Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Holographic hydrogel bandage sensor for continual monitoring of wound healing†
Yihan
Zhang
 a,
Yubing
Hu
a,
Yubing
Hu
 a,
Zhenkang
Zhu
b,
Yunuen
Montelongo
c,
Yanting
Liu
f,
Shihabuddeen
Waqar
a,
Yoon Soo
Park
d,
Leon CZ
Chan
e,
Nan
Jiang
a,
Zhenkang
Zhu
b,
Yunuen
Montelongo
c,
Yanting
Liu
f,
Shihabuddeen
Waqar
a,
Yoon Soo
Park
d,
Leon CZ
Chan
e,
Nan
Jiang
 fg and
Ali K.
Yetisen
fg and
Ali K.
Yetisen
 *a
*a
aDepartment of Chemical Engineering, Imperial College London, South Kensington, London, SW7 2BU, UK. E-mail: a.yetisen@imperial.ac.uk
bDepartment of Chemistry, Imperial College London, South Kensington, London, SW7 2AZ, UK
cDepartment of Engineering Science, University of Oxford, Oxford, OX1 3PJ, UK
dDepartment of Medicine, Imperial College London, South Kensington, London SW7 2AZ, UK
eAgency for Science, Technology and Research (A*STAR), Singapore Institute of Manufacturing Technology (SIMTech), 2 Fusionopolis Way, Innovis #08-04, Singapore 138634, Republic of Singapore
fWest China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu 610041, China
gJinfeng Laboratory, Chongqing 401329, China
First published on 2nd July 2025
Abstract
Chronic wounds pose serious health and economic challenges. A low calcium (Ca2+) ion concentration during the early stage often indicates infections. Holographic hydrogel sensors offer label-free sensing platforms, providing real-time and continuous detections of analytes upon diffractive wavelength changes detectable by the naked eye or spectrophotometers, improving the Ca2+ ion concentration quantification accessibility. Herein, we present a holographic Ca2+ ion bandage sensor using carboxylate-containing hydrogels on polydimethylsiloxane (PDMS) substrates for real-time wound-healing assessment through smartphone readout. Simulations are conducted to investigate the effects of mechanical strength on sensitivity. The holographic Ca2+ ion sensor replays blueshifts of 35 nm (hue value change of 7) with 0–4 mmol L−1 Ca2+ ions, changing colors from dark red to red within 7 minutes. It can accurately and stably (over 24 hours) measure Ca2+ ions when bent. The stiffness of PDMS was tuned to balance comfort and sensitivity. In point-of-care settings, holographic bandage sensors, comprising the holographic hydrogel sensor, a backing layer, and a dark cotton layer, can continuously monitor Ca2+ ions over 10 hours via a smartphone application using hue values. A guiding square in the application assists users in capturing pictures within the inherently narrow viewing angle range of 20–33°. This holographic Ca2+ ion bandage sensor facilitates personalized wound assessment through colorimetric interrogation via smartphone readout.
1. Introduction
Wound healing is a highly complex biological process affected by numerous factors, such as bacterial colonization, gender, health conditions, and the extent of the injury.1–3 These factors can prevent normal tissue regeneration and may fail to heal within three months. Globally, 1–2% of the population will experience a chronic wound in their lifetime and impose significant socioeconomic burdens.4–10 Early intervention and timely treatment can prevent the deterioration of wound conditions and reduce the need for debridement, biological interventions, and amputations. However, conventional wound assessment methods rely on visual inspections and are limited to subjective error and the experience of clinicians. Therefore, a strategy to quantify wound-healing-related biomarkers in the wound milieu is needed. Wound dressings are an ideal biosensor platform due to their mechanical flexibility and ease of operation, which helps reduce patients' pain. The integrated wound dressing sensors should be flexible, breathable, and capable of accurately, timely, and continuously quantifying biomarkers in wound exudates.11Ca2+ ions, regulating intracellular signals, are essential electrolytes for cell metabolism and mineral homeostasis.12,13 Unlike pH level monitoring,14 which can provide information on the inflammation and infection status, Ca2+ ion concentration monitoring can provide more insight into key healing steps, such as fibroblast activities, keratinocyte proliferation, barrier formation, and coagulation at the early stage of the wound-healing process (Fig. 1a).15 At the point of injury, the concentration increases to 1.84–2.72 mmol L−1, persists during the hemostasis, inflammation, and proliferation phases, and returns to the normal concentration of 1.2 mmol L−1 during the remodeling phase.13,16 Decreases in the Ca2+ ion concentration during the early stages often indicate possible infections and hinder healing, as insufficient concentrations of Ca2+ ions may inhibit keratinocyte proliferation, migration toward the wound area, and slow wound healing.13,17 Wearable Ca2+ ion sensing technologies predominantly focus on colorimetric, fluorescence, and electrochemical sensing, but signal drifting and the need for power sources are significant issues for electrochemical sensors, photobleaching and signal interference might affect the accuracy of fluorescence sensors, and the irreversibility of colorimetric sensors also limits broader applications (Table S1†). The need to develop a stable and power-free wearable sensor for accurate, continuous, and real-time wound Ca2+ ions monitoring is unmet.
Holographic hydrogel sensors offer a label-free and low-cost optical sensing approach, providing high sensitivity, direct visualization, and long-term stability. They are widely applied for continuous biomarker monitoring.18–20 Periodic variations of the refractive index (RI) inside holographic sensors are achieved by grating structures of nanocomposites,21–23 nanoparticles,24–26 or secondary polymer layers.18,27 Recently, double-photopolymerization provided a biocompatible nanoparticle-free method to fabricate pH19 and glucose18 sensors by creating alternating nanoscale layers with different RIs within functionalized hydrogels. Integrating these sensors with flexible substrates is preferred for medical devices due to their disposability and flexibility. However, previous studies demonstrated that the flexibility of substrates has a significant impact on the sensitivity of holographic sensors.14,28 This is a tradeoff where the need for conformability must be compromised to maintain the sensitivity, while ensuring it is soft enough to be worn. PDMS has been widely applied in biomedical and biosensing fields as a substrate due to its good mechanical properties, stability, ease of fabrication, oxygen permeability, and optical properties. The tuning of mechanical properties can be done by adjusting the ratio between the elastomer and the curing agent. To overcome this drawback, the mechanical properties of holographic sensor substrates must be tuned to meet both sensitivity and conformability requirements.
Smartphone-based sensing systems have been studied, as smartphones can be used to improve the accessibility of sensors. Smartphones can acquire data in various ways, such as Bluetooth,29,30 near-field communication,31,32 cellular networks,33 and cameras.34,35 The wavelength shift of holographic sensors can give colorimetric responses, which can be detected by complementary metal-oxide semiconductor (CMOS) sensors quantitatively. Additionally, embedded mobile processing units allow biosensing data to be processed and analyzed directly after readout, providing timely diagnosis and alert.36 As the data processing power is improving, image processing has become a means to process optical biosensing signals, such as surface plasmon resonance37 and fluorescence.34,35 In our previous study,14 the colorimetric responses can be captured by smartphone cameras, as the color change gave a significant hue value change in captured photographs. This provided an opportunity for smartphone readout to be applied to holographic sensors, where colorimetric signals can be captured by smartphone cameras and then processed to automatically give biomarker levels.
In our previous study, we demonstrated a conformable holographic pH-sensing bandage sensor using a poly(2-hydroxyethyl methacrylate-co-ethylene glycol dimethacrylate-co-dimethylaminoethyl acrylate) hydrogel on a polyurethane substrate.14 Based on this foundation, we developed a holographic hydrogel bandage sensor that reversibly and continuously quantifies Ca2+ ion concentrations in wound exudates during inflammatory and proliferation phases to provide more clinical insights into the wound-healing process. In this project, the sensor was developed by i) replacing the pH-sensitive functional monomer dimethylaminoethyl acrylate with methacrylic acid (MAA) to enable Ca2+ ion sensing; ii) studying the effect of adding inhibitors to optimize the diffraction efficiency; iii) testing the impact of substrate mechanical strength on the sensitivity with PDMS; iv) incorporating the smartphone readout application. Computational simulations facilitated the development of holographic Ca2+ ion bandage sensors. Integrating holographic Ca2+ ion sensors with PDMS allows bandage sensors to be mounted on wound areas. The selectivity of the bandage was tested across the physiological range of pH and interference concentrations in chronic wounds. A smartphone application was developed to support the readout of holographic sensors via smartphone cameras, enabling effective wound assessment in point-of-care (POC) settings.
2. Results and discussion
2.1. Design of holographic hydrogel Ca2+ ion sensor
The holographic hydrogel Ca2+ ion sensor was fabricated using two monomer solutions as the responsive matrix and interference layers (Fig. 1b). The primary monomer solution (P1) contains 2-hydroxyethyl methacrylate (HEMA), 2–6 mol% polyethylene glycol diacrylate (PEGDA 700), 5–40 mol% MAA, and 1 mol% 2-hydroxy-2-methylpropiophenone (HMPP) (Fig. 1bi). The secondary monomer solution contains 55 mol% HEMA, 40 mol% ethylene glycol dimethacrylate (EGDMA), and 5 mol% HMPP (Fig. 1bii). The responsive matrix was fabricated on a silanized glass slide using the free-radical polymerization of the primary monomer solution to immobilize MAA in polyHEMA hydrogels, generating a responsive matrix sensitive to varying Ca2+ ion levels (Fig. 1c). Given the alkaline nature of chronic wounds, the responsive matrix can deprotonate to form carboxylate-rich hydrogels that could bind with Ca2+ ions, resulting in a volumetric response. Diffraction gratings were imprinted within the responsive matrix via a single-flash Nd:YAG laser (355 nm, 5 ns, 30 μs delay) exposure, which generated periodic differences in RIs as interference layers, thereby creating holographic Ca2+ ion sensors. In basic environments of wounds with pH 7.15–8.50, the responsive matrix swells to the largest extent due to deprotonation.24,38 Two carboxylate groups can bind with one Ca2+ ion, increasing the crosslinking density of the responsive matrix. As the responsive matrix shrinks with increasing Ca2+ ion concentration, the distance between interference layers decreases, leading to a blue shift in the diffracted light.2.2. Computational simulation of flexible holographic sensors readout
Simulations facilitated the design of holographic Ca2+ ion sensors by visualizing laser exposure and interference layers at the nanoscale using MATLAB R2021b. A graphic process unit accelerated the large-scale simulation via parallel computing. In the holographic recording simulation, a planar wave propagated through the simulated hydrogel and was reflected by a planar mirror at the bottom tilted at 5° to match the actual laser optical setup used in experiments (Fig. 2a). This laser interference generated periodic antinode stripes, triggering diffraction gratings formation with varying RIs (Fig. 2b). Fig. 2ci demonstrates the simulation of the holographic sensor readout process using a scanning light source of wavelengths 300–800 nm. The hologram interrogation was tested under 100–150% swelling ratios, resulting in a Bragg peak shift from 406 nm to 588 nm. (Fig. 2cii and iii). Furthermore, simulations allowing for three-dimensional expansion facilitated the design of flexible holographic sensors (Fig. 2di). Under the same swelling ratio increase of 100–150%, the sensitivity of the flexible holographic sensors decreased by approximately three folds, with the Bragg peak shifting from 419 nm to 480 nm (Fig. 2dii and iii). The simulation results theoretically corroborated the observed decrease in the sensitivity of holographic sensors on flexible substrates.14,28 Nevertheless, the three-dimensional volumetric change shifted wavelength linearly, confirming the feasibility of flexible holographic sensors for quantifying analytes.2.3. Fabrication of holographic hydrogel Ca2+ ion sensors
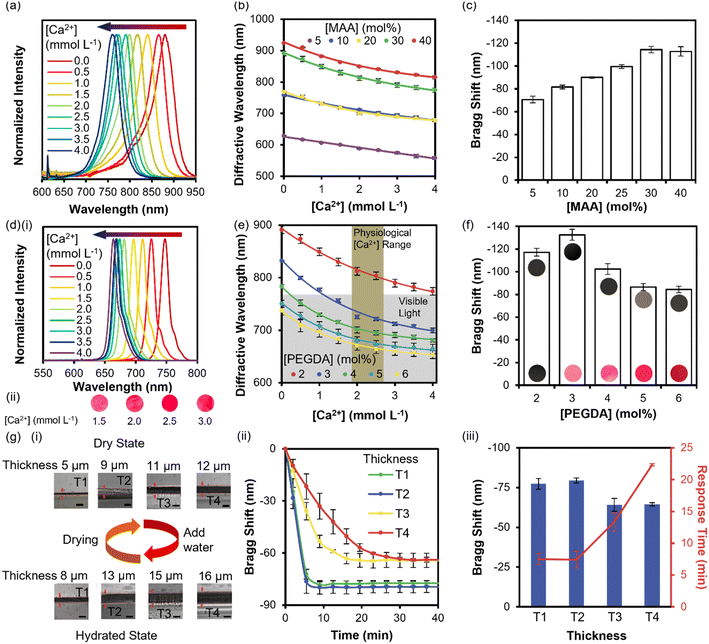
To maximize the sensitivity to Ca2+ ions, holographic sensors were fabricated using 5, 10, 20, 30, and 40 mol% MAA and 2 mol% PEGDA 700. Fig. 3a demonstrates normalized diffractive spectra for holographic sensors with 30 mol% MAA and 2 mol% PEGDA 700 taken by spectrophotometers. The sensitivity increased with the MAA composition in the responsive matrix from 5–30 mol%, and no further increase from a blue shift of 120 nm for 0–4 mmol L−1 of Ca2+ ions was observed with more than 30 mol% of MAA (Fig. 3b and c). The carboxylic acid group deprotonation in the responsive matrix significantly swelled the hydrogel. The holographic sensors replayed a peak at 880 nm with 0 mmol L−1 Ca2+ ions and pH 8. The whole physiological range lies within the infrared spectrum, illustrating no distinguishable colorimetric response to the naked eye. 2–6 mol% PEGDA 700 were investigated to alter the replay wavelength range to the visible light. Fig. 3di illustrates the normalized diffractive spectra of the holographic sensors with 30 mol% MAA and 3 mol% of PEGDA 700. Fig. 3dii demonstrated the color change in response to 1.5–3.0 mmol L−1 Ca2+ ions. The sensitivity reached its maximum at 3 mol% PEGDA 700 and decreased as the composition of PEGDA 700 increased (Fig. 3e and f). The total shift and replay wavelengths remained similar at 85 nm and in the range of 653–750 nm after the PEGDA 700 composition reached 5 mol%.The thickness of the responsive matrix affects the biomarker diffusion through the hydrogel, influencing the response time and sensitivity of holographic hydrogel sensors.18 To characterize the impact of hydrogel thickness, the P1 solution with 64 mol% HEMA, 30 mol% MAA, and 5 mol% PEGDA 700 was prepared, and the thickness of the hydrogel was controlled by different P1 solution volumes (20–100 μL). Fig. 3gi demonstrates micrographs of the obtained hydrogels of different thicknesses in dry and swollen states. The Bragg peak shift was taken every 2.5 minutes when the Ca2+ ion concentration changed from 0 to 4 mmol L−1 (Fig. 3gii). Thicker hydrogels showed decreased sensitivity towards Ca2+ ions and a slower response (Fig. 3giii). No significant difference in response time was observed when the P1 volume was less than 50 μL, implying that the binding of Ca2+ ions to the carboxylate groups in the responsive matrix became the rate-determining step. The minimum sample volume was tested by pipetting 100, 50, and 25 μL of TRIS buffer solution with 0 and 4 mmol L−1 Ca2+ ion concentrations, respectively (Fig. S1a†). The minimum sample volume was determined to be 50 μL, where there are minimal impacts on the readout (Fig. S1b†).
Since the environment and the healing process can affect the wound bed temperature varying between 30.2–39.5 °C,39–43 the temperature impacts on holographic Ca2+ ion sensors were investigated in a temperature-controlled water bath (Fig. 4a). Fig. 4b and c demonstrate a 12-nm increase in replay wavelengths of holographic Ca2+ ion sensors at 30–45 °C, and the holographic sensor only shifted 6 nm at the temperature of 30–40 °C, resulting in negligible differences in the Ca2+ ion quantification accuracy within the physiological wound bed temperature. Viewing angle dependence was measured using a rotational stage to vary the angle between the incident light and the receiver fiber optic. The overlay contour plot shows the viewing angle dependence of the holographic Ca2+ ion sensor in TRIS buffer with 0, 1, 2, and 4 mmol L−1 Ca2+ ions, demonstrating good visibility within the range 20–33° (Fig. 4d). The inherently narrow viewing angle range can be overcome by being recorded over a spherical mirror.14Fig. 4e demonstrates the reversibility test of the holographic Ca2+ ion sensor in the TRIS buffer solution. During 15 cycles of transferring between Ca2+ ion concentrations of 0 and 4 mmol L−1, the sensor showed good stability with less than 10 nm difference in the Bragg shift. Moreover, since carboxylic acid groups deprotonation in the responsive matrix can be affected by pH (7.15–8.50) and ionic strength (133–161 mmol L−1)16 in the wound exudates, selectivity tests using TRIS buffer solution with ionic strengths 50–300 mmol L−1 and pH 6.0–9.0 were conducted. Higher ionic strength decreased the total blueshift from 80 nm at 50 mmol L−1 to only 37 nm at 300 mmol L−1 (Fig. 4f). The holographic Ca2+ ion sensor also showed a decrease in the total shift and replay wavelengths at a pH lower than 7.5 caused by fewer carboxylate groups (Fig. 4g). Other interferences were added to the TRIS buffer with 150 mmol L−1 ionic strength and pH 8. Adding sodium ions, uric acid, urea, lactate, glucose, and albumin only caused a diffractive wavelength difference within 10 nm for both 0 and 4 mmol L−1 Ca2+ ions since they cannot interact with carboxylate groups in the responsive matrix (Fig. 4h). Mg2+ ions are also divalent ions. They exist in a vast amount within the wound milieu (0.75–1.24 mmol L−1) due to the release of Mg2+ ions from broken cells. With the presence of 0.94 mmol L−1 Mg2+ ions, the total blue shift decreased to 21 nm. The binding of Mg2+ ions to carboxylate groups shrank the responsive matrix, and therefore, a decrease in replay wavelengths was observed. Since its lower affinity to carboxylate groups, the shrinkage caused by the binding of Mg2+ ions was smaller than that of Ca2+ ions.44 To further characterize the impact of Mg2+ ion level in the wound milieu on the accuracy of Ca2+ ion quantification, the holographic Ca2+ ion sensor was tested in TRIS buffer solution at pH 8, 150 mmol L−1 ionic strength, 0.5–1.5 mmol L−1 Mg2+ ions, and 0–3 mmol L−1 Ca2+ ions (Fig. 4i). The Mg2+ ions variation has minimal impact on the sensitivity, but the replay wavelength of the holographic Ca2+ ion sensor decreases by approximately 6 nm as the Mg2+ ion concentration increases by 0.5 mmol L−1. However, since Mg2+ ion concentrations in the wound milieu remain steady, the sensor needs to be recalibrated when Mg2+ ions are present. Moreover, the biocompatibility of the materials was tested using cell counting kit-8 (CCK-8) and Live/dead staining assay. The NIH-3T3 was cultured in the responsive matrix extract, the holographic sensor extract, and the Dulbecco's modified Eagle medium (DMEM)-only media, respectively. The biocompatibility of the responsive matrix and holographic sensor was evaluated based on cell growth on days 1, 4, and 7 (Fig. S2).† In the CCK-8 assay, there is no significant difference between the experimental and control groups. The Live/dead staining assay result showed more than 95% cell viability in culture media with the holographic sensor after seven days of culture.
 | ||
| Fig. 4 Characterizations of holographic Ca2+ ion sensor. (a) The photo of the holographic sensor interrogation setup at controlled temperature for angle dependence measurements (scale bar = 5 mm). (b) Normalized spectra of holographic Ca2+ ion sensors at different temperatures in TRIS buffer solution without Ca2+ ions. (c) The diffractive wavelength of holographic Ca2+ ion sensors to varying temperatures in TRIS buffer solution with Ca2+ ion concentrations of 0 and 4 mmol L−1. The gray-shaded region indicates the temperature range of the wound bed. Error bars represent calculated standard error based on three independent readings (n = 3) (d) the angle dependence contour plot of the holographic Ca2+ ion sensor at Ca2+ ion concentrations of 0–4 mmol L−1, respectively. (e) The reversibility test of holographic Ca2+ ion sensor in TRIS buffer solution between Ca2+ ion concentrations of 0 and 4 mmol L−1. (f) Ionic strength effect of TRIS buffer solution in the range of 50–250 mmol L−1 on the total Bragg shift of holographic Ca2+ ion sensors at pH 8. Error bars represent calculated standard error based on three independent readings (n = 3). (g) The pH effect of TRIS buffer solution in the range of 6–9 on the total Bragg shift of holographic Ca2+ ion sensors. Error bars represent calculated standard error based on three independent readings (n = 3). (h) The holographic Ca2+ ion sensor was tested in TRIS buffer solution with common interference compounds in TRIS buffer at physiological concentrations (Table S2†). Error bars represent calculated standard error based on three independent readings (n = 3). (i) The impact of Mg2+ ion level variations of 0.5–1.5 mmol L−1 on the holographic Ca2+ ion sensor in TRIS buffer solution. Error bars represent calculated standard error based on three independent readings (n = 3). | ||
2.4. Characterizations of flexible holographic Ca2+ ion sensor
The holographic hydrogel Ca2+ ion sensors should be fabricated onto flexible, optically inactive, and non-birefringent substrates to act as wearable analytical devices.20 PDMS was chosen as substrates due to their excellent biocompatibility and mechanical and optical properties. PDMS is widely used in biomedical applications and as flexible substrates for wearable biosensors, making it an ideal platform to integrate holographic Ca2+ ion sensors. Fig. 5a demonstrates the fabrication process of the flexible holographic Ca2+ ion sensor. The 160 μm thick PDMS substrates were fabricated by mixing elastomer and the curing agent and cured at 80 °C for an hour. The fabricated PDMS substrates were treated with air plasma and silanized overnight using 3-(trimethoxysilyl) propyl methacrylate in acetone at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v). Meanwhile, a free-standing holographic Ca2+ ion sensor was fabricated on an untreated glass slide and then attached to the treated PDMS surface using an adhesive monomer solution of PEGDA 700 and 3 vol% HMPP in DI water at 2
50 (v/v). Meanwhile, a free-standing holographic Ca2+ ion sensor was fabricated on an untreated glass slide and then attached to the treated PDMS surface using an adhesive monomer solution of PEGDA 700 and 3 vol% HMPP in DI water at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v. The sample was placed under 4 UV(A) strips for 15 minutes and then washed in 50 vol% methanol for 30 minutes to remove unpolymerized monomers. Fig. 5b shows the side view of the flexible holographic Ca2+ ion sensor on PDMS substrates before and after use for 24 hours, indicating a reliable bonding between PDMS substrates and sensors. However, since the stretchability of PDMS allows expansions along the substrate, the sensitivity of flexible holographic sensors is lower than those bonded on rigid glass substrates.14,28 Adjustments in the composition of PDMS were necessary to balance mechanical strength and sensor sensitivity. PDMS substrates with curing-agent-to-base ratios ranging from 1
1 v/v. The sample was placed under 4 UV(A) strips for 15 minutes and then washed in 50 vol% methanol for 30 minutes to remove unpolymerized monomers. Fig. 5b shows the side view of the flexible holographic Ca2+ ion sensor on PDMS substrates before and after use for 24 hours, indicating a reliable bonding between PDMS substrates and sensors. However, since the stretchability of PDMS allows expansions along the substrate, the sensitivity of flexible holographic sensors is lower than those bonded on rigid glass substrates.14,28 Adjustments in the composition of PDMS were necessary to balance mechanical strength and sensor sensitivity. PDMS substrates with curing-agent-to-base ratios ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 1
10 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were tested (Fig. 5c and d). Increasing the curing agent percentage made the PDMS substrates more rigid, restraining the expansion of holographic sensors along the substrate and increasing their total Bragg blue shift from 17 nm to 35 nm. The sensor demonstrated good conformability on curved surfaces with 30–70 mm diameters (Fig. 5e). Bending has minimal impact on flexible holographic sensors at 0–4 mmol L−1 Ca2+ ions (Fig. 5f). The operation time of flexible holographic Ca2+ ion sensors was then tested in buffer solutions with 0, 2, and 4 mmol L−1 Ca2+ ions, respectively. The flexible holographic sensors demonstrated consistent readout over 24 hours, indicating their reliability in quantifying Ca2+ ion levels for wound monitoring at POC settings.
2 were tested (Fig. 5c and d). Increasing the curing agent percentage made the PDMS substrates more rigid, restraining the expansion of holographic sensors along the substrate and increasing their total Bragg blue shift from 17 nm to 35 nm. The sensor demonstrated good conformability on curved surfaces with 30–70 mm diameters (Fig. 5e). Bending has minimal impact on flexible holographic sensors at 0–4 mmol L−1 Ca2+ ions (Fig. 5f). The operation time of flexible holographic Ca2+ ion sensors was then tested in buffer solutions with 0, 2, and 4 mmol L−1 Ca2+ ions, respectively. The flexible holographic sensors demonstrated consistent readout over 24 hours, indicating their reliability in quantifying Ca2+ ion levels for wound monitoring at POC settings.
2.5. Smartphone readout of conformable Ca2+ ion bandage sensors
The popularity of smartphones has stimulated the development of smartphone-based diagnosis devices due to their high accessibility and improved computational power.36 A dark cotton layer constructed the three-layer sensing bandage, the flexible holographic Ca2+ ion sensor, and a breathable cotton-fabric backing layer (Fig. S3†). Dark cotton layers were used to reduce the white scattering from skin surfaces and improve the signal-to-noise ratio. The backing layer can attach the sensor to the skin surface like a plaster (Fig. 6ai). Smartphone cameras can detect the color changes of the bandage sensor (Fig. 6aii). Color information is stored in picture format using HSV, where hue quantifies colors. Fig. 6b demonstrates the smartphone application flowchart for the sensor readout. Pictures can be imported from internal storage or captured via camera. The selected picture was white-balanced to remove the impact of different light sources and ambient lighting conditions with the equation below: | (1) |
![[b with combining macron]](https://www.rsc.org/images/entities/i_char_0062_0304.gif) are the average a, b values of the original image, and a, b, and L are three channels of CIELAB colorspace. Then, it was processed to remove glares and reflections from the surface of the bandage sensor using Gaussian blur and grayscale thresholding. Pictures without glare can be Gaussian blurred and eroded to denoise the taken pictures. The denoised pictures can then be thresholded to extract the region of interest (ROI) containing the hologram, where the average hue value was extracted and converted to Ca2+ ion concentration in wound exudates, providing a quantitative wound assessment.
are the average a, b values of the original image, and a, b, and L are three channels of CIELAB colorspace. Then, it was processed to remove glares and reflections from the surface of the bandage sensor using Gaussian blur and grayscale thresholding. Pictures without glare can be Gaussian blurred and eroded to denoise the taken pictures. The denoised pictures can then be thresholded to extract the region of interest (ROI) containing the hologram, where the average hue value was extracted and converted to Ca2+ ion concentration in wound exudates, providing a quantitative wound assessment.
 | ||
| Fig. 6 Smartphone readout of holographic Ca2+ ion sensors. (a) The schematic of smartphone readout of holographic Ca2+ ion sensors. i, The top view of the holographic Ca2+ ion bandage sensor. ii, Schematic of smartphone capturing pictures of the holographic Ca2+ ion bandage sensor. (b) The flowchart of the smartphone readout process. (c) The user interface of a developed smartphone application for Ca2+ ion concentration quantification, including i, start screen, ii, camera activity for picture taking, iii, loaded picture preview, iv, ROI detection results preview and display of readout result and wound assessment, and v, Display of historical results. (d) The demonstration of smartphone readout of conformable holographic Ca2+ ion sensing bandages. i, The demonstration of ROI detection at different Ca2+ ion concentrations of 0–4 mmol L−1 (scale bar = 1 mm). ii, The average hue value change of detected ROI at Ca2+ ion concentrations of 0–4 mmol L−1, where error bars represent eight independent readings (n = 8). The Ca2+ ion concentration ranges in infected and healing wounds are illustrated in red and gray shades, respectively.16,17 (e) Continuous monitoring of Ca2+ ion concentration in artificial wound exudate (AWE) simulating an infected wound, where error bars represent three independent readings (n = 3). | ||
As shown in Fig. 6ci, on the Start user interface, users can choose to start a new test, view historical results, and read the instructions to facilitate the readout process. To start a new test, the application creates a camera activity allowing users to take pictures of the holographic Ca2+ ion bandage sensor or a media selector to load pictures from the internal storage (Fig. 6cii). A red square is provided during the picture-taking to help users locate the sensor from a narrow viewing angle. After loading the picture, the ROI detection algorithm can automatically detect and display the sensing region on the screen (Fig. 6ciii and iv). Once the results are obtained, quantitative wound assessment results are displayed on the analyzed concentration (Fig. 6cv). Results can then be stored in the internal storage and viewed in the future (Fig. 6cvi). Fig. 6di demonstrates that the algorithm can accurately detect the sensing region at 1–4 mmol L−1 Ca2+ ion concentrations. The smartphone camera readout can be affected by using different camera parameters during the readout. Therefore, camera parameters were fixed during the calibration, (e.g. exposure −2.0 using Samsung Galaxy S22). The obtained calibration curve was embedded in the application to automatically convert the extracted hue into Ca2+ ion concentrations, allowing for a quantitative assessment of wound healing as the hue value is directly related to human color perception and the dominant wavelength of spectra (Fig. 6dii).45 The uniformness of grating nanostructure in holographic Ca2+ ion sensors ensured diffractive peaks with short full-width at half maximum so that the dominant wavelength can directly interpret the peak wavelength shift and, therefore, the hue value.46 The sensor was then tested for continuous monitoring via smartphone readout of AWE, simulating an infected wound over 10 hours (Fig. 6e). At the point of injury, the calcium level decreased due to bacterial colonization in the wound milieu. The Ca2+ ion level remains low in the range of 1–2 mmol L−1 until the wound starts to heal after the treatment, where Ca2+ ion concentration starts to increase above 2 mmol L−1. Fig. S4† compares the readout results of Ca2+ ion concentration from the smartphone readout and the actual Ca2+ concentration, demonstrating high consistency between the actual Ca2+ ion concentration and the readout results. The results demonstrated good stability and accuracy in monitoring Ca2+ ion concentration in AWE over 10 hours, which can provide users with information on the wound healing progression and early diagnosis.
3. Conclusion
In this work, a flexible holographic Ca2+ ion bandage sensor was developed on a PDMS substrate supported by a smartphone readout for quantitative wound assessment. The holographic Ca2+ ion sensor was fabricated using a double photopolymerization method using carboxylate-rich hydrogels, demonstrating high sensitivity towards Ca2+ ions in wound exudates. The sensing system efficiently quantified 0–4 mmol L−1 Ca2+ ions. The optimized conformable holographic Ca2+ ion sensing bandage demonstrated a sensitivity of −8.53 nm mmol−1 L in the replay wavelength and a total hue change of 6 in the 0–4 mmol L−1 Ca2+ ions. The simulation of holographic sensors on rigid and flexible substrates facilitated the fabrication of PDMS substrates to maintain good conformability while constraining the hydrogel from expanding in different directions. Investigating the effect of the mechanical strength of PDMS substrates on sensitivity has provided more insights into the fabrication method of conformable holographic sensors. The conformability of the bandage allows for mounting on body surfaces, providing patients with accurate quantification of Ca2+ ions in wound exudates. Integrating smartphone readout further improved accessibility, allowing quantitative wound assessment in POC settings.However, the integration of flexible substrates decreased the sensitivity of the holographic sensor, resulting in a limited hue value change of 6 when Ca2+ ion concentration changes from 0–4 mmol L−1. In addition, the temperature dependence of the sensor might affect the accuracy of readout when the bandage is mounted on the wound surface, since the environment and the healing process can affect the wound bed temperature, varying between 30.2–39.5 °C.39–43 Moreover, the inherently restricted viewing angle might affect the readout accuracy when taking pictures from different viewing angles. The background color also affects the readout accuracy, as the reflection light from the surface behind the holographic grating can increase the signal-to-noise ratio (SNR).14 Current mitigations include a dark backing layer and an on-screen alignment guide, with future enhancements such as fixed-angle mounting frames and advanced image-processing algorithms. In this work, we mainly focused on Ca2+ ion sensing, while multiplexed sensing (pH and proteases) can be used to get a deeper insight into the wound healing process. Ambient lighting variability also poses a challenge for optical biosensing; our smartphone app already uses white-balance correction during image processing. However, thanks to the advances of machine learning and artificial intelligence algorithms, the impact of ambient lighting conditions can be reduced by using a convolutional neural network.47 Finally, this study focuses solely on Ca2+ ion monitoring. Future efforts will develop multiplexed holographic arrays capable of simultaneous pH, protease, and other biomarker detection to provide a more comprehensive wound-bed profile.
Moreover, the accuracy of wound healing monitoring can be further enhanced by multiplexed monitoring, such as pH, interleukins, and matrix metalloproteinases. By miniaturizing multiple holographic sensors14 using methods like text patterning,18 multiplexed holographic bandage sensors can be fabricated to monitor multiple biomarkers simultaneously. Advances in mobile processing units allow efficient processing of high-dimensional biosensing data, provide patients with timely alerts when abnormalities are found, and integrate with artificial intelligence to facilitate personal diagnosis and wound assessment, improving diagnosis accuracy.36 Moreover, hydrogels have been used for medication delivery to the wound bed during detection.48,49 The swelling and shrinkage of the hydrogel matrix can control the release of drugs, such as bioactive extracts,50 anti-inflammatories,51 and growth factors,52 offering an opportunity to apply controlled drug delivery with the holographic Ca2+ sensor during continual monitoring. The integration of drug delivery to the wound bed during detection enabled on-demand therapy and therefore enhanced wound healing.
4. Experimental section
Materials
All chemicals were analytical grade. PDMS was purchased from Dow and Corning. 3-(trimethoxy silyl)-propyl methacrylate, acetone, hydroxy ethyl methyl methacrylate (HEMA), ethylene glycol dimethacrylate (EGDMA), poly(ethylene glycol) diacrylate (Mn 700; PEGDA 700), methacrylic acid (MAA), 2-hydroxy-2-methylpropiophenone (HMPP), isopropanol, tris(hydroxymethyl)amino methane (TRIS), TRIS hydrochloride (TRIS HCl), potassium chloride, calcium chloride, magnesium chloride, sodium bicarbonate, urea, uric acid, sodium lactate, and hydrochloric acid (1 mol L−1) were purchased from Sigma Aldrich. Methanol, glucose, and sodium chloride were purchased from VWR. Aluminized polyester films were purchased from HiFi Industrial Film Ltd. Cotton layers were purchased from Synergy Health (UK) Ltd. For biocompatibility tests, HEMA and PEGDA 700 were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (China), HMPP, MAA, methanol, hydroquinone, and isopropanol were obtained from Shanghai Titan Technology Co., Ltd. (China), and EGDMA was purchased from Shanghai Sahn Chemical Technology Co., Ltd. (China). CCK-8, Calcein AM, and propidium iodide (PI) were obtained from APExBIO Technology Co., Ltd. (China). NIH-3T3 fibroblast cells were obtained from the National Collection of Authenticated Cell Cultures (China).Equipment
Microscope slides were purchased from Fisher Scientific, cover glass slides from VWR and a UVP crosslinker from Analytik Jena. Nd: YAG frequency tripled Quantel Q-smart (5 ns, 355 nm) solid-state laser purchased from Lumibird, France. The drying oven UN30 used for hydrogel drying was purchased from Memmert. The plano-concave lens (−75.0 mm, Ø1′′ UV fused silica plano-concave lens, uncoated), optical posts (Ø12.7 mm, L = 20 mm), pedestal post holder (L = 20 mm, Ø12.7 mm), power and energy meter interface (PM100USB), UV extended Si photodiode, motorized precision rotation stage (Ø1′′), collimated laser-diode-dumped DPSS laser module (532 nm, 4.5 mW), laser diode module mounting kit (Ø11 mm), and iris (mounted standard iris, Ø12 mm max aperture, TR3 Post) were purchased from Thorlabs, United States. A 25 mm dielectric 355 nm Nd:YAG laser line mirror was purchased from Edmund Optics, UK. Fisherbrand classic vortex mixer was purchased from Fisher Scientific, Bishop Meadow, UK. The Mettler Toledo FiveEasy Plus™ pH benchtop meter was purchased from Mettler-Toledo Ltd. Orion Star A212 Benchtop Conductivity Meter from ThermoFisher. Holographic responses were analyzed using UV-vis bifurcated fiber optical cables, a 20 W tungsten halogen broadband light source, Flame-S-VIS-NIR-ES spectrophotometer, and Oceanview software (2.0.8) purchased from Ocean Insight. Liquid PDMS was degassed using a vacuum desiccator purchased from Thermo Scientific. PDMS substrate surface treatment was performed using Zepto low-pressure plasma cleaner purchased from Diener Electronic GmbH & Co. KG.Holographic hydrogel Ca2+ ion sensor fabrication
Unless mentioned otherwise, all procedures were carried out in light-tight conditions. Glass slides were silanized using a 3-(trimethoxysilyl) propyl methacrylate in acetone at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) overnight. The optimized formulation and thickness of the holographic calcium ion sensor were used for fabrication. On the polyester side of a flat aluminized polyester film, 50 μL of the primary monomer solution was pipetted, and a treated glass slide was placed onto the droplet. It was then exposed under 4 UV (A) strip lights for 30 minutes. The polymerized primary hydrogel layer was then removed from the polyester film and washed in methanol and water at a ratio of 1
50 (v/v) overnight. The optimized formulation and thickness of the holographic calcium ion sensor were used for fabrication. On the polyester side of a flat aluminized polyester film, 50 μL of the primary monomer solution was pipetted, and a treated glass slide was placed onto the droplet. It was then exposed under 4 UV (A) strip lights for 30 minutes. The polymerized primary hydrogel layer was then removed from the polyester film and washed in methanol and water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) for 30 minutes to remove any by-products and unpolymerized monomers from the hydrogel layer. The hydrogel was then wiped dry, and 150 μL of the secondary monomer solution was pipetted onto a clean, flat, aluminized polyester film. The hydrogel side was facing downward, and the slide was then placed on the droplet to allow the hydrogel to be soaked in the secondary monomer solution for 10 minutes. The hydrogel surface was then given a single wipe to remove excessive secondary monomer solution. The hydrogel was then dried in an oven at 55 °C for 4.5 minutes. The hydrogel was then left to cool down to 25 °C. Since the thickness of hydrogel at laser exposure is a critical factor affecting replay wavelengths of holographic sensors, these steps must be maintained at the same temperature and humidity. Slides were kept under safe lighting and exposed to a single UV pulse from an Nd: YAG laser (355 nm, 5 ns, 30 μs delay) on the planar mirror with the hydrogel side facing downwards at a tilted angle of 5° from the mirror. While maintaining safe lighting, hydrogels were washed in methanol and DI water (1
1 (v/v) for 30 minutes to remove any by-products and unpolymerized monomers from the hydrogel layer. The hydrogel was then wiped dry, and 150 μL of the secondary monomer solution was pipetted onto a clean, flat, aluminized polyester film. The hydrogel side was facing downward, and the slide was then placed on the droplet to allow the hydrogel to be soaked in the secondary monomer solution for 10 minutes. The hydrogel surface was then given a single wipe to remove excessive secondary monomer solution. The hydrogel was then dried in an oven at 55 °C for 4.5 minutes. The hydrogel was then left to cool down to 25 °C. Since the thickness of hydrogel at laser exposure is a critical factor affecting replay wavelengths of holographic sensors, these steps must be maintained at the same temperature and humidity. Slides were kept under safe lighting and exposed to a single UV pulse from an Nd: YAG laser (355 nm, 5 ns, 30 μs delay) on the planar mirror with the hydrogel side facing downwards at a tilted angle of 5° from the mirror. While maintaining safe lighting, hydrogels were washed in methanol and DI water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) overnight.
1 v/v) overnight.
Minimal volume for detection
Place the holographic sensor in a petri dish with the hydrogel side facing upward. 20, 10, 5 μL of sample solution was pipetted onto the sensor area, and then a cover slip was placed on top of the droplet. Between each measurement, the sensor was washed in DI water for 3 minutes and then dried with an air flow.Biocompatibility test
The culture medium consists of 10% FBS, 1% penicillin–streptomycin solution, and 89% DMEM-HG. The responsive matrix hydrogels and holographic sensors were soaked in culture medium, respectively, for 24 hours to obtain the extract with a concentration of 0.1 g mL−1. 300 NIH-3T3 fibroblasts and 100 μL culture medium were added to each well of the 96-hole plate. After 24 hours, the culture medium was replaced by the responsive matrix extract and the holographic sensor extract, respectively. Live/dead staining and CCK-8 test were used to test cell viability and function at 1, 4, and 7 days.Live/dead staining
Wash the NIH-3T3 fibroblasts 3 times with PBS buffer to remove dead or damaged cells. Calcein AM and PI were added 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to the buffer. After 15 minutes of incubation, the samples were acquired and observed on a fluorescence microscope. Under the microscope, the final signal is observed using blue and green light. By comparing the fluorescence intensities of green fluorescence emitted by calcein AM and red fluorescence emitted by PI, the quantitative relationship between the total number of cells, the number of viable cells and the number of dead cells can be obtained.
1 to the buffer. After 15 minutes of incubation, the samples were acquired and observed on a fluorescence microscope. Under the microscope, the final signal is observed using blue and green light. By comparing the fluorescence intensities of green fluorescence emitted by calcein AM and red fluorescence emitted by PI, the quantitative relationship between the total number of cells, the number of viable cells and the number of dead cells can be obtained.
CCK-8
The CCK-8 was mixed with the culture medium at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, and 100μL of the mixed solution was added to the 96-well cell culture plate and continued to incubate in the 37 °C incubator for 0.5–1 hours. 450 nm single wavelength detection was performed by an enzyme-labeled instrument.
1000, and 100μL of the mixed solution was added to the 96-well cell culture plate and continued to incubate in the 37 °C incubator for 0.5–1 hours. 450 nm single wavelength detection was performed by an enzyme-labeled instrument.
Free-standing holographic calcium ion sensor fabrication
On the polyester side of a flat aluminized polyester film, 50 μL of the primary monomer solution was pipetted, and a clean, untreated glass slide was placed onto the droplet. It was then exposed under 4 UV (A) strip lights for 30 minutes. The polymerized primary hydrogel layer was then removed from the polyester film and washed in methanol and water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) for 30 minutes to remove any by-products and unpolymerized monomers from the hydrogel layer. The hydrogel was then wiped dry, and 150 μL of the secondary monomer solution was pipetted onto the hydrogel surface to allow the hydrogel to be soaked in the secondary monomer solution for 10 minutes. The hydrogel surface was then given a single wipe to remove excessive secondary monomer solution. The hydrogel was then dried in an oven at 55 °C for 4.5 minutes. The hydrogel was then left to cool down to 25 °C. Since the thickness of hydrogel at laser exposure is a critical factor affecting replay wavelengths of holographic sensors, these steps must be maintained at the same temperature and humidity. Slides were kept under safe lighting and exposed to a single UV pulse from an Nd:YAG laser (355 nm, 5 ns, 30 μs delay) on the planar mirror with the hydrogel side facing downwards with a tilted angle of 5° from the mirror. While maintaining safe lighting, hydrogels were washed in methanol and DI water (1
1 (v/v) for 30 minutes to remove any by-products and unpolymerized monomers from the hydrogel layer. The hydrogel was then wiped dry, and 150 μL of the secondary monomer solution was pipetted onto the hydrogel surface to allow the hydrogel to be soaked in the secondary monomer solution for 10 minutes. The hydrogel surface was then given a single wipe to remove excessive secondary monomer solution. The hydrogel was then dried in an oven at 55 °C for 4.5 minutes. The hydrogel was then left to cool down to 25 °C. Since the thickness of hydrogel at laser exposure is a critical factor affecting replay wavelengths of holographic sensors, these steps must be maintained at the same temperature and humidity. Slides were kept under safe lighting and exposed to a single UV pulse from an Nd:YAG laser (355 nm, 5 ns, 30 μs delay) on the planar mirror with the hydrogel side facing downwards with a tilted angle of 5° from the mirror. While maintaining safe lighting, hydrogels were washed in methanol and DI water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) overnight.
1 v/v) overnight.
PDMS substrates fabrication
The siloxane was mixed with the curing agent at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was degassed using a vacuum desiccator, poured into a mold, and covered using clean microscope glass slides to fabricate a PDMS sheet with a thickness of 80 μm. The mold was then placed in an oven at 80 °C for 1 hour. The fabricated PDMS sheet was then removed from the mold, treated using the plasma cleaner, and silanized using a 3-(trimethoxysilyl) propyl methacrylate in acetone at 1
1. The mixture was degassed using a vacuum desiccator, poured into a mold, and covered using clean microscope glass slides to fabricate a PDMS sheet with a thickness of 80 μm. The mold was then placed in an oven at 80 °C for 1 hour. The fabricated PDMS sheet was then removed from the mold, treated using the plasma cleaner, and silanized using a 3-(trimethoxysilyl) propyl methacrylate in acetone at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) overnight.
50 (v/v) overnight.
Attach free-standing holographic sensor on PDMS substrates
Both free-standing holographic calcium sensors and PDMS substrates were rinsed with methanol to clean surfaces. An adhesive monomer solution was prepared by mixing PEGDA 700 and 3 vol% HMPP in DI water at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v. The diluted PEGDA 700 solution was pipetted on the free-standing holographic calcium sensor surface. The PDMS substrate was then placed on the PEGDA 700 solution droplet. A pressure of 1 kPa was applied to facilitate the attachment. The sample was placed under 4 UV (A) strip lights for 15 minutes. The PDMS substrate holographic calcium sensor was carefully removed from glass slides and washed with methanol and DI water (1
1 v/v. The diluted PEGDA 700 solution was pipetted on the free-standing holographic calcium sensor surface. The PDMS substrate was then placed on the PEGDA 700 solution droplet. A pressure of 1 kPa was applied to facilitate the attachment. The sample was placed under 4 UV (A) strip lights for 15 minutes. The PDMS substrate holographic calcium sensor was carefully removed from glass slides and washed with methanol and DI water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for 30 minutes.
1 v/v) for 30 minutes.
Selectivity testing
The holographic sensor was tested in TRIS buffer solution containing sodium chloride (128.83 mmol L−1), magnesium chloride (0.94 mmol L−1), calcium chloride (0.00–4.00 mmol L−1), sodium bicarbonate (19.00 mmol L−1), glucose (1.80 mmol L−1), albumin (22 g L−1), potassium chloride (4.40 mmol L−1), urea (8.90 mmol L−1), uric acid (0.35 mmol L−1), and sodium lactate (10.90 mmol L−1), respectively. TRIS and TRIS HCl were added by maintaining TRIS concentration at 50 mmol L−1 at a constant pH of 8.00 to stabilize the pH. 3 mmol L−1 citric acid was added to stabilize the calcium concentration in AWE. The pH value was corrected using 1 mol L−1 hydrochloric acid and confirmed using the pH meter.Portable spectrophotometer development
The holographic sensors underwent a color change when the calcium ion level changed in the wound milieu. Smartphone-taken pictures can detect these color changes. To improve the accuracy of ROI detection, white balance, glare removal, and denoising (including Gaussian blur and image erosion) were deployed. The ROI detection method was based on the boundary detection algorithm. The ROI was then converted to HSV color space; therefore, hue can be analyzed and corresponded to specific calcium ion concentrations. The calibration curve was embedded in the APP.Statistical analysis
Spectra taken by the spectrophotometer were firstly processed by the Savitzky–Golay filter and then subtracted by broadband light spectra taken before the measurement. The processed spectra were then normalized to [0,1]. All replay wavelength and Bragg peak shift data were expressed as mean ± standard error. All the above mentioned data processing was carried out using Origin 2020. The overlay contour plots for angle dependence measurements were processed by subtracting previously taken broadband light spectra and then normalized to [0,1]. The data was processed using Python 3.9.7 with Matplotlib and NumPy libraries.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
A. K. Y. and Y. H. acknowledge the Engineering and Physical Sciences Research Council (EPSRC) (EP/T013567/1). N. J. acknowledges the National Natural Science Foundation of China (82102182), the Fundamental Research Funds for the Central Universities (YJ202152), and JinFeng Laboratory, Chongqing, China (jfkyjf202203001). Y. M. thanks the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement (896410).References
- S. Guo and L. A. Dipietro, J. Dent. Res., 2010, 89, 219–229 CrossRef CAS PubMed.
- N. X. Landen, D. Li and M. Stahle, Cell. Mol. Life Sci., 2016, 73, 3861–3885 CrossRef CAS PubMed.
- A. C. Panayi, C. Reitblat and D. P. Orgill, in Total Scar Management, Springer Singapore, 2020, ch. 1, pp. 3–16, DOI:10.1007/978-981-32-9791-3_1.
- X. Xiao, X. Xiao, A. Nashalian, A. Libanori, Y. Fang, X. Li and J. Chen, Adv. Healthcare Mater., 2021, 10, e2100975 CrossRef PubMed.
- K. Jarbrink, G. Ni, H. Sonnergren, A. Schmidtchen, C. Pang, R. Bajpai and J. Car, Syst. Rev., 2016, 5, 152 CrossRef PubMed.
- K. Jarbrink, G. Ni, H. Sonnergren, A. Schmidtchen, C. Pang, R. Bajpai and J. Car, Syst. Rev., 2017, 6, 15 CrossRef PubMed.
- S. R. Nussbaum, M. J. Carter, C. E. Fife, J. DaVanzo, R. Haught, M. Nusgart and D. Cartwright, Value Health, 2018, 21, 27–32 CrossRef PubMed.
- V. Falanga, R. R. Isseroff, A. M. Soulika, M. Romanelli, D. Margolis, S. Kapp, M. Granick and K. Harding, Nat. Rev. Dis. Primers, 2022, 8, 50 CrossRef PubMed.
- C. K. Sen, Adv. Wound Care, 2023, 12, 657–670 CrossRef PubMed.
- C. K. Sen, G. M. Gordillo, S. Roy, R. Kirsner, L. Lambert, T. K. Hunt, F. Gottrup, G. C. Gurtner and M. T. Longaker, Wound Repair Regen., 2009, 17, 763–771 CrossRef PubMed.
- A. Pal, D. Goswami, H. E. Cuellar, B. Castro, S. Kuang and R. V. Martinez, Biosens. Bioelectron., 2018, 117, 696–705 CrossRef CAS PubMed.
- H. Y. Nyein, W. Gao, Z. Shahpar, S. Emaminejad, S. Challa, K. Chen, H. M. Fahad, L. C. Tai, H. Ota, R. W. Davis and A. Javey, ACS Nano, 2016, 10, 7216–7224 CrossRef CAS PubMed.
- T. Subramaniam, M. B. Fauzi, Y. Lokanathan and J. X. Law, Int. J. Mol. Sci., 2021, 22, 6486 CrossRef CAS PubMed.
- Y. Zhang, Y. Hu, Y. Montelongo, M. Hsu, J. Blyth, N. Jiang and A. K. Yetisen, Adv. Funct. Mater., 2024, 34, 2308490 CrossRef CAS.
- P. Salvo, V. Dini, F. Di Francesco and M. Romanelli, Wound Medicine, 2015, 8, 15–18 CrossRef.
- N. J. Trengove, S. R. Langton and M. C. Stacey, Wound Repair Regen., 1996, 4, 234–239 CrossRef CAS PubMed.
- Z. Liu, J. Liu, T. Sun, D. Zeng, C. Yang, H. Wang, C. Yang, J. Guo, Q. Wu, H. J. Chen and X. Xie, ACS Sens., 2021, 6, 3112–3124 CrossRef CAS PubMed.
- S. Davies, Y. Hu, J. Blyth, N. Jiang and A. K. Yetisen, Adv. Funct. Mater., 2023, 33, 2214197 CrossRef CAS.
- S. Davies, Y. Hu, N. Jiang, Y. Montelongo, A. Richardson, J. Blyth and A. K. Yetisen, Biosens. Bioelectron., 2022, 207, 114206 CrossRef CAS PubMed.
- A. K. Yetisen, I. Naydenova, F. da Cruz Vasconcellos, J. Blyth and C. R. Lowe, Chem. Rev., 2014, 114, 10654–10696 CrossRef CAS PubMed.
- E. Leite, I. Naydenova, S. Mintova, L. Leclercq and V. Toal, Appl. Opt., 2010, 49, 3652–3660 CrossRef CAS PubMed.
- O. Sakhno, P. Yezhov, V. Hryn, V. Rudenko and T. Smirnova, Polymers, 2020, 12, 480 CrossRef CAS PubMed.
- M. Irfan, S. Martin, M. A. Obeidi, S. Miller, F. Kuster, D. Brabazon and I. Naydenova, Polymers, 1858, 2022, 14 Search PubMed.
- A. J. Marshall, J. Blyth, C. A. Davidson and C. R. Lowe, Anal. Chem., 2003, 75, 4423–4431 CrossRef CAS PubMed.
- A. G. Mayes, J. Blyth, R. B. Millington and C. R. Lowe, Anal. Chem., 2002, 74, 3649–3657 CrossRef CAS PubMed.
- S. Kabilan, A. J. Marshall, F. K. Sartain, M. C. Lee, A. Hussain, X. Yang, J. Blyth, N. Karangu, K. James, J. Zeng, D. Smith, A. Domschke and C. R. Lowe, Biosens. Bioelectron., 2005, 20, 1602–1610 CrossRef CAS PubMed.
- N. Jiang, S. Davies, Y. Jiao, J. Blyth, H. Butt, Y. Montelongo and A. K. Yetisen, ACS Sens., 2021, 6, 915–924 CrossRef CAS PubMed.
- A. K. Yetisen, H. Butt and S.-H. Yun, ACS Sens., 2016, 1, 493–497 CrossRef CAS.
- J. Kim, S. Imani, W. R. de Araujo, J. Warchall, G. Valdes-Ramirez, T. R. Paixao, P. P. Mercier and J. Wang, Biosens. Bioelectron., 2015, 74, 1061–1068 CrossRef CAS PubMed.
- T. Arakawa, K. Tomoto, H. Nitta, K. Toma, S. Takeuchi, T. Sekita, S. Minakuchi and K. Mitsubayashi, Anal. Chem., 2020, 92, 12201–12207 CrossRef CAS PubMed.
- G. Xu, C. Cheng, Z. Y. Liu, W. Yuan, X. Z. Wu, Y. L. Lu, S. S. Low, J. L. Liu, L. H. Zhu, D. Z. Ji, S. Li, Z. T. Chen, L. S. Wang, Q. G. Yang, Z. Cui and Q. J. Liu, Adv. Mater. Technol., 2019, 4, 1800658 CrossRef CAS.
- S. G. Kang, M. S. Song, J. W. Kim, J. W. Lee and J. Kim, Sensors, 2021, 21, 703 CrossRef CAS PubMed.
- J. Guo, S. Chen, S. Tian, K. Liu, J. Ni, M. Zhao, Y. Kang, X. Ma and J. Guo, Biosens. Bioelectron., 2021, 181, 113160 CrossRef CAS PubMed.
- Y. Shi, Y. Zhang, Y. Hu, R. Moreddu, Z. Fan, N. Jiang and A. K. Yetisen, Sens. Actuators, B, 2023, 378, 133128 CrossRef CAS.
- Y. Wu, Y. H. Zhang, Y. B. Hu, N. Jiang, R. P. Patel, A. K. Yetisen and M. F. Cordeiro, Adv. Mater. Technol., 2024, 9, 2400238 CrossRef CAS.
- Y. Zhang, Y. Hu, N. Jiang and A. K. Yetisen, Biosens. Bioelectron., 2023, 219, 114825 CrossRef CAS PubMed.
- Y. Liu, Q. Liu, S. Chen, F. Cheng, H. Wang and W. Peng, Sci. Rep., 2015, 5, 12864 CrossRef CAS PubMed.
- B. Greener, A. A. Hughes, N. P. Bannister and J. Douglass, J. wound care, 2005, 14, 59–61 CrossRef CAS PubMed.
- M. Fierheller and R. G. Sibbald, Adv. Skin Wound Care, 2010, 23, 369-379; quiz 380-361 Search PubMed.
- G. Gethin, J. D. Ivory, D. Sezgin, H. Muller, G. O'Connor and A. Vellinga, Wound Repair Regen., 2021, 29, 843–847 CrossRef PubMed.
- D. Lou, Q. Pang, X. Pei, S. Dong, S. Li, W. Q. Tan and L. Ma, Biosens. Bioelectron., 2020, 162, 112275 CrossRef CAS PubMed.
- G. Power, Z. Moore and T. O'Connor, J. wound care, 2017, 26, 381–397 CrossRef CAS PubMed.
- Y. Zhang, B. Lin, R. Huang, Z. Lin, Y. Li, J. Li and X. Li, Microb. Biotechnol., 2021, 14, 1566–1579 CrossRef CAS PubMed.
- D. Wang, A. F. Wallace, J. J. De Yoreo and P. M. Dove, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21511–21516 CrossRef CAS PubMed.
- R. G. Kuehni, Color Res. Appl., 2011, 37, 424–428 CrossRef.
- Q. Yao, J. Ju, R. Liang, D. Chen and H. Zhao, Leukos, 2013, 10, 11–18 CrossRef.
- Z. Wang, Y. Dong, X. Sui, X. Shao, K. Li, H. Zhang, Z. Xu and D. Zhang, npj Flexible Electron., 2024, 8, 35 CrossRef CAS.
- N. H. Thang, T. B. Chien and D. X. Cuong, Gels, 2023, 9, 523 CrossRef CAS PubMed.
- R. Marroquin-Garcia, J. Royakkers, M. Gagliardi, R. Arreguin-Campos, T. J. Cleij, K. Eersels, N. M. S. van den Akker, D. G. M. Molin, B. van Grinsven and H. Diliën, ACS Appl. Polym. Mater., 2022, 4, 2871–2879 CrossRef CAS.
- A. Kowalczyk, A. Kraskiewicz, M. Weisbrodt and K. Kowalczyk, Polimery, 2023, 68, 461–472 CrossRef CAS.
- H. T. T. Nguyen, N. H. N. Do, H. D. Lac, P. L. N. Nguyen and P. K. Le, J. Porous Mater., 2022, 30, 655–670 CrossRef.
- B. H. Shan and F. G. Wu, Adv. Mater., 2024, 36, e2210707 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sd00047e |
| This journal is © The Royal Society of Chemistry 2025 |