Open Access Article
Open Access ArticleHeavy-atom effect regulating room temperature phosphorescence in hybrid metal halide glasses
Linyuan
Lian
,
Ming
Ai
,
Daming
Xiong
,
Tao
Liang
,
Jibin
Zhang
 ,
Mochen
Jia
,
Mochen
Jia
 ,
Ying
Liu
,
Zhuangzhuang
Ma
,
Ying
Liu
,
Zhuangzhuang
Ma
 ,
Xu
Chen
,
Xu
Chen
 ,
Yanbing
Han
,
Yanbing
Han
 ,
Yongtao
Tian
,
Yongtao
Tian
 ,
Xinjian
Li
and
Zhifeng
Shi
,
Xinjian
Li
and
Zhifeng
Shi
 *
*
Key Laboratory of Materials Physics of Ministry of Education, School of Physics, Zhengzhou University, Zhengzhou 450052, China. E-mail: shizf@zzu.edu.cn
First published on 3rd November 2025
Abstract
Room-temperature phosphorescence (RTP) in hybrid metal halide glasses offers promising applications in optoelectronics and anti-counterfeiting, yet achieving tunable RTP properties remains challenging. Here, we report a solvent-assisted rapid evaporation method to synthesize a series of zero-dimensional (0D) butyltriphenylphosphonium-based (BuTPP+) hybrid metal halide glasses with the composition (BuTPP)2MCl2X2 (M = Zn, Cd; X = Cl, Br, I). By incorporating optically inert heavy-atom-containing metal halide units [MCl2X2]2−, we demonstrate precise regulation of RTP lifetimes via the heavy-atom effect, with lifetimes decreasing from 608.6 ms for (BuTPP)2ZnCl4 to 146 ms for (BuTPP)2CdCl2Br2 as the atomic number of inorganic metal halide units increases. Notably, when the atomic number exceeds 170 (e.g., [ZnCl2I2]2− and [CdCl2I2]2−), self-trapped exciton (STE) emissions dominate, completely suppressing organic afterglow. Furthermore, (BuTPP)2ZnCl4 glass exhibits excitation-dependent multicolor phosphorescence due to aggregate cluster luminescence. These glasses showcase dual-mode emissions (RTP/STE) and are successfully applied in shape-controllable anti-counterfeiting and high-resolution X-ray scintillation imaging (10 lp mm−1). This work provides a facile vitrification strategy and design principles for hybrid RTP materials with tailored photophysical properties.
1 Introduction
Room temperature phosphorescence (RTP) is a fascinating optical phenomenon characterized by long-lived emission that can persist from several microseconds to even seconds under ambient conditions after the cessation of excitation.1–4 Owing to its distinct photophysical features, such as extended luminescence decay lifetime and large Stokes shift, it has garnered significant attention for a wide range of applications, including anti-counterfeiting, bioimaging, and light-emitting devices.5–8 Over the last few years, pure organic phosphors have been extensively studied due to their tunable molecular structures, easy modification and processing, excellent biocompatibility, and high mechanical flexibility.1,3,9–11 Nevertheless, the intrinsic weak spin–orbit coupling (SOC) effect and low radiative recombination rate of organic phosphors lead to low phosphorescence efficiency, which limits their practical applications.Recently, zero-dimensional (0D) organic–inorganic hybrid metal halides have emerged as promising candidates for RTP materials by integrating the advantages of both organic and inorganic constituents.12–19 In particular, the intrinsic RTP characteristics arising from triplet excitons of organic cations contribute significantly to the diversification of luminescent properties of hybrid metal halides. Meanwhile, the optically inert metal halide units provide a rigid structural framework and introduce the heavy-atom effect, which effectively enhances the afterglow emission of the organic components in these hybrid metal halides.12,13 Benefiting from these combined optical and structural merits, 0D organic–inorganic hybrid metal halides are considered to be one of the most desirable RTP materials. For example, Ma et al. reported a series of 0D TPP2ZnX4 (X = Cl, Br) exhibiting efficient RTP with tunable lifetimes ranging from 215.5 to 37.2 ms.13 Similarly, Yan and co-workers developed a 0D (Ph4P)2Cd2Br6, which demonstrates excellent luminescent stability over a broad temperature range (100–320 K), along with a high RTP quantum yield of 62.79% and a lifetime of 37.85 ms.15 To date, hybrid metal halide RTP materials have predominantly existed in the form of single crystals and powders.12,13 However, these forms suffer from poor processability, and face challenges in constructing large-scale, shape-controllable devices required for practical applications.
Hybrid metal halide glasses, are an emerging class of luminescent materials, offer distinct advantages over their crystalline counterparts.14,20–22 Notably, they feature high transparency, excellent processability, high mechanical rigidity and durability, making them highly attractive in photonic fields.20,21 In addition, the closely packed three-dimensional (3D) networks in these hybrid glasses provide a rigid and confined microenvironment that effectively suppresses non-radiative relaxation of triplet excitons, thereby promoting RTP.21,23–25 Moreover, hybrid glasses can serve as prospective host matrices with high structural tolerance, enabling the incorporation of various metal halide structural units.20,22,26–28 For example, the emissive metal halide structure units, such as [MnBr4]2− tetrahedrons and [SbCl5]2− pyramids, have been successfully incorporated into the hybrid glasses, exhibiting bright green and orange emissions, respectively.20,22,26,29 However, as long as the emissive metal halide units are present in 0D hybrid glasses, the energy transfer from organic triplet states to inorganic emission centers with shorter lifetimes will occur, as a result, the afterglow emission of organic components is suppressed in these glasses. Therefore, introducing the optically inert metal halide units to them is beneficial to realize the RTP in 0D hybrid metal halide glasses. Furthermore, these hybrid glasses provide an excellent platform for tuning the intrinsic RTP properties of organic cations by leveraging the heavy-atom effect of inert inorganic metal halide units.13 However, the on-demand manipulation of RTP properties to achieve desirable phosphorescent performance remains a formidable challenge. Therefore, in depth studies on how inorganic heavy-atom units influence the RTP properties of organic components are essential for the rational design of advanced RTP materials.
However, the fabrication of glassy hybrid metal halides remains challenging due to their relatively weak glass-forming ability.30–32 This limitation primarily arises from the premature dissociation of organic components before the melting of precursors using the mainstream melt-quenching method, as well as a strong propensity for recrystallization during the quenching process.32 Consequently, the development of alternative vitrification strategies, particularly those that circumvent the melting process, is of great significance for advancing multifunctional hybrid metal halide glasses.
In this work, we present a facile solvent-assisted rapid evaporation method to synthesis a series of 0D butyltriphenylphosphonium-based (BuTPP+) hybrid metal halide glasses with a well-defined composition of (BuTPP)2MCl2X2. The BuTPP+ is selected as the organic cation, due to its characteristic long-lived phosphorescence emission, a series of optically inert, heavy-atom-containing metal halide anions [MCl2X2]2− (such as [ZnCl4]2−, [CdCl4]2−, [ZnCl2Br2]2−, [CdCl2Br2]2−, [ZnCl2I2]2−, and [CdCl2I2]2−), are introduced to tune the RTP properties of (BuTPP)2MCl2X2 glasses. Significantly, as the atomic number of inorganic metal halide units increases, the phosphorescence decay lifetime of the triplet state of BuTPP+ gradually decreases due to the heavy-atom effect. More importantly, when the atomic number of inorganic metal halide unit exceeds 170, (BuTPP)2MCl2X2 glasses (as in the cases of [ZnCl2I2]2−, and [CdCl2I2]2−) exhibit the self-trapped exciton (STE) emissions with faster decay lifetimes, while the afterglow emission of BuTPP+ is completely suppressed. Interestingly, aggregate cluster luminescence is observed in (BuTPP)2ZnCl4 glass, exhibiting excitation-dependent multicolor phosphorescence. These novel 0D hybrid metal halide glasses, featuring both long-lived RTP and short-lived STE emissions, are demonstrated in applications such as shape-controllable anti-counterfeiting and X-ray scintillation imaging. Fig. 1d summarized the key features of this study. This work not only provides an effective strategy for synthesizing hybrid metal halide glasses but also offers valuable insights for designing hybrid RTP materials with tunable photophysical properties.
2 Results and discussion
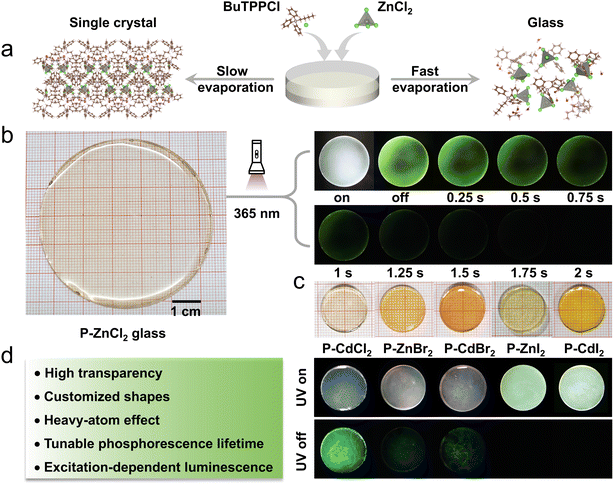
In this work, we present a solvent-assisted rapid evaporation method that bypasses the conventional melting process for the fabrication of glass materials. As a representative system, (BuTPP)2ZnCl4 was selected to investigate the glass formation behavior (Fig. 1a). In a typical synthesis, BuTPPCl and ZnCl2 precursors at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
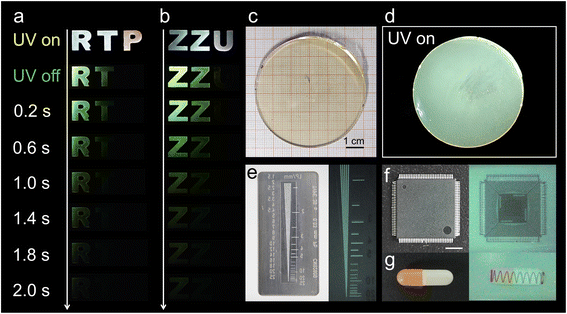
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio were dissolved in a mixed solvent of dimethyl sulfoxide (DMSO) and deionized water, yielding a homogeneous and transparent precursor solution. This solution was then transferred to a glass dish and heated to 200 °C, triggering rapid solvent evaporation. The glass formation process proceeds through the following three distinct stages: (1) solution concentration: initially, at 200 °C, rapid solvent evaporation reduces the solvent content, drawing the building blocks into closer proximity and facilitating non-covalent interactions such as hydrogen bonding and ionic interactions. (2) Viscoelastic transition: continued evaporation increases the solution's viscosity, transitioning it into a viscoelastic glassy liquid analogous to a supercooled liquid in the melt-quenching process (Fig. S1a).33 This elevated viscosity kinetically inhibits crystallization by limiting molecular mobility. (3) Vitrification: ongoing solvent removal ultimately yields a homogeneous, transparent glass with a diameter of 6 cm (Fig. 1b). This strategy enables the rapid and straightforward synthesis of a series of hybrid metal halide glasses with high transparency and tunable RTP properties by incorporating various optically inert metal halide units (Fig. 1c). Control experiments reveal that slower evaporation at room temperature leads to crystallization of (BuTPP)2ZnCl4 (Fig. 1a), underscoring the critical role of evaporation rate in achieving vitrification.
1 molar ratio were dissolved in a mixed solvent of dimethyl sulfoxide (DMSO) and deionized water, yielding a homogeneous and transparent precursor solution. This solution was then transferred to a glass dish and heated to 200 °C, triggering rapid solvent evaporation. The glass formation process proceeds through the following three distinct stages: (1) solution concentration: initially, at 200 °C, rapid solvent evaporation reduces the solvent content, drawing the building blocks into closer proximity and facilitating non-covalent interactions such as hydrogen bonding and ionic interactions. (2) Viscoelastic transition: continued evaporation increases the solution's viscosity, transitioning it into a viscoelastic glassy liquid analogous to a supercooled liquid in the melt-quenching process (Fig. S1a).33 This elevated viscosity kinetically inhibits crystallization by limiting molecular mobility. (3) Vitrification: ongoing solvent removal ultimately yields a homogeneous, transparent glass with a diameter of 6 cm (Fig. 1b). This strategy enables the rapid and straightforward synthesis of a series of hybrid metal halide glasses with high transparency and tunable RTP properties by incorporating various optically inert metal halide units (Fig. 1c). Control experiments reveal that slower evaporation at room temperature leads to crystallization of (BuTPP)2ZnCl4 (Fig. 1a), underscoring the critical role of evaporation rate in achieving vitrification.
The synthesis route followed the same procedure as that for the (BuTPP)2ZnCl4 glass, except that ZnCl2 was excluded. In this case, glass formation was not achieved, and the material crystallized instead (Fig. S1b), indicating that the A-site organic molecules alone cannot form a glassy phase. Additionally, we attempted to directly prepare the glass by uniformly mixing BuTPPCl and ZnCl2, followed by heating the mixture to 200 °C. However, as shown in Fig. S1c, the mixture did not fully melt under these conditions, and no transparent glass was obtained after cooling to room temperature, indicating that the conventional direct melt-quenching method is insufficient to achieve vitrification in this system. The glass-forming ability of P-ZnCl2 can be attributed to three main factors: (1) Hydrogen bonding interactions: strong multiple hydrogen bonds among DMSO, H2O, BuTPP+, and [ZnCl4]2− stabilize the disordered network. Rapid solvent evaporation prevents molecular rearrangement, favoring glass formation.33 (2) Bulky organic cations: the glass-forming ability is strongly correlated with crystallization resistance. Incorporating large organic cations with high steric hindrance effectively suppresses crystallization.22 Previous studies have shown that small cations promote nucleation and crystallization during quenching, whereas bulky cations favor glass formation.26 In this work, bulky organic cations BuTPP+, containing three benzene rings and one branched group, provides significant steric hindrance that inhibits crystallization. (3) High viscosity: the high viscosity of the glass liquid further restricts the movement of organic and inorganic species, preventing crystallization during quenching. These three factors collectively enable the successful formation of the P-ZnCl2 glass.
Fig. 1b and c show images of the as-prepared (BuTPP)2ZnCl4 (abbreviated as P-ZnCl2), (BuTPP)2CdCl4 (P-CdCl2), (BuTPP)2ZnCl2Br2 (P-ZnBr2), (BuTPP)2CdCl2Br2 (P-CdBr2), (BuTPP)2ZnCl2I2 (P-ZnI2), and (BuTPP)2CdCl2I2 (P-CdI2) glasses under ambient light, ultraviolet (UV) irradiation (365 nm), and UV off. Upon UV irradiation, P-ZnCl2, P-CdCl2, P-ZnBr2, and P-CdBr2 exhibit bright white-light emission, whereas P-ZnI2 and P-CdI2show cyan emission. Notably, once the UV lamp is turned off, the emissions disappeared immediately for P-ZnI2 and P-CdI2 glasses, while the green afterglow emissions from P-ZnCl2, P-CdCl2, P-ZnBr2, and P-CdBr2 were observed with naked eyes. These observations highlight the critical influence of the heavy-atom effect from the inorganic metal halide units on the emission characteristics, particularly in modulating RTP behavior in hybrid metal halide glasses. The crystal structure of P-ZnCl2 single crystals was determined by single-crystal X-ray diffraction (SCXRD). As shown in Fig. 1a, P-ZnCl2 crystal crystallizes in the orthorhombic space group Pca21, in which the [ZnCl4]2− tetrahedrons are completely isolated from each other and periodically embedded in the matrix of large organic cations BuTPP+, forming a 0D crystal structure at the molecular level. The Zn2+ ion adopts a typical tetrahedral coordination geometry, bonded to four Cl atoms with an average Zn–Cl bond length of 2.28 Å and an average Cl–Zn–Cl bond angle of 109.47°, consistent with previously reported [ZnCl4]2− tetrahedrons in other Zn-based metal halides.16,17 More detailed crystal diffraction data are summarized in Table S1.
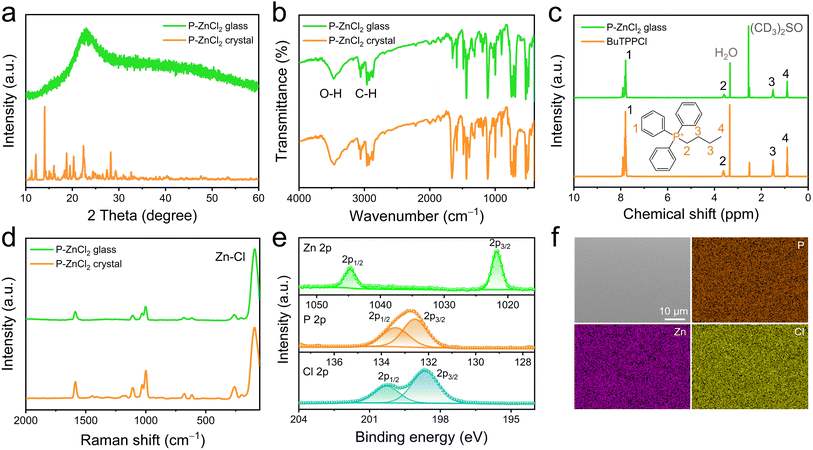
The formation of P-ZnCl2 glass was confirmed by powder X-ray diffraction (PXRD). As shown in Fig. 2a, in contrast to the crystal counterpart that displays sharp characteristic diffraction peaks, the as-prepared P-ZnCl2 glass exhibits a broad diffuse diffraction band, indicating the highly disordered amorphous structure.20 To further investigate the organic molecular structure within the P-ZnCl2 glass, Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H NMR) spectroscopy were conducted. The FTIR spectra of the P-ZnCl2 glass and crystal samples are nearly identical (Fig. 2b), suggesting that the chemical structure of the organic cations BuTPP+ remains intact after vitrification. Specifically, the absorption bands between 400 and 1600 cm−1 are assigned to the C–H stretching vibrations of phenyl rings, while the peaks near 3000 cm−1 correspond to the sp3-hybridized C–H bonds in butyl chains.34 Additionally, a broad band centered at 3470 cm−1 is attributed to the O–H group vibrations, indicating the presence of hydrogen bonding interactions within the glass matrix.2 The 1H NMR spectrum further confirms the structural integrity of organic cations BuTPP+ in P-ZnCl2 glass, as shown in Fig. 2c. After excluding the signals from the solvent ((CD3)2SO) and water (H2O), the spectrum of P-ZnCl2 glass exhibits four distinct proton environments. These are attributed to aromatic protons (δ1 = 7.5–8.1 ppm), and adjacent to the triphenylphosphonium core protons (δ2 = 3.4–3.7), interstitial protons (δ3 = 1.3–1.7), and terminal protons (δ4 = 0.7–1.0) of the butyl chain, with integration areas of 15.00, 2.00, 4.00, and 3.00, respectively. The integration ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:4
2:4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 is consistent with the theoretical proton counts, confirming that the structure of organic cations BuTPP+ is preserved during glass formation.35
3 is consistent with the theoretical proton counts, confirming that the structure of organic cations BuTPP+ is preserved during glass formation.35
Raman spectroscopy was employed to investigate the chemical bonding characteristics in P-ZnCl2 glass. As shown in Fig. 2d, the nearly identical peak patterns in the Raman spectra for both the glass and crystal phases further confirm the similarity in the chemical environment of their inorganic [ZnCl4]2− tetrahedrons. Clearly, the Raman spectrum of the P-ZnCl2 glass retains the characteristic vibrational features associated with both the inorganic [ZnCl4]2− tetrahedrons and the carbon backbone structure of organic cations BuTPP+ (Fig. 2d). In detail, the Raman shift in the 50–300 cm−1 range corresponds to the Zn–Cl bond stretching vibrations from the [ZnCl4]2− tetrahedrons,36 while the 500–1700 cm−1 range is assigned to the alkyl C–C bonds vibrations from the organic cations BuTPP+, including a prominent benzene ring vibration mode at 1000 cm−1.34 Moreover, to confirm the chemical composition and valence states of the elements in P-ZnCl2 glass, X-ray photoelectron spectroscopy (XPS) measurements were carried out and revealed the existence of P, Zn, and Cl elements in the P-ZnCl2 glass. As shown in Fig. 2e, the high-resolution Zn 2p spectrum displays double characteristic peaks at 1044.8 eV (2p1/2) and 1021.7 eV (2p3/2) with an energy separation of 23.1 eV, demonstrating the divalent state of Zn in the P-ZnCl2 glass.16 In addition, the corresponding binding energies of P 2p and Cl 2p spectra are consistent with the characters of P+ and Cl− in the P-ZnCl2 glass.37,38 These complementary spectroscopic analyses provide conclusive evidence for the successful formation of P-ZnCl2 glass, clearly demonstrating that both the structural integrity of the organic cation BuTPP+ and the characteristic tetrahedral coordination geometry of the inorganic [ZnCl4]2− anions are well-preserved in the resulting amorphous phase.
The micromorphology and elemental distribution of the as-prepared P-ZnCl2 glass were further characterized using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). As shown in Fig. 2f, the SEM image reveals a compact and smooth surface, indicating a high-quality amorphous phase. EDS elemental mapping confirms the homogeneous distribution of P, Zn, and Cl throughout the P-ZnCl2 glass. Moreover, the quantitative EDS elemental analysis yields an average atomic ratio of P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl = 27.87
Cl = 27.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56.56
56.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.57 (Fig. S2), which is consistent with the stoichiometric ratio of P-ZnCl2. This characteristic surface structure effectively minimizes light scattering caused by surface pores or crystal boundaries, thereby contributing to the high optical transparency of the glass.20 The thermal behavior of P-ZnCl2 glass was evaluated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). As shown in the TGA curve (Fig. S3), the P-ZnCl2 glass exhibits a high decomposition temperature (Td) of 280 °C, demonstrating its excellent thermal stability. The glass transition temperature Tg is the characteristic temperature at which an amorphous material transitions from a hard and brittle glassy state to a soft and rubbery or viscous supercooled liquid state upon heating. The DSC curve reveals a clear glass transition temperature (Tg) at 49.5 °C (Fig. S4), further confirming the successful vitrification of the material and its amorphous nature.27 From a practical perspective, it is worth noting that further enhancement of the Tg in hybrid metal halide glasses represents an essential direction for future research to improve their thermal stability and broaden their potential applications. As shown in Fig. S5, the ultraviolet-visible (UV-vis) absorption spectra of these hybrid metal halide glasses exhibit strong absorption in the range of 200–400 nm, consistent with their appearance under natural light. The high energy absorption peaks at around 276 nm can be attributed to the π–π* transitions of the phenyl moieties in the BuTPP+ cations.12 The observed low energy absorption bands spanning 300–410 nm can be attributed to the intermolecular charge transfer state, with these UV-vis absorption characteristics demonstrating the coexistence of multiple distinct electronic transition processes.12 Moreover, these hybrid metal halide glasses display only a broad peak (Fig. S6), confirming their fully amorphous structure, which arises from random molecular arrangements within the glass matrix.14
15.57 (Fig. S2), which is consistent with the stoichiometric ratio of P-ZnCl2. This characteristic surface structure effectively minimizes light scattering caused by surface pores or crystal boundaries, thereby contributing to the high optical transparency of the glass.20 The thermal behavior of P-ZnCl2 glass was evaluated using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). As shown in the TGA curve (Fig. S3), the P-ZnCl2 glass exhibits a high decomposition temperature (Td) of 280 °C, demonstrating its excellent thermal stability. The glass transition temperature Tg is the characteristic temperature at which an amorphous material transitions from a hard and brittle glassy state to a soft and rubbery or viscous supercooled liquid state upon heating. The DSC curve reveals a clear glass transition temperature (Tg) at 49.5 °C (Fig. S4), further confirming the successful vitrification of the material and its amorphous nature.27 From a practical perspective, it is worth noting that further enhancement of the Tg in hybrid metal halide glasses represents an essential direction for future research to improve their thermal stability and broaden their potential applications. As shown in Fig. S5, the ultraviolet-visible (UV-vis) absorption spectra of these hybrid metal halide glasses exhibit strong absorption in the range of 200–400 nm, consistent with their appearance under natural light. The high energy absorption peaks at around 276 nm can be attributed to the π–π* transitions of the phenyl moieties in the BuTPP+ cations.12 The observed low energy absorption bands spanning 300–410 nm can be attributed to the intermolecular charge transfer state, with these UV-vis absorption characteristics demonstrating the coexistence of multiple distinct electronic transition processes.12 Moreover, these hybrid metal halide glasses display only a broad peak (Fig. S6), confirming their fully amorphous structure, which arises from random molecular arrangements within the glass matrix.14
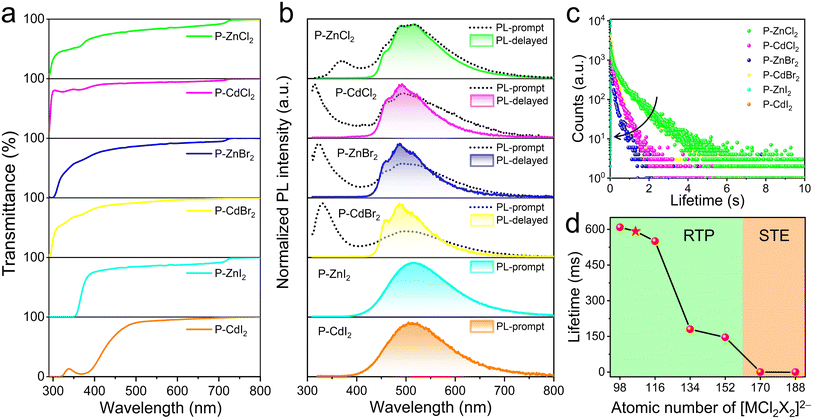
The resulting six hybrid metal halide glasses exhibit high optical transparency, as shown in Fig. 3a, with over 80% transmittance in the range of 400–800 nm. To investigate how the metal halide species influence RTP behavior, the photophysical properties of these glasses were systematically studied. The prompt and delayed (acquired after 5 ms of excitation) PL spectra were recorded under 300 nm excitation (Fig. 3b). As shown in Fig. S7, the organic molecule BuTPPCl exhibits dual-band emission characteristics, featuring a fluorescence peak at 337 nm with a short lifetime of 1.87 ns, and a phosphorescence peak at 492 nm with a longer lifetime of 1032 ms.13 Upon incorporating BuTPP+ with optically inert metal halide units (such as [ZnCl4]2−, [CdCl4]2−, [ZnCl2Br2]2−, and [CdCl2Br2]2−), the resulting four hybrid metal halide glasses display prompt PL spectra similar to that of organic molecule BuTPPCl, with only slight variations in fluorescence and phosphorescence intensities and peak positions (Fig. 3b). The delayed PL spectra of these hybrid glasses also show the same phosphorescence profiles as BuTPPCl, indicating that the afterglow emissions originate primarily from the organic cations BuTPP+. Time-resolved PL decay curves show that the emission lifetimes of the four hybrid glasses, monitored at 492 nm, decrease significantly with the incorporation of heavier metal halide species (Fig. 3c). Specifically, as shown in Fig. 3d, the PL decay lifetimes decrease from 608.6 ms for P-ZnCl2 to 550.3 ms for P-CdCl2, 179.8 ms for P-ZnBr2, and 146 ms for P-CdBr2. This reduction in phosphorescence lifetime is attributed to the heavy-atom effect: the inclusion of heavier metal halide units enhances spin–orbit coupling, which in turn facilitates intersystem crossing (ISC) rate kISC from 5.69 × 107 s−1 to 8.32 × 107 s−1 (Table S2).13 As a result, the radiative decay rate kp from the triplet state is accelerated (Table S2), leading to shorter phosphorescence lifetimes.39 The fluorescence lifetimes of these hybrid metal halide glasses are shown in Fig. S8. The luminescence kinetic parameters of these hybrid glasses are summarized in Table S2.
Further density functional theory (DFT) calculation was performed to rationally predict the energy gaps among the ground state (S0), singlet (S1) and triplet (T1) states, which can be used to evaluate the heavy-atom effect and ISC rate. According to the perturbation theory, the ISC rate is inversely proportional to the energy gap between singlet and triplet excited state, that is, small energy gap is in favor of ISC rate. Based on the calculated excited structures (Fig. S9), the energy gap between S1 and T1 of P-ZnBr2 (0.164 eV) is smaller than that of P-ZnCl2 (0.566 eV), indicating that the reduced gap in P-ZnBr2 facilitates ISC and enhances phosphorescence generation. Similarly, the energy gap between S1 and T1 of P-CdBr2 (0.321 eV) is smaller than that of P-CdCl2 (0.632 eV). Moreover, the calculated SOC coefficients between S1 and T1 in (BuTPP)2MCl2X2 increase with the atomic number of the inorganic metal halide units, suggesting that heavier inorganic metal halide units induce stronger SOC interactions and thus higher ISC rates. These theoretical results clearly demonstrate that the incorporation of inorganic metal halide units introduces a pronounced heavy-atom effect, promoting ISC and enabling efficient phosphorescence generation in (BuTPP)2MCl2X2 glasses, which is consistent with the experimental observations. The pronounced heavy-atom effect from the inorganic metal halide species provides a reliable approach for predicting and tuning the phosphorescence lifetimes. The modulation of inorganic metal halide species in 0D hybrid metal halide glasses is an effective strategy to design RTP materials with anticipated phosphorescence lifetimes. Based on this strategy, we deliberately selected [SrCl4]2− with an atomic number of 106 as an intermediate between [ZnCl4]2− and [CdCl4]2− to construct (BuTPP)2SrCl4 (P-SrCl2) glass, anticipating an intermediate phosphorescence lifetime. Remarkably, the prepared P-SrCl2 glass not only retained the characteristic dual-band emission of BuTPPCl but also exhibited a phosphorescence lifetime of 592 ms (Fig. S10), perfectly matching our predicted lifetime range (Fig. 3d), thereby validating our design principle.
Distinct from the triplet phosphorescence spectrum characterized by three sublevels, the broad Gaussian-shaped prompt PL emission, large Stokes shift, and microsecond lifetimes observed in P-ZnI2 and P-CdI2 glasses (Fig. 3b and c), which are characteristic features of STE emission.40–42 The identical wavelength-dependent PLE and PL spectra further confirm that these PL emissions originate from the relaxation of the same excited state (Fig. S11 and S12).43 Additionally, the PL intensities of P-ZnI2 and P-CdI2 glasses gradually increase with decreasing temperature (Fig. S13a and S14a). Meanwhile, the thermal activation energy (Ea) is considered to evaluate the exciton binding energy, which can be estimated by Arrhenius equation.38 The Ea values for P-ZnI2 and P-CdI2 were calculated to be 67.18 and 66.16 meV (Fig. S13b and S14b), respectively. The high values of Ea in both samples indicate the formation of stable STEs. Due to the strong and fast nature of STE emission, it dominates the prompt PL spectra (Fig. 3b).
Based on the above results, the possible energy transfer pathways are illustrated in Fig. 4. The excited state energy levels and emission energy levels are calculated based on the excitation and emission wavelengths of these materials (Fig. S15). As for organic molecule BuTPPCl, photoexcitation promotes electrons to the singlet state S1, from which some electrons relax to the ground state S0, producing fluorescence. Others undergo an ISC process to the triplet states (T1, T2, T3), resulting in afterglow emission. For P-ZnCl2, P-CdCl2, P-ZnBr2, and P-CdBr2, the observed triplet phosphorescence originates not only from the singlet state of organic cation BuTPP+, but also from the energy transfer involving the inorganic metal halide units. As shown in Fig. S7a, the delayed PL spectra of BuTPPCl molecule reveal three triplet sublevels (T1, T2, and T3), with the T2 emission located at 492 nm. The excitation spectrum monitored at 492 nm matches that of the singlet state (Fig. S7b), indicating that the triplet emission in BuTPPCl primarily arises from the singlet state. Upon incorporation of ZnCl2, the resulting P-ZnCl2 glass exhibits a similar triplet phosphorescence profile to that of BuTPPCl (Fig. 3b). However, the excitation spectrum monitored at 492 nm for the triplet emission not only overlaps with the singlet state excitation spectrum but also displays an additional low-energy broadband excitation peak at 426 nm (Fig. S15a), suggesting that the triplet luminescence originates from both the singlet state and newly introduced inorganic metal halide units (Fig. 4). For P-CdCl2, P-ZnBr2, and P-CdBr2, the photoluminescence excitation (PLE) spectra also exhibit the same characteristics, that is the PLE spectrum not only overlaps with the singlet state excitation spectrum, but also exhibits a new low-energy broadband excitation peak (Fig. S15b–d), indicating that the triplet luminescence not only originates from the singlet state, but also from newly introduced inorganic metal halide units [MCl2X2]2− (Fig. 4). The harvested excitons from [MCl2X2]2− can efficiently populate the triplet states of BuTPP+, thereby enhancing its phosphorescence. Substitution of Cl with Br and Zn with Cd in [ZnCl2X2]2− introduces heavy-atom effect and enhances SOC, accelerating ISC and reducing the phosphorescence lifetimes. Consequently, P-CdBr2 exhibits the shortest afterglow lifetime among the four (BuTPP)2MCl2X2 glasses.
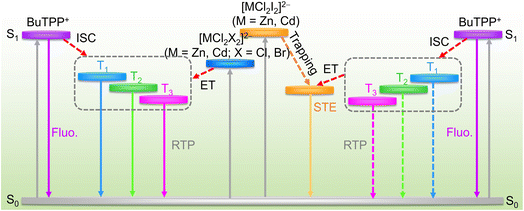 | ||
| Fig. 4 Schematic illustrations of the excited states alignments, energy transfer and relaxation paths of excited states in (BuTPP)2MCl2X2 glasses. | ||
In the case of P-ZnI2 and P-CdI2 glasses, the PL spectra exhibit Gaussian-shaped emission profiles, which differs from the triplet phosphorescence spectrum of BuTPPCl containing three sublevels (Fig. 3b). The time-resolved PL decay spectrum of P-ZnI2 (P-CdI2) glass monitored at 492 nm emission shows a lifetime of 2.18 (2.13) µs (Fig. S16a and b), characteristic of STE state. To investigate whether long-lived triplet luminescence exists in P-ZnI2 (P-CdI2) glass, we tested the time-resolved PL decay spectrum at 492 nm with a 1 ms delay to eliminate the contribution from STE emission. As shown in Fig. S16c and d, after a delay of 1 ms, almost no photon signal was detected, indicating the absence of triplet state luminescence. This suggests efficient energy transfer from the triplet excitons of BuTPP+ to the STE states of the inorganic metal halide units, resulting in detectable emission solely from STE excitons. Furthermore, temperature-dependent time-resolved PL decay curves of P-ZnI2 glass monitored at 492 and 516 nm emission were measured. As shown in Fig. S17, as the temperature increases from 80 to 300 K, the decay lifetime remains in the microsecond range, confirming that STE dominates the emission even at low temperatures. Theoretically, the energy transfer efficiency (ηET) from BuTPP+ to STE state is calculated by following equation,
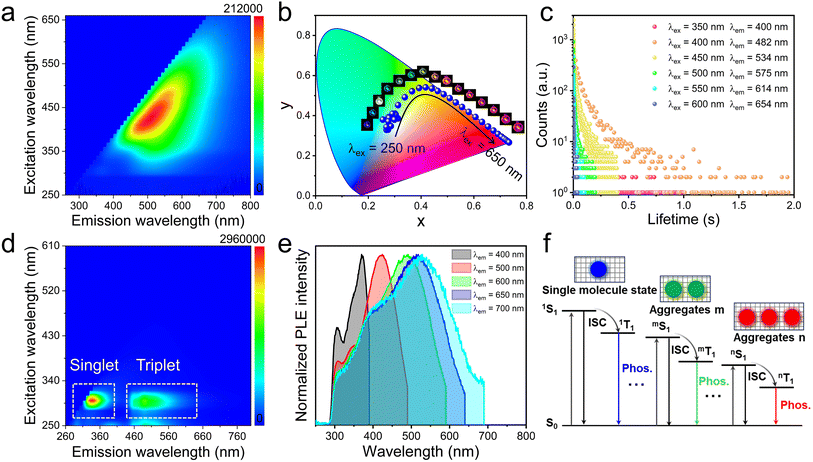
Interestingly, the experimental results revealed that P-ZnCl2 glass exhibits excitation wavelength-dependent multicolor luminescence. This phenomenon was quantitatively recorded by the two-dimensional (2D) excitation–emission matrix spectra (Fig. 5a). For instance, under 350 nm excitation, P-ZnCl2 glass shows a maximum emission peak at 518 nm, whereas shifting the excitation to 600 nm results in a redshifted emission peak at 659 nm (Fig. S18). The color variations of the P-ZnCl2 glass under different excitation wavelengths were illustrated in the Commission Internationale de l’Eclairage (CIE) chromaticity coordinate diagram (Fig. 5b), showing a continuous shift in emission color from blue to white to red as the excitation wavelength increased from 250 to 650 nm. To the best of our knowledge, such a broad range of excitation-dependent colorful luminescence is rarely reported in hybrid metal halides. This excitation-dependent emission could be attributed to the presence of multiple luminescent centers within the glass matrix.41,44 Time-resolved PL decay measurements further revealed that the excited state lifetimes of P-ZnCl2 glass was dependent on the excitation wavelength, exhibiting tunable phosphorescence lifetimes ranging from 0.11 to 15.34 ms as the excitation wavelength shifts from 350 to 600 nm (Fig. 5c). These distinct variations in excited state lifetimes further confirmed the existence of disparate emissive species.4,8,45,46 In contrast, no excitation-dependent emission behavior is observed in BuTPPCl molecule (Fig. 5d), indicating its isolated luminescent feature.2 Moreover, the excitation spectra of P-ZnCl2 glass exhibit a progressive redshift with increasing emission wavelength (Fig. 5e), suggesting that the excitation-dependent multicolor phosphorescence arises from multiple aggregate clusters in P-ZnCl2 glass.33,46,47 These diverse aggregates likely form cascade excited states that give rise to the observed multicolor emissions (Fig. 5f).
The PL spectra of P-ZnCl2 glass under the variable excitation light power were measured. As shown in Fig. S19, the PL intensity of P-ZnCl2 glass exhibited a linear dependence on excitation power, ruling out the possibility of defect state luminescence. The delayed PL spectra of P-ZnCl2 glass with delay times ranging from 1 to 80 ms were recorded, as shown in Fig. S20. As the delay time increases, the intensity of different luminescent centers gradually decreases, indicating the existence of different luminescent species. Additionally, to further investigate the cluster structure of P-ZnCl2 glass, 2D grazing-incidence wide-angle X-ray scattering (GiWAXS) patterns and one-dimensional (1D) scattering profiles along the qz direction were conducted. P-ZnCl2 glass exhibits an intensified central scattering signal (Fig. S21a), suggesting strong molecular aggregation. Distinct scattering peaks appear at q = 0.74, 0.95, 1.42, 1.67 Å−1 (Fig. S21b), corresponding to π–π stacking distances of 8.49, 6.61, 4.42, 3.76 Å, respectively, as calculated using Bragg's equation d = 2π/q.47 These peaks originate from multiple aggregated clusters within the glass matrix. At q values above 1.75 Å−1, the scattering intensity rapidly decays, indicating the absence of long-range structural periodicity at the molecular level. This observation is consistent with the amorphous structural characteristics of P-ZnCl2 glass. The 2D excitation–emission matrix spectra of the other three glasses, P-CdCl2, P-ZnBr2, and P-CdBr2, were also recorded. All of them exhibit excitation-dependent multicolor luminescence (Fig. S22), similar to that observed for the P-ZnCl2 glass.
Benefiting from the moldability of glasses and time-resolved RTP properties of these hybrid metal halide glasses, these materials exhibit great potential for multifunctional anti-counterfeiting applications. As illustrated in Fig. 6a, a series of hybrid metal halide glasses were shaped into distinct letters, including “R” from P-ZnCl2, “T” from P-CdCl2, and “P” from P-ZnBr2. Under 365 nm UV lamp irradiation, these patterns emit bright white-light emissions. Once the UV light is turned off, the letters “R” and “T” immediately display green afterglow emissions, while only the letter “R” remains visible after more than 1.4 s. This enables the design of customized shapes and multiple anti-counterfeiting features by selecting appropriate material combinations. A similar phenomenon is observed with the “ZZU” letter pattern (Fig. 6b), demonstrating the reproducibility and versatility of this strategy. Furthermore, by taking advantage of the facile processibility, excellent glass-forming ability, and rapid emission characteristics of P-ZnI2, we successfully fabricated a large-area glass scintillation screen with a diameter of 6 cm. As shown in Fig. 6c, the resulting P-ZnI2 glass scintillation screen is highly transparent under ambient light and displays a uniform bright greenish white light emission under 365 nm UV irradiation (Fig. 6d). Spatial resolution, a key parameter for assessing X-ray imaging performance, was evaluated using a standard line-pair card.48 As shown in Fig. 6e, the spatial resolution of the P-ZnI2 glass scintillation screen reaches approximately 10 lp mm−1, indicating good X-ray imaging capability. To demonstrate its practical applicability, a series of X-ray imaging experiments were conducted. As shown in Fig. 6f and g, fine structural details, such as the internal circuits of an electronic chip and the metallic spring inside a capsule, are clearly visible using the P-ZnI2 glass scintillation screen, highlighting its strong potential for high-resolution X-ray imaging applications.49
Furthermore, we characterized the scintillation properties of P-ZnI2 glass. Fig. S23a shows the X-ray attenuation efficiencies of P-ZnI2 glass and Bi4Ge3O12 (BGO) as a function of thickness at an X-ray photon energy of 22 keV, and the X-ray attenuation efficiency of BGO scintillator is superior to that of P-ZnI2 glass. To accurately estimate the light yield of P-ZnI2 glass, a commercial BGO scintillator (light yield: 8500 photons MeV−1) was used as a reference. By calibrating the radioluminescence (RL) intensity, the light yield of P-ZnI2 glass was determined to be 2296 photons MeV−1 (Fig. S23b). To demonstrate the linear response range to X-ray dose rates, the RL spectra of the P-ZnI2 glass under various dose rates was examined. As shown in Fig. S23c, the RL intensity of P-ZnI2 glass monotonically increases with dose rates ranging from 603 to 6100 µGy s−1, and exhibits an excellent linear response (Fig. S23d). When the signal-to-noise ratio is 3, the linear fitting of the RL intensity yields a detection limit of 10.37 µGy s−1 for P-ZnI2 glass (Fig. S23d). The X-ray irradiation stability of P-ZnI2 glass was further examined under continuous exposure at a high dose rate of 12.7 mGy s−1 for 1092 s, during which the RL intensity retained over 91% of its initial value (Fig. S23e), confirming its better stability. Moreover, the RL response profile exhibited a rise time of 20 ms and a fall time of 20 ms (Fig. S23f), demonstrating prompt radiation response and rapid signal decay, both essential characteristics for practical scintillation applications. Therefore, these novel types of hybrid metal halide glasses have great potentials towards information security and X-ray imaging applications.
3 Conclusion
In conclusion, we developed a series of 0D (BuTPP)2MCl2X2 hybrid metal halide glasses via a solvent-assisted rapid evaporation method, overcoming the limitations of conventional melt-quenching. The heavy-atom effect from inorganic metal halide anions [MCl2X2]2− was leveraged to systematically tune RTP lifetimes, with heavier atoms accelerating ISC and reducing phosphorescence decay lifetimes. Unique dual-mode emission, combining long-lived RTP (organic triplet states) and short-lived STE (inorganic excitons), was achieved in P-ZnI2 and P-CdI2 glasses. Additionally, P-ZnCl2 glass displayed excitation-dependent multicolor phosphorescence, attributed to multiple aggregate cluster luminescence. The glasses' excellent processability enabled applications in shape-controlled anti-counterfeiting and X-ray imaging with high spatial resolution. This study not only advances the synthesis of hybrid metal halide glasses but also establishes a framework for designing RTP materials with programmable lifetimes and emission properties, paving the way for future optoelectronic and security technologies.Author contributions
L. Y. L. and Z. F. S. initiated and guided the research. L. Y. L. wrote the manuscript, L. Y. L. and Z. F. S. revised it. L. Y. L. and M. A. performed material synthesis, spectra measurements, and data analysis. D. M. X., T. L., J. B. Z., M. C. J., Y. L., Z. Z. M., and X. C. assisted with luminescence properties measurements and data analysis. Y. B. Han, Y. T. T., and X. J. Li performed the X-ray scintillation properties characterization and data analysis. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the findings of this paper are available from the corresponding author upon reasonable request.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details, characterization data (EDS, TGA, DSC, UV-vis absorption spectra, PXRD, PL spectra, timed-resolved PL spectra, DFT calculation, temperature-dependent PL spectra, 2D GiWAXS, X-ray scintillation properties, crystal data table, and luminescence kinetic parameters table). See DOI: https://doi.org/10.1039/d5sc06898c.
Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2022YFB28033900), National Natural Science Foundation of China (No. 12304457, 12074347, 61935009, 12404466), the Postdoctoral Fellowship Program of CPSF (No. GZB20230673), the Key Research Projects of Higher Education Institutions in Henan Province (No. 24A140020), the China Postdoctoral Innovative Talent Support Program (Grant No. BX20230330), the China Postdoctoral Science Foundation (No. 2024M752915), the Support Program for Scientific and Technological Innovation Teams of Higher Education in Henan Province (No. 23IRTSTHN011), the Natural Science Foundation of Henan Province (No. 252300421236), and the Science and Technology Research and Development Program Joint Fund of Henan Province (No. 232301420002).References
- L. Gu, H. Shi, L. Bian, M. Gu, K. Ling, X. Wang, H. Ma, S. Cai, W. Ning, L. Fu, H. Wang, S. Wang, Y. Gao, W. Yao, F. Huo, Y. Tao, Z. An, X. Liu and W. Huang, Nat. Photonics, 2019, 13, 406–411 CrossRef CAS.
- L. Li, J. Zhou, J. Han, D. Liu, M. Qi, J. Xu, G. Yin and T. Chen, Nat. Commun., 2024, 15, 3846 CrossRef CAS PubMed.
- J. Wei, C. Liu, J. Duan, A. Shao, J. Li, J. Li, W. Gu, Z. Li, S. Liu, Y. Ma, W. Huang and Q. Zhao, Nat. Commun., 2023, 14, 627 CrossRef CAS PubMed.
- F. Nie and D. Yan, Nat. Commun., 2024, 15, 9491 CrossRef CAS PubMed.
- X. Wang, H. Shi, H. Ma, W. Ye, L. Song, J. Zan, X. Yao, X. Ou, G. Yang, Z. Zhao, M. Singh, C. Lin, H. Wang, W. Jia, Q. Wang, J. Zhi, C. Dong, X. Jiang, Y. Tang, X. Xie, Y. Yang, J. Wang, Q. Chen, Y. Wang, H. Yang, G. Zhang, Z. An, X. Liu and W. Huang, Nat. Photonics, 2021, 15, 187–192 CrossRef CAS.
- N. Gan, X. Zou, M. Dong, Y. Wang, X. Wang, A. Lv, Z. Song, Y. Zhang, W. Gong, Z. Zhao, Z. Wang, Z. Zhou, H. Ma, X. Liu, Q. Chen, H. Shi, H. Yang, L. Gu, Z. An and W. Huang, Nat. Commun., 2022, 13, 3995 CrossRef CAS PubMed.
- M. Ren, S. Zhang, J. Wu, Q. Hu, S. Cao, B. Zou, Z. Yin, W. W. Yu and R. Zeng, Adv. Funct. Mater., 2025, 35, 2420362 CrossRef CAS.
- Y. Shen, Z. An, H. Liu, B. Yang and Y. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202214483 CrossRef CAS PubMed.
- X. Li, W. Li, Z. Deng, X. Ou, F. Gao, S. He, X. Li, Z. Qiu, R. T. K. Kwok, J. Sun, D. L. Phillips, J. W. Y. Lam, Z. Guo and B. Z. Tang, J. Am. Chem. Soc., 2025, 147, 14198–14210 CrossRef CAS PubMed.
- H. Li, J. Gu, Z. Wang, J. Wang, F. He, P. Li, Y. Tao, H. Li, G. Xie, W. Huang, C. Zheng and R. Chen, Nat. Commun., 2022, 13, 429 CrossRef CAS PubMed.
- S. Feng, Q. Huang, S. Yang, Z. Lin and Q. Ling, Chem. Sci., 2021, 12, 14451–14458 RSC.
- Z. Luo, Y. Liu, Y. Liu, C. Li, Y. Li, Q. Li, Y. Wei, L. Zhang, B. Xu, X. Chang and Z. Quan, Adv. Mater., 2022, 34, 2200607 CrossRef CAS PubMed.
- L.-J. Xu, A. Plaviak, X. Lin, M. Worku, Q. He, M. Chaaban, B. J. Kim and B. Ma, Angew. Chem., Int. Ed., 2020, 59, 23067–23071 CrossRef CAS PubMed.
- F. Nie and D. Yan, Nat. Commun., 2024, 15, 5519 CrossRef CAS PubMed.
- S. Liu, X. Fang, B. Lu and D. Yan, Nat. Commun., 2020, 11, 4649 CrossRef CAS PubMed.
- J.-Q. Zhao, D.-Y. Wang, T.-Y. Yan, Y.-F. Wu, Z.-L. Gong, Z.-W. Chen, C.-Y. Yue, D. Yan and X.-W. Lei, Angew. Chem., Int. Ed., 2024, 63, e202412350 CrossRef CAS PubMed.
- Y.-H. Liu, B.-L. Zhang, Y.-J. Wang, X.-Y. Zhang, Y.-B. Shang, T.-C. Liu, X.-W. Lei, Z.-W. Chen and C.-Y. Yue, Adv. Opt. Mater., 2024, 12, 2303285 CrossRef CAS.
- L. Zhou, K. Li, Y. Chang, Y. Yao, Y. Peng, M. Li and R. He, Chem. Sci., 2024, 15, 10046–10055 RSC.
- Y. Peng, J. Ma, Y. Zhao, D. You, Y. Yao, Z. Deng, J. Liao, Y. Chang, W. Shen, M. Li, R. He and L. Zhou, Adv. Funct. Mater., 2025, 35, 2420311 CrossRef CAS.
- B. Li, Y. Wang, Y. Xu and Z. Xia, Adv. Mater., 2025, 37, 2415483 CrossRef CAS PubMed.
- B. Zhou and D. Yan, Matter, 2024, 7, 1950–1976 CrossRef.
- J.-B. Luo, J.-H. Wei, Z.-Z. Zhang, Z.-L. He and D.-B. Kuang, Angew. Chem., Int. Ed., 2023, 62, e202216504 CrossRef CAS PubMed.
- B. Zhou, Z. Qi and D. Yan, Angew. Chem., Int. Ed., 2022, 61, e202208735 CrossRef CAS PubMed.
- Z. Xu, W. Chen, K. Chen, S. Lin, Z. Wu, G. Deng, J. Chen, M. Tayyab, Y. Xiong, M.-D. Li, D. Wang, Z. An and B. Z. Tang, Adv. Mater., 2025, 37, 2418778 CrossRef CAS PubMed.
- F. Nie and D. Yan, Sci. China Mater., 2024, 67, 3531–3536 CrossRef.
- B. Li, J. Jin, M. Yin, K. Han, Y. Zhang, X. Zhang, A. Zhang, Z. Xia and Y. Xu, Chem. Sci., 2023, 14, 12238–12245 RSC.
- B. Li, J. Jin, X. Liu, M. Yin, X. Zhang, Z. Xia and Y. Xu, ACS Mater. Lett., 2024, 6, 1542–1548 CrossRef CAS.
- B. Li, J. Jin, M. Yin, X. Zhang, M. S. Molokeev, Z. Xia and Y. Xu, Angew. Chem., Int. Ed., 2022, 61, e202212741 CrossRef CAS PubMed.
- M. Yin, B. Li, Z. Yi, Y. Zhang, Z. Xia and Y. Xu, Chem. Commun., 2023, 59, 11361–11364 RSC.
- Z.-Z. Zhang, Z.-L. He, Q.-P. Peng, J.-H. Chen, B. Lan and D.-B. Kuang, J. Mater. Chem. C, 2024, 12, 17411–17418 RSC.
- T. Wang, X. Xu, S. Yao, J. Yu, Z. Yang, J. Qiu, Y. Yang and X. Yu, Adv. Opt. Mater., 2024, 12, 2302203 CrossRef CAS.
- W. Wang, C.-D. Liu, C.-C. Fan, X.-B. Fu, C.-Q. Jing, M.-L. Jin, Y.-M. You and W. Zhang, J. Am. Chem. Soc., 2024, 146, 9272–9284 CrossRef CAS PubMed.
- X. Li, Y. Yang, Z. Zhao, S. Bai, Q. Li and J. Li, Angew. Chem., Int. Ed., 2025, 64, e202422272 CrossRef CAS PubMed.
- R. Zhang, H. Xie, W. Liu, K. Zhan, H. Liu, Z. Tang and C. Yang, ACS Appl. Mater. Interfaces, 2023, 15, 47238–47249 CrossRef CAS PubMed.
- Y. Xu, Z. Li, C. Shi, Y. Li, Y. Lei, G. Peng, T. Yu, H. Ren, H. Wang, H. Fan, Y. Zhang, Z. Ci, Q. Wang and Z. Jin, Adv. Mater., 2024, 36, 2406128 CrossRef CAS.
- S. Zaghden, H. Ben Attia, M. S. M. Abdelbaky, A. Oueslati, S. García-Granda, M. Dammak and L. Ktari, ACS Omega, 2024, 9, 47597–47612 CrossRef CAS PubMed.
- G. Zhou, Y. Mao, J. Zhang, Q. Ren, M. S. Molokeev, Z. Xia and X.-M. Zhang, Adv. Funct. Mater., 2025, 35, 2413524 CrossRef CAS.
- L. Lian, D. Xiong, J. Zhang, M. Jia, Y. Liu, Z. Ma, X. Chen, Y. Han, Y. Tian, X. Li, J. Zhang and Z. Shi, Laser Photonics Rev., 2025, e00509 CrossRef CAS.
- Z.-Y. Li, R. Feng, S.-S. Huang, W. Li and X.-H. Bu, J. Am. Chem. Soc., 2025, 147, 7017–7027 CrossRef CAS.
- L. Lian, M. Zheng, P. Zhang, Z. Zheng, K. Du, W. Lei, J. Gao, G. Niu, D. Zhang, T. Zhai, S. Jin, J. Tang, X. Zhang and J. Zhang, Chem. Mater., 2020, 32, 3462–3468 CrossRef CAS.
- L. Lian, P. Zhang, G. Liang, Y. Xia, J. Gao, D. Zhang and J. Zhang, ACS Appl. Mater. Interfaces, 2024, 16, 9030–9038 CrossRef CAS PubMed.
- L. Lian, X. Wang, P. Zhang, J. Zhu, X. Zhang, J. Gao, S. Wang, G. Liang, D. Zhang, L. Gao, H. Song, R. Chen, X. Lan, W. Liang, G. Niu, J. Tang and J. Zhang, J. Phys. Chem. Lett., 2021, 12, 6919–6926 CrossRef CAS PubMed.
- L. Lian, M. Zheng, W. Zhang, L. Yin, X. Du, P. Zhang, X. Zhang, J. Gao, D. Zhang, L. Gao, G. Niu, H. Song, R. Chen, X. Lan, J. Tang and J. Zhang, Adv. Sci., 2020, 7, 2000195 CrossRef CAS PubMed.
- L. Kang, J. Xu, J. Wu, Q. Ling and Z. Lin, Inorg. Chem. Front., 2025, 12, 5133–5142 RSC.
- L. Hou, T. Wang, S. Yu, X. Xu and X. Yu, Aggregate, 2025, 6, e70071 CrossRef CAS.
- J. Zhang, Z. Xiong, H. Zhang and B. Z. Tang, Nat. Commun., 2025, 16, 3910 CrossRef CAS PubMed.
- H. Wang, H. Shi, W. Ye, X. Yao, Q. Wang, C. Dong, W. Jia, H. Ma, S. Cai, K. Huang, L. Fu, Y. Zhang, J. Zhi, L. Gu, Y. Zhao, Z. An and W. Huang, Angew. Chem., Int. Ed., 2019, 58, 18776–18782 CrossRef CAS PubMed.
- L. Lian, D. Xiong, J. Zhang, M. Jia, Y. Liu, Z. Ma, X. Chen, Y. Han, Y. Tian, X. Li, J. Zhang, S. Qian, C. Shan and Z. Shi, Appl. Phys. Rev., 2025, 12, 031409 CAS.
- L. Lian, D. Xiong, J. Zhang, M. Jia, Y. Liu, Z. Ma, X. Chen, Y. Han, Y. Tian, X. Li, J. Zhang, P. Zhang and Z. Shi, Laser Photonics Rev., 2025, e01538 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |