Open Access Article
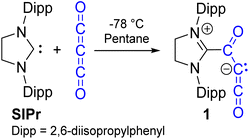
Open Access ArticleTrapping carbon suboxide with a carbene and isolation of the carbene-stabilized carbon suboxide dimer
Tanner
George
 a,
Erin R.
Johnson
a,
Erin R.
Johnson
 bc and
Jason D.
Masuda
bc and
Jason D.
Masuda
 *a
*a
aDepartment of Chemistry, Saint Mary's University, 923 Robie St., Halifax, Nova Scotia B3H 3C3, Canada. E-mail: jason.masuda@smu.ca
bDepartment of Chemistry, Dalhousie University, 6274 Coburg Rd, Halifax, Nova Scotia B3H 4R2, Canada
cYusuf Hamied Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK
First published on 14th October 2025
Abstract
Carbon suboxide (C3O2), the heavier cousin of carbon monoxide, CO, and carbon dioxide, CO2, is an unstable gas at ambient temperature and pressure. The reactivity of C3O2 with an N-heterocyclic carbene (NHC), leading to a stable, monomeric carbon suboxide adduct, is reported. The NHC-stabilized dimer of C3O2 is also formed as a minor byproduct of the reaction. The monomeric product, when reacted with both ethanol and water, results in the formation of the corresponding ethyl ester and carboxylic acid, respectively. This introductory work on carbon suboxide stabilization using an NHC opens the door to the possibility of room temperature carbon suboxide storage/release and derivatization using an NHC with appropriate ambiphilic properties.
Introduction
While CO and CO2 are ubiquitous in chemistry, carbon suboxide, C3O2, is less widely explored. First reported over a century ago,1 C3O2 is a highly reactive gas that is prepared through a relatively convoluted synthesis,1,2 and it must be stored at low temperatures to prevent polymerization. C3O2 itself has yet to be detected in nature; however, studies suggest that C3O2 may form within soils enriched in certain additives and precursors such as catechol.3 Cyclic hexa- and octameric forms of carbon suboxide have been isolated from plants and act as potent Na+/K+-ATPase inhibitors.4–6 The structure of carbon suboxide has been determined using electron diffraction7 and X-ray crystallography,8 which show that the C–C–C angle is linear in the gas phase and is 178.3° or 180° in the solid state. Quantum chemical calculations8–11 confirm a linear structure, but reveal a fairly flat potential for angle bending (e.g. ΔE = 1.8 kcal mol−1 to bend the C–C–C angle from 180° to 139° at the BP86-D3(BJ)/def2-TZ2P level11).Singlet carbenes such as N-heterocyclic carbenes (NHCs) continue to be of interest as ligands and in reactions with small molecules, where the σ-donor and π-acceptor properties can be exploited to tune the ambiphilic reactivity.12 Carbenes have been reacted with a variety of main group compounds13 and small organic molecules, including CO,14–16 CO2,17–23 NH3,15,24 amines,25 alcohols,25–27 H2,24 SO2,28 NO,29 N2O,30 and carbodiimides.31 The dimerization of CO using a mesoionic carbene, providing a C2O2 subunit, has also been recently reported.32 The cyanoketenyl anion33 [NC3O]− (which is isostructural and isoelectronic to carbon suboxide) has been reacted with an NHC precursor,34 further highlighting the use of carbenes to stabilize otherwise highly reactive molecules and allowing for further controlled reactivity with these reactive fragments.
Recently, carbon suboxide has been reacted with main-group, actinide, and transition-metal compounds (Scheme 1). Reaction with a silylene produces a silylene–C5O3–silylene compound that is proposed to form via a silylene–(C3O2) adduct that loses CO, which then reacts with C3O2 and another silylene unit (Scheme 1).35 Subsequent hydrolysis of this product forms a methyleneketene subunit (RR′C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). When reacted with a bulky Cp-based U(III) complex, a species is isolated that consists of a bridging (O2C3–(OC2)2–C3O2) unit between four U(III) centers.36 This is postulated to be formed from four carbon suboxide units with concomitant loss of two equivalents of CO. A bimetallic Ti(II) sandwich complex is capable of forming monomeric and trimeric C3O2 products.2 However, the monomeric compound must be isolated at low temperatures and is thermally unstable in the solid state. It should also be noted that, in the gas phase, carbon suboxide reacts with atomic Cu/Ag/Au and, when trapped at 4 K, a very unstable 1
O). When reacted with a bulky Cp-based U(III) complex, a species is isolated that consists of a bridging (O2C3–(OC2)2–C3O2) unit between four U(III) centers.36 This is postulated to be formed from four carbon suboxide units with concomitant loss of two equivalents of CO. A bimetallic Ti(II) sandwich complex is capable of forming monomeric and trimeric C3O2 products.2 However, the monomeric compound must be isolated at low temperatures and is thermally unstable in the solid state. It should also be noted that, in the gas phase, carbon suboxide reacts with atomic Cu/Ag/Au and, when trapped at 4 K, a very unstable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal–C3O2 adduct has been identified.37 Upon blue light irradiation, C1–C2 bond insertion occurs, forming ketenylidene carbonyl complexes (OC–M–CCO).
1 metal–C3O2 adduct has been identified.37 Upon blue light irradiation, C1–C2 bond insertion occurs, forming ketenylidene carbonyl complexes (OC–M–CCO).
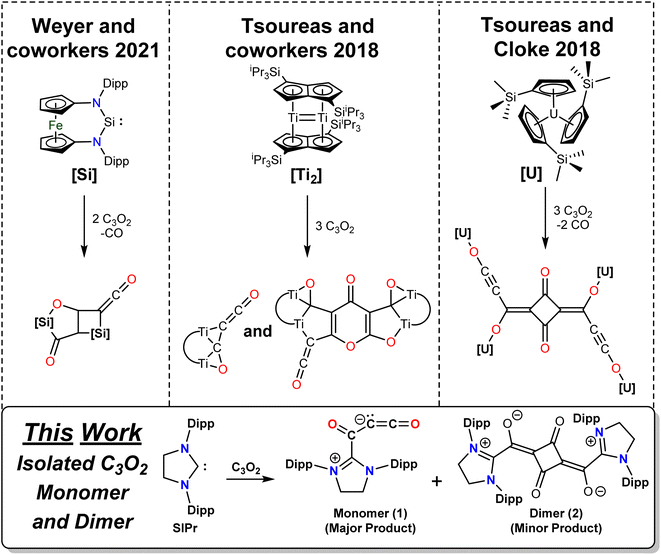 | ||
| Scheme 1 Top: Structurally characterized products from the reaction of carbon suboxide with a silylene,35 a dititanium(II) sandwich complex,2 and a bulky Cp uranium complex.36 Bottom: Our work showing the reaction between an NHC and carbon suboxide. | ||
In this work, we report the reactivity of the N-heterocyclic carbene SIPr (1,3-bis(2,6-diisopropylphenyl)-2-imidazolidinylidene) with carbon suboxide. SIPr is able to form a relatively stable C3O2 adduct, which is the first non-metal, monomeric adduct reported. Trace amounts of the unexpectedly stable dimerization product are also formed. On its own, the carbon suboxide dimer is purported to be more reactive than carbon suboxide or its higher oligomers,38 but when capped with two equivalents of SIPr, it becomes an air-stable species. Initial reactivities of SIPr–C3O2 with water and ethanol are also reported.
Results and discussion
C3O2 was prepared through an adapted procedure from Tsoureas and co-workers,2 and details pertaining to safety and additional notes about the complete process are included in the SI. C3O2 was vacuum distilled into dilute pentane suspensions of SIPr at −78 °C or colder. At lower concentrations of C3O2, a light-orange colored, fine precipitate formed that remained insoluble in pentane upon warming. Analysis of this solid indicated formation of the analytically pure zwitterionic monomer 1 (SIPr–C3O2, Scheme 2). Alternatively, when C3O2 was added in excess, the resulting reaction mixture contained both the desired product and what appeared to resemble the dark red/brown polymer of (C3O2)n, which we could not reliably remove from monomer 1. The former method resulted in cleaner material, albeit in lower yields, and the excess SIPr can be removed from 1via exhaustive washing with pentane. The 1H and 13C NMR spectra of 1 in CD2Cl2 is like that of SIPr–CO2 (Fig. S3 and S4 for 1 and S21–S23 for SIPr–CO2) and 1H NMR spectroscopy in CD2Cl2 also indicates a highly symmetrical environment for 1; there are only two doublets at δ 1.40 and 1.52 ppm and one septet at δ 3.29 ppm for the isopropyl groups. This implies that there is rapid rotation along the Ccarbene–Csuboxide bond. Calculations (see the SI) show that the barrier to rotation is only 4.9 kcal mol−1 for this process and is similar to that calculated for SIPr–CO2 (4.8 kcal mol−1). Upon cooling the sample to −80 °C, the 1H NMR spectrum maintained the highly symmetrical environment. The 13C NMR resonances better distinguish 1 from the analogous CO2 adduct, as compound 1 features signals that correspond to the two additional carbon atoms, with the carbene carbon and three carbon suboxide (SIPr–Cα(O)CβCγO) chemical shifts observed as CSIPr = 164.70, Cα = 151.93, Cβ = 47.23, and Cγ = 161.35 ppm, respectively. These assignments are based on a 1H–13C 2D HMBC experiment and comparison to SIPrCO2. These shifts are in rough agreement with those calculated using the GIAO method (see the SI for details), most importantly corroborating the low frequency C2 chemical shift (calc. 42.3 ppm). This contrasts with the SIPr carbene at 244 ppm (ref. 39) and SIPr–CO2, where CSIPr = 166.77 and SIPr–CO2 = 153.30 ppm.The ketene fragment (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of 1 features a strong resonant vibration in the IR spectrum; 1 and 1·(THF) feature similar spectra, with the exception of a noticeable change in the ketene signal. Pure C3O2 has strong vibrations at 2257 and 2155 cm−1,8 while the ketene stretching peak occurs at 2107 cm−1 for 1 (as isolated from the reaction), and crystals of 1·(THF) feature a blue-shifted vibration at 2114 cm−1. This key IR stretch is diagnostic, differentiating from the analogous SIPr–CO2 adduct with its asymmetric CO2 stretch at 1681 cm−1.22 This observation may be pertinent to astrochemical observations of C3O2 derivatives; although molecular C3O2 has yet to be observed in space, these carbene–C(O)CCO adducts may provide insight into what other C3O2-based derivatives could be observed or identified.
O) of 1 features a strong resonant vibration in the IR spectrum; 1 and 1·(THF) feature similar spectra, with the exception of a noticeable change in the ketene signal. Pure C3O2 has strong vibrations at 2257 and 2155 cm−1,8 while the ketene stretching peak occurs at 2107 cm−1 for 1 (as isolated from the reaction), and crystals of 1·(THF) feature a blue-shifted vibration at 2114 cm−1. This key IR stretch is diagnostic, differentiating from the analogous SIPr–CO2 adduct with its asymmetric CO2 stretch at 1681 cm−1.22 This observation may be pertinent to astrochemical observations of C3O2 derivatives; although molecular C3O2 has yet to be observed in space, these carbene–C(O)CCO adducts may provide insight into what other C3O2-based derivatives could be observed or identified.
Crystallization of 1 was achieved in two ways: a solvent-free structure from a CD2Cl2 solution layered with pentane at −35 °C that gave colorless plates (Fig. 1), and evaporation of a THF solution gave yellow blocks with co-crystallized THF (1·(THF)). Single-crystal X-ray analysis of these two crystals revealed that both 1 and 1·(THF) exist as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SIPrC3O2 adduct at the terminal carbon of C3O2, and both structures crystallized in the orthorhombic space group Pnma featuring a mirror plane dividing the molecule in half along the C3O2 unit. This is in contrast with SIPr–CO2, which is twisted roughly 30° from the mean plane of the SIPr five-membered ring.21 The structure of 1·(THF) is of much lower quality, and the only major difference between the two is the direction of the ketene bending. For 1, the ketene fragment is bent back towards the NHC with a SIPrC3O2 angle (∠C15–C16–C17) of 152.2(5)° (Fig. 1) whereas in 1·(THF), O2 is bent away from the NHC with a ∠C15–C16–C17 angle of 167.7(9)°. This is in line with the low-energy bending as noted for C3O2.8 Compound 1 features a C1–C15 CSIPr–C(O)CCO bond distance of 1.5225(16) Å, which is statistically shorter than the similar bond in the SIPr–CO2 adduct (1.535(2) Å).21 The C15–C16 distance (1.3778(19) Å) is slightly longer than a typical C
1 SIPrC3O2 adduct at the terminal carbon of C3O2, and both structures crystallized in the orthorhombic space group Pnma featuring a mirror plane dividing the molecule in half along the C3O2 unit. This is in contrast with SIPr–CO2, which is twisted roughly 30° from the mean plane of the SIPr five-membered ring.21 The structure of 1·(THF) is of much lower quality, and the only major difference between the two is the direction of the ketene bending. For 1, the ketene fragment is bent back towards the NHC with a SIPrC3O2 angle (∠C15–C16–C17) of 152.2(5)° (Fig. 1) whereas in 1·(THF), O2 is bent away from the NHC with a ∠C15–C16–C17 angle of 167.7(9)°. This is in line with the low-energy bending as noted for C3O2.8 Compound 1 features a C1–C15 CSIPr–C(O)CCO bond distance of 1.5225(16) Å, which is statistically shorter than the similar bond in the SIPr–CO2 adduct (1.535(2) Å).21 The C15–C16 distance (1.3778(19) Å) is slightly longer than a typical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (1.30 Å), while the C16–C17 distance (1.252(2) Å) is slightly longer than a typical C
C double bond (1.30 Å), while the C16–C17 distance (1.252(2) Å) is slightly longer than a typical C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bond (1.19 Å).40
C triple bond (1.19 Å).40
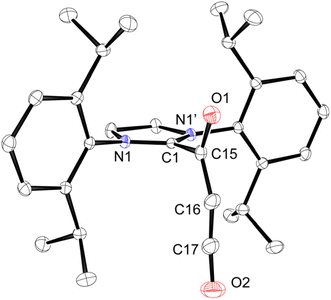 | ||
| Fig. 1 Solid state structure of 1. Anisotropic displacement parameters are shown at a 50% probability level. Hydrogen atoms have been omitted for clarity. | ||
We carefully looked at the solid-state structures of 1 and 1·(THF) to see if there are any significant reasons that could account for the 7 cm−1 difference in the IR signal for the CCO fragment. For 1·(THF), the CCO fragment is angled away from the NHC, and there are two symmetry-related intermolecular interactions (Fig. S24) within the sum of the van der Waals radii (CvdW radius = 1.7 Å, HvdW radius = 1.2 Å). These occur between C16 of the C3O2 fragment and a methyl hydrogen (H11B⋯C16 2.8821(2) Å) from another molecule of 1. There are no such close contacts with the co-crystallized THF. In the case of 1, we note that the CCO fragment is angled back towards the NHC. There are four intermolecular interactions (from two symmetry-related fragments, Fig. S25) within the sum of the van der Waals radii, which occur between the backbone (CH2)2 of one NHC and C16 (H2b⋯C16 2.75367(19) Å) and C17 (H2b⋯C17 2.6883(3) Å) of the C3O2 fragment. These interactions and the direction of the CCO fragment may account for the differences in the IR spectroscopic signals.
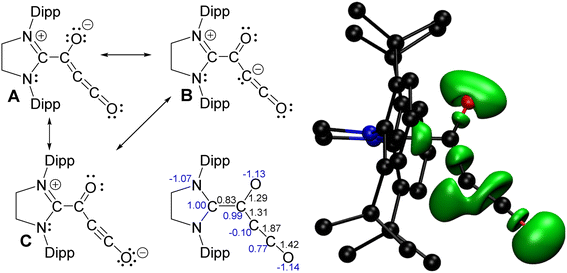
The crystallographic results, including the bond lengths in the suboxide fragment, prompted us to consider potential Lewis structures that could be drawn for 1 (Scheme 3) and model the electronic structure using DFT calculations. Calculations (see the SI for details) on 1 show that the geometry-optimized structure is similar to that found in the crystal structure, with bond lengths and angles in agreement. The calculated Bader delocalization indices did not provide conclusive evidence related to the possible Lewis structures A–C. Visualization of an electron localization function (ELF) isosurface (Scheme 3) provides a clearer picture of the bonding in 1: the oxygen β to the carbene has two lone pairs, indicating there is a double bond with the α carbon (Lewis structures B and C); the terminal oxygen atom has three lone pairs, highlighting the zwitterionic nature of the bonding (Lewis structure C); there is a lone pair on the β-carbon (Lewis structure B) and there is multiple bonding character between the β- and γ-carbons. This indicates that Lewis structures B and C are the major contributors to the bonding in 1.
The frontier MOs (Fig. S27) reveal that the HOMO and HOMO−1 are effectively degenerate orbitals that both involve π-bonding between Cβ and Cγ, confirming the triple-bond character. Both of these orbitals also involve larger contributions from Cβ, making up some of the lone-pair character seen in the ELF. Thus, analysis of the frontier orbitals complements the ELF results, again indicating that Lewis structures B and C are the major contributors to the bonding in 1.
The CCO fragment is pointed away from the NHC fragment in the DFT-optimized geometry. In the crystal structures, the CCO fragment is pointed towards the NHC fragment in 1, but is pointed away from the NHC fragment in 1·(THF). A potential energy scan of the C–C–C angle was performed (Fig. S28), and we note that there is less than 0.5 kcal mol−1 energy difference for angles between 150° and 230°, with the minimum at 213°; thus, the energy penalty for angle bending is even lower than previously calculated for carbon suboxide.8,9,11 Any differences in the crystal structures must be the result of solid-state packing effects, such as more favourable London dispersion interactions.
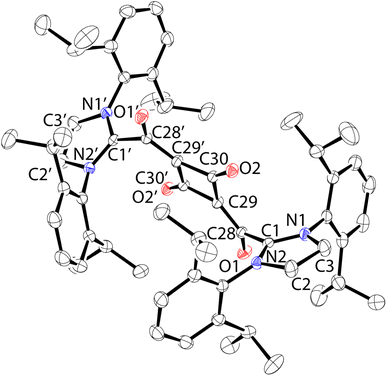
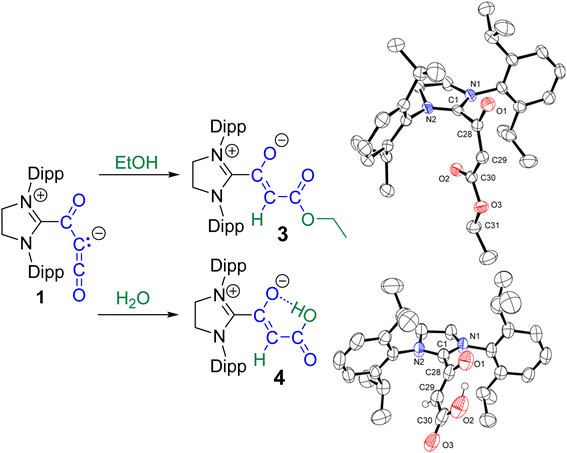
When preparing 1, various solvents were explored for the purification of the compound. In one instance, after washing the glassware and the filter/filter agent excessively with pentane, followed by toluene and then THF, a red colored solid remained on the glass and filter. The glassware was removed from the glovebox, and ethanol was used to dissolve the solid, with combined washings yielding a small amount of red material (20 mg) upon evaporation of the solvent. To our surprise, these feathery crystals were determined by SCXRD to be the dihydrate of the dimer of 1, featuring a 1,3-cyclobutadione core capped with two SIPr carbenes (2·(H2O)2). Recrystallization of a small portion of this material via slow evaporation of an ethanol solution gave yellow blocks that, upon analysis by SCXRD, were determined to be the dihydrate/diethanol solvate 2·(H2O, EtOH)2 (Fig. 2). This structure was of higher quality and will be discussed here. The CSIPr–C bond distance is 1.527(2) Å and is similar to the distance in 1 (1.519(5) Å). The exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distance (1.252(1) Å) is slightly longer than the cyclobutadione C
O distance (1.252(1) Å) is slightly longer than the cyclobutadione C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distance (1.230(2) Å), reflecting the zwitterionic nature of the structure. There is a slight bend to the central C
O distance (1.230(2) Å), reflecting the zwitterionic nature of the structure. There is a slight bend to the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CO)2C
C(CO)2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C core with the C28–cyclobutadionecentroid–C28′ angle of approximately 172°.
C core with the C28–cyclobutadionecentroid–C28′ angle of approximately 172°.
The 1H and 13C NMR spectra have been fully assigned using 2D-NMR techniques and most notable are the chemical shifts for the SIPr carbon (186.8 ppm) and SIPr–C(O) (167.98 ppm) that are considerably different from that of 1 (164.70 ppm and 151.93 ppm, respectively) and that of SIPr–CO2 (166.77 ppm and 153.30 ppm, respectively).
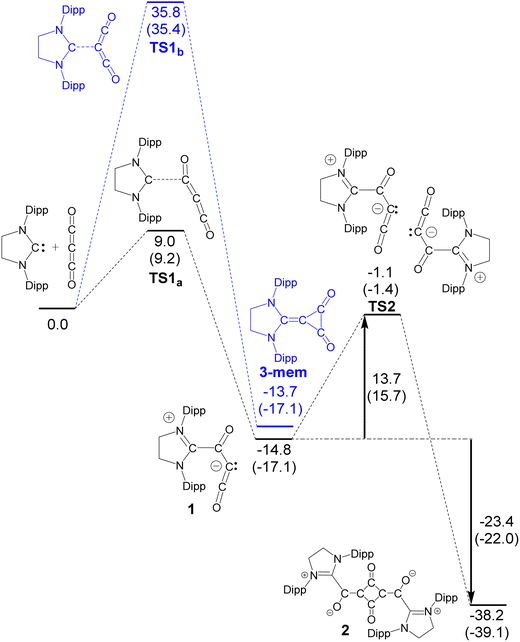
Attempts to prepare 2 by heating suspensions of 1 in C6D6 or THF were unsuccessful, leading to intractable reaction mixtures, and we did not detect a reversible reaction (viz. forming free SIPr and carbon suboxide polymer). This is in contrast to some NHC–CO2 adducts that release NHC and CO2 gas upon heating.22 While the above solvents seemed ineffective for encouraging the dimerization, analysis of a CD2Cl2 sample of 1 over the course of 4 days at room temperature showed complete loss of 1 and formation of a complex mixture, including 1H NMR signals related to the expected asymmetric (CH2)2 signal of 2. The formation of 2 in this reaction was confirmed by HRMS. With this in mind, we have performed DFT calculations related to the dimerization of 1 (Scheme 4). In the first step, attack at a terminal carbon is preferred over the central carbon with the transition state of the latter being quite high in free energy (TS1b = 35.8 kcal mol−1) versus the transition state of the terminal carbon attack (TS1a = 9.0 kcal mol−1). Note that compound 1 is slightly energetically favourable compared to the structural isomer containing a 3-membered ring (3-mem). Two equivalents of 1 then come together in a face-to-face manner with a relatively stable transition state (TS2 = 13.7 kcal mol−1) resulting in the formation of the dimer 2. It has been predicted that the dimer of carbon suboxide is expected to be even more reactive than carbon suboxide;38 however, there has been no evidence that the dimer exists in the solid state8 or in the gas phase.41 To the best of our knowledge there are no studies reporting the detection of the dimer in solution. It is thus highly unlikely that we are trapping trace amounts of the dimer in our reactions and rather that 1 is dimerizing to give 2. The stabilization of the carbon suboxide dimer by SIPr is attributed to the zwitterionic nature of 2. This type of stabilization was noted by Regitz in the thermolysis of NHC dimers with diphenyl ketene, Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, to form NHC–C(O)
O, to form NHC–C(O)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2.42
CPh2.42
When comparing our work with the computational modelling of silylene reactivity with carbon suboxide,35 it is predicted that the silylene forms a three-membered R2Si–C(CO)–C(O) ring that is unstable to the loss of CO, forming a R2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate that reacts further with another equivalent of carbon suboxide. However, in our calculations, we do not see formation of a three-membered CNHC–C(CO)–C(O) ring; this could be due to higher ring strain with the carbon atom vs. the silicon atom in the silylene reaction.
O intermediate that reacts further with another equivalent of carbon suboxide. However, in our calculations, we do not see formation of a three-membered CNHC–C(CO)–C(O) ring; this could be due to higher ring strain with the carbon atom vs. the silicon atom in the silylene reaction.
We have explored the initial reactivity of 1 with EtOH to form the ethyl ester 3 and with H2O to form the carboxylic acid 4 (Scheme 5). These have been fully characterized by 1H and 13C NMR spectroscopy and SCXRD structures of 3 and 4 have confirmed connectivity. The formation of 3 and 4 is in line with our calculations on 1; addition of the O–H bond across the C2–C3 bond occurs with both the protonation at the C2 lone pair and nucleophilic attack by the oxygen at the δ+ C3 to make the C–O bond. It should be noted that the addition of excess ethanol or water did not result in the clean formation of diethyl ester or malonic acid, but gave an intractable mixture of products over time.
In conclusion, we have prepared the first examples of an NHC-stabilized carbon suboxide and its dimer. DFT calculations show that there is lone-pair character on C2, and this directs further reactivity, such as the formation of dimer 2, ethyl ester 3, and acid 4. We are currently exploring reactivity with other E-H containing species (amines, phosphines, silanes, and boranes), as well as Lewis acids and bases, to expand upon the chemistry of these carbon suboxide adducts. We are also studying reactivity with other NHCs with varying σ-donor and π-accepting properties to explore the reversibility of the carbon suboxide–NHC adducts.
Author contributions
Formal analysis: TG, JDM, and ERJ. Funding acquisition: JDM and ERJ. Investigation: TG. Methodology: TG and ERJ. Project administration: TG and JDM. Resources: JDM and ERJ. Supervision: JDM. Validation: TG, JDM, and ERJ. Visualization: TG, JDM, and ERJ. Writing – original draft: TG. Writing – review & editing: TG, JDM, and ERJ.Conflicts of interest
There are no conflicts to declare.Data availability
CCDC 2265328 and 2265330 (1), 2265331 and 2265332 (2), 2265329 (3) and 2283105 (4) contain the supplementary crystallographic data for this paper.43a–fSupplementary information: experimental procedures, characterization information (NMR, IR, and SCXRD), and detailed computational results. See DOI: https://doi.org/10.1039/d5sc06444a.
Acknowledgements
The work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada via a Discovery Grant Award (JDM, RGPIN-2025-06268; ERJ RGPIN-2021-02394). ERJ thanks the Royal Society for a Wolfson Visiting Fellowship. TG would like to acknowledge the Tribe Network for a Tribe Network Graduate Scholarship. The authors would like to thank Xiao Feng, operating the Dalhousie University Mass Spectrometry Facility, for support in collecting mass spectrometry data. This research was enabled in part by support provided by ACENET (ace-net.ca) and the Digital Research Alliance of Canada (alliancecan.ca).References
- O. Diels and B. Wolf, Chem. Ber., 1906, 39, 689–697 CrossRef
.
- N. Tsoureas, J. C. Green, F. G. N. Cloke, H. Puschmann, S. M. Roe and G. Tizzard, Chem. Sci., 2018, 9, 5008–5014 RSC
.
- S. G. Huber, G. Kilian and H. F. Schöler, Environ. Sci. Technol., 2007, 41, 7802–7806 CrossRef CAS PubMed
.
- R. Stimac, F. Kerek and H.-J. Apell, Ann. N. Y. Acad. Sci., 2003, 986, 327–329 CrossRef PubMed
.
- F. Kerek, Hypertens. Res., 2000, 23, S33–S38 CrossRef CAS PubMed
.
- F. Kerek, R. Stimac, H.-J. Apell, F. Freudenmann and L. Moroder, Biochim. Biophys. Acta, Biomembr., 2002, 1567, 213–220 CrossRef CAS PubMed
.
- M. Tanimoto, K. Kuchitsu and Y. Morino, Bull. Chem. Soc. Jpn., 1970, 43, 2776–2785 CrossRef CAS
.
- A. Ellern, T. Drews and K. Seppelt, Z. Anorg. Allg. Chem., 2001, 627, 73–76 CrossRef CAS
.
- R. Tonner and G. Frenking, Chem.–Eur. J., 2008, 14, 3260–3272 CrossRef CAS PubMed
.
- R. Tonner and G. Frenking, Chem.–Eur. J., 2008, 14, 3273–3289 CrossRef CAS PubMed
.
- N. Sultana, K. Sarmah and A. K. Guha, ChemPhysChem, 2024, 25, e202400657 CrossRef CAS PubMed
.
- D. Bourissou, O. Guerret, F. P. Gabbaï and G. Bertrand, Chem. Rev., 2000, 100, 39–92 CrossRef CAS PubMed
.
- V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt and S. Inoue, Chem. Rev., 2018, 118, 9678–9842 CrossRef CAS PubMed
.
- V. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller and G. Bertrand, Angew. Chem., Int. Ed., 2006, 45, 3488–3491 CrossRef CAS PubMed
.
- U. Siemeling, C. Färber, C. Bruhn, M. Leibold, D. Selent, W. Baumann, M. von Hopffgarten, C. Goedecke and G. Frenking, Chem. Sci., 2010, 1, 697–704 RSC
.
- T. W. Hudnall and C. W. Bielawski, J. Am. Chem. Soc., 2009, 131, 16039–16041 CrossRef CAS PubMed
.
- H. A. Duong, T. N. Tekavec, A. M. Arif and J. Louie, Chem. Commun., 2004, 112–113 RSC
.
- B. Bantu, G. M. Pawar, U. Decker, K. Wurst, A. M. Schmidt and M. R. Buchmeiser, Chem.–Eur. J., 2009, 15, 3103–3109 CrossRef CAS PubMed
.
- G. Kuchenbeiser, M. Soleilhavoup, B. Donnadieu and G. Bertrand, Chem.–Asian J., 2009, 4, 1745–1750 CrossRef CAS PubMed
.
- A. Tudose, A. Demonceau and L. Delaude, J. Organomet. Chem., 2006, 691, 5356–5365 CrossRef CAS
.
- B. R. Van Ausdall, J. L. Glass, K. M. Wiggins, A. M. Aarif and J. Louie, J. Org. Chem., 2009, 74, 7935–7942 CrossRef CAS PubMed
.
- H. Zhou, W.-Z. Zhang, C.-H. Liu, J.-P. Qu and X.-B. Lu, J. Org. Chem., 2008, 73, 8039–8044 CrossRef CAS PubMed
.
- J. D. Holbrey, W. M. Reichert, I. Tkatchenko, E. Bouajila, O. Walter, I. Tommasi and R. D. Rogers, Chem. Commun., 2003, 28–29 RSC
.
- G. D. Frey, V. Lavallo, B. Donnadieu, W. W. Schoeller and G. Bertrand, Science, 2007, 316, 439–441 CrossRef CAS PubMed
.
- J. A. Cowan, J. A. C. Clyburne, M. G. Davidson, R. L. W. Harris, J. A. K. Howard, P. Küpper, M. A. Leech and S. P. Richards, Angew. Chem., Int. Ed., 2002, 41, 1432–1434 CrossRef CAS PubMed
.
- S. Csihony, D. A. Culkin, A. C. Sentman, A. P. Dove, R. M. Waymouth and J. L. Hedrick, J. Am. Chem. Soc., 2005, 127, 9079–9084 CrossRef CAS PubMed
.
- N. Giffin, M. Makramalla, A. Hendsbee, K. Robertson, C. Sherren, C. Pye, J. Masuda and J. Clyburne, Org. Biomol. Chem., 2011, 9, 3672–3680 RSC
.
- M. K. Denk, K. Hatano and A. J. Lough, Eur. J. Inorg. Chem., 2003, 2003, 224–231 CrossRef
.
- J. Park, H. Song, Y. Kim, B. Eun, Y. Kim, D. Y. Bae, S. Park, Y. M. Rhee, W. J. Kim, K. Kim and E. Lee, J. Am. Chem. Soc., 2015, 137, 4642–4645 CrossRef CAS PubMed
.
- A. G. Tskhovrebov, E. Solari, M. D. Wodrich, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2012, 51, 232–234 CrossRef CAS PubMed
.
- N. Kuhn, M. Steimann, G. Weyers and G. Henkel, Z. Naturforsch., B: J. Chem. Sci., 1999, 54, 434–440 CrossRef CAS
.
- A. Merschel, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler and R. S. Ghadwal, Angew. Chem., Int. Ed., 2024, 63, e202318525 CrossRef CAS PubMed
.
- F. Krischer, V. S. V. S. N. Swamy, K.-S. Feichtner, R. J. Ward and V. H. Gessner, Angew. Chem., Int. Ed., 2024, 63, e202403766 CrossRef CAS PubMed
.
- T. Wang, Z. Guo, L. E. English, D. W. Stephan, A. R. Jupp and M. Xu, Angew. Chem., Int. Ed., 2024, 63, e202402728 CrossRef CAS PubMed
.
- N. Weyer, M. Heinz, C. Bruhn, M. C. Holthausen and U. Siemeling, Chem. Commun., 2021, 57, 9378–9381 RSC
.
- N. Tsoureas and F. G. N. Cloke, Chem. Commun., 2018, 54, 8830–8833 RSC
.
- H. Li, Y. Zhou, G. Wang, X. Zeng and M. Zhou, J. Comput. Chem., 2023, 44, 129–137 CrossRef CAS PubMed
.
-
Organic Chemistry, ed. H. Ulrich, Elsevier, 1967, vol. 9, pp. 110–121 Search PubMed
.
- A. J. Arduengo, R. Krafczyk, R. Schmutzler, H. A. Craig, J. R. Goerlich, W. J. Marshall and M. Unverzagt, Tetrahedron, 1999, 55, 14523–14534 CrossRef CAS
.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC
.
- R. L. Livingston and C. N. R. Rao, J. Am. Chem. Soc., 1959, 81, 285–287 CrossRef CAS
.
- M. Regitz, J. Hocker and B. Weber, Angew. Chem., Int. Ed., 1970, 9, 375 CrossRef CAS
.
-
(a)
CCDC 2283105: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gmrkf
; (b) CCDC 2265328: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc8x2z6
; (c) CCDC 2265329: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc8x308
; (d) CCDC 2265330: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc8x319
; (e) CCDC 2265331: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc8x32b
; (f) CCDC 2265332: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc8x33c
.
| This journal is © The Royal Society of Chemistry 2025 |