 as a catalyst during the HTL of spruce wood was tested. The catalytic HTL of spruce wood with 5 wt% K2CO3 and NaOH loading yielded 25.4 and 27 wt% biocrude with 17 and 22 wt% oxygen and 15.6 wt% and 11.4 wt% hydrochar yield respectively. The results agreed with the conclusions as stated in the previous literature that alkaline–thermal treatment led to positive influences on the liquefaction of biomass through an increase in the production of the biocrude with lower oxygen content and limiting char formation.26,51 The noncatalytic HTL conducted at 300 °C achieved 19.1 wt% biocrude with 23.8 wt% oxygen content and 31.4 wt% hydrochar yield. The results show that catalytic liquefaction significantly improves biocrude yield and decreases hydrochar formation compared with the noncatalytic HTL studies. This could be because the base catalyst is favorable for liquefying high lignin-containing biomass. It also suppresses coke formation and promotes retro aldol cleavage and condensation thereby stabilizing the products.37 However, NaOH is a strong base and corrosive to the reactor compared to K2CO3. HTL of spruce wood with K2CO3 catalyst gives a similar biocrude yield (25.4 wt%) with lower oxygen content (17 wt%) compared to NaOH. The pre-treatment of biomass in an alkaline medium was indeed investigated during the initial hydrothermal liquefaction (HTL) experiments. However, these preliminary studies revealed that pretreatment did not have a significant impact on either the quantity or quality of the resulting biocrude. Consequently, all HTL experiments reported in the manuscript were conducted without pre-treating the biomass, using K2CO3 as the catalyst.
The HTL experiment using pure water as a solvent (0 vol% ethanol) at 300 °C yielded 25.1 wt% biocrude with 20.5 wt% oxygen. The results in Fig. 3 clearly show that as the concentration of co-solvent ethanol gradually increases from 0 to 30 vol% the biocrude yield also increases from 25 to 30 wt% with a decrease in oxygen content from 21 to 18 wt%. Another significant observation is that the use of ethanol as a co-solvent suppresses the hydrochar formation. As the ethanol ratio changes from 0%, 10%, 20%, 30%, and 40% the hydrochar amount varies from 15.6, 9.1, 7.2, 3.8, and 2 wt% respectively. The hot compressed water helps the liquefaction of lignocellulosic biomass during HTL. However, the alcohol–water mixture showed a synergistic effect on biomass direct liquefaction. The low dielectric constant and improved diffusivity of the mixed ethanol–water solvent increased the permeation of the solvent into the lignocellulose biomass structure and improved their depolymerization.
Ethanol also increased the solubility of liquefaction intermediates, hence preventing the repolymerization of the reaction intermediates by dissolving the depolymerized products and resulting in improved biocrude yield. The combined effect of an alkaline catalyst and ethanol as a co-solvent may promote the in situ hydrogen production and promote the hydrodeoxygenation of biocrude which might be a reason for the decreased oxygen content in the biocrude obtained by catalytic HTL.37 The binary alcohol–water solvent mixture has a lower critical temperature (T) and pressure (P) (∼311.3 °C and 10.2 MPa or ∼1484 psi) than water alone (374.1 °C and 22.1 MPa or ∼3200 psi) making it a highly reactive medium.34 Further increasing the ethanol percentage to 40 vol% decreases the biocrude yield to 24 wt%. Decreased biochar yield and increased gas formation (final reaction pressure of 2000 psi) were also observed under these conditions. A possible reason for this can be that at 40% ethanol in water, the supercritical conditions of the ethanol–water mixture can be achieved under the given reaction conditions (280 °C and 2000 psi). This could lead to enhanced solubility of the intermediates in the aqueous phase and gasification reactions, whereas at lower concentrations of ethanol (≤30%), the supercritical conditions might not have been achieved in the reaction.35 Ethanol–water mixture with 30 vol% co-solvent ratio was identified as the most effective solvent concentration for improved biocrude production from spruce sawdust under the reaction conditions.
Fig. 4 shows that as the temperature increases from 260 °C to 280 °C the biocrude yield gradually increases from 27 wt% to 31 wt%. Furthermore, an increase in temperature to 300 and 320 °C slightly decreases the biocrude yield. The lower biocrude yield at a lower reaction temperature (260 °C) might be due to the incomplete depolymerization of biomass components.52 which is evident from the increased hydrochar yield (∼20 wt%). Furthermore, increasing the temperature (280 °C) enhances the fracture of chemical bonds and the depolymerization of biomass. The hydrochar yield obtained under this condition was 7.2 wt%. After the biocrude yield reaches the maximum value, further increasing the temperature (300–320 °C) could inhibit biocrude production. The observed increment in hydrochar (14 wt%), as well as the gas formation at the elevated reaction temperature, might be due to (1) the secondary decomposition of the produced biocrude and Boudouard gas reactions which leads to gaseous products or (2) recombination/condensation reactions of intermediates (free radicals) to generate hydrochar.21,25,53Fig. 4 shows that the oxygen content in the biocrude steadily decreases with increasing temperature. Thus, increasing the temperature leads to a higher calorific value of the produced crude owing to the decreased oxygen content and increased carbon content due to deoxygenation reactions.54 Based on the results, 280 °C is selected as the optimum reaction temperature for further studies.
| K2CO3 + H2O → KHCO3 + KOH |
The presence of an alkaline catalyst, during the ethanol-assisted HTL of spruce, can weaken structural linkages between cellulose and lignin, resulting in a decrease in the activation energy of biomass bond cleavage and thereby improving the biocrude yield.25 Besides, the reaction rate of carbon–carbon scission was improved in the case of higher alkali concentration, which might be favourable to gas formation as well as more water-soluble products thereby leading to lower biocrude yield as observed with 7.5 wt% K2CO3 concentration.
The summarized data in Table S6† illustrate that the combination of K2CO3 and ethanol in the HTL of spruce wood biomass under optimum reaction conditions could significantly enhance the efficiency and effectiveness of the process. This synergistic approach leads to higher yields and better quality of biocrude, with lower hydrochar formation. Catalytic enhancement by K2CO3 accelerates key reactions, such as hydrolysis, decarboxylation, and dehydration, leading to more efficient conversion of biomass into bio-crude.23,37 Ethanol improves the solubility of biomass and intermediate products, ensuring better contact between the biomass and the catalyst. Ethanol can also alter the phase behavior of the reaction mixture, potentially lowering the necessary temperature and pressure for effective biomass conversion. As a hydrogen donor, ethanol stabilizes free radicals and promotes hydrogenation reactions, which are crucial for the formation of high-quality bio-crude.26,30,31 The enhanced catalytic activity and improved solubilization minimize the formation of hydrochar, which is a solid by-product of incomplete biomass conversion. The catalytic action of K2CO3, along with ethanol's role as a hydrogen donor, reduces the oxygen content in the bio-crude, resulting in a higher energy density.
| Extraction solvent | Relative polarity | Biocrude yield (wt%) | Oxygen content (wt%) |
|---|---|---|---|
a Average values are reported in the table. Reaction conditions: 70 g feedstock, biomass to solvent ratio (W/V) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 30 vol% ethanol co-solvent, 5 wt% K2CO3 catalyst T = 280 °C, initial reaction pressure = 100 psi, final pressure: 1500 psi. 10, 30 vol% ethanol co-solvent, 5 wt% K2CO3 catalyst T = 280 °C, initial reaction pressure = 100 psi, final pressure: 1500 psi.
|
|||
| Ethanol | 0.65 | 31 | 21 |
| Acetonitrile | 0.46 | 25 | 24 |
| Acetone | 0.36 | 35 | 29 |
| Ethyl acetate | 0.23 | 33 | 13 |
3.3 Optimization of process parameters for the improved biocrude yield from low lignin woody biomass (poplar wood)
Further experiments were devoted to optimizing process conditions for improved biocrude yield from low-lignin woody biomass poplar. Initial experiments were carried out at varying reaction temperatures (260, 280, and 300 °C). Poplar liquefaction at 260 °C for 30 minute retention time using 30 vol% ethanol and 5 wt% K2CO3 yielded 36.3 wt% of biocrude with 27.6 wt% of oxygen. A decrease in biocrude yield was observed upon further increase in HTL temperature. An elevated biocrude yield of poplar wood at lower HTL temperature might be due to its lower lignin, higher cellulose, and hemicellulose content. Poplar liquefaction was conducted for 15 minutes by keeping all other parameters constant and the obtained 21 wt% of biocrude with 32 wt% of oxygen indicates that lowering retention time does not favour the biocrude yield and quality. The effect of co-solvent ethanol concentration in the aqueous phase for poplar liquefaction at 260 °C was optimized by varying the concentration of ethanol from 0 to 30 vol%. Liquefaction of poplar in the presence of 10 vol% co-solvent ethanol at 260 °C for 30 minutes yielded 38 wt% of biocrude with 31 wt% of oxygen. Further increase in ethanol ratio resulted in a decrease in the biocrude yield maybe due to the increased solubility of intermediates in the aqueous phase. Compared to the HTL of high lignin woody biomass spruce, a lower reaction temperature (260 °C) and lower ethanol concentration (10 vol%) for 30 min retention time are the optimum conditions for higher biocrude production from low lignin woody biomass poplar.3.4 Effects of co-liquefaction of high-lignin (spruce) and low-lignin (poplar) woody biomass
The present study focused on the optimization of the relevant process parameters to obtain the maximum biocrude production via co-hydrothermal liquefaction of high lignin (spruce) and low lignin (poplar) woody biomass by varying the weight percentage of the species from 0 to 100 under optimized reaction conditions at 260 °C (Fig. 8). The co-liquefaction was conducted at a lower reaction temperature (260 °C), the temperature where poplar gave maximum biocrude yield with an assumption that the free radical produced during the poplar liquefaction can facilitate the liquefaction of spruce wood at a lower temperature. Hydrothermal co-liquefaction of spruce and poplar wood showed a potential synergistic effect on crude yield and oxygen content. Co-liquefaction of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% of spruce and poplar using 30 vol% ethanol shows the highest biocrude yield (35 wt%) with 29 wt% of oxygen at a reduced reaction temperature of 260 °C. This reduction in temperature is a significant advancement, as it lowers the energy and operational costs of the HTL process. The hydrochar yield was found to be 11.4 wt%. An increase in the yield of hydrochar was observed as the ratio of spruce (high lignin woody biomass) in the feedstock increased. The obtained values of biocrude yield and oxygen content lie in between those of the individual species at this temperature. Furthermore, the co-liquefaction of 50
50 wt% of spruce and poplar using 30 vol% ethanol shows the highest biocrude yield (35 wt%) with 29 wt% of oxygen at a reduced reaction temperature of 260 °C. This reduction in temperature is a significant advancement, as it lowers the energy and operational costs of the HTL process. The hydrochar yield was found to be 11.4 wt%. An increase in the yield of hydrochar was observed as the ratio of spruce (high lignin woody biomass) in the feedstock increased. The obtained values of biocrude yield and oxygen content lie in between those of the individual species at this temperature. Furthermore, the co-liquefaction of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% spruce and poplar was also conducted at 260 °C using 10 vol% ethanol, and a lower biocrude yield of 32 wt% with 32 wt% oxygen was obtained. Also, the co-liquefaction of 50
50 wt% spruce and poplar was also conducted at 260 °C using 10 vol% ethanol, and a lower biocrude yield of 32 wt% with 32 wt% oxygen was obtained. Also, the co-liquefaction of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt% spruce and poplar was conducted at 280 °C using 30 vol% ethanol, the temperature where spruce gave maximum biocrude yield, and a biocrude yield of 38.3 wt% with 32 wt% of oxygen was obtained. The study suggests that the co-liquefaction shows a synergistic effect on the biocrude yield, however, it did not lower the oxygen content significantly in the biocrude.
50 wt% spruce and poplar was conducted at 280 °C using 30 vol% ethanol, the temperature where spruce gave maximum biocrude yield, and a biocrude yield of 38.3 wt% with 32 wt% of oxygen was obtained. The study suggests that the co-liquefaction shows a synergistic effect on the biocrude yield, however, it did not lower the oxygen content significantly in the biocrude.
4 Characterization of the biocrudes, hydrochars, and gases
Tables S7 and S8† represent the classification of chemical compounds detected by GC-MS analysis of the biocrude obtained by the HTL of spruce and poplar wood. The most common compounds identified were alcohols, aldehydes, acids, ketones, esters, and phenols. Among them, phenols are the major class of compounds found in both the biocrudes. Possible decomposition pathways of lignocellulosic biomass macromolecules, including cellulose, hemicellulose, and lignin, under HTL conditions are represented in Fig. S1 and S2.† The long-chain fatty acid esters, alcohols and hydrocarbons (such as 3-hexenoic acid, ethyl ester, hexadecanoic acid ethyl ester, ethyl oleate, octadecanoic acid ethyl ester, 1,2 nonadecanediol, and heptadecane as evident from GCMS analysis (data obtained from GCMS analysis are reported in Tables S7 and S8†)) were formed by the complex hydrolysis and dehydration reactions of the cellulose and hemicellulose (Fig. S1†).61 The presence of ethyl esters of long-chain fatty acids in the biocrude can indeed be attributed to esterification reactions between carboxylic acids present in the biocrude and ethanol present in the co-solvent mixture. Phenol derivatives could originate from cleavage of ether bonds or C–C linkage in lignin through hydrolysis followed by complex demethoxylation and demethylation reactions (Fig. S2†).61–63 2-Methoxy phenol, 4-ethyl-2-methoxy phenol, 2-methoxy-4-(1-propenyl)-, (Z)-, 4-butyl-2-methoxyphenol are the major compounds identified in softwood (spruce) biocrude, which originated from guacyl(G)-units, whereas homosyringaldehyde, syringyl acetone, 2′,5′-dihydroxy-4′-methoxyacetophenone, 2-methoxy phenol, and 2,6-dimethoxy-4-[(E)-prop-1-enyl] phenol are the major compounds identified in hardwood (poplar) biocrude and they mostly originated from syringyl and guacyl (S/G)-units as they are identified as the major chemical compounds in the hardwood. The results are in line with the chemical and structural characterization of softwood and hardwood as reported in the literature by Maria et al.11 The higher oxygen content in the poplar biocrude might be due to the presence of a higher syringyl derivative which has more oxygen functionalities compared to the guacyl unit in the spruce wood biocrude.
Table 2 summarizes the higher heating value (HHV), moisture content, and total acid number of biocrudes obtained from spruce and poplar wood under optimum HTL conditions. The spruce wood has an HHV of 16.8 MJ kg−1 and the biocrudes obtained from spruce wood under optimum reaction conditions at 280 and 260 °C have HHVs of 27.3 and 26.4 MJ kg−1 respectively. The energy recovery rate in both cases is 57.4% and 43.8% respectively. Poplar wood has an HHV of 18.1 MJ kg−1 and the biocrude obtained from poplar wood has an HHV of 27.8 MJ kg−1. The energy recovery in this case is 55.8%. The biocrudes obtained by the co-liquefaction of spruce wood and poplar wood at a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
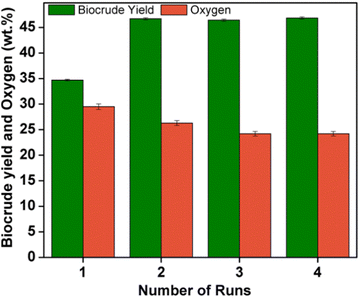
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 weight ratio at 260 °C and 280 °C show HHVs of 27 and 26.2 MJ kg−1 respectively. These values are found to be close to the calorific values of the biocrudes of the individual species. The calorific values of the biocrudes are inversely proportional to their oxygen content. The biocrude obtained by the fourth HTL aqueous phase recycling experiment shows a higher calorific value (29.9 MJ kg−1) owing to their lower oxygen content (24.2 wt%), and the energy recovery, in this case, is 83.4%. The aqueous phase recycling improved the biocrude yield and energy recovery. However, the corresponding biocrude contains 1 wt% ash content due to the increased mineral matter content. The ICP-OES analysis (data obtained from ICP-OES analysis are reported in Table S9†) of spruce wood, poplar wood, and their biocrude show the presence of inorganic elements such as Li, Na, Mg, K, Ca, Fe, Al, Zn, Ti, Si, and S. The huge content of K (87 ppm) observed in the ICP analysis of spruce wood biocrude obtained from the aqueous phase recycling experiment might be due to the increased concentration of K2CO3 catalyst in the recycled water which agrees with the previously reported literature.59 Therefore, HTL aqueous phase recycling shows an increasing effect of the inorganic element content and thereby leads to an increase in ash content. Poplar wood biocrude exhibited an ash content of 0.75 wt% and the biocrude obtained by co-liquefaction of spruce and poplar wood has an ash content of 0.79 wt%. The ICP analysis of the poplar wood biocrude as well as the biocrude obtained from the co-liquefaction experiment exhibited the presence of the above-mentioned inorganic elements with higher quantities of potassium (35.8 ppm and 76.6 ppm respectively). The large numbers of potassium ions detected in all the biocrudes might be due to the use of K2CO3 as a catalyst during liquefaction, which implies that their use provides a negative effect and increases the ash content. The bound water in the biocrude was analyzed via Karl Fischer titration and is included in Table 2. The moisture content of the obtained biocrudes is in the range of 1.6–2.1 wt%.
50 weight ratio at 260 °C and 280 °C show HHVs of 27 and 26.2 MJ kg−1 respectively. These values are found to be close to the calorific values of the biocrudes of the individual species. The calorific values of the biocrudes are inversely proportional to their oxygen content. The biocrude obtained by the fourth HTL aqueous phase recycling experiment shows a higher calorific value (29.9 MJ kg−1) owing to their lower oxygen content (24.2 wt%), and the energy recovery, in this case, is 83.4%. The aqueous phase recycling improved the biocrude yield and energy recovery. However, the corresponding biocrude contains 1 wt% ash content due to the increased mineral matter content. The ICP-OES analysis (data obtained from ICP-OES analysis are reported in Table S9†) of spruce wood, poplar wood, and their biocrude show the presence of inorganic elements such as Li, Na, Mg, K, Ca, Fe, Al, Zn, Ti, Si, and S. The huge content of K (87 ppm) observed in the ICP analysis of spruce wood biocrude obtained from the aqueous phase recycling experiment might be due to the increased concentration of K2CO3 catalyst in the recycled water which agrees with the previously reported literature.59 Therefore, HTL aqueous phase recycling shows an increasing effect of the inorganic element content and thereby leads to an increase in ash content. Poplar wood biocrude exhibited an ash content of 0.75 wt% and the biocrude obtained by co-liquefaction of spruce and poplar wood has an ash content of 0.79 wt%. The ICP analysis of the poplar wood biocrude as well as the biocrude obtained from the co-liquefaction experiment exhibited the presence of the above-mentioned inorganic elements with higher quantities of potassium (35.8 ppm and 76.6 ppm respectively). The large numbers of potassium ions detected in all the biocrudes might be due to the use of K2CO3 as a catalyst during liquefaction, which implies that their use provides a negative effect and increases the ash content. The bound water in the biocrude was analyzed via Karl Fischer titration and is included in Table 2. The moisture content of the obtained biocrudes is in the range of 1.6–2.1 wt%.
| Sample | Reaction conditions | Elemental analysis (wt%) | H/C ratio | O/C ratio | HHV (MJ kg−1) | Moisture (wt%) | TAN (mg KOH per g) | ||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | |||||||
| a Average values are reported in the table. The values of N and S are less than 0.1 wt%. | |||||||||
| Spruce wood | 51.2 | 6.7 | 42.0 | 1.57 | 0.62 | 16.8 | 6.2 | — | |
| Poplar wood | 46.2 | 6.4 | 47.4 | 1.66 | 0.77 | 18.1 | 6.5 | — | |
| Spruce wood biocrude | 280 °C, 30 min, 30 vol% EtOH, 5 wt% K2CO3 | 65.4 | 7.1 | 27.5 | 1.22 | 0.26 | 27.3 | 1.6 | 34 |
| Poplar wood biocrude | 260 °C, 30 min, 10 vol% EtOH, 5 wt% K2CO3 | 66 | 6.4 | 27.6 | 1.16 | 0.30 | 27.8 | 1.4 | 37 |
| Spruce wood biocrude | 260 °C, 30 min, 30 vol% EtOH, 5 wt% K2CO3 | 62.7 | 6.5 | 30.7 | 1.24 | 0.37 | 26.4 | 1.9 | 27 |
Co-liquefaction biocrude (poplar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) spruce = 50 spruce = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt%) 50 wt%) |
260 °C, 30 min, 30 vol% EtOH, 5 wt% K2CO3 | 63.7 | 7.2 | 29.1 | 1.33 | 0.34 | 27.0 | 1.8 | 31 |
Co-liquefaction biocrude (poplar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) spruce = 50 spruce = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt%) 50 wt%) |
280 °C, 30 min, 30 vol% EtOH, 5 wt% K2CO3 | 60.6 | 7.2 | 32.2 | 1.8 | 0.5 | 26.2 | 2.1 | 35 |
| Aqueous phase recycling (4th cycle) | 280 °C, 30 min, 30 vol% EtOH, 5 wt% K2CO3 | 69. 3 | 6.5 | 24.2 | 1.13 | 0.26 | 29.9 | 1.5 | 21.5 |
The observed high total acid number (TAN) of the produced biocrudes (27–37 mg KOH per g) under optimum conditions is directly related to their high oxygen content. The presence of highly oxygenated compounds in the biocrude such as alcohols, aldehydes, acids, ketones, esters, and phenols (as evident from GCMS analysis (data obtained from GCMS analysis are reported in Tables S7 and S8†)) results in lower calorific value and increased TAN, viscosity, and density of the biocrude.19 The biocrude obtained in the present study was a highly viscous dark-colored semi-solid. The HHV and TAN of the produced biocrudes were nowhere near the crude oil which is ∼40–44 MJ kg−1 and ∼1.7 mg KOH per g respectively. High TAN values in biocrude indicate acidic compounds that cause corrosion of infrastructure, increase maintenance costs, and shorten lifespan. They also lead to chemical instability, forming gums or sludges that clog pipelines and hinder handling. High TAN is associated with high oxygen content, reducing energy density and necessitating costly upgrading processes like hydrotreatment, which require significant hydrogen and elevate production costs and carbon footprint of the fuel.19
Table S10† summarizes the HHV and textural properties of hydrochar obtained by the HTL of spruce wood and poplar wood under optimum HTL conditions. The spruce and poplar wood hydrochar show a heating value of 27.3 and 22.9 MJ kg−1 respectively. The values are higher than those of their corresponding feedstocks suggesting that the hydrochar obtained can be used as solid fuel pellets. The textural properties of the hydrochar were studied after removing the residual oil by calcination at 550 °C for 3 h in a nitrogen atmosphere. Spruce wood and poplar wood hydrochar exhibited a surface area of 329.9 and 267.4 m2 g−1 respectively with pore size in the mesopore range (3–3.5 nm). The observed high surface area of these materials offers use as a support for the metal catalyst which can be used for the catalytic upgrading of the produced biocrudes.62–64
The non-condensable gases produced during the hydrothermal liquefaction of the spruce wood and poplar wood were collected in a tedlar bag and analyzed by gas chromatography. The results in Table S11† show that the yields of gaseous products contained mainly CO2, CO, O2, H2, CH4, and trace amounts of acetylene. CO2 (∼65–68 mol%) was the primary component in gas yields in both cases followed by CO (∼18–22 mol%), H2 (∼8–11 mol%), O2 (0.8–5 mol%), CH4 (0.2–0.3 mol%) and acetylene (0.02–0.06 mol%) respectively. The presence of considerable amounts of combustible gases (CO, H2, CH4, and acetylene) offers the opportunity to use the gases for harnessing energy in a biorefinery concept.65
5 Conclusions
This study establishes the technological feasibility of producing biocrude through hydrothermal liquefaction (HTL) of Canadian softwood and hardwood species, specifically spruce and poplar. These species were identified as the most promising feedstocks based on their availability, biocrude yield, and quality. Process optimization studies revealed that HTL using an ethanol–water co-solvent system and K2CO3 as a catalyst significantly enhanced biocrude yield and reduced oxygen content, leading to higher energy density.Key findings include the production of 35.3 wt% biocrude with a calorific value of 27.3 MJ kg−1 from spruce at 280 °C, and 36 wt% biocrude with a calorific value of 27.8 MJ kg−1 from poplar at 260 °C. Recycling the aqueous phase further boosted biocrude yield and quality, with spruce yielding 47 wt% biocrude and a calorific value of 29.9 MJ kg−1 under optimized conditions. However, this approach also increased ash content and potassium concentration in the biocrude, underscoring the need for additional processing to address ash-related challenges.
The ethanol–water co-solvent system proved to be a suitable and innovative approach, offering a synergistic effect that not only enhances biocrude yield but also simplifies the process by serving as an in situ hydrogen source. This method effectively reduces the need for external hydrogen supply, making it economically viable and operationally efficient.
The co-liquefaction of spruce and poplar (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 wt%) demonstrated a synergistic effect, achieving 35 wt% biocrude yield and a calorific value of 27 MJ kg−1 at a reduced reaction temperature of 260 °C. This reduction in operating temperature is particularly significant, as it sustains high biocrude yield and quality while reducing energy requirements and operational costs, contributing to the economic feasibility of biofuel production.
50 wt%) demonstrated a synergistic effect, achieving 35 wt% biocrude yield and a calorific value of 27 MJ kg−1 at a reduced reaction temperature of 260 °C. This reduction in operating temperature is particularly significant, as it sustains high biocrude yield and quality while reducing energy requirements and operational costs, contributing to the economic feasibility of biofuel production.
Future research will focus on the detailed characterization of the aqueous phase to better understand its role in enhancing biocrude yield and quality. Additionally, exploring strategies to mitigate ash content and investigating the scalability of the HTL process for woody biomass feedstocks will be crucial for further development.
This study provides valuable insights into the valorization of Canada's abundant forest residues, paving the way for their use in sustainable bio refineries. By producing high-quality transportation fuels from industrially available feedstocks, this work supports the economic viability of biofuel production and contributes to a sustainable energy future.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Sreenavya Awadakkam: conceptualization; reaction methodology; preparation of materials and formal analysis; roles/writing – original draft; writing – review & editing; visualization. Vasu Chaudhary: methodology; roles/writing – original draft; writing – review & editing. Ramesh Kalagnanam: reaction methodology; preparation of materials and formal analysis. Venu Babu Borugadda: investigation; resources; software; supervision; validation; visualization; writing – review & editing. Ajay K. Dalai: project administration; resources; software; supervision; funding acquisition; investigation.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the studies reported in this paper.Acknowledgements
The authors thank the industrial partner Tidewater Renewables Ltd, Calgary, Canada and the Natural Sciences and Engineering Research Council of Canada (NSERC) (Alliance) for providing financial support.References
- J. L. Holechek, H. M. E. Geli, M. N. Sawalhah and R. Valdez, Sustainability, 2022, 14, 4792 CrossRef.
- K. Alper, K. Tekin, S. Karagöz and A. J. Ragauskas, Sustainable Energy Fuels, 2020, 4(9), 4390–4414 RSC.
- A. Mathanker, D. Pudasainee, A. Kumar and R. Gupta, Fuel, 2020, 271, 117534 CrossRef CAS.
- J. Y. Kim, H. W. Lee, S. M. Lee, J. Jae and Y. K. Park, Bioresour. Technol., 2019, 279, 373–384 CrossRef CAS PubMed.
- Implementation of Bioenergy in Canada-2021 Update, https://www.ieabioenergy.com/wpcontent/uploads/2021/11/CountryReport2021_Canada_final.pdf, accessed March 2024 Search PubMed.
- What are the Clean Fuel Regulations?, https://www.canada.ca/en/environment-climate-change/services/managing-pollution/energy-production/fuel-regulations/clean-fuel-regulations/about.htmltoc1, accessed March 2024 Search PubMed.
- K. Srirangan, L. Akawi, M. Moo-Young and C. P. Chou, Appl. Energy, 2012, 100, 172–186 CrossRef.
- N. M. Clauser, F. E. Felissia, M. C. Area and M. E. Vallejos, Renewable Sustainable Energy Rev., 2021, 139, 110687 CrossRef.
- Statistical Data, https://cfs.nrcan.gc.ca/statsprofile/inventory/canada, accessed March 2024 Search PubMed.
- A. Yousuf, D. Pirozzi and F. Sannino, in Lignocellulosic Biomass to Liquid Biofuels, ed. A. Yousuf, D. Pirozzi and F. Sannino, Elsevier, United Kingdom, 2020, ch.1, pp. 1–15 Search PubMed.
- M. J. Suota, T. A. da Silva, S. F. Zawadzki, G. L. Sassaki, F. A. Hansel, M. Paleologou and L. P. Ramos, Ind. Crops Prod., 2021, 173, 114138 CrossRef CAS.
- H. S. Han, A. Jacobson, E. T. Bilek and J. Sessions, Appl. Eng. Agric., 2018, 34(1), 5–10 Search PubMed.
- Forest Bioeconomy, Bioenergy, and Bioproducts, https://natural-resources.canada.ca/our-natural-resources/forests/industry-and-trade/forest-bioeconomy-bioenergy-bioproducts/bioenergy-biomass/13323, accessed March 2024 Search PubMed.
- H. Wang, X. Bi and R. Clift, Renewable Sustainable Energy Rev., 2021, 150, 111480 Search PubMed.
- Saskatchewan Wood Biomass Overview, https://www.saskatchewan.ca/government/government-structure/ministries/energy-and-resources, accessed March 2024 Search PubMed.
- N. Jokinen, E. Eronen, A. Salami, M. Hyttinen, J. Jänis, J. Vepsäläinen, R. Lappalainen and L. Tomppo, Bioresour. Technol. Rep., 2023, 21, 101385 Search PubMed.
- S. Fridolfsson, PhD thesis, Umeå University, 2022.
- C. U. Jensen, J. K. Rodriguez Guerrero, S. Karatzos, G. Olofsson and S. B. Iversen, Biomass Convers. Biorefin., 2017, 7(4), 495–509 CrossRef CAS.
- J. P Lange, ChemSusChem, 2018, 11(6), 997–1014 Search PubMed.
- P. Batalha, A. Kumar and T. Bhaskar, Komarova, Renewable Sustainable Energy Rev., 2019, 115, 109400 Search PubMed.
- N. Sharma, K. K. Jaiswal, V. Kumar, M. S. Vlaskin, M. Nanda, I. Rautela, M. S. Tomar and W. Ahmad, Renewable Energy, 2021, 174, 810–822 CrossRef CAS.
- S. Nagappan, R. R. Bhosale, D. D. Nguyen, N. T. L. Chi, V. K. Ponnusamy, C. S. Woong and G. Kumar, Fuel, 2021, 285, 119053 Search PubMed.
- A. A. Shah, K. Sharma, M. S. Haider, S. S. Toor, L. A. Rosendahl, T. H. Pedersen and D. Castello, Processes, 2022, 10(2), 207 CrossRef CAS.
- M. K. Jindal and M. K. Jha, Indian Chem. Eng., 2016, 58, 157–171 Search PubMed.
- Z. Zhu, S. S. Toor, L. Rosendahl, D. Yu and G. Chen, Energy, 2015, 80, 284–292 Search PubMed.
- L. Nazari, Z. Yuan, S. Souzanchi, M. B. Ray and C. C. Xu, Fuel, 2015, 162, 74–83 Search PubMed.
- Z. Cui, F. Cheng, J. M. Jarvis, C. E. Brewer and U. Jena, Bioresour. Technol., 2020, 310, 123454 Search PubMed.
- B. Zhao, Y. Hu, L. Qi, J. Gao, G. Zhao, M. B. Ray and C. C. Xu, Fuel, 2021, 285, 119150 Search PubMed.
- J. Yamazaki, E. Minami and S. Saka, J. Wood Sci., 2006, 52(6), 527–532 Search PubMed.
- G. van Rossum, W. Zhao, M. Castellvi Barnes, J. P. Lange and S. R. Kersten, ChemSusChem, 2014, 7(1), 253–259 CrossRef CAS PubMed.
- H. J. Huang and X. Z. Yuan, Prog. Energy Combust. Sci., 2015, 49, 59–80 Search PubMed.
- C. Xu and T. Etcheverry, Fuel, 2008, 87(3), 335–345 Search PubMed.
- S. Cheng, C. Wilks, Z. Yuan, M. Leitch and C. C. Xu, Polym. Degrad. Stab., 2012, 97, 839–848 CrossRef CAS.
- U. Jena, B. E. Eboibi and K. C. Das, Fuels, 2022, 3(2), 326–341 CrossRef CAS.
- S. Cheng, I. D'cruz, M. Wang, M. Leitch and C. Xu, Energy Fuels, 2010, 24(9), 4659–4667 CrossRef CAS.
- H. Wu, U. Shakeel, Q. Zhang, K. Zhang, X. Xu and J. Xu, Energies, 2022, 15(9), 305 Search PubMed.
- S. J. Kim, G. H. Kim and B. H. Um, Energy, 2022, 249, 123509 CrossRef CAS.
- G. Zhang, K. Wang, Q. Liu, L. Han and X. Zhang, Int. J. Environ. Res. Public Health, 2022, 19(17), 10499 CrossRef CAS PubMed.
- Q. Lang, Y. Guo, Q. Zheng, Z. Liu and C. Gai, Bioresour. Technol., 2018, 266, 242–248 CrossRef CAS PubMed.
- A. Sahoo, K. Saini, M. Jindal, T. Bhaskar and K. K. Pant, Bioresour. Technol., 2021, 342, 125948 CrossRef CAS PubMed.
- Q. Li, X. Yuan, X. Hu, E. Meers, H. C. Ong, W. H. Chen and Y. S. Ok, Renewable Sustainable Energy Rev., 2022, 154, 111814 CrossRef CAS.
- C. Gai, Y. Li, N. Peng, A. Fan and Z. Liu, Bioresour. Technol., 2015, 185, 240–245 CrossRef CAS PubMed.
- T. H. Seehar, S. S. Toor, A. A. Shah, T. H. Pedersen and L. A. Rosendahl, Energies, 2020, 13, 3114 CrossRef CAS.
- P. Biller, I. Johannsen, J. S. D. Passos and L. D. M. Ottosen, Water Res., 2018, 130, 58–68 CrossRef CAS PubMed.
- L. Leng, J. Li, X. Yuan, J. Li, P. Han, Y. Hong and W. Zhou, Bioresour. Technol., 2018, 251, 49–56 CrossRef CAS PubMed.
- A. A. Shah, S. S. Toor, T. H. Seehar, K. K. Sadetmahaleh, T. H. Pedersen, A. H. Nielsen and L. A. Rosendahl, Fuel, 2021, 288, 119407 CrossRef.
- K. Sharma, A. A. Shah, S. S. Toor, T. H. Seehar, T. H. Pedersen and L. A. Rosendahl, Energies, 2021, 14(6), 1708 CrossRef CAS.
- F. Güleç, L. M. G. Riesco, O. Williams, E. T. Kostas, A. Samson and E. Lester, Fuel, 2021, 302, 121166 CrossRef.
- Z. Shi, Y. Liu, H. Xu, Q. Yang, C. Xiong, S. Kuga and Y. Matsumoto, Ind. Crops Prod., 2018, 118, 48–52 CrossRef CAS.
- Z. Shi, Q. Yang, S. Kuga and Y. Matsumoto, J. Agric. Food Chem., 2015, 63(27), 6113–6119 CrossRef CAS PubMed.
- K. Khampuang, N. Boreriboon and P. Prasassarakich, Biomass Bioenergy, 2015, 83, 460–466 CrossRef CAS.
- Y. Yu, X. Lou and H. Wu, Energy Fuels, 2008, 22(1), 46–60 CrossRef CAS.
- Z. Abu El-Rub, E. A. Bramer and G. Brem, Ind. Eng. Chem. Res., 2004, 43(22), 6911–6919 CrossRef CAS.
- R. B. Carpio, Y. Zhang, C. T. Kuo, W. T. Chen, L. C. Schideman and R. de Leon, Bioresour. Technol., 2021, 14, 100679 CrossRef CAS.
- R. B. Madsen and M. Glasius, Ind. Eng. Chem. Res., 2019, 58(37), 17583–17600 CrossRef CAS.
- Z. Liu and F. S. Zhang, Energy Convers. Manage., 2008, 49(12), 3498–3504 CrossRef CAS.
- P. Biller, R. B. Madsen, M. Klemmer, J. Becker, B. B. Iversen and M. Glasius, Bioresour. Technol., 2016, 220, 190–199 CrossRef CAS PubMed.
- A. B. Ross, J. M. Jones, M. L. Kubacki and T. Bridgeman, Bioresour. Technol., 2008, 99(14), 6494–6504 CrossRef CAS PubMed.
- S. Yin, N. Zhang, C. Tian, W. Yi, Q. Yuan, P. Fu and Z. Li, Energies, 2021, 14(2), 285 CrossRef CAS.
- C. Tian, B. Li, Z. Liu, Y. Zhang and H. Lu, Renewable Sustainable Energy Rev., 2014, 38, 933–950 CrossRef CAS.
- Z. Zhu, L. Rosendahl, S. S. Toor and G. Chen, Sci. Total Environ., 2018, 630, 560–569 CrossRef CAS PubMed.
- Q. Yan, Y. Lu, F. To, Y. Li and F. Yu, Catal. Sci. Technol., 2015, 5, 3270–3280 RSC.
- Y. Y. Wang, L. L. Ling and H. Jiang, Green Chem., 2016, 18, 4032–4041 RSC.
- J. R. Kastner, S. Mani and A. Juneja, Fuel Process. Technol., 2015, 130, 31–37 CrossRef CAS.
- T. H. Pedersen, N. H. Hansen, O. M. Pérez, D. E. V. Cabezas and L. A. Rosendahl, Biofuels, Bioprod. Biorefin., 2018, 12, 213–223 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se01347f |
| This journal is © The Royal Society of Chemistry 2025 |