Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Pronounced electronic modulation of geometrically-regulated metalloenediyne cyclization
Sarah E.
Lindahl
a,
Erin M.
Metzger
a,
Chun-Hsing
Chen
b,
Maren
Pink
b and
Jeffrey M.
Zaleski
*a
aDepartment of Chemistry, Indiana University, Bloomington, IN 47405, USA. E-mail: zaleski@iu.edu
bMolecular Structure Center, Indiana University, Bloomington, IN 47405, USA
First published on 10th July 2025
Abstract
Correction for ‘Pronounced electronic modulation of geometrically-regulated metalloenediyne cyclization’ by Sarah E. Lindahl et al., Chem. Sci., 2025, 16, 255–279, https://doi.org/10.1039/D4SC05396F.
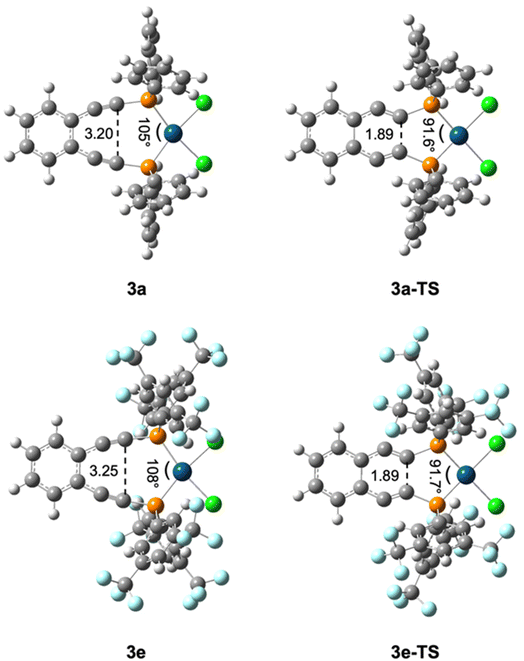
The authors regret that the experimental values of ΔG‡ for the Bergman cyclization kinetics in their published work are incorrect due to a unit conversion error. Table 2 and Fig. 4, 7, and 8 are hereby corrected to the values as given below. Table 18S of the ESI has also been corrected. Despite the unit conversion error, all experimental trends observed in the cyclization kinetics are accurately reported in the original publication.
The published computational studies were performed with the pure density functional BPW91, which was chosen based on its ability to accurately model the experimental activation barriers to Bergman cyclization. After correcting the unit conversion error in the experimental ΔG‡ values, the BPW91 functional now underestimates the activation barriers to Bergman cyclization by 5–6 kcal mol−1; however, all computational trends originally reported are still consistent with the corrected experimental data.
After correcting the experimental unit conversion error, the B3PW91 functional accurately models activation barriers to within <2 kcal mol−1. Therefore, we have elected to update Fig. 4, 7, and 8 to include the B3PW91 data. All trends reported in these figures with the updated B3PW91 data are consistent with those originally published with BPW91 computed structures. Table 18S has also been added to summarize the B3PW91 computational results simply for completeness.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2025 |