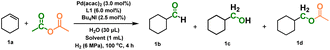Open Access Article
Open Access ArticleAcetic anhydride as a versatile carbon source in carbonylation reactions
Yanru
Zhang†
abc,
Ying
Wang†
 *a,
Junfeng
Xiang
d,
Yanyan
Wang
ac,
Longbo
Zhang
ac,
Jun
He
a,
Chenglong
Yu
ac,
Jia
Guo
ac,
Jie
Cui
d,
Xing
Tong
ac,
Ziwei
Zhao
ac,
Tianbin
Wu
a,
Qingli
Qian
*a,
Junfeng
Xiang
d,
Yanyan
Wang
ac,
Longbo
Zhang
ac,
Jun
He
a,
Chenglong
Yu
ac,
Jia
Guo
ac,
Jie
Cui
d,
Xing
Tong
ac,
Ziwei
Zhao
ac,
Tianbin
Wu
a,
Qingli
Qian
 *ac and
Buxing
Han
*ac and
Buxing
Han
 *ace
*ace
aBeijing National Laboratory for Molecular Sciences, Key Laboratory of Colloid and Interface and Thermodynamics, Center for Carbon Neutral Chemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: wangying2016@iccas.ac.cn; qianql@iccas.ac.cn; hanbx@iccas.ac.cn
bResearch Institute of Petroleum Processing, SINOPEC, Beijing 100083, China
cSchool of Chemical Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
dCenter for Physicochemical Analysis Measurements, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
eShanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, China
First published on 31st October 2025
Abstract
Acetic anhydride (Ac2O) is a cheap and easily available chemical feedstock that is widely used as an acetylating or dehydrating agent in organic synthesis. Here, we report Ac2O as a novel and versatile carbon source in carbonylation reactions. Notably, besides CO, the acetic acid (AcOH), decomposed from the Ac2O molecule, could participate in the reaction and simultaneously act as a necessary cosolvent. Acetate esters, aldehydes, and carboxylic acids could be produced separately by simply regulating the Pd-catalyzed reaction of olefins, Ac2O and H2. Remarkably, the reaction is applicable to various olefins, and high yields (up to 95%) as well as good regioselectivities of the acetate esters and aldehydes could be achieved. The outstanding results of acetate ester synthesis represent a successful and innovative cascade reaction, which demonstrates good atom economy. The mechanism of the reactions, especially the Ac2O decomposition, has been studied by control experiments and DFT calculations. This work opens a new avenue for carbonylation reactions.
Introduction
Carbonylation reactions are of great significance in chemistry and the chemical industry.1–3 In the past few decades, their versatility and increasing elaboration as synthetic tools have led to broader applications.4 Carbonyl-containing chemicals (such as aldehydes, carboxylic acids and esters) are widely used as raw materials and solvents for producing medicines, pesticides, cosmetics, perfumes, shampoos, lubricants, food additives, etc. As a traditional feedstock, CO is widely used in carbonylation reactions,1,5,6 but it is a flammable and toxic gas that encounters various inconveniences in many cases.To address these problems, developing benign and safe CO surrogates has gained much attention in synthetic chemistry and catalysis.7–9 The known CO surrogates include carbon dioxide (CO2),10–14 formic acid,15,16 formates,17,18 aldehydes,19,20 formamides,21,22 alcohols,23,24 metal carbonyls,25,26 chloroform,27 silacarboxylic acid,28,29 glyoxylic acid and its acetal derivatives,30,31 acid chlorides32,33 and so on. In addition, a two-chamber reactor was developed, where CO was generated by controlled activation of a solid CO surrogate in one chamber and delivered to another chamber for carbonylation reactions.28,33,34 In a pioneering study, in the presence of a sterically congested hydrosilane, aroyl chloride was used as a carbon electrophile and CO source for stereoselective carboformylation of alkynes, which was further extended to four chemodivergent carbonylation reactions.32
Ac2O is a cheap and readily available chemical feedstock, and its annual world production is about 3 million tons. To our knowledge, direct valorization of Ac2O as a CO surrogate in carbonylation reactions has not been reported yet. In this work, we disclosed Ac2O as an unprecedented CO surrogate as well as a versatile carbon source, which was applied in Pd-catalyzed olefin carbonylation reactions (Fig. 1). Interestingly, by only subtle modification of the catalytic system, three categories of important carbonylation products (acetate esters, aldehydes and carboxylic acids) could be produced separately with moderate to excellent yields. It is worth noting that Ac2O could not only act as a versatile feedstock but also engender necessary solvent conditions during the reaction.
Results and discussion
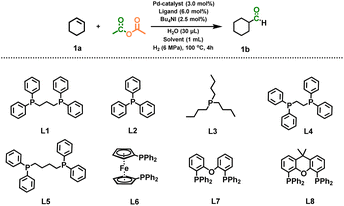
Catalytic systems
Different parts of the Ac2O molecule can be in situ transferred to a new carbonyl-containing molecule in the presence of an appropriate catalytic system. In this work, we started with the carbonylation of cyclohexene with Ac2O and H2. The results of catalyst screening are presented in Table 1. High yield (81%) of 1b was achieved when the reaction was promoted by a Pd(acac)2 catalyst, L1 ligand and Bu4NI additive in 2-methyltetrahydrofuran (2-MTHF) solvent. CO and methane (CH4) were observed after the reaction, which may be from decomposition of Ac2O (Fig. S1). Obviously, CO participated in the hydroformylation of cyclohexene. No 1b was observed in the absence of H2 because H2 is necessary for the decomposition of Ac2O as well as the hydroformylation of the olefin. Other Pd complexes were also tested, but the yields were lower (Pd(OAc)2, Pd(TFA)2 and Pd2(dba)3) or the reaction could hardly proceed (PdCl2 and PdI2) (Table S1). When other transition metal compounds (Co, Mn, Ru, Rh, Ni, Pt, and Ir) were used as catalysts, the target reaction did not occur. 1b was not detected without the bidentate ligand L1. We tried monodentate ligands (L2 and L3) as well, but they were ineffective. Other bidentate phosphine ligands such as L4, L5, L6, L7, and L8 were also screened in the reaction. The results suggested that the distance between the phosphorus coordination sites and the rigidity of ligands significantly affected the reaction yields.| Entry | Catalyst | Ligandb | Solvent | Yieldc [%] |
|---|---|---|---|---|
| a Reaction conditions: 30 μmol Pd catalyst, 60 μmol ligand, 25 μmol Bu4NI, 30 μL H2O, 1 mL solvent, 1 mmol cyclohexene, 4 mmol Ac2O, 6 MPa H2, 100 °C, 4 h. b L1, 1,3-bis(diphenylphosphino)propane (DPPP); L2, triphenylphosphine (PPh3); L3, tri-n-butylphosphine (PnBu3), L4, 1,2-bis(diphenylphosphino)ethane (DPPE); L5, 1,4-bis(diphenylphosphino)butane (DPPB); L6, 1,1′-bis(diphenylphosphino)ferrocene (DPPF); L7, (oxybis(2,1-phenylene))bis-(diphenylphosphane) (DPEPhos); L8, (9,9- dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphine) (Xantphos). c The yields of 1b were GC yields, which were calculated on the basis of the cyclohexene substrate. d Remarkable cyclohexylmethyl acetate (1d, yield of 14%) was observed after the reaction, as shown in Table 2, and optimization of the conditions for this product is given in Table 3. e If no additive was added before the reaction, cyclohexanecarboxylic acid (1e, yield of 3%) was observed, and the yield could reach 69% when the reaction time was extended to 48 h. Abbreviations: dba (dibenzylideneacetone), TFA (trifluoroacetic acid), Bu4NI (tetrabutylammonium iodide), Bu4NBr (tetrabutylammonium bromide), Bu4NCl (tetrabutylammonium chloride), 2,5-DMTHF (2,5-dimethyltetrahydrofuran). | ||||
| 1 | Pd(acac)2 | L1 | 2-MTHF | 81 |
| 2 | Pd(OAc)2 | L1 | 2-MTHF | 68 |
| 3 | PdI2 | L1 | 2-MTHF | 0 |
| 4 | Pd(acac)2 | — | 2-MTHF | 0 |
| 5 | Pd(acac)2 | L2 | 2-MTHF | <1 |
| 6 | Pd(acac)2 | L3 | 2-MTHF | <1 |
| 7 | Pd(acac)2 | L4 | 2-MTHF | <1 |
| 8 | Pd(acac)2 | L5 | 2-MTHF | 35 |
| 9 | Pd(acac)2 | L6 | 2-MTHF | <1 |
| 10 | Pd(acac)2 | L7 | 2-MTHF | 28 |
| 11 | Pd(acac)2 | L8 | 2-MTHF | 0 |
| 12 | Pd(acac)2 | L1 | THF | 22 |
| 13 | Pd(acac)2 | L1 | 2,5-DMTHF | 73 |
| 14 | Pd(acac)2 | L1 | 1,2-Dichloroethane | <1 |
| 15 | Pd(acac)2 | L1 | AcOH | <1 |
| 16 | Pd(acac)2 | L1 | NMP | <1 |
| 17 | Pd(acac)2 | L1 | H2O | 0 |
| 18 | Pd(acac)2 | L1 | Toluene | 54d |
| 19 | Pd(acac)2 | L1 | 2-MTHF | 1e |
The iodide additive was necessary in the reaction, while the performances of its bromide and chloride counterparts were poor. Other iodides (NIS, NaI, and KI) were not as effective as Bu4NI, while molecular iodine did not work at all (Table S2). Thus, the iodide anion was indispensable, and the cation of the additive may be responsible for the increased solubility of the reaction components. A notable solvent effect was observed, and 2-MTHF gave the best result in the reaction. Interestingly, the methyl group (–CH3) on the THF ring had a remarkable promotive influence on the catalytic result, and the reaction yield decreased from 81% to 22% when THF was used as the solvent. However, the introduction of two methyl groups on the THF ring (2,5-DMTHF) also depressed the reaction rate. Some other ethers were also effective solvents, albeit with lower reaction yields than 2-MTHF (Table S3), while the commonly used solvents such as 1,2-dichloroethane, AcOH and NMP (N-methyl-2-pyrrolidone) were hardly usable. Although trace water (H2O) was needed in the reaction, H2O itself was not an effective solvent for the reaction. In short, the catalytic system consisting of Pd(acac)2, L1, Bu4NI and 2-MTHF was suitable to accelerate the reaction.
Based on the optimized catalytic system for the synthesis of aldehydes, we conducted the reaction at different temperatures and found that 100 °C was appropriate (Fig. S2). The impact of dosages of the catalytic components on the hydroformylation was also systematically studied (Tables S4 and S5). The amount of the Pd catalyst and L1 ligand had a marked impact on the reaction yields. In addition, the ratio of their dosages, which may change the coordination state of the metal center, was also a key factor for the catalytic performance. The optimal dosage of Bu4NI was slightly less than that of the Pd catalyst, which suggested that the halide-promoting effect only existed at one or several step(s) in the catalytic cycle.35 Interestingly, the addition of a small amount of H2O was needed for the reaction, and 30 μL (1.7 mmol) H2O engendered the optimal result. Further studies indicated that the suitable amounts of Ac2O and H2 were 4 mmol and 6 MPa, respectively (Table S6). Within only 4 h, the reaction proceeded quickly and highly selectively to give 1b, where only trace byproducts were observed (Fig. S3). Therefore, the conditions in entry 1 of Table 1 were the optimal conditions for the synthesis of aldehyde by carbonylation of olefin using Ac2O as the carbonyl source.
Very interestingly, when the solvent was altered from 2-MTHF to toluene, the production of 1b was restrained and a remarkable amount of acetate ester was observed (Table 1, entry 18). It was found that toluene as a solvent may promote the formation of cyclohexylmethanol more effectively, which may react in situ with acetic acid from Ac2O to generate 1d (Table 2). Synthesis of higher acetate esters from olefins is an interesting topic, and the previous reports generally utilized Rh or Ru catalysts, which encountered multi-step operations, low yields/regioselectivities and/or involvement of strong acidic conditions.36–38 We further screened the different catalytic systems for this reaction (Table 3). The choice of catalyst precursors and ligands remarkably influences the generation of acetate esters. Through systematic screening, we identified Pd(acac)2 as the optimal catalyst and L1 as the most effective ligand. Building on this finding, we observed that variations in the solvent have a considerable impact on the formation of acetate esters. To facilitate a more comprehensive comparison of solvent effects, we conducted our studies with an extended reaction time of 16 h. Notably, aromatic solvents with structures analogous to toluene also demonstrated favorable outcomes, although they were not as good as toluene. The catalytic results with different dosages of reactants, temperature and reaction time are shown in Table S7. Under the optimal conditions, the yield of 1d was 90%. It was found that the yield of 1e showed an upward trend when the Bu4NI additive was removed from the typical conditions of Table 1. When we extended the reaction time to 48 h, the yield of the acid product increased to 69%. In this way, acetate esters, aldehydes and carboxylic acids could be separately and efficiently produced from olefin, Ac2O and H2 by simply adjusting the catalytic system and the reaction conditions.
| Entry | Solvent | Yieldb [%] | ||
|---|---|---|---|---|
| 1b | 1c | 1d | ||
| a Reaction conditions: 30 μmol Pd(acac)2, 60 μmol L1, 25 μmol Bu4NI, 30 μL H2O, 1 mL solvent, 1 mmol cyclohexene, 4 mmol Ac2O, 6 MPa H2, 100 °C, 4 h. b The yields of 1b, 1c and 1e were GC yields, which were calculated on the basis of the cyclohexene substrate. | ||||
| 1 | Toluene | 54% | 15% | 14% |
| 2 | 2-MTHF | 81% | 8% | 2% |
| Entry | Catalyst | Ligand | Reaction time [h] | Solvent | Yieldb [%] |
|---|---|---|---|---|---|
| a Reaction conditions: 30 μmol Pd catalyst, 60 μmol ligand, 25 μmol Bu4NI, 30 μL H2O, 1 mL solvent, 1 mmol cyclohexene, 4 mmol Ac2O, 6 MPa H2, 100 °C. b The yields of 1d were GC yields, which were calculated based on the cyclohexene substrate. c Reaction conditions: 30 μmol Pd(acac)2, 60 μmol L1, 25 μmol Bu4NI, 40 μL H2O, 1 mL toluene, 1 mmol cyclohexene, 8 mmol Ac2O, 6 MPa H2, 110 °C. | |||||
| 1 | Pd(acac)2 | L1 | 4 | 2-MTHF | 2 |
| 2 | PdI2 | L1 | 4 | 2-MTHF | 0 |
| 3 | Pd(dba)2 | L1 | 4 | 2-MTHF | <1 |
| 4 | Pd(acac)2 | L4 | 4 | 2-MTHF | 0 |
| 5 | Pd(acac)2 | L5 | 4 | 2-MTHF | <1 |
| 6 | Pd(acac)2 | L6 | 4 | 2-MTHF | 0 |
| 7 | Pd(acac)2 | L7 | 4 | 2-MTHF | 0 |
| 8 | Pd(acac)2 | L8 | 4 | 2-MTHF | 0 |
| 9 | Pd(acac)2 | L1 | 4 | Toluene | 14 |
| 10 | Pd(acac)2 | L1 | 16 | Toluene | 69 |
| 11 | Pd(acac)2 | L1 | 16 | p-Xylene | 54 |
| 12 | Pd(acac)2 | L1 | 16 | m-Xylene | 43 |
| 13 | Pd(acac)2 | L1 | 16 | Mesitylene | 46 |
| 14 | Pd(acac)2 | L1 | 16 | 2-MTHF | 21 |
| 15 | Pd(acac)2 | L1 | 16 | Toluene | 90c |
Synthesis of aldehydes
As can be seen in Fig. 2, the typical catalytic system for the hydroformylation can be directly applied to various olefin substrates, including linear olefins and cyclic olefins (1a–13a), where corresponding aldehydes were produced with yields up to 86%. Furthermore, excellent regioselectivities (up to l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b = 91
b = 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) could be obtained with linear olefins as substrates. It is noteworthy that internal olefins from petroleum refineries, in particular the mixtures of their isomers, are cheap and readily available feedstocks for carbonylation reactions. However, internal alkenes are more stable and often less reactive than terminal ones.39 To our delight, high yields of aldehydes and excellent regioselectivities for linear aldehydes can be obtained whether terminal olefins (5a, 7a, 10a and 12a) or internal olefins (6a, 8a, 9a, 11a and 13a) were used as substrates. This suggested that our catalytic system possesses both high olefin isomerization activity and efficient synergy among different catalytic steps.40
9) could be obtained with linear olefins as substrates. It is noteworthy that internal olefins from petroleum refineries, in particular the mixtures of their isomers, are cheap and readily available feedstocks for carbonylation reactions. However, internal alkenes are more stable and often less reactive than terminal ones.39 To our delight, high yields of aldehydes and excellent regioselectivities for linear aldehydes can be obtained whether terminal olefins (5a, 7a, 10a and 12a) or internal olefins (6a, 8a, 9a, 11a and 13a) were used as substrates. This suggested that our catalytic system possesses both high olefin isomerization activity and efficient synergy among different catalytic steps.40
Synthesis of acetate esters
We are also pleased to find that high to excellent yields of acetate esters could be produced by simply switching the reaction solvent from 2-MTHF to toluene and increasing the temperature from 100 °C to 110 °C. As a whole, the yields of the acetate esters were obviously higher than those of the corresponding aldehydes discussed above. The regioselectivities of the linear acetate esters were also high in the case of linear olefins, whether they were terminal or internal ones, further demonstrating the efficient olefin isomerization capability of the catalytic system. This strategy exhibits good atom economy because most atoms of the Ac2O molecule could be incorporated into the products (Fig. 3).Synthesis of carboxylic acids
Previous studies showed that solvent was an important factor for the competition between hydroformylation and hydrocarboxylation,16 while we discovered that the iodide additive can act as a switch for the reaction between hydroformylation and hydrocarboxylation in 2-MTHF solvent. As discussed above, aldehydes of moderate to high yields could be generated with the Bu4NI additive (1b–8b). Interestingly, without Bu4NI, the production of aldehydes was almost completely suppressed and the corresponding carboxylic acids were formed with moderate yields (1e–4e), albeit with a longer reaction time and decreased regioselectivity (3e, 4e). The mass and NMR spectra of the target products in Fig. 2 are shown in Fig. S26–S44.In short, by subtle adjustments of the reaction parameters, three categories of important products could be successfully synthesized via the carbonylation of olefins using Ac2O as the versatile carbon feedstock and H2 as the reductant. This discovery represents a significant advance in synthetic chemistry.
Mechanistic study of the carbonylation reactions
We started the mechanistic study with the synthesis of aldehyde from cyclohexene, Ac2O and H2. As mentioned above, the reaction could hardly occur without adding H2O as a cosolvent (1.7 mmol). Additionally, the control experiments using an equivalent molar amount of DMSO (1.7 mmol) or 4 mmol AcOH instead of H2O displayed a similar catalytic performance (Table S8), which may be ascribed to their comparable polarities. To further understand the role of the cosolvent, we carried out the hydroformylation of cyclohexene with CO and H2, which revealed that H2O was not needed in the presence of a certain carboxylic acid (Table S9). These results suggest that the role of the cosolvent may be to promote Ac2O decomposition to generate CO and AcOH. The necessity of an acid cocatalyst in the Pd catalyzed hydroformylation has been explained elsewhere.41To elucidate the molecular transformation pathway, a series of isotope labeling tests were carried out using (CH313CO)2O, (13CH3CO)2O, (CD3CO)2O, H218O, D2, D2O and THF-d8, respectively (Fig. S4–S11), and the results are summarized in Fig. 4. It suggests that CO, decomposed from Ac2O, participated in the formation of 1bvia hydroformylation. CH4 was generated synchronously by decomposition of the acetyl group (CH3CO–) in the Ac2O molecule [(CH3CO)2O + H2 → CO + CH4 + CH3COOH]. In addition, remarkable H–D exchange was observed when the deuterated reagents ((CD3CO)2O, D2 and D2O) were utilized. We further conducted the in situ1H NMR and 31P{1H} NMR studies of the reaction process. The results suggest that at the start of the reaction, considerable AcOH was produced, indicating it was a quick step (Fig. S12). With the accumulation of CO as well as the activation of the catalyst, 1b was observed, and its amount increased gradually. As the reaction progressed, the peak of H2 became weaker because of its consumption during the reaction. In the NMR tube, the cyclohexene is in excess compared to H2. Evident Pd–H signals were found (Fig. S13), which is a typical feature of a hydroformylation catalyst. Observation of a quintet hydride signal suggested the presence of binuclear Pd hydrides.42 The in situ31P{1H} NMR spectra demonstrated that DPPP coordinated with Pd in the reaction (Fig. S14).16,35,43
Based on the above results, we can deduce the reaction pathway for the synthesis of acetate esters, aldehydes and carboxylic acids using olefins, Ac2O and H2, as depicted in Fig. 5. The transformation of olefins follows the sequence: the isomerization of internal olefins to terminal ones, the hydrocarboxylation of terminal olefins to carboxylic acids or the hydroformylation of terminal olefins to aldehydes, the hydrogenation of aldehydes to alcohols, and the esterification of alcohols to acetate esters. These cascade steps could be verified by monitoring the reactive intermediates during the reaction (Fig. S15). Compared to 2-MTHF, toluene as a solvent may simultaneously and more effectively accelerate these steps (Tables 2 and 3).
 | ||
| Fig. 5 Pd-catalyzed synthesis of acetate esters, aldehydes and carboxylic acids with Ac2O as a versatile reactant. | ||
The role of the iodide additive in switching between hydroformylation and hydrocarboxylation reactions is shown in Fig. 1. The halide anion could assist the heterolytic splitting of dihydrogen in the Pd-acyl species and promote the Pd-acyl hydrogenolysis step of the hydroformylation catalytic cycle, which is the rate-determining step.40 Without the iodide additive, the hydroformylation reaction was inhibited, and the hydrocarboxylation reaction became predominant over a longer reaction time (48 h).
Besides Ac2O, we also tried various other anhydrides, including n-anhydrides, iso-anhydrides and cyclic anhydrides, as the potential carbonyl source for the synthesis of 1b (Table S10). It was found that most other anhydrides were ineffective for the reaction. Interestingly, acetic propionic anhydride could also engender good results, but the result using propanoic anhydride was poor. Furthermore, the acetyl group in acetaldehyde, acetate and acetyl iodide was not an effective CO source either (Table S11). Thus, the CH3CO– group in the anhydride is key for the generation of CO. We also tried several other common carbonyl-containing chemicals (formic acid, paraformaldehyde, acetone, acetylacetone, oxalic acid, pyruvic acid, etc.), but they did not work. These results demonstrated that Ac2O was a unique CO source.
Ac2O is a low-toxicity, easy-to-store, and transportable liquid. As a CO surrogate, it may significantly enhance operational safety by eliminating the need for high-pressure equipment and handling of the toxic CO gas. More importantly, Ac2O played a multifunctional role in our system. It served as both the CO source and the carboxylate reagent for in situ ester formation, while the acetic acid byproduct provided the necessary acidic environment to promote the carbonylation reactions. This integrated design allows for an efficient process under mild conditions, improving the accessibility of this transformation for synthetic applications.
To our knowledge, this is the first report of Ac2O as a versatile CO and carbon source. Therefore, a detailed study of the Ac2O decomposition process in the reaction is highly desirable.
Mechanistic study of Ac2O decomposition
Based on the systematic study of CO production from the reaction of Ac2O and H2, we found that the simplest catalytic components to decompose Ac2O were Pd(acac)2 and L1 (Table S12). The decomposition rate increased quickly with increasing temperature, and the impact of temperature became less significant at above 110 °C (Fig. 6). The in situ1H NMR characterization was conducted to further monitor the Ac2O decomposition process (Fig. S16). At the beginning of the reaction, AcOH and CH4 were observed, which should form synchronously with CO. The analysis of the time course indicated that Ac2O decomposition was a quick process (Fig. S17). CH4 and CO of similar amounts were detected, formed by C–C bond breaking of the CH3CO– group in the presence of H2. Besides CO and methane, equal moles of AcOH to Ac2O could be produced via Ac2O decomposition without H2O. As discussed above, a cosolvent such as H2O or DMSO was required in the hydroformylation of olefin with Ac2O and H2. This is mainly because the iodide additive of the catalytic system could significantly inhibit Ac2O decomposition, which may be overcome by the use of a cosolvent with high polarity (Table S12, entries 7–10). | ||
| Fig. 6 Effect of temperature on Ac2O decomposition. Reaction conditions: 30 μmol Pd(acac)2, 60 μmol L1, 4 mmol Ac2O, 1 mL 2-MTHF, 6 MPa H2, 4 h. | ||
The in situ31P{1H} NMR study of the Ac2O decomposition showed that the active center of the reaction was mainly the Pd(0) complex formed by Pd(acac)2 and a L1 ligand (Fig. S18). Formation of minor hemioxide and oxide of L1 was found, which may be ascribed to the redox reaction between the Pd(II) precursor and the ligand.44 The tendency to form a Pd(0) active center was further confirmed by a control experiment with another Pd(II) precursor, Pd(OAc)2(DPPP) (Fig. S19).45 To learn more about the catalyst, we analyzed the solution quenching at 0.5 h of reaction by matrix-assisted laser desorption ionization Fourier transform ion cyclotron resonance mass spectrometry (MALDI-FT-ICR-MS), and [Pd(DPPP)(H)]+ was observed at m/z 519.06241 (Fig. S20). The D2 labeling test proved that the H+ of the [Pd(DPPP)(H)]+ was from the matrix dithranol rather than H2 (Fig. S21). At 0.5 h, minor [Pd(DPPP)(CH3)]+ and [Pd(DPPP)(OAc)]+ were also detected at m/z 533.07807 and m/z 577.06799, respectively (Fig. S20). No catalytic species containing CO was observed, which suggested that the release of CO from the species was a quick step. After 4 h of reaction, [Pd(DPPP)(CH3)]+ disappeared, which suggested the completion of the catalytic cycle for Ac2O decomposition (Fig. S22).
According to the results in Table S12, the necessary catalytic components to decompose Ac2O were Pd(acac)2 and L1. Based on this fact and the literature,42,46,47 we proposed the possible pathway of Ac2O decomposition (Fig. 7). The rationality of the pathway as well as the related transition states was further verified by DFT calculations of solvated free energy (ΔG) (Fig. 8 and S23–S25). The initial active species IM2 of the catalytic cycle was Pd(DPPP), formed by coordination of the Pd precursor and the L1 ligand. Firstly, Ac2O underwent oxidative addition to Pd(DPPP) via the transition state (TS2-4), forming a four-coordinated intermediate IM4, Pd(DPPP)(Ac)(OAc). Secondly, the C–C bond of the acetyl group (Ac) broke, and a subsequent rearrangement occurred via the transition state (TS4-6), forming a five-coordinated intermediate IM6, Pd(DPPP)(OAc)(Me)(CO). Thirdly, CO was generated by dissociation from IM6, giving a four-coordinated species IM7, namely, Pd(DPPP)(OAc)(Me). Subsequently, in the presence of H2, the –OAc group dissociated from IM7via the transition state (TS7-11), where AcOH was produced and the four-coordinate species IM11 formed, namely, Pd(DPPP)(Me)(H). Finally, CH4 dissociated from IM11 through the transition state (TS11-2), where the initial active species IM2 was regenerated and the next catalytic cycle began. In Fig. 8, the proposed pathway (green curves) was also compared with various other possible decomposition routes, such as different modes of oxidative addition of Ac2O (dark blue curves), and different detachment orders of groups on the intermediates (purple curves and brown curves). The results demonstrated that the pathway in Fig. 7 was reasonable. Besides Pd(DPPP) (IM2), other common initial Pd species such as Pd(DPPP)(OAc)− (IM1) and Pd(DPPP)(OAc)(H) (IM3) were also evaluated by DFT calculations (Fig. S23). The results suggested that Pd(DPPP) was the more favorable initial active species of the reaction. In a word, the proposed pathway of the Ac2O decomposition was reasonable.
 | ||
| Fig. 7 The proposed pathway of Ac2O decomposition. In the figure, the structural formula of the bidentate phosphine ligand DPPP is simplified and presented within the box. | ||
Conclusions
In summary, we have discovered that Ac2O can be used as a CO surrogate and a versatile carbon source in carbonylation reactions. A Pd-based catalytic system was designed to selectively synthesize aldehydes, acetate esters and carboxylic acids from olefins, Ac2O and H2, where the type of the main product could be tuned by simply changing the reaction solvent or regulating the iodide additive. The yields of acetate esters, aldehydes and carboxylic acids could be as high as 95%, 86% and 69%, respectively. The catalytic system could be applied to various olefin substrates, including linear olefins (terminal and internal) and cyclic olefins. In addition, both terminal and internal alkenes could serve as substrates, yielding acetates or aldehydes with good regioselectivity. In some cases, the linear-to-branched ratio reached up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The outstanding results of acetate esters synthesis displayed good atom economy and successful cascade reactions. Notably, Ac2O not only participated in these carbonylation reactions as a feedstock, but also provided the necessary acidic solvent environment. The mechanism of Ac2O decomposition was proposed based on a series of control experiments and DFT calculations. We believe this discovery represents a novel paradigm for carbonylation reactions and will trigger more research in this area.
1. The outstanding results of acetate esters synthesis displayed good atom economy and successful cascade reactions. Notably, Ac2O not only participated in these carbonylation reactions as a feedstock, but also provided the necessary acidic solvent environment. The mechanism of Ac2O decomposition was proposed based on a series of control experiments and DFT calculations. We believe this discovery represents a novel paradigm for carbonylation reactions and will trigger more research in this area.
Author contributions
Y. R. Z. and Y. W. contributed equally. Y. R. Z., Y. W., Q. L. Q. and B. X. H. proposed the project, designed the experiments, and wrote the manuscript. Y. R. Z. performed all the experiments. J. F. X. and J. C. conducted the high-pressure NMR studies and offered the operation guidance for the in situ NMR test. Y. Y. W., L. B. Z., J. H., C. L. Y., J. G., X. T., Z. W. Z. and T. B. W. conducted some characterization studies and assisted in analyzing the experimental data. Q. L. Q. and B. X. H. supervised the whole project.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental methods, figures, tables, and DFT calculations. See DOI: https://doi.org/10.1039/d5sc06323j.Acknowledgements
The work was financially supported by the National Key Research and Development Program of China (2024YFE0206500), the National Science Foundation of China (22033009, 22072156, 22073104, and 22121002) and Science and Technology Bureau of Tai'an City (2022ZDZX031).Notes and references
- J. B. Peng, F. P. Wu and X. F. Wu, First-Row Transition-metal-catalyzed carbonylative transformations of carbon electrophiles, Chem. Rev., 2018, 119, 2090–2127 CrossRef PubMed.
- L. Wu, X. Fang, Q. Liu, R. Jackstell, M. Beller and X. F. Wu, Palladium-catalyzed carbonylative transformation of C(sp3)-X bonds, ACS Catal., 2014, 4, 2977–2989 CrossRef.
- R. Franke, D. Selent and A. Börner, Applied hydroformylation, Chem. Rev., 2012, 112, 5675–5732 CrossRef PubMed.
- J. Li and Y. Shi, Progress on transition metal catalyzed asymmetric hydroesterification, hydrocarboxylation, and hydroamidation reactions of olefins, Chem. Soc. Rev., 2022, 51, 6757–6773 RSC.
- X. F. Wu, X. Fang, L. Wu, R. Jackstell, H. Neumann and M. Beller, Transition-Metal-Catalyzed Carbonylation Reactions of Olefins and Alkynes: A Personal Account, Acc. Chem. Res., 2014, 47, 1041–1053 CrossRef PubMed.
- T. Tewari, R. Kumar and S. H. Chikkali, Iron-catalysed highly selective hydroalkoxycarbonylation of alkynes using CO as C1 source, Catal. Sci. Technol., 2023, 13, 5549–5555 RSC.
- M. V. Khedkar, S. R. Khan, T. L. Lambat, R. G. Chaudhary and A. A. Abdala, CO surrogates: a green alternative in palladium-catalyzed CO gas free carbonylation reactions, Curr. Org. Chem., 2020, 24, 2588–2600 CrossRef CAS.
- P. Gautam and B. M. Bhanage, Recent advances in the transition metal catalyzed carbonylation of alkynes, arenes and aryl halides using CO surrogates, Catal. Sci. Technol., 2015, 5, 4663–4702 RSC.
- L. Wu, Q. Liu, R. Jackstell and M. Beller, Carbonylations of alkenes with CO surrogates, Angew. Chem., Int. Ed., 2014, 53, 6310–6320 CrossRef CAS.
- L. Fu, S. Li, Z. Cai, Y. Ding, X. Q. Guo, L. P. Zhou, D. Yuan, Q. F. Sun and G. Li, Ligand-enabled site-selectivity in a versatile rhodium(II)-catalysed aryl C–H carboxylation with CO2, Nat. Catal., 2018, 1, 469–478 CrossRef CAS.
- M. He, Y. Sun and B. Han, Green carbon science: Efficient carbon resource processing, utilization, and recycling towards carbon neutrality, Angew. Chem., Int. Ed., 2022, 61, e202112835 CrossRef CAS PubMed.
- B. B. A. Bediako, Q. Qian and B. Han, Synthesis of C2+ chemicals from CO2 and H2 via C–C bond formation, Acc. Chem. Res., 2021, 54, 2467–2476 CrossRef.
- S. Wang and C. Xi, Recent advances in nucleophile-triggered CO2 -incorporated cyclization leading to heterocycles, Chem. Soc. Rev., 2019, 48, 382–404 RSC.
- L. Song, Y. X. Jiang, Z. Zhang, Y. Y. Gui, X. Y. Zhou and D. G. Yu, CO2 = CO + [O]: recent advances in carbonylation of C-H bonds with CO2, Chem. Commun., 2020, 56, 8355–8367 RSC.
- R. Sang, P. Kucmierczyk, K. Dong, R. Franke, H. Neumann, R. Jackstell and M. Beller, Palladium-catalyzed selective generation of CO from formic acid for carbonylation of alkenes, J. Am. Chem. Soc., 2018, 140, 5217–5223 CrossRef CAS.
- W. Ren, W. Chang, J. Dai, Y. Shi, J. Li and Y. Shi, An effective Pd-catalyzed regioselective hydroformylation of olefins with formic acid, J. Am. Chem. Soc., 2016, 138, 14864–14867 CrossRef CAS PubMed.
- Y. Hoshimoto, T. Ohata, Y. Sasaoka, M. Ohashi and S. Ogoshi, Nickel(0)-catalyzed [2+2+1] carbonylative cycloaddition of imines and alkynes or norbornene leading to γ-lactams, J. Am. Chem. Soc., 2014, 136, 15877–15880 CrossRef CAS PubMed.
- H. Li, H. Neumann, M. Beller and X. F. Wu, Aryl formate as bifunctional reagent: Applications in palladium-catalyzed carbonylative coupling reactions using in situ generated CO, Angew. Chem., Int. Ed., 2014, 53, 3183–3186 CrossRef CAS.
- S. K. Murphy, J. W. Park, F. A. Cruz and V. M. Dong, Rh-catalyzed C-C bond cleavage by transfer hydroformylation, Science, 2015, 347, 56–60 CrossRef CAS.
- T. Morimoto, K. Fuji, K. Tsutsumi and K. Kakiuchi, CO-transfer carbonylation reactions. A catalytic Pauson-Khand-type reaction of enynes with aldehydes as a source of carbon monoxide, J. Am. Chem. Soc., 2002, 124, 3806–3807 CrossRef PubMed.
- T. Tewari, R. Kumar and S. H. Chikkali, Iron-catalyzed magnesium-mediated formal hydroformylation of alkynes and alkenes, ChemCatChem, 2023, 15, e202201394 CrossRef.
- K. Hosoi, K. Nozaki and T. Hiyama, Carbon monoxide free aminocarbonylation of aryl and alkenyl iodides using DMF as an amide source, Org. Lett., 2002, 4, 2849–2851 CrossRef PubMed.
- J. H. Park, Y. Cho and Y. K. Chung, Rhodium-catalyzed Pauson–Khand-type reaction using alcohol as a source of carbon monoxide, Angew. Chem., Int. Ed., 2010, 49, 5138–5141 CrossRef PubMed.
- Q. Liu, K. Yuan, P. B. Arockiam, R. Franke, H. Doucet, R. Jackstell and M. Beller, Regioselective Pd-catalyzed methoxycarbonylation of alkenes using both paraformaldehyde and methanol as CO surrogates, Angew. Chem., Int. Ed., 2015, 54, 4493–4497 CrossRef PubMed.
- L. Åkerbladh, P. Nordeman, M. Wejdemar, L. R. Odell and M. Larhed, Synthesis of 4-quinolones via a carbonylative sonogashira cross-coupling using molybdenum hexacarbonyl as a CO source, J. Org. Chem., 2015, 80, 1464–1471 CrossRef PubMed.
- J. Chen, K. Natte, A. Spannenberg, H. Neumann, M. Beller and X. F. Wu, Palladium-catalyzed carbonylative [3+2+1] annulation of N-aryl-pyridine-2-amines with internal alkynes by C-H activation facile synthesis of 2-quinolinones, Chem.–Eur. J., 2014, 20, 14189–14193 CrossRef.
- G. Sun, M. Lei and L. Hu, A facile and efficient method for the synthesis of alkynone by carbonylative Sonogashira coupling using CHCl3as the CO source, RSC Adv., 2016, 6, 28442–28446 RSC.
- S. D. Friis, R. H. Taaning, A. T. Lindhardt and T. Skrydstrup, Silacarboxylic acids as efficient carbon monoxide releasing molecules: Synthesis and application in palladium-catalyzed carbonylation reactions, J. Am. Chem. Soc., 2011, 133, 18114–18117 CrossRef PubMed.
- S. K. Pedersen, H. G. Gudmundsson, D. U. Nielsen, B. S. Donslund, H. C. D. Hammershøj, K. Daasbjerg and T. Skrydstrup, Main element chemistry enables gas-cylinder-free hydroformylations, Nat. Catal., 2020, 3, 843–850 CrossRef.
- Y. Xiang, G. Zeng, X. Sang, X. Li, Q. Ding and Y. Peng, Decarboxylative coupling of glyoxylic acid and its acetal derivatives: A unique C1 formylation synthon, Tetrahedron, 2021, 91, 132193 CrossRef.
- H. Huang, C. Yu, Y. Zhang, Y. Zhang, P. S. Mariano and W. Wang, Chemo- and regioselective organo-photoredox catalyzed hydroformylation of styrenes via a radical pathway, J. Am. Chem. Soc., 2017, 139, 9799–9802 CrossRef PubMed.
- Y. H. Lee, E. H. Denton and B. Morandi, Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source, Nat. Chem., 2021, 13, 123–130 CrossRef PubMed.
- P. Hermange, A. T. Lindhardt, R. H. Taaning, K. Bjerglund, D. Lupp and T. Skrydstrup, Ex situ generation of stoichiometric and substoichiometric 12CO and 13CO and its efficient incorporation in palladium catalyzed Aminocarbonylations, J. Am. Chem. Soc., 2011, 133, 6061–6071 CrossRef PubMed.
- S. D. Friis, A. T. Lindhardt and T. Skrydstrup, The development and application of two-chamber reactors and carbon monoxide precursors for safe carbonylation reactions, Acc. Chem. Res., 2016, 49, 594–605 CrossRef PubMed.
- M. Sigrist, Y. Zhang, C. Antheaume and P. Dydio, Isoselective hydroformylation of propylene by iodide-assisted palladium catalysis, Angew. Chem., Int. Ed., 2022, 61, e202116406 CrossRef.
- R. Dühren, P. Kucmierczyk, C. Schneider, R. Jackstell, R. Franke and M. Beller, Ruthenium-catalysed domino hydroformylation–hydrogenation–esterification of olefins, Catal. Sci. Technol., 2021, 11, 5777–5780 RSC.
- A. O. Dias, F. G. Delolo, J. A. Avendano-Villarreal, E. N. dos Santos and E. V. Gusevskaya, Production of diacetates from propenylphenols: A one-pot two-step approach involving hydroformylation/hydrogenation/O-acylation, Appl. Catal., A, 2023, 665, 119369 CrossRef.
- F. G. Delolo, T. P. Moreira, A. O. Dias, E. N. dos Santos and E. V. Gusevskaya, Replacing syngas by formic acid and acetic anhydride with advantages: tandem carbonylation of renewable terpenes and propenylbenzenes, J. Catal., 2024, 432, 115437 CrossRef.
- G. Occhialini, V. Palani and A. E. Wendlandt, Catalytic, contra-Thermodynamic Positional Alkene Isomerization, J. Am. Chem. Soc., 2022, 144, 145–152 CrossRef PubMed.
- D. Konya, K. Q. A. Lenero and E. Drent, Highly selective halide anion-promoted palladium-catalyzed hydroformylation of internal alkenes to linear alcohols, Organometallics, 2006, 25, 3166–3174 CrossRef.
- R. Jennerjahn, I. Piras, R. Jackstell, R. Franke, K. D. Wiese and M. Beller, Palladium-Catalyzed Isomerization and Hydroformylation of Olefins, Chem. Eur. J., 2009, 15, 6383–6388 CrossRef.
- R. Geitner, A. Gurinov, T. Huang, S. Kupfer, S. Gräfe and B. M. Weckhuysen, Reaction mechanism of Pd-catalyzed “CO-Free” carbonylation reaction uncovered by in situ spectroscopy: The formyl mechanism, Angew. Chem., Int. Ed., 2020, 60, 3422–3427 CrossRef.
- Y. Zhang, S. Torker, M. Sigrist, N. Bregović and P. Dydio, Binuclear Pd(I)–Pd(I) catalysis assisted by iodide ligands for selective hydroformylation of alkenes and alkynes, J. Am. Chem. Soc., 2020, 142, 18251–18265 CrossRef PubMed.
- C. Amatore, A. Jutand and A. Thuilliez, Formation of palladium(0) complexes from Pd(OAc)2 and a bidentate phosphine ligand (dppp) and their reactivity in oxidative addition, Organometallics, 2001, 20, 3241–3249 CrossRef.
- Z. Csákai, R. Skoda-Földes and L. Kollár, NMR investigation of Pd(II)-Pd(0) reduction in the presence of mono- and ditertiary phosphines, Inorg. Chim. Acta, 1999, 286, 93–97 CrossRef.
- A. Yamamoto, Design of catalytic processes based on studies of elementary processes, J. Org. Chem., 2004, 689, 4499–4510 CrossRef.
- F. Zhao, L. Han and T. Liu, Mechanistic insight into the ligand-controlled regioselective hydrocarboxylation of aryl olefins with palladium catalyst: A computational study, J. Org. Chem., 2023, 989, 122645 CrossRef.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |