 Open Access Article
Open Access ArticleA non-invasive device for glucose monitoring through saliva – a paradigm shift in diabetes care†
Shweta
Panwar
 a,
D. Syed
Kasim
a,
Harpreet
Singh
a,
Akanksha
Priya
b,
K. K.
Deepak
ac,
Shyam
Prakash
b and
Sandeep Kumar
Jha
a,
D. Syed
Kasim
a,
Harpreet
Singh
a,
Akanksha
Priya
b,
K. K.
Deepak
ac,
Shyam
Prakash
b and
Sandeep Kumar
Jha
 *ac
*ac
aCentre for Biomedical Engineering, Indian Institute of Technology Delhi, New Delhi, 110016, India. E-mail: sandeepjha@iitd.ac.in; Tel: +91 11 2659 1119
bDepartment of Laboratory Medicine, All India Institute of Medical Sciences, New Delhi, 110029, India
cDepartment of Biomedical Engineering, All India Institute of Medical Sciences, New Delhi, 110029, India
First published on 30th May 2025
Abstract
Diabetes mellitus requires consistent monitoring to prevent complications, yet conventional self-monitoring of blood glucose (SMBG) through finger-pricking can be cumbersome and invasive. This study explores the potential of a saliva-based glucose monitoring biosensor as a non-invasive alternative. The developed biosensor utilizes immobilized glucose oxidase (GOD), peroxidase (POD) and a chromogenic dye (4-amino antipyrine and phenol) to produce a detectable colour change in response to salivary glucose, which is measured using an RGB sensor. The biosensor demonstrated a detection range of 14.5–213 mg dL−1, with a sensitivity of 10.6 count per mg dL−1 and a response time of under 3 minutes. The biosensor was clinically validated against a commercial glucometer to compare salivary glucose level (SGL) to blood glucose level (BGL), and a strong correlation coefficient between the two methods was established for diabetic patients as 0.97 and 0.9 under fasting and postprandial conditions, respectively. For non-diabetic subjects the values were 0.58 and 0.87. Gender-wise, significant correlations were observed under postprandial conditions for diabetic and non-diabetic males (R2 = 0.98, 0.81) and females (R2 = 0.89, 0.85). In fasting conditions, diabetic males (R2 = 0.98) and females (R2 = 0.90), as well as non-diabetic males (R2 = 0.74) and females (R2 = 0.66), exhibited strong correlations. The biosensor, designed with an improved optical detection system, offers a practical, non-invasive approach for diabetes monitoring, making it particularly suitable for populations seeking alternatives to SMBG.
1. Introduction
The traditional self-monitoring of blood glucose (SMBG) for diabetes management relies on finger-prick tests, which can be painful, costly, and impractical for frequent monitoring. This often leads to poor adherence to routine glycaemic monitoring among patients, particularly those who require multiple daily measurements. As a result, there is a need for non-invasive alternatives that provide accurate glucose measurements without the discomfort of conventional needle prick-based methods.1–5 Saliva has emerged as a promising medium for glucose monitoring, reflecting changes in blood glucose levels and offering a pain-free, easy-to-collect alternative.6–8 Previous studies, including our own,9–11 have demonstrated the potential of salivary glucose sensors, but challenges remain in terms of limits of detection, accuracy, interference from environmental factors, and device usability.12In saliva-based glucose sensors, both enzymatic and non-enzymatic approaches have been explored by various groups in past, with organic electrochemical transistors (OECTs) showing promise. Caizhi Liao and colleagues 2014,13 designed a PEDOT:PSS-based OECT with platinum (Pt) electrodes on polyethylene terephthalate (PET) substrates for the detection of H2O2 produced during glucose oxidation. The selectivity was enhanced by coating the electrodes with graphene, Nafion, and polyaniline.14 Such device achieved a detection limit of 30 nM and could also detect uric acid and cholesterol. Elkington and colleagues further reduced the device's response time to 500 s using inkjet printing and design optimizations.15 Moreover, Ko A. et al., explained that hydrogels have recently gained attention as biocompatible, moisture-retentive wound dressings that enhance diabetic foot ulcer healing by supporting key cellular processes such as proliferation, migration, and angiogenesis.16
Upon further advancements in such technology, a low-cost, disposable test strip made from filter paper and integrated with a smartphone was developed for glucose detection,17–19 using pH-indicating dye and glucose oxidase. The colour change was analysed through the RGB profile, revealing an exponential relationship with a linear range of 50–540 mg dL−1 and a detection limit of 24.6 mg dL−1. The correlation between blood and salivary glucose levels was found to be 0.44 for non-diabetics, 0.64 for pre-diabetics, and 0.94 for diabetics. An Android app was created to analyse the detection zone by calculating pixel slope changes. However, factors such as changing camera specifications with iterative smartphone versions, the need to continuously upgrade the software as per Android system upgrades, and requiring re-calibration and presence of interference from ambient light, were identified as significant sources of error. Some of these issues could be addressed using standalone devices developed by many groups where they used a colorimetric bienzymatic paper-based strip that changed colour in the presence of salivary glucose, allowing for visual detection20,21 or by using an office scanner to quantify the glucose levels. The sensor was made from paraffin-coated Whatman paper, with hydrophobic barriers created using a hot metal mold to form the detection zone.22 The detection zone and the colour calibration were specifically optimized to ensure accurate glucose quantification from the colorimetric response. The device had a detection limit of 0.37 mg dL−1, with a linear range of 1 mg dL−1 to 22.5 mg dL−1. However, narrow dynamic range was an impediment to the clinical suitability of this technology. Similarly, another study was conducted on a similar concept; however, it also had the limitations in terms of narrow dynamic detection range.10
Researchers recently developed a portable, reliable, non-enzymatic lab-on-a-chip (LOC) device using MEMS technology.23 It consisted of three zones: a pre-treatment zone for glucose reactions, a mixing zone for combining H2O2 and saliva with a colouring agent, and a measurement zone with an LED and photodiode for glucose detection. Glucose was detected by the decomposition of H2O2, which causes a colour change, and the absorbance was measured.24 Increasing glucose concentrations led to higher absorbance and lower output current. However, a microchip-based sensing system always has the burden of higher manufacturing cost and low environmental sustainability. Additionally, an optical fibre-based detector using long-period grating (LPG) technology detected glucose with a detection limit of 10 μM and reliable performance at concentrations as low as 0.1 mmol L−1.25 This also appears to be a viable alternative, but it cannot serve as a POC device due to the bulkiness of device having complex on board optical components.
While the above solutions proposed by various researchers did not yield a viable working alternative to SMBGs due to lack of portability, accuracy or other associated problems, we had in the past attempted to develop a simple paper-strip-based optical biosensor for detecting SGL11 by way of monitoring colour change on the strip due to enzymatic reaction followed by pH change. Although we transitioned to a handheld biosensing device to eliminate reliance on smartphone cameras, which had been a limitation in our initial attempt.26–28 The paper-based strips had exposed regions where the indicator solution was drop-casted. This could be a reason behind the decreased efficiency of the enzymes or dye, thereby decreasing the strips' efficiency and shelf life. Those strips indicated colour change corresponding to the change in the pH of the detection zone because of the formation of gluconic acid under enzymatic reaction with immobilized GOD, hence the technology was highly dependent on the intrinsic pH of the saliva sample. Thus, the presence of interferants or the patient being uncompliant to the standard operating procedure (SOP) of sample collection might produce false results. Hence, the technology had to be refined that not only allowed for an increased shelf life of strips due to the shielding of the enzymes and dye in the detection region from oxidation or encountering moisture but also made the protocol independent of major interferences.
In this work, we have introduced a refined handheld salivary glucometer that overcomes these earlier limitations and offers a clinically viable alternative to conventional SMBGs. The novelty lies in combining an enzyme-mediated colorimetric reaction with a dedicated optical detection system, bypassing smartphone dependencies and interference-prone open-strip designs. Our approach uniquely integrates a statistically validated SGL-to-BGL conversion model with a machine-coded microcontroller algorithm, enabling real-time estimation of blood glucose levels from saliva. Unlike non-enzymatic or spectrometric methods, which either require alkaline media or complex optics,29,30 our sensor operates under physiological conditions using a shielded cellulose matrix to filter debris, stabilize enzyme activity, and maintain strip integrity. Clinically, this innovation holds particular relevance for vulnerable populations, such as elderly patients, children, and individuals with needle phobia, offering a painless, portable, and accessible option for frequent glucose monitoring. This device promotes better glycaemic adherence and could help delay or prevent diabetes-related complications.
The biosensor featured a red-green-blue (RGB) colour sensor coupled with a high-power specific wavelength light emitting diode (LED) for precise detection of colour changes on enzyme-coated strips. Additionally, the device included a cellulose-based paste to filter out particulate matter, starch and froth, thereby improving the accuracy of detection with saliva samples. Our study compares the performance of this biosensor against both the 3,5-dinitrosalicylic acid (DNS) assay method and a commercially available blood glucose meter, highlighting its clinical applicability and potential advantages over existing salivary glucose monitoring methods. The results show a significant correlation between salivary and blood glucose levels across different patient groups, positioning this biosensor as a practical tool for non-invasive diabetes monitoring. The present combination of hardware, software coding implementation, strip composition and assay protocols not only was able to enhance the dynamic range and shelf life but also weeded out interference and enhanced the accuracy, thereby bridging the gap for an alternate to fingerprick-based SMBG technology.
2. Materials and methods
Horseradish peroxidase (POx), glucose oxidase (GOx), glucose, 4-aminoantipyrine, phenol, and all the chemicals required for the experiment were purchased from Merck (Darmstadt, Germany). The extinction coefficient of the chromogenic dye was determined at 505 nm in phosphate buffer (PB) at 7.4 pH and room temperature of 25 °C. All the statistical analyses were performed using Microsoft Excel and Origin Pro 2022b and the results were shown as average ± standard deviation. Unless otherwise mentioned, all experiments were performed at least in triplicates.2.1. Preparation of test strips
The glucose test strips (Fig. 1 and 2) having dimensions of 52.88 × 11.5 mm and a thickness of 1.2 mm were fabricated using a Raise 3D Pro2 3D printer. The same can also be fabricated using injection moulding as well and the process is compliant with industrial standards. The strip acted as a supporting layer for the filling material in the strip. The rear side of the strip consisted of two holes, one at the collection zone and another at the detection zone having 2 mm and 1 mm diameters respectively. It also consisted of a notch of dimension 2 mm × 1 mm at the bottom of the strip, which was used to align the strip in the device. The strip was printed with black colour 1.75 mm PLA (polylactic acid) filament to avoid any interference of light during the measurement of saliva.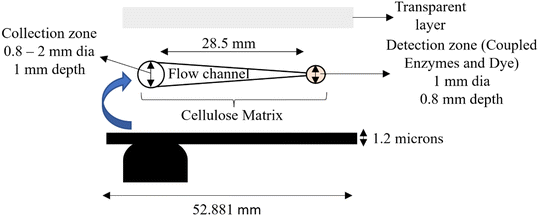 | ||
| Fig. 1 Schematics and dimensions used in fabrication of salivary glucometer strips, its different layers and their geometries. | ||
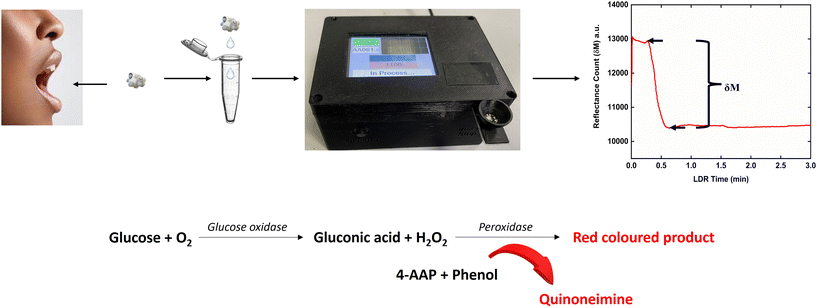 | ||
| Fig. 2 Schematic representation of the sample collection, detection zone on the strip and the representative sensor response curve and chemistry involved in the reaction. | ||
After 3D printing the strips, it has been filled with cellulose powder mixed along with 1% glycerol, polyethylene glycol (PEG), 1% polyvinyl acetate (PVA), and distilled water. All the ingredients were prepared in 100 mM PB buffer of pH 7.4 and mixed homogeneously to obtain a paste. The freshly prepared paste was filled in the groove of the strips and was left to dry at room temperature for overnight. Then, the peripheral area of the strips was cleaned with isopropyl alcohol (IPA).
For immobilization on a batch of 20 strips, 100 μL of the enzyme–dye solution was prepared by mixing 5.46 mg of GOx, and 1.32 mg of POx, 25 mM 4-aminoantipyrine and 125 mM phenol indicator dye in 100 mM phosphate buffer of pH 7.4. A 0.5% BSA was also used added in this solution along with 1 mM of β-mercaptoethanol (β-ME) to increase the stability of strips and the solution was mixed thoroughly without vortexing. A 5 μL of the obtained solution was then immobilized onto the detection zone slowly so that there is no backflow of solution towards the sample application zone. These strips were then left to dry at room temperature for 30 min. Later, adhesive glue was applied to the peripheral area of the strip followed by pasting a transparent cello tape over the entire layer to prevent leakage of saliva through the surface. The prepared strips were stored at 4 °C overnight. They were used for the experiment the next day or stored at 4 °C under desiccated conditions until further use. The gold standard DNS assay method was used for the estimation of glucose present in a saliva sample. Spectroscopic measurements for the dinitro salicylic acid (DNS) based assay of glucose in saliva samples have been conducted with a U-5100 Spectrophotometer, Hitachi.29
2.2. Fabrication of the standalone glucometer
The chassis of the standalone handheld glucometer was designed using Autodesk Fusion 360 software and 3D printed using a Prusa i3 MK3 3D printer with black colour 1.75 mm polylactic acid (PLA) filament. The circuit used in the equipment was designed using EAGLE software from Autodesk and the double-sided printed circuit board (PCB) was outsourced to a local market on which different electronic components such as the light-dependent resistor (LDR), white light emitting diode (LED), RGB colour sensor (S11059-02DT/-03DS from Hamamatsu) and 505 nm single colour LED were assembled. The circuitry also included an Atmega MEGA2560 Pro 16 AUCHIP high performance low power microchip, which contained an 8-bit RISC-based microcontroller. A USB slot was used to transfer the data from the device to a laptop/PC. Once the strip was inserted into the device, a frontal notch provided its proper alignment in the strip slot (ESI† Fig. S1). When the saliva sample was added into the collection well of the strip, the sample travelled towards the detection well and on the way an LDR-white LED pair in reflectance mode sensed the travelling of the sample. A dip in the LDR sensor output indicated that an ample amount of the sample had entered the fluidic path, and this condition was used to initiate data acquisition on the device and was the starting point of the sensor response curve (Fig. 3). Then the sample finally entered the detection zone where the primary LED-RGB sensor was aligned in reflectance mode and if it detected change in absorbance from the strip, as indicated by a dip (minima of Fig. 3) due to the reaction of glucose present in the sample with the enzyme pair and chromogenic dye, followed by the saturation of the sensor response (maxima Fig. 3), the differential reflectance count (δM) was calculated for maxima – minima and stored in the device memory card. The response values obtained in terms of δM was plotted with respect to glucose concentration (intrinsic salivary glucose concentration plus the glucose spiked saliva samples) to get the biosensor calibration curve. The equation from the calibration curve was fed into the Arduino microcontroller program to deduce the unknown concentration of glucose from clinical samples and SGL values were displayed on the alphanumeric liquid crystal display (LCD) screen. To personalize the data of the user, a 32 GB Multimedia Memory Card (MMC) having a serial peripheral interface (SPI) protocol was used to store the data with date and time. This device was run on a low-cost, high-energy-density 7.4 V 4000 mA h rechargeable lithium-ion battery. The stored data could be transferred from the device to the laptop/mobile phone using a USB type B connector.2.3. SOP for saliva collection
All experiments were performed in accordance with the guidelines “Indian Council of Medical Research (ICMR) National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017)”, and approved by the ethics committee at “All India Institute of Medical Sciences (AIIMS), New Delhi (reference number IEC-656/07.12.2018)”. Informed consents were obtained from human participants of this study.In order to minimize variations, a standard operating procedure (SOP) was followed for saliva collection. Patients were recruited from Central Collection facility Lab no. 27, Department of Laboratory Medicine, All India Institute of Medical Sciences (AIIMS), New Delhi, after getting written consent from them to participate in the clinical trial. The ethical clearance was obtained from the AIIMS ethics committee. The saliva sample was collected from the frenulum of the tongue of the subject using a medical grade sterilized cotton ball weighing 33 mg so that only 100 μL sample can be collected and transferred to a microcentrifuge tube and latter pipetted onto the collection zone of the test strip. After adding the saliva, the mesh in the collection zone followed by the sieving action of the cellulose infill material in the fluidic channel of the strip separated the salivary debris and larger proteins and then the sample moved towards the detection zone via capillary action. Upon reaching the detection zone, the glucose in saliva initiated the GOx–POx reaction, leading to the formation of a red coloured dye intermediate. This change in colour intensity was recorded by the RGB colour sensor.30 The reaction chemistry occurring at the detection zone is illustrated in Fig. 2. The hydrogen peroxide generated during the GOx reaction led to the formation of oxygen free radicals, which reacted with the chromogenic dye and produced the red colour. The intensity of the colour was directly proportional to the glucose concentration as shown in ESI† Fig. S2.
2.4. Clinical validation of the biosensor with real samples
To verify the accuracy of the biosensor with clinical samples, saliva was collected from healthy donors. Each sample was collected after the donor rinsed their mouth with drinking water to reduce potential interferences from oral substances like ascorbate and lactate. Fresh saliva was used within 5 minutes of collection for each analysis. The glucose levels measured by the biosensor were cross-validated with readings from a commercially available AccuChek Active glucometer (Roche, Basel Switzerland). Correlation between the two methods was analyzed using a t-test using Microsoft Excel, and direct correlation equations were generated between blood and salivary glucose levels for fasting and postprandial states among non-diabetic, pre-diabetic, and diabetic subjects. These equations were integrated into the biosensor software to calculate and display blood glucose equivalent (BGL) values, allowing the device to identify diabetic or non-diabetic status based on detected glucose ranges. To cross-verify the results, we plotted Bland–Altman plots to evaluate the agreement between the two measurement methods by analyzing the differences against their averages. Each plot represents different conditions or groups, specifically in the context of glucose measurements for diabetic and non-diabetic individuals, as shown in ESI† Fig. S3.In the Bland–Altman plots for non-diabetic and prediabetic/diabetic fasting groups, most data points fall within the limits of agreement (LOA), represented by the dashed orange lines. The solid blue line indicates the mean difference between the two methods, which is close to zero, demonstrating strong agreement between the developed device and the Accu-Chek Active.
For the non-diabetic and prediabetic/diabetic post-prandial groups, the distribution of points shows slightly greater variability compared to fasting levels. However, the majority of data points still lie within the LOA, indicating good agreement between the two methods even under post-prandial conditions.
To ensure data reliability, patients with poor oral hygiene or dental/gum diseases were excluded from the study, and repeatability and reproducibility tests were conducted by measuring glucose levels multiple times on the same sample and on three different samples at different time intervals using the biosensor.
3. Results and discussion
3.1. Biosensor measurements
The calibration curve of the developed instrument was obtained using the saliva sample from a healthy donor, which was spiked with a 10% v/v of known concentrations of glucose prepared in 100 mM phosphate buffer 7.4 pH to obtain higher concentrations. The spiking ratio was restricted to 10% to avoid any significant change in viscosity and other salivary parameters. The standard DNS method was used to estimate the amount of intrinsic glucose present in a healthy individual's saliva sample. The calibration curve thus contained data pertaining to intrinsic glucose concentration in saliva plus the spiked concentration. For the sensor measurements, 100 μL of the spiked sample was added into the collection zone on fabricated strips aged for one day and brought down to room temperature using a pipette. The saliva passed through the mesh present in the collection zone of strip to remove froth and large food debris. Upon further movement of saliva in the fluidic channel filled with cellulose-based infill material, the LDR-white LED pair detected the change in reflectance and initiated sensor response curve recording. Once the reflectance change was observed in the detection zone, it confirmed the arrival of saliva and initiation of enzyme–dye chemistry (observed via a dip in sensor baseline, termed ‘maxima’ region in Fig. 3). The colour change in this region on strip was detected by using an RGB colour sensor (Part no. S11059-02DT/-03DS) from Hamamatsu, which is a highly sensitive 16-bit colour sensor among the family of RGB sensors. A single wavelength LED of 505 nm wavelength was aligned along with the RGB colour sensor on the same PCB so that the detection was performed in reflectance mode at the bottom of the strip through a transparent hole covered with cello tape. The color intensity increased proportionally with the glucose concentration once the reaction was completed, as observed with a ‘minimum’ (Fig. 3). The response time of the sensor varied for different glucose concentrations and on an average, it was up to 3 minutes for the reaction to complete, which includes both the time required for the saliva to flow between the LDR detector and main detection region on the strip. Fig. 3 shows the time response curve of the biosensor for different glucose concentrations (14.5–213 mg dL−1). The lowest concentration of glucose was obtained after prolonged fasting by the volunteer followed by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dilution in PBS buffer, as removal of intrinsic glucose from the sample was practically not possible owing to very short stability period for saliva. It was important that from the time of collecting saliva the biosensor measurements were completed within 5 min, else, saliva properties start changing drastically, as was repeatedly observed by our group in the past, although the lowest concentrations were statistically insignificant considering that the LOD of the sensor was calculated at 3.3 × SD/slope from the sensor and was 14.5 mg dL−1. To evaluate the sensor response in blank samples, the saliva strips were heated in an oven at 85 °C for 24 h to deactivate glucose oxidase and peroxidase without affecting the dye. The δM, i.e. the difference in maxima and minima, has been taken from the individual response curves and was plotted against the glucose concentration in Origin software version 2020b (Fig. 4). The experiment was performed in triplicate for each concentration, with standard deviation represented as error bars.
1 dilution in PBS buffer, as removal of intrinsic glucose from the sample was practically not possible owing to very short stability period for saliva. It was important that from the time of collecting saliva the biosensor measurements were completed within 5 min, else, saliva properties start changing drastically, as was repeatedly observed by our group in the past, although the lowest concentrations were statistically insignificant considering that the LOD of the sensor was calculated at 3.3 × SD/slope from the sensor and was 14.5 mg dL−1. To evaluate the sensor response in blank samples, the saliva strips were heated in an oven at 85 °C for 24 h to deactivate glucose oxidase and peroxidase without affecting the dye. The δM, i.e. the difference in maxima and minima, has been taken from the individual response curves and was plotted against the glucose concentration in Origin software version 2020b (Fig. 4). The experiment was performed in triplicate for each concentration, with standard deviation represented as error bars.
 | ||
| Fig. 4 Calibration curve of the biosensor plotted from the sensor response (δM) at a response time, which varied for glucose concentrations and automatically sensed using the Arduino algorithm. | ||
The response curve of the biosensor showed two graphs with one as a baseline, the graph plotted as blank, and the rest were of different glucose concentrations. However, the baseline values were not all equal; this may be due to the non-specific adsorption of the sample or some ambiguity in the system,31 but the variation was concurrent to 5% standard deviation allowed in quality control (QC) of the strip before selecting them for studies. This QC was carried out by measuring the sensor signal for each enzyme–dye immobilized strips without addition of saliva. This is the reason behind the drift in the biosensor reading after the sample spreads over the detection zone. Another ambivalence in the system was that the viscosity of the saliva sample varies from person to person or in the same person at a different interval along the day time, which leads to the variation in time required for the transportation of the saliva sample.32,33 To address these limitations, the instrument was programmed to record the sensor response curve only after the saliva reached the detection zone. This setup also ensures that the adequate amount of saliva was provided by the user. As we had encountered a major issue pertaining to change in saliva pH naturally upon storage for more than 5 min in previous attempts,11,26,27 this limitation was not present in the current work as our enzyme–dye system was independent of small pH variations.
The calibration curve shown in Fig. 4 was derived from the response curve while automatically recording δM values and was fitted with straight line equation (eqn (1)).
| δM = 10.61 × Glucose, mg dL−1 + 927.65 | (1) |
3.2. Clinical validation of biosensor
Clinical saliva samples from subjects, both diabetic and non-diabetic, were used for validation of the fabricated instrument, with an attempt to determine a correlation and agreement between saliva and blood glucose levels. While SGL values were shown on the device screen upon biosensing, the BGL results were obtained using an FDA approved commercial and amongst highly accurate optical glucometer from Roche with brand name Accuchek Active.34 The correlation pattern was demonstrated using the student's t-test for both the diabetic/pre-diabetic (n = 26) and non-diabetic (n = 77) subject samples, showing a P value and Pearson's R-value of 0.0098 and 0.97; 0.0059 and 0.9 for diabetic or pre-diabetic subjects under fasting and post-prandial conditions respectively (Fig. 5). For non-diabetic subject samples, the corresponding values were 0.0046 and 0.58; 0.0030 and 0.87 respectively. The results suggested a good agreement in t-test for correlation between BGL and SGL and also matched with our previously reported work. Thus, after a successful correlation, the correlation equations were obtained for each conditions (Fig. 5), namely diabetic and prediabetic under fasting and postprandial conditions and same for non-diabetic subjects. These equations provided a range of sensor response δM corresponding to the WHO recommended cutoff for BGL to be classified as non-diabetic/prediabetic or diabetic conditions. The range of glucose under these conditions are the glucose concentration <99 mg dL−1 as non-diabetic under fasting conditions and <140 mg dL−1 under post prandial conditions. A glucose level of 100 mg dL−1 or above under fasting conditions and >140 mg dL−1 under post prandial conditions classifies it as pre-diabetic or diabetic conditions. These correlation equations and range of δM were entered into our Arduino algorithm and only two choices were shown on the first screen of device to select the sample to be tested as ‘fasting’ and ‘post-prandial’. Thereafter, the device could recalculate the BGL equivalent of glucose and display it onto device screen to complete the analysis. | ||
| Fig. 5 Statistical correlation analysis between the SGL and BGL in clinical samples of (A and B) diabetic and (C and D) non-diabetic subjects, under both fasting and postprandial conditions. | ||
In case of gender wise distribution, male and female subjects both showed good correlations between BGL and SGL as shown in the ESI† Fig. S6 and S7 and Table 1 below:
| Diabetic and prandial status | Male | Female |
|---|---|---|
| Non-diabetic post prandial | n = 9 | n = 38 |
| R 2 = 0.81 | R 2 = 0.85 | |
| Diabetic/prediabetic post prandial | n = 5 | n = 11 |
| R 2 = 0.98 | R 2 = 0.89 | |
| Non-diabetic fasting | n = 7 | n = 12 |
| R 2 = 0.74 | R 2 = 0.66 | |
| Diabetic/prediabetic fasting | n = 3 | n = 7 |
| R 2 = 0.98 | R 2 = 0.90 |
Further, to measure the accuracy of the developed instrument, Clarke's error grid analysis (EGA) had to be undertaken while comparing its calculated BGL equivalent with BGL data from the commercial Accuchek Active glucometer (Fig. 6). The results plotted for all clinical samples showed values were in zones A, thereby confirming that the efficacy of the developed instrument was fit for commercialization and medical use.
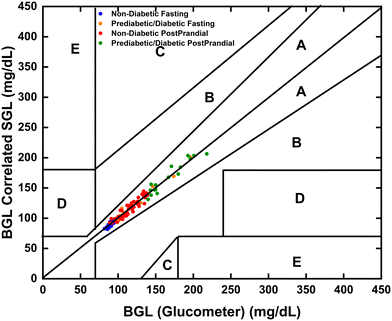 | ||
| Fig. 6 Clarke error grid analysis shows the correlation between BGL (glucose level estimated by commercial glucometer) and SGL (calculated by the developed device). | ||
3.3. Interferent study
These glucose strips were based on cellulose fluidics, but the sample did not evaporate as used to happen in our previous work.11 We used the 3D printed strips along with cellulose in-fill solution, which mimics the property of paper-based strips and avert the complex steps of making paper-based glucose strips. The detection zone and optical detector were housed in a closed chassis to isolate them from light and wind, ensuring no interference was observed. Therefore, the developed instrument has proven to be better as compared to other optical detector-based systems.20Another factor that could affect the biosensor response was the presence of common interferents in saliva such as lactic acid, ascorbic acid, lactose, sugary drinks, and acidic food remnants. Interferent studies were conducted to assess how the mentioned interferents impacted the sensor's response. There was a SOP that was established, which require the user to rinse their mouth with water and avoid eating or drinking for at least 30 minutes prior to the collection of the saliva sample. Interferent study with different interferents at 5 mM concentrations each along with same spiked salivary glucose concentration of 94 mg dL−1, showed no interference (ESI† Fig. S8), as the signal in the presence of these interferents was lower than the LOD of device, which clearly represents that our biosensor was specific for the glucose sample only.
3.4. Reproducibility and shelf-life studies
The reproducibility of the biosensor was evaluated using four different samples at four different time intervals. The biosensor demonstrated high accuracy throughout the day, particularly with fasting, post-breakfast, and post-lunch samples. However, slight variability was observed in the evening random samples, though this remained within the acceptable tolerance level of ±10% for these handmade or lab-made strips. The minimum variability observed was ±5.68%, while maintaining a quality control level of ±5% variation between strips without the addition of any sample (ESI† Fig. S10).The shelf life of the biosensor strip was evaluated over a period of 20 days under refrigerated storage (4 °C, desiccated) with and without the addition of 1 mM β-mercaptoethanol (β-ME) during enzyme–dye immobilization. The strips containing β-ME retained their performance consistently throughout the test period, with negligible variation in sensor output for a fixed glucose concentration (100 mg dL−1). In contrast, strips without β-ME showed a noticeable decline in signal after 10 days, confirming the β-ME's critical role in enzyme stabilization (ESI† Fig. S9). Both types of strips were kept in a desiccated state at 4 °C during the shelf-life evaluation. Notably, all strips exhibited a slight loss in activity immediately after preparation (day 0), which stabilized from day 1 onward, likely due to the drying and curing period post-fabrication. Consequently, strips used for further studies, including calibration curve preparation and clinical validation, were those stored after day 1 of preparation. In addition to refrigerated storage, we also evaluated the short-term performance of the biosensor strips at room temperature (∼25 °C). While refrigeration remained the primary storage conditions, the strips retained acceptable enzyme activity and sensor output (within ±5%) for up to 48 hours at room temperature. However, beyond this period, significant signal degradation was observed. This analysis further supports the adoption of refrigerated, desiccated storage (4 °C) as the standard protocol to ensure optimal biosensor performance and stability.
The inclusion of bovine serum albumin (BSA) in the enzyme solution also helped prevent non-specific protein binding. Additionally, the cellulose in-fill solution of the strips, consisting of polyvinyl alcohol (PVA) and polyethylene glycol (PEG), which are hydrophilic materials, enhanced adsorption stability and prevented the desorption of co-immobilized glucose oxidase (GOx), peroxidase (POx), and the chromogenic dye. The sensor's repeatability was assessed by measuring the salivary glucose concentration of a healthy donor's saliva sample five times consecutively (n = 5), with a glucose concentration of 88 mg dL−1. The standard deviation of these measurements was ±5, indicating a repeatability of ±94.3%.
4. Conclusion
In this study, a handheld optical biosensor for detecting glucose levels from saliva samples was developed. Glucose oxidase (GOD) and peroxidase (POD), along with the yellow dye 4-aminoantipyrine and phenol, were co-immobilized onto the strip's detection zone. The strip featured a fluidic channel filled with a cellulose-based paste designed to remove food debris and certain protein interferents from the saliva sample, while also controlling the flow of saliva toward the detection zone. When salivary glucose reacted with the enzyme–dye system, an oxygen-free radical produced from the coupled enzyme activity reacted with the indicator dye, 4-aminoantipyrine, in the presence of phenol to produce a red-coloured byproduct. This colour intensity was detected using a single-wavelength LED coupled with an RGB optical sensor in reflectance mode.Initially, the device displayed the salivary glucose level (SGL). Later, when clinical correlation equations were programmed into the device's software, it could predict the equivalent blood glucose level (BGL) and display it on the screen. Other notable features of this system include a standard operating procedure (SOP), a user-friendly saliva collection method, a clinically relevant dynamic detection range of 14.5–213 mg dL−1 for SGL, high sensitivity of 10.6 sensor counts per mg dL−1, and a lower limit of detection at 14.5 mg dL−1, which is well below the hypoglycaemic threshold for uncontrolled diabetic patients. The biosensor also provided results within a tolerable response time of 5 minutes.
The biosensor was clinically validated against a commercial glucometer to compare SGL with BGL, demonstrating a strong correlation coefficient for diabetic patients (0.97) and non-diabetic subjects under fasting (0.9) and postprandial conditions (0.58 and 0.87, respectively). Gender-wise, significant correlations were observed under postprandial conditions for diabetic and non-diabetic males (R2 = 0.98, 0.81) and females (R2 = 0.89, 0.85). In fasting conditions, diabetic males (R2 = 0.98) and females (R2 = 0.90), as well as non-diabetic males (R2 = 0.74) and females (R2 = 0.66), exhibited strong correlations. The device also passed the Clarke error grid analysis in the A zone, along with the Bland–Altman analysis, and met all the WHO-recommended ‘ASSURED’ criteria: affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable to end-users, making it a viable standalone point-of-care device with commercialization potential. Future research could focus on enhancing the biosensor's sensitivity and specificity, expanding its capability to detect multiple biomarkers, and validating its effectiveness through clinical trials across diverse populations. Additionally, integrating the biosensor with mobile health platforms and cloud-based data storage would enable remote patient monitoring, facilitate data sharing with healthcare providers, and enhance personalized diabetes management. This biosensor could significantly aid elderly diabetic patients by offering a convenient, painless alternative to traditional blood glucose monitoring, improving their daily management of diabetes without frequent finger pricks.
However, one limitation of the current study is that the clinical validation was conducted on a demographically homogeneous group, without representation from diverse ethnicities or age categories. Further large-scale studies across varied populations are needed to fully establish the device's generalizability and real-world performance.
Ethical approval and consent
The Institute Ethical Committee approved the protocol (reference number IEC-656/07.12.2018).Data availability
No code was used in this study. All relevant data are included within the manuscript.Conflicts of interest
We have received consent from all the authors and no conflict of interest is relevant to this paper.Acknowledgements
Our sincere gratitude is to the Indian Institute of Technology Delhi and All India Institute of Medical Sciences, New Delhi for providing the infrastructure facility. We are thankful to the funding agencies IIT Delhi-AIIMS multidisciplinary collaborative institutional fund (MIFIRP-MI01913) and BIRAC PACE (grant no. BT/AIR0647/PACE-16/18) for carrying out this research work.References
- M. Mobasseri, M. Shirmohammadi, T. Amiri, N. Vahed, H. H. Fard and M. Ghojazadeh, Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis, Health Promot. Perspect., 2020, 10, 98 CrossRef PubMed.
- A. St John and C. P. Price, Existing and emerging technologies for point-of-care testing, Clin. Biochem. Rev., 2014, 35, 155 Search PubMed.
- P. Jain, A. M. Joshi, S. P. Mohanty and L. R. Cenkeramaddi, Non-invasive Glucose Measurement Technologies: Recent Advancements and Future Challenges, IEEE Access, 2024, 61907–61936 Search PubMed.
- C. Sabu, T. Henna, V. Raphey, K. Nivitha and K. Pramod, Advanced biosensors for glucose and insulin, Biosens. Bioelectron., 2019, 141, 111201 CrossRef CAS PubMed.
- S. Liakat, K. A. Bors, L. Xu, C. M. Woods, J. Doyle and C. F. Gmachl, Noninvasive in vivo glucose sensing on human subjects using mid-infrared light, Biomed. Opt. Express, 2014, 5, 2397–2404 CrossRef PubMed.
- A. L. Blotsky, E. Rahme, M. Dahhou, M. Nakhla and K. Dasgupta, Gestational diabetes associated with incident diabetes in childhood and youth: a retrospective cohort study, CMAJ, 2019, 191, E410–E417 Search PubMed.
- A. Ko and C. Liao, Salivary glucose measurement: a holy ground for next generation of non-invasive diabetic monitoring, Hybrid Advances., 2023, 3, 100052 CrossRef.
- X. Qian, A. Ko, H. Li and C. Liao, Flexible non-enzymatic glucose strip for direct non-invasive diabetic management, Microchem. J., 2024, 197, 109818 CrossRef CAS.
- A. Soni and S. K. Jha, Smartphone based non-invasive salivary glucose biosensor, Anal. Chim. Acta, 2017, 996, 54–63 CrossRef CAS PubMed.
- S. Panwar, P. Sarkar, D. S. Kasim, R. Anand, A. Priya and S. Prakash, et al., Portable optical biosensor for point-of-care monitoring of salivary glucose using a paper-based microfluidic strip, Biosens. Bioelectron.: X, 2024, 17, 100452 CAS.
- A. K. Singh and S. K. Jha, Fabrication and validation of a handheld non-invasive, optical biosensor for self-monitoring of glucose using saliva, IEEE Sens. J., 2019, 19, 8332–8339 CAS.
- Y.-S. Hwang, E. Y.-C. Kang, C.-R. Shen, W.-H. Hong and W.-C. Wu, Noncontact optical measurement of aqueous humor glucose levels and correlation with serum glucose levels in rabbit, Biosensors, 2021, 11, 387 CrossRef CAS PubMed.
- C. Liao, High performance biological sensors based on organic electrochemical transistors (OECTs), M.Phil, Department of Applied Physics, Hong Kong Polytechnic University, 2014 Search PubMed.
- C. Liao and F. Yan, Organic semiconductors in organic thin-film transistor-based chemical and biological sensors, Polym. Rev., 2013, 53, 352–406 CrossRef CAS.
- D. Elkington, M. Wasson, W. Belcher, P. Dastoor and X. Zhou, Printable organic thin film transistors for glucose detection incorporating inkjet-printing of the enzyme recognition element, Appl. Phys. Lett., 2015, 106, 263301 CrossRef.
- A. Ko and C. Liao, Hydrogel wound dressings for diabetic foot ulcer treatment: status-quo, challenges, and future perspectives, BMEMat, 2023, 1, e12037 CrossRef.
- A. Soni and S. K. Jha, A paper strip based non-invasive glucose biosensor for salivary analysis, Biosens. Bioelectron., 2015, 67, 763–768 CrossRef CAS PubMed.
- A. Soni, R. K. Surana and S. K. Jha, Smartphone based optical biosensor for the detection of urea in saliva, Sens. Actuators, B, 2018, 269, 346–353 CrossRef CAS.
- A. Soni and S. K. Jha, Saliva based noninvasive optical urea biosensor, in 2017 IEEE sensors, IEEE, 2017, pp. 1–3 Search PubMed.
- H. J. Chun, Y. M. Park, Y. D. Han, Y. H. Jang and H. C. Yoon, Based glucose biosensing system utilizing a smartphone as a signal reader, BioChip J., 2014, 8, 218–226 CrossRef CAS.
- M. A. Abbasi, Cellulose through the lens of microfluidics: a review, Appl. Biosci., 2022, 1, 1–37 CrossRef.
- W. Tan, L. Zhang, P. Jarujamrus, J. C. Doery and W. Shen, Improvement strategies on colorimetric performance and practical applications of Paper-based analytical devices, Microchem. J., 2022, 180, 107562 CrossRef CAS.
- Y. Du, W. Zhang and M. L. Wang, An on-chip disposable salivary glucose sensor for diabetes control, J. Diabetes Sci. Technol., 2016, 10, 1344–1352 CrossRef CAS PubMed.
- I. R. Ilyasov, V. L. Beloborodov, I. A. Selivanova and R. P. Terekhov, ABTS/PP decolorization assay of antioxidant capacity reaction pathways, Int. J. Mol. Sci., 2020, 21, 1131 CrossRef CAS PubMed.
- T. Libish, J. Linesh, M. Bobby, B. Nithyaja, S. Mathew and C. Pradeep, et al., Glucose concentration sensor based on long period grating fabricated from hydrogen loaded photosensitive fiber, Sens. Transducers J., 2011, 129, 142 CAS.
- A. Soni and S. K. Jha, A paper strip based non-invasive glucose biosensor for salivary analysis, Biosens. Bioelectron., 2015, 67, 763–768 CrossRef CAS PubMed.
- A. Soni and S. K. Jha, Smartphone based non-invasive salivary glucose biosensor, Anal. Chim. Acta, 2017, 996, 54–63 CrossRef CAS PubMed.
- M. Elsherif, M. U. Hassan, A. K. Yetisen and H. Butt, Wearable contact lens biosensors for continuous glucose monitoring using smartphones, ACS Nano, 2018, 12, 5452–5462 CrossRef CAS PubMed.
- A. Machado, R. Maneiras, A. A. Bordalo and R. B. R. Mesquita, Monitoring glucose, calcium, and magnesium levels in saliva as a non-invasive analysis by sequential injection multi-parametric determination, Talanta, 2018, 186, 192–199 CrossRef CAS PubMed.
- P. Dak, A. Ebrahimi, V. Swaminathan, C. Duarte-Guevara, R. Bashir and M. A. Alam, Droplet-based biosensing for lab-on-a-chip, open microfluidics platforms, Biosensors., 2016, 6, 14 CrossRef PubMed.
- A. K. Ellerbee, S. T. Phillips, A. C. Siegel, K. A. Mirica, A. W. Martinez and P. Striehl, et al., Quantifying colorimetric assays in paper-based microfluidic devices by measuring the transmission of light through paper, Anal. Chem., 2009, 81, 8447–8452 CrossRef CAS PubMed.
- B. Brena, P. González-Pombo and F. Batista-Viera, Immobilization of enzymes: a literature survey, Immobilization of Enzymes and Cells, 3rd edn, 2013, pp. 15–31 Search PubMed.
- A. Tripathi and J. S. Melo, Immobilization strategies: biomedical, bioengineering and environmental applications, Springer Nature, 2020 Search PubMed.
- A. Waghmare, F. S. Parizi, J. Hoffman, Y. Wang, M. Thompson and S. Patel, Glucoscreen: A smartphone-based readerless glucose test strip for prediabetes screening, Proc. ACM Interact. Mob. Wearable Ubiquitous Technol., 2023, 7, 1–20 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sd00027k |
| This journal is © The Royal Society of Chemistry 2025 |

