Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective synthesis of 1,2-disubstituted thiocyclobutanes via Michael addition†
Emma G. L.
Robert
 and
Jerome
Waser
and
Jerome
Waser
 *
*
Laboratory of Catalysis and Organic Synthesis, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne, 1015 Lausanne, Switzerland. E-mail: jerome.waser@epfl.ch
First published on 23rd May 2025
Abstract
We report the diastereoselective and enantioselective synthesis of thio-substituted cyclobutanes via a sulfa-Michael addition using cyclobutenes. In the presence of DBU, various thio-cyclobutane esters and amides were obtained in up to quantitative yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr. Using a chiral chinchona-based squaramide bifunctional acid–base catalyst and an N-acyl-oxazolidinone-substituted cyclobutene, thio-cyclobutanes were obtained with high yield and enantioselectivity (er up to 99.7
5 dr. Using a chiral chinchona-based squaramide bifunctional acid–base catalyst and an N-acyl-oxazolidinone-substituted cyclobutene, thio-cyclobutanes were obtained with high yield and enantioselectivity (er up to 99.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3).
0.3).
Introduction
The incorporation of strained saturated rings into drug candidates has been widely adopted in medicinal chemistry.1 This approach gives access to compounds with enhanced molecular rigidity and often improved metabolic stability and solubility due to an increased fraction of sp3-hybridized carbons (Fsp3). Among strained rings, cyclobutanes have established themselves as important structural motifs in drug development,2 and accessing di-substituted derivatives with high diastereoselectivity and enantioselectivity has become an area of intense research in synthetic chemistry (Scheme 1A).3 In addition, sulfur atoms are widely utilized in pharmaceuticals due to their unique electronic and structural properties, appearing in various functional groups such as thioethers and sulfones.4 In this regard, sulfur-substituted cyclobutanes have been investigated for their conformational rigidity, particularly in comparison to non-cyclic derivatives such as the drug captopril.5 The configuration of thio-containing stereocenters in 1,2-disubstituted cyclobutanes has been shown to significantly influence their bioactivity. Nevertheless, the thio-substituted cyclobutane motif remains largely underexplored in medicinal chemistry and drug development, with only a few examples reported in the patent literature.6 This can be attributed to the limited synthetic strategies available to synthesize this motif, especially in an enantiopure form, reinforcing the need for further exploration and development in this area. | ||
| Scheme 1 S-Substituted cyclobutanes in medicinal chemistry (A), synthetic strategies (B), and this work (C). | ||
There are currently four main strategies for accessing sulfur-substituted cyclobutanes: cyclization of acyclic precursors by deprotonation in the α-position to a sulfone, sulfoximine or sulfoxide and the subsequent substitution reaction (Scheme 1 and eqn (1));7 thermal-catalyzed,8 Lewis acid-catalyzed9 or photo-catalyzed10 [2 + 2] cycloaddition (Scheme 1 and eqn (2)); incorporating a sulfur group into an existing cyclobutene, either via radical intermediates (Scheme 1 and eqn (3))11–14 or through Michael addition of a thio nucleophile (Scheme 1 and eqn (4)).5b,6,8,15,16 The ring expansion of cyclopropanes to sulfur-substituted cyclobutanes was also explored, but it occurred with low efficiency.17
Concerning the radical sulfur addition pathway, Witiak and co-workers reported in 1972 the first two examples of Giese addition of thioacetic acid and benzyl thiol radicals generated by light irradiation of cyclobut-1-ene-1-carboxylic acid.11 These conditions led to a mixture of isomers in the case of benzyl thiol. In 2020, Gouverneur and co-workers used sulfonyl12 and sulfamoyl13 chlorides for the hydrosulfonylation and hydrosulfamoylation of activated alkenes under visible light catalysis. They described five examples using cyclobutene esters and amides as alkenes to obtain sulfoxide-substituted cyclobutanes majorly with a cis-configuration. In the same year, Landais and co-workers reported the sulfonylcyanation of chiral cyclobutenes under light irradiation.14 In this case, a trans-relationship between the N-acyl-oxazolidinone and the sulfonyl group was obtained.
Surprisingly, although the Michael addition of nitrogen nucleophiles to cyclobutenes has been exploited,18 its application to sulfur-based nucleophiles has remained largely unexplored. Ciabatti and co-workers reported that the addition of thioacetic acid to cyclobut-1-ene-1-carboxylic acid was also possible without generation of the radical but proceeded with only 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dr.5b Probably, due to the low selectivity, this method found only occasional use for the generation of simple building blocks for medicinal chemistry projects.6 Besides addition to cyclobut-1-ene-1-carboxylic acid, the only other cases of Michael addition involve a non-selective addition of glutathione to a conjugated ketone in the context of metabolic intermediate studies,15a as well as additions to cyclobutene esters on a biased bicyclic system15b and on a polyhalogenated derivative.8 In 2019, Aitken and co-workers reported access to β-sulfinyl cyclobutane amides via a rearrangement of α-sulfinyl precursors.16 The scope was limited to aryl sulphoxides, and the products were obtained in a diastereomeric mixture due to the stereogenic sulphoxide. Overall, highly diastereoselective Michael additions of thio-nucleophiles to cyclobutene esters remain extremely rare, and no enantioselective method has been reported yet. This is surprising considering that numerous examples of enantioselective sulfa-Michael additions using less strained substrates have been reported over the past two decades, primarily relying on the use of chiral metal complexes or organocatalysts.19
3 dr.5b Probably, due to the low selectivity, this method found only occasional use for the generation of simple building blocks for medicinal chemistry projects.6 Besides addition to cyclobut-1-ene-1-carboxylic acid, the only other cases of Michael addition involve a non-selective addition of glutathione to a conjugated ketone in the context of metabolic intermediate studies,15a as well as additions to cyclobutene esters on a biased bicyclic system15b and on a polyhalogenated derivative.8 In 2019, Aitken and co-workers reported access to β-sulfinyl cyclobutane amides via a rearrangement of α-sulfinyl precursors.16 The scope was limited to aryl sulphoxides, and the products were obtained in a diastereomeric mixture due to the stereogenic sulphoxide. Overall, highly diastereoselective Michael additions of thio-nucleophiles to cyclobutene esters remain extremely rare, and no enantioselective method has been reported yet. This is surprising considering that numerous examples of enantioselective sulfa-Michael additions using less strained substrates have been reported over the past two decades, primarily relying on the use of chiral metal complexes or organocatalysts.19
Herein, we describe the first highly diastereoselective (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) and enantioselective (up to 99.7
5 dr) and enantioselective (up to 99.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 er) synthesis of thio-cyclobutanes. Starting from commercially available thiols and readily available cyclobutenes, our method enables the efficient formation of a wide range of thio-substituted cyclobutane esters and amides. High diastereoselectivities were first achieved just by using DBU as a base. With a chiral chinchona squaramide catalyst, thio-cyclobutanes were then accessed with high enantioselectivity. This novel method therefore offers for the first time control over both diastereoselectivity and enantioselectivity for the synthesis of 1,2-substituted thio-cyclobutane esters, offering efficient access to a currently underexploited chemical space for applications in medicinal chemistry.
0.3 er) synthesis of thio-cyclobutanes. Starting from commercially available thiols and readily available cyclobutenes, our method enables the efficient formation of a wide range of thio-substituted cyclobutane esters and amides. High diastereoselectivities were first achieved just by using DBU as a base. With a chiral chinchona squaramide catalyst, thio-cyclobutanes were then accessed with high enantioselectivity. This novel method therefore offers for the first time control over both diastereoselectivity and enantioselectivity for the synthesis of 1,2-substituted thio-cyclobutane esters, offering efficient access to a currently underexploited chemical space for applications in medicinal chemistry.
Results and discussion
Reaction design and optimization
We began our investigation with the reaction of 2-bromothiophenol (1a) and readily accessible benzyl ester cyclobutene 2a,20 using K2CO3 as a base in MeCN at room temperature for 18 hours (Table 1, entry 1). To our satisfaction, the corresponding thio-cyclobutane ester 3a was obtained in 72% yield, but no diastereoselectivity was observed (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dr). Switching K2CO3 for NEt3 (entry 2) or TMG (entry 3) increased the yield but resulted in a low dr (95% yield, 51
50 dr). Switching K2CO3 for NEt3 (entry 2) or TMG (entry 3) increased the yield but resulted in a low dr (95% yield, 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 dr and 90% yield, 64
49 dr and 90% yield, 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 dr, respectively). TBD offered the desired product in 69% yield and 72
36 dr, respectively). TBD offered the desired product in 69% yield and 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 dr (entry 4). In contrast, DBU provided the trans-product in quantitative yield and >95
28 dr (entry 4). In contrast, DBU provided the trans-product in quantitative yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr (entry 5). Changing the solvent from MeCN to EtOAc resulted in the same yield but lower dr (82
5 dr (entry 5). Changing the solvent from MeCN to EtOAc resulted in the same yield but lower dr (82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18) (entry 6). Lowering the reaction time to 1 or 2 hours did not affect the yield but lowered the diastereoselectivity to 64
18) (entry 6). Lowering the reaction time to 1 or 2 hours did not affect the yield but lowered the diastereoselectivity to 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 after 1 hour (entry 7) and to 71
36 after 1 hour (entry 7) and to 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 (entry 8) after 2 hours, suggesting thermodynamic control of the dr. Finally, conducting the reaction under an air atmosphere (entry 9) or with HPLC-grade MeCN (entry 10) resulted in a lower diastereoselectivity (86
29 (entry 8) after 2 hours, suggesting thermodynamic control of the dr. Finally, conducting the reaction under an air atmosphere (entry 9) or with HPLC-grade MeCN (entry 10) resulted in a lower diastereoselectivity (86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 dr and 89
14 dr and 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr, respectively), probably due to the presence of water, which can slow down the epimerization.
11 dr, respectively), probably due to the presence of water, which can slow down the epimerization.
| Entry | Base | Solvent | Time | Yieldb | drc |
|---|---|---|---|---|---|
| a 1.0 equiv. thiol 1a (0.1 mmol), 1.1 equiv. cyclobutene 2a, 1.1 equiv. base, rt. b 1H NMR of the crude mixture with dibromomethane as an internal standard. c Measured from the crude 1H NMR. | |||||
| 1 | K2CO3 | MeCN | 18 h | 72% | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 2 | NEt3 | MeCN | 18 h | 95% | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
| 3 | TMG | MeCN | 18 h | 90% | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
| 4 | TBD | MeCN | 18 h | 69% | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 5 | DBU | MeCN | 18 h | Quant. |
>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5 5
|
| 6 | DBU | EtOAc | 18 h | Quant. | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 7 | DBU | MeCN | 1 h | Quant. | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
| 8 | DBU | MeCN | 2 h | 98% | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
| 9 | DBU | MeCN + air | 18 h | 81% | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 10 | DBU | HPLC-MeCN | 18 h | 97% | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
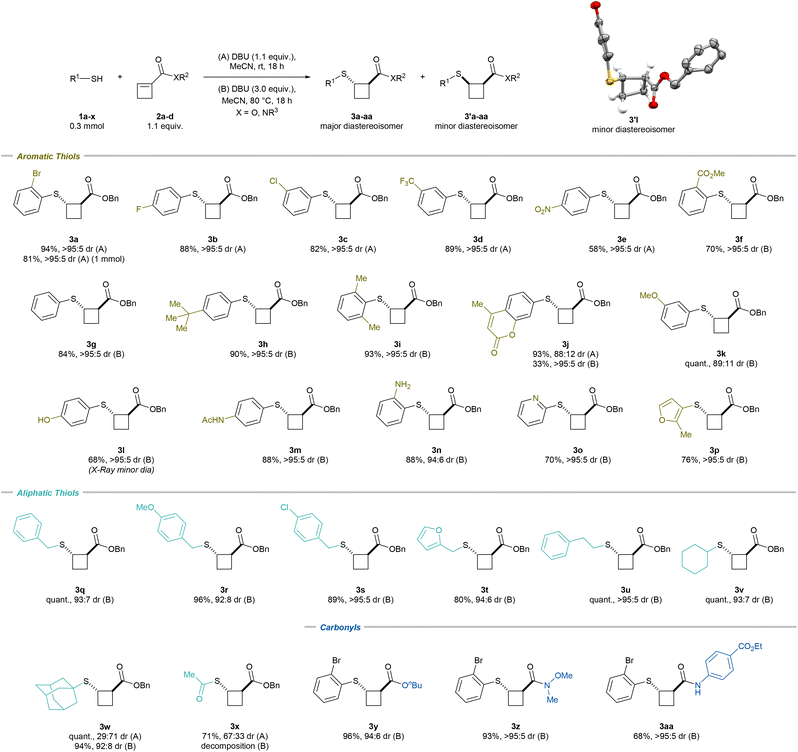
Scope of the diastereoselective Michael addition
With the optimized conditions in hand, we began investigating the scope of aromatic thiols (Scheme 2). The model substrate 3a was obtained in 94% isolated yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr on a 0.3 mmol scale. Scaling up the reaction to 1 mmol offered the desired product in 81% yield and an identical dr. A 4-fluorine or 3-chlorine substituent on the benzene moiety was well tolerated and led to 3b (88% yield and >95
5 dr on a 0.3 mmol scale. Scaling up the reaction to 1 mmol offered the desired product in 81% yield and an identical dr. A 4-fluorine or 3-chlorine substituent on the benzene moiety was well tolerated and led to 3b (88% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) and 3c (82% yield and >95
5 dr) and 3c (82% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr), respectively. Other electron-withdrawing groups could be introduced, such as a CF3 (3d; 89% yield and >95
5 dr), respectively. Other electron-withdrawing groups could be introduced, such as a CF3 (3d; 89% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr), a NO2 (3e; 58% yield and >95
5 dr), a NO2 (3e; 58% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) or a methyl ester (3f; 70% yield and >95
5 dr) or a methyl ester (3f; 70% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) group. For the latter, 3.0 equivalents of DBU and heating to 80 °C were necessary to obtain the desired product with high diastereoselectivity (method (B)). These conditions showed improved diastereoselectivity for all the thiols not bearing an electron-withdrawing group. Simple thiophenol provided 3g in 84% yield and >95
5 dr) group. For the latter, 3.0 equivalents of DBU and heating to 80 °C were necessary to obtain the desired product with high diastereoselectivity (method (B)). These conditions showed improved diastereoselectivity for all the thiols not bearing an electron-withdrawing group. Simple thiophenol provided 3g in 84% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr. tert-Butylthiophenol led to 3h in 90% yield and >95
5 dr. tert-Butylthiophenol led to 3h in 90% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, while 2,6-dimethylthiophenol offered 3i in 93% yield and >95
5 dr, while 2,6-dimethylthiophenol offered 3i in 93% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr. A chromenone-substituted thiol was tolerated, and 3j was obtained in 93% yield and 88
5 dr. A chromenone-substituted thiol was tolerated, and 3j was obtained in 93% yield and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 dr with method (A) and in 33% yield and >95
12 dr with method (A) and in 33% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr with method (B). Methoxy-substituted thiophenol provided 3k in quantitative yield and 89
5 dr with method (B). Methoxy-substituted thiophenol provided 3k in quantitative yield and 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr. A free alcohol, an acetamide and a free amine led to 3l (68% yield and >95
11 dr. A free alcohol, an acetamide and a free amine led to 3l (68% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr), 3m (88% yield and >95
5 dr), 3m (88% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) and 3n (88% yield and 94
5 dr) and 3n (88% yield and 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr), respectively. The X-ray structure of 3'l confirmed the molecular structure and relative configuration of the minor cis-diastereoisomer.21
6 dr), respectively. The X-ray structure of 3'l confirmed the molecular structure and relative configuration of the minor cis-diastereoisomer.21
Heteroaromatic-substituted thiols could also be introduced; 2-pyridine as well as furan-substituted thiol provided 3o (70% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) and 3p (76% yield and >95
5 dr) and 3p (76% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr), respectively. Moreover, benzyl thiol provided 3q in quantitative yield and 93
5 dr), respectively. Moreover, benzyl thiol provided 3q in quantitative yield and 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr. Similarly, methoxy- and chloro-substituted benzyl thiols could be introduced, giving 3r (96% yield and 92
7 dr. Similarly, methoxy- and chloro-substituted benzyl thiols could be introduced, giving 3r (96% yield and 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr) and 3s (89% yield and >95
8 dr) and 3s (89% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr), respectively. Furan-2-ylmethanethiol provided 3t (80% yield and 94
5 dr), respectively. Furan-2-ylmethanethiol provided 3t (80% yield and 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr). A primary alkyl-substituted thiol led to 3u (quant. yield and >95
6 dr). A primary alkyl-substituted thiol led to 3u (quant. yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr), while a secondary thiol gave 3v (quant. yield, 93
5 dr), while a secondary thiol gave 3v (quant. yield, 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr). The sterically hindered adamantyl thiol led to 3w in quantitative yield and 29
7 dr). The sterically hindered adamantyl thiol led to 3w in quantitative yield and 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 dr with method (A) and 94% yield and 92
71 dr with method (A) and 94% yield and 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr with method (B). Thioacetic acid provided 3x in 71% yield and 67
8 dr with method (B). Thioacetic acid provided 3x in 71% yield and 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 dr with method (A). In this case, the use of method (B) could not improve the diastereoselectivity as decomposition was observed. Finally, replacement of the benzyl ester was implemented with an n-butyl ester (3y; 96% yield and 94
33 dr with method (A). In this case, the use of method (B) could not improve the diastereoselectivity as decomposition was observed. Finally, replacement of the benzyl ester was implemented with an n-butyl ester (3y; 96% yield and 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr), a Weinreb amide (3z; 93% yield and >95
6 dr), a Weinreb amide (3z; 93% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr) or a benzocaine-substituted amide (3aa; 68% yield and >95
5 dr) or a benzocaine-substituted amide (3aa; 68% yield and >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr).
5 dr).
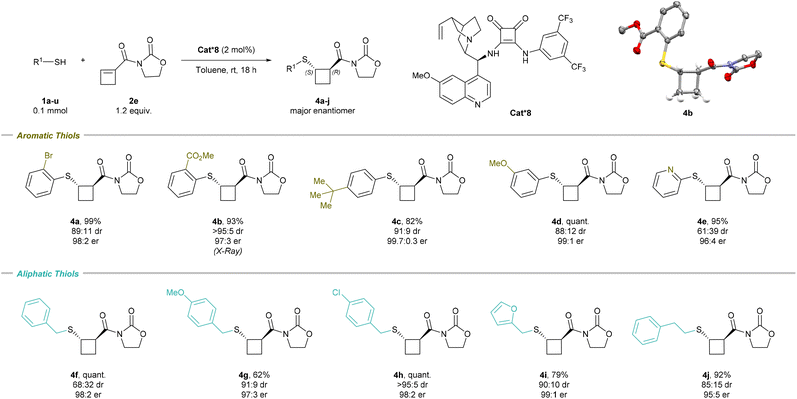
Development of the enantioselective reaction
For our investigation of the enantioselective sulfa-Michael addition of thiols onto cyclobutenes, we turned our attention to cyclobutene 2e substituted with a simple oxazolidinone auxiliary, which has demonstrated its versatility in asymmetric reactions (Scheme 3).19 Based on previous reports,19j–l chiral bifunctional acid–base catalysts Cat*1–9 containing thiourea, urea and squaramides as well as chinchona alkaloids or chiral diamines were selected (Fig. 1).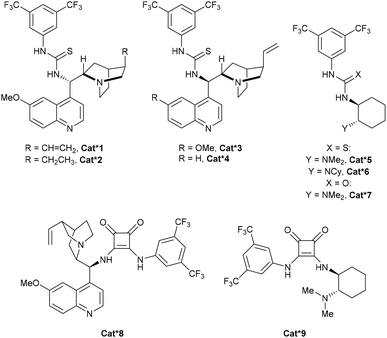 | ||
| Fig. 1 Structures of chiral bifunctional organocatalysts used in the optimization of the enantioselective sulfa-Michael addition. | ||
Using 2 mol% of the commercially available thiourea cinchona catalyst Cat*1 in toluene at room temperature overnight, we were pleased to obtain the desired product in 92% yield, modest diastereoselectivity of 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 and good enantiomeric ratio of 96
21 and good enantiomeric ratio of 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for the major diastereoisomer (Scheme 3 and Table 2, entry 1), while the er of the minor diastereoisomer was lower (59
4 for the major diastereoisomer (Scheme 3 and Table 2, entry 1), while the er of the minor diastereoisomer was lower (59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 er). To improve the dr, 3.0 equivalents of DBU were added and the reaction mixture was heated to 70 °C for one hour. An improvement of dr was observed (94
41 er). To improve the dr, 3.0 equivalents of DBU were added and the reaction mixture was heated to 70 °C for one hour. An improvement of dr was observed (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr compared to 79
6 dr compared to 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 dr), but the er dropped to 57
21 dr), but the er dropped to 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43, suggesting that the epimerization occurred via a retro-Michael/Michael-addition process rather than a simple deprotonation. This hypothesis was confirmed by adding a second thiol to the crude reaction mixture after full conversion, resulting in a mixture of the two thiol-substituted products (see Section 4.1 in the ESI†).
43, suggesting that the epimerization occurred via a retro-Michael/Michael-addition process rather than a simple deprotonation. This hypothesis was confirmed by adding a second thiol to the crude reaction mixture after full conversion, resulting in a mixture of the two thiol-substituted products (see Section 4.1 in the ESI†).
| Entry | Catalyst | Yieldb | drc | erd |
|---|---|---|---|---|
| a 1.0 equiv. thiol 1a (0.1 mmol), 1.2 equiv. cyclobutene 2e, 2 mol% Cat*, toluene [0.1 M], rt, 18 h. b 1H NMR of the crude mixture with dibromomethane as an internal standard. c Measured from the crude 1H NMR. d Measured from the SFC chromatogram. | ||||
| 1 | Cat*1 | 92% | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 2 | Cat*2 | Quant. | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 3 | Cat*3 | Quant. | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94 94 |
| 4 | Cat*4 | 86% | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
| 5 | Cat*5 | 98% | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 6 | Cat*6 | Quant. | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
| 7 | Cat*7 | 84% | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 8 | Cat*8 | 99% |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 11 11
|
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2
|
| 9 | Cat*9 | 98% | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
We then turned our attention to other chiral organocatalysts in order to further improve the diastereoselectivity under kinetic control. Cat*2 with a hydrogenated quinuclidine substituent led to an improvement in dr (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) and the same er (Table 2, entry 2). Cat*3 with an inverted urea stereocenter gave a quantitative yield, but lower dr (71
15) and the same er (Table 2, entry 2). Cat*3 with an inverted urea stereocenter gave a quantitative yield, but lower dr (71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29) and er (6
29) and er (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94) than Cat*1. Removing the methoxy group of the quinoline backbone (Cat*4) led to a low dr (entry 4). Takemoto's thiourea (Cat*5–6) and urea (Cat*7) type of catalysts led to a decrease in diastereoselectivity and enantioselectivity (entries 5–7). Squaramide-based cinchona Cat*8 gave the best results with 99% yield, 89
94) than Cat*1. Removing the methoxy group of the quinoline backbone (Cat*4) led to a low dr (entry 4). Takemoto's thiourea (Cat*5–6) and urea (Cat*7) type of catalysts led to a decrease in diastereoselectivity and enantioselectivity (entries 5–7). Squaramide-based cinchona Cat*8 gave the best results with 99% yield, 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr and 98
11 dr and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er (entry 8). Replacement of the chinchona by a chiral diamine moiety (Cat*9) gave a similar yield (98%), a higher dr (91
2 er (entry 8). Replacement of the chinchona by a chiral diamine moiety (Cat*9) gave a similar yield (98%), a higher dr (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), but a lower enantioselectivity (90
9), but a lower enantioselectivity (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 er) (entry 9). Cat*8 was then selected as the best catalyst, and further fine-tuning of the conditions was performed, but no significant improvement of neither the dr nor the er could be obtained (see the ESI†). In addition, performing the reaction with ester-substituted cyclobutene 2a led to the formation of thiocyclobutane 3a in a racemic form, confirming the essential role of the oxazolidinone auxiliary.
10 er) (entry 9). Cat*8 was then selected as the best catalyst, and further fine-tuning of the conditions was performed, but no significant improvement of neither the dr nor the er could be obtained (see the ESI†). In addition, performing the reaction with ester-substituted cyclobutene 2a led to the formation of thiocyclobutane 3a in a racemic form, confirming the essential role of the oxazolidinone auxiliary.
Scope of the enantioselective reaction
The scope of the thiols was then examined for the enantioselective transformation (Scheme 4). Scaling up the reaction to 0.8 mmol led to the formation of 4a in similar yield, increased dr, and identical er (95% yield, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 dr, and 98
9 dr, and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er). Among aromatic thiols, an ester electron-withdrawing group, a neutral bulky tert-butyl group, and a methoxy electron-donating group all smoothly underwent the transformation with high diastereo- and enantioselectivity; 4b (93% yield, >95
2 er). Among aromatic thiols, an ester electron-withdrawing group, a neutral bulky tert-butyl group, and a methoxy electron-donating group all smoothly underwent the transformation with high diastereo- and enantioselectivity; 4b (93% yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, and 97
5 dr, and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er), 4c (82% yield, 91
3 er), 4c (82% yield, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 dr, and 99.7
9 dr, and 99.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 er), and 4d (quant. yield, 88
0.3 er), and 4d (quant. yield, 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 dr, and 99
12 dr, and 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er) were obtained, respectively. The X-ray structure of 4b confirmed the molecular structure and absolute configuration of the major trans-enantiomer as (S-,R-), in accordance with Houk's Brønsted acid–hydrogen bonding stereoinduction model (see the ESI, Fig. S1†).22 2-Pyridine-substituted thiol produced 4e (95% yield, 61
1 er) were obtained, respectively. The X-ray structure of 4b confirmed the molecular structure and absolute configuration of the major trans-enantiomer as (S-,R-), in accordance with Houk's Brønsted acid–hydrogen bonding stereoinduction model (see the ESI, Fig. S1†).22 2-Pyridine-substituted thiol produced 4e (95% yield, 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 dr, and 96
39 dr, and 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er). Aliphatic thiols were also suitable in the enantioselective transformation. Benzyl thiol 4f (quant. yield, 68
4 er). Aliphatic thiols were also suitable in the enantioselective transformation. Benzyl thiol 4f (quant. yield, 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 dr, and 98
32 dr, and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er) and substituted benzyl thiol 4g (62% yield, 91
2 er) and substituted benzyl thiol 4g (62% yield, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 dr, and 97
9 dr, and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er) and 4h (quant. yield, >95
3 er) and 4h (quant. yield, >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dr, and 98
5 dr, and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er) were tolerated. Furan-2-ylmethanethiol produced 4i (79% yield, 90
2 er) were tolerated. Furan-2-ylmethanethiol produced 4i (79% yield, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr, and 99
10 dr, and 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er), while 2-phenylethane-1-thiol gave 4j (92% yield, 85
1 er), while 2-phenylethane-1-thiol gave 4j (92% yield, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 dr, and 95
15 dr, and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). However, bulkier thiols such as cyclohexyl thiol and adamantyl thiol were not compatible with the reaction and provided the desired product in low yields (14% and <5%, respectively).
5 er). However, bulkier thiols such as cyclohexyl thiol and adamantyl thiol were not compatible with the reaction and provided the desired product in low yields (14% and <5%, respectively).
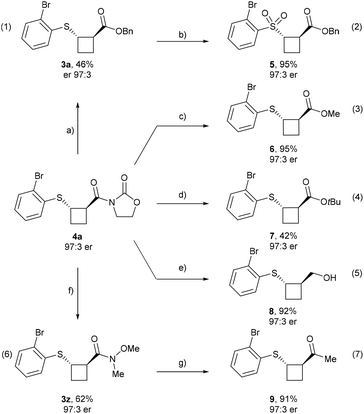
Product modifications
Finally, we explored the functionalization of the obtained products (Scheme 5). The oxazolidinone auxiliary was successfully replaced by a benzyl ester to afford enantioenriched 3a in 46% yield with preservation of the enantiomeric ratio (eqn (1)). Treatment of 3a with a slight excess of mCPBA furnished sulfone 5, a motif frequently encountered in medicinal chemistry, in 95% yield and without erosion of enantiopurity (eqn (2)).Moreover, other ester derivatives were synthesized from enantioenriched 4a, including methyl ester 6 (95% yield and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er) (eqn (3)) and the tert-butyl ester 7, obtained via saponification followed by esterification in 42% yield and 97
3 er) (eqn (3)) and the tert-butyl ester 7, obtained via saponification followed by esterification in 42% yield and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er (eqn (4)). Enantioenriched alcohol 8 can be obtained in 92% yield and with unchanged er by reduction of the oxazolidinone auxiliary using NaBH4 (eqn (5)). Furthermore, the Weinreb amide analog 3z was obtained by reaction with the amine chloride salt in the presence of base and a catalytic amount of Yb(OTf)3 (eqn (6)) and was subsequently converted into the corresponding methyl ketone 9 in 91% yield without loss of er (eqn (7)).
3 er (eqn (4)). Enantioenriched alcohol 8 can be obtained in 92% yield and with unchanged er by reduction of the oxazolidinone auxiliary using NaBH4 (eqn (5)). Furthermore, the Weinreb amide analog 3z was obtained by reaction with the amine chloride salt in the presence of base and a catalytic amount of Yb(OTf)3 (eqn (6)) and was subsequently converted into the corresponding methyl ketone 9 in 91% yield without loss of er (eqn (7)).
Conclusions
In summary, we have developed a diastereoselective sulfa-Michael addition using cyclobutene derivatives, enabling the synthesis of thio-substituted cyclobutane esters and amides. Furthermore, the enantioselective version of the reaction was achieved, delivering thio-cyclobutanes with high enantioselectivity using a chiral cinchona squaramide organocatalyst. The synthetic versatility of the obtained compounds was demonstrated through oxidation of the sulfur atom to sulfone and transformation of the ester functionality into an alcohol or a carboxylic acid. Finally, the oxazolidinone auxiliary was successfully converted into various esters, a Weinreb amide, and a ketone, all whilemaintaining enantiopurity.Data availability
ESI† is available as a pdf file, including general methods, experimental procedures, compound characterization data and copies of NMR spectra for new compounds. Raw data for compound characterization will be available with free access at https://doi.org/10.5281/zenodo.15490161 after final publication of the work. The authors have cited additional references within the ESI.†23–25Author contributions
E. G. L. R. conceived the project, optimized the reaction, performed the investigation on the scope of the reaction, the modification of the products and prepared the experimental parts and first draft of the manuscript. J. W. supervised the project, edited the manuscript, and proofread the experimental part.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank EPFL and the Swiss National Science Foundation (Grant number: 200020_212129) for providing financial support. Dr Farzaneh Fadaei Tirani and Dr Rosario Scopelliti (ISIC, EPFL) are acknowledged for performing the X-ray analysis.Notes and references
- (a) A. F. Stepan, C. Subramanyam, I. V. Efremov, J. K. Dutra, T. J. O'Sullivan, K. J. DiRico, W. S. McDonald, A. Won, P. H. Dorff, C. E. Nolan, S. L. Becker, L. R. Pustilnik, D. R. Riddell, G. W. Kauffman, B. L. Kormos, L. Zhang, Y. Lu, S. H. Capetta, M. E. Green, K. Karki, E. Sibley, K. P. Atchison, A. J. Hallgren, C. E. Oborski, A. E. Robshaw, B. Sneed and C. J. O'Donnell, J. Med. Chem., 2012, 55, 3414 CrossRef CAS PubMed; (b) M. V. Westphal, B. T. Wolfstädter, J.-M. Plancher, J. Gatfield and E. M. Carreira, ChemMedChem, 2015, 10, 461 CrossRef CAS PubMed; (c) P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839 RSC; (d) M. R. Bauer, P. D. Fruscia, S. C. C. Lucas, I. N. Michaelides, J. E. Nelson, R. I. Storer and B. C. Whitehurst, RSC Med. Chem., 2021, 12, 448 RSC.
- (a) M. L. Wrobleski, G. A. Reichard, S. Paliwal, S. Shah, H.-C. Tsui, R. A. Duffy, J. E. Lachowicz, C. A. Morgan, G. B. Varty and N.-Y. Shih, Bioorg. Med. Chem. Lett., 2006, 16, 3859 CrossRef CAS PubMed; (b) T. T. Wager, B. A. Pettersen, A. W. Schmidt, D. K. Spracklin, S. Mente, T. W. Butler, H. J. Howard, D. J. Lettiere, D. M. Rubitski, D. F. Wong, F. M. Nedza, F. R. Nelson, H. Rollema, J. W. Raggon, J. Aubrecht, J. K. Freeman, J. M. Marcek, J. Cianfrogna, K. W. Cook, L. C. James, L. A. Chatman, P. A. Iredale, M. J. Banker, M. L. Homiski, J. B. Munzner and R. Y. Chandrasekaran, J. Med. Chem., 2011, 54, 7602 CrossRef CAS PubMed; (c) B. Cox, K. I. Booker-Milburn, L. D. Elliott, M. Robertson-Ralph and V. Zdorichenko, ACS Med. Chem. Lett., 2019, 10, 1512 CrossRef CAS PubMed; (d) M. R. van der Kolk, M. A. C. H. Janssen, F. P. J. T. Rutjes and D. Blanco-Ania, ChemMedChem, 2022, 17, e202200020 CrossRef CAS PubMed.
- (a) E. Lee-Ruff and G. Mladenova, Chem. Rev., 2003, 103, 1449 CrossRef CAS PubMed; (b) K.-G. Wen, Y.-Y. Peng and X.-P. Zeng, Org. Chem. Front., 2020, 7, 2576 RSC.
- (a) K. Tanaka, A. Kaji, in Sulphones and Sulphoxides, John Wiley & Sons, Ltd, 1988 Search PubMed; (b) E. A. Ilardi, E. Vitaku and J. T. Njardarson, J. Med. Chem., 2014, 57, 2832 CrossRef CAS PubMed; (c) M. Feng, B. Tang, S. H. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200 CrossRef CAS PubMed; (d) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed; (e) C. Zhao, K. P. Rakesh, L. Ravidar, W.-Y. Fang and H.-L. Qin, Eur. J. Med. Chem., 2019, 162, 679 CrossRef CAS PubMed.
- Selected examples: (a) D. T. Witiak, B. K. Sinha, R. R. Ruffolo Jr and P. N. Patil, J. Med. Chem., 1973, 16, 232 CrossRef CAS PubMed; (b) R. Ciabatti, G. Padova, E. Bellasio, G. Tarzia, A. Depaoli, F. Battaglia, M. Cellentani, D. Barone and E. Baldoli, J. Med. Chem., 1986, 29, 411 CrossRef CAS PubMed.
- (a) J. Deng, H. Lei, X. Ma and X. Lin, Novel Compounds, WO 2015/180612A1, 2015; (b) B. Hu, V. Verma, M. Volgraf, A. Estrada and J. Lyssikatos, Sulfonylcycloalkyl Carboxamide Compounds as Trpa1 Modulators, WO 2018/015411A1, 2018.
- Selected examples: (a) J. F. Bunnett and J. A. Skorcz, J. Org. Chem., 1962, 27, 3836 CrossRef CAS; (b) Y. Gaoni, Tetrahedron, 1989, 45, 2819 CrossRef CAS; (c) M. Iwao, J. Org. Chem., 1990, 55, 3622 CrossRef CAS; (d) H. J. Monteiro, Synlett, 2002, 1992, 990 CrossRef; (e) D. F. Taber, Y. He and M. Xu, J. Am. Chem. Soc., 2004, 126, 13900 CrossRef CAS PubMed; (f) M. Harmata, W. Ying, X. Hong and C. L. Barnes, Synthesis, 2008, 2008, 594 CrossRef; (g) T. Satoh, T. Kasuya, M. Ishigaki, M. Inumaru, T. Miyagawa, N. Nakaya and S. Sugiyama, Synthesis, 2011, 2011, 397 CrossRef.
- Selected example: M.-A. Plancquaert, P. François, R. Merényi and H. G. Viehe, Tetrahedron Lett., 1991, 32, 7265 CrossRef CAS.
- Selected examples: (a) K. Narasaka, Y. Hayashi, H. Shimadzu and S. Niihata, J. Am. Chem. Soc., 1992, 114, 8869 CrossRef CAS; (b) T. Fujiwara, K. Sawabe and T. Takeda, Tetrahedron, 1997, 53, 8349 CrossRef CAS.
- Selected examples: (a) F. Strieth-Kalthoff, C. Henkel, M. Teders, A. Kahnt, W. Knolle, A. Gómez-Suárez, K. Dirian, W. Alex, K. Bergander, C. G. Daniliuc, B. Abel, D. M. Guldi and F. Glorius, Chem, 2019, 5, 2183 CrossRef CAS; (b) D. Dibchak, V. Shcherbacova, A. V. Denisenko and P. K. Mykhailiuk, Org. Lett., 2019, 21, 8909 CrossRef CAS PubMed; (c) N. Jeremias, L.-M. Mohr and T. Bach, Org. Lett., 2021, 23, 5674 CrossRef CAS PubMed.
- D. T. Witiak, B. K. Sinha, O. S. Lee and D. R. Feller, J. Med. Chem., 1972, 15, 803 CrossRef CAS PubMed.
- S. M. Hell, C. F. Meyer, A. Misale, J. B. I. Sap, K. E. Christensen, M. C. Willis, A. A. Trabanco and V. Gouverneur, Angew. Chem., Int. Ed., 2020, 59, 11620 CrossRef CAS PubMed.
- S. M. Hell, C. F. Meyer, G. Laudadio, A. Misale, M. C. Willis, T. Noël, A. A. Trabanco and V. Gouverneur, J. Am. Chem. Soc., 2020, 142, 720 CrossRef CAS PubMed.
- V. Pirenne, I. Traboulsi, L. Rouvière, J. Lusseau, S. Massip, D. M. Bassani, F. Robert and Y. Landais, Org. Lett., 2020, 22, 575 CrossRef CAS PubMed.
- Selected examples: (a) A. Mutlib, H. Chen, J. Shockcor, R. Espina, S. Chen, K. Cao, A. Du, G. Nemeth, S. Prakash and L.-S. Gan, Chem. Res. Toxicol., 2000, 13, 775 Search PubMed; (b) H. Hongo, K. Iwasa, C. Kabuto, H. Matsuzaki and H. Nakano, J. Chem. Soc., Perkin Trans. 1, 1997, 1747 RSC.
- L. Ghisu, N. Melis, L. Serusi, A. Luridiana, F. Soddu, F. Secci, P. Caboni, R. Guillot, D. J. Aitken and A. Frongia, Org. Biomol. Chem., 2019, 17, 6143 RSC.
- L. A. Spurlock, R. K. Porter and W. G. Cox, J. Org. Chem., 1972, 37, 1162 CrossRef CAS.
- E. G. L. Robert and J. Waser, Chem. Eur. J., 2025, 31, e202403986 CrossRef CAS PubMed.
- Review: (a) D. Enders, K. Lüttgen and A. A. Narine, Synthesis, 2007, 2007, 959 CrossRef; (b) P. Chauhan, S. Mahajan and D. Enders, Chem. Rev., 2014, 114, 8807 CrossRef CAS PubMed . Selected examples of asymmetric sulfa-Michael on α,β-unsaturated N-acylated oxazolidin-2-ones: ; (c) S. Kanemasa, Y. Oderaotoshi and E. Wada, J. Am. Chem. Soc., 1999, 121, 8675 CrossRef CAS; (d) S. Kobayashi, C. Ogawa, M. Kawamura and M. Sugiura, Synlett, 2001, 2001, 983 CrossRef; (e) K. Matsumoto, A. Watanabe, T. Uchida, K. Ogi and T. Katsuki, Tetrahedron Lett., 2004, 45, 2385 CrossRef CAS; (f) S. J. K. Sauerland, E. Kiljunen and A. M. P. Koskinen, Tetrahedron Lett., 2006, 47, 1291 CrossRef CAS; (g) A. M. M. Abe, S. J. K. Sauerland and A. M. P. Koskinen, J. Org. Chem., 2007, 72, 5411 CrossRef CAS PubMed; (h) M. Kawatsura, Y. Komatsu, M. Yamamoto, S. Hayase and T. Itoh, Tetrahedron Lett., 2007, 48, 6480 CrossRef CAS; (i) M. Kawatsura, Y. Komatsu, M. Yamamoto, S. Hayase and T. Itoh, Tetrahedron, 2008, 64, 3488 CrossRef CAS; (j) Y. Liu, B. Sun, B. Wang, M. Wakem and L. Deng, J. Am. Chem. Soc., 2009, 131, 418 CrossRef CAS PubMed; (k) L. Dai, H. Yang and F. Chen, Eur. J. Org Chem., 2011, 2011, 5071 CrossRef CAS; (l) N. K. Rana and V. K. Singh, Org. Lett., 2011, 13, 6520 CrossRef CAS PubMed; (m) A. C. Breman, J. M. M. Smits, R. de Gelder, J. H. van Maarseveen, S. Ingemann and H. Hiemstra, Synlett, 2012, 23, 2195 CrossRef CAS; (n) R. A. Unhale, N. K. Rana and V. K. Singh, Tetrahedron Lett., 2013, 54, 1911 CrossRef CAS; (o) A. C. Breman, S. E. M. Telderman, R. P. M. van Santen, J. I. Scott, J. H. van Maarseveen, S. Ingemann and H. Hiemstra, J. Org. Chem., 2015, 80, 10561 CrossRef CAS PubMed; (p) S. Lauzon, H. Keipour, V. Gandon and T. Ollevier, Org. Lett., 2017, 19, 6324 CrossRef CAS PubMed; (q) S. Lauzon, L. Schouwey and T. Ollevier, Org. Lett., 2022, 24, 1116 CrossRef CAS PubMed.
- A. A. Homon, O. V. Hryshchuk, S. Trofymchuk, O. Michurin, Y. Kuchkovska, D. S. Radchenko and O. O. Grygorenko, Eur. J. Org Chem., 2018, 2018, 5596 CrossRef CAS.
- Deposition Number 2383043 for 3′l and 2415842 for 4b contain the supplementary crystallographic data for this paper.†.
- (a) M. N. Grayson and K. N. Houk, J. Am. Chem. Soc., 2016, 138, 1170 CrossRef CAS PubMed; (b) J. Guo and M. W. Wong, J. Org. Chem., 2017, 82, 4362 CrossRef CAS PubMed.
- E. G. L. Robert, V. Pirenne, M. D. Wodrich and J. Waser, Angew. Chem., Int. Ed., 2023, 62, e202302420 CrossRef CAS PubMed.
- H. Xu, W. Zhang, D. Shu, J. B. Werness and W. Tang, Angew. Chem., Int. Ed., 2008, 47, 8933 CrossRef CAS PubMed.
- J. M. Robinson, S. F. Tlais, J. Fong and R. L. Danheiser, Tetrahedron, 2011, 67, 9890 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Optimization details, experimental procedures, characterization data and NMR spectra of new compounds. Raw data for NMR, IR, and HRMS will be made freely available on the platform Zenodo. CCDC 2383043 and 2415842. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01727k |
| This journal is © The Royal Society of Chemistry 2025 |