Open Access Article
Open Access ArticleA cation gating–breathing synergetic mechanism in K-MER-2.0 zeolite enables unprecedented selective CO2 separation from hydrocarbon gas streams
Renhao
Li†
a,
Chenxu
Liu†
a,
Wenli
Bao
b,
Lin
Li
c,
Jingfeng
Han
d,
Xiaoxin
Zou
 a,
Xiaowei
Song
a,
Xiaowei
Song
 *a,
Donghai
Mei
*a,
Donghai
Mei
 *b and
Zhiqiang
Liang
*b and
Zhiqiang
Liang
 *a
*a
aState Key Lab of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: xiaoweisong@jlu.edu.cn; liangzq@jlu.edu.cn
bSchool of Materials Science and Engineering, Tiangong University, Tianjin, 300387, P. R. China. E-mail: dhmei@tiangong.edu.cn
cElectron Microscopy Centre, Jilin University, Changchun 130012, P. R. China
dNational Engineering Research Center of Lower-Carbon Catalysis Technology, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China
First published on 14th October 2025
Abstract
The ubiquitous presence of CO2 impurities in industrial hydrocarbon streams underscores the critical need for developing efficient CO2-selective adsorbents. Herein, we synthesized a flexible K-MER-2.0 (a silica–alumina ratio of 2.0) zeolite without using an organic template, which demonstrates exceptional selective adsorption performance in CO2/hydrocarbon systems, and in particular achieves the unprecedented reverse separation of CO2/C2H2. K-MER exhibits a CO2 adsorption capacity of 70.9 cm3 g−1 under ambient conditions, accompanied by a remarkable CO2/C2H2 uptake ratio (13.4). Breakthrough experiments conducted with a CO2/C2H2 (50/50, v/v) mixture reveal that K-MER-2.0 delivers a high dynamic CO2 adsorption capacity (65.1 cm3 g−1) and separation factor (12.1), and in particular a high C2H2 yield of 1872 mmol kg−1, establishing a benchmark for CO2/C2H2 reverse separation. The rapid adsorption kinetics, excellent regeneration and robust moisture resistance of K-MER-2.0 further confirmed its application potential under harsh practical conditions. Mechanistic analyses, including CO2/C2H2 adsorption isotherms, in situ CO2 powder X-ray diffraction (PXRD) patterns and periodic density functional theory (DFT) calculations, reveal a unique CO2-triggered cation gating—breathing synergetic mechanism in K-MER-2.0 zeolite. This stimulus-responsive mechanism facilitates selective framework expansion during CO2 adsorption while preserving a constricted pore geometry that excludes C2H2. Such discriminative structural adaptability drives the record-breaking separation performance of the material. Additionally, the unique recognition ability for CO2 endows K-MER-2.0 with good dynamic separation ability for binary mixtures of CO2/hydrocarbons.
Introduction
The strategic significance of developing advanced CO2/hydrocarbon separation technologies arises from two fundamental industrial considerations: (1) hydrocarbon gases (including C2H2, C2H4, C2H6, C3H6, C3H8, and CH4) serve as critical feedstocks and energy vectors, and (2) CO2 invariably coexists in hydrocarbon product streams as a ubiquitous impurity.1 C2H2/CO2 separation represents one of the most challenging gas purification processes in petrochemical systems, primarily due to their nearly identical molecular properties, including comparable polarities (33.3 × 10−25 cm3 for C2H2vs. 29.1 × 10−25 cm3 for CO2), similar kinetic diameters (3.3 Å for both), and overlapping adsorption affinities.2–4 Conventional industrial methods predominantly employ organic-solvent-based absorption systems and cryogenic distillation operations, which suffer from significant energy consumption.4 This has driven the increasing adoption of physisorption-based separation platforms that offer distinct advantages in energy efficiency (>30% reduction in energy consumption), rapid adsorption–desorption kinetics, and exceptional cycling stability.5–7 Notably, the energy efficiency of CO2-selective adsorbents (CO2/C2H2 reverse separation) increases by 40% compared to that of C2H2-selective alternatives (C2H2/CO2 positive separation) in separation processes, highlighting the potential for sustainable hydrocarbon purification.1 This advantage arises primarily from CO2/C2H2 reverse separation, which facilitates the direct acquisition of pure C2H2 product while obviating the need for energy-intensive multistep desorption processes.The key to achieving efficient CO2/C2H2 reverse separation is the design and synthesis of advanced porous adsorbents. In the past decade, metal–organic frameworks (MOFs) have attracted considerable research interest for CO2/C2H2 reverse separation, with notable examples including Zn-ox-mtz,8 MUF-16,9 PCP-NH2-bdc,10 and PMOF-1.11 However, their practical implementation in gas separation technologies has been constrained by persistently high synthesis costs and relatively poor stability.12 This economic limitation has driven the increasing attention paid to zeolite-based materials, which offer distinct advantages in terms of cost-effectiveness, industrial scalability, structural stability, and high thermal/hydrothermal stability.4 Recent studies have demonstrated the potential of zeolites in this domain, exemplified by Sr/K-HEU achieving a dynamic CO2/C2H2 separation factor of 48.0 and CO2 dynamic uptake of 21.5 cm3 g−1.13 Furthermore, Na-GIS(3.1) has shown selective CO2 adsorption in CO2/C2H2 mixtures, accompanied by a pure C2H2 yield of 100 mmol kg−1.14 In addition, NaAlO2@MOR(0.5) also exhibited a remarkable kinetic separation selectivity of 534.3.15 Among zeolitic materials, K-MER has emerged as particularly promising due to its flexible framework and unique adsorption mechanisms, particularly for CO2/N2 and CO2/CH4 mixed systems. In situ CO2 variable-pressure synchrotron powder X-ray diffraction coupled with Rietveld refinement have revealed that K-MER exhibited a unique cation gating–breathing synergistic mechanism during CO2 adsorption.16–19 This dual-action mechanism differs from the “trapdoor” effect observed in Sr/K-HEU,13 the monofunctional breathing behaviour of Na-GIS(3.1),14 and the kinetic separation of NaAlO2@MOR(0.5).15 However, it has rarely been reported that the pore architecture of K-MER retains closure not only towards low-polar gases (N2, CH4) but also extends to high-polar hydrocarbons (e.g., C2H2, C2H4, C2H6, C3H6, and C3H8). Despite this critical property, systematic investigation of the potential of K-MER for CO2/hydrocarbon separation applications remains notably underexplored, creating a substantial gap in the understanding of its gas separation applications.
Herein, we report an organic-template-free synthesized K-MER-2.0 zeolite (a silica–alumina ratio of 2.0) that exhibits a remarkable CO2 static adsorption capacity of 70.9 cm3 g−1 at 298 K and 101 kPa, significantly outperforming its hydrocarbon adsorption capacities, and thereby demonstrating exceptional CO2/hydrocarbon separation performance. This material achieves an ideal adsorbed solution theory (IAST) selectivity of 3056 for CO2/C2H2 (50/50, v/v) mixtures. Notably, K-MER-2.0 enables the single-step purification of acetylene with a remarkably high C2H2 productivity of 1872 mmol kg−1 in breakthrough experiments, eliminating the need for multi-stage desorption processes and serving as a benchmark for a zeolite-based adsorbent. Meanwhile, the dynamic adsorption capacity of CO2 reached 65.1 cm3 g−1, accompanied by a separation factor of 12.1. The material demonstrates rapid adsorption kinetics (1.7 min to reach CO2 uptake of 43.7 mg g−1), exceptional moisture tolerance (73.9% C2H2 production under 50% relative humidity), and a low regeneration temperature (353 K). Comparative analyses of CO2/C2H2 adsorption isotherms, in situ CO2 powder X-ray diffraction (PXRD) patterns, and periodic density functional theory (DFT) calculations reveal a unique CO2-triggered cation gating–breathing synergetic mechanism. This stimuli-responsive framework maintains a rigid structure for hydrocarbons while exhibiting CO2-induced framework flexibility, enabling molecular discrimination through dynamic pore modulation. This work advances a fundamental understanding of cation-mediated framework flexibility and establishes a novel strategy for developing smart zeolite materials with selective framework-breathing and gate-opening functionalities for challenging gas separations.
Results and discussion
Characterizations of K-MER-2.0 zeolites
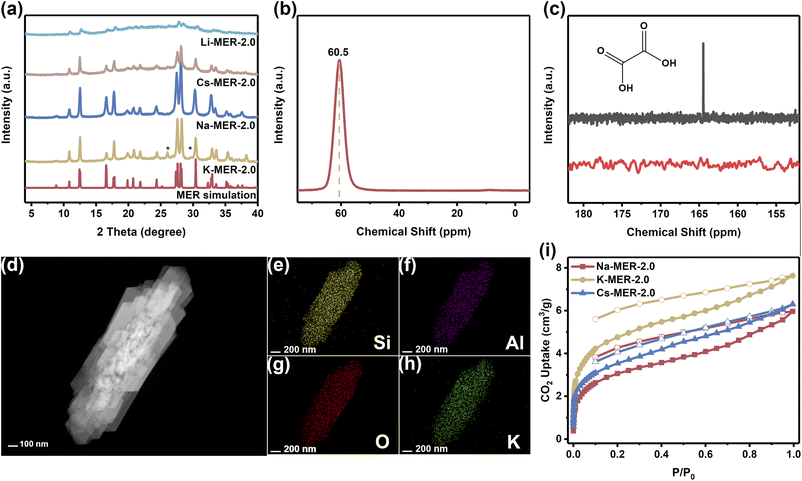
Utilizing KOH as the exclusive alkaline medium, K-MER-2.0 zeolite was successfully synthesized in an organic-template-free system. The powder X-ray diffraction (PXRD) pattern of as-synthesized K-MER-2.0 zeolite was almost consistent with a simulated one of MER-type zeolite, except for the distinctive diffraction peaks at 26.0° and 29.7° (Fig. 1a), which were induced by symmetry reduction from the Immm space group (no. 71) of a typical MER framework to a lower-symmetry Pnnm space group (no. 58), rather than arising from phase impurities.20,21 Scanning/transmission electron microscopy (SEM/TEM) images showed the as-synthesized K-MER-2.0 zeolite existed in the form of micron-sized rod-like aggregations (Fig. S1, S2 and Table S1). The coordination environment of framework elements was elucidated through 29Si/27Al solid-state magic angle spinning nuclear magnetic resonance (MAS NMR) analysis (Fig. 1b and S3). Four distinct strong signals at −91, −96, −101, and −107 ppm in the 29Si MAS NMR spectrum were attributed to Si(OSi)4 (Q4), Si(OSi)3(OAl) (Q4), Si(OSi)2(OAl)2 (Q4), and Si(OSi)(OAl)3 (Q4), respectively, indicating the good crystallization of K-MER-2.0. The 27Al solid-state MAS NMR spectrum demonstrated that the aluminum species in the as-synthesized K-MER-2.0 existed exclusively in a tetrahedral coordination state (60.5 ppm) without the presence of extra-framework aluminum species (0 ppm). Complementary 13C solid-state CP/MAS NMR spectrum (Fig. 1c) and thermogravimetric analysis (Fig. S4) confirmed no residual oxalic acid in the as-synthesized K-MER-2.0 zeolite. These findings suggest that oxalic acid functions primarily as a pH modulator and mineralizer complexing agent (with K+), rather than acting as a structure-directing template.22 Elemental mapping via TEM-EDS (Fig. 1d–h) further verified the homogeneous distribution of K+ within the pore channels. To investigate cation effects on structural stability and gas adsorption properties, ion-exchanged derivatives (Li-MER-2.0, Na-MER-2.0, and Cs-MER-2.0) were prepared from K-MER-2.0 through cation exchange followed by calcination (Fig. 1a and Table S1). Na-MER-2.0 maintained both MER topology and crystallinity comparable to the parent K-MER-2.0, while Cs-MER-2.0 exhibited minor framework degradation during the ion exchange process. In contrast, Li-MER-2.0 showed significant attenuation in PXRD peak intensity, attributed to the insufficient framework stabilization by smaller Li+ ions in the flexible MER architecture.17,18 Consequently, subsequent analyses focused on the more stable Na-MER-2.0, K-MER-2.0, and Cs-MER-2.0 variants. Meanwhile, for Na-MER-2.0 and Cs-MER-2.0, SEM images confirmed the preservation of morphological features during ion exchange (Fig. S5), and 27Al solid-state MAS NMR spectra confirmed the absence of extra-framework Al species (Fig. S6 and S7).It is well known that the adsorption capacity of porous materials is critically influenced by their specific surface area and pore volume.23 However, the adsorption isotherms of N2 (d = 0.36 nm) at 77 K and Ar (d = 0.34 nm) at 87 K revealed that only a few N2 and Ar molecules could be adsorbed into the micropores of K-MER-2.0, Na-MER-2.0, and Cs-MER-2.0 (Fig. S8 and S9). This observation suggests that the flexible framework of K-MER-2.0 maintains a contracted configuration under nitrogen or argon atmospheres.16 To further investigate this size exclusion effect, we conducted CO2 (d = 0.33 nm) adsorption measurements at 195 K. Surprisingly, despite its smaller kinetic diameter, the CO2 adsorption amounts of all three MER-type zeolites were still less than 8 cm3 g−1 (Fig. 1i). We propose that this anomalous exclusion behavior stems primarily from the suppressed thermal motion of charge-balancing cations within the zeolite pores at cryogenic temperatures. The reduced cation vibration elevates the energy barrier for cation migration, effectively maintaining a rigid pore structure that prevents gas molecule penetration. This phenomenon aligns with the temperature-dependent adsorption behavior observed in LTA-type zeolites, which adsorb N2 at room temperature but exhibit non-adsorbent characteristics at 77 K.24
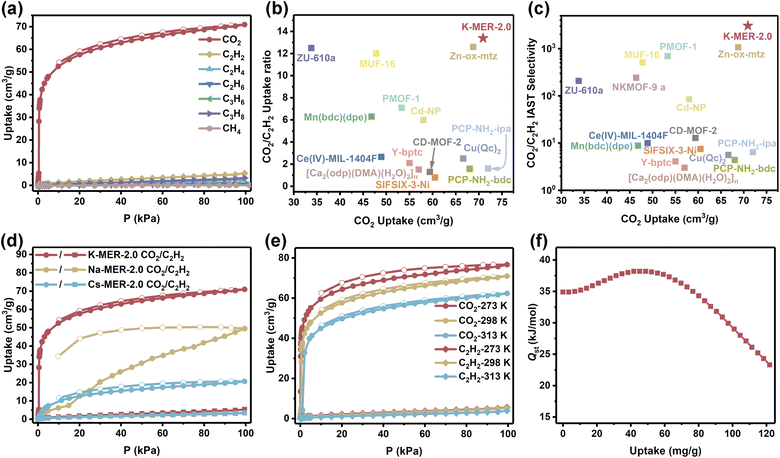
Compared to the CO2 adsorption isotherm observed at 195 K, the K-MER-2.0 zeolite demonstrated significantly enhanced CO2 adsorption performance at 298 K with a remarkable adsorption capacity of 70.9 cm3 g−1 at 101 kPa and a steep adsorption curve within the low-pressure region (1 kPa: 37.8 cm3 g−1, 10 kPa: 52.6 cm3 g−1) (Fig. 2a and Table S2). The quasi-reversible nature of CO2 adsorption was confirmed by the nearly overlapping adsorption–desorption isotherms. Simultaneously, a critical structural transition was identified at 0.4 kPa (298 K), marked by an inflection point in the CO2 adsorption curve and a subsequent sharp increase in adsorption capacity (Fig. S10). This phenomenon indicates a pressure-induced framework transformation from a contracted to an expanded state in the K-MER-2.0 structure, which has been observed in PHI topology molecular sieves.25 In stark contrast, K-MER-2.0 displayed ultra-low adsorption capacities for various hydrocarbon gases at 298 K and 101 kPa, with uptakes of 5.3 cm3 g−1 for C2H2, 1.3 cm3 g−1 for C2H4, 0.6 cm3 g−1 for C2H6, 2.7 cm3 g−1 for C3H6, 3.3 cm3 g−1 for C3H8, and 0.04 cm3 g−1 for CH4 (Fig. 2a and Table S2). The ultra-low adsorption capacities and the absence of inflection points in these hydrocarbon isotherms suggest that the framework maintains its contracted conformation during hydrocarbon gas adsorption. This salient adsorption selectivity establishes K-MER-2.0 as an exceptional molecular sieve material for CO2/hydrocarbon separation, with impressive adsorption capacity ratios at 298 K and 101 kPa: 13.4 (CO2/C2H2), 54.5 (CO2/C2H4), 118.2 (CO2/C2H6), 26.3 (CO2/C3H6), 21.3 (CO2/C3H8), and 1772.5 (CO2/CH4) (Table S2).
It is well established that among various hydrocarbons, C2H2 exhibits physicochemical properties and a kinetic diameter most closely resembling those of CO2.4,12 The high adsorption capacity ratio of CO2/C2H2 indicates that the K-MER-2.0 zeolite is particularly suitable for selective CO2 capture from the CO2/C2H2 separation system to produce pure C2H2. Remarkably, K-MER-2.0 demonstrated exceptional separation efficiency with an IAST selectivity of 3056 for the CO2/C2H2 (50/50, v/v) system (Fig. S11). As shown in Fig. 2b, c and Table S4, K-MER-2.0 exhibited superior performance metrics in reverse CO2/C2H2 separation: CO2 uptake (70.9 cm3 g−1), uptake ratio (13.4), and IAST selectivity (3056), significantly surpassing current benchmark materials. These values notably exceed those of state-of-the-art materials, including Zn-ox-mtz (68.78 cm3 g−1; 12.6; 1064.9),8 MUF-16 (48 cm3 g−1; 12.0; 510),8 and PMOF-1 (53.3 cm3 g−1; 7.1; 694).11 Comparative analysis with other cation-exchanged MER frameworks revealed distinct differences in performance (Fig. 2d). K-MER-2.0 demonstrated a CO2 adsorption capacity of 70.9 cm3 g−1 with reversible adsorption and favorable low-pressure adsorption characteristics, while Na-MER-2.0 showed reduced capacity (49.6 cm3 g−1) accompanied by an adsorption isotherm inflection point shift to 7.5 kPa and significant adsorption–desorption hysteresis. These characteristics substantially compromised the separation performance of Na-MER-2.0, despite its comparable uptake ratio (14.6) to K-MER-2.0. Cs-MER-2.0 exhibited even poorer performance with CO2 uptake of 20.6 cm3 g−1 and a low uptake ratio of 5.9 (Table S3).
Thermodynamic analysis through variable-temperature adsorption isotherms revealed a zero-coverage CO2 adsorption enthalpy (Qst) of 34.9 kJ mol−1 for K-MER-2.0 (Fig. 2e and f). This physisorption-dominated mechanism, evidenced by the moderate Qst value, facilitates material regeneration compared to frameworks with higher enthalpies, such as NKMOF-9a (69.5 kJ mol−1)26 and CD-MOF-2 (67.2 kJ mol−1)27 (Table S4). Notably, the CO2 adsorption enthalpy profile of K-MER-2.0 exhibited an atypical trend of an initial increase followed by a gradual decline (Fig. 2f), which we attribute to the limited pore accessibility and shielding effects on high-energy adsorption sites at low CO2 loadings. The temperature-dependent adsorption behavior showed systematic shifts in isotherm inflection points from 0.04 kPa (273 K) to 1.0 kPa (313 K) (Fig. S12), consistent with established gas adsorption thermodynamics.28 This predictable temperature response further supports the potential of K-MER-2.0 for practical separation applications under varying operational conditions.
The CO2 adsorption kinetics of K-MER-2.0 were systematically investigated under varying pressure conditions (15, 50 and 96 kPa), as shown in Fig. S13. Under low-pressure conditions (15 kPa), the adsorption capacity demonstrated rapid initial uptake, reaching 43.7 mg g−1 (22.2 cm3 g−1) within 1.7 minutes, followed by gradual equilibration to a maximum capacity of 102.2 mg g−1 (52.0 cm3 g−1) over 15 minutes. In contrast, when subjected to elevated pressures (50 and 96 kPa), the system exhibited instantaneous equilibrium characteristics upon pressure stabilization, achieving CO2 adsorption capacities of 119.6 mg g−1 (60.9 cm3 g−1) and 128.7 mg g−1 (65.5 cm3 g−1), respectively. This pressure-dependent kinetic behavior highlights the rapid adsorption response of the material at higher pressures, which significantly enhances separation efficiency by minimizing the unnecessary consumption of selective adsorption capacity during non-productive stages involving simultaneous gas permeation.23 The distinct kinetic profiles observed under different pressure conditions provide critical insights for optimizing operational parameters in gas separation applications.
To investigate the impact of the Si/Al ratio on CO2/C2H2 separation performance in K-MER, we synthesized K-MER samples with other Si/Al ratios of 2.7 and 3.8, denoted K-MER-2.7 and K-MER-3.8 (Fig. S14 and Table S1). The decreased K+ content resulting from higher Si/Al ratios increases the void volume of MER zeolites, boosting the static CO2 adsorption capacity from 70.9 cm3 g−1 (K-MER-2.0) to 75.9 cm3 g−1 (K-MER-2.7) and 85.2 cm3 g−1 (K-MER-3.8) (Fig. S15). Meanwhile, the reduction of K+ content in the pore of the MER zeolite weakens the interactions between K+ and the zeolite framework, causing the CO2 adsorption inflection point to shift from 0.4 kPa (K-MER-2.0) to 0.1 kPa (K-MER-2.7), with a further decline to below 0.02 kPa for K-MER-3.8 (Fig. S16). However, the C2H2 adsorption capacity significantly increases from 5.3 cm3 g−1 (K-MER-2.0) to 11.3 cm3 g−1 (K-MER-2.7) and 52.3 cm3 g−1 (K-MER-3.8) (Fig. S15). Consequently, the CO2/C2H2 adsorption ratio decreases from 13.4 (K-MER-2.0) to 6.7 (K-MER-2.7) and 1.6 (K-MER-3.8) (Table S5). The increase in Si/Al ratios reduces pore blockage in K-MER zeolites, allowing C2H2 adsorption to occur—a phenomenon consistent with previous literature.22 Overall, K-MER-2.0 exhibited significantly superior separation performance compared to K-MER-2.7 and K-MER-3.8.
To evaluate the practical separation performance of K-MER-2.0 under realistic flow conditions, dynamic breakthrough experiments were conducted using a CO2/C2H2 (50/50, v/v) gas mixture at a total flow rate of 4 mL min−1, following established protocols in gas separation studies (Fig. 3a).29 The breakthrough curves revealed distinct adsorption behaviors: C2H2 exhibited rapid breakthrough at 1.0 min g−1 with subsequent saturation, while CO2 demonstrated prolonged retention with a breakthrough time of 24.4 min g−1. Quantitative analysis yielded dynamic adsorption capacities of 65.1 cm3 g−1 for CO2 and 5.4 cm3 g−1 for C2H2, corresponding to an impressive separation factor (α) of 12.1 (Table S6). Notably, K-MER-2.0 achieved the direct production of high-purity C2H2 (99.6%) with a yield of 1872 mmol kg−1 through single-step adsorption, eliminating the requirement for energy-intensive thermal desorption processes. Comparative performance analysis with state-of-the-art adsorbents highlights the superior separation characteristics of K-MER-2.0. Although Zn-ox-mtz (a leading MOF material) shows marginally higher C2H2 productivity (2091 mmol kg−1),18 K-MER-2.0 demonstrates a two-fold enhancement in separation factor (12.1 vs. 6.24), significantly reducing acetylene loss during separation cycles (Fig. 3h and Table S7). Furthermore, K-MER-2.0 outperforms other benchmark materials, including Ce(IV)-MIL-140-4F (1370 mmol kg−1, α = 4.9),30 Y-bptc (1520 mmol kg−1, α = 5),31 MFU-4 (1320 mmol kg−1, α = 5.8),32 and Cd-NP (1242 mmol kg−1, α = 4),33 establishing it as the current performance leader among previously reported porous adsorbents, including MOFs and zeolites. (Table S7). Regeneration studies demonstrated exceptional desorption properties: complete CO2 release occurred within 10 min under He purging at 353 K, with C2H2 signals dissipating immediately upon initiation of desorption (Fig. 3b). Subsequent temperature-programmed desorption to 473 K confirmed the absence of residual adsorbates via mass spectrometry, demonstrating full regenerability. This facile regeneration behavior aligns with the low CO2 adsorption enthalpy of the material and reversible adsorption isotherms, suggesting minimal energy requirements for cyclic operation, which is a critical advantage for industrial implementation.
Compared to K-MER-2.0 (23.4 min g−1), the CO2/C2H2 breakthrough time differentials for Na-MER-2.0 and Cs-MER-2.0 significantly decreased to 0.35 and 1.79 min g−1, respectively (Fig. 3c). Notably, K-MER-2.0 demonstrated superior separation performance with a pure C2H2 yield of 1872 mmol kg−1 and separation factor of 12.1, substantially exceeding those of Na-MER-2.0 (0.015 mmol kg−1, 1.4) and Cs-MER-2.0 (47 mmol kg−1, 3.6). These remarkable disparities established K-MER-2.0 as the optimal candidate for subsequent investigations. Systematic evaluation revealed that K-MER-2.0 maintained consistent CO2 dynamic adsorption capacities between 63.8 and 65.1 cm3 g−1 across flow rates spanning 2–8 mL min−1 (Fig. 3d). Thermal stability tests showed that the C2H2 productivity remained 1291 mmol kg−1 (69.0% retention) even at 333 K (Fig. 3e). Addressing the well-documented moisture sensitivity of zeolites,23 K-MER-2.0 exhibited exceptional humidity tolerance, retaining 80.0% of its CO2 breakthrough time and 73.9% C2H2 production capacity under 50% relative humidity (Fig. 3f). Furthermore, the material demonstrated excellent regeneration stability through seventeen consecutive adsorption–desorption cycles (desorption at 353 K), with negligible variation in CO2/C2H2 breakthrough profiles (Fig. 3g). The excellent cycling stability of K-MER-2.0 was also preserved under humid conditions (Fig. S17).
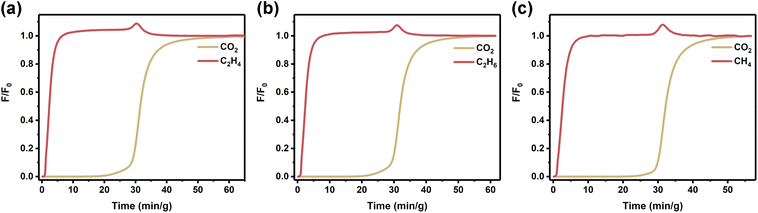
To systematically validate the universality of K-MER-2.0 in CO2/hydrocarbon separation, we performed dynamic breakthrough experiments using isovolumetric binary mixtures of CO2/C2H4, CO2/C2H6, and CO2/CH4 under a constant total flow rate of 4 mL min−1 (Fig. 4a–c and Table S8). The separation performance exhibited remarkable consistency across different hydrocarbon systems. For the CO2/C2H4 separation system, K-MER-2.0 demonstrated a CO2 dynamic adsorption capacity of 64.8 cm3 g−1, achieving ethylene purification with 99.6% purity (1663 mmol kg−1 yield) and a separation factor of 27.3. In the CO2/C2H6 system, comparable CO2 uptake capacity (65.1 cm3 g−1) was observed while maintaining an ethane purity of 99.6% (1789 mmol kg−1 yield), with a separation factor of 19.3. Similarly, in the CO2/CH4 separation experiments, the material showed enhanced CO2 capture capacity (66.1 cm3 g−1) accompanied by methane purification to 99.6% purity (1992 mmol kg−1 yield) and a separation factor of 13.9. These comprehensive results, supported by both static adsorption isotherms and dynamic breakthrough analyses, establish that K-MER-2.0 possesses exceptional versatility in gas separation applications. The material not only enables the reverse separation of CO2/C2H2, but it also demonstrates superior separation performance for CO2 removal from various hydrocarbon systems, including light alkenes (C2H4), alkanes (C2H6), and methane (CH4).
The adsorption mechanisms of CO2 in K-MER-2.0 have been systematically investigated through in situ CO2 powder X-ray diffraction coupled with the static CO2 adsorption isotherms. Notably, the flexible framework of K-MER-2.0 demonstrates a distinct structural transition from a contracted to an expanded state during CO2 adsorption, as evidenced by characteristic inflection points in both static adsorption isotherms and in situ CO2 PXRD patterns (Fig. S10 and S18). Meanwhile, it has been covered extensively in previous reports that this structural transformation is accompanied by specific cation migration events: K+ ions relocate from initial positions at sites Ia (within the d8r cage) and sites II (in the s8rs between pau and ste cages) to energetically favorable positions at sites I (in the s8rs between d8r and pau cages) and sites III (in the s8rs between two adjacent ste cages) (Fig. S19).17,18,34–36 This concerted framework-cation interaction has been formally described as a cooperative cation-gated breathing mechanism.17 In striking contrast, C2H2 adsorption studies reveal fundamentally different behavior. The K-MER-2.0 framework maintains its contracted configuration throughout C2H2 exposure, as demonstrated by both the absence of inflection points in static adsorption isotherms and the negligible C2H2 uptake capacity of the material (5.3 cm3 g−1) (Fig. 2a and Table S2). Periodic density functional theory (DFT) calculations were used to further explain the unique recognition ability of K-MER-2.0 towards CO2. As shown in Fig. S20, the energy barrier for the transition from C2H2 adsorption on the contracted K-MER-2.0 framework to the expanded framework was 191.0 kJ mol−1. In contrast, the energy barrier associated with the transition from the contracted K-MER-2.0 framework to the expanded framework for CO2 adsorption was only −37.6 kJ mol−1, significantly lower than that for C2H2. These theoretical models were based on a previously reported K-MER structure with a closely matched Si/Al ratio.17 These computational findings were in good agreement with the experimental observations, demonstrating that K-MER-2.0 undergoes selective framework expansion during CO2 adsorption while maintaining a constricted pore geometry that effectively excludes C2H2. Notably, the K-MER-2.0 framework remains in an expanded state throughout the CO2/C2H2 dynamic breakthrough process due to the presence of CO2. However, C2H2 remains difficult to adsorb by the swollen K-MER-2.0 framework (C2H2 dynamic uptake: 5.38 cm3 g−1). To further investigate this phenomenon, we conducted additional DFT calculations based on the expanded structural model of the K-MER framework. It is well established that the K-MER structure contains three distinct gas diffusion pathways: (i) from the d8r cage to the pau cage (denoted Pathway I), (ii) between adjacent ste cages (denoted Pathway II), and (iii) from the ste cage to the pau cage (denoted Pathway III) (Fig. S21). All cage-type structures along the diffusion pathways are interconnected through an 8-membered ring (8 MR). Meanwhile, although the K-MER framework remains in an expanded state during CO2/C2H2 dynamic breakthrough, the potassium (K+) ions located within the 8 MR continue to play a “trapdoors” role throughout the breakthrough process. As illustrated in Fig. 5a–f and Table S9, the energy barriers for CO2 to displace K+ ions along all three diffusion pathways (Pathway I: 22.0 kJ mol−1; Pathway II: 13.9 kJ mol; Pathway III: 22.5 kJ mol−1) are significantly lower than those for C2H2 (Pathway I: 83.2 kJ mol−1; Pathway II: 29.1 kJ mol−1; Pathway III: 42.9 kJ mol−1). These DFT calculations reveal that, even when the K-MER framework is in an expanded state, C2H2 remains more difficult to adsorb by K-MER compared to CO2, which is consistent with the ultra-low C2H2 uptake (5.38 cm3 g−1) and remarkable CO2/C2H2 separation factor (12.1) observed in the CO2/C2H2 (50/50, v/v, 4 mL min−1) breakthrough experiments at 298 K. The cation gating–breathing synergetic mechanism of the material enables preferential accommodation of CO2 molecules while effectively excluding C2H2, establishing a novel mechanism for gas separation that combines molecular sieving with framework adaptability.
Conclusions
In summary, we have successfully demonstrated the organic-template-free synthesis of a K-MER-2.0 zeolite that exhibits unprecedented CO2/C2H2 reverse separation performance via a unique cation-gating and breathing synergistic mechanism. The material exhibits a CO2 adsorption capacity of 70.9 cm3 g−1 at 298 K and 101 kPa, along with exceptional separation metrics, including a CO2/C2H2 uptake ratio of 13.4 and record-breaking IAST selectivity of 3056. More importantly, the K-MER-2.0 zeolite delivers a high dynamic adsorption capacity of 65.1 cm3 g−1 and separation factor of 12.1, which enable single-step C2H2 production of 1872 mmol kg−1 with 99.6% purity, surpassing conventional energy-intensive purification methods. Mechanistic studies reveal CO2-triggered cooperative cation-gated breathing behavior, where selective pore activation occurs exclusively for CO2 adsorption, while also maintaining pore closure against C2H2 molecules. The excellent selective adsorption capacity could be further extended to other CO2/hydrocarbon systems. The practical viability of the K-MER-2.0 zeolite is further enhanced by efficient adsorption kinetics, exceptional moisture stability, and outstanding regenerability. Critically, the organic-template-free synthesis protocol enables cost-efficient production, positioning K-MER-2.0 as a viable industrial candidate for scalable applications. This work establishes a new paradigm for gas separation through stimulus-responsive pore modulation, simultaneously advancing both industrial gas purification technologies and a fundamental understanding of dynamic molecular sieving with zeolitic materials.Author contributions
R. L. and C. L. conceived the research design and drafted the initial manuscript; L. L. and J. H. performed sample characterization; D. M., W. B., and X. Z. carried out theoretical calculations; and X. S. and Z. L. supervised the project and revised the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary Information: experimental details and characterization of the adsorbent. See DOI: https://doi.org/10.1039/d5sc03281d.Acknowledgements
This work was supported by the National Key Research and Development Program (no. 2023YFB3810700), the National Natural Science Foundation of China (22375070 and 22288101), and the “111 Center” (B17020). We also thank the Shanghai Synchrotron Radiation Facility of BL17UM (https://cstr.cn/31124.02.SSRF.BL17UM) for assistance with experimental measurements.References
- Z. Zhang, Z. Deng, H. A. Evans, D. Mullangi, C. Kang, S. B. Peh, Y. Wang, C. M. Brown, J. Wang, P. Canepa, A. K. Cheetham and D. Zhao, Exclusive Recognition of CO2 from Hydrocarbons by Aluminum Formate with Hydrogen-Confined Pore Cavities, J. Am. Chem. Soc., 2023, 145, 11643–11649 CrossRef CAS PubMed.
- B. Ma, D. Li, Q. Zhu, Y. Li, W. Ueda and Z. Zhang, A Zeolitic Octahedral Metal Oxide with Ultra-Microporosity for Inverse CO2/C2H2 Separation at High Temperature and Humidity, Angew. Chem., Int. Ed., 2022, 61, e202209121 CrossRef CAS PubMed.
- K. J. Chen, H. S. Scott, D. G. Madden, T. Pham, A. Kumar, A. Bajpai, M. Lusi, K. A. Forrest, B. Space, J. J. Perry and M. J. Zaworotko, Benchmark C2H2/CO2 and CO2/C2H2 Separation by Two Closely Related Hybrid Ultramicroporous Materials, Chem, 2016, 1, 753–765 CAS.
- S. Liu, X. Han, Y. Chai, G. Wu, W. Li, J. Li, I. Da Silva, P. Manuel, Y. Cheng, L. L. Daemen, A. J. Ramirez-Cuesta, W. Shi, N. Guan, S. Yang and L. Li, Efficient Separation of Acetylene and Carbon Dioxide in a Decorated Zeolite, Angew. Chem., Int. Ed., 2021, 60, 6526–6532 CrossRef CAS PubMed.
- S. Liu, X. Lian, B. Yue, S. Xu, G. Wu, Y. Chai, Y. Zhang and L. Li, Control of Zeolite Local Polarity toward Efficient Xenon/Krypton Separation, J. Am. Chem. Soc., 2024, 146, 8335–8342 CrossRef CAS PubMed.
- Y. Zhou, J. Zhang, L. Wang, X. Cui, X. Liu, S. S. Wong, H. An, N. Yan, J. Xie, C. Yu, P. Zhang, Y. Du, S. Xi, L. Zheng, X. Cao, Y. Wu, Y. Wang, C. Wang, H. Wen, L. Chen, H. Xing and J. Wang, Self-Assembled Iron-Containing Mordenite Monolith for Carbon Dioxide Sieving, Science, 2021, 373, 315–320 CrossRef CAS PubMed.
- S. Foorginezhad, F. Weiland, Y. Chen, S. Hussain and X. Ji, Review and Analysis of Porous Adsorbents for Effective CO2 Capture, Renewable Sustainable Energy Rev., 2025, 215, 115589 CrossRef CAS.
- S. Q. Yang, R. Krishna, H. Chen, L. Li, L. Zhou, Y. F. An, F. Y. Zhang, Q. Zhang, Y. H. Zhang, W. Li, T. L. Hu and X. H. Bu, Immobilization of the Polar Group into an Ultramicroporous Metal–Organic Framework Enabling Benchmark Inverse Selective CO2/C2H2 Separation with Record C2H2 Production, J. Am. Chem. Soc., 2023, 145, 13901–13911 CrossRef CAS PubMed.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Selective Capture of Carbon Dioxide from Hydrocarbons Using a Metal–Organic Framework, Nat. Commun., 2021, 12, 197 CrossRef CAS PubMed.
- Y. Gu, J. Zheng, K. Otake, M. Shivanna, S. Sakaki, H. Yoshino, M. Ohba, S. Kawaguchi, Y. Wang, F. Li and S. Kitagawa, Host–Guest Interaction Modulation in Porous Coordination Polymers for Inverse Selective CO2/C2H2 Separation, Angew. Chem., Int. Ed., 2021, 60, 11688–11694 CrossRef CAS PubMed.
- L. Cai, Z. Yao, S. Lin, M. Wang and G. Guo, Photoinduced Electron-Transfer (PIET) Strategy for Selective Adsorption of CO2 over C2H2 in a MOF, Angew. Chem., Int. Ed., 2021, 60, 18223–18230 CrossRef CAS PubMed.
- X. Han and S. Yang, Molecular Mechanisms behind Acetylene Adsorption and Selectivity in Functional Porous Materials, Angew. Chem., Int. Ed., 2023, 62, e202218274 CrossRef CAS PubMed.
- J. Jia, N. Yan, X. Lian, S. Liu, B. Yue, Y. Chai, G. Wu, J. Xu and L. Li, Molecular Trapdoor in HEU Zeolite Enables Inverse CO2-C2H2 Separation, Angew. Chem., Int. Ed., 2025, 64, e202419091 CrossRef CAS PubMed.
- G. Deng, K. Cheng, B. Meng, X. Shi, X. Liu, Y. Zhou and J. Wang, Regulating the Si/Al Ratio of GIS Zeolite with Bulky Primary Particles for Selective CO2 Capture from Hydrocarbons, Sep. Purif. Technol., 2024, 340, 126764 CrossRef CAS.
- X. Zhang, Y. Wang, L. Yang, X. Lu, X. Suo, X. Cui and H. Xing, Efficient Kinetic Separation of Carbon Dioxide from Acetylene Using Mordenites Featuring Modified 1D Channels with Excellent Selectivity and Diffusion, Adv. Mater., 2025, 37, 2501870 CrossRef CAS PubMed.
- V. M. Georgieva, E. L. Bruce, M. C. Verbraeken, A. R. Scott, W. J. Casteel, S. Brandani and P. A. Wright, Triggered Gate Opening and Breathing Effects during Selective CO2 Adsorption by Merlinoite Zeolite, J. Am. Chem. Soc., 2019, 141, 12744–12759 CrossRef CAS PubMed.
- H. J. Choi, D. Jo, J. G. Min and S. B. Hong, The Origin of Selective Adsorption of CO2 on Merlinoite Zeolites, Angew. Chem., Int. Ed., 2021, 60, 4307–4314 CrossRef CAS PubMed.
- E. L. Bruce, V. M. Georgieva, M. C. Verbraeken, C. A. Murray, M.-F. Hsieh, W. J. Casteel, A. Turrina, S. Brandani and P. A. Wright, Structural Chemistry, Flexibility, and CO2 Adsorption Performance of Alkali Metal Forms of Merlinoite with a Framework Si/Al Ratio of 4.2, J. Phys. Chem. C, 2021, 125, 27403–27419 CrossRef CAS.
- H. Lee, D. Xie, S. I. Zones and A. Katz, CO2 Desorbs Water from K-MER Zeolite under Equilibrium Control, J. Am. Chem. Soc., 2024, 146, 68–72 CrossRef CAS PubMed.
- MER: XPD Pattern, https://europe.iza-structure.org/IZA-SC/pow_pat.php?ID=178, accessed April 21, 2025.
- B. M. Skofteland, O. H. Ellestad and K. P. Lillerud, Potassium Merlinoite: Crystallization, Structural and Thermal Properties, Microporous Mesoporous Mater., 2001, 43, 61–71 CrossRef CAS.
- X. Tang, M. Wei, X. Bai, J. Li and J. Yang, Precise Pore Size Modulation of K-MER Zeolites for N2 Trapping, Sep. Purif. Technol., 2024, 339, 126601 CrossRef CAS.
- D. Fu and M. E. Davis, Carbon Dioxide Capture with Zeotype Materials, Chem. Soc. Rev., 2022, 51, 9340–9370 RSC.
- F. Akhtar, Q. Liu, N. Hedin and L. Bergström, Strong and Binder Free Structured Zeolite Sorbents with Very High CO2-over-N2 Selectivities and High Capacities to Adsorb CO2 Rapidly, Energy Environ. Sci., 2012, 5, 7664 RSC.
- H. J. Choi, E. L. Bruce, K. S. Kencana, J. Hong, P. A. Wright and S. B. Hong, Highly Cooperative CO2 Adsorption via a Cation Crowding Mechanism on a Cesium-Exchanged Phillipsite Zeolite, Angew. Chem., Int. Ed., 2023, 62, e202305816 CrossRef CAS PubMed.
- S. Geng, H. Xu, C. Cao, T. Pham, B. Zhao and Z. Zhang, Bioinspired Design of a Giant [Mn86] Nanocage-Based Metal–Organic Framework with Specific CO2 Binding Pockets for Highly Selective CO2 Separation, Angew. Chem., Int. Ed., 2023, 62, e202305390 CrossRef CAS PubMed.
- L. Li, J. Wang, Z. Zhang, Q. Yang, Y. Yang, B. Su, Z. Bao and Q. Ren, Inverse Adsorption Separation of CO2/C2H2 Mixture in Cyclodextrin-Based Metal–Organic Frameworks, ACS Appl. Mater. Interfaces, 2019, 11, 2543–2550 CrossRef CAS PubMed.
- S.-M. Wang, M. Shivanna, S. T. Zheng, T. Pham, K. A. Forrest, Q. Y. Yang, Q. Guan, B. Space, S. Kitagawa and M. J. Zaworotko, Ethane/Ethylene Separations in Flexible Diamondoid Coordination Networks via an Ethane-Induced Gate-Opening Mechanism, J. Am. Chem. Soc., 2024, 146, 4153–4161 CrossRef CAS PubMed.
- Y. Chai, X. Han, W. Li, S. Liu, S. Yao, C. Wang, W. Shi, I. da-Silva, P. Manuel, Y. Cheng, L. D. Daemen, A. J. Ramirez-Cuesta, C. C. Tang, L. Jiang, S. Yang, N. Guan and L. Li, Control of Zeolite Pore Interior for Chemoselective Alkyne/Olefin Separations, Science, 2020, 368, 1002–1006 CrossRef CAS PubMed.
- Z. Zhang, S. B. Peh, R. Krishna, C. Kang, K. Chai, Y. Wang, D. Shi and D. Zhao, Optimal Pore Chemistry in an Ultramicroporous Metal–Organic Framework for Benchmark Inverse CO2/C2H2 Separation, Angew. Chem., Int. Ed., 2021, 60, 17198–17204 CrossRef CAS PubMed.
- C. He, P. Zhang, Y. Wang, Y. Zhang, T. Hu, L. Li and J. Li, Thermodynamic and Kinetic Synergetic Separation of CO2/C2H2 in an Ultramicroporous Metal–Organic Framework, Sep. Purif. Technol., 2023, 304, 122318 CrossRef CAS.
- Q. Liu, S. G. Cho, J. Hilliard, T. Wang, S. Chien, L. Lin, A. C. Co and C. R. Wade, Inverse CO2/C2H2 Separation with MFU-4 and Selectivity Reversal via Postsynthetic Ligand Exchange, Angew. Chem., Int. Ed., 2023, 62, e202218854 CrossRef CAS PubMed.
- Y. Xie, H. Cui, H. Wu, R. Lin, W. Zhou and B. Chen, Electrostatically Driven Selective Adsorption of Carbon Dioxide over Acetylene in an Ultramicroporous Material, Angew. Chem., Int. Ed., 2021, 60, 9604–9609 CrossRef CAS PubMed.
- H. June Choi, D. Jo and S. Bong Hong, Effect of Framework Si/Al Ratio on the Adsorption Mechanism of CO2 on Small-Pore Zeolites: II. Merlinoite, Chem. Eng. J., 2022, 446, 137100 CrossRef CAS.
- V. M. Georgieva, E. L. Bruce, R. G. Chitac, M. M. Lozinska, A. M. Hall, C. A. Murray, R. I. Smith, A. Turrina and P. A. Wright, Cation Control of Cooperative CO2 Adsorption in Li-Containing Mixed Cation Forms of the Flexible Zeolite Merlinoite, Chem. Mater., 2021, 33, 1157–1173 CrossRef CAS.
- D. Yang, H. V. Doan, U. O'Hara, D. Reed, J. Hungerford, J. C. Eloi, N. E. Pridmore, P. F. Henry, S. Rochat, M. Tian and V. P. Ting, Impact of Cations and Framework on Trapdoor Behavior: A Study of Dynamic and In Situ Gas Analysis, Langmuir, 2024, 40, 12394–12406 CrossRef CAS PubMed.
Footnote |
| † R. Li and C. Liu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |