Open Access Article
Open Access ArticleA dual-excitation ratiometric NIR-II fluorescent nanoplatform enables high contrast in vivo imaging†
Yongchao Liu *ab,
Lili Tenga,
Bo Zhanga,
Xiao-Bing Zhang
*ab,
Lili Tenga,
Bo Zhanga,
Xiao-Bing Zhang b and
Guosheng Song
b and
Guosheng Song *b
*b
aState Key Laboratory of New Pharmaceutical Preparations and Excipients, Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, College of Chemistry and Materials Science, Hebei University, Baoding, 071002, China. E-mail: liuyongchao@hnu.edu.cn
bState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082, China. E-mail: songgs@hnu.edu.cn
First published on 24th June 2025
Abstract
Ratiometric fluorescent probes in the second near-infrared window (NIR-II) with a self-calibration function are sought after for reliable imaging of physiological and pathological processes. Nevertheless, current ratiometric NIR-II fluorescent probes usually show severe spectral overlap in the emission channel, resulting in inevitable sacrifice of the emission intensity of the probe and compromised imaging quality in the NIR-II region. To address these challenges, we developed a novel dual-excitation ratiometric NIR-II fluorescence nanoplatform (DERF-NP), in which the intensity ratio of the same full-wavelength emission from 1000 to 1700 nm under two non-overlapping monochrome excitations with distinct responses is conventionally defined as the quantification parameter. As a proof of concept, DERF-NO, our test case of a ratiometric NIR-II nanoprobe for NO imaging with high sensitivity and quality, was developed based on an energy transfer strategy, which showed increased emission with excitation at 660 nm and constant emission with excitation at 808 nm upon activation of NO. In vivo ratiometric NIR-II fluorescence imaging with DERF-NO successfully tracked macrophage polarization and lymphatic metastasis, suggesting the extensive distribution and critical role of macrophages in tumorigenesis and progression. This dual-excitation ratiometric imaging strategy may provide a novel approach for designing ratiometric NIR-II fluorescent probes and has great application potential for intravital imaging analysis.
Introduction
Fluorescence imaging in the second near-infrared window (NIR-II, 1000–1700 nm) has attracted increasing attention owing to its high imaging depth, signal-to-background ratio (SBR), and spatial resolution.1–3 As a result, it can compensate for the deficiency of fluorescence imaging in the first near-infrared window (NIR-I, 700–1000 nm), including tissue absorption, scattering, and interference from the biological background.4,5 The significantly improved imaging quality makes NIR-II fluorescence imaging a powerful alternative for intravital imaging, such as vascular imaging,6,7 lymph node imaging,8,9 tumor surgical navigation,10,11 disease diagnosis,12,13 etc. As the most promising optical imaging technology, NIR-II fluorescence imaging shows great potential for high-performance imaging research of biological events in vivo.14,15Current NIR-II fluorescence imaging probes usually exhibit “off–on” signal conversion based on the single-excitation and single-emission mode, which may be easily affected by the external environment, including the detection conditions, photobleaching, and probe concentration in vivo.16–19 In contrast, ratiometric NIR-II fluorescent probes with a built-in self-calibration function can eliminate or weaken the false signal caused by the external environment, which is conducive to the accurate detection of specific targets in deep tissues compared to the “off–on” probes.20 As a result, in vivo ratiometric NIR-II fluorescence imaging can directly provide reliable visualization information about relevant pathophysiological processes.21,22 A general strategy for developing ratiometric NIR-II fluorescent probes mainly depends on the imaging mode of single-excitation and double-emission, that is, the ratio of two separate fluorescence emissions in different channels under single-wavelength excitation is defined as a sensitive parameter for quantitative analysis.23,24 Nevertheless, emission channels usually show severe spectral overlap and crosstalk, and it is an intractable problem to completely separate the two fluorescence emission channels,25,26 which may result in poor change in the fluorescence ratio and greatly reduce the sensitivity of the probe. Importantly, in order to achieve the ratiometric imaging in two specific channels, multiple filters are usually required to separate the emission channels,27,28 which inevitably sacrifices the emission intensity of the probe and reduces the imaging quality in the NIR-II window. Consequently, it is urgent to develop novel ratiometric NIR-II fluorescence imaging strategies and probes for high-sensitivity quantitative detection and high-quality intravital imaging, which is of great significance for further promoting the development of NIR-II fluorescence imaging technology in high-performance biosensing and imaging.
In order to overcome the limitations of ratiometric NIR-II fluorescent probes with single-excitation, in this work, we have developed a novel dual-excitation ratiometric NIR-II fluorescence nanoplatform (DERF-NP), in which the intensity ratio (FL660ex/FL808ex) in the NIR-II region under two nonoverlapping monochrome excitations (660 and 808 nm) is conventionally defined as a sensitive parameter for quantitative analysis. Compared with the previously reported ratiometric NO probes that depend on the imaging mode of dual-emission (Table S1†), DERF-NO can effectively overcome the spectral overlap of emission channels and significantly improve the SBR, as it relies on the single-emission imaging mode in the full-wavelength NIR-II region from 1000 to 1700 nm. Additionally, due to the self-calibration of FL660ex and FL808ex in the NIR-II window, this ratiometric imaging strategy of DERF-NP could provide reliable quantitative analysis, and the intensity ratio (FL660ex/FL808ex) is independent of interference factors, including probe concentration, acquisition time, and imaging depth. As a biological proof of concept, DERF-NO, our test case of an NO-responsive NIR-II nanoprobe, was developed based on the dual-excitation ratiometric imaging strategy, which showed increased and constant NIR-II intensity with excitation at 660 nm and 808 nm, respectively, upon the activation of NO. Next, benefiting from the high SBR and resolution in vivo imaging, we verified the ability of DERF-NO to specifically detect NO in activated macrophages and metastatic sentinel lymph nodes. We found that the intensity ratio (FL660ex/FL808ex) could, indeed, be used as a noninvasive predictor for real-time evaluation of macrophage polarization in tumor and lymphatic metastasis, indicating that this dual-excitation ratiometric imaging strategy has great application potential for intravital imaging analysis.
Results and discussion
Development of a dual-excitation ratiometric NIR-II fluorescence imaging nanoplatform
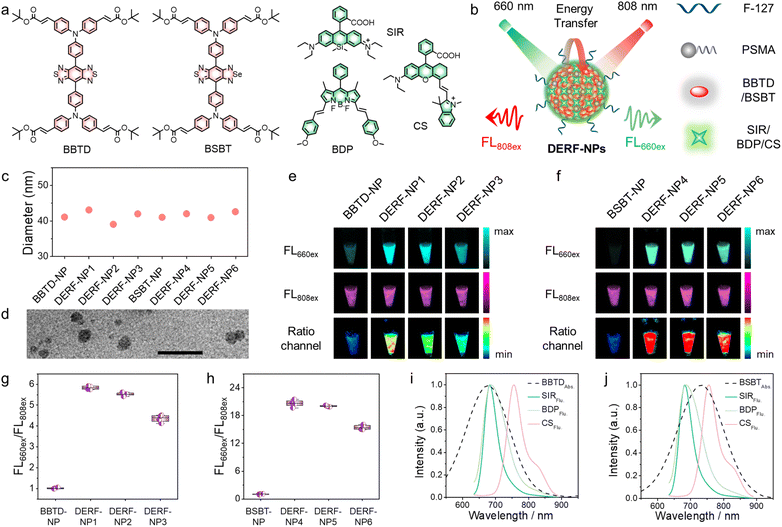
Among the many NIR-II organic chromophores, the donor–acceptor–donor (D–A–D)-type chromophores with benzo[1,2-c:4,5-c′]bis([1,2,5]thiadiazole) (BBTD) as an acceptor unit have been widely applied in intravital NIR-II fluorescence imaging due to their excellent photophysical properties.29 Furthermore, another selenium analogue (BSBT) was chosen because the replacement of sulfur with selenium usually leads to further band gap reduction and a longer NIR-II emission wavelength (Fig. 1a). Based on this, two NIR-II model chromophores (BBTD and BSBT) were synthesized, and their photophysical properties were investigated. As shown in Fig. S1,† both BBTD and BSBT showed obvious NIR-II emission, and the almost constant fluorescence under irradiation with an 808 nm laser indicated their excellent fluorescence stability compared to ICG. Although the fluorescence quantum yields (QYs) of BBTD and BSBT may not have an advantage compared with many organic molecules or inorganic nanoparticles, they still have great potential in NIR-II fluorescence imaging due to their high photostability and chemical stability.30 In addition, their extinction coefficient (∼104 M−1 cm−1) appeared insufficient by recording their maximum absorbance at 700 and 756 nm, respectively (Table S1†), suggesting their low photon-harvesting capacity, which is not beneficial for NIR-II fluorescence imaging with high brightness. Additionally, owing to their inherent structural inertness, insufficient response sites are available to design activatable NIR-II fluorescent probes for different biological species or physiological processes.We hypothesized the development of a novel NIR-II fluorescence imaging platform based on BBTD and BSBT chromophores that would (1) improve their photon-harvesting capacity for high-quality intravital imaging and (2) allow for the customization of activatable NIR-II fluorescent probes for quantitative analysis of specific analytes. Currently, Förster resonance energy transfer (FRET) is a flexible probe design strategy because of the diversity of donors and acceptors in the hybrid system,31,32 which can meet the design requirements for developing ratiometric fluorescent probes. Therefore, we attempted to develop a dual-excitation ratiometric fluorescence nanoplatform (DERF-NP) based on the FRET mechanism using two different fluorophores loaded in a nanoparticle to obtain the ratiometric NIR-II signal. In order to pair up with the absorption of energy acceptors (BBTD or BSBT), three NIR-I chromophores (SIR, BDP and CS) were selected as the representative energy donors because of their molecular designability, high extinction coefficient (∼105 M−1 cm−1), quantum yields and excellent photophysical properties33 (Fig. 1a, Table S2†). The synthetic route is outlined in Scheme S1,† and the new compound structures were confirmed. In the DERF-NP system, energy donors (SIR, BDP and CS) can relay their NIR-I fluorescence to the BBTD or BSBT components to emit NIR-II fluorescence through energy transfer upon excitation at 660 nm, termed FL660ex, and another NIR-II emission (FL808ex) of BBTD or BSBT components served as an internal reference upon excitation at 808 nm. Thus, dual-excitation ratiometric fluorescence imaging can be implemented by self-calibration of the two same full-wavelength NIR-II emissions (FL660ex and FL808ex) from 1000 to 1700 nm upon excitation at 660 nm and 808 nm, respectively (Fig. 1b).
Then, the DERF-NPs were prepared by encapsulation of the model energy donors (SIR, BDP and CS) and acceptors (BBTD and BSBT) with F-127 and PSMA as surfactants (Fig. S2†). As shown in Fig. 1c and d, dynamic light scattering (DLS) revealed similar hydrodynamic diameters of the DERF-NPs, and transmission electron microscopy (TEM) revealed the spherical morphology of these DERF-NPs. And NIR-II fluorescence imaging of DERF-NPs from 1000 to 1700 nm was performed upon excitation at 660 nm and 808 nm, respectively. As shown in Fig. 1e and f, upon excitation at 660 nm, the NIR-II fluorescence can be barely detectable for the DERF-NPs consisting of only BBTD or BSBT, whereas strong NIR-II fluorescence was observed in SIR-, BDP-, and CS-doped DERF-NPs, indicating that the introduction of NIR-I chromophores into DERF-NPs is beneficial for the enhancement of NIR-II fluorescence intensity. The absorption spectra showed that the absorbance of SIR, BDP and CS was significantly higher than that of BBTD and BSBT at the same molar concentrations (Fig. S3†), which suggests the higher photon-harvesting capacity of SIR, BDP and CS. Importantly, almost constant NIR-II fluorescence of DERF-NPs was observed upon excitation at 808 nm (Fig. 1e and f), which was ascribed to the inherent NIR-II emission of BBTD and BSBT. The obviously decreased NIR-I fluorescence of SIR, BDP, and CS, and increased NIR-II fluorescence of DERF-NPs upon excitation at 660 nm after doping with BBTD or BSBT, collectively indicated the effective energy transfer between the energy donors (SIR, BDP and CS) and acceptors (BBTD and BSBT) (Fig. S4†). In addition, to reveal the influence of tail peaks of SIR, BDP, and CS in the NIR-II region on the fluorescence of DERF-NPs, the NIR-II fluorescence spectra of SIR-NPs, BDP-NPs, CS-NPs, and DERF-NPs under 660/808 nm excitation were measured. As shown in Fig. S5,† the NIR-II fluorescence spectra of these DERF-NPs under 808 nm excitation remain constant due to the exceeded excitation wavelength for SIR, BDP, and CS. In contrast, the fluorescence tail peak of SIR, BDP, and CS under 660 nm excitation does indeed have an impact on the spectra of DERF-NPs, for example, altering their wavelength and shape (especially for DERF-NP3 and DERF-NP6). Fortunately, the fluorescence tail peaks of SIR, BDP, and CS under 660 nm excitation are mainly located in the region from 800 to 1000 nm (Fig. S6†), and their influence on the fluorescence of DERF-NPs in the NIR-II window (1000–1700 nm) is negligible.
The ratiometric fluorescence images and normalized intensity ratio (FL660ex/FL808ex) clearly revealed the fluorescence enhancement of DERF-NPs after doping with SIR, BDP and CS (Fig. 1g and h). It is worth mentioning that DERF-NPs doped with BSBT showed a higher NIR-II fluorescence intensity ratio (FL660ex/FL808ex) compared with BBTD-doped ones, which may be attributed to the low absorbance and fluorescence of BSBT upon excitation at 660 nm. Among these nanoparticles, DERF-NPs consisting of BSBT and SIR (termed DERF-NP4) showed the highest NIR-II fluorescence intensity ratio (FL660ex/FL808ex) compared to the BDP and CS doped ones, which was 21-fold higher than that of the BSBT-only nanoparticles (Fig. 1h). In order to verify the fluorescence enhancement mechanism of DERF-NPs upon excitation at 660 nm, we compared the extinction coefficients of the donor and acceptor. As shown in Table S1,† the extinction coefficients of SIR, BDP and CS, which are an order of magnitude higher than those of BBTD and BSBT, are sufficient to justify their high photon-harvesting capacity, which results in a significant enhancement of fluorescence of DERF-NPs upon excitation at 660 nm. Additionally, the large spectral overlap between the absorption spectra of energy acceptors and the emission spectra of energy donors can also ensure high efficiency energy transfer in DERF-NPs (Fig. 1i and j). Consequently, based on the energy transfer mechanism, our strategy successfully endowed the resultant DERF-NPs with ratiometric imaging, distinctly broader absorption, great light-harvesting capacity and high NIR-II emission efficiency.
Reliable ratiometric imaging of DERF-NPs both in vitro and in vivo
Due to the excellent photophysical properties of DERF-NP4, we then applied it for further ratiometric NIR-II fluorescence imaging research in subsequent experiments both in vitro and in vivo. Generally, the NIR-II fluorescence intensity is dependent on probe concentrations and exposure time. It was assumed that the ratios (FL660ex/FL808ex) of two NIR-II fluorescence intensities upon the excitation at 660 nm and 808 nm would be independent of those interference factors, thereby enhancing reliability for accurate quantification. To confirm this supposition, we systematically investigated the NIR-II fluorescence intensity ratio of DERF-NP4 under different concentrations (Fig. 2a) and exposure time (Fig. 2c) upon excitation at 660 nm and 808 nm, respectively. As expected, the NIR-II fluorescence intensities of both FL660ex and FL808ex were proportional to the probe concentrations (from 2 μg mL−1 to 10 μg mL−1) and exposure time (from 20 ms to 100 ms). Surprisingly, the fluorescence intensity ratio (FL660ex/FL808ex) was constant and independent of them (Fig. 2b and d). Furthermore, we explored the luminescence ability of DERF-NP4 in tissues with different thicknesses (Fig. 2e). When intralipid or chicken tissues were placed on top of DERF-NP4, the decreased fluorescence signals (FL660ex and FL808ex) were observed (Fig. 2f and h). Importantly, the fluorescence intensity ratios (FL660ex/FL808ex) were constant with depth of intralipid from 0 to 0.8 cm (Fig. 2g), or chicken tissues from 0 to 0.4 cm (Fig. 2i). Furthermore, we explored the ability of DERF-NP4 for ratiometric imaging of tumor by recording the fluorescence intensities upon excitation at 660 nm and 808 nm at the indicated time points (Fig. 2j). Notably, both the fluorescence intensities upon excitation at 660 nm and 808 nm exhibited a gradual increase from 3 to 24 h, suggesting the accumulation of DERF-NP4 in tumors through the enhanced permeability and retention (EPR) effect. Importantly, the fluorescence intensity ratios (FL660ex/FL808ex) were constant, indicating that they were not affected by the enrichment of concentration and heterogeneous distribution of DERF-NP4 in tumors.In order to further evaluate the imaging resolution, we have performed lymphatic drainage and angiography in mice as a function of DERF-NP4 administration time. As shown in Fig. S7,† mice were i.d. injected with DERF-NP4 at the rear paw for NIR-II fluorescence imaging. The popliteal and sciatic lymph nodes were clearly identified from all the images acquired post-injection in two emission channels (660 and 808 nm excitation) and their corresponding ratio channel (Fig. 2l). After 10 min post-injection, the afferent and efferent lymphatic vessels connecting the injection site with the two lymph nodes were gradually distinguished. The ratiometric signals of popliteal lymph nodes in DERF-NP4-treated mice showed no obvious change from 10 to 40 min post-injection (Fig. 2m), which demonstrated the ability of DERF-NP4 for reliable imaging of lymph nodes in living mice through ratiometric NIR-II fluorescence imaging. Similarly, carotid and thigh arteries of mice upon excitation at 660 nm and 808 nm can be clearly identified from the acquired fluorescence images by using DERF-NP4 (Fig. 2n), indicating the high-resolution imaging capability of DERF-NP4. The peritoneal blood vessels of mice were also distinguished using filters of different wavelengths, and the filters with longer wavelengths showed weaker brightness and longer exposure time (Fig. S8†), which confirms that the separation of the NIR-II fluorescence emission channel is not beneficial for the acquisition of images with high SBR. It also indicated the inevitability of imaging in the full-band NIR-II region. The above results indicated that dual-excitation ratiometric fluorescence imaging is reliable when imaging the deep-sited tissues, and the main reason for the constant intensity ratios may be attributed to the low background signal of tissues in the NIR-II region.
Activatable dual-excitation ratiometric NIR-II nanoprobe for NO analysis
As a biological proof of concept, our test case of an NO-responsive ratiometric NIR-II fluorescent nanoprobe (DERF-NO) was designed for specific quantification of NO through integrating the NO-responsive molecular probe (SIR-NO), NIR-II chromophore (BSBT), and surfactants (F-127) via a self-assembly strategy (Fig. S9a†). The synthetic route of SIR-NO is outlined in Scheme S3.† The fluorescence spectra of SIR-NO after incubation with NO indicated its excellent responsivity to NO, and the spectral overlap of SIR-NO after reaction with NO and BSBT also suggests the efficient energy transfer between them (Fig. S10†). First, we investigated the influence of pH values of buffer solution on the responsivity of DERF-NO toward NO. The absorbance and NIR-II fluorescence of DERF-NO did not change significantly in different pH buffer solutions, indicating that DERF-NO was not sensitive to pH (Fig. S11†). When DERF-NO was incubated with different concentrations of NO in pH 7.0 buffer solution, the absorbance and NIR-II fluorescence showed almost negligible variations, suggesting that DERF-NO cannot detect NO under neutral conditions (Fig. S12†). Afterwards, we attempted to detect NO in acidic buffer solutions, and the results showed that only when the pH of the buffer solution reduced to 5.5 or even 5.0, we can observe the obviously increased absorbance at 660 nm and bright NIR-II fluorescence under 660 nm excitation (Fig. 3c and S13†), which demonstrated that the activation of DERF-NO requires the participation of both NO and acid. In order to reduce the dependence on acid of DERF-NO in NO responsivity, we tried to dope the acidic polymer (polystyrene-co-maleic anhydride, PSMA) during the preparation of the nanoprobe (Fig. S9b†), termed DERF-NO (+PSMA). As shown in Fig. 3d and S14,† DERF-NO (+PSMA) showed significantly increased absorbance at 660 nm, and NIR-II fluorescence under 660 nm excitation was observed when the pH value of the buffer solution was reduced to 6.0 or even 6.5. As a comparison, a neutral polymer (polystyrene, PS)-doped DERF-NO was prepared, which still showed the pH-dependent responsivity to NO (Fig. S15†). Given the acidity of PSMA within the nanoprobe DERF-NO (+PSMA), we considered that the introduction of PSMA can greatly relieve the pH-dependent responsivity of SIR-NO toward NO (Fig. 3e). Thus, the PSMA-doped DERF-NO was selected for NO detection in the next experiments. And the DLS and TEM images showed the size and spherical morphology of DERF-NO in diameter (Fig. 3b).After optimizing parameters for the synthesis of DERF-NO, such as surfactants (F127 or DSPE-PEG) and doping amount of BSBT/SIR-NO (from 25% to 200%), the detection ability of PSMA-doped DERF-NO toward NO was then systematically investigated (Fig. S16 and S17†). As expected, with the increased concentrations of NO, the absorbance of DERF-NO gradually increased at 660 nm while remained almost constant at 808 nm (Fig. 3f). Similarly, the fluorescence of DERF-NO at 1028 nm increased significantly upon excitation at 660 nm while remained almost constant upon excitation at 808 nm (Fig. 3g and h). And the quantitative detection of NO could be performed by calculating the fluorescence intensity ratio (FL660ex/FL808ex). As shown in Fig. 3i, the fluorescence intensity ratio (FL660ex/FL808ex) was linearly correlated with NO concentrations from 0 to 6 μM, and the detection limit (3σ/slope) of DERF-NO for NO was calculated to be 45.8 nM, strongly suggesting that this ratiometric strategy of DERF-NO could provide quantitative analysis by self-calibration of the emissions upon excitation at 660 nm and 808 nm, respectively.
The ratiometric NIR-II fluorescence imaging ability of DERF-NO for NO was then investigated. As expected, the NIR-II fluorescence of DERF-NO showed brighter FL660ex and constant FL808ex with the increase of NO concentration upon 660 nm and 808 nm excitation, respectively (Fig. 3j). And the resulting enhanced ratiometric signal of DERF-NO when the concentration of NO was gradually increased also demonstrated that DERF-NO is capable of ratiometric imaging of NO. Additionally, we measured the specificity of DERF-NO and found that only NO could induce the significant enhancement of FL660ex/FL808ex ratios, indicating that DERF-NO was a specific probe for the detection of NO (Fig. 3k and S18†). The selective recognition ability of SIR-NO (the response unit) for NO, and the chemical stability of BSBT (the internal reference unit) in the presence of NO or other interferents collectively result in the specificity of DERF-NO for the detection of NO. In addition, we also investigated the photostability and colloidal stability of DERF-NO. It was clear that fluorescent signals collected at 660 nm and 808 nm of DERF-NO after incubation with NO were comparatively stable (Fig. S19a†), proving that DERF-NO is photostable under physiological conditions. And DERF-NO exhibited good dispersion in PBS, FBS, and DMEM with diameters of ∼45 nm during 3 days of measurement (Fig. S19b†), indicating its colloidal stability. These results indicated that DERF-NO was an excellent nanoprobe for ratiometric NIR-II fluorescence imaging of NO with high sensitivity and specificity.
In vivo ratiometric NIR-II fluorescence imaging of macrophage polarization in tumors
Tumor-associated macrophages (TAMs) are a type of immune cell infiltrating the tumor microenvironment, which can exert immunosuppressive properties by polarizing from the M2-phenotype to the anti-tumorigenic M1-phenotype to achieve anticancer efficacy.34,35 Thus, real-time imaging of macrophage polarization is critical for comprehending the interaction between macrophages and cancer cells, which is of great significance for the evaluation of macrophage function in immunotherapy.36,37 Owing to the excellent properties of DERF-NO for reliable imaging of NO in solution, we then applied it to evaluate macrophage polarization by real-time ratiometric NIR-II fluorescence imaging of M1-phenotype macrophage-released proinflammatory cytokines (Fig. 4a), such as NO, a hallmark of the polarization of TAMs to the M1-phenotype. Interferon-γ (IFN-γ), a classic macrophage polarization modulator, can stimulate proinflammatory macrophages, induce NO diffusion and inhibit tumor growth.38 After intratumoral injection of IFN-γ, 4T1 tumor-bearing mice were subsequently i.v.-injected with DERF-NO, and NIR-II fluorescence images of tumors at different post-injection time points were recorded (Fig. 4b). NIR-II fluorescence images showed enhanced fluorescence in both PBS- and IFN-γ treated tumors within 24 h, indicating the accumulation of DERF-NO. Furthermore, IFN-γ-treated tumors maintained consistently strong NIR-II fluorescence upon excitation at 660 nm, which may be ascribed to the rapid activation of DERF-NO due to its higher NO concentration in tumors (Fig. 4c). Ratiometric NIR-II fluorescence imaging results showed higher intensity ratios in IFN-γ-treated mice than in control mice, indicating that IFN-γ could induce a higher level of endogenous NO.Specifically, at 24 h post-injection of IFN-γ, the fluorescence intensity ratios (FL660ex/FL808ex) were 1.23 for the control group and 1.71 for the IFN-γ group, respectively (Fig. 4d). After separating the tumor tissues, ex vivo flow cytometric analysis was performed to provide further mechanistic validation of the results obtained using NIR-II fluorescence imaging in vivo. As shown in Fig. 4e, all tumors treated with IFN-γ showed higher levels of tumor-associated macrophages with increased M1-phenotype markers (CD80+ F4/80+), corresponding to the higher fluorescence intensity ratio (FL660ex/FL808ex). These results validated the correlation between ratiometric fluorescence intensity and macrophage polarization, demonstrating the potential of DERF-NO as a promising visualization tool for predicting macrophage polarization and screening drugs for macrophage-modulated immunotherapy.
In vivo NIR-II fluorescence imaging of lymph nodes
To further verify the high resolution and sensitivity of DERF-NO for ratiometric NIR-II fluorescence imaging of NO, we then applied it to evaluate the dynamic change of NO in lymph nodes using an animal model of lymphatic inflammation induced by intradermal (i.d.) injection of lipopolysaccharide (LPS). Mice were i.d. injected with LPS or PBS in the rear paw, followed by an additional i.d. injection of DERF-NO after 4 h for NO detection through ratiometric NIR-II fluorescence imaging (Fig. 5a). Significantly, popliteal and sciatic lymph nodes were clearly identified from all NIR-II images upon excitation at 660 and 808 nm and their corresponding ratio images. As shown in Fig. 5b, 30 min post-injection, the afferent and efferent lymphatic vessels connecting the injection site to the two lymph nodes were gradually distinguished. The ratiometric signals of popliteal lymph nodes in LPS-treated mice increased by approximately 1.39-fold compared to the control group of PBS-treated mice (Fig. 5c). The results demonstrate the ability of DERF-NO to detect NO with high resolution in inflammatory tissues in living mice through ratiometric NIR-II fluorescence imaging.It has been demonstrated that the phagocytes residing in sentinel lymph nodes (SLNs) can be activated during tumor lymphatic metastasis.39,40 Nitric oxide activity is highly upregulated during the activation of inflammatory cells such as macrophages, which play an important role in the invasion and metastasis of tumor cells.41,42 Consequently, real-time monitoring of the level changes of NO in SLNs is key to realizing the visualization of macrophage activity and metastatic imaging of tumor lymph nodes. Furthermore, to visualize and track tumor metastasis in the lymph nodes, we used DERF-NO to investigate macrophage activity in lymphatic metastasis, and in vivo NIR-II fluorescence imaging of metastatic tumors in the lymph nodes was performed after intradermal (i.d.) injection of DERF-NO (Fig. 5d). When DERF-NO was injected into the left and right hind paws of 4T1 tumor-bearing mice, obvious NIR-II fluorescence was observed in the popliteal and sciatic lymph nodes of the tumor side (right 1 and 2), whereas the lymph nodes on the normal side (left 1 and 2) of the mice exhibited weak fluorescence (Fig. 5e). In comparison, only weak fluorescence signals were detected in the lymph nodes of the normal sides (left) of mice. The ratiometric fluorescence imaging results and higher intensity ratios were observed in the SLN of the tumor side, indicating that metastatic lymph nodes could generate a higher level of NO. The quantized average intensity ratio (FL660ex/FL808ex) showed that the signal in the area of popliteal lymph nodes on the tumor side was stronger than that on the normal side, indicating that metabolic migration of cancer cells through the lymphatic system indeed occurred (Fig. 5f). H&E staining of the two excised lymph nodes confirmed the existence of metastatic tumors in both of them, and immunofluorescence staining (Ki67 and F4/80) demonstrated an extensive distribution of tumor cells and macrophages in both lymph nodes (Fig. 5g), indicating that the NIR-II fluorescence of the two lymph nodes resulted from metastatic tumors and the polarization of macrophages. Collectively, this study provides preclinical proof of concept for a ratiometric imaging platform to accurately evaluate lymph node status.
Conclusions
In summary, we have developed a novel dual-excitation ratiometric NIR-II fluorescence imaging nanoplatform (DERF-NP) for in vivo imaging and molecular sensing. Because the acquired emission channel of DERF-NP covers the full-wavelength range of the NIR-II region, we can achieve ratiometric NIR-II imaging with high quality upon two nonoverlapping monochrome excitations (660 nm and 808 nm), respectively. Such a ratiometric strategy could provide reliable and quantitative analysis by self-calibration of the NIR-II emission under two separate excitations. Theoretically, we can customize various ratiometric imaging probes by introducing responsive units that match the spectrum of the NIR-II fluorophore. For instance, our test case of an activatable nanoprobe (DERF-NO) was developed for NO imaging with high sensitivity and resolution because of the significantly improved SBR and imaging quality in the NIR-II region. This nanoprobe can monitor the fluctuations of NO in macrophage polarization and lymphatic metastasis, which provides a reliable parameter for real-time dynamic evaluation of tumorigenesis and progression. Our work thus opens up a de novo ratiometric imaging platform to design various activatable probes for high-performance imaging of biomarkers in vivo, which shows great potential in clinical research, including pathogenesis, high-throughput drug screening, etc.Ethical statement
All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Hunan University, and the experiments were approved by the Animal Ethics Committee of the College of Biology (Hunan University).Data availability
All experimental supporting data and procedures are available in the ESI.†Author contributions
Y. Liu, L. Teng, B. Zhang, G. Song, and X-B. Zhang conceived and designed the experiments; Y. Liu and L. Teng performed the experiments; Y. Liu and G. Song performed data analysis and wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (22304043 and 22307032), Natural Science Foundation of Hebei Province (B2023201033), and Science Research Project of Hebei Education Department (BJ2025085 and QN2025171).Notes and references
- B. Li, M. Zhao, J. Lin, P. Huang and X. Chen, Chem. Soc. Rev., 2022, 51, 7692–7714 RSC.
- F. Wang, Y. Zhong, O. Bruns, Y. Liang and H. Dai, Nat. Photonics, 2024, 18, 535–547 CrossRef CAS.
- J. Mu, M. Xiao, Y. Shi, X. Geng, H. Li, Y. Yin and X. Chen, Angew. Chem., Int. Ed., 2022, 61, e202114722 CrossRef CAS PubMed.
- Y. Sun, P. Sun, Z. Li, L. Qu and W. Guo, Chem. Soc. Rev., 2022, 51, 7170–7205 RSC.
- Z. Liu, L. Luo and R. Jin, Adv. Mater., 2023, 36, 2309073 CrossRef PubMed.
- B. Li, M. Zhao, L. Feng, C. Dou, S. Ding, G. Zhou, L. Lu, H. Zhang, F. Chen, X. Li, G. Li, S. Zhao, C. Jiang, Y. Wang, D. Zhao, Y. Cheng and F. Zhang, Nat. Commun., 2020, 11, 3012 CrossRef PubMed.
- D. Liu, Z. He, Y. Zhao, Y. Yang, W. Shi, X. Li and H. Ma, J. Am. Chem. Soc., 2021, 143, 17136–17143 CrossRef CAS PubMed.
- A. Baghdasaryan, F. Wang, F. Ren, Z. Ma, J. Li, X. Zhou, L. Grigoryan, C. Xu and H. Dai, Nat. Commun., 2022, 13, 5613 CrossRef CAS PubMed.
- R.-X. Wang, Y. Ou, Y. Chen, T.-B. Ren, L. Yuan and X.-B. Zhang, J. Am. Chem. Soc., 2024, 146, 11669–11678 CrossRef CAS PubMed.
- C. Li, J. Du, G. Jiang, J. Gong, Y. Zhang, M. Yao, J. Wang, L. Wu and B. Z. Tang, Nat. Commun., 2024, 15, 5832 CrossRef CAS PubMed.
- L. He, Y. Li, C. Zhang, X. Zhang, B. Wang, T. Ren and L. Yuan, Coord. Chem. Rev., 2025, 533, 216549 CrossRef CAS.
- J. Chen, L. Chen, Y. Wu, Y. Fang, F. Zeng, S. Wu and Y. Zhao, Nat. Commun., 2021, 12, 6870 CrossRef CAS PubMed.
- Y. Chen, P. Pei, Z. Lei, X. Zhang, D. Yin and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 15809–15815 CrossRef CAS PubMed.
- Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 16294–16308 CrossRef CAS PubMed.
- Z. Chen, Y. Zhou, L. Li, W. Ma, Y. Li and Z. Yang, Small, 2024, 21, 2411787 CrossRef PubMed.
- Y. Tang, Y. Li, C. He, Z. Wang, W. Huang, Q. Fan and B. Liu, Nat. Commun., 2025, 16, 278 CrossRef PubMed.
- Z. Qin, T. B. Ren, H. Zhou, X. Zhang, L. He, Z. Li, X. B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2022, 61, e202201541 CrossRef CAS PubMed.
- L. He, L. H. He, S. Xu, T. B. Ren, X. X. Zhang, Z. J. Qin, X. B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2022, 61, e202211409 CrossRef CAS PubMed.
- X. Zhang, Y. Chen, H. He, S. Wang, Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 26337–26341 CrossRef CAS PubMed.
- M. Zhao, B. Li, H. Zhang and F. Zhang, Chem. Sci., 2021, 12, 3448–3459 RSC.
- B. Hu, Q. Liu, Y. Jiang, Y. Huang, H. Ji, J. Zhang, X. Wang, X. C. Shen and H. Chen, Angew. Chem., Int. Ed., 2024, 64, e202418378 CrossRef PubMed.
- F. Chu, B. Feng, Y. Zhou, M. Liu, H. Zhang, M. Liu, Q. Chen, S. Zhang, Y. Ma, J. Dong, F. Chen and W. Zeng, Chem. Sci., 2025, 16, 4490–4500 RSC.
- Y. Liu, L. Zhang, Y. Chen, H. Sun, J. Chen, A. M. El-Toni, A. Khan, Z. Lei and F. Zhang, Adv. Opt. Mater., 2023, 11, 2301144 CrossRef CAS.
- X. Ge, Y. Lou, L. Su, B. Chen, Z. Guo, S. Gao, W. Zhang, T. Chen, J. Song and H. Yang, Anal. Chem., 2020, 92, 6111–6120 CrossRef CAS PubMed.
- Q. Lan, P. Yu, K. Yan, X. Li, F. Zhang and Z. Lei, J. Am. Chem. Soc., 2022, 144, 21010–21015 CrossRef CAS PubMed.
- P. Yu, K. Yan, S. Wang, C. Yao, Z. Lei, Y. Tang and F. Zhang, Nano Lett., 2022, 22, 9732–9740 CrossRef CAS PubMed.
- M. Zhao, J. Wang, Z. Lei, L. Lu, S. Wang, H. Zhang, B. Li and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 5091–5095 CrossRef CAS PubMed.
- Z. Lei, C. Sun, P. Pei, S. Wang, D. Li, X. Zhang and F. Zhang, Angew. Chem., Int. Ed., 2019, 58, 8166–8171 CrossRef CAS PubMed.
- L. Wang, N. Li, W. Wang, A. Mei, J. Shao, W. Wang and X. Dong, ACS Nano, 2024, 18, 4683–4703 CrossRef CAS PubMed.
- Y. Liu, Y. Li, S. Koo, Y. Sun, Y. Liu, X. Liu, Y. Pan, Z. Zhang, M. Du, S. Lu, X. Qiao, J. Gao, X. Wang, Z. Deng, X. Meng, Y. Xiao, J. S. Kim and X. Hong, Chem. Rev., 2021, 122, 209–268 CrossRef PubMed.
- W. R. Algar, N. Hildebrandt, S. S. Vogel and I. L. Medintz, Nat. Methods, 2019, 16, 815–829 CrossRef CAS PubMed.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X.-P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110–5139 RSC.
- S. Samanta, K. Lai, F. Wu, Y. Liu, S. Cai, X. Yang, J. Qu and Z. Yang, Chem. Soc. Rev., 2023, 52, 7197–7261 RSC.
- S.-L. Li, H.-Y. Hou, X. Chu, Y.-Y. Zhu, Y.-J. Zhang, M.-D. Duan, J. Liu and Y. Liu, ACS Nano, 2024, 18, 7769–7795 CrossRef CAS PubMed.
- C. Liang, Y. Zhang, S. Wang, W. Jiao, J. Guo, N. Zhang and X. Liu, J. Mater. Chem. B, 2024, 12, 4809–4823 RSC.
- N. D. Barth, F. J. Van Dalen, U. Karmakar, M. Bertolini, L. Mendive-Tapia, T. Kitamura, M. Verdoes and M. Vendrell, Angew. Chem., Int. Ed., 2022, 61, e202207508 CrossRef CAS PubMed.
- J. Huang, M. Xu, P. Cheng, J. Yu, J. Wu and K. Pu, Angew. Chem., Int. Ed., 2024, 63, e202319780 CrossRef CAS PubMed.
- C. W. Shields IV, M. A. Evans, L. L.-W. Wang, N. Baugh, S. Lyer, D. Wu, Z. Zhao, A. Pusuluri, A. Ukidve, D. C. Pan and S. Mitragotri, Sci. Adv., 2020, 6, eaaz6579 CrossRef PubMed.
- Z. Wang, H. Xia, B. Chen, Y. Wang, Q. Yin, Y. Yan, Y. Yang, M. Tang, J. Liu, R. Zhao, W. Li, Q. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 14512–14520 CrossRef CAS PubMed.
- S. Jalkanen and M. Salmi, Nat. Rev. Immunol., 2020, 20, 566–578 CrossRef CAS PubMed.
- P. Yuan, X. Xu, D. Hu, Y. Chen, J. Fan, S. Yao, Y. Piao, Z. Zhou, S. Shao, N. K. H. Slater, Y. Shen and J. Tang, J. Am. Chem. Soc., 2023, 145, 7941–7951 CrossRef CAS PubMed.
- C. Lu, S. Liao, B. Chen, L. Xu, N. Wu, D. Lu, H. Kang, X.-B. Zhang and G. Song, Nat. Mater., 2024, 24, 133–142 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02705e |
| This journal is © The Royal Society of Chemistry 2025 |