Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed asymmetric carbonylative hydroallylation of vinylarenes†
Sufang
Shao‡
ab,
Yang
Yuan‡
a,
Alban
Schmoll
a and
Xiao-Feng
Wu
 *abc
*abc
aDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: xwu2020@dicp.ac.cn
bLeibniz-Institut für Katalyse, Rostock 18059, Germany
cState Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071, China
First published on 14th May 2025
Abstract
We describe a novel and efficient copper-catalyzed carbonylative hydroallylation of vinylarenes, providing a direct route to chiral α,β-unsaturated ketones, which are important compounds in organic synthesis and bioactive molecules. The method employs readily accessible vinylarenes and allylic phosphates, utilizing carbon monoxide as the carbonyl source under mild reaction conditions. The reaction demonstrates a broad substrate scope, including diverse vinylarenes with various functional groups, as well as vinylarenes derived from natural products. Additionally, all four stereoisomers of a chiral allylic alcohol were prepared by employing this strategy, showcasing its versatility in stereodivergent synthesis.
Introduction
α,β-Unsaturated carbonyl compounds are important intermediates in organic chemistry, extensively used in synthetic transformations and the production of fine and commodity chemicals.1 Traditionally, these compounds have been prepared through methods such as the Horner–Wadsworth–Emmons reaction,2 aldol condensation,3 Knoevenagel condensation,4 and Saegusa oxidation.5 More recently, alternative methods, including dehydrogenation reactions,6 coupling reactions,7 and other strategies,8 have also been developed for the synthesis of α,β-unsaturated carbonyl compounds. However, most of these methods require a pre-existing carbonyl group in the reactant, which somewhat limits the substrate scope. Consequently, developing practical and efficient methods for their synthesis is still important and desired.Carbonylation, a process that introduces carbon monoxide (CO) into molecules, has become one of the most direct and efficient methods for synthesizing carbonyl compounds.9 Among these, α,β-unsaturated ketones are important intermediates in organic synthesis, widely utilized in various transformations such as Michael addition,10 Baylis–Hillman reaction,11 epoxidation,12 Simmons–Smith reaction,13 Diels–Alder reaction,14 hydrogenation,15 hydroalkoxylation,16 and aldol addition.17 Additionally, they serve as key structural elements in numerous drug molecules and bioactive compounds (Fig. 1a).18 In contrast to other α,β-unsaturated derivatives, such as α,β-unsaturated amides and α,β-unsaturated esters, the carbonylative synthesis of α,β-unsaturated ketones remains relatively challenging and underdeveloped, primarily due to the generally lower reactivity of carbon-based nucleophiles.19 The two most important carbonylative methods for synthesizing α,β-unsaturated ketones are the Pauson–Khand reaction20 and carbonylative Heck reactions21 (Fig. 1b). In addition, Mankad and co-workers presented a copper-catalyzed four-component coupling reaction of alkynes, alkyl halides, B2pin2, and CO enables modular synthesis of β-borylated tetrasubstituted enones.22 Alexanian group reported a cobalt-catalyzed stereospecific carbonylative coupling of alkyl tosylates and dienes producing enantioenriched enones.23
Recently, our group developed copper-catalyzed hydroaminocarbonylation of alkenes with hydroxylamine electrophiles to provide α-chiral amides, β-chiral amides, and γ-chiral amides.24 Given the rapid advancements in copper-catalyzed enantioselective hydrofunctionalization and allylic substitution reactions in recent years,25 as well as our ongoing interest in carbonylation reactions, we hypothesized that a similar approach could be employed to construct enantioenriched α,β-unsaturated ketones using a suitable allylic agent. Herein, we report a copper-catalyzed carbonylative hydroallylation of vinylarenes with allylic electrophiles, using carbon monoxide (CO) as the carbonyl source under mild conditions. This approach provides a highly efficient strategy for the synthesis of valuable chiral α,β-unsaturated ketones with good functional group tolerance (Fig. 1c).
Results and discussion
In the initial phase of our research, we studied the reaction of vinylarene (1k) with 2-methylallyl diphenyl phosphate (2k) in THF at 50 °C under 10 bar CO, using (MeO)2MeSiH as the hydrogen source. Xantphos was used as the ligand, and various bases were screened. The results showed that LiOMe, NaOtBu, and CsF produced trace amounts of the desired product 3k (Table 1, entries 1, 3 and 4). However, using LiOtBu as the base improved the yield slightly (Table 1, entry 2). Subsequently, with LiOtBu showing the most promise, we evaluated different ligands. BINAP, (R)-Segphos, and (R)-DTBM-Segphos exhibited similar low reactivity to Xantphos, yielding very low yields of the product (entries 5–7). The use of (S,S)-Ph-BPE as the ligand significantly improved the yield to 44% with 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 enantiomeric ratio (entry 8). The examination of the solvent effects revealed that 1,2-dichloroethane (DCE) was the optimal choice, affording the product in 89% yield and high enantioselectivity (entry 9). Despite this, DCE led to the formation of side product 3k′ (9% yield), which posed a considerable challenge to the purification and isolation. This problem was resolved by employing a mixed solvent system of DCE and 1,4-dioxane, which yielded a single product 3k in 84% isolated yield with 92
16 enantiomeric ratio (entry 8). The examination of the solvent effects revealed that 1,2-dichloroethane (DCE) was the optimal choice, affording the product in 89% yield and high enantioselectivity (entry 9). Despite this, DCE led to the formation of side product 3k′ (9% yield), which posed a considerable challenge to the purification and isolation. This problem was resolved by employing a mixed solvent system of DCE and 1,4-dioxane, which yielded a single product 3k in 84% isolated yield with 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 enantiomeric ratio (Table 1, entry 12).
8 enantiomeric ratio (Table 1, entry 12).
| Entry | Ligand | Base | Solvent | Yield (%) | er |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 1b (0.2 mmol), CuCl (10 mol%), L (10 mol%), base (2.0 equiv.), (MeO)2MeSiH (2.0 equiv.) and CO (10 bar) in solvent (1 mL) at 50 °C for 24 h. Yields are determined by GC-FID with n-hexadecane as the internal standard, enantiomeric ratios were determined by chiral HPLC. b Isolated yield. c Mixed with 9% yield of 3k′. | |||||
| 1 | Xantphos | LiOMe | THF | Trace | — |
| 2 | Xantphos | LiOtBu | THF | <10 | — |
| 3 | Xantphos | NaOtBu | THF | Trace | — |
| 4 | Xantphos | CsF | THF | 0 | — |
| 5 | BINAP | LiOtBu | THF | <10 | — |
| 6 | (R)-Segphos | LiOtBu | THF | <10 | — |
| 7 | (R)-DTBM-Segphos | LiOtBu | THF | 13 | — |
| 8 | (S,S)-Ph-BPE | LiOtBu | THF | 44 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 9 | (S,S)-Ph-BPE | LiOtBu | PhMe | 54 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 10 | (S,S)-Ph-BPE | LiOtBu | Dioxane | 75 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 11 | (S,S)-Ph-BPE | LiOtBu | DCE | 89c | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 12 | (S,S)-Ph-BPE | LiOtBu | Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCE = 1 DCE = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88 (84)b | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
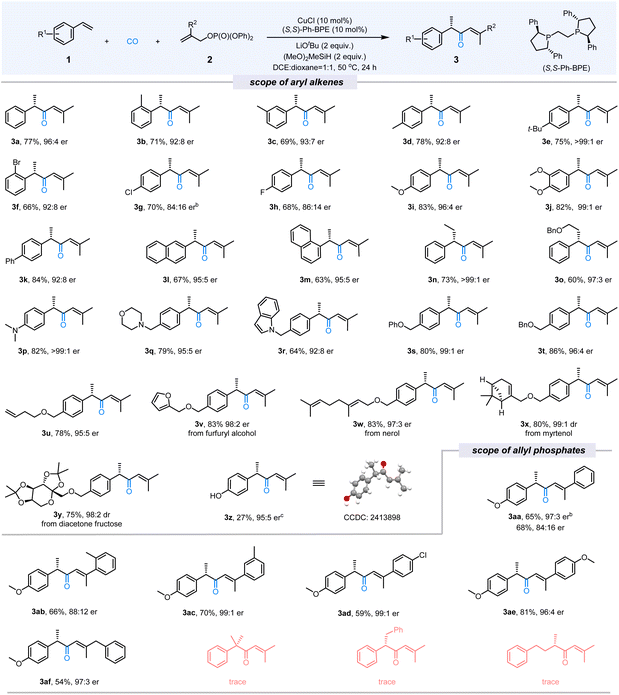
After establishing the optimal conditions for the carbonylative hydroallylation reaction, an extensive investigation into the substrate scope of olefins was conducted. The results, summarized in the Fig. 2, demonstrate that a wide variety of aryl olefins can produce the corresponding α,β-unsaturated ketones with excellent yields (up to 84%) and enantiomeric ratios (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). Specifically, variations in the position of alkyl substituents on the aromatic ring had a negligible effect on reaction outcomes, as evident in examples (3a–3d, 69–78% yield, 92
1 er). Specifically, variations in the position of alkyl substituents on the aromatic ring had a negligible effect on reaction outcomes, as evident in examples (3a–3d, 69–78% yield, 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–96
8–96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er). Substrates with electron-donating groups on the aromatic ring (3e, 3i, 3j) exhibited improvements in both yield and enantioselectivity, achieving yields of up to 83% and enantiomeric ratios of >99
4 er). Substrates with electron-donating groups on the aromatic ring (3e, 3i, 3j) exhibited improvements in both yield and enantioselectivity, achieving yields of up to 83% and enantiomeric ratios of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er. Halogen-substituted olefins (3f–3h) afforded the desired products with slightly lower enantioselectivity, as shown by yields of 66–84% and er values of 84
1 er. Halogen-substituted olefins (3f–3h) afforded the desired products with slightly lower enantioselectivity, as shown by yields of 66–84% and er values of 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16–96
16–96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Notably, internal aryl olefins, such as 3n and 3o, were well-tolerated, achieving enantiomeric ratios of up to >99
4. Notably, internal aryl olefins, such as 3n and 3o, were well-tolerated, achieving enantiomeric ratios of up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er. Olefins containing heteroatoms (N, O) exhibited excellent reactivity and stereoselectivity, with yields ranging from 64% to 86% and er values of up to >99
1 er. Olefins containing heteroatoms (N, O) exhibited excellent reactivity and stereoselectivity, with yields ranging from 64% to 86% and er values of up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (3p–3u). Furthermore, natural product-derived olefins were explored. Substrates derived from furfuryl alcohol (3v), nerol (3w), myrtenol (3x), and diacetone fructose (3y) produced the desired products in high yields. It should be noted that when 4-vinylphenyl acetate was used as the substrate, only 3z can be detected and isolated in 27% yield with 95
1 (3p–3u). Furthermore, natural product-derived olefins were explored. Substrates derived from furfuryl alcohol (3v), nerol (3w), myrtenol (3x), and diacetone fructose (3y) produced the desired products in high yields. It should be noted that when 4-vinylphenyl acetate was used as the substrate, only 3z can be detected and isolated in 27% yield with 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er. The absolute configuration of 3z was determined to be S through X-ray crystallographic analysis.
5 er. The absolute configuration of 3z was determined to be S through X-ray crystallographic analysis.
Then, we investigated the scope of electrophilic reagents. Allyl phosphates with various substituents at the 2-position, including aryl, benzyl, and related groups, were compatible with the reaction. These substrates produced the desired α,β-unsaturated ketones (3aa–3af) with yields up to 81% and enantiomeric ratios of up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er. α-Methylstyrene, (E)-stilbene, and 4-phenyl-1-butene were also subjected to the reaction under standard conditions, unfortunately, no desired ketone products can be detected.
1 er. α-Methylstyrene, (E)-stilbene, and 4-phenyl-1-butene were also subjected to the reaction under standard conditions, unfortunately, no desired ketone products can be detected.
To gain deeper insight into the reaction pathway, a series of experiments was conducted. First, deuterated diphenylsilane was synthesized, and a deuterium-labelling experiment was performed under standard conditions. Using Ph2SiD2 as the hydrogen source, product 3i-D was obtained 36% yield, and 93% deuterium incorporation was observed at the β-position (Fig. 3a). To investigate whether the reaction proceeds through a radical-based mechanism, two experiments were conducted. First, TEMPO (3 equiv.) was employed as a radical trapping reagents, and the product 3k was obtained in 58% yield, indicating that the reaction was not significantly inhibited. Next, a radical clock experiment was performed using (1-cyclopropylvinyl)benzene as an additional substrate. In this case, the desired product 3k was formed in 68% yield, and (1-cyclopropylvinyl)benzene remained intact. These results indicate that no radical intermediates were involved, suggesting that the reaction likely proceeds through a non-radical pathway (Fig. 3b and c).
Based on the experimental results presented above and previous literature,25,26 we proposed the following plausible reaction mechanism (Fig. 3d). The cycle begins with the insertion of olefin into the key catalyst L*CuH Int-I, generating an enantiomerically enriched intermediate, Int-II. Subsequently, CO and the allyl electrophilic reagent coordinate with Int-II, leading to the formation of intermediate Int-III. Following this, CO insertion, SN2′-allylic substitution, and reductive elimination occur, affording product A, which undergoes isomerization to form the final α,β-unsaturated ketone (see ESI†). Finally, in the presence of a metal alkoxide and hydrosilane, Int-IV facilitates the regeneration of LCuH, completing the catalytic cycle.
In biological systems, the chiral environment plays a crucial role, as drugs with different stereochemical configurations can exhibit varying therapeutic efficacies or cause adverse reactions. Despite many advances in the field of asymmetric catalytic reactions, the construction of multiple consecutive chiral centers continues to be a challenge.27 Therefore, we wondered whether we could identify reaction conditions suitable for the stereodivergent construction of all four stereoisomers of 5. Initially, following literature precedent,28 we subjected racemic 3j to reduction using the Cu(OAc)2/DTBM-Segphos catalytic system, obtaining racemic 5 in 81% isolated yield (Fig. 4a). Next, we prepared α,β-unsaturated ketones (R)-3j and (S)-3j by using (R,R)-L1 and (S,S)-L1 as the ligands, respectively. Notably, by subjecting (R)-3j and (S)-3j to the reaction under the specified conditions with (R)-L2 or (S)-L2-based catalysts, all four stereoisomeric chiral alcohols (5a–5d) were obtained in high yields (72–89%) with excellent enantioselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr),as confirmed by the HPLC traces in Fig. 4c.
1 dr),as confirmed by the HPLC traces in Fig. 4c.
 | ||
| Fig. 4 (a and b) Synthesis of all stereoisomeric chiral alcohols; (c) HPLC traces of all stereoisomeric chiral alcohols. | ||
Conclusions
In conclusion, we have developed an efficient copper-catalyzed carbonylative hydroallylation methodology for the enantioselective synthesis of α,β-unsaturated ketones. This approach utilizes mild reaction conditions, demonstrating high functional group tolerance and excellent enantioselectivity across a broad substrate scope. Moreover, the stereodivergent construction of all four stereoisomers of a chiral allylic alcohol was achieved, highlighting the method's versatility for synthesizing potential multiple contiguous stereocentres biologically relevant molecules in organic and medicinal chemistry.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
XW and YY directed the project and revised the manuscript. SS performed most of the experiments and prepared the manuscript. AS supported in substrates preparation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the financial support provided by National Key R&D Program of China (2023YFA1507500), National Natural Science Foundation of China (22302198) and the International Partnership Program of the Chinese Academy of Sciences (Grant No. 028GJHZ2023045FN).Notes and references
- (a) L. V. Reddy, V. Kumar, R. Sagar and A. K. Shaw, Chem. Rev., 2013, 113, 3605–3631 CrossRef CAS; (b) M. Schroeder, M. Mathys, N. Ehrensperger and M. Büchel, Chem. Biodiversity, 2014, 11, 1651–1673 CrossRef CAS; (c) J. W. Choi, B. K. Jang, N. C. Cho, J. H. Park, S. K. Yeon, E. J. Ju, Y. S. Lee, G. Han, A. N. Pae, D. J. Kim and K. D. Park, Bioorg. Med. Chem., 2015, 23, 6486–6496 CrossRef CAS; (d) R. Buffa, P. Sedova, I. Basarabova, M. Moravcova, L. Wolfova, T. Bobula and V. Velebny, Carbohydr. Polym., 2015, 134, 293–299 CrossRef CAS; (e) B. Hu, T. X. Liu, P. Zhang, Q. Liu, J. Bi, L. Shi, Z. Zhang and G. Zhang, Org. Lett., 2018, 20, 4801–4805 CrossRef CAS.
- (a) L. Horner, H. Hoffmann and H. G. Wippel, Chem. Ber., 1958, 91, 61–63 CrossRef CAS; (b) W. S. Wadsworth and W. D. Emmons, J. Am. Chem. Soc., 1961, 83, 1733–1738 CrossRef CAS.
- F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry: Part B: Reactions and Synthesis, Springer, 3rd edn, 1993, pp. 65–137 Search PubMed.
- E. Knoevenagel, Ber. Dtsch. Chem. Ges., 1898, 31, 2596–2619 CrossRef CAS.
- Y. Ito, T. Hirao and T. Saegusa, J. Org. Chem., 1978, 43, 1011–1013 CrossRef CAS.
- (a) K. Nicolaou, Y.-L. Zhong and P. Baran, J. Am. Chem. Soc., 2000, 122, 7596–7597 CrossRef CAS; (b) M. A. Bigi and M. C. White, J. Am. Chem. Soc., 2013, 135, 7831–7834 CrossRef CAS; (c) M.-M. Wang, X.-S. Ning, J.-P. Qu and Y.-B. Kang, ACS Catal., 2017, 7, 4000–4003 CrossRef CAS.
- (a) T. S. Jiang and J. H. Li, Chem. Commun., 2009, 7236–7238 RSC; (b) J. Wang, C. Liu, J. Yuan and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 2256–2259 CrossRef CAS.
- (a) S. Mandal, S. Mahato and C. K. Jana, Org. Lett., 2015, 17, 3762–3765 CrossRef CAS; (b) D. Kaiser, L. F. Veiros and N. Maulide, Adv. Synth. Catal., 2017, 359, 64–77 CrossRef CAS.
- (a) W. Bertleff, M. Roeper and X. Sava. Carbonylation, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2007 Search PubMed; (b) Q. Liu, H. Zhang and A. Lei, Angew. Chem., Int. Ed., 2011, 50, 10788–11079 CrossRef CAS; (c) J.-B. Peng, H.-Q. Geng and X.-F. Wu, Chem, 2019, 5, 526–552 CrossRef CAS.
- H. Pellissier, Adv. Synth. Catal., 2015, 357, 2745–2780 CrossRef CAS.
- J. Karabline-Kuks, P. Ramesh and M. Portnoy, Adv. Synth. Catal., 2016, 358, 3541–3554 CrossRef CAS.
- M. Bougauchi, S. Watanabe, T. Arai, H. Sasai and M. Shibasaki, J. Am. Chem. Soc., 1997, 119, 2329–2330 CrossRef CAS.
- L. Pontini, J. A. Leitch and D. L. Browne, Green Chem., 2023, 25, 4319–4325 RSC.
- A. B. Northrup and D. W. MacMillan, J. Am. Chem. Soc., 2002, 124, 2458–2460 CrossRef CAS.
- N. J. A. Martin and B. List, J. Am. Chem. Soc., 2006, 128, 13368–13369 CrossRef CAS PubMed.
- J.-J. Yun, M.-L. Zhi, W.-X. Shi, X.-Q. Chu, Z.-L. Shen and T.-P. Loh, Adv. Synth. Catal., 2018, 360, 2632–2637 CrossRef CAS.
- S. Crotti, G. Belletti, N. Di Iorio, E. Marotta, A. Mazzanti, P. Righi and G. Bencivenni, RSC Adv., 2018, 8, 33451–33458 RSC.
- (a) Z. Nowakowska, Eur. J. Med. Chem., 2007, 42, 125–137 CrossRef CAS PubMed; (b) D. Kumar, N. M. Kumar, K. Akamatsu, E. Kusaka, H. Harada and T. Ito, Bioorg. Med. Chem. Lett., 2010, 20, 3916–3919 CrossRef CAS PubMed; (c) R. G. Damazio, A. P. Zanatta, L. H. Cazarolli, L. D. Chiaradia, A. Mascarello, R. J. Nunes, R. A. Yunes and F. R. M. B. Silva, Eur. J. Med. Chem., 2010, 45, 1332–1337 CrossRef CAS; (d) N. K. Sahu, S. S. Balbhadra, J. Choudhary and D. V. Kohli, Curr. Med. Chem., 2012, 19, 209–255 CrossRef CAS.
- S. Zhang, H. Neumann and M. Beller, Chem. Soc. Rev., 2020, 49, 3187–3210 RSC.
- (a) I. U. Khand, G. R. Knox, P. L. Pauson, W. E. Watts and M. I. Foreman, J. Chem. Soc., Perkin Trans. 1, 1973, 977–981 RSC; (b) O. Geis and H.-G. Schmalz, Angew. Chem., Int. Ed., 1998, 37, 911–914 CrossRef CAS; (c) J. Blanco-Urgoiti, L. AÇorbe, L. PØrez-Serrano, G. Domínguez and J. PØrez-Castells, Chem. Soc. Rev., 2004, 33, 32–42 RSC; (d) E. Cristóbal-Lecina, A. R. Costantino, A. Grabulosa, A. Riera and X. Verdaguer, Organometallics, 2015, 34, 4989–4993 CrossRef.
- (a) X.-F. Wu, H. Neumann, A. Spannenberg, T. Schulz, H. Jiao and M. Beller, J. Am. Chem. Soc., 2010, 132, 14596–14602 CrossRef CAS; (b) X.-F. Wu, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 5284–5288 CrossRef CAS; (c) X.-F. Wu, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 122, 5412–5416 CrossRef; (d) X.-F. Wu, H. Neumann and M. Beller, Chem. Soc. Rev., 2011, 40, 4986–5009 RSC.
- L.-J. Cheng and N. P. Mankad, Angew. Chem., Int. Ed., 2018, 57, 10328–10332 CrossRef CAS.
- B. T. Sargent and E. J. Alexanian, J. Am. Chem. Soc., 2017, 139, 12438–12440 CrossRef CAS.
- (a) Y. Yuan, F.-P. Wu, C. Schünemann, J. Holz, P. C. J. Kamer and X.-F. Wu, Angew. Chem., Int. Ed., 2020, 59, 22441–22445 CrossRef CAS; (b) Y. Yuan, F. Zhao and X.-F. Wu, Chem. Sci., 2021, 12, 12676–12681 RSC; (c) Y. Yuan, Y. Zhang, W. Li, Y. Zhao and X.-F. Wu, Angew. Chem., Int. Ed., 2023, 62, e202309993 CrossRef CAS; (d) Y. Yuan, Y. Zhang and X.-F. Wu, Nat. Commun., 2024, 15, 6705–6712 CrossRef CAS PubMed.
- (a) Y.-M. Wang and S. L. Buchwald, J. Am. Chem. Soc., 2016, 138, 5024–5027 CrossRef CAS; (b) R. Y. Liu and S. L. Buchwald, Acc. Chem. Res., 2020, 53, 1229–1243 CrossRef CAS; (c) S. Krautwald and E. M. Carreira, J. Am. Chem. Soc., 2017, 139, 5627–5639 CrossRef CAS PubMed; (d) Z.-Y. Yi, H. Xu, X. Chang, Y. Dang, X.-Q. Dong and C.-J. Wang, ACS Catal., 2024, 14, 14058–14068 CrossRef CAS.
- N. Kim, J. T. Han, D. H. Ryu and J. Yun, Org. Lett., 2017, 19, 6144–6147 CrossRef CAS PubMed.
- S.-L. Shi, Z. L. Wong and S. L. Buchwald, Nature, 2016, 532, 353–356 CrossRef CAS PubMed.
- R. Moser, Ž. V. Bošković, C. S. Crowe and B. H. Lipshutz, J. Am. Chem. Soc., 2010, 132, 7852–7853 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: General comments, general procedures, analytic data and NMR spectra. CCDC 2413898. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc02421h |
| ‡ They contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |