Open Access Article
Open Access ArticleStrain-release driven spirocyclization of bicyclo[1.1.0]butanes: access to 6,7-diazaspiro[3.4]octanes†
Qin
Jiang
,
Jianyang
Dong
*,
Fang
Lei
,
Dejiang
Yu
,
Ting
Li
,
Huaming
Sun
and
Dong
Xue
 *
*
Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an, 710062, China. E-mail: jydong@snnu.edu.cn; xuedong_welcome@snnu.edu.cn
First published on 5th June 2025
Abstract
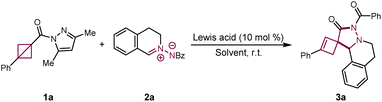
The use of ring strain in bicyclo[1.1.0]butanes (BCBs) to facilitate organic transformations is a powerful strategy in organic synthesis. However, their application to the synthesis of spirocyclic scaffolds has remained challenging. Spirocyclobutanes have attracted considerable interest in medicinal chemistry due to their diverse biological activities and potential clinical applications. In this work, we present a method for scandium-catalyzed spirocyclization of simple BCBs with azomethine imines, creating a new platform for the synthesis of previously inaccessible 6,7-diazaspiro[3.4]octanes. We demonstrate the utility of this approach by performing scaled-up reactions and transforming the products, highlighting its synthetic potential.
Introduction
Strain energy in organic molecules serves as a powerful driving force that enhances reactivity by facilitating the release of ring strain. This energy release enables a wide range of valuable transformations with broad applications across various research fields, including total synthesis, bioisosterism, and polymer science.1 Bicyclo[1.1.0]butanes (BCBs), strained carbocycles consisting of two fused cyclopropane rings, have become well-established building blocks in organic synthesis, medicinal chemistry, and chemical biology.2 This is primarily due to the unique reactivity of their inter-bridgehead C1–C3 bond (Scheme 1A), which exhibits almost entirely p-character.3 The ring-opening chemistry of this high-energy bond (with a ring strain of 64 kcal mol−1) has been successfully harnessed using a variety of reagents, including nucleophiles,4 radicals,5 electrophiles,6 and transition metal catalysts.7 Beyond ring-opening, the ring expansion of BCBs to bicyclo[n.1.1]alkanes through formal one-,8 two-,9 three-,10 or four-atom11 stepwise insertion processes has emerged as a particularly valuable method, owing to the relevance of these scaffolds in drug discovery. Additionally, metalation of the acidic C–H bonds of BCBs provides further opportunities for scaffold diversification.12 Despite this rich chemistry, the exploration of BCBs in spirocyclization reactions remains limited, likely due to the ease with which the aforementioned reactions proceed.13 However, developing novel spirocyclization strategies would offer rapid access to functionalized spirocyclobutane derivatives, which are increasingly valuable in pharmaceutical applications.Over the past few decades, spirocyclic scaffolds have emerged as privileged pharmacophores in medicinal chemistry, with the discovery of numerous spirocyclic compounds exhibiting significant biological activities.14 These scaffolds are now successfully incorporated into various approved drugs and drug candidates used to treat a wide range of diseases, including infections, neurological and metabolic disorders, and cancer.15 Notably, spiro[3.4]octane-containing compounds stand out for their remarkable anticancer activity (Scheme 1B).16 Despite their promising potential, the exploration of spirocyclic chemical space remains in its early stages, largely due to the limited variety of synthetic methodologies available for their construction. Research in this area typically focuses on the development of molecules with specific targets, underscoring the increasing demand for general, modular methods for the synthesis of highly functionalized spirocycle-based structures.17
As part of our ongoing efforts to develop new chemistry for BCBs,18 we reasoned that a strain-release-driven spirocyclization strategy could provide a straightforward route to spiro[3.4]octanes (Scheme 1C). We hypothesized that activation of BCBs by a Lewis acid would generate the carbanionic intermediate A. This intermediate could then react with electrophilic moiety of dipole to form intermediate B. Finally, intramolecular nucleophilic substitution of B would yield the spirocyclobutane. We identified two key challenges for the successful realization of this spirocyclization: (1) the intramolecular nucleophilic substitution of B must occur rapidly to prevent the formation of the bicyclo[n.1.1]alkane cycloaddition product, and (2) the leaving group must be sufficiently labile to facilitate nucleophilic substitution, avoiding the formation of cyclobutene products. Guided by this hypothesis, we turned to C,N-cyclic azomethine imines, which are known for their high reactivity as 1,3-dipoles in annulation reactions.19 However, research on azomethine imines has largely focused on their role in constructing various nitrogen heterocycles via [3 + 2],20 [3 + 3]21 and [3 + 4]22 cycloaddition reactions. To date, spirocyclization reactions involving these compounds to form spirocyclic nitrogen heterocycles have not been explored. In light of this, we investigated the potential for strain-release-driven spirocyclization between BCBs and azomethine imines as a means to efficiently access 6,7-diazaspiro[3.4]octanes. Herein, we report the first examples of scandium-catalyzed spirocyclization between simple BCBs and azomethine imines, establishing a new platform for the synthesis of previously inaccessible 6,7-diazaspiro[3.4]octane (Scheme 1D).
Results and discussion
To assess the feasibility of the spirocyclization reaction and optimize the conditions, we used pyrazole amide-substituted BCB (1a) and C,N-cyclic azomethine imine (2a) as standard substrates. Initial screening of various metal Lewis acids in THF at room temperature under argon for 12 hours (entries 1–6) revealed that the desired 6,7-diazaspiro[3.4]octane 3a was obtained in good yield and with low diastereoselectivity (78% yield, 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. as determined by 1H NMR spectroscopy) when Sc(OTf)3 or Zn(OTf)2 was used as the Lewis acid (entries 1 and 5). Other Lewis acids provided lower yields, and no cycloaddition products were detected. Subsequent solvent screening, using Sc(OTf)3 as the catalyst, showed that dichloroethane was the optimal solvent, yielding 3a in 87% yield and 1.7
1 d.r. as determined by 1H NMR spectroscopy) when Sc(OTf)3 or Zn(OTf)2 was used as the Lewis acid (entries 1 and 5). Other Lewis acids provided lower yields, and no cycloaddition products were detected. Subsequent solvent screening, using Sc(OTf)3 as the catalyst, showed that dichloroethane was the optimal solvent, yielding 3a in 87% yield and 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. (entries 7–12). Lowering the reaction temperature to 0 °C resulted in a reduced yield but improved diastereoselectivity (entry 13). Increasing the temperature to 60 °C did not affect the yield, but it led to poorer diastereoselectivity (entry 14). Control experiments demonstrated that no reaction occurred in the absence of Sc(OTf)3 (entry 15). Ultimately, the optimal conditions were determined to be: 1a (0.1 mmol, 1.0 equiv.), 2a (0.15 mmol, 1.5 equiv.), and Sc(OTf)3 (10 mol%) in dichloroethane (2 mL) at room temperature under argon for 12 hours (entry 12). The structure of 3a was confirmed by X-ray diffraction analysis of a single crystal (Scheme 2 and Table 1).
1 d.r. (entries 7–12). Lowering the reaction temperature to 0 °C resulted in a reduced yield but improved diastereoselectivity (entry 13). Increasing the temperature to 60 °C did not affect the yield, but it led to poorer diastereoselectivity (entry 14). Control experiments demonstrated that no reaction occurred in the absence of Sc(OTf)3 (entry 15). Ultimately, the optimal conditions were determined to be: 1a (0.1 mmol, 1.0 equiv.), 2a (0.15 mmol, 1.5 equiv.), and Sc(OTf)3 (10 mol%) in dichloroethane (2 mL) at room temperature under argon for 12 hours (entry 12). The structure of 3a was confirmed by X-ray diffraction analysis of a single crystal (Scheme 2 and Table 1).
 | ||
| Scheme 2 Exploration of substrate scope. Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), Sc(OTf)3 (0.02 mmol, 10 mol%), DCE (4.0 mL), Ar, r.t., 12 h. For details, see ESI.† | ||
| Entry | Lewis acid | Solvent | d.r. | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.15 mmol), Lewis acid (10 mol%), solvent (2.0 mL), Ar, room temperature (r.t.), 12 h. Yields were determined by 1H NMR spectroscopy with CH2Br2 as an internal standard. The d.r. values were measured by 1H NMR. NR, no reaction. b Isolated yield. c Reaction temperature, 0 °C. d Reaction temperature, 60 °C. | ||||
| 1 | Sc(OTf)3 | THF | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 |
| 2 | Cu(OTf)2 | THF | 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63 |
| 3 | Yb(OTf)3 | THF | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
57 |
| 4 | Mg(OTf)2 | THF | — | Trace |
| 5 | Zn(OTf)2 | THF | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 |
| 6 | Eu(OTf)3 | THF | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72 |
| 7 | Sc(OTf)3 | 1,4-Dioxane | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 |
| 8 | Sc(OTf)3 | MeOH | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
58 |
| 9 | Sc(OTf)3 | MeCN | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75 |
| 10 | Sc(OTf)3 | EtOAc | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
69 |
| 11 | Sc(OTf)3 | DCM | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 |
| 12 | Sc(OTf) 3 | DCE |
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
87 (84b) |
| 13c | Sc(OTf)3 | DCE | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
58 |
| 14d | Sc(OTf)3 | DCE | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 |
| 15 | — | DCE | — | NR |
Using the optimized conditions, we evaluated the substrate scope with respect to the BCB (Scheme 2, top panel). Pyrazole amide-substituted BCBs, featuring electron-neutral, electron-donating, or electron-withdrawing substituents on the aryl ring at the bridgehead position, proved reactive, delivering the desired products (3a–3i) in yields ranging from 53% to 86%. Specifically, phenyl BCBs with a methyl group, halogen atom, or trifluoromethoxy group at the para position gave the corresponding products 3b–3e in 61–86% yields. Additionally, phenyl BCBs with meta-methyl or -methoxy substituents were also suitable, providing 3f and 3g in 67% yields. Multi-substituted phenyl BCBs afforded 6,7-diazaspiro[3.4]octanes 3h and 3i in 63% and 53% yields, respectively. Different leaving groups, such as ester or amide-substituted BCB substrates were also tested, yielding no products, with significant amounts of starting material remaining (see ESI†). Next, we explored the substrate scope with respect to various structurally diverse C,N-cyclic azomethine imines (Scheme 2, middle panel). C,N-Cyclic azomethine imines with electron-neutral, electron-donating, or electron-withdrawing substituents on the phenyl ring of the benzoyl moiety were reactive, yielding products 3j–3u in 31–82% yields. Specifically, C,N-cyclic azomethine imines with methyl, methoxy, halogen, trifluoromethyl, or nitro groups in the para position of the benzoyl moiety gave the corresponding products 3j–3p in yields ranging from 31% to 82%. Additionally, C,N-cyclic azomethine imines with meta-bromine or -nitro substituents were also effective, producing 3q and 3r in 71% and 38% yields, respectively. Notably, ortho-chlorine and trifluoromethyl-substituted C,N-cyclic azomethine imines afforded the desired 6,7-diazaspiro[3.4]octanes 3s and 3t in 60% and 58% yields, respectively. Furthermore, multi-substituted C,N-cyclic azomethine imines gave 6,7-diazaspiro[3.4]octane 3u in 55% yield. In addition to C,N-cyclic azomethine imines with the benzoyl group, C,N-cyclic azomethine imines bearing an N-sulfonyl group were also suitable, yielding 6,7-diazaspiro[3.4]octanes 3v–3x in 57–62% yields. Subsequently, various functional groups including methyl and halogen atoms were successfully introduced into the isoquinoline ring moiety without significantly compromising reaction efficiency, affording the desired products 3y–3ac in satisfactory yields. After successfully establishing the non-asymmetric spirocyclization of BCBs with azomethine imines, we wish to describe our preliminary results on the asymmetric version of the reaction. The desired product 3a was obtained in 66% yield and the highest enantioselectivity (1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r., 82% and 63% ee) when using BINOL-derived CPA as the catalyst and 1,4-dioxane as the solvent (Scheme 2, bottom panel).
1 d.r., 82% and 63% ee) when using BINOL-derived CPA as the catalyst and 1,4-dioxane as the solvent (Scheme 2, bottom panel).
To demonstrate the utility of our spirocyclization method, we scaled up the reaction to 4 mmol and successfully generated 6,7-diazaspiro[3.4]octanes 3v, with only a slight decrease in yield (Scheme 3A). Additionally, we carried out several transformations of 3v (Scheme 3B). Specifically, deprotection of 3v with Mg/MeOH yielded compound 4 in 69% yield. Furthermore, the carbonyl group of 3v was reduced to the corresponding alcohol, resulting in the formation of 5 in 54% yield. Subsequently, compound 3v underwent successful hydrogenation under reducing conditions using Pd/C as the catalyst, affording product 6 in 64% yield. Finally, halohydrin 7 was synthesized via the reaction of 3v with N-bromosuccinimide (NBS) in the presence of water. These transformation products are valuable heterocyclic structures found in both natural and synthetic bioactive compounds.
After exploring the reaction's substrate scope and demonstrating the utility of the products in various transformations, we shifted our focus to investigating the reaction mechanism (Scheme 4). First, when 2a was subjected to the optimized conditions in the absence of pyrazole amide-substituted BCB (1a), no product was formed, and a significant amount of starting material remained (Scheme 4A). However, when 1a was treated under the same conditions in the absence of C,N-cyclic azomethine imine (2a), cyclobutene 8 was obtained in 10% yield (Scheme 4B), suggesting that 1a is likely activated directly by Sc(OTf)3 to form a cationic intermediate. Finally, the addition of the radical-trapping agent TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl) to the reaction mixture containing 1a and 2a did not significantly affect the yield of 3a (Scheme 4C), indicating that a radical pathway is not involved in the mechanism.
Based on our mechanistic studies, we propose the mechanism outlined in Scheme 5. Initially, substrate 1a is activated through coordination of Sc(OTf)3 to the carbonyl group, forming the cationic intermediate I. Elimination of intermediate I produces intermediate II. Next, nucleophilic addition of II to substrate 2avia intermediate III results in the formation of intermediate IV. This key intermediate undergoes substitution, with the nitrogen atom attacking the pyrazole amide group, yielding the desired 6,7-diazaspiro[3.4]octane product 3a and regenerating the Sc(OTf)3 catalyst.
Conclusions
In summary, we have developed a scandium-catalyzed spirocyclization method for simple BCBs, providing a versatile platform for the de novo construction of highly sought-after 6,7-diazaspiro[3.4]octanes. This method enables spirocyclizations of a broad range of BCBs with azomethine imines, granting access to 6,7-diazaspiro[3.4]octane frameworks for the first time. The synthetic utility of this approach was demonstrated through gram-scale reactions and subsequent transformations of the products. Notably, this method not only expands the toolbox for BCB spirocyclizations but also holds great potential for accelerating the exploration of novel 6,7-diazaspiro[3.4]octanes in drug discovery. Ongoing research in our laboratory is focused on extending this methodology to other spirocyclic scaffolds and exploring their applications.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 3a (CCDC 2427337†) have been deposited at the Cambridge Crystallographic Data Centre.Author contributions
J. Y. D. and D. X. conceived and directed the project. Q. J. discovered and developed the reaction. Q. J., F. L., D. J. Y., T. L. and H. M. S. performed the experiments and collected the data. All authors discussed and analyzed the data. J. Y. D. and D. X. wrote the manuscript with contribution from other authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the National Natural Science Foundation of China (22171174, 22401177), the Fundamental Research Funds for the Central Universities (GK202401008), the Innovation Capability Support Program of Shaanxi (2023-CX-TD-28), the Fundamental Science Research Project of Shaanxi for Chemistry, Biology (22JHZ002), the Young Talent Fund of Association for Science and Technology in Shaanxi, China (20240606), Key Research and Development Program of Shaanxi (2024SF2-GJHX32), Major Scientific and Technological Achievements Transformation Project of Xianyang (L2024-ZDKJ-ZDCGZH-0015).Notes and references
-
(a) J. Turkowska, J. Durka and D. Gryko, Chem. Commun., 2020, 56, 5718–5734 RSC
; (b) J. L. Tyler and V. K. Aggarwal, Chem.–Eur. J., 2023, 29, e202300008 CrossRef CAS PubMed
; (c) C. B. Kelly, J. A. Milligan, L. J. Tilley and T. M. Sodano, Chem. Sci., 2022, 13, 11721–11737 RSC
; (d) P. Bellotti and F. Glorius, J. Am. Chem. Soc., 2023, 145, 20716–20732 CrossRef CAS PubMed
.
-
(a) M. Golfmann and J. C. L. Walker, Commun. Chem., 2023, 6, 9 CrossRef PubMed
; (b) C. B. Kelly, J. A. Milligan, L. J. Tilley and T. M. Sodano, Chem. Sci., 2022, 13, 11721–11737 RSC
; (c) A. Fawcett, Pure Appl. Chem., 2020, 92, 751–765 CrossRef
.
-
(a) J. M. Schulman and M. D. Newton, J. Am. Chem. Soc., 1974, 96, 6295–6297 CrossRef CAS
; (b) D. R. Whitman and J. F. Chiang, J. Am. Chem. Soc., 1972, 94, 1126–1129 CrossRef CAS
; (c) M. Pomerantz and E. W. Abrahamson, J. Am. Chem. Soc., 1966, 88, 3970–3972 CrossRef CAS
.
-
(a) L. Guo, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2021, 60, 212–216 CrossRef CAS PubMed
; (b) J. M. Lopchuk, K. Fjelbye, Y. Kawamata, L. R. Malins, C.-M. Pan, R. Gianatassio, J. Wang, L. Prieto, J. Bradow, T. A. Brandt, M. R. Collins, J. Elleraas, J. Ewanicki, W. Farrell, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, R. Oliver, N. W. Sach, J. K. Smith, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 3209–3226 Search PubMed
; (c) R. Gianatassio, J. M. Lopchuk, J. Wang, C. Pan, L. R. Malins, L. Prieto, T. A. Brandt, M. R. Collins, G. M. Gallego, N. W. Sach, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, Science, 2016, 351, 241–246 CrossRef CAS PubMed
; (d) R. A. Panish, S. R. Chintala and J. M. Fox, Angew. Chem., Int. Ed., 2016, 55, 4983–4987 CrossRef CAS
; (e) R. Panish, S. R. Chintala, D. T. Boruta, Y. Fang, M. T. Taylor and J. M. Fox, J. Am. Chem. Soc., 2013, 135, 9283–9286 CrossRef
; (f) Y. Gaoni, Tetrahedron, 1989, 45, 2819–2840 CrossRef
; (g) Y. Gaoni and A. Tomazic, J. Org. Chem., 1985, 50, 2948–2957 Search PubMed
; (h) A. Kaur, W. Lin, V. Dovhalyuk, L. Driutti, M. L. Di Martino, M. Vujasinovic, J. M. Löhr, M. E. Sellin and D. Globisch, Chem. Sci., 2023, 14, 5291–5301 Search PubMed
; (i) B. D. Schwartz, A. P. Smyth, P. E. Nashar, M. G. Gardiner and L. R. Malins, Org. Lett., 2022, 24, 1268–1273 Search PubMed
; (j) K. Tokunaga, M. Sato, K. Kuwata, C. Miura, H. Fuchida, N. Matsunaga, S. Koyanagi, S. Ohdo, N. Shindo and A. Ojida, J. Am. Chem. Soc., 2020, 142, 18522–18531 Search PubMed
; (k) P. Zhang, R. Zhuang, X. Wang, H. Liu, J. Li, X. Su, X. Chen and X. Zhang, Bioconjugate Chem., 2018, 29, 467–472 Search PubMed
; (l) J. M. Lopchuk, K. Fjelbye, Y. Kawamata, L. R. Malins, C. M. Pan, R. Gianatassio, J. Wang, L. Prieto, J. Bradow, T. A. Brandt, M. R. Collins, J. Elleraas, J. Ewanicki, W. Farrell, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, R. Oliver, N. W. Sach, J. K. Smith, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 3209–3226 Search PubMed
.
-
(a) C. J. Pratt, R. A. Aycock, M. D. King and N. T. Jui, Synlett, 2020, 31, 51–54 CrossRef
; (b) M. Silvi and V. K. Aggarwal, J. Am. Chem. Soc., 2019, 141, 9511–9515 CrossRef PubMed
.
-
(a) A. Guin, S. Bhattacharjee, M. S. Harariya and A. T. Biju, Chem. Sci., 2023, 14, 6585–6591 RSC
; (b) M. J. Kerner and P. Wipf, Org. Lett., 2021, 23, 3615–3619 CrossRef PubMed
; (c) S. H. Bennett, A. Fawcett, E. H. Denton, T. Biberger, V. Fasano, N. Winter and V. K. Aggarwal, J. Am. Chem. Soc., 2020, 142, 16766–16775 CrossRef
; (d) A. Dasgupta, S. Bhattacharjee, Z. Tong, A. Guin, R. E. McNamee, K. E. Christensen, A. T. Biju and E. A. Anderson, J. Am. Chem. Soc., 2024, 146, 1196–1203 CrossRef PubMed
; (e) S. Lin, Y. Chen, H. Liu, S. Xiang and B. Tan, J. Am. Chem. Soc., 2023, 145, 21152–21158 Search PubMed
.
-
(a) B. Wölfl, N. Winter, J. Li, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2023, 62, e202217064 CrossRef PubMed
; (b) Z. Zhang and V. Gevorgyan, J. Am. Chem. Soc., 2022, 144, 20875–20883 CrossRef PubMed
; (c) T. Pinkert, M. Das, M. L. Schrader and F. Glorius, J. Am. Chem. Soc., 2021, 143, 7648–7654 CrossRef PubMed
; (d) M. Ociepa, A. J. Wierzba, J. Turkowska and D. Gryko, J. Am. Chem. Soc., 2020, 142, 5355–5361 CrossRef
; (e) A. Fawcett, T. Biberger and V. K. Aggarwal, Nat. Chem., 2019, 11, 117–122 CrossRef PubMed
; (f) M. A. A. Walczak, T. Krainz and P. Wipf, Acc. Chem. Res., 2015, 48, 1149–1158 CrossRef
; (g) M. A. A. Walczak and P. Wipf, J. Am. Chem. Soc., 2008, 130, 6924–6925 CrossRef
.
-
(a) R. Bychek and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2022, 61, e202205103 CrossRef
; (b) X. Ma, W. Pinto, L. N. Pham, D. L. Sloman and Y. Han, Eur. J. Org Chem., 2020, 2020, 4581–4605 CrossRef
; (c) X. Ma, D. L. Sloman, Y. Han and D. J. Bennett, Org. Lett., 2019, 21, 7199–7203 CrossRef PubMed
; (d) R. M. Bychek, V. Hutskalova, Y. P. Bas, O. A. Zaporozhets, S. Zozulya, V. V. Levterov and P. K. Mykhailiuk, J. Org. Chem., 2019, 84, 15106–15117 CrossRef
; (e) D. E. Applequist, T. L. Renken and J. W. Wheeler, J. Org. Chem., 1982, 47, 4985–4995 CrossRef
.
-
(a) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 535–541 CrossRef CAS PubMed
; (b) R. Kleinmans, S. Dutta, K. Ozols, H. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2023, 145, 12324–12332 CrossRef CAS PubMed
; (c) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc and F. Glorius, Nature, 2022, 605, 477–482 CrossRef CAS PubMed
; (d) R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef CAS
; (e) Y. Liang, R. Kleinmans, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2022, 144, 20207–20213 CrossRef CAS
; (f) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. C. Leitch, Angew. Chem., Int. Ed., 2022, 61, e202204719 Search PubMed
; (g) J. Su, J. Zhang, Z. Xin, H. Li, H. Zheng and W. Deng, Org. Chem. Front., 2024, 11, 4539–4545 RSC
.
-
(a) T. Yu, J. Yang, Z. Wang, Z. Ding, M. Xu, J. Wen, L. Xu and P. Li, J. Am. Chem. Soc., 2023, 145, 4304–4310 CrossRef CAS
; (b) J. Zhang, J. Y. Su, H. L. Zheng, H. Li and W. P. Deng, Angew. Chem., Int. Ed., 2024, e202318476 Search PubMed
; (c) Y. Zheng, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 23685–23690 CrossRef CAS
; (d) J. Zhang, J. Su, H. Zheng, H. Li and W. Deng, ACS Catal., 2024, 14, 17837–17849 CrossRef CAS
.
-
(a) J. J. Wang, L. Tang, Y. J. Xiao, W. B. Wu, G. Q. Wang and J. J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202405222 Search PubMed
; (b) X. Y. Gao, L. Tang, X. Zhang and J. Feng, Chem. Sci., 2024, 15, 13942–13948 RSC
; (c) S. Deswal, A. Guin and A. T. Biju, Angew. Chem., Int. Ed., 2024, 63, e202408610 Search PubMed
; (d) S. Nicolai and J. Waser, Chem. Sci., 2024, 15, 10823–10829 Search PubMed
; (e) T. Yu, X. Zhao, Z. Nie, L. Qin, Z. Ding, L. Xu and P. Li, Angew. Chem., Int. Ed., 2025, 64, e202420831 Search PubMed
; (f) K. Zhang, Z. Gao, Y. Xia, P. Li, P. Gao, X. Hua and L. Guo, Chem. Sci., 2025, 16, 1411–1416 RSC
.
-
(a) R. E. McNamee, M. M. Haugland, J. Nugent, R. Chan, K. E. Christensen and E. A. Anderson, Chem. Sci., 2021, 12, 7480–7485 RSC
; (b) R. E. McNamee, A. L. Thompson and E. A. Anderson, J. Am. Chem. Soc., 2021, 143, 21246–21251 CrossRef
; (c) J. L. Tyler and V. K. Aggarwal, Chem.–Eur. J., 2023, 29, e202300008 CrossRef PubMed
.
-
(a) K. Das, A. Pedada, T. Singha and D. P. Hari, Chem. Sci., 2024, 15, 3182–3186 RSC
; (b) T. Singha, N. A. Bapat, S. K. Mishra and D. P. Hari, Org. Lett., 2024, 26, 6396–6399 CrossRef PubMed
; (c) Y. Xiao, L. Tang, X. Yang, N. Wang, J. Zhang, W. Deng and J. Feng, CCS Chem., 2024 DOI:10.31635/ccschem.025.202505825
; (d) X. Yang, J. Wang, Y. Xiao and J. Feng, Angew. Chem., Int. Ed., 2025, e202505803 Search PubMed
; (e) P. Bellotti and F. Glorius, J. Am. Chem. Soc., 2023, 145, 20716–20732 CrossRef
.
-
(a) E. Chupakhin, O. Babich, A. Prosekov, L. Asyakina and M. Krasavin, Molecules, 2019, 24, 4165–4180 CrossRef PubMed
; (b) A. Ding, M. Meazza, H. Guo, J. W. Yang and R. Rios, Chem. Soc. Rev., 2018, 47, 5946–5996 RSC
; (c) S. Nasri, M. Bayat and F. Mirzaei, Top. Curr. Chem., 2021, 379, 25–50 CrossRef
; (d) M. Liu, X. Zhang and G. Li, Chin. J. Chem., 2022, 40, 1867–1889 CrossRef
; (e) X. Y. Xu, S. W. Tsang, Y. F. Guan, K. L. Liu, W. H. Pan, C. S. Lam, K. M. Lee, Y. X. Xia, W. J. Xie, W. Y. Wong, M. M. L. Lee, W. C. S. Tai and H. J. Zhang, J. Med. Chem., 2019, 62, 1541–1561 CrossRef CAS PubMed
.
-
(a) V. F. Batista, D. Pinto and A. M. S. Silva, Expet Opin. Drug Discov., 2022, 17, 603–618 CrossRef CAS PubMed
; (b) D. Bora, A. Kaushal and N. Shankaraiah, Eur. J. Med. Chem., 2021, 215, 113263 CrossRef CAS PubMed
; (c) M. Benabdallah, O. Talhi, F. Nouali, N. Choukchou-Braham, K. Bachari and A. M. S. Silva, Curr. Med. Chem., 2018, 25, 3748–3767 CrossRef CAS PubMed
; (d) P. Zhao, L. Zhuang, X. Wang, S. Huang, H. Wu, Y. Zhou, Y. Yan, F. Zhang, R. Shen, J. Li, S. Liu, R. Zhang, P. Dong, Y. Mao, Y. Fan, C. He, J. Sun, L. Zhang, Q. Hu, H. Wan, J. Feng, C. Bai, F. He and W. Tao, Eur. J. Med. Chem., 2022, 228, 114040 CrossRef CAS PubMed
; (e) L. Yu, A. Dai, W. Zhang, A. Liao, S. Guo and J. Wu, J. Agric. Food Chem., 2022, 70, 10693–10707 CrossRef PubMed
; (f) A. A. Braddock and E. A. Theodorakis, Mar. Drugs, 2019, 17, 232 CrossRef
; (g) S. Ikeda, H. Sugiyama, H. Tokuhara, M. Murakami, M. Nakamura, Y. Oguro, J. Aida, N. Morishita, S. Sogabe, D. R. Dougan, S. C. Gay, L. Qin, N. Arimura, Y. Takahashi, M. Sasaki, Y. Kamada, K. Aoyama, K. Kimoto and M. Kamata, J. Med. Chem., 2021, 64, 11014–11044 CrossRef
.
-
(a) H. Cao, G. Zhu, L. Sun, G. Chen, X. Ma, X. Luo and J. Zhu, Eur. J. Med. Chem., 2019, 183, 111694 CrossRef PubMed
; (b) Z. Ding, W. Pan, Y. Xiao, B. Cheng, G. Huang and J. Chen, Eur. J. Med. Chem., 2022, 237, 114401 CrossRef PubMed
; (c) B. K. Chan, E. Seward, M. Lainchbury, T. F. Brewer, L. An, T. Blench, M. W. Cartwright, G. K. Y. Chan, E. F. Choo, J. Drummond, R. L. Elliott, E. Gancia, L. Gazzard, B. Hu, G. E. Jones, X. Luo, A. Madin, S. Malhotra, J. G. Moffat, J. Pang, L. Salphati, C. J. Sneeringer, C. E. Stivala, B. Wei, W. Wang, P. Wu and T. P. Heffron, ACS Med. Chem. Lett., 2022, 13, 84–91 CrossRef
; (d) A. Aguilar, J. Lu, L. Liu, D. Du, D. Bernard, D. McEachern, S. Przybranowski, X. Li, R. Luo, B. Wen, D. Sun, H. Wang, J. Wen, G. Wang, Y. Zhai, M. Guo, D. Yang and S. Wang, J. Med. Chem., 2017, 60, 2819–2839 CrossRef
; (e) N. J. Clegg, J. Wongvipat, J. D. Joseph, C. Tran, S. Ouk, A. Dilhas, Y. Chen, K. Grillot, E. D. Bischoff, L. Cai, A. Aparicio, S. Dorow, V. Arora, G. Shao, J. Qian, H. Zhao, G. Yang, C. Cao, J. Sensintaffar, T. Wasielewska, M. R. Herbert, C. Bonnefous, B. Darimont, H. I. Scher, P. Smith-Jones, M. Klang, N. D. Smith, E. De Stanchina, N. Wu, O. Ouerfelli, P. J. Rix, R. A. Heyman, M. E. Jung, C. L. Sawyers and J. H. Hager, Cancer Res., 2012, 72, 1494–1503 CrossRef PubMed
.
-
(a) C. L. Manach, J. Dam, J. G. Woodland, G. Kaur, L. P. Khonde, C. Brunschwig, M. Njoroge, K. J. Wicht, A. Horatscheck, T. Paquet, G. A. Boyle, L. Gibhard, D. Taylor, N. Lawrence, T. Yeo, S. Mok, R. T. Eastman, D. Dorjsuren, D. C. Talley, H. Guo, A. Simeonov, J. Reader, M. Watt, E. Erlank, N. Venter, J. W. Zawada, A. Aswat, L. Nardini, T. L. Coetzer, S. B. Lauterbach, B. C. Bezuidenhout, A. Theron, D. Mancama, L. L. Koekemoer, L.-M. Birkholtz, S. Wittlin, M. Delves, S. Ottilie, E. A. Winzeler, T. W. von Geldern, D. Smith, D. A. Fidock, L. J. Street, G. S. Basarab, J. Duffy and K. Chibale, J. Med. Chem., 2021, 64, 2291–2309 CrossRef PubMed
; (b) H. Zhu, X. Song, Y. Pan, M. Li, L. Chen, P. Xiao, R. Du, Z. Dong and C.-G. Yang, Eur. J. Med. Chem., 2023, 258, 115595 CrossRef PubMed
; (c) K. Y. Lee, H. S. Lee and J. N. Kim, Tetrahedron Lett., 2007, 48, 8583–8586 CrossRef
; (d) M. J. Kerner and P. Wipf, Org. Lett., 2021, 23, 3615–3619 CrossRef
; (e) J. L. Tyler, D. A. Noble and P. V. K. Aggarwal, Angew. Chem., Int. Ed., 2021, 60, 11824–11829 CrossRef
.
-
(a) H. Liao, J. Dong, X. Zhou, Q. Jiang, Z. Lv, F. Lei and D. Xue, Chem. Sci., 2025, 16, 4654–4660 RSC
; (b) Q. Jiang, J. Dong, X. Zhou, H. Liao, J. Zhou and D. Xue, Org. Lett., 2024, 26, 9311–9315 CrossRef PubMed
; (c) X. Zhou, J. Dong, H. Liao, Q. Jiang, B. Zhang, T. Li, F. Lei, H. Sun and D. Xue, Org. Lett., 2025, 27, 3571–3577 CrossRef PubMed
.
- T. Hashimoto, Y. Maeda, M. Omote, H. Nakatsu and K. Maruoka, J. Am. Chem. Soc., 2010, 132, 4076–4084 CrossRef PubMed
.
- The C,N-cyclic azomethine imines as 1,3-dipoles for [3 + 2] annulations, see:
(a) X. Liu, D. Yang, K. Wang, J. Zhang and R. Wang, Green Chem., 2017, 19, 82–87 RSC
; (b) Y. Wang, Q. Wang and J. Zhu, Chem.–Eur. J., 2016, 22, 8084–8088 CrossRef
; (c) D. Wang, Y. Lei, Y. Wei and M. Shi, Chem.–Eur. J., 2014, 20, 15325–15329 CrossRef PubMed
; (d) S. Milosevic and A. Togni, J. Org. Chem., 2013, 78, 9638–9646 CrossRef PubMed
; (e) X. Liu, Y. Wang, D. Yang, J. Zhang, D. Liu and W. Su, Angew. Chem., Int. Ed., 2016, 55, 8100–8103 CrossRef
; (f) F. Li, J. Chen, Y. Hou, Y. Li, X.-Y. Wu and X. Tong, Org. Lett., 2015, 17, 5376–5379 CrossRef
; (g) B.-S. Li, Y. Wang, Z. Jina and Y. R. Chi, Chem. Sci., 2015, 6, 6008–6012 RSC
; (h) C. Jing, R. Na, B. Wang, H. Liu, L. Zhang, J. Liu, M. Wang, J. Zhong, O. Kwon and H. Guo, Adv. Synth. Catal., 2012, 354, 1023–1034 CrossRef CAS
; (i) Q. Jia, L. Chen, G. Yang, J. Wang, J. Wei and Z. Du, Tetrahedron Lett., 2015, 56, 7150–7153 CrossRef CAS
; (j) C. Izquierdo, F. Esteban, A. Parra, R. Alfaro, J. Aleman, A. Fraile and J. L. Garcia Ruano, J. Org. Chem., 2014, 79, 10417–10433 CrossRef CAS
; (k) T. Hashimoto, M. Omote and K. Maruoka, Angew. Chem., Int. Ed., 2011, 50, 3489–3492 CrossRef CAS PubMed
; (l) Z.-H. Gao, X.-Y. Chen, J.-T. Cheng, W.-L. Liao and S. Ye, Chem. Commun., 2015, 51, 9328–9331 RSC
.
- The C,N-cyclic azomethine imines as 1,3-dipoles for [3 + 3] annulations, see:
(a) Y.-Y. Zhou, J. Li, L. Ling, S.-H. Liao, X.-L. Sun, Y.-X. Li, L.-J. Wang and Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 1452–1456 CrossRef CAS
; (b) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316–4319 CrossRef CAS
; (c) C. Yuan, H. Liu, Z. Gao, L. Zhou, Y. Feng, Y. Xiao and H. Guo, Org. Lett., 2015, 17, 26–29 CrossRef CAS PubMed
.
- The C,N-cyclic azomethine imines as 1,3-dipoles for [3 + 4] annulations, see:
(a) L. Chen, G. M. Yang, J. Wang, Q. F. Jia, J. Wei and Z. Y. Du, RSC Adv., 2015, 5, 76696–76699 RSC
; (b) C. H. Yuan, L. J. Zhou, M. R. Xia, Z. H. Sun, D. Q. Wang and H. C. Guo, Org. Lett., 2016, 18, 5644–5647 CrossRef PubMed
; (c) J. F. Xu, S. R. Yuan, J. Y. Peng, M. Z. Miao, Z. K. Chen and H. J. Ren, Org. Biomol. Chem., 2017, 15, 7513–7517 RSC
; (d) Y. Wang, L. Zhu, M. Wang, J. Xiong, N. Chen, X. Feng, Z. Xu and X. Jiang, Org. Lett., 2018, 20, 6506–6510 CrossRef CAS PubMed
; (e) X.-Q. Hu, J.-R. Chen, S. Gao, B. Feng, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2013, 49, 7905–7907 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2427337. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03141a |
| This journal is © The Royal Society of Chemistry 2025 |