Open Access Article
Open Access ArticleChemo- and atroposelective Boc protection for asymmetric synthesis of NH2-free axially chiral biaryl amino phenols
Yangyang
Wang
a,
Yike
Wang
a,
Zhe
Xu
a,
Kexin
Chen
a,
Ying-guo
Liu
 *b,
Xingkuan
Chen
*b,
Xingkuan
Chen
 *c,
Hongwei
Zhou
*c,
Hongwei
Zhou
 *d and
Jianfeng
Xu
*d and
Jianfeng
Xu
 *a
*a
aSchool of Chemistry and Chemical Engineering, Zhejiang Sci-Tech University, Hangzhou 310018, China. E-mail: jfxu@zstu.edu.cn
bHenan Institute of Advanced Technology and Pingyuan Laboratory, Zhengzhou University, Zhengzhou 450001, China. E-mail: liuyg@zzu.edu.cn
cDepartment of Chemistry, College of Chemistry and Materials Science, Jinan University, Guangzhou 510632, China. E-mail: xkchen@jnu.edu.cn
dCollege of Biological, Chemical Science and Engineering, Jiaxing University, Jiaxing 314001, China. E-mail: zhouhw@zju.edu.cn
First published on 27th October 2025
Abstract
Enantioselective protection strategies have emerged as powerful tools for creating high-value optically active compounds. Herein, we report an efficient and direct Lewis base-catalyzed enantioselective tert-butoxycarbonyl (Boc) protection strategy for the construction of axial chirality. Employing a chiral isothiourea (ITU) catalyst, a series of amino bisphenols undergo chemo- and atroposelective O-Boc protection with Boc anhydride (Boc2O), delivering NH2-free axially chiral biaryl amino phenols with moderate yields and high enantioselectivities. This desymmetrization protocol is scalable to gram level and enables facile downstream transformations of the chiral products. Computational studies reveal that the amino (NH2) group on the naphthalene ring facilitates intramolecular proton transfer of the hydroxyl (OH) group. Moreover, an unprecedented S⋯C–NH3+ electrostatic interaction, in combination with π–π stacking, is identified as a key stabilizing factor in the transition states.
Introduction
The strategic use of protecting groups is essential for synthesizing complex molecules containing multiple reactive functionalities.1 By integrating chiral catalysts with rationally designed substrates, conventional protection methods can be adapted for asymmetric transformations, opening new avenues for the construction of optically active compounds. In 2006, Snapper, Hoveyda, and co-workers pioneered an amino acid-derived imidazole-catalyzed asymmetric silylation of meso diols to afford secondary alcohols with high enantioselectivities (Scheme 1a).2 Inspired by this elegant work, the research groups of Snapper and Hoveyda,3a–c Oestreich,3d–g Song,3h He,3i List,3j and Franz3k subsequently developed a range of Lewis base-, transition-metal-, and Brønsted acid-catalyzed enantioselective O-silyl protection protocols to generate carbon-, silicon-, and axial chirality. Despite these advances, there remains an urgent need for the development of versatile enantioselective protection systems. Boc anhydride (Boc2O), also known as di-tert-butyl dicarbonate, is an easily available and synthetically versatile reagent that has been widely employed for amine protection in peptide, pharmaceutical, and natural product synthesis.1,4 Occasionally, Boc2O is also used for the protection of alcohols and thiols (Scheme 1b, left).5 We envision that a chiral Lewis base could activate Boc2O to form a reactive intermediate within a chiral environment, thereby enabling either the kinetic resolution of racemic substrates or the desymmetrization of prochiral substrates to establish new stereogenic centers (Scheme 1b, right). This approach could diversify catalytic asymmetric protection strategies beyond traditional silylation methods.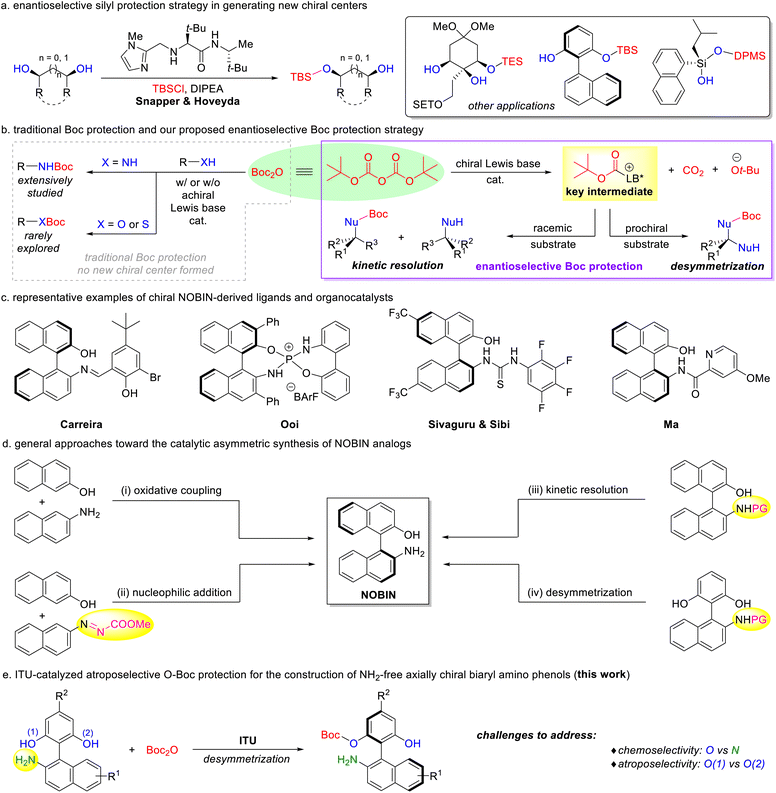 | ||
| Scheme 1 Enantioselective protection strategy and our protocol to directly access NH2-free NOBIN analogs. | ||
Atropisomeric 2-amino-2′-hydroxy-1,1′-binaphthyl (NOBIN) and its analogs are recognized as privileged scaffolds broadly utilized in the development of chiral ligands and catalysts (Scheme 1c).6 Consequently, significant efforts have been devoted to the stereoselective preparation of these frameworks.7–12 To date, there are four primary catalytic asymmetric strategies toward the synthesis of axially chiral NOBIN analogs (Scheme 1d): (i) transition-metal-catalyzed oxidative coupling of 2-naphthols and 2-naphthylamines;8 (ii) Brønsted/Lewis acid-catalyzed nucleophilic addition of one aryl fragment to another;9 (iii) organocatalytic kinetic resolution of racemic NOBINs;10 and (iv) N-heterocyclic carbene (NHC)-catalyzed desymmetrization of prochiral amino bisphenols.11 While those protocols are well-established, most of them lead to N-protected axially chiral biaryl amino phenols as final products, necessitating an additional deprotection step that limits their synthetic utility. Our group aims to advance catalytic systems for constructing synthetically valuable molecules.13 We envisage that a Lewis base-catalyzed enantioselective O-Boc protection of unprotected biaryl amino bisphenols could offer a highly efficient and direct route to chiral NOBIN analogs. Achieving such a transformation requires addressing two key challenges: chemoselectivity between O-Boc and N-Boc protection, and atroposelectivity between the two identical hydroxy groups. Herein, we disclose an isothiourea (ITU)-promoted chemo- and atroposelective Boc protection of amino bisphenols, allowing for the rapid access to NH2-free axially chiral amino phenols with excellent enantiocontrol (Scheme 1e).
Results and discussion
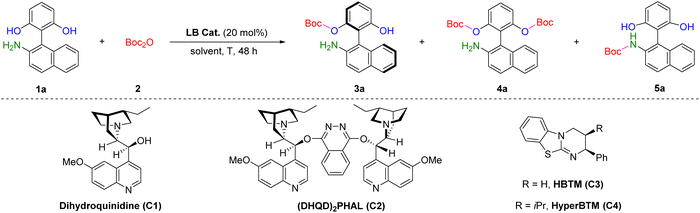
We began our investigation by selecting amino bisphenol 1a as the model substrate to react with Boc2O 2 in dichloromethane (CH2Cl2) at 25 °C for 48 hours, with key results summarized in Table 1. The use of cinchona alkaloid-derived Lewis base catalysts C1 and C2 efficiently generated the desired O-Boc protected amino phenol 3a in moderate yields and enantioselectivities, accompanied by side products including the di-O-Boc and N-Boc protected amino phenols (4a and 5a) (entries 1 and 2). To our delight, when isothiourea (ITU) catalysts C3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and C4
14 and C4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 were employed, formation of the undesired N-Boc product 5a was completely suppressed. Under these conditions, 3a was obtained in 35% yield with 56% ee and 53% yield with 69% ee, respectively (entries 3 and 4). Utilizing C4 as the optimal catalyst, we next evaluated a variety of solvents (entries 5–9). Both polar and non-polar solvents were compatible, with diethyl ether (Et2O) providing the best result, affording 3a in 44% yield and 72% ee (entry 6). Remarkably, lowering the reaction temperature to −20 °C significantly enhanced enantioselectivity, furnishing 3a with excellent 98% ee (entries 10–12). Further optimization of stoichiometry and catalyst loading revealed that using 1.1 equiv. of 2 and 5 mol% of C4 was sufficient to deliver the expected axially chiral biaryl amino phenol 3a in 62% yield and 98% ee (entries 13 and 14).
15 were employed, formation of the undesired N-Boc product 5a was completely suppressed. Under these conditions, 3a was obtained in 35% yield with 56% ee and 53% yield with 69% ee, respectively (entries 3 and 4). Utilizing C4 as the optimal catalyst, we next evaluated a variety of solvents (entries 5–9). Both polar and non-polar solvents were compatible, with diethyl ether (Et2O) providing the best result, affording 3a in 44% yield and 72% ee (entry 6). Remarkably, lowering the reaction temperature to −20 °C significantly enhanced enantioselectivity, furnishing 3a with excellent 98% ee (entries 10–12). Further optimization of stoichiometry and catalyst loading revealed that using 1.1 equiv. of 2 and 5 mol% of C4 was sufficient to deliver the expected axially chiral biaryl amino phenol 3a in 62% yield and 98% ee (entries 13 and 14).
| Entry | Lewis base cat. | Solvent | T (°C) | Yield of 3ab (%) | Yield of 4ab (%) | Yield of 5ab (%) | ee of 3ac (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions are as follows: 1a (0.2 mmol), 2 (0.3 mmol), Lewis base cat. (0.04 mmol), and solvent (2 mL) at a specific temperature for 48 h. b Isolated yield of product based on 1a. c Enantiomeric excess of 3a, determined via chiral-phase HPLC analysis. d 96 h. e 0.22 mmol of 2 was used. f 0.01 mmol of C4 was used. THF = tetrahydrofuran. | |||||||
| 1 | C1 | CH2Cl2 | 25 | 32 | 55 | 6 | 53 |
| 2 | C2 | CH2Cl2 | 25 | 39 | 40 | 4 | 66 |
| 3 | C3 | CH2Cl2 | 25 | 35 | 57 | Trace | 56 |
| 4 | C4 | CH2Cl2 | 25 | 53 | 40 | Trace | 69 |
| 5 | C4 | THF | 25 | 49 | 47 | Trace | 69 |
| 6 | C4 | Et2O | 25 | 44 | 37 | Trace | 72 |
| 7 | C4 | EtOAc | 25 | 46 | 42 | Trace | 63 |
| 8 | C4 | Toluene | 25 | 35 | 21 | Trace | 67 |
| 9 | C4 | CH3CN | 25 | 43 | 34 | Trace | 20 |
| 10 | C4 | Et2O | 0 | 49 | 47 | Trace | 94 |
| 11 | C4 | Et2O | −20 | 48 | 50 | Trace | 98 |
| 12d | C4 | Et2O | −40 | 48 | 50 | Trace | 98 |
| 13e | C4 | Et2O | −20 | 63 | 18 | Trace | 98 |
| 14e , | C4 | Et 2 O | −20 | 62 | 16 | Trace | 98 |
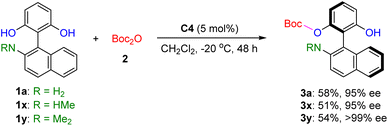
Having established the optimized reaction conditions (Table 1, entry 14), we then explored the generality of this enantioselective protection strategy. As shown in Table 2, a series of amino bisphenols 1 bearing diverse substitution patterns were examined. Substrates containing 6-methyl and 6-cyclopropyl groups on the naphthyl ring delivered the corresponding O-Boc protected products 3b and 3c in 57% yield with >99% ee and 53% yield with 97% ee, respectively. Halogen substituent such as 6-bromo was accommodated to afford 3d in 54% yield and 98% ee, indicating the potential for further elaboration of the axially chiral amino phenol scaffold. In contrast, the substrate bearing an electron-withdrawing COOMe group at the 6-position of the naphthyl ring formed the desired product 3e in only 16% yield and 90% ee. When amino bisphenols possessing 6-aryl and 6-(2-naphthyl) groups were subjected to the reaction, the desired products 3f–3i were obtained smoothly in 41–65% yields and 95 to >99% ee. Notably, replacing the aryl group with heteroaryl units had negligible effect on the reaction outcome, both 6-(2-furyl) and 6-(2-thienyl) substituted reactants efficiently furnished products 3j and 3k with moderate yields and outstanding enantioselectivities. Importantly, amino bisphenols comprising functional groups such as phenylethynyl, styryl, and vinyl were all competent substrates, providing the target axially chiral products 3l–3n in 55–65% yields and 98–99% ee. Substitution at the 7-position of the naphthyl ring was equally compatible, giving rise to products 3o–3q with decent yields and high enantioselectivities. Furthermore, when methyl groups were installed at the 5- or 4-position of the naphthyl ring, products 3r and 3s were isolated in approximately 50% yield with 98% ee. However, introducing a methyl group at the 3-position led to a significant drop in yield for product 3t, although high enantioselectivity was retained. Substituents on the bisphenol moiety were also well-tolerated. Reactions of substrates featuring methyl or phenyl groups at the 4′-position offered products 3u and 3v in 55% yield with 98% ee and 58% yield with 98% ee, respectively. However, when a trifluoromethyl group was introduced at the 4′-position, both the yield and enantioselectivity of the resulting product 3w decreased dramatically. Finally, the employment of ent-C4 as the catalyst successfully delivered ent-3a in 60% yield and −99% ee, suggesting that both of the enantiomers were readily accessible in our catalytic system. The absolute configurations of these axially chiral amino phenols were assigned as (S) by analogy to product 3a and 3k, whose structure was unequivocally confirmed through X-ray crystallographic analysis.16
| a Reaction conditions are as follows: 1 (0.2 mmol), 2 (0.22 mmol), C4 (0.01 mmol), and Et2O (2 mL) at −20 °C for 48 h. Isolated yield of product 3 based on 1. The enantiomeric excess of 3 were determined via chiral-phase HPLC analysis. b ent-C4 was used as the catalyst. c CH2Cl2 was used as the solvent. d 72 h. |
|---|
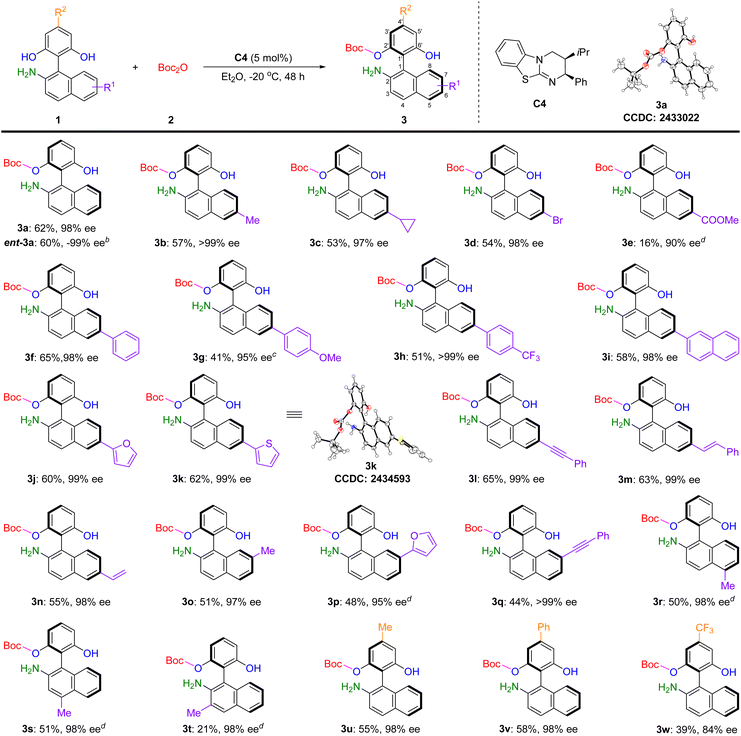
|
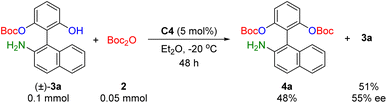
Since for all the substrates examined in Table 2, the formation of di-O-Boc protected amino phenol 4 was consistently observed as a side product, which largely limited the yield of the desired product 3, we sought to further investigate the role of the second O-Boc protection step. To this end, we conducted an additional experiment to determine whether this second protection event functions as a kinetic resolution (Scheme 2). Under the optimized reaction conditions, treatment of racemic 3a with 2 (0.5 equiv.) led to the recovery of chiral 3a in 51% yield (based on racemic 3a) and 55% ee, along with the generation of di-O-Boc product 4a in 48% yield. These results indicate that the high enantioselectivity observed in our catalytic system primarily originates from the initial desymmetrizing O-Boc protection step. Additionally, the second O-Boc protection may contribute positively to the final enantioselectivity outcome, albeit at the expense of product yield.
Experimentally, the asymmetric Boc protection of aminophenol 1a using isothiourea catalyst C4 predominantly yielded the S-configured product 3a, without requiring additional bases. Notably, the phenol group was selectively acylated over the amino group. To gain further mechanistic insight into the origin of chemo- and atroposelectivity, density functional theory (DFT) calculations were performed, focusing on the key transition state. A simplified model was employed to probe the reaction pathway, highlighting the role of the amino group as an intramolecular base.
As depicted in Fig. 1, the most favorable transition state, TS_(S), exhibited the lowest energy barrier (27.4 kcal mol−1), which was 1.3 kcal mol−1 lower than TS_N (28.7 kcal mol−1) and 2.2 kcal mol−1 lower than TS_(R) (29.6 kcal mol−1). This energy difference is consistent with the experimentally observed high enantioselectivity (98% ee), although trace amounts of the N-acylated byproduct 5a was detected. Interestingly, despite TS_(R) having a slightly lower relative Gibbs free energy—0.3 kcal mol−1 below TS_(S)—its corresponding starting substrate complex (R-complex) was 2.5 kcal mol−1 more stable than the S-complex. This energy landscape may help explain the minor formation of the di-O-Boc protected byproduct 4a, even though the S-configured product 3a remains predominant.
Non-covalent interactions were analyzed using the independent gradient model based on Hirshfeld partition (IGMH).17 Contrary to previous reports,18 no O⋯S chalcogen bonding (nO – σ*S–C) was identified in either TS_(S) or TS_(R), both of which led to the O-acylated product (S)-3a and (R)-3a. Instead, the S-atom of the Boc-C4 intermediate engaged in electrostatic interaction with the amino carbon of substrate 1a (S⋯C–NH3+). This interaction is likely driven by intramolecular proton transfer from the OH group to the NH2 group. In contrast, TS_N, which leads to N-acylation, exhibited O⋯S chalcogen bonding, contributing to its conformational stability. Additionally, π–π stacking interactions played a significant role in stabilizing all three key transition states.
To determine whether the NH2 group is essential for maintaining a stable conformation and achieving high enantioselectivity, we conducted three control experiments. As illustrated in Scheme 3, the secondary (NHMe) and tertiary amine (NMe2) substituted substrates afforded the corresponding products 3x and 3y in moderate yields with excellent enantioselectivities. These results indicate that protonation of the nitrogen atom facilitates electrostatic interactions between the sulfur and phenyl carbon, which are crucial regardless of whether the nitrogen substituent is NH2, NHMe, or NMe2. Further transition-state calculations for 3x and 3y also reveal the presence of such noncovalent interactions (please see SI for details). It is also noteworthy that, in the case of 3y, the lower reaction energy barrier (21.0 kcal mol−1) compared to TS_(S) may be attributed to its higher enantioselectivity (ee > 99%).
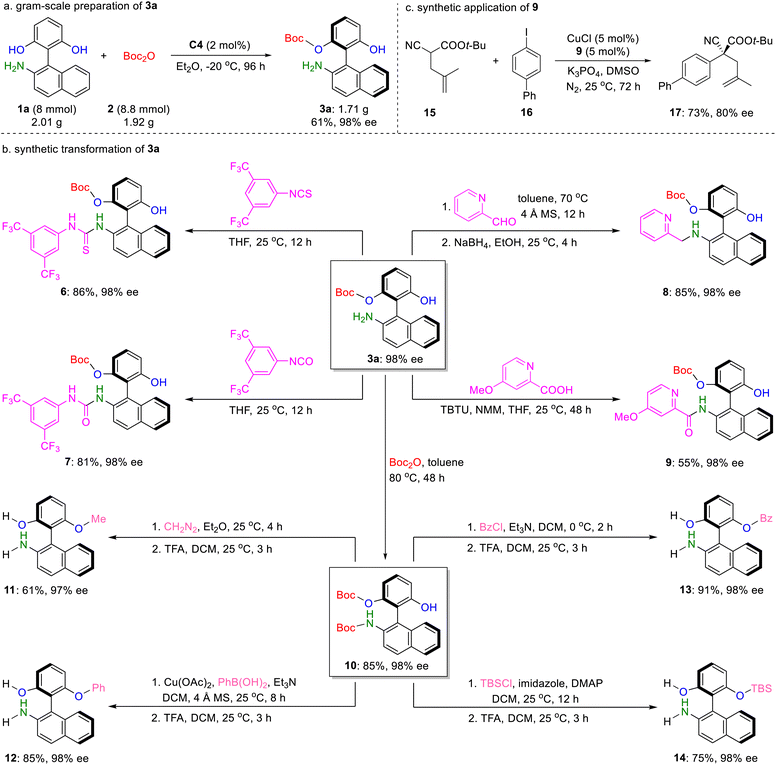
Finally, the practicality and applicability of the developed protocol were demonstrated by gram-scale preparation and downstream transformations of 3a. Under the optimized catalytic conditions, the reaction was efficiently scaled up to an 8 mmol scale using only 2 mol% of C4, affording 1.71 g of 3a without any loss in enantiopurity (Scheme 4a). The versatility of 3a was showcased through various derivatizations. Treatment of enantioenriched 3a with isothiocyanate and isocyanate smoothly delivered the corresponding thiourea 6 and urea 7 in 86% yield with 98% ee and 81% yield with 98% ee, respectively. Reactions of 3a with pyridine derivatives provided the chiral amino phenol analogs 8 and 9 in acceptable yields with retention of the enantioselectivities. Moreover, condensation of 3a with Boc2O 2 in the absence of a catalyst offered N-Boc protected amino phenol 10. Subsequent O-functionalization of 10, including O-methylation, O-phenylation, O-benzoylation, and O-silylation, followed by Boc deprotection, furnished the anticipated derivatives 11–14 in moderate to good yields and excellent enantioselectivities, thereby significantly enriching the library of axially chiral amino phenols (Scheme 4b). To further highlight the synthetic potential of this methodology, a Cu-catalyzed enantioselective coupling reaction of α-substituted cyanoacetate 15 with aryl iodide 16 was carried out utilizing the previously synthesized amino phenol derivative 9 as a chiral ligand (Scheme 4c).6d Gratifyingly, preliminary result showed that the corresponding product 17 bearing an all carbon quaternary stereocenter could be obtained in 73% yield with a promising 80% ee, revealing the utility of these axially chiral amino phenols in asymmetric catalysis.
Conclusions
In summary, we have developed the first enantioselective Boc protection strategy for the construction of optically pure compounds. In the presence of a chiral isothiourea catalyst, a broad range of bisphenols featuring diverse substitution patterns undergo reaction with Boc anhydride to afford NH2-free axially chiral biaryl amino phenols with excellent enantiocontrol. This operationally simple and efficient method shows strong potential for application in preparative synthesis, and the resulting chiral products can be readily derivatized into valuable functional molecules. Complementary DFT studies provide mechanistic insight into the origins of the observed chemo- and atroposelectivities. Further investigations aimed at extending this enantioselective Boc protection strategy to the synthesis of additional types of chiral elements are currently underway in our laboratory.Author contributions
Y. W. performed and analysed the experiments. Y. W., Z. X. and K. C. contributed to the data analysis. Y.-G. L. conducted the DFT calculations. X. C. and J. X. conceptualized and directed the project. H. Z. and J. X. drafted the manuscript. All authors contributed to the discussions.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental procedures and characterization data. See DOI: https://doi.org/10.1039/d5sc06233k.CCDC 2433022 and 2434593 contain the supplementary crystallographic data for this paper.16a,b
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (Grant No. 21602203, 22071082, 22101266), the Natural Science Foundation of Guangdong Province (No. 2024A1515011116), and the Pearl River talent program of Guangdong Province (Youth Top-Notch Talent, 2021QN020472), the Excellent Youth Program of Hennan Province (242300421119), China Scholarship Council and Zhengzhou University for financial support.Notes and references
- P. G. M. Wuts, Greene's Protective Groups in Organic Synthesis, Wiley, Hoboken, 2025 Search PubMed.
- Y. Zhao, J. Rodrigo, A. H. Hoveyda and M. L. Snapper, Nature, 2006, 443, 67 CrossRef CAS PubMed.
- (a) Y. Zhao, A. W. Mitra, A. H. Hoveyda and M. L. Snapper, Angew. Chem., Int. Ed., 2007, 46, 8471 CrossRef CAS PubMed; (b) Z. You, A. H. Hoveyda and M. L. Snapper, Angew. Chem., Int. Ed., 2009, 48, 547 CrossRef CAS PubMed; (c) N. Manville, H. Alite, F. Haeffner, A. H. Hoveyda and M. L. Snapper, Nat. Chem., 2013, 5, 768 CrossRef CAS PubMed; (d) J. Seliger, X. Dong and M. Oestreich, Angew. Chem., Int. Ed., 2019, 58, 1970 CrossRef CAS PubMed; (e) J. Seliger and M. Oestreich, Angew. Chem., Int. Ed., 2021, 60, 247 CrossRef CAS PubMed; (f) M. Zhu, H. J. Jiang, I. Sharanov, E. Irran and M. Oestreich, Angew. Chem., Int. Ed., 2023, 62, e202304475 CrossRef CAS PubMed; (g) M. Zhu and M. Oestreich, ACS Catal., 2023, 13, 10244 CrossRef CAS; (h) S. Y. Park, J.-W. Lee and C. E. Song, Nat. Commun., 2015, 6, 7512 CrossRef PubMed; (i) J. Gao, P.-L. Mai, Y. Ge, W. Yuan, Y. Li and C. He, ACS Catal., 2022, 12, 8476 CrossRef CAS; (j) J. T. Han, H. Zhou and B. List, Synlett, 2023, 34, 2393 CrossRef CAS; (k) J. J. Dalton, A. Bernal Sanchez, A. T. Kelly, J. C. Fettinger and A. K. Franz, ACS Catal., 2024, 14, 1005 CrossRef CAS PubMed.
- S. W. Pedersen, C. J. Armishaw and K. Strømgaard, in Peptide Synthesis and Applications, ed. K. J. Jensen, P. T. Shelton and S. L. Pedersen, Springer, New York, 2013, pp. 65–80 Search PubMed.
- (a) Z. Cheraiet, S. Hessainia, S. Ouarna, M. Berredjem and N.-E. Aouf, Green Chem. Lett. Rev., 2013, 6, 211 CrossRef CAS; (b) Y.-L. Xu, J.-M. Qi, F.-F. Sun and N. Ma, Tetrahedron Lett., 2015, 56, 2744 CrossRef CAS; (c) A. Guardia, G. Gulten, R. Fernandez, J. Gomez, F. Wang, M. Convery, D. Blanco, M. Martinez, E. Perez-Herran, M. Alonso, F. Ortega, J. Rullas, D. Calvo, L. Mata, R. Young, J. C. Sacchettini, A. Mendoza-Losana, M. Remuinan, L. Ballell Pages and J. Castro-Pichel, ChemMedChem, 2016, 11, 687 CrossRef CAS PubMed.
- (a) E. M. Carreira, R. A. Singer and W. Lee, J. Am. Chem. Soc., 1994, 116, 8837 CrossRef CAS; (b) D. Uraguchi, N. Kinoshita and T. Ooi, J. Am. Chem. Soc., 2010, 132, 12240 CrossRef CAS PubMed; (c) N. Vallavoju, S. Selvakumar, S. Jockusch, M. P. Sibi and J. Sivaguru, Angew. Chem., Int. Ed., 2014, 53, 5604 CrossRef CAS PubMed; (d) R. Zhang, Q. Zhou, X. Wang, L. Xu and D. Ma, Angew. Chem., Int. Ed., 2023, 62, e202312383 CrossRef CAS PubMed.
- (a) K. Ding, X. Li, B. Ji, H. Guo and M. Kitamura, Curr. Org. Synth., 2005, 2, 499 CrossRef CAS; (b) J. K. Cheng, S. H. Xiang, S. Li, L. Ye and B. Tan, Chem. Rev., 2021, 121, 4805 CrossRef CAS PubMed.
- (a) M. Smrcina, M. Lorenc, V. Hanus, P. Sedmera and P. Kocovsky, J. Org. Chem., 1992, 57, 1917 CrossRef CAS; (b) X.-J. Zhao, Z.-H. Li, T.-M. Ding, J.-M. Tian, Y.-Q. Tu, A.-F. Wang and Y.-Y. Xie, Angew. Chem., Int. Ed., 2021, 60, 7061 CrossRef CAS PubMed; (c) A. Dyadyuk, V. Vershinin, H. Shalit, H. Shalev, N. Y. More and D. Pappo, J. Am. Chem. Soc., 2022, 144, 3676 CrossRef CAS PubMed.
- (a) Y.-H. Chen, L.-W. Qi, F. Fang and B. Tan, Angew. Chem., Int. Ed., 2017, 56, 16308 CrossRef CAS PubMed; (b) L.-W. Qi, S. Li, S.-H. Xiang, J. Wang and B. Tan, Nat. Catal., 2019, 2, 314 CrossRef CAS; (c) W.-Y. Ding, P. Yu, Q.-J. An, K. L. Bay, S.-H. Xiang, S. Li, Y. Chen, K. N. Houk and B. Tan, Chem, 2020, 6, 2046 CrossRef CAS; (d) S. Cen, N. Huang, D. Lian, A. Shen, M.-X. Zhao and Z. Zhang, Nat. Commun., 2022, 13, 4735 CrossRef CAS PubMed.
- (a) S. Shirakawa, X. Wu and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 14200 CrossRef CAS PubMed; (b) S. Lu, S. V. H. Ng, K. Lovato, J.-Y. Ong, S. B. Poh, X. Q. Ng, L. Kurti and Y. Zhao, Nat. Commun., 2019, 10, 3061 CrossRef PubMed; (c) W. Liu, Q. Jiang and X. Yang, Angew. Chem., Int. Ed., 2020, 59, 23598 CrossRef CAS PubMed.
- (a) G. Yang, D. Guo, D. Meng and J. Wang, Nat. Commun., 2019, 10, 3062 CrossRef PubMed; (b) S. Lu, S. B. Poh, Z. Rong and Y. Zhao, Org. Lett., 2019, 21, 6169 CrossRef CAS PubMed; (c) X. Yang, L. Wei, Y. Wu, L. Zhou, X. Zhang and Y. R. Chi, Angew. Chem., Int. Ed., 2023, 62, e202211977 CrossRef CAS PubMed; (d) Y. G. Liu, Z. Zhong, Y. Tang, H. Wang, S. V. C. Vummaleti, X. Peng, P. Peng, X. Zhang and Y. R. Chi, Nat. Commun., 2025, 16, 54 CrossRef PubMed.
- L. Wei, J. Li, Y. Zhao, Q. Zhou, Z. Wei, Y. Chen, X. Zhang and X. Yang, Angew. Chem., Int. Ed., 2023, 62, e202306864 CrossRef CAS PubMed.
- (a) C. He, Z. Li, H. Zhou and J. Xu, Org. Lett., 2019, 21, 8022 CrossRef CAS PubMed; (b) Z. Li, J. Peng, C. He, J. Xu and H. Ren, Org. Lett., 2020, 22, 5768 CrossRef CAS PubMed; (c) Z. Li, H. Zhou and J. Xu, Org. Lett., 2021, 23, 6391 CrossRef CAS PubMed; (d) H. Liu, P. He, X. Liao, Y. Zhou, X. Chen, W. Ou, Z. Wu, C. Luo, L. Yang and J. Xu, ACS Catal., 2022, 12, 9864 CrossRef CAS; (e) X. Liao, H. Zhou, X. Chen and J. Xu, Org. Lett., 2023, 25, 3099 CrossRef CAS PubMed; (f) Y. Wang, K. Chen, H. Zhou, X. Chen and J. Xu, Org. Lett., 2025, 27, 7064 CrossRef CAS PubMed.
- V. B. Birman and X. Li, Org. Lett., 2008, 10, 1115 CrossRef CAS PubMed.
- C. Joannesse, C. P. Johnston, C. Concellón, C. Simal, D. Philp and A. D. Smith, Angew. Chem., Int. Ed., 2009, 48, 8914 CrossRef CAS PubMed.
- (a) CCDC: 2433022 (for 3a): Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2mnrln; (b) CCDC: 2434593 (for 3k): Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2mqd81.
- (a) T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580 CrossRef CAS PubMed; (b) T. Lu and Q. Chen, J. Comput. Chem., 2022, 43, 539 CrossRef CAS PubMed; (c) T. Lu, J. Chem. Phys., 2024, 161, 082503 CrossRef CAS PubMed.
- (a) C. M. Young, A. Elmi, D. J. Pascoe, R. K. Morris, C. McLaughlin, A. M. Woods, A. B. Frost, A. d. l. Houpliere, K. B. Ling, T. K. Smith, A. M. Z. Slawin, P. H. Willoughby, S. L. Cockroft and A. D. Smith, Angew. Chem., Int. Ed., 2020, 59, 3705 CrossRef CAS PubMed; (b) E. S. Munday, M. A. Grove, T. Feoktistova, A. C. Brueckner, D. M. Walden, C. M. Young, A. M. Z. Slawin, A. D. Campbell, P. H.-Y. Cheong and A. D. Smith, Angew. Chem., Int. Ed., 2020, 59, 7897 CrossRef CAS PubMed; (c) S. K. Agrawal, P. K. Majhi, A. S. Goodfellow, R. K. Tak, D. B. Cordes, A. P. McKay, K. Kasten, M. Buhl and A. D. Smith, Angew. Chem., Int. Ed., 2024, 63, e202402909 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |