Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A versatile NIR probe for multifunctional detection of tumors, fatty liver, and liver injury†
Qihang
Ding‡
 ab,
Jiqiang
Liu‡
ab,
Jiqiang
Liu‡
 cd,
Xinyue
Zhang‡
a,
Jun
Li
b,
Keith Man-Chung
Wong
cd,
Xinyue
Zhang‡
a,
Jun
Li
b,
Keith Man-Chung
Wong
 c,
Amit
Sharma
c,
Amit
Sharma
 e,
Pengfei
Zhang
e,
Pengfei
Zhang
 d,
Quli
Fan
d,
Quli
Fan
 a,
Chao
Yin
a,
Chao
Yin
 *a,
Hui
Zhou
*a,
Hui
Zhou
 *a,
Tony D.
James
*a,
Tony D.
James
 *fg and
Jong Seung
Kim
*fg and
Jong Seung
Kim
 *b
*b
aState Key Laboratory of Flexible Electronics (LoFE), Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamhzhou@njupt.edu.cn; iamcyin@njupt.edu.cn
bDepartment of Chemistry, Korea University, Seoul 02841, Korea. E-mail: jongskim@korea.ac.kr
cDepartment of Chemistry, Southern University of Science and Technology, 1088 Xueyuan Blvd, Shenzhen 518055, China
dGuangdong Key Laboratory of Nanomedicine, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China
eAmity School of Chemical Sciences, Amity University Punjab, Sector 82A, Mohali, Punjab 140306, India
fDepartment of Chemistry, University of Bath, Bath BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
gSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 12th June 2025
Abstract
Abnormal viscosity, reduced pH, and elevated levels of superoxide anion (O2˙−) in living cells are often associated with various biological dysfunctions and oxidative stress. Although some studies have reported probes capable of detecting one or two of these biomarkers, achieving simultaneous, rapid, and convenient detection of all three remains a significant challenge. Herein, we present a rhodamine based probe, DM301, which selectively activates fluorescence in three distinct channels in response to pH, viscosity, and O2˙− respectively. Systematic spectroscopic analyses demonstrated DM301's exceptional response to these biomarkers, while in vitro experiments confirmed its mitochondrial specificity at the cellular level. To validate the diagnostic potential of DM301, we employed various disease models, including 4T1 tumors, fatty liver, and drug-induced liver injury, all associated with these abnormal biomarkers. In vivo, experiments further established the safety of DM301 and demonstrated its specificity to pH, viscosity, and O2˙− across different pathological conditions in living organisms. We anticipate that these findings will offer practical and effective strategies for investigating the physiological roles of biomarkers and analytes in diverse biological systems.
Introduction
The concentrations of various bioactive substances are tightly regulated to maintain homeostasis in normal cells and tissues.1 However, abnormal levels of certain biomarkers, including enzymes, lipids, pH, viscosity, reactive oxygen species (ROS), and reactive nitrogen species, are often associated with diseases and pathological processes, leading to significant fluctuations in these molecules.2 For example, cancer is marked by an acidic tumor microenvironment, non-alcoholic fatty liver disease is linked to increased viscosity, and superoxide anion (O2˙−), an important ROS, is implicated in liver injury.3 Since pH, viscosity, and superoxide anion are three critical indicators of various diseases and cellular dysfunction, precise detection of these biomarkers is crucial for unraveling disease mechanisms and identifying potential therapeutic targets.4 Fluorescence imaging has become a widely used technique for visualizing bioactive molecules in living organisms, offering high sensitivity, excellent specificity, and low detection limits.5 Its superior spatial and temporal resolution enables accurate real-time imaging of these molecules both in situ and in vivo.6 While numerous fluorescent probes have been developed to independently detect pH, viscosity, and O2˙−, they often face challenges such as limited imaging contrast and an increased risk of false-positive signals in abnormal cells or tissues.7 To address these limitations, dual-stimuli responsive probes targeting multiple biomarkers have been developed, offering a more effective approach to enhance imaging resolution and sensitivity both in vitro and in vivo, thereby improving disease diagnosis. For example, several dual-function fluorescent probes have been designed to simultaneously target two analytes, such as pH and viscosity,8 pH and O2˙−,9 and viscosity and O2˙−.4b However, no probe has yet been reported that can simultaneously respond to all three analytes—pH, viscosity, and O2˙−. There is a pressing need to develop probes capable of responding to these three biomarkers with exceptional sensitivity, selectivity, and visualization capabilities. Such advancements would not only improve the detection of disease biomarkers but also reduce costs by minimizing the number of probes and associated reagents required.Herein, we engineered a fluorescent probe featuring three spectrally distinct switch-on modes that responds to pH, viscosity, and O2˙−. The probe molecule is comprised of two fluorescent functional moieties linked by a single covalent bond, imparting viscosity-sensitive characteristics. The fluorescein moiety exhibits sensitivity to both pH and O2˙−, endowing the probe with the ability to respond to changes in these two factors. Moreover, the probe is designed to target the mitochondria, facilitating near-infrared (NIR) imaging. Targeting the mitochondria enables a more precise investigation of mitochondria-related biological processes and pathological alterations. Systematic spectroscopic analysis confirmed the high sensitivity of the probe under various conditions. Furthermore, cell and animal experiments demonstrated its superior performance in live-cell imaging and various disease models, including 4T1 tumors, fatty liver, and drug-induced liver injury (DILI). This versatile probe holds significant potential for applications in biomedical research and diagnostics.
Results and discussion
Synthesis, structural optimization, and multi-responsive fluorescence mechanisms of DM301
The synthetic strategy for the fluorescent probe DM301 is illustrated in Scheme 1. The rhodamine and fluorescein moieties were synthesized independently. The rhodamine component, Rho-Br, was first converted to a boronate ester via Miyaura borylation and subsequently coupled with Fluo-Br using Suzuki cross-coupling. The resulting probe was fully characterized by 1H NMR, 13C NMR, 19F NMR, 31P NMR, and HRMS (Fig. S1–S11†). Calculations were conducted to simulate the multi-responsive behavior of DM301. Theoretical calculations of DM301's geometric and electronic structures under acidic and basic conditions (Fig. S12†) indicated that the optimized dihedral angles for C18–C13–C3–C4 (rhodamine/phenyl groups) and C10–C11–C37–C38 (fluorescein/pyridine groups) in both protonated (acidic) and deprotonated (basic) states remained similar to those in the neutral form. However, the C4–C5–C7–N8 angle (phenyl/pyridine groups) was reduced to 13.8° and 9.3° in basic and acidic conditions, respectively, compared to 23.5° in the neutral form. Additionally, the energy gap (ΔE) decreased to 0.24 eV (acidic) and 1.56 eV (basic) from the neutral form (1.75 eV), suggesting a strong intramolecular charge transfer (ICT) effect between the rhodamine and fluorescein moieties.10 This ICT effect enhances fluorescence and enables the pH response by facilitating the transition between the hydroxyl group and oxygen anion in the fluorescein moiety (Fig. 1).Similarly, upon interaction with O2˙−, the optimized dihedral angle of C4–C5–C7–N8 (phenyl/pyridine groups) decreased to 7.8°, (Fig. 1G), compared to 23.5° in the neutral form, further strengthening the ICT effect. The O2˙− then oxidized the hydroxyl group (fluorescein moiety) to a ketocarbonyl, thereby enhancing the Förster Resonance Energy Transfer (FRET) process. This reaction decreased the ΔE to 1.34 eV, resulting in enhanced, red-shifted fluorescence and increased emission (Fig. 1I and H). To further investigate DM301's response to viscosity, a solvation model density simulation was performed in glycerol. Results revealed that the HOMO–LUMO transition dominated DM301's excitation from S0 to S1 in a viscous medium. As depicted in Fig. 1J and K, the dihedral angles C18–C13–C3–C4 were set to 0° and 90° to assess fluorescence behavior from the rhodamine moiety. In the orthogonal conformation (90°), LUMO electron density was confined to the fluorescein moiety, while the HOMO was localized in the rhodamine moiety, reflecting charge separation during twisted intramolecular charge transfer (TICT) excitation.11 Conversely, in the planar conformation (0°), both HOMO and LUMO were primarily localized on the rhodamine with slight LUMO extension toward the phenyl ring, corresponding to local excitation (LE) and enhanced fluorescence. The calculated oscillator strengths (f) were 1.0545 at 0° and 0.0057 at 90°, indicating that restricted rotation in high-viscosity environments limits the TICT state, supporting the observed fluorescence enhancement.
Viscosity, superoxide, and pH-responsive optical properties of DM301 for versatile biological sensing
Fluo-Br, Rho-Br, and DM301 exhibited maximum absorption wavelengths at 463 nm, 562 nm, and 561 nm, respectively, with corresponding peak emission wavelengths at 530 nm, 575 nm, and 576 nm. Their molar extinction coefficients were determined to be 3.17 × 104, 4.45 × 104, and 4.75 × 104 M−1 cm−1, respectively (Fig. 2A, B, and Table S1†). Interestingly, DM301 exhibited optical properties derived from its rhodamine moiety through a FRET process, as evidenced by a consistent emission profile under various excitation wavelengths (Fig. S13†). The energy gap of DM301 was comparable to that of Rho-Br (1.79 eV) and significantly smaller than that of Fluo-Br (3.11 eV) (Fig. S14†), aligning with the observed fluorescence emission phenomenon originating solely from the rhodamine moiety.The optical properties of DM301 were evaluated in various solvents, including CH3CN, DMSO, MeOH, EtOH, and glycerol (Fig. 2C and D). While its absorbance remained largely unchanged across solvents, fluorescence emission was significantly enhanced in the highly viscous solvent glycerol, with a quantum yield of 0.104, substantially higher than in other solvents (Table S2†). These results suggest that DM301 exhibits strong potential as a viscosity-responsive probe. To further explore its spectroscopic response to viscosity, DM301 was examined in varying water/glycerol mixtures with different volume ratios. A pronounced red shift in absorption was observed, shifting from 510 nm in pure water to 560 nm in 100% glycerol (Fig. 2E). Additionally, DM301 exhibited weak fluorescence in pure water, but its intensity progressively increased with higher glycerol content (Fig. 2F and G). This effect is likely due to glycerol's high viscosity, restricting molecular rotation in DM301, leading to both a red shift in its absorbance as well as enhanced fluorescence intensity.
We then investigated DM301's response to O2˙−. O2˙− was generated through KO2 using a previously reported method12 and confirmed via the DHR123 assay. A notable increase in the absorption peak of DHR123 at 525 nm was observed with increasing KO2 concentrations ranging from 0 to 105 μM, confirming the successful preparation of O2˙−. Using this method, DM301 was employed for O2˙− detection, exhibiting a progressive increase in fluorescence intensity with rising KO2 levels (Fig. 2I). A strong linear relationship was observed between the fluorescence intensity at 586 nm and KO2 concentrations from 0 to 105 μM, with the intensity plateauing between 105 and 120 μM (Fig. 2J). The limit of detection (LOD) for O2˙− was determined to be as low as 124 nM, calculated using the formula 3σ/K, where σ represents the standard deviation of the blank sample and K is the slope of the calibration curve.12 This result indicates the exceptional sensitivity of DM301 for the in vitro detection of O2˙−. To investigate the pH response of DM301, it was dissolved in Britton–Robinson buffer across a pH range from 3 to 10. As shown in Fig. 2K, the primary absorption peak at approximately 500 nm over a pH range from 7–10 gradually redshifted to 560 nm as the pH decreased. Fitting the pH response to the classical Henderson–Hasselbalch equation yielded an apparent pKa value of 6.0 for DM301 (Fig. S15†). Additionally, the fluorescence intensity significantly increased under acidic conditions (Fig. 2L).
To evaluate whether viscosity affects the response of DM301 to O2˙−, fluorescence measurements were performed in water/glycerol mixtures with varying viscosities (30%, 60%, 90%) in the presence of KO2. As shown in Fig. S16,†DM301 exhibited strong and consistent fluorescence enhancement upon KO2 addition (∼586 nm) across all viscosities, indicating that superoxide-triggered activation is not hindered by high viscosity. Next, to investigate the combined influence of pH and viscosity on the probe's fluorescence response, DM301 was evaluated in Britton–Robinson buffer solutions with varying glycerol content at pH 3, 7, and 10. As shown in Fig. S17,†DM301 exhibited viscosity-dependent fluorescence enhancement under the three representative pH conditions. Notably, under acidic conditions (pH 3), the sensitivity to glycerol content was markedly higher than that observed at neutral or basic pH, suggesting a possible synergistic effect between protonation and viscosity-induced restriction of intramolecular motion. These results indicate that DM301 is capable of simultaneously responding to both pH and viscosity changes, demonstrating its utility as a multi-stimuli-responsive probe.
To further mimic physiologically relevant conditions where multiple environmental stimuli may coexist, we evaluated the integrated response of DM301 under simultaneous exposure to low pH, high viscosity, and elevated superoxide levels. Specifically, the probe (1 μM) was incubated in a medium containing 40% glycerol (v/v), buffered at pH 4, and supplemented with 35 μM KO2 as a superoxide source. As shown in Fig. S18,†DM301 exhibited a significantly enhanced fluorescence intensity at ∼586 nm compared to conditions involving any individual stimulus or dual combinations. This result confirms that DM301 remains responsive and functional under complex microenvironmental conditions, and that its activation mechanisms do not interfere with one another. Such robustness makes DM301 particularly suitable for biological applications where pH fluctuations, redox stress, and viscosity changes often coexist. Furthermore, to assess the selectivity of DM301, we evaluated its fluorescence response toward a series of potential biological interferents, including reactive oxygen species (1O2, H2O2, ˙OH, and O2˙−), proteins (BSA, IgG), metal ions (Ca2+, Cu2+, Fe2+, Fe3+), anions (NO2−, HSO3−, CN−), amino acids (Lys, Gly, Cys), and hypochlorous acid (HClO). As shown in Fig. S19,†DM301 exhibited a pronounced fluorescence increase specifically in response to O2˙−. While minor increases were observed for 1O2, H2O2, ˙OH, and HClO, the signal was significantly weaker. No notable fluorescence changes were detected with the other analytes. These results confirm the high selectivity of DM301 toward superoxide over other biologically relevant species.
Moreover, the stability of DM301 was assessed in PBS and DMEM. As shown in Fig. S20a and b,† no significant changes were observed in the absorption spectra of DM301 under continuous white light irradiation (1.0 W cm−2) over 30 minutes, confirming its good photostability in both media. Additionally, long-term stability was confirmed by monitoring the absorption over five days (Fig. S20c and d†), with no noticeable degradation. These results demonstrate that DM301 is stable and photostable under physiologically relevant conditions.
Mitochondria-targeted and multi-responsive DM301 for visualizing pH, viscosity, and superoxide dynamics in living cells
Next, the cytotoxicity of DM301 was assessed using the MTT method. After 24 hours of incubation with DM301 (320 nM), over 80% of both normal (3T3) and cancerous (4T1) cells remained (Fig. S21 and S22†), indicating low cytotoxicity. Since, mitochondria, the energy factories of cells, are crucial for respiration, metabolism, and signaling, developing small-molecule probes targeting mitochondria is vital for detecting physiological changes and enabling early disease diagnosis. The mitochondrial-targeting ability of DM301 was evaluated via co-localization experiments with the commercial dye MTR-green in 4T1 and HeLa cells. A strong overlap was observed with Pearson correlation coefficients of approximately 0.89 and 0.90, confirming effective mitochondrial targeting (Fig. 3A–C and S23†). Additionally, co-incubation of DM301 with LysoTracker Green in 4T1, HeLa and 3T3 cells further confirmed that DM301 selectively targeted mitochondria rather than lysosomes (Pearson correlation coefficients are 0.52, 0.51 and 0.4 in 4T1, HeLa, and 3T3 cells, respectively) (Fig. 3D–F, and S24†).Next, the pH-responsive behavior of DM301 was evaluated in two normal cell lines (3T3 and AML12) and two cancer cell lines (HeLa and 4T1). Both HeLa and 4T1 cells exhibited strong red fluorescence, whereas minimal fluorescence was detected in normal cells (3T3 and AML12) (Fig. 3G and H), likely due to the higher acidity in cancer cells. Notably, the fluorescence was more intense in 4T1 cells than in HeLa cells, consistent with reports indicating greater acidity in 4T1 cells. To simulate acidic and alkaline conditions, 4T1 cells were incubated with buffer solutions at pH 5.0, 6.8, and 8.0 before treatment with DM301. The strongest fluorescence signal was observed at pH 5.0, confirming DM301's effectiveness in visualizing pH fluctuations in mitochondria (Fig. 3I and S25†). To evaluate DM301's effectiveness in detecting viscosity changes, an antifungal drug, nystatin, which is known to disrupt ionic balance, induce mitochondrial dysfunction, and increase intracellular viscosity was used.13 After nystatin treatment, AML12 cells incubated with DM301 exhibited a significant increase in red fluorescence compared to the control group, demonstrating DM301's ability to detect endogenously induced viscosity changes in living cells (Fig. 3J and S26†). Finally, the response of DM301 to O2˙− was examined in AML12 cells. O2˙− production was stimulated using 2-methylestradiol (2-ME), an inhibitor of superoxide dismutase.12 After a 1-hour incubation with 2-ME, DM301-treated AML12 cells showed a 2.8-fold increase in red fluorescence compared to the control group. However, when cells were pretreated with N-acetylcysteine (NAC), an O2˙− scavenger, before 2-ME incubation, the fluorescence intensity significantly decreased (Fig. 3K and S27†). These results confirm that DM301 is an effective tool for real-time visualization and monitoring of endogenous O2˙− in hepatocytes. Furthermore, to quantitatively assess the cellular response of DM301 to superoxide anions, we performed flow cytometric analysis using AML12 cells under chemically induced oxidative stress. Cells were divided into four groups: (i) untreated control; (ii) DM301 only (50 nM); (iii) 10 μM 2-ME pre-treatment followed by DM301 incubation; and (iv) 20 μM 2-ME pre-treatment followed by DM301. As shown in Fig. S28,† the fluorescence intensity was significantly increased in 2-ME-treated cells in a dose-dependent manner, with the 20 μM group exhibiting the highest signal. In contrast, the DM301-only group exhibited minimal fluorescence, indicating low background activation in the absence of oxidative stimuli. These results confirm that DM301 is capable of selectively detecting elevated intracellular superoxide levels in live cells.
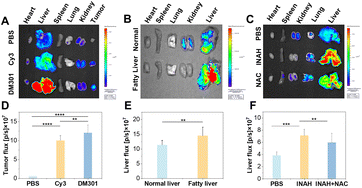
In vivo evaluation of DM301 for multi-responsive detection of tumor acidity, liver viscosity, and superoxide in disease models
Encouraged by the promising in vitro response of DM301 to the three biomarkers, we expanded our investigation to assess the in vivo potential across various disease models. To verify DM301's pH response, 4T1 tumor-bearing mice were intravenously injected with PBS (phosphate buffered saline, control), Cy3, or DM301. Cy3, a commonly used cyanine dye with absorption and emission wavelengths similar to DM301, served as a positive control. Organs and tumors were collected and imaged 20 minutes post-injection. The fluorescence intensity in the tumor region of the DM301 treatment group was 1.2 times higher than that of the Cy3 group, indicating the good sensitivity of DM301 for tumor imaging (Fig. 4A and D). Next, we examined DM301's viscosity-sensing capability in a fatty liver model induced by a methionine- and choline-deficient diet over five weeks. In the control group, normal livers appeared bright red, whereas fatty livers in the experimental group showed yellow, uneven surfaces, visually confirming successful establishment of the model (Fig. S29†). This was further validated by H&E staining (Fig. S30†). After injecting DM301 into both groups, ex vivo results exhibited significantly stronger fluorescence in the fatty liver group than in controls, indicating higher DM301 accumulation (Fig. 4B and E) in response to elevated viscosity. Finally, DM301 was employed to detect O2˙− in DILI models, as oxidative stress and ROS production, including O2˙−, are hallmark features of DILI. The tuberculosis drug, isoniazid (INAH) was used to induce liver injury, and the establishment of the model was confirmed by H&E staining (Fig. S31†). After 12 hours post-injection of DM301, significant fluorescence was observed in the DILI group compared to the control group (healthy mice without DILI). Notably, pretreatment of DILI mice with NAC, an effective ROS inhibitor, resulted in reduced NIR fluorescence signals, confirming DM301's specificity for O2˙− detection (Fig. 4C and F). Collectively, these findings highlight DM301's strong potential for diagnosing diseases like cancer, fatty liver, and liver injury.Conclusions
In summary, we have developed a triple-responsive probe, DM301 integrating fluorescein and rhodamine units to sensitively monitor changes in pH, viscosity, and O2˙−. Theoretical calculations were used to simulate DM301's response to various stimuli, and in vitro studies confirmed significant fluorescence changes in response to these different biomarkers. Cellular experiments confirmed DM301's strong mitochondrial targeting and its capability to visualize pH, viscosity, and O2˙− fluctuations. Furthermore, DM301 exhibited reliable fluorescent signals for detecting tumors, liver injuries, and fatty liver in mouse models, establishing it as a powerful imaging tool for various diseases. This study offers a promising approach for utilizing a simple, easily synthesized fluorescent probe for precise diagnosis and image-guided therapy.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Q. D., J. L., and X. Z. equally contributed to this work. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Research Foundation of Korea (2018R1A3B1052702 to JSK), the National Natural Science Foundation of China (52373142, 22475105 and 22405132), the Natural Science Foundation of Jiangsu Province (BK20240655), the Project of Jiangsu Specially-Appointed Professor (RK030STP22003), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (23KJB150024), the Project of State Key Laboratory of Organic Electronics and Information Displays, the Natural Science Research Start-up Foundation of Recruiting Talents of Nanjing University of Posts and Telecommunications (NY222066), and the China Scholarship Council (No. 202106270027 to QD). A. S. thanks the Prestigious DBT-Ramalingaswami Fellowship (BT/RLF/Re-entry/59/2018) and SERB (CRG/2021/0022476) for financial assistance. T. D. J. wishes to thank the University of Bath and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (2020ZD01) for support.References
- R. Li, K. Liu, X. Huang, D. Li, J. Ding, B. Liu and X. Chen, Adv. Sci., 2022, 9, 2105152 CrossRef CAS PubMed.
- (a) Y. Zhou, X. Yang, X. Wei, S. S. Zhang and M. Yan, Coord. Chem. Rev., 2024, 513, 215864 CrossRef CAS; (b) Y. Geng, Z. Wang, J. Zhou, M. Zhu, J. Liu and T. D. James, Chem. Soc. Rev., 2023, 52, 3873 RSC; (c) J. Yin, J. Zhan, Q. Hu, S. Huang and W. Lin, Chem. Soc. Rev., 2023, 52, 2011 RSC; (d) A. Kumar, J. M. George, S. Sharma, S. Koyyadi, S. K. Sharma, P. Verwilst, A. Bhatia, S. K. Sahoo, A. Aggarwal, S. Gupta, S. Sharma and A. Sharma, ACS Appl. Bio Mater., 2024, 7, 8517 CrossRef CAS PubMed; (e) Q. Ding, L. Ding, C. Xiang, C. Li, E. Kim, C. Yoon, H. Wang, M. Gu, P. Zhang, L. Wang, B. Z. Tang and J. S. Kim, Angew. Chem., Int. Ed., 2025, e202506505 Search PubMed.
- (a) C. Ma, A. H. Kesarwala, T. Eggert, J. M. Echeverz, D. E. Kleiner, P. Jin, D. F. Stroncek, M. Terabe, V. Kapoor, M. ElGindi, M. Han, A. M. Thornton, H. Zhang, M. Egger, J. Luo, D. W. Felsher, D. W. McVicar, A. Weber, M. Heikenwalder and T. F. Greten, Nature, 2016, 531, 253–257 CrossRef CAS PubMed; (b) W. Quan, W. Song, Q. Zhang, H. Huang and W. Lin, Coord. Chem. Rev., 2023, 497, 215407 CrossRef CAS; (c) Z. Chen, Y. Zhou, L. Li, W. Ma, Y. Li and Z. Yang, Small, 2024, 21, 2411787 CrossRef PubMed.
- (a) X. Yang, D. Zhang, Y. Ye and Y. Zhao, Coord. Chem. Rev., 2022, 453, 214336 CrossRef CAS; (b) J. Ma, R. Sun, K. Xia, Q. Xia, Y. Liu and X. Zhang, Chem. Rev., 2024, 124, 1738 CrossRef CAS PubMed; (c) Q. Ding, C. Wang, H. Wang, C. Xiang, Z. Wang, Y. Wang, L. Zhao, M. Vendrell and J. S. Kim, J. Am. Chem. Soc., 2025, 147, 16661–16673 CrossRef CAS PubMed.
- (a) J. Yao, M. Yang and Y. Duan, Chem. Rev., 2014, 114, 6130 CrossRef CAS PubMed; (b) L. Gao, W. Wang, X. Wang, F. Yang, L. Xie, J. Shen, M. A. Brimble, Q. Xiao and S. Q. Yao, Chem. Soc. Rev., 2021, 50, 1219 RSC; (c) C. Ding and T. Ren, Coord. Chem. Rev., 2023, 482, 215080 CrossRef CAS; (d) J. Liu, W. Zhang, C. Zhou, M. Li, X. Wang, W. Zhang, Z. Liu, L. Wu, T. D. James and P. Li, J. Am. Chem. Soc., 2022, 144, 13586 CrossRef CAS PubMed; (e) Q. Ding, X. Xu, Y. Li, B. Li, Q. Saiding, M. Gu, W. Tao, B. Z. Tang and J. S. Kim, Chem, 2024, 10, 2031 CrossRef CAS; (f) Q. Ding, X. Wang, Y. Luo, X. Leng, X. Li, M. Gu and J. S. Kim, Coord. Chem. Rev., 2024, 508, 215772 CrossRef CAS.
- (a) X. Wu, H. Li, E. Lee and J. Yoon, Chem, 2020, 6, 2893 CrossRef CAS; (b) X. Wu, R. Wang, N. Kwon, H. Ma and J. Yoon, Chem. Soc. Rev., 2022, 51, 450 RSC; (c) Z. Wu, M. Liu, Z. Liu and Y. Tian, J. Am. Chem. Soc., 2020, 142, 7532 CrossRef CAS PubMed; (d) W. Gong, L. Jiang, Y. Zhu, M. Jiang, D. Chen, Z. Jin, S. Qin, Z. Yu and Q. He, Angew. Chem., Int. Ed., 2022, 134, e202114594 CrossRef; (e) Q. Ding, Z. Zhang, M. Li, J.-H. Zhu, W. Fu, M. He, Y. Bai, Z. Zhang, S. Li, L. Wang, C. Deng, X. Hong, Y. Xiao and J. S. Kim, Cell. Biomater., 2025, 1, 100001 CrossRef; (f) Q. Ding, A. Guo, S. Zhang, C. Gu, X. Wang, X. Li, M. Gu and J. S. Kim, Biomaterials, 2025, 316, 123012 CrossRef CAS PubMed.
- (a) L. Wu, J. Huang, K. Pu and T. D. James, Nat. Rev. Chem., 2021, 5, 406–421 CrossRef CAS PubMed; (b) Z. Zeng, S. S. Liew, X. Wei and K. Pu, Angew. Chem., Int. Ed., 2021, 133, 26658 CrossRef; (c) D. Samanta, S. B. Ebrahimi and C. A. Mirkin, Adv. Mater., 2020, 32, 1901743 CrossRef CAS PubMed.
- Y. Song, H. Zhang, X. Wang, X. Geng, Y. Sun, J. Liu and Z. Li, Anal. Chem., 2020, 93, 1786 CrossRef PubMed.
- (a) W. Zhang, X. Wang, P. Li, H. Xiao, W. Zhang, H. Wang and B. Tang, Anal. Chem., 2017, 89, 6840 CrossRef CAS PubMed; (b) H. Huang, F. Dong and Y. Tian, Anal. Chem., 2016, 88, 12294 CrossRef CAS PubMed.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899 CrossRef PubMed.
- H. Xiao, W. Zhang, P. Li, W. Zhang, X. Wang and B. Tang, Angew. Chem., Int. Ed., 2020, 132, 4244 CrossRef.
- J. Liu, W. Zhang, X. Wang, Q. Ding, C. Wu, W. Zhang, L. Wu, T. D. James, P. Li and B. Tang, J. Am. Chem. Soc., 2023, 145, 19662 CrossRef CAS PubMed.
- (a) B. Chen, S. Mao, Y. Sun, L. Sun, N. Ding, C. Li and J. Zhou, Chem. Comm., 2021, 57, 4376 RSC; (b) F. Peng, X. Ai, J. Sun, L. Yang and B. Gao, Chem. Commun., 2024, 60, 2994 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc01433f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: