Open Access Article
Open Access ArticleEngineering d–p orbital hybridization of single-atom Fe sites via axial B-mediation for the oxygen reduction reaction
Xiaoqin Xua,
Tianmi Tanga,
Xue Baia,
Tao Gan*b and
Jingqi Guan *a
*a
aInstitute of Physical Chemistry, College of Chemistry, Jilin University, Changchun 130021, PR China. E-mail: guanjq@jlu.edu.cn
bShanghai Synchrotron Radiation Facility, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai, PR China. E-mail: gant@sari.ac.cn
First published on 12th November 2025
Abstract
Single-atom Fe–N–C catalysts have demonstrated promising potential in the oxygen reduction reaction (ORR), yet their intrinsic activity remains less than ideal. Orbital hybridization provides a versatile means to modulate the thermodynamic and kinetic properties during electrochemical processes. In this study, we adopt an “axial ligand boron-modulation” strategy to regulate the electronic structure of single-atom Fe sites through d–p orbital hybridization. The synthesized FeN4–B/NC demonstrates exceptional ORR activity with a half-wave potential of 0.915 V, surpassing planar FeN4/NC and commercial Pt/C. In situ XAS results reveal the dynamic stretching of Fe–N/O and Fe–B bonds during the ORR process, providing an intuitive confirmation that the single-atom sites undergo reversible structural changes to optimize the adsorption of reaction intermediates. Theoretical investigations combined with zero-field cooling temperature dependence analyses demonstrate that in the intermediate spin state, hybridization occurs between the central Fe's 3d orbitals and B's 2p orbitals, which results in increased eg orbital occupancy and positions the d-band center closer to the Fermi level, which enhances charge transfer efficiency and O2 adsorption capabilities. Furthermore, the newly developed FeN4–B/NC catalyst shows remarkable performance in liquid and quasi-solid-state zinc–air batteries.
Introduction
With the advancement of flexible and wearable electronic devices, zinc–air batteries (ZABs) with high energy density, low cost, and safety demonstrate significant potential for practical applications.1,2 The oxygen reduction reaction (ORR) plays a pivotal role in ZAB systems. Although platinum-based catalysts are widely regarded as the optimal choice for the ORR due to their exceptional catalytic activity, the prohibitively expensive nature, scarce resources, limited stability, and poor tolerance to CO/methanol significantly hinder their large-scale implementation.3 Therefore, it is highly desirable to explore low-cost, non-precious metal-based catalysts with remarkable catalytic performance.Single-atom catalysts (SACs), characterized by their atomically dispersed metal active sites, represent promising experimental and theoretical models for elucidating catalytic mechanisms and guiding the rational design of ORR catalysts.4 This is attributed to their high metal atom efficiency, unique electronic structure, and uniformly tunable active sites.5,6 In this regard, Fe-based catalysts with square-planar Fe–N4 centers are widely regarded as promising candidates for achieving high-efficiency ORR activity and replacing Pt-based catalysts.7,8 However, the strong binding affinity of Fe–N4 centers towards *OH intermediates hinders the desorption of OH− or H2O, thereby limiting their catalytic activity and kinetics.9,10 Recent studies have demonstrated that the incorporation of foreign atoms at the fifth coordination position, such as Cl,11,12 O,13 S,10 or I,14 can regulate the electronic properties of Fe-based active sites. This modulation occurs because the Fe–O bonds formed between ORR intermediates and Fe–N4 sites involve axial orbital overlap relative to the Fe–N4 plane, thereby significantly improving the ORR performance.15 In addition, Fe, as a transition metal with localized d orbitals, exhibits the unique ability to engage in d–p orbital hybridization with heteroatoms that possess p orbitals. Through this d–p orbital hybridization, Fe improves its charge transfer capabilities by modifying the electronic environment and optimizing the energy levels of relevant orbitals. This enhancement facilitates more efficient participation of electrons in electrochemical reactions.16 However, due to the ionic nature of their axial ligands binding to Fe–N4 sites, such ligands tend to leach during the ORR process.17 Establishing stable charge distribution at Fe–N4 sites is therefore fundamentally and practically significant. If such stability can be achieved, it would pave the way for progressive enhancement in the ORR activity of Fe–N4 SACs.
Here, we introduce a novel d–p orbital hybridization approach facilitated by the axial coordination of B to enhance the intrinsic ORR activity. Utilizing a molecular-cage encapsulation approach combined with ligation strategies, we synthesized two Fe SACs with distinct active centers on N-doped carbon supports, including square-planar FeN4 and quasi-octahedral geometry FeN4–B configurations. Notably, the FeN4–B catalyst demonstrated superior ORR performance in alkaline media, significantly outperforming FeN4-based catalysts. Density functional theory (DFT) calculations revealed that the interactions between B's 2p orbitals and Fe's 3d orbitals (dz2, dxz, and dyz) establish both bonding and antibonding interactions. This orbital coupling induces an upshift of the d-band center, which facilitates *OH adsorption and consequently reduces the energy barrier for the rate-determining step (RDS) of the ORR. To further evaluate the practical application potential of FeN4–B, we incorporated it as the cathode in a ZAB system with a solid-state configuration. The FeN4–B/NC-based ZAB exhibited exceptional performance, delivering higher power density and energy density compared to the commercial Pt/C + RuO2-based ZAB, demonstrating its immense potential for practical applications.
Results and discussion
Structure and composition analyses
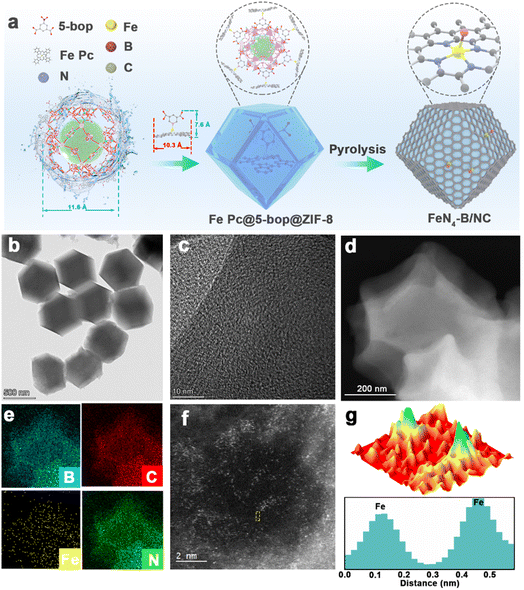
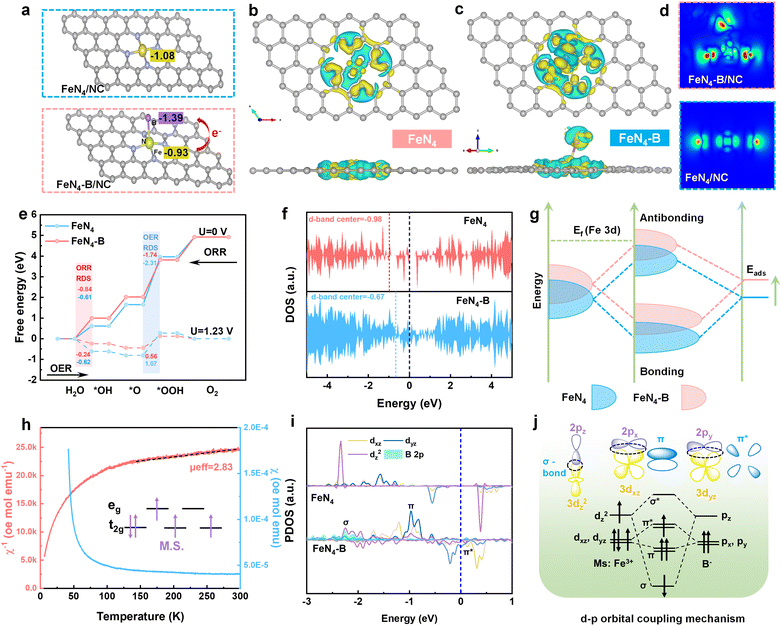
The synthesis procedure for the carbon nanocage catalyst incorporating single-atom FeN4–B sites is illustrated in Fig. 1a. To construct FeN4–B/NC, we employed a molecular cage encapsulation strategy. Iron(II) phthalocyanine (Fe Pc), which possesses a condensed aromatic ring framework and well-defined molecular structure, was utilized as the metal precursor. 5-Borondiphenic acid (5-bop) served as the boron source. The synthesis proceeded through three key steps. First, Fe Pc and 5-bop underwent Lewis acid–base interactions to form Fe Pc@5-bop complexes (10.3 Å × 7.6 Å). These complexes were then immersed in a methanol solution containing Zn2+ ions and 2-methylimidazole to encapsulate them within ZIF-8 nanocages (11.6 Å). Finally, high-temperature annealing was conducted to obtain the final FeN4–B/NC material.It was observed via scanning electron microscopy (SEM) in Fig. S1 that the annealed FeN4–B/NC exhibited a consistent morphology with Fe Pc@5-bop@ZIF-8, indicating that no collapse occurred during the process. Transmission electron microscopy (TEM), high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and energy-dispersive X-ray spectroscopy (EDS) mapping of Fe Pc@5-bop@ZIF-8 show that Fe Pc@5-bop is encapsulated in ZIF-8 and the dodecahedral appearance of ZIF-8 is not compromised, and Fe and B are uniformly distributed in ZIF-8 (Fig. S2). TEM reveals that FeN4–B/NC retains the regular rhombic dodecahedral structure of ZIF-8 (Fig. 1b). No clusters or nanoparticles were observed in the high resolution-TEM (HR-TEM) image (Fig. 1c), while clear lattice fringes corresponding to graphitized carbon structures were evident. The HAADF-STEM image of the sample does not show visible iron-based nanoparticles (Fig. 1d). EDS mapping (Fig. 1e) demonstrates uniform distribution of Fe, C, N, and B elements within the FeN4–B/NC polyhedral framework. The face-scanning analysis using HAADF-STEM combined with EDS shows that the elemental content of Fe in the FeN4–B/NC catalyst is 0.49 wt% (Fig. S3). This further indicates that the encapsulation–thermal decomposition strategy successfully anchored the atomically dispersed Fe sites in the carbon substrate and achieved a considerable active site density. Aberration-corrected (AC) HAADF-STEM imaging allows atomic-level observation of the FeN4–B/NC structure (Fig. 1f). Numerous isolated points are identified, which correspond to iron atoms as clearly visualized in the corresponding 3D representation (Fig. 1g).
X-ray diffraction (XRD) patterns of Fe Pc@ZIF-8 and Fe Pc@5-bop@ZIF-8 are consistent with those of ZIF-8, indicating that the incorporation of Fe Pc and Fe Pc@5-bop does not disrupt the structural integrity of ZIF-8 (Fig. S4a). Furthermore, no Fe metal signals were detected in the XRD patterns of these samples after thermal decomposition (Fig. S4b). Additionally, the graphitic characteristics of the catalysts were systematically investigated using Raman spectroscopy to evaluate their degree of graphitization. In the Raman spectra, the D peak corresponds to defects while the G peak represents the graphitization degree. As shown in Fig. S5, the FeN4–B/NC sample exhibits a lower ID/IG ratio (1.01), indicating a higher degree of graphitization and consequently enhanced electrical conductivity.
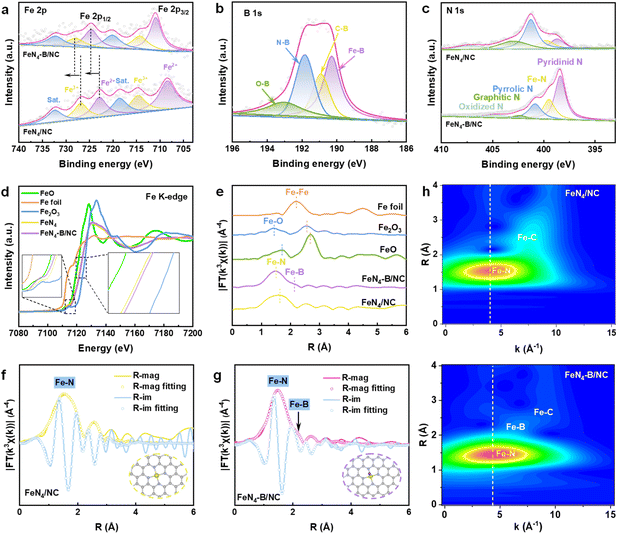
To investigate the chemical states of FeN4–B/NC and FeN4/NC, X-ray photoelectron spectroscopy (XPS) analysis was conducted. The survey spectra reveal the presence of C, N, O, and Fe elements in both samples (Fig. S6a). Additionally, a distinct B peak was observed for FeN4–B/NC, confirming the successful incorporation of B atoms within the carbon nanocage framework. As shown in Fig. S6b, the high-resolution C 1s XPS spectrum was deconvoluted into three characteristic peaks: C–C (284.8 eV), C–N (285.6 eV), and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (286.9 eV).18 The Fe 2p spectra for both samples are shown in Fig. 2a. The Fe 2p spectra exhibit four prominent peaks at binding energies of 708.4 eV (Fe2+ 2p3/2), 714.7 eV (Fe3+ 2p3/2), 722.9 eV (Fe2+ 2p1/2), and 726.9 eV (Fe3+ 2p1/2).19 Relative to FeN4/NC, the binding energy shifts of both the Fe 2p1/2 and 2p3/2 orbitals in FeN4–B/NC exhibited higher values. This observation suggests an electron transfer from Fe to B, accompanied by an increase in electron density around the Fe center.20 As shown in Fig. 2b, the B 1s XPS spectrum of FeN4–B/NC reveals four distinct boron species: Fe–B (∼190.3 eV), B–C (∼190.9 eV), B–N (∼191.8 eV), and B–O (∼193.1 eV).21 The presence of the Fe–B bond provides direct evidence of chemical bonding between boron and iron, ruling out the possibility of mere π–π stacking interactions. Based on the N 1s spectroscopy analysis (Fig. 2c), both FeN4–B/NC and FeN4/NC samples exhibit pyridinic N (398.4 eV), Fe–N (399.5 eV), pyrrolic N (400.9 eV), graphitic N (402.5 eV), and oxidized N (404.7 eV) species.22 However, significant differences in their nitrogen coordination environments are observed: FeN4/NC predominantly features pyrrolic N, whereas FeN4–B/NC shows a preference for pyridinic N. This indicates that FeN4/NC adopts a wrinkled structure, while FeN4–B/NC exhibits a planar tetragonal FeN4 coordination geometry.23 The structural modification arises from the incorporation of B, which strengthens the Fe–N interaction and facilitates the conversion of pyrrolic N to more stable pyridinic N and modulates the FeN4–B/NC's electronic properties and optimizes the distribution of active sites. This structural optimization ultimately enhances both catalytic performance and operational durability.24,25
O (286.9 eV).18 The Fe 2p spectra for both samples are shown in Fig. 2a. The Fe 2p spectra exhibit four prominent peaks at binding energies of 708.4 eV (Fe2+ 2p3/2), 714.7 eV (Fe3+ 2p3/2), 722.9 eV (Fe2+ 2p1/2), and 726.9 eV (Fe3+ 2p1/2).19 Relative to FeN4/NC, the binding energy shifts of both the Fe 2p1/2 and 2p3/2 orbitals in FeN4–B/NC exhibited higher values. This observation suggests an electron transfer from Fe to B, accompanied by an increase in electron density around the Fe center.20 As shown in Fig. 2b, the B 1s XPS spectrum of FeN4–B/NC reveals four distinct boron species: Fe–B (∼190.3 eV), B–C (∼190.9 eV), B–N (∼191.8 eV), and B–O (∼193.1 eV).21 The presence of the Fe–B bond provides direct evidence of chemical bonding between boron and iron, ruling out the possibility of mere π–π stacking interactions. Based on the N 1s spectroscopy analysis (Fig. 2c), both FeN4–B/NC and FeN4/NC samples exhibit pyridinic N (398.4 eV), Fe–N (399.5 eV), pyrrolic N (400.9 eV), graphitic N (402.5 eV), and oxidized N (404.7 eV) species.22 However, significant differences in their nitrogen coordination environments are observed: FeN4/NC predominantly features pyrrolic N, whereas FeN4–B/NC shows a preference for pyridinic N. This indicates that FeN4/NC adopts a wrinkled structure, while FeN4–B/NC exhibits a planar tetragonal FeN4 coordination geometry.23 The structural modification arises from the incorporation of B, which strengthens the Fe–N interaction and facilitates the conversion of pyrrolic N to more stable pyridinic N and modulates the FeN4–B/NC's electronic properties and optimizes the distribution of active sites. This structural optimization ultimately enhances both catalytic performance and operational durability.24,25
To probe the intricate details of local coordination geometry and electronic states, X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopic analyses were employed. As shown in Fig. 2d, the absorption edge of Fe in FeN4–B/NC is positioned between those of FeO and Fe2O3, indicating that the average oxidation state of Fe is between +2 and +3.26 Compared to FeN4/NC, a noticeable positive shift in the Fe K-edge suggests a change in the electronic structure of Fe sites, which means an increase in the oxidation state of Fe. This is likely due to the B axial coordination leading to electron transfer from Fe to B,27 consistent with previous XPS observations.11 In the inset of Fig. 2d, after B incorporation, the intensity of the pre-edge peak decreases (related to the 1s–4pz dipolar transition in a planar D4h configuration), indicating that the local symmetry transitions from planar D4h to C4v.28,29 Fourier-transformed k3-weighted EXAFS spectra were plotted. As shown in Fig. 2e, FeN4 exhibits a prominent peak centered at 1.63 Å in the R-space, corresponding to the Fe–N scattering path. Notably, a shoulder peak at 2.1 Å is observed for FeN4–B/NC, which corresponds to Fe–B backscattering, further confirming the presence of an Fe–B axial interaction.30 The absence of Fe–Fe features in FeN4–B/NC and FeN4/NC samples indicates that Fe sites are atomically dispersed within the carbon framework. To elucidate the coordination environment around Fe sites, quantitative least-squares EXAFS curve fitting was performed. In the FeN4/NC structure, iron centers are each bonded to four nitrogen atoms in the first coordination shell, with a mean bond distance of 1.92 Å (Fig. 2f and Table S3). In contrast, Fe in FeN4–B/NC is bonded to four N atoms and one B atom (FeN4–B), with an average bond length of 1.96 Å and 2.15 Å, respectively (Fig. 2g). Importantly, the FT k3-weighted EXAFS spectra match perfectly with the simulated EXAFS spectra obtained from DFT, further validating the proposed FeN4–B structure. Additionally, in the wavelet transform (WT)-EXAFS plots, we observe minor peak shifts in the k-space between FeN4/NC and FeN4–B/NC (Fig. 2h), indicating subtle changes in their atomic coordination environments.21
Electrochemical performances
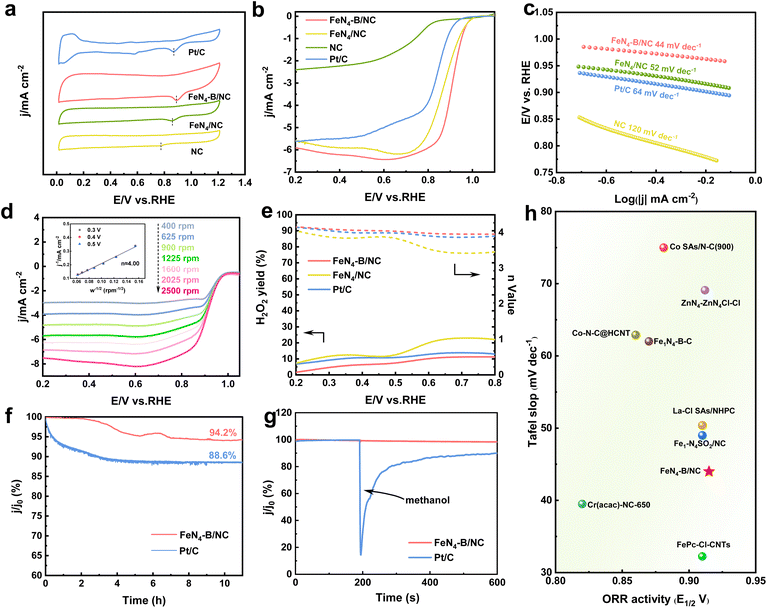
The ORR electrocatalytic performance was evaluated using a rotating disk electrode (RDE) in an O2-saturated 0.1 M KOH solution. As shown by the comparative cyclic voltammetry (CV) curves, all synthesized catalysts exhibit distinct reduction peaks (Fig. 3a).31 Notably, FeN4–B/NC achieves the most positive peak potential, indicating its potentially superior ORR activity. Linear scan voltammetry (LSV) curves (Fig. 3b) reveal that FeN4–B/NC delivers an outstanding half-wave potential (E1/2) of 0.915 V vs. RHE, surpassing those of FeN4/NC (0.866 V), NC (0.748 V), and commercial Pt/C (0.850 V). This improvement demonstrates that the B-axial coordination in FeN4 configuration enhances the ORR performance through synergistic electronic modulation. Furthermore, as shown in Fig. 3c, the Tafel slope of FeN4–B/NC (44 mV dec−1) is significantly lower than those of FeN4/NC, NC, and Pt/C, suggesting that the incorporation of B-axial coordination at the Fe site effectively accelerates ORR kinetics. Fitting the LSV curves at various rotation speeds with the K–L equation reveals that the electron transfer number of FeN4–B/NC is approximately 4.0, indicating a 4-electron ORR process (Fig. 3d and S7). RRDE tests further demonstrate that FeN4–B/NC exhibits a higher electron transfer number closer to 4 than FeN4/NC. The introduction of axial B-coordination reduces the H2O2 yield from 11% to 1%, suggesting enhanced O2 dissociation and superior ORR activity for FeN4–B/NC (Fig. 3e). Furthermore, the turnover frequency (TOF) of FeN4–B/NC at 0.8 V is 0.982 O2 s−1, which is much higher than that of Pt/C (0.049 O2 s−1), indicating that the iron-related active sites in FeN4–B/NC have high efficiency in the ORR.32In addition to its excellent ORR activity, stability of a catalyst is crucial for practical applications. The long-term durability of FeN4–B/NC for the ORR was evaluated using chronoamperometric measurements. Notably, FeN4–B/NC shows a minimal current decay of only 5.8% over 11 hours of continuous operation, significantly outperforming Pt/C, which exhibits an 11.4% current loss (Fig. 3f). Furthermore, in methanol tolerance tests conducted after 200 seconds of potentiostatic measurement (Fig. 3g), commercial Pt/C displays severe current fluctuations and a noticeable drop upon addition of 1 mL methanol, whereas FeN4–B/NC maintains relatively stable current responses, demonstrating strong resistance to methanol poisoning.33 Compared to other SACs reported previously, FeN4–B/NC achieves top-tier activity (Fig. 3h and Table S3). The accelerated durability test (ADT) results reveal that after 5000 cycles of testing, the half-wave potential in alkaline electrolyte decreases by 22 mV for FeN4–B/NC, whereas a more significant loss of 38 mV is observed for Pt/C (Fig. S8). These findings demonstrate the superior stability of FeN4–B/NC during the ORR. We further evaluated the oxygen evolution reaction (OER) performance of FeN4–B/NC in 1 M KOH solution. As shown in Fig. S9, the FeN4–B/NC exhibited an overpotential of 370 mV at a current density of 10 mA cm−2, comparable to that of RuO2 and superior to that of FeN4/NC (380 mV).34
Study on the oxygen electrocatalysis mechanisms
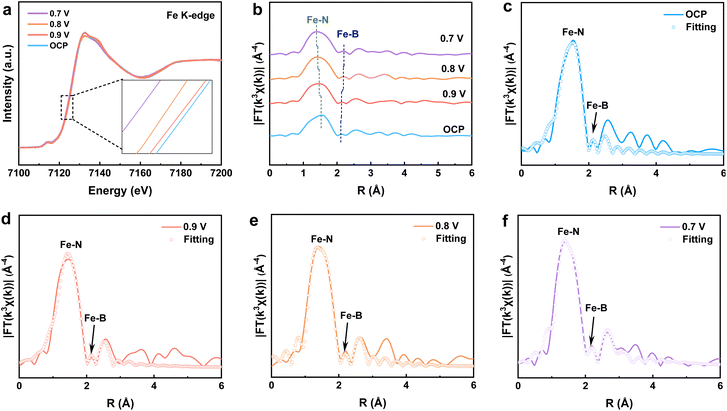
To investigate the structural evolution of FeN4–B/NC during the ORR process, in situ XAS experiments were conducted. The XAS spectra of FeN4–B/NC were collected at different potentials in O2-saturated 0.1 M KOH solution. As shown in Fig. 4a, a negative shift in the absorption edge energy was observed with decreasing applied potential, providing evidence for the occurrence of the ORR.35 To further probe the structural changes at the active sites during the ORR, EXAFS oscillations were analyzed. The Fe K-edge FT-EXAFS spectra (Fig. 4b) reveal the structural stability of FeN4–B/NC to retain its initial structural integrity under operating conditions. Specifically, as the applied voltage decreases, the Fe–N/O bond length shortens from 1.52 to 1.39 Å, while the Fe–B bond length extends from 2.11 to 2.21 Å, demonstrating that at different potentials, both Fe–N/O and Fe–B bonds undergo stretching due to intermediate adsorption at the active centers.36 Additionally, variations in the intensities of Fe–N/O and Fe–B bonds were observed at different potentials, likely attributed to redox processes involving Fe atoms and structural rearrangements. These changes were further influenced by the adsorption and desorption of reaction intermediates, suggesting dynamic structural modifications of FeN4–B during the ORR. The FT-EXAFS fitting results at various potentials showed an increase in the coordination number of Fe–N/O bonds (Fig. 4c–f and Table S2), indicating that Fe atoms serve as the active centers for the ORR. Furthermore, Fig. S10 displays the WT plots of FeN4–B/NC at different potentials, showing partial shifts in Fe–N/O scattering paths, which further confirms that Fe atoms are the active sites for the ORR.Theoretical calculations revealed that the axial coordination of B to FeN4 significantly influences both the electronic structure and ORR performance. The optimized structures of FeN4 and FeN4–B are presented in Fig. 5a. It can be seen that establishing the axial coordination of B at the Fe–N4 locus causes the distortion of the square-plane field around Fe–N4, which is transformed into a quasi-octahedral geometry. Bader charge analysis reveals the net charge on individual atoms, where positive values indicate electron loss (oxidation) and negative values reflect electron gain (reduction).37 As evidenced by the calculations (Fig. 5a), the Bader charges for Fe in FeN4 and FeN4–B systems are determined to be −1.39 and −0.93, respectively. This suggests an electronic transfer from Fe to B, leading to an increase in Fe's valence state in agreement with the aforementioned XPS and XAS results.38 As shown by differential charge density plots (Fig. 5b–d), electron transfer in symmetric FeN4 occurs exclusively within the FeN4 plane, with a symmetrical distribution of electron clouds. In contrast, the axial B atom in FeN4–B interacts with the Fe metal center, inducing pronounced charge redistribution in the axial region and breaking the symmetry of charge distribution within the FeN4 plane. Free energy diagrams for the ORR on both catalysts are plotted in Fig. S11 and S12. At U = 0 V (Fig. 5e), the RDS of FeN4–B exhibits a higher energy barrier (ΔG = −0.84 eV) compared to FeN4 (ΔG = −0.61 eV). Conversely, at U = 1.23 V (Fig. 5e), the ΔG for the rate-determining step in FeN4 (−0.64 eV) is significantly higher than that of FeN4–B (−0.24 eV).39 These results indicate that introducing axially coordinated B atoms at the FeN4 site can effectively reduce the energy barrier, thereby enhancing the intrinsic ORR activity.40 Moreover, FeN4–B shows a lower initial oxygen activation energy barrier compared to FeN4, indicating enhanced oxygen adsorption capability.
As revealed by the density of states (DOS) analysis (Fig. 5f and g), the incorporation of an axial B atom causes a shift in the d-band center from −0.98 eV for the FeN4 to −0.67 eV for the FeN4–B.41 The elevation of the d-band center facilitates electron excitation to anti-bonding orbitals, increasing their occupation and thereby enhancing the O2 adsorption capability at the Fe site.42,43 This upward shift is attributed to the increased occupancy of eg orbitals due to the introduction of a high-energy p-band, leading to enhanced unpaired electrons, which facilitate charge transfer and improve reaction kinetics.44 To investigate the spin states of FeN4–B/NC, we employed zero-field-cooled temperature-dependent (ZFC-T) magnetic susceptibility (χm) measurements. Through analysis of the temperature-dependent χ−1 data (Fig. 5h), we determined that FeN4–B exhibits an effective magnetic moment of 2.83 µB, with a t2g4eg1 electron configuration, revealing a middle-spin state (M.S.).45 The d-orbitals split into four distinct states: dx2−y2, dxy, and two-fold degenerate dxz/dyz. The dz2 and dxz/dyz orbitals undergo hybridization with the p orbitals of B atoms, resulting in bonding (σ and π) and antibonding (σ* and π*) states.46 In contrast, the horizontal d-orbitals (dx2−y2 and dxy) remain non-binding due to their inactivity.47 As shown in Fig. 5i, the projected density of states (PDOS) analysis reveals a strong orbital overlap between Fe 3d (dz2/dxz/dyz) and B 2p orbitals in FeN4–B, indicating a significant d–p coupling effect that generates both bonding (σ and π) and antibonding (σ* and π*) states (Fig. 5i).48,49 Based on the orbital hybridization theory and the observed PDOS overlap, we determine the optimal electron occupancy across these bonding and antibonding states.50 As illustrated in Fig. 5j, the vacant 2pz orbital of B− can accept an electron from the 3dz2 orbital of middle-spin Fe3+.51 This process involves the delocalization of electrons into σ orbitals, thereby preventing excessive occupancy of the antibonding σ* orbital and facilitating the reaction progress.52 Additionally, the half-filled 2px and 2py orbitals of B− enable moderate π* coupling with the 3dxz and 3dyz orbitals of Fe3+.53,54 This coupling stabilizes the Fe sites without fully occupying the d-electrons, thus optimizing the adsorption energies for *OOH and *OH species.55
Application of FeN4–B/NC in ZABs
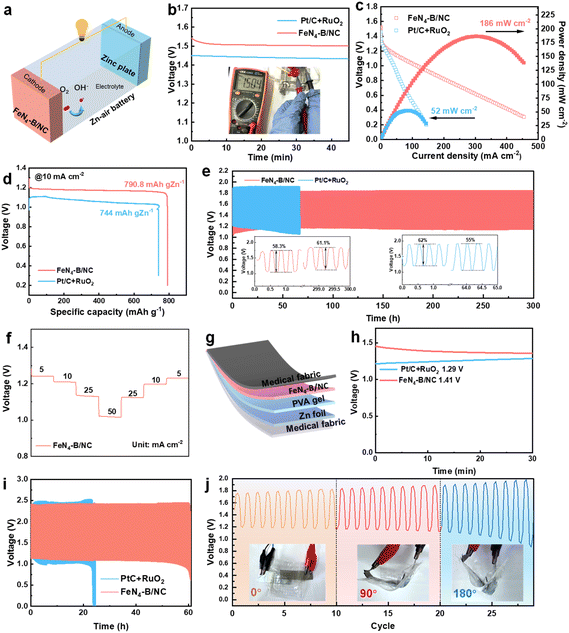
Motivated by the remarkable ORR and OER performance of FeN4–B/NC, liquid and flexible ZABs were developed by utilizing FeN4–B/NC as the air cathode and Zn foil as the anode (Fig. 6a). As illustrated in Fig. 6b, the ZAB utilizing FeN4–B/NC as the cathode demonstrates an open-circuit voltage (OCV) of 1.504 V, which exceeds that of the ZAB with a Pt/C + RuO2 cathode (1.450 V) within a 50-minute period. This reduction is attributed to the enhanced charge and discharge capabilities of FeN4–B/NC. Furthermore, the FeN4–B/NC-based ZAB achieves a maximum power density of 186 mW cm−2 (Fig. 6c), which considerably surpasses that of the commercial Pt/C + RuO2-based ZAB (52 mW cm−2). The FeN4–B/NC-based ZAB demonstrates a remarkable specific capacity of 790 mAh gZn−1, which not only meets the required standards, but also surpasses that of Pt/C + RuO2-based ZAB (744 mAh gZn−1) (Fig. 6d). The FeN4–B/NC-based ZAB demonstrates minimal decline in discharge/charge performance over 300-hour of cycling at 10 mA cm−2, with a 10-minute discharge and 10-minute charge process. In contrast, the Pt/C + RuO2-based ZAB experiences significant degradation within 65 hours, further validating the superior ORR/OER stability of the FeN4–B/NC-based cathode (Fig. 6e). In addition, the FeN4–B/NC-based ZAB demonstrates a notable and consistent round-trip efficiency, increasing from an initial 58.3% to a final 61.1%, highlighting its exceptional recharging performance (inset of Fig. 6e). According to Fig. 6f, the discharge voltages of FeN4–B/NC-based batteries at current densities of 5, 10, 25, and 50 mA cm−2 are 1.24, 1.20, 1.12, and 1.02 V, respectively. The outstanding stability of the FeN4–B/NC catalyst is the result of the inherent rigidity of the FeN4 structure, the additional stability provided by the axial B ligands, the efficient charge transfer ability, and the ideal adsorption strength ensured by the resulting intermediate spin state, all of which work together in synergy. Even after the current density is returned to 5 mA cm−2, the system sustains a stable discharge voltage of 1.24 V, highlighting its remarkable rate performance. Compared with other Fe SACs, the FeN4–B/NC-based ZABs exhibit superior performance and stability as evidenced in Table S4.To explore the feasibility of the FeN4–B/NC electrocatalyst in flexible and wearable electronics, an all-solid-state flexible ZAB (FZAB) was assembled, as shown in Fig. 6g. To simplify the testing procedure, we assembled a sandwich-type FZAB using a piece of zinc foil, an electrolyte membrane with a hydrogel, and a cathode with FeN4–B/NC through a layer-by-layer method. The flexible ZAB with the FeN4–B/NC cathode achieves a stable OCV of ∼1.41 V, surpassing the performance of the Pt/C + RuO2-based counterpart (Fig. 6h). Furthermore, the cycling stability of the FeN4–B based FZAB reached 62 hours at a current density of 2 mA cm−2 and a charge–discharge rate of 4 min per cycle (Fig. 6i), which exceeded that of the Pt/C based FZAB (26 hours). The FeN4–B/NC-based flexible ZAB exhibited stable charge/discharge voltages at various bending states (0°, 90° and 180°), further demonstrating the practical application potential of the FeN4–B/NC cathode (Fig. 6j).56
Conclusions
In summary, a “B-mediation” strategy has been developed to transform planar FeN4 into a pseudo-octahedral FeN4–B configuration, where an axial B atom coordinates with the FeN4 center. This approach modulates the electronic structure of Fe single atoms through d–p orbital hybridization, achieving an ORR half-wave potential of 0.915 V for FeN4–B/NC and an OER overpotential of 370 mV at 10 mA cm−2. In situ XAS confirmed the dynamic structural changes of the Fe sites during the reaction process, providing direct evidence for its outstanding performance. DFT calculations revealed that d–p orbital hybridization between the axial B atom and Fe centers shifts the d-band center closer to the Fermi level and optimizes *OH intermediate adsorption, thereby reducing the reaction energy barrier for the RDS and facilitating both ORR and OER processes. Experimental validation using a liquid ZAB setup demonstrated the practical potential of FeN4–B/NC, delivering remarkable power density and capacity (186 mW cm−2 and 790.8 mAh gZn—1, respectively), along with robust stability over 300 hours of operation. Furthermore, FeN4–B/NC showed promise for flexible ZABs with an open-circuit voltage of 1.41 V and stable charge–discharge cycling performance under various bending angles.Author contributions
All of the authors contributed to the literature search, writing and editing of this review.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental methods, electrochemical evaluation, Zn–air battery test, DFT methods, Fig. S1–S12 and Tables S1–S4. See DOI: https://doi.org/10.1039/d5sc07064c.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22075099), and the Education Department of Jilin Province (No. JJKH20250070BS). The authors acknowledge the BL11B beamline (31124.02.SSRF.BL11B) of the Shanghai Synchrotron Radiation Facility for providing the XAS beamtime.Notes and references
- Y. Wang, J. Wu, S. Tang, J. Yang, C. Ye, J. Chen, Y. Lei and D. Wang, Synergistic Fe–Se atom pairs as bifunctional oxygen electrocatalysts boost low-temperature rechargeable zn–air battery, Angew. Chem., Int. Ed., 2023, 62, e202219191 CrossRef CAS PubMed.
- T. Tang, W.-J. Jiang, X.-Z. Liu, J. Deng, S. Niu, B. Wang, S.-F. Jin, Q. Zhang, L. Gu, J.-S. Hu and L.-J. Wan, Metastable rock salt oxide-mediated synthesis of high-density dual-protected M@NC for long-life rechargeable zinc–air batteries with record power density, J. Am. Chem. Soc., 2020, 142, 7116–7127 CrossRef CAS PubMed.
- J. Fu, R. Liang, G. Liu, A. Yu, Z. Bai, L. Yang and Z. Chen, Recent progress in electrically rechargeable zinc–air batteries, Adv. Mater., 2019, 31, 1805230 CrossRef PubMed.
- J. Li, S. Chen, N. Yang, M. Deng, S. Ibraheem, J. Deng, J. Li, L. Li and Z. Wei, Ultrahigh-loading zinc single-atom catalyst for highly efficient oxygen reduction in both acidic and alkaline media, Angew. Chem., Int. Ed., 2019, 58, 7035–7039 CrossRef CAS PubMed.
- A. Han, W. Sun, X. Wan, D. Cai, X. Wang, F. Li, J. Shui and D. Wang, Construction of Co4 atomic clusters to enable Fe–N4 motifs with highly active and durable oxygen reduction performance, Angew. Chem., Int. Ed., 2023, 62, e202303185 CrossRef CAS PubMed.
- Z. Miao, S. Li, C. Priest, T. Wang, G. Wu and Q. Li, Effective approaches for designing stable M–Nx/C oxygen-reduction catalysts for proton-exchange-membrane fuel cells, Adv. Mater., 2022, 34, 2200595 CrossRef CAS PubMed.
- X. Wan, X. Liu, Y. Li, R. Yu, L. Zheng, W. Yan, H. Wang, M. Xu and J. Shui, Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells, Nat. Catal., 2019, 2, 259–268 CrossRef CAS.
- H. Zhang, H.-C. Chen, S. Feizpoor, L. Li, X. Zhang, X. Xu, Z. Zhuang, Z. Li, W. Hu, R. Snyders, D. Wang and C. Wang, Tailoring oxygen reduction reaction kinetics of Fe–N–C catalyst via spin manipulation for efficient zinc–air batteries, Adv. Mater., 2024, 36, 2400523 CrossRef CAS PubMed.
- Z. Yang, Y. Wang, M. Zhu, Z. Li, W. Chen, W. Wei, T. Yuan, Y. Qu, Q. Xu, C. Zhao, X. Wang, P. Li, Y. Li, Y. Wu and Y. Li, Boosting oxygen reduction catalysis with Fe–N4 sites decorated porous carbons toward fuel cells, ACS Catal., 2019, 9, 2158–2163 CrossRef CAS.
- Q. Qu, Y. Mao, S. Ji, J. Liao, J. Dong, L. Wang, Q. Wang, X. Liang, Z. Zhang, J. Yang, H. Li, Y. Zhou, Z. Wang, G. I. N. Waterhouse, D. Wang and Y. Li, Engineering the lewis acidity of fe single-atom sites via atomic-level tuning of spatial coordination configuration for enhanced oxygen reduction, J. Am. Chem. Soc., 2025, 147, 6914–6924 CrossRef CAS PubMed.
- M. Liu, Y. Liu, X. Zhang, L. Li, X. Xue, M. Humayun, H. Yang, L. Sun, M. Bououdina, J. Zeng, D. Wang, R. Snyders, D. Wang, X. Wang and C. Wang, Altering the symmetry of Fe–N–C by axial cl-mediation for high-performance zinc–air batteries, Angew. Chem., Int. Ed., 2025, 64, e202504923 CrossRef CAS PubMed.
- J. Qiao, C. Lu, L. Kong, J. Zhang, Q. Lin, H. Huang, C. Li, W. He, M. Zhou and Z. Sun, Spin engineering of Fe–N–C by axial ligand modulation for enhanced bifunctional oxygen catalysis, Adv. Funct. Mater., 2024, 34, 2409794 CrossRef CAS.
- L. Peng, J. Yang, Y. Yang, F. Qian, Q. Wang, D. Sun-Waterhouse, L. Shang, T. Zhang and G. I. N. Waterhouse, Mesopore-rich Fe–N–C catalyst with FeN4–O–NC single-atom sites delivers remarkable oxygen reduction reaction performance in alkaline media, Adv. Mater., 2022, 34, 2202544 CrossRef CAS PubMed.
- X. Wang, Z.-Y. Yi, Y.-Q. Wang and D. Wang, Molecular evidence for the axial coordination effect of atomic iodine on Fe–N4 sites in oxygen reduction reaction, Angew. Chem., Int. Ed., 2025, 64, e202413673 CrossRef CAS PubMed.
- K.-M. Zhao, S. Liu, Y.-Y. Li, X. Wei, G. Ye, W. Zhu, Y. Su, J. Wang, H. Liu, Z. He, Z.-Y. Zhou and S.-G. Sun, Insight into the mechanism of axial ligands regulating the catalytic activity of Fe–N4 sites for oxygen reduction reaction, Adv. Energy Mater., 2022, 12, 2103588 CrossRef CAS.
- X.-W. Lv, J. Gong, S. Wang, X. Yan, C. Sun, X. Hu, Z. Lai, Y. Liu, H. Wang, Z.-Y. Yuan and J. Geng, Engineering orbital hybridization in advanced electrocatalysts for energy conversion: fundamentals, modulations, and perspectives, Adv. Energy Mater., 2025, 15, 2501129 CrossRef CAS.
- A. Zitolo, V. Goellner, V. Armel, M.-T. Sougrati, T. Mineva, L. Stievano, E. Fonda and F. Jaouen, Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials, Nat. Mater., 2015, 14, 937–942 CrossRef CAS PubMed.
- L. Wang, L. Yang, X. Zhao, H. Ma, B. Pang, L. Duan, K. Zeng, L. Liu, A. Chen and H. Guo, Using CrN4 moiety to weaken the dissociation barrier of hydroxyl on adjacent single iron atom for efficient oxygen reduction, Energy Storage Mater., 2025, 74, 103927 CrossRef.
- C. Liu, R. Yang, J. Wang, B. Liu, X. Chang, P. Feng, X. Zhang, L. Zhong, X. Zhao, L. Niu, S. Gan, Y. Xi, M. Huang and H. Wang, Synergistic catalysts with Fe single atoms and fe3c clusters for accelerated oxygen adsorption kinetics in oxygen reduction reaction, Angew. Chem., Int. Ed., 2025, 64, e202501266 CrossRef CAS PubMed.
- S. Zhang, J. Wu, M. Zheng, X. Jin, Z. Shen, Z. Li, Y. Wang, Q. Wang, X. Wang, H. Wei, J. Zhang, P. Wang, S. Zhang, L. Yu, L. Dong, Q. Zhu, H. Zhang and J. Lu, Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia, Nat. Commun., 2023, 14, 3634 CrossRef CAS PubMed.
- W. Liu, E. Shi, H. Wu, Y. Liang, M. Chen, H. Zhang, R. Zhang, X. Li, Y. Wang and L. Zhang, Spatially axial boron coordinated single-atom nanozymes with boosted multi-enzymatic performances for periodontitis treatment, Adv. Funct. Mater., 2024, 34, 2403386 CrossRef CAS.
- Y. Zhou, G. Chen, Q. Wang, D. Wang, X. Tao, T. Zhang, X. Feng and K. Müllen, Fe–N–C electrocatalysts with densely accessible Fe–N4 sites for efficient oxygen reduction reaction, Adv. Funct. Mater., 2021, 31, 2102420 CrossRef CAS.
- Y. Tan, J. Fu, T. Luo, K. Liu and M. Liu, Theoretical insights into the selectivity of single-atom Fe–N–C catalysts for electrochemical nox reduction, J. Am. Chem. Soc., 2025, 147, 4937–4944 CrossRef CAS PubMed.
- P. Yin, T. Yao, Y. Wu, L. Zheng, Y. Lin, W. Liu, H. Ju, J. Zhu, X. Hong, Z. Deng, G. Zhou, S. Wei and Y. Li, Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts, Angew. Chem., Int. Ed., 2016, 55, 10800–10805 CrossRef CAS PubMed.
- S. Wu, X. Liu, H. Mao, J. Zhu, G. Zhou, J. Chi, Z. Wu and L. Wang, Unraveling the tandem effect of nitrogen configuration promoting oxygen reduction reaction in alkaline seawater, Adv. Energy Mater., 2024, 14, 2400183 CrossRef CAS.
- Y. Hu, S. Niu, Z. Zhang, T. Chao, T. Zhao, G. Yu, F. Zhou, X. Liang, H. Jin, Z. Yang, D. Wang, W. Chen and Y. Li, Axial chlorine engineering of p-block antimony atomic sites boosts oxygen reduction, J. Am. Chem. Soc., 2025, 147(24), 21231–21240 CrossRef CAS PubMed.
- M. Tong, F. Sun, G. Xing, C. Tian, L. Wang and H. Fu, Potential dominates structural recombination of single atom Mn sites for promoting oxygen reduction reaction, Angew. Chem., Int. Ed., 2023, 62, e202314933 CrossRef CAS PubMed.
- B. Tang, Q. Ji, X. Zhang, R. Shi, J. Ma, Z. Zhuang, M. Sun, H. Wang, R. Liu, H. Liu, C. Wang, Z. Guo, L. Lu, P. Jiang, D. Wang and W. Yan, Symmetry breaking of FeN4 moiety via edge defects for acidic oxygen reduction reaction, Angew. Chem., Int. Ed., 2025, 64, e202424135 CrossRef CAS PubMed.
- Y. Dai, B. Liu, Z. Zhang, P. Guo, C. Liu, Y. Zhang, L. Zhao and Z. Wang, Tailoring the d-orbital splitting manner of single atomic sites for enhanced oxygen reduction, Adv. Mater., 2023, 35, 2210757 CrossRef CAS PubMed.
- F. Wang, R. Zhang, Y. Zhang, Y. Li, J. Zhang, W. Yuan, H. Liu, F. Wang and H. L. Xin, Modulating electronic structure of atomically dispersed nickel sites through boron and nitrogen dual coordination boosts oxygen reduction, Adv. Funct. Mater., 2023, 33, 2213863 CrossRef CAS.
- C. Hu, G. Xing, W. Han, Y. Hao, C. Zhang, Y. Zhang, C.-H. Kuo, H.-Y. Chen, F. Hu, L. Li and S. Peng, Inhibiting demetalation of Fe–N–C via mn sites for efficient oxygen reduction reaction in zinc–air batteries, Adv. Mater., 2024, 36, 2405763 CrossRef CAS PubMed.
- J. Zhang, J. Chen, Y. Jing, X. Xu, C. Liu and J. Jia, Fe, Co, N-doped composite as an efficient catalyst for oxygen reduction and oxygen evolution reaction, Mater. Today Chem., 2025, 44, 102531 CrossRef CAS.
- X. Wang, S. Zhang, M. Li, Y. Wan, Z. Sun, R. Li, Z. Zhu, H. Wu, Z. Zhao, S. Hu, F. Bu, D. Chao and W. Luo, Tailored design of mesoporous metal organic framework single crystals by kinetics-mediated micelle assembly for efficient asymmetrical single-atom catalysis, Adv. Mater., 2025, 37, 2500370 CrossRef CAS PubMed.
- H. Niu, L. Huang, Y. Qin, R. Qi, B. Mei, D. Wu, F.-M. Li, B. You, Q. Li, Y. Yao, Z. Wang, T. Yao, S. Ding, W. Guo, Y. Chen, Y. Su, F. Song and B. Y. Xia, Hydrogen peroxide spillover on platinum–iron hybrid electrocatalyst for stable oxygen reduction, J. Am. Chem. Soc., 2024, 146, 22650–22660 CrossRef CAS PubMed.
- T. Tang, X. Xu, X. Bai, C. Hou, T. Gan, Z. Wang and J. Guan, A triatomic cobalt catalyst for oxygen electrocatalysis, Angew. Chem., Int. Ed., 2025, 64, e202503019 CrossRef CAS PubMed.
- Y. Zhao, H. Wu, Y. Wang, L. Liu, W. Qin, S. Liu, J. Liu, Y. Qin, D. Zhang, A. Chu, B. Jia, X. Qu and M. Qin, Sulfur coordination engineering of molybdenum single-atom for dual-functional oxygen reduction/evolution catalysis, Energy Storage Mater., 2022, 50, 186–195 CrossRef.
- Y. Yang, Y. Kang, H. Zhao, X. Dai, M. Cui, X. Luan, X. Zhang, F. Nie, Z. Ren and W. Song, An interfacial electron transfer on tetrahedral NiS2/NiSe2 heterocages with dual-phase synergy for efficiently triggering the oxygen evolution reaction, Small, 2020, 16, 1905083 CrossRef CAS PubMed.
- Y. Yan, Z. Ran, T. Zeng, X. Wen, H. Xu, R. Li, C. Zhao and C. Shu, Interfacial electron redistribution of hydrangea-like Nio@Ni2p heterogeneous microspheres with dual-phase synergy for high-performance lithium–oxygen battery, Small, 2022, 18, 2106707 CrossRef CAS PubMed.
- J.-Y. Yue, Z.-X. Pan, Y. Guo, P. Yang and B. Tang, Distinct oxygen reduction pathways for solar H2O2 production by regulating unsaturated bonds in covalent organic frameworks, Chem. Sci., 2025, 16, 13883–13892 RSC.
- Z. Li, S. Ji, C. Xu, L. Leng, H. Liu, J. H. Horton, L. Du, J. Gao, C. He, X. Qi, Q. Xu and J. Zhu, Engineering the electronic structure of single-atom iron sites with boosted oxygen bifunctional activity for zinc–air batteries, Adv. Mater., 2023, 35, 2209644 CrossRef CAS PubMed.
- X. Wang, J. Wang, P. Wang, L. Li, X. Zhang, D. Sun, Y. Li, Y. Tang, Y. Wang and G. Fu, Engineering 3d–2p–4f gradient orbital coupling to enhance electrocatalytic oxygen reduction, Adv. Mater., 2022, 34, 2206540 CrossRef CAS PubMed.
- J.-H. Zheng, G. Li, J.-M. Zhang, N. Cheng, L.-F. Ji, J. Yang, J. Zhang, B.-W. Zhang, Y.-X. Jiang and S.-G. Sun, General strategy for evaluating the d-band center shift and ethanol oxidation reaction pathway towards pt-based electrocatalysts, Sci. China: Chem., 2023, 66, 279–288 CrossRef CAS.
- L. Yang, R. Grzeschik, S. Schlücker and W. Xie, Contact electrification as an emerging strategy for controlling the performance of metal nanoparticle catalysts, Chem.–Eur. J., 2024, 30, e202401718 CrossRef CAS PubMed.
- R. Cheng, X. He, M. Jiang, X. Shao, W. Tang, B. Ran, H. Li and C. Fu, F–p–d gradient orbital coupling induced spin state enhancement of atomic fe sites for efficient and stable oxygen reduction reaction, Adv. Funct. Mater., 2025, 35, 2425138 CrossRef CAS.
- Z. Li, Z. Zhuang, F. Lv, H. Zhu, L. Zhou, M. Luo, J. Zhu, Z. Lang, S. Feng, W. Chen, L. Mai and S. Guo, The marriage of the fen4 moiety and mxene boosts oxygen reduction catalysis: Fe 3d electron delocalization matters, Adv. Mater., 2018, 30, 1803220 CrossRef PubMed.
- Y. Zhou, K. Yin, Y. Huang, J. Li, A. Zhu, D. Lin, G. Gan, J. Zhang, K. Liu, T. Zhang, K. Liu, C. Luan, H. Yang, H. Chen, S. Guo, W. Zhang and G. Hong, D-orbital reconstruction achieves low charge overpotential in li-oxygen batteries, Nat. Commun., 2025, 16, 3353 CrossRef CAS PubMed.
- Z. Han, S. Zhao, J. Xiao, X. Zhong, J. Sheng, W. Lv, Q. Zhang, G. Zhou and H.-M. Cheng, Engineering d–p orbital hybridization in single-atom metal-embedded three-dimensional electrodes for li–s batteries, Adv. Mater., 2021, 33, 2105947 CrossRef CAS PubMed.
- H. Cai, S. He, H. Yang, Q. Huang, F. Luo, Q. Hu, X. Zhang and C. He, Highly exposed ultra-small high-entropy sulfides with d–p orbital hybridization for efficient oxygen evolution, Adv. Mater., 2025, 37, 2508610 CrossRef CAS PubMed.
- M. Tamtaji, Q. Peng, T. Liu, X. Zhao, Z. Xu, P. R. Galligan, M. D. Hossain, Z. Liu, H. Wong, H. Liu, K. Amine, Y. Zhu, W. A. Goddard Iii, W. Wu and Z. Luo, Non-bonding interaction of dual atom catalysts for enhanced oxygen reduction reaction, Nano Energy, 2023, 108, 108218 CrossRef CAS.
- H. Long, X. Zhang, Z. Zhang, J. Zhang, J. Yu and H. Yu, Fine-tuning d–p hybridization in Ni–Bx cocatalyst for enhanced photocatalytic H2 production, Nat. Commun., 2025, 16, 946 CrossRef CAS PubMed.
- B. Jing, Q. Zhang, M. Liu, S. Yang, J. Zhang, S. Qiu, I. Sirés and F. Deng, Magnetically-altered eg-orbital occupancy to boost the two-electron oxygen reduction electrocatalysis for faster water decontamination, Appl. Catal., B, 2025, 369, 125149 CrossRef CAS.
- X. Yue, L. Cheng, E. Baráth, R. V. Jagadeesh and Q. Xiang, Deciphering orbital hybridization in heterogeneous catalysis, Electron, 2024, 2, e16 CrossRef CAS.
- Y. Qi, K. Song, Q. Liang, X. Zhou, M. Liu, W. Li, F. Liu, Z. Jiang, Q. Gu, Z. Chen, B. Zhang and W. Zhang, Asymmetric b-coordination stimulated high-spin cobalt boosts orr, Nano Res., 2025, 18, 94907278 CrossRef.
- X. Wang, J. Zhang, P. Wang, L. Li, H. Wang, D. Sun, Y. Li, Y. Tang, X. F. Lu, Y. Wang and G. Fu, Terbium-induced cobalt valence-band narrowing boosts electrocatalytic oxygen reduction, Energy Environ. Sci., 2023, 16, 5500–5512 RSC.
- S. Ning, M. Li, X. Wang, D. Zhang, B. Zhang, C. Wang, D. Sun, Y. Tang, H. Li, K. Sun and G. Fu, Importing antibonding-orbital occupancy through Pd–O–Gd bridge promotes electrocatalytic oxygen reduction, Angew. Chem., Int. Ed., 2023, 62, e202314565 CrossRef CAS PubMed.
- X. Zhong, Z. Zheng, J. Xu, X. Xiao, C. Sun, M. Zhang, J. Ma, B. Xu, K. Yu, X. Zhang, H.-M. Cheng and G. Zhou, Flexible zinc–air batteries with ampere-hour capacities and wide-temperature adaptabilities, Adv. Mater., 2023, 35, 2209980 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |