Open Access Article
Open Access ArticleCu(I)-catalyzed enantioselective and stereospecific borylative annulation of cyclic 1,3-dione-tethered 1,3-enynes†
G. Raghu
Ramudu‡
ac,
Vaibhav B.
Patil‡
a,
Jagadeesh Babu
Nanubolu
 bc and
Rambabu
Chegondi
bc and
Rambabu
Chegondi
 *ac
*ac
aDepartment of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad 500007, India. E-mail: rchegondi@iict.res.in; cramhcu@gmail.com; Web: https://www.cramhcu.wixsite.com/rambabu-chegondi
bDepartment of Analytical and Structural Chemistry, CSIR-Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad 500007, India
cAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201 002, India
First published on 14th July 2025
Abstract
A copper(I)-catalyzed, highly enantioselective, and diastereoselective borylative cyclization of prochiral cyclic 1,3-dione-tethered 1,3-enynes is reported. This stereospecific transformation exhibits a broad substrate scope, enabling access to bicyclic organoboron products containing four contiguous stereocenters with excellent enantioselectivity. Notably, the reaction rate is significantly influenced by the substrate's stereochemistry, with the (Z)-isomer undergoing borylative cyclization much faster than the (E)-isomer due to reduced steric interactions during C–C bond formation. Furthermore, treatment of the resulting products with sodium perborate yields the corresponding alcohols without compromising enantiomeric excess.
Introduction
Conjugated enynes are highly reactive and valuable substrates in modern organic chemistry, widely utilized in the synthesis of complex aromatic molecules and materials.1 While 1,3-enyne motifs can be readily accessed through several efficient methods, the catalytic cross-coupling of terminal alkynes stands out as the most convenient approach.2 Recently, copper-catalyzed hydro- and borofunctionalizations, multicomponent reactions, radical functionalizations, and cyclizations of these π-systems have garnered significant attention from organic and medicinal chemists.3 Among these methods, enantioselective Cu-catalyzed borylation of 1,3-enynes has also emerged as an elegant strategy, providing access to a diverse range of chiral organoboranes.4 The resulting C–B bonds can be conveniently converted into C–C, C–O, and C–N bonds through stereospecific 1,2-migration.5 However, achieving asymmetric chemo- and regioselective borylative difunctionalization of 1,3-enynes remains highly challenging due to the presence of multiple reactive sites.In general, two pathways are possible for 1,3-enynes during hydro- or borofunctionalization. The reaction at the olefin can yield propargyl or allene products via the ene-pathway (Scheme 1a), whereas the reaction at the alkyne results in diene products through the yne-pathway.3 In 2011, Ito and co-workers reported that the regioselectivity of borocupration is influenced by the nature of the ligand employed in the reaction and steric hindrance around the olefinic functionality of the enyne.6 The first enantioselective Cu-catalyzed borylative 1,2-difunctionalization of 1,3-enynes was reported by the Hoveyda group in 2014 via the ene-pathway (Scheme 1b).7 The borocupration of 1,3-enynes enables the formation of a chiral allenylcopper complex, which is readily captured by aldehydes to provide propargylated products with excellent diastereo- and enantioselectivities. In light of this report, Yin and co-workers disclosed similar consecutive protocols on ketones.8 Later, Procter and co-workers disclosed a highly enantio- and diastereoselective borylative 1,2-coupling of 1,3-enynes with imines to provide chiral homopropargyl amines with excellent diastereo- and enantioselectivity.9 Recently, Yun and co-workers reported a highly enantioselective 1,2-borylation/conjugate addition with β-substituted alkylidene malonates, enabling organoboranes bearing adjacent stereocentres.10 In 2020, Hu, Wang, Liao, and co-workers developed a cooperative Cu/Pd-catalyzed 1,4-boroarylation, wherein arylation occurs via transmetalation with palladium to access chiral tri- and tetra-substituted allenes.11 Shortly thereafter, Xu et al. reported the 1,4-boroprotonation of trifluoromethyl-substituted conjugated enynes to access enantioenriched homoallenyboronates.12 Similarly, CuH-catalyzed hydrofunctionalization of 1,3-enynes also allows the generation of chiral allenylcopper intermediates, which are readily captured by various electrophiles to yield the corresponding propargylic products and allenes with excellent stereoselectivities.13,14 Among these elegant methods, 1,2-borofunctionalization is generally limited to terminal 1,3-enynes, with only two reports9,10 exploring internal enynes due to their lower reactivity and other challenges related to regioselectivity and stereoselectivity. Here, E/Z-selectivity of the double bond controls the diastereoselectivity of the corresponding product in a stereospecific manner.
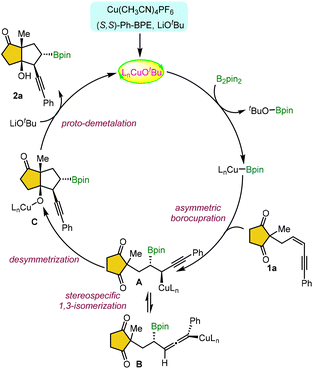
To the best of our knowledge, there are no intramolecular cyclizations reported via Cu-catalyzed borylative 1,2-difunctionalization of 1,3-enynes with internal electrophiles. Based on our interest in the area of enantioselective Cu-catalysis,15 herein, we report a stereospecific annulation of 1,3-enyne-tethered cyclic 1,3-enones (Scheme 1c). This work describes an unprecedented intramolecular Cu(I)-catalyzed borylative difunctionalization of 1,3-enynes, delivering products with excellent diastereo- and enantioselectivity. The E/Z configuration of the substrates significantly influences the reaction rate and diastereoselectivity of products. We envisioned that stereospecific syn-addition of a borylcopper(I) intermediate on the double bond of (Z/E)-1 could provide intermediate A/B. Subsequent intramolecular nucleophilic attack of organocuprate A/B could afford the corresponding product anti-2 or syn-2, respectively with the retention of the configuration at the C–B bond. Notably, the reaction proceeds through highly regioselective 1,2-borocupration on the double bond adjacent to the sterically demanding quaternary prochiral center.
Results and discussion
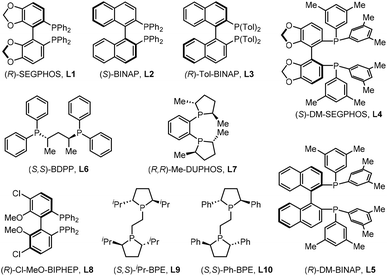
We began our investigation on Cu(I)-catalyzed borylative cyclization of Z-selective 1,3-enyne 1a as a model substrate in the presence of bis(pinacolato)diboron as the borylation source with various chiral bidentate phosphine ligands (Table 1). The reaction was initially conducted with 2.5 mol% of Cu(CH3CN)4PF6, 5 mol% of ligand, and 2.0 equivalents of base in THF at −78 °C for 2 hours. Notably, the BINAP ligands provided the desired product anti-2a in moderate yield with enantioselectivity ranging from 37% to 92%, whereas SEGPHOS ligands resulted in only trace amounts of the product (entries 1–5). In addition, the BDPP ligand L6, DUPHOS ligand L7, BIPHEP ligand L8, and iPr-BPE ligand L9 proved ineffective in the model reaction, resulting in no product formation. Fortunately, the reaction with (S,S)-Ph-BPE ligand L10 afforded 2a in high yield with excellent enantioselectivity (>99% ee). The exclusive diastereoselectivity suggests that the borylative cyclization of (Z)-1a proceeds with high stereocontrol. The relative stereochemistry of anti-2a was confirmed by single crystal X-ray diffraction analysis (see Table 2).16| Entry | Ligand | Yield [%] | 2a (ee) |
|---|---|---|---|
a Reaction conditions: 1a (50 mg, 0.2 mmol), B2(pin)2 (60 mg, 0.24 mmol), Cu(CH3CN)4PF6 (1.9 mg, 2.5 mol%), ligand (5.0 mol%), tBuOH (38 μL, 0.4 mmol), LiOtBu (36 μL, 0.4 mmol, 1.0 M THF solution), in THF solvent (3 mL, 0.1 M).
b Isolated yields.
c Enantiomeric ratio (er) was determined by HPLC analysis using a chiral stationary phase.
d >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr was observed from 1H NMR analysis. 1 dr was observed from 1H NMR analysis.
|
|||
| 1 | (R)-SEGPHOS, L1 | <5 | — |
| 2 | (S)-BINAP, L2 | 54 | 92% |
| 3 | (R)-Tol-BINAP, L3 | 36 | 69% |
| 4 | (S)-DM-SEGPHOS, L4 | <5 | — |
| 5 | (R)-DM-BINAP, L5 | 31 | 37% |
| 6 | (S,S)-BDPP, L6 | <5 | — |
| 7 | (R,R)-Me-DUPHOS, L7 | — | — |
| 8 | (R)-Cl-MeO-BIPHEP, L8 | — | — |
| 9 | (S,S)-iPr-BPE, L9 | — | — |
| 10 | (S,S)-Ph-BPE- L10 | 82% | >99% |
|
|||
a Reaction conditions: 1 (0.3 mmol), B2(pin)2 (91 mg, 0.36 mmol), Cu(CH3CN)4PF6 (2.8 mg, 2.5 mol%), (S,S)-Ph-BPE (7.6 mg, 5.0 mol%), tBuOH (57 μL, 0.6 mmol), LiOtBu (54 μL, 0.6 mmol, 1.0 M THF solution), in THF solvent (3 mL, 0.1 M).
b Isolated yields after column chromatography.
c Enantiomeric ratio (er) was determined by chiral HPLC analysis.
d >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr was observed (unless otherwise mentioned) through 1H NMR analysis of a crude reaction mixture. SM = Starting material. 1 dr was observed (unless otherwise mentioned) through 1H NMR analysis of a crude reaction mixture. SM = Starting material.
|
|---|

|
Under standard conditions, the borylative cyclization of 1,3-enyne (Z)-1a proceeded with complete anti-selectivity, affording the bicyclic product 2a in 82% yield with >99% enantioselectivity (Scheme 2). In contrast, the reaction of 1,3-enyne (E)-1a yielded the syn-selective bicyclic product 2a with moderate yield and 92% enantioselectivity. Besides, prolonging the reaction time did not enhance the conversion, and over 35% of the starting material (E)-1a remained unreacted in both cases. The reaction rates and syn/anti selectivity of the reaction were significantly influenced by the E or Z configuration of the 1,3-enyne substrates.17 Notably, the borylative cyclization of (Z)-1a proceeded much faster than that of (E)-1a, affording the desired product in high yield with exclusive enantioselectivity. This enhanced reactivity is likely attributed to reduced steric interaction between the Bpin and alkyne groups in Int-A compared to Int-B during C–C bond formation (see Scheme 1c).
Later, the scope of the borylative cyclization in the presence of B2pin2 was explored using various prochiral (Z)-selective 1,3-enyne-tethered cyclic 1,3-diones 1 under optimized reaction conditions, unless otherwise specified for the Z/E ratio of the substrate. Our initial investigation focused on phenyl-substituted enynes with various substituents at the all-carbon prochiral center, and the results are summarized in Table 2. Substrates bearing sterically diverse alkyl groups and a cinnamyl group at the quaternary center were well-tolerated, undergoing borylative enantioselective desymmetrization to afford the corresponding products 2a–2e in 67–82% yield with excellent enantioselectivity. Next, a range of benzyl groups with electronically and sterically tuned substituents at the prochiral center were evaluated. These 1,3-enyne-tethered cyclopenta-1,3-diones proved to be highly suitable, delivering bicyclo[3.3.0]octane products 2f–2v in good yields with uniformly >99% ee across all cases. Particularly, the reaction was carried out on the substrates as an inseparable mixture of (Z/E)-isomers, with the major (Z)-1,3-enyne predominantly yielding the corresponding products anti-2n and anti-2p, along with trace amounts of syn-products derived from the (E)-isomer. Beyond benzyl groups, a similar range of yields and enantioselectivities was observed with other substituents, including 1-naphthyl and thiophen-2-ylmethylene groups at the quaternary prochiral center, furnishing products 2w and 2x, respectively. Next, the generality of the present approach was evaluated using Z-selective enyne-tethered cyclohexa-1,3-diones under standard conditions, which provided the corresponding products 2y–2ac in comparable yields and enantioselectivities. However, the cycloheptane-1,3-dione substrate failed to afford the desired product (2ad), and most of the starting material was recovered. The absolute stereochemistry of the bicyclic ketone anti-2o was unambiguously established by single-crystal X-ray diffraction analysis.16 The stereochemical assignments of the remaining products were assumed by analogy.
Next, we explored the scope of 1,3-enynes bearing various aromatic substituents (Table 3). The reaction was carried out on a mixture of (Z/E)-isomers under standard conditions, yielding product anti-2 predominantly from (Z)-1,3-enyne as the major diastereomer due to its higher reaction rate. Only trace amounts of the syn-product 2 (<5% yield) were identified from (E)-1 in a few examples, highlighting the strong stereochemical influence on the reaction outcome. The transformation proceeded with good diastereoselectivity, and excellent enantioselectivity. Aromatic 1,3-enynes with diverse substituents, regardless of their electronic properties, were well-tolerated under the standard conditions. Substituents such as alkyl, alkoxy, and halogen groups at the para-, meta-, or ortho-positions of the phenyl ring afforded the corresponding products (2ae–2ao) in moderate yields with excellent enantioselectivity (97–99% ee). Additionally, 9-phenanthryl and 1-cyclohexenyl groups on 1,3-enynes were readily converted into the desired products (2ap and 2aq) in good yields with >99% enantioselectivity. Unfortunately, 3-enyne substrates bearing aliphatic substituents failed to afford the desired products, and most of the starting material was recovered (entries 2ar and 2as).
a Reaction conditions same as in Table 2.
b Isolated yields of the major product anti-2.
c Diastereoselectivity was observed to be >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the major isomer, which was confirmed by 1H NMR analysis.
d Unable to find the ratio of major and minor products in the crude 1H NMR analysis.
e Enantioselectivity of the major isomer. SM = starting material. 1 for the major isomer, which was confirmed by 1H NMR analysis.
d Unable to find the ratio of major and minor products in the crude 1H NMR analysis.
e Enantioselectivity of the major isomer. SM = starting material.
|
|---|

|
The enantioselective desymmetrization of α,α-disubstituted 1,3-indandione (Z/E)-3a (dr = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) proceeded efficiently under the optimized reaction conditions, affording the desired product 4a as a mixture of inseparable diastereomers in a 1.5
1) proceeded efficiently under the optimized reaction conditions, affording the desired product 4a as a mixture of inseparable diastereomers in a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with a 71% yield (Scheme 3). Subsequent oxidation of the Bpin group using NaBO3·4H2O yielded the corresponding alcohols anti-5a (56%) and syn-5a (35%) as separable diastereomers, which were easily purified via simple column chromatography. It is interesting to observe that the (E)-isomer of the 1,3-indandione substrate reacts just as effectively as the (Z)-isomer.
1 ratio with a 71% yield (Scheme 3). Subsequent oxidation of the Bpin group using NaBO3·4H2O yielded the corresponding alcohols anti-5a (56%) and syn-5a (35%) as separable diastereomers, which were easily purified via simple column chromatography. It is interesting to observe that the (E)-isomer of the 1,3-indandione substrate reacts just as effectively as the (Z)-isomer.
For ease of handling, we performed a one-pot borylative cyclization/oxidation of 1,3-indandiones under standard reaction conditions, followed by the sequential addition of NaBO3·4H2O (Table 4). We briefly screened this reaction with substrates featuring various benzyl groups at the prochiral quaternary center, yielding separable diastereomeric products anti-5 and syn-5. Electronically diverse substituents on the benzyl group provided high yields and excellent enantioselectivities (5a–5d). However, a sterically demanding ortho-substituted benzyl group resulted in only a trace amount of syn-product from the (E)-isomer and predominantly anti-5e from the (Z)-isomer.
| a Reaction conditions: Same as in Table 2 and then NaBO3·4H2O (150 mg, 1.5 mmol, 5.0 equiv.) was added in the same reaction. b Isolated yields of respective diastereomers. c Enantiomeric excess (ee) was determined by chiral HPLC analysis. d The dr was observed from 1H NMR analysis of the crude reaction mixture. SM = starting material. |
|---|

|
Gram-scale reactions using (Z)-1a and (Z)-1y were carried out with reduced catalyst loading under standard conditions, affording the desired products anti-2a and anti-2y, respectively, without any significant loss in yield or enantioselectivity (Scheme 4a). The one-pot borylative cyclization, followed by the sequential addition of the mild oxidizing agent sodium perborate, afforded the corresponding alcohol 6 in a similar yield with exclusive enantioselectivity (Scheme 4b). Interestingly, the reaction with the strong oxidizing agent sodium periodate also afforded alcohol 6 in high yield, instead of undergoing boronic ester hydrolysis (Scheme 4c). The Pd/C-catalyzed hydrogenolysis of compound 2a successfully reduces the triple bond, providing bicyclic ketone 7 in 88%yield. The Fischer cyclization of compound 2y with phenylhydrazine furnished a highly functionalized tetracyclic fused indole 8 in 72% yield. Subsequent oxidation of 8 with sodium perborate afforded the corresponding alcohol 9 in 84% yield. Interestingly, direct oxidation of 2y with sodium perborate yielded alcohol 10, which upon Dess–Martin periodinane (DMP) oxidation provided the α-alkynyl enone 11via sequential alcohol oxidation and tert-alcohol elimination in good yield. This highly reactive, fused, electron-deficient enyne 11 serves as a promising intermediate for diverse cycloaddition reactions. In addition, Au(I)-catalyzed hydration of enyne 11 in methanol afforded arylketone 12 in 57% yield.
Based on the proposed copper catalytic cycle, the ligated copper alkoxide complex LnCuOtBu is generated in situ in the presence of a chiral ligand and base (Scheme 5). This complex undergoes σ-bond metathesis with a diboron reagent, yielding the LnCu–Bpin species. The enantioselective and regioselective 1,2-syn-addition of LnCu–Bpin to the 1,3-enyne-tethered cyclic-1,3-dione 1a leads to the formation of a propargylic copper intermediate A. This intermediate exists in equilibrium with an axially chiral allenyl–copper intermediate B via a stereospecific isomerization. Subsequent desymmetrization through annulation of propargylic intermediate A furnishes the desired product 2a, while the catalytic cycle is completed by regenerating LnCu–OtBu via a copper alkoxide intermediate C in the presence of a base.
Conclusions
In summary, we have developed a stereospecific, enantioselective copper(I)-catalyzed borylative cyclization of prochiral 1,3-enyne-tethered cyclic-1,3-diones with excellent diastereoselectivity. This asymmetric desymmetrization reaction efficiently delivers highly functionalized chiral octahydropentalenes bearing four contiguous stereocenters. Notably, the use of a BPE-ligand resulted in >99% ee for most examples. Additionally, (Z)-1,3-enyne substrates react more rapidly than their (E)-isomers, affording borylation products in high yields under standard reaction conditions. Ongoing studies in our laboratory are focused on further Cu(I)-catalyzed stereoselective transformations.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
G. R. and V. B. P. performed the experiments and analyzed the experimental data; J. B. N. conducted the single X-ray crystallographic analysis; R. C. designed and supervised the project and wrote the manuscript with the assistance of co-authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the SERB Core Research Grant (CRG/2022/001419) and the SERB-STAR Award (STR/2022/000007) from ANRF, New Delhi, for financial support. The authors thank CSIR-IICT for research facilities and CSIR, New Delhi for research fellowships. IICT Manuscript Communication Number: IICT/Pubs./2025/107.Notes and references
- (a) S. Saito and Y. Yamamoto, Chem. Rev., 2000, 100, 2901–2916 CrossRef CAS PubMed; (b) P. Wessig and G. Müller, Chem. Rev., 2008, 108, 2051–2063 CrossRef CAS PubMed; (c) K. Campbell, C. J. Kuehl, M. J. Ferguson, P. J. Stang and R. R. Tykwinski, J. Am. Chem. Soc., 2002, 124, 7266–7267 CrossRef CAS PubMed.
- (a) B. M. Trost and J. T. Masters, Chem. Soc. Rev., 2016, 45, 2212–2238 RSC; (b) E.-i. Negishi and L. Anastasia, Chem. Rev., 2003, 103, 1979–2018 CrossRef CAS PubMed.
- Q. Dherbassy, S. Manna, F. J. T. Talbot, W. Prasitwatcharakorn, G. J. P. Perry and D. J. Procter, Chem. Sci., 2020, 11, 11380–11393 RSC.
- (a) A. Whyte, A. Torelli, B. Mirabi, A. Zhang and M. Lautens, ACS Catal., 2020, 10, 11578–11622 CrossRef CAS; (b) M. Holmes, L. A. Schwartz and M. J. Krische, Chem. Rev., 2018, 118, 6026–6052 CrossRef CAS PubMed; (c) for the Cu-catalyzed borylative difunctionalization of π-systems, see: S. K. Dorn and M. K. Brown, ACS Catal., 2022, 12, 2058–2063 CrossRef CAS PubMed; (d) W. Xue and M. Oestreich, ACS Cent. Sci., 2020, 6, 1070–1081 CrossRef CAS PubMed; (e) G. J. P. Perry, T. Jia and D. J. Procter, ACS Catal., 2020, 10, 1485–1499 CrossRef CAS; (f) D. Hemming, R. Fritzemeier, S. A. Westcott, W. L. Santos and P. G. Steel, Chem. Soc. Rev., 2018, 47, 7477–7494 RSC; (g) for other selected asymmetric functionalizations on 1,3-enynes, see: J. W. Han, N. Tokunaga and T. Hayashi, J. Am. Chem. Soc., 2001, 123, 12915–12916 CrossRef CAS PubMed; (h) N. J. Adamson, H. Jeddi and S. J. Malcolmson, J. Am. Chem. Soc., 2019, 141, 8574–8583 CrossRef CAS PubMed; (i) Y. Zhang, J. Yang, Y. L. Ruan, L. Liao, C. Ma, X. S. Xue and J. S. Yu, Chem. Sci., 2023, 14, 12676 RSC; (j) L. Li, S. Wang, A. Jakhar and Z. Shao, Green Synth. Catal., 2023, 4, 124–134 CAS.
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; (b) C. Sandford and V. K. Aggarwal, Chem. Commun., 2017, 53, 5481–5494 RSC.
- Y. Sasaki, Y. Horita, C. Zhong, M. Sawamura and H. Ito, Angew. Chem., Int. Ed., 2011, 50, 2778–2782 CrossRef CAS PubMed.
- F. Meng, F. Haeffner and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 11304–11307 CrossRef CAS PubMed.
- (a) X.-C. Gan and L. Yin, Org. Lett., 2019, 21, 931–936 CrossRef CAS PubMed; (b) X. C. Gan, Q. Zhang, X.-S. Jia and L. Yin, Org. Lett., 2018, 20, 1070–1073 CrossRef CAS PubMed; (c) for 1,2-borylacylation, see: X.-L. Liu, L. Li, H.-Z. Lin, J.-T. Deng, X.-Z. Zhang and J.-B. Peng, Chem. Commun., 2022, 58, 5968–5971 RSC.
- S. Manna, Q. Dherbassy, G. J. P. Perry and D. J. Procter, Angew. Chem., Int. Ed., 2020, 59, 4879–4882 CrossRef CAS PubMed.
- W. S. Yoon, W. J. Jang, W. Yoon, H. Yun and J. Yun, Nat. Commun., 2022, 13, 2570 CrossRef CAS.
- Y. Liao, X. Yin, X. Wang, W. Yu, D. Fang, L. Hu, M. Wang and J. Liao, Angew. Chem., Int. Ed., 2020, 59, 1176–1180 CrossRef CAS PubMed.
- C. Yang, Z.-L. Liu, D.-T. Dai, Q. Li, W.-W. Ma, M. Zhao and Y.-H. Xu, Org. Lett., 2020, 22, 1360–1367 CrossRef CAS.
- For CuH catalyzed 1,2-hydrofunctionalizations, see: (a) Y. Yang, I. B. Perry, G. Lu, P. Liu and S. L. Buchwald, Science, 2016, 353, 144–150 CrossRef CAS PubMed; (b) Y. Zhou, L. Zhou, L. T. Jesikiewicz, P. Liu and S. L. Buchwald, J. Am. Chem. Soc., 2020, 142, 9908–9914 CrossRef CAS PubMed.
- For CuH catalyzed 1,4-hydrofunctionalizations, see: (a) L. Bayeh-Romero and S. L. Buchwald, J. Am. Chem. Soc., 2019, 141, 13788–13794 CrossRef CAS; (b) S. Yu, H. L. Sang, S.-Q. Zhang, X. Hong and S. Ge, Commun. Chem., 2018, 1, 64 CrossRef; (c) for enantioselective Cu-catalyzed hydroboration of 1,3-enynes, see: Y. Huang, J. Pozo, S. Torker and A.-H. Hoveyda, J. Am. Chem. Soc., 2018, 140, 2643–2655 CrossRef CAS PubMed; (d) H.-J. Sang, S. Yu and S. Ge, Org. Chem. Front., 2018, 5, 1284–1287 RSC; (e) D.-W. Gao, Y. Xiao, M. Liu, M.-K. Karunananda, J.-S. Chen and K.-M. Engle, ACS Catal., 2018, 8, 3650–3654 CrossRef CAS PubMed.
- (a) S. Maurya, N. Navaneetha, P. Behera, J. B. Nanubolu, L. Roy and R. Chegondi, Angew. Chem., Int. Ed., 2025, 64, e202420106 CrossRef CAS; (b) N. Navaneetha, S. Maurya, P. Behera, S. B. Jadhav, L. R. Magham, J. B. Nanubolu, L. Roy and R. Chegondi, Chem. Sci., 2024, 15, 20379–20387 RSC; (c) V. B. Patil, S. B. Jadhav, J. B. Nanubolu and R. Chegondi, Org. Lett., 2022, 24, 8233–8238 CrossRef CAS PubMed; (d) S. B. Jadhav, S. R. Dash, S. Maurya, J. B. Nanubolu, K. Vanka and R. Chegondi, Nat. Commun., 2022, 13, 854 CrossRef CAS PubMed.
- CCDC-2443592 (compound 2a) and CCDC-2443593 (compound 2o) contain the supplementary crystallographic data for this paper..
- (a) H. Ito, Y. Kosaka, K. Nonoyama, Y. Sasaki and M. Sawamura, Angew. Chem., Int. Ed., 2008, 47, 7424–7427 CrossRef CAS PubMed; (b) H. Ito, T. Toyoda and M. Sawamura, J. Am. Chem. Soc., 2010, 132, 5990–5992 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2443592 and 2443593. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03007b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |