Open Access Article
Open Access ArticleDisentangling chemical pressure and superexchange effects in lanthanide–organic valence tautomerism†
Anton
Viborg
 a,
Maja A.
Dunstan
a,
Maja A.
Dunstan
 *a,
Nathan J.
Yutronkie
*a,
Nathan J.
Yutronkie
 b,
Amit
Chanda
b,
Amit
Chanda
 d,
Felix
Trier
d,
Felix
Trier
 d,
Nini
Pryds
d,
Fabrice
Wilhelm
b,
Andrei
Rogalev
d,
Nini
Pryds
d,
Fabrice
Wilhelm
b,
Andrei
Rogalev
 b,
Dawid
Pinkowicz
b,
Dawid
Pinkowicz
 c and
Kasper S.
Pedersen
c and
Kasper S.
Pedersen
 *a
*a
aDepartment of Chemistry, Technical University of Denmark, 2800 Kgs, Lyngby, Denmark. E-mail: kastp@kemi.dtu.dk; majdu@kemi.dtu.dk
bEuropean Synchrotron Radiation Facility, 38000 Grenoble, France
cFaculty of Chemistry, Jagiellonian University, 30-387 Kraków, Poland
dDepartment of Energy Conversion and Storage, Technical University of Denmark, 2800 Kgs, Lyngby, Denmark
First published on 7th March 2025
Abstract
Valence tautomerism in molecule-based f-block materials remains virtually elusive. As a result, the effects driving and controlling the valence conversion phenomenon are poorly understood. Herein, we unravel these fundamental factors by systematic chemical modification of a bona fide lanthanide coordination solid, SmI2(pyrazine)2(tetrahydrofuran), in which a complete, temperature-driven conversion between Sm(II) and Sm(III) occurs abruptly around 200 K. Solid solutions incorporating either divalent, diamagnetic metal ions or Sm(III) ions feature disparate behavior. Substitution with redox-inactive, divalent metal ions invariably leads to lower conversion temperatures and reduced cooperativity. In contrast, incorporation of redox-inactive Sm(III) ions leads to trapped pyrazine anion radicals in the ligand scaffold, shifting the valence tautomeric conversion phenomenon towards higher temperature with virtually no loss of cooperativity. These materials are rare examples of lanthanide–organic materials hosting mixed valency in both the lanthanide and organic scaffold, affording switchable conductivity associated with the valence tautomeric conversion.
Introduction
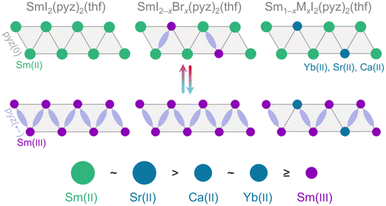
Materials with intrinsic switchability of physical properties are receiving increasing attention due to their potential applications in future electronics,1 catalysis,2 and sensing.3–5 A given material can convert between states through a range of different mechanisms. One mechanism for switching is stimulated electron transfer between a metal ion and an organic ligand, leading to two (or more) valence tautomers existing in equilibrium.6,7 The conversion between the valence tautomers may be actuated by temperature alterations through the competition of changes in enthalpy (ΔH) and entropy (ΔS). In transition metal chemistry, the thermodynamics of valence tautomeric (VT) conversions have been studied extensively since their discovery in 1980,8 and design principles for these materials have been developed.9–11 In contrast, for the f-block elements, this level of understanding has not been achieved. Temperature-driven valence changes in lanthanide compounds date back more than a hundred years with their observation in elemental Ce, wherein the electron transfer is instead a promotion of a localized 4f electron to the s–d conduction band.12–14 This behavior was later found to be paralleled in several inorganic solids, such as SmS and Sm2.75C60, which, however, seldom attain the limiting di- or trivalent states but reside in intermediate oxidation states at ambient pressure.15,16 As such, molecular materials remain conceptually different from these purely inorganic f-block compounds. We recently reported a unique example of a lanthanide-based coordination solid, SmI2(pyz)3 (pyz = pyrazine), exhibiting a quantitative VT transition between integer oxidation states.17 At high temperature, Sm(II) is present, but below ∼190 K, Sm(II) reversibly and cooperatively transfers a single electron to a bridging pyz ligand yielding a Sm(III)-pyz(·−) tautomer (Fig. 1). This abrupt VT conversion is accompanied by both a structural change in the first coordination sphere and a significant change in the magnetic moment. Additionally, the VT event can be pushed to lower temperature by substituting Yb(II) into the material, which are trapped in the Yb(II)-pyz(0) state at all temperatures. The Yb(II) impurities compromise the cooperativity and exert chemical pressure which destabilizes the low temperature phase and thus decreases the VT conversion temperature. Herein, we provide new synthetic guidelines for these unique materials by uncovering the size dependence of divalent impurities and demonstrating how persistent Sm(III)-pyz(·−) impurities shift the VT transition towards room temperature while fully conserving cooperativity.Results and discussion
Synthesis and structural characterisation
The occurrence of the VT event is fundamentally related to the reduction potential of the metal ion node. This, in turn, can be modified by the ancillary ligands, as illustrated by SmI2 (E1/2 = −0.89 V vs. SCE) and SmBr2 (E1/2 = −1.6 V) in tetrahydrofuran (thf).18We attempted to synthesize SmBr2(pyz)3 analogously to SmI2(pyz)3 by addition of SmBr2(thf)2 in acetonitrile to molten pyrazine, which was unsuccessful due to the poor solubility of the starting material. By performing the reaction in thf or thf/dimethoxyethane, both SmBr2(pyz)2(thf) and SmI2(pyz)2(thf) could be obtained. For SmI2(pyz)2(thf) (0%, Fig. 2a), single crystal X-ray diffraction (SCXRD) reveals the compound to be structurally analogous to the parent SmI2(pyz)3.17 Upon cooling from 230 K to 170 K, the average Sm–I and Sm–N bond lengths shrink significantly by 3% and 6%, respectively, accompanied by an 8% deviation of the ∠I–Sm–I bond angle from linearity (Table S1†). These structural changes suggest a Sm(II)-to-Sm(III) valence change, akin to that observed in SmI2(pyz)3. Furthermore, inspection of the pyz bond lengths suggests the presence of zig-zag chains of pyz(·−) radicals (Fig. 1).19,20 Single crystals of SmBr2(pyz)2(thf) (100%) suitable for SCXRD were not obtained, and the structure was determined by Rietveld refinement of room temperature powder X-ray diffraction (PXRD) data (Fig. 3). 100% is isomorphous to the low temperature (LT) phase of 0% (Fig. 2bc and S1†). The average Sm–N (2.52 Å) bond length in 100% is similar to that found in LT-0% (2.55 Å), suggesting the presence of the Sm(III)-pyz(·−) tautomer at room temperature in 100%.
Characterisation of the valence conversion
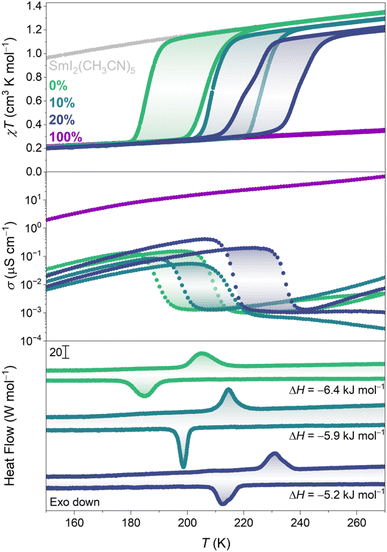
The temperature dependence of the magnetic susceptibility–temperature product (χT) of 0% and 100% is presented in Fig. 4. 0% has a χT value of 1.35 cm3 K mol−1 at room temperature, as expected for Sm(II).21 Around , χT rapidly decreases before it plateaus, reaching a value of ∼0.05 cm3 K mol−1 at 3 K (Fig. S15†). Ignoring the finite magnetic moment of Sm(III) at room temperature,22 the χT product of LT-0% can be modelled as an infinite chain of antiferromagnetically coupled pyz(·−), yielding J ≈ −75 cm−1 (Fig. S14†), as a lower bound of the actual pyz(·−)⋯pyz(·−) superexchange coupling constant.17,23 On heating, the reverse conversion occurs at
, χT rapidly decreases before it plateaus, reaching a value of ∼0.05 cm3 K mol−1 at 3 K (Fig. S15†). Ignoring the finite magnetic moment of Sm(III) at room temperature,22 the χT product of LT-0% can be modelled as an infinite chain of antiferromagnetically coupled pyz(·−), yielding J ≈ −75 cm−1 (Fig. S14†), as a lower bound of the actual pyz(·−)⋯pyz(·−) superexchange coupling constant.17,23 On heating, the reverse conversion occurs at  , behaving almost identically to SmI2(pyz)3 (Fig. S13†). This observation is substantiated by the fact that the VT conversion does not involve significant change to the Sm–N bond of the exo-chain pyrazine in SmI2(pyz)3. This contrasts with the sensitivity of the other reported molecular lanthanide compounds, where minute changes in the local coordination sphere are detrimental to the VT event.24,25 In contrast, 100% shows no signs of conversion up to the decomposition temperature (∼420 K, Fig. S20†), with a magnetic profile up to 400 K corresponding to the Sm(III)-pyz(·−) tautomer.
, behaving almost identically to SmI2(pyz)3 (Fig. S13†). This observation is substantiated by the fact that the VT conversion does not involve significant change to the Sm–N bond of the exo-chain pyrazine in SmI2(pyz)3. This contrasts with the sensitivity of the other reported molecular lanthanide compounds, where minute changes in the local coordination sphere are detrimental to the VT event.24,25 In contrast, 100% shows no signs of conversion up to the decomposition temperature (∼420 K, Fig. S20†), with a magnetic profile up to 400 K corresponding to the Sm(III)-pyz(·−) tautomer.
Kahn's notion of molecular alloying elucidated that a solid solution of two spin crossover (SCO) complexes assumes a SCO conversion temperature in between those of the pristine phases.26–28 As no VT conversion is observed in 100% below the decomposition temperature, we adopt Kahn's approach and hypothesize that alloying 0% with 100% shifts the VT conversion towards room temperature. The addition of a solution containing both SmI2 and SmBr2(thf)2 to molten pyrazine leads to a single polycrystalline phase (Fig. 3, S4–S7†) for molar percentages of SmBr2(thf)2 up to 20% (SmI1.8Br0.2(pyz)2(thf), 10% and SmI1.6Br0.4(pyz)2(thf), 20%). Analysis of the powder X-ray diffractograms reveals that 10% and 20% are isomorphous to HT-0% at room temperature. Attempts to incorporate more than 20% Br invariably led to isolation of a mixture of two crystallographic phases (isomorphous to 0% and 100%), as observed by PXRD (Fig. S8†).
For 0%, 10%, and 20%, the unit cell volume contracts with increasing Br content (Table S4†). Br and I elemental analysis of 10% and 20% confirms the composition of each alloy (cf. Experimental section, ESI†). The temperature dependence of the χT product of 10% reveals an upward shift in  by 21 K and a slightly smaller hysteresis width (18 K, Fig. 4, S15 and S18†). This shift is accompanied by a reduction in the χT product of the HT phase compared to 0% (vide infra). In 20%, T* is shifted an additional 13 K with conserved hysteresis width (19 K, Fig. S16 and S19†). Additionally, the VT conversion shows a slight step at approximately 50% conversion (Fig. S17†) and a further reduction in the magnetic moment of the HT phase.
by 21 K and a slightly smaller hysteresis width (18 K, Fig. 4, S15 and S18†). This shift is accompanied by a reduction in the χT product of the HT phase compared to 0% (vide infra). In 20%, T* is shifted an additional 13 K with conserved hysteresis width (19 K, Fig. S16 and S19†). Additionally, the VT conversion shows a slight step at approximately 50% conversion (Fig. S17†) and a further reduction in the magnetic moment of the HT phase.
The progressive drop in the magnetic moment of the HT phases casts ambiguity on the Sm oxidation state at these temperatures. The room temperature and 50 K Sm L2-edge X-ray absorption near edge structure (XANES) spectra (Fig. 5 and S21†) of 100% are identical, corroborating the conclusion that only Sm(III) is present at all temperatures. Conversely, in HT-0%, at room temperature, the dipole-allowed 2s → 5d, 6s ‘white line’ is red-shifted by 7.6 eV from that of 100%, consistent with a Sm(II) oxidation state (Fig. 5 and S24†). At 50 K, the spectrum becomes virtually identical to that of 100%, with no indication of residual Sm(II). In stark contrast, 10% and 20% show a primary feature at the Sm(II) white line photon energy, with an additional minor feature at the energy corresponding to Sm(III). The progressive increase in intensity of the Sm(III) peak with increasing Br content is paralleled by a decrease in the intensity of the Sm(II) peak. At 50 K, however, the spectra overlap with 100%, demonstrating complete conversion to Sm(III). Upon reheating, the room temperature spectra are recovered, suggesting full reversibility. Notably, the room temperature spectra can be reproduced by a linear combination of the room temperature spectra of 0% and 100% with relative weights corresponding to the Br content (Fig. S22 and S23†).
While the thermodynamics of both the transition metal spin crossover and VT phenomena have been studied in detail, there are no experimental reports on the enthalpy and entropy changes associated with VT conversions in molecule-based lanthanide compounds. The differential scanning calorimetry (DSC) data of 0%, 10%, and 20% are shown in Fig. 4 (Fig. S26†). The phase transitions observed by DSC correspond to the conversions observed by magnetometry. For 0%, the enthalpy change was determined to be −6.4 kJ mol−1, which is comparable to spin crossover complexes.29,30 The values of ΔH for VT conversions in transition metal compounds are similar.31 ΔH for 10% and 20% decreases to 5.9 kJ mol−1 and 5.2 kJ mol−1, respectively, which constitutes reductions by 8% and 19%, consistent with the decrease in I content (Table S6†).
Local coordination environments
The analyses of the XANES spectra and the DSC data independently point towards ∼10% and ∼20% of the Sm centers in 10% and 20%, respectively, not undergoing a VT conversion. The alloyed phases could contain {SmI2}, {SmBr2}, or {SmIBr} nodes. To ascertain the actual composition of the alloys, we consider a fully random statistical mixture. In the case of 20%, this gives a distribution of 64% {SmI2}, 4% {SmBr2}, and 32% {SmIBr}. We assume, based on the experimental findings in 0% and 100%, that {SmI2} and {SmBr2} exist solely as Sm(II) and Sm(III) at room temperature. If mixed {SmIBr} nodes contain Sm(II), the ratio of Sm(II) to Sm(III) is 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Conversely, if they contain Sm(III) the ratio is 64
4. Conversely, if they contain Sm(III) the ratio is 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36. Both of these ratios are incompatible with the relative Sm(II) and Sm(III) peak intensities of 20% found by XANES spectroscopy. The fully analogous situation is observed in 10%. The experimental data, therefore, point towards conserved {SmI2} and {SmBr2} nodes, with no evidence of {SmIBr}. Further evidence for this conclusion is provided by the analysis of the aforementioned reductions in the χT product of 10% and 20% relative to 0% at 270 K, which reveal approximately 14% Sm(III) in 20% and 6% in 10%.
36. Both of these ratios are incompatible with the relative Sm(II) and Sm(III) peak intensities of 20% found by XANES spectroscopy. The fully analogous situation is observed in 10%. The experimental data, therefore, point towards conserved {SmI2} and {SmBr2} nodes, with no evidence of {SmIBr}. Further evidence for this conclusion is provided by the analysis of the aforementioned reductions in the χT product of 10% and 20% relative to 0% at 270 K, which reveal approximately 14% Sm(III) in 20% and 6% in 10%.
Electrical conductivity
Not only do 0%, 10%, 20% and 100% show mixed valency in the ligand scaffold, but 10% and 20% are also rare examples of compounds featuring lanthanide ions present in different oxidation states.32–34 Mixed valency in ligand35–37 and/or metal ion scaffolds38–40 has been shown to be at the origin of high electrical conductivity in transition metal-based coordination solids. The 270 K-conductivity of 100% amounts to ∼0.15 mS cm−1 (Fig. 4 and S25†) and decreases to reach ∼20 μS cm−1 at 67 K, comparable to many conductive transition metal coordination polymers.41,42 In contrast, 0% exhibits conductivity three to four orders of magnitude lower at room temperature (∼1 nS cm−1), which increases sharply at and assumes a temperature dependence similar to that of 100%. A similar abrupt change in conductivity linked to the VT conversion is observed for 10% and 20% at the corresponding T*s. The behavior of the Sm(III)-pyz(·−) phases is comparable to that of some recent lanthanide-based metal–organic frameworks, which show conductivity in the range of ∼90 μS cm−1 to 50 mS cm−1.43,44
and assumes a temperature dependence similar to that of 100%. A similar abrupt change in conductivity linked to the VT conversion is observed for 10% and 20% at the corresponding T*s. The behavior of the Sm(III)-pyz(·−) phases is comparable to that of some recent lanthanide-based metal–organic frameworks, which show conductivity in the range of ∼90 μS cm−1 to 50 mS cm−1.43,44
M(II) alloys
The substitution of {SmI2} nodes with {SmBr2} nodes containing a trapped Sm(III) oxidation state has the opposite effect to that of substituting {YbI2} nodes into SmI2(pyz)3, where the VT conversion decreases in temperature and loses cooperativity. The effective size, reflected by the average metal–N distance, of Sm(III) in a {SmBr2} node (2.52(1) Å) is 4% smaller than that of Yb(II) in a {YbI2} node (2.63(2) Å;17 Table S3†). In contrast to Yb(II), which has an intermediate size to that of Sm(II) (2.75(1) Å in HT-0%) and Sm(III) (2.55(1) Å in LT-0%) in a {SmI2} node, the Sm(III) ion in a {SmBr2} node is smaller than both. To further elucidate the apparent strong size-dependence of the dopant ion on T*, the analogous SrI2(pyz)2(thf) (Sr-100%) and CaI2(pyz)2(thf) (Ca-100%) were synthesized from SrI2 and CaI2 (Fig. S2 and S3†). The effective sizes of Sr(II) and Ca(II) are again inferred by the metal–N bond lengths of 2.77(1) Å and 2.66(1) Å, respectively. Ca(II) is virtually identical in size to Yb(II), while Sr(II) is identical in size to Sm(II). The isomorphism of Sr-100% and Ca-100% to 0% allows the substitution of Sm(II) in 0% by Sr(II) and Ca(II), in phases entitled Ca-10%, Ca-20%, Sr-7.5%, and Sr-15% (Tables S2, S5, S6, Fig. S9–S12†).The temperature dependences of the χT products for the Sr(II) and Ca(II) alloyed phases are shown in Fig. 6. All M(II) alloys display a shift of T* towards lower temperatures. While the Ca(II) impurity concentration dependence of T* mirrors that of Yb(II), as expected for the similar effective volume, the Sr(II) substitution has a more pronounced concentration dependence on T*. In Sr-7.5% and Sr-15%, the larger Sr(II) ions stabilize the HT Sm(II) phase and push T* towards lower temperatures. Ca(II) and Yb(II), having effective sizes between those of Sm(II) and Sm(III) also stabilize the HT Sm(II) phase. The increasing substitution percentage of Sr(II) and Ca(II) is accompanied by a gradual loss of cooperativity, as reflected in the loss of abruptness of the VT conversion and a narrowing of the thermal hysteresis, due to the increased concentration of non-participating impurities (Fig. 1). In contrast, the substitution of Sm(II) with Sm(III) obtained upon introduction of {SmBr2} nodes is fundamentally different. The effective size of Sm(III) in {SmBr2} is identical to that of Sm(III) found in the {SmI2} nodes in the LT-0% phase. Thus, following the same argument as above, the presence of valence trapped Sm(III) substituents will destabilize the HT phase and push T* towards higher temperatures. More remarkably, the alloying with Sm(III) nodes is accompanied by only a minute loss in cooperativity, even at a 20% substitution level, diametrically opposed to the alloys featuring divalent substituents, where the hysteresis is fully closed at such impurity concentrations.
 | ||
| Fig. 6 Magnetic susceptibility–temperature product of 0%, Ca-10%, and Ca-20% (top) and of 0%, Sr-7.5%, and Sr-15% (bottom). | ||
Effects of superexchange
Despite the conceptual resemblance of the observed behavior of the {SmI2}/{SmBr2} alloys to SCO materials, it is rare for metal ion substitution in SCO materials to maintain the cooperativity of the parent compound.45,46 However, the VT phenomenon in a polymeric network presents an important difference. The introduction of Sm(III) is unavoidably accompanied by the introduction of valence trapped pyz(·−) along the chain direction. Therefore, the charge distribution around an Sm(II) situated next to a valence-trapped Sm(III) resembles that of an Sm(II) in the pristine phase facing the domain wall of a cooperative VT conversion. Additionally, given the strong pyz(·−)⋯pyz(·−) superexchange coupling, previously predicted by DFT calculations (J = −750 cm−1), the associated energy of ∼|2JS2| = 4.5 kJ mol−1 is similar to that of the VT conversion, ΔH = −6.4 kJ mol−1. The formation of pyz(·−)⋯pyz(·−) superexchange pathways may, therefore, be hypothesized to drive the VT conversion. The majority of coordination networks displaying a cooperative VT conversion feature only weak magnetic exchange coupling, so at this moment, further confirmation of this hypothesis is precluded. There is some evidence from computational studies of Fe(II) SCO coordination polymers that magnetic exchange coupling in the high-spin phase could be an important factor for the observed cooperativity, in addition to the elastic interactions.47 In the case of the strong superexchange interactions presented here, we believe the effect cannot be neglected. This is consistent with the observation of reduced cooperativity when diamagnetic M(II) impurities are incorporated into the LT phases, whereas alloying with {SmBr2} nodes does not affect the antiferromagnetically coupled radical chains in the LT phases, leading to conservation of the cooperativity and hysteresis width.Conclusions
We find the novel phenomenon of valence tautomerism in molecule-based lanthanide materials to be robust and tunable in terms of conversion temperature and cooperativity. The enthalpy of the VT conversion is found to be comparable to that of transition metal analogues and SCO materials, although the underlying mechanism remains fundamentally different. The reversible charge injection into the organic scaffold results in increased electrical conductivity and the opening of strong superexchange channels, whose energy is comparable to that of the VT conversion enthalpy and thereby conserves the cooperativity in the alloyed phases. Such chemical tailoring of the local charge distribution to control the VT event is unprecedented despite theoretical predictions of strong sensitivity to applied electric fields in transition metal-based VT materials.48Data availability
The data supporting this article have been included as part of the ESI.† Deposition numbers 2420160–2420164 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via the joint Cambridge Crystallographic Data Centre (CCDC) and Fachinformationszentrum Karlsruhe Access Structures service. X-ray spectroscopy was performed at the European Synchrotron Radiation Facility at beamline ID12 and data are available at DOI: https://doi.org/10.15151/ESRF-ES-1435808919.Author contributions
K. S. P., M. A. D. and A. V. conceived and planned the research project. A. V. and M. A. D. developed and performed the synthesis and the crystallographic analysis. M. A. D., K. S. P. and A. V. performed the characterization of the magnetic properties. N. J. Y., F. W. and A. R. acquired and analysed the X-ray spectroscopic data. M. A. D., A. C., F. T., N. P., and A. V. acquired and analyzed the resistivity data. D. P. acquired and analysed the differential scanning calorimetry data. The manuscript was written by K. S. P., M. A. D. and A. V. with input from all coauthors. All authors have given their consent to the publication of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Anna S. Manvell for helpful discussions. The X-ray spectroscopy experiments were performed at the ID12 beamline of the European Synchrotron Radiation Facility (Grenoble, France). We thank the Danish Agency for Science, Technology, and Innovation for funding the instrument center Danscatt. K. S. P. thanks the VILLUM Foundation for a VILLUM Young Investigator+ (42094) grant, the Independent Research Fund Denmark for a DFF-Sapere Aude Starting grant (no. 0165-00073B), and the Carlsberg Foundation for a research infrastructure grant (no. CF17-0637). A. C. and F. T. acknowledge support by research grant 37338 (SANSIT) from the VILLUM Foundation. D. P. acknowledges the financial support of the National Science Centre Poland within the Opus project 2020/37/B/ST5/02735.Notes and references
- B. Lowe, B. Field, J. Hellerstedt, J. Ceddia, H. L. Nourse, B. J. Powell, N. V. Medhekar and A. Schiffrin, Nat. Commun., 2024, 15, 3559 CrossRef CAS PubMed
.
- N. Das and C. Maity, Commun. Chem., 2022, 5, 115 CrossRef CAS PubMed
.
- W. Lee, D. Kim, S. Lee, J. Park, S. Oh, G. Kim, J. Lim and J. Kim, Nano Today, 2018, 23, 97–123 CrossRef CAS
.
- O. Sato, Nat. Chem., 2016, 8, 644–656 CrossRef CAS PubMed
.
- O. Sato, J. Tao and Y.-Z. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2152–2187 Search PubMed
.
- T. Tezgerevska, K. G. Alley and C. Boskovic, Coord. Chem. Rev., 2014, 268, 23–40 CrossRef CAS
.
- M. A. Dunstan and K. S. Pedersen, Chem. Commun., 2025, 61, 627–638 Search PubMed
.
- R. M. Buchanan and C. G. Pierpont, J. Am. Chem. Soc., 1980, 102, 4951–4957 CrossRef CAS
.
- E. Evangelio and D. Ruiz-Molina, Eur. J. Inorg. Chem., 2005, 15, 2957–2971 CrossRef
.
- G. K. Gransbury, M. E. Boulon, S. Petrie, R. W. Gable, R. J. Mulder, L. Sorace, R. Stranger and C. Boskovic, Inorg. Chem., 2019, 58, 4230–4243 CrossRef CAS PubMed
.
- G. K. Gransbury, B. N. Livesay, J. T. Janetzki, M. A. Hay, R. W. Gable, M. P. Shores, A. Starikova and C. Boskovic, J. Am. Chem. Soc., 2020, 142, 10692–10704 CrossRef CAS PubMed
.
- M. Owen, Ann. Phys., 1912, 37, 657 CrossRef CAS
.
- J. M. Robinson, Phys. Rep., 1979, 51, 1–62 CrossRef CAS
.
- J. M. Lawrence and R. D. Parks, J. Phys. Colloq., 1976, 37, 249–253 CrossRef
.
-
M. Loewenhaupt and K. H. Fischer, in Handbook on the Physics and Chemistry of Rare earths, ed. K. A. Gschneider Jr and L. Eyring, Elsevier, Amsterdam, 1993, ch. 1, vol. 16, pp. 1–105 Search PubMed
.
- J. Arvanitidis, K. Papagelis, S. Margadonna, K. Prassides and A. N. Fitch, Nature, 2003, 425, 599–602 CrossRef CAS PubMed
.
- M. A. Dunstan, A. S. Manvell, N. J. Yutronkie, F. Aribot, J. Bendix, A. Rogalev and K. S. Pedersen, Nat. Chem., 2024, 16, 735–740 CrossRef CAS PubMed
.
- M. Szostak, M. Spain and D. J. Procter, J. Org. Chem., 2014, 79, 2522–2537 CrossRef CAS PubMed
.
- W. Kaim, Angew. Chem., Int. Ed., 1983, 22, 171–190 Search PubMed
.
- N. Bajaj, N. Mavragani, A. A. Kitos, D. Chartrand, T. Maris, A. Mansikkamäki and M. Murugesu, Chem, 2024, 10, 2484–2499 Search PubMed
.
- M. Kubus, L. Voigt and K. S. Pedersen, Inorg. Chem. Commun., 2020, 114, 107819 Search PubMed
.
-
L. Sorace and D. Gatteschi, in Lanthanides and Actinides in Molecular Magnetism, ed. R. A. Layfield and M. Murugesu, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Germany, 2015, ch. 1, pp. 1–26 Search PubMed
.
- J. C. Bonner and M. E. Fisher, Phys. Rev., 1964, 135, A640 CrossRef
.
- I. L. Fedushkin, O. V. Maslova, A. G. Morozov, S. Dechert, S. Demeshko and F. Meyer, Angew. Chem., Int. Ed., 2012, 51, 10584–10587 CrossRef CAS PubMed
.
- D. A. Lukina, A. A. Skatova, R. V. Rumyantcev, S. V. Demeshko, F. Meyer and I. L. Fedushkin, Dalton Trans., 2024, 53, 8850–8856 RSC
.
- O. Kahn and J. Kröber, Adv. Mater., 1992, 4, 718–728 CrossRef CAS
.
- J. Kröber, E. Codjovi, O. Kahn, F. Grolière and C. Jay, J. Am. Chem. Soc., 1993, 115, 9810–9811 CrossRef
.
- Y.-Y. Wu, Z.-Y. Li, S. Peng, Z.-Y. Zhang, H.-M. Cheng, H. Su, W.-Q. Hou, F.-L. Yang, S.-Q. Wu, O. Sato, J.-W. Dai, W. Li and X.-H. Bu, J. Am. Chem. Soc., 2024, 146, 8206–8215 CrossRef CAS PubMed
.
- K. P. Kepp, Inorg. Chem., 2016, 55(6), 2717–2727 CrossRef CAS PubMed
.
- S. Vela, M. Fumanal, J. Cirera and J. Ribas-Arino, Phys. Chem. Chem. Phys., 2020, 22, 4938–4945 RSC
.
-
D. A. Schultz, in Magnetism: Molecules to materials II: Models and Experiments, ed. J. S. Miller and M. Drillon, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Germany, 2001, ch. 8, pp. 281–306 Search PubMed
.
- C. A. Gould, K. R. McClain, D. Reta, J. G. Kragskow, D. A. Marchiori, E. Lachman, E.-S. Choi, J. G. Analytis, R. D. Britt, N. F. Chilton, B. G. Harvey and J. R. Long, Science, 2022, 375, 198–202 CrossRef CAS PubMed
.
- M. D. Roy, T. P. Gompa, S. M. Greer, N. Jiang, L. S. Nassar, A. Steiner, J. Basca, B. W. Stein and H. S. La Pierre, J. Am. Chem. Soc., 2024, 146, 5560–5568 CrossRef CAS PubMed
.
- T. J. Obey, M. K. Singh, A. B. Canaj, G. S. Nichol, E. K. Brechin and J. B. Love, J. Am. Chem. Soc., 2024, 146, 28658–28662 CrossRef CAS PubMed
.
- K. S. Pedersen, P. Perlepe, M. L. Aubrey, D. N. Woodruff, S. E. Reyes-Lillo, A. Reinholdt, L. Voigt, Z. Li, K. Borup, M. Rouzières, D. Samohvalov, F. Wilhelm, A. Rogalev, J. B. Noeaton, J. R. Long and R. Clérac, Nat. Chem., 2018, 10, 1056–1061 CrossRef CAS PubMed
.
- P. Perlepe, I. Oyarzabal, A. Mailman, M. Yquel, M. Platunov, I. Dovgaliuk, M. Rouzières, P. Négrier, D. Mondieig, E. A. Suturina, M.-A. Dourges, S. Bonhommeau, R. A. Musgrave, K. S. Pedersen, D. Chernyshov, F. Wilhelm, A. Rogalev, C. Mathonière and R. Clérac, Science, 2020, 370, 587–592 CrossRef CAS PubMed
.
- F. Aribot, L. Voigt, M. A. Dunstan, W. Wan, J. N. McPherson, M. Kubus, N. J. Yutronkie, C. J. McMonagle, M. Coletta, A. S. Manvell, A. Viborg, S. Wong, K. A. Stampe, V. Baran, A. Senyshyn, M. D. Le, H. C. Walker, A. Chanda, F. Trier, N. Pryds, F. Wilhelm, M. R. Probert, N. B. Christensen, E. K. Brechin, A. Rogalev and K. S. Pedersen, ChemRxiv, 2024 DOI:10.26434/chemrxiv-2024-rlsf8
.
- L. E. Darago, M. L. Aubrey, C. J. Yu, M. I. Gonzalez and J. R. Long, J. Am. Chem. Soc., 2015, 137, 15703–15711 Search PubMed
.
- J. A. DeGayner, I. R. Jeon, L. Sun, M. Dincă and T. D. Harris, J. Am. Chem. Soc., 2017, 139, 4175–4184 Search PubMed
.
- R. Murase, C. F. Leong and D. M. D'Alessandro, Inorg. Chem., 2017, 56, 14373–14382 CrossRef CAS PubMed
.
- M. Wang, Z.-Y. Li, R. Ishikawa and M. Yamashita, Coord. Chem. Rev., 2021, 435, 213819 Search PubMed
.
- G. Givaja, P. Amo-Ochoa, C. J. Gómez-García and F. Zamora, Chem. Soc. Rev., 2012, 41, 115–147 Search PubMed
.
- G. Skorupskii, B. A. Trump, T. W. Kasel, C. M. Brown, C. H. Hendon and M. Dincă, Nat. Chem., 2020, 12, 131–136 CrossRef CAS PubMed
.
- F. Manna, M. Oggianu, P. Auban-Senzier, G. Novitchi, E. Canadell, M. L. Mercuri and N. Avarvari, Chem. Sci., 2024, 15, 19247–19263 RSC
.
- A. Moneo-Corcuera, D. Nieto-Castro, J. Cirera, V. Gómez, J. Sanjosé-Orduna, C. Casadevall, G. Molnár, A. Bousseksou, T. Parella, J. M. Martínez-Agudo, J. Lloret-Fillol, M. H. Pérez-Temprano, E. Ruiz and J. R. Galán-Mascarós, Chem, 2023, 9, 377–393 CAS
.
- M. A. Halcrow, Dalton Trans., 2024, 53, 13694 RSC
.
- H. Banerjee, M. Kumar and T. Saha-Dasgupta, Phys. Rev. B:Condens. Matter Mater. Phys., 2014, 90, 174433 CrossRef
.
- A. Droghetti and S. Sanvito, Phys. Rev. Lett., 2011, 107, 047201 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, experimental procedures, single crystal X-ray diffraction data, magnetic susceptibility and magnetization, PXRD and Rietveld refinement, electrical conductivity and differential scanning calorimetry. CCDC 2420160–2420164. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01246e |
| This journal is © The Royal Society of Chemistry 2025 |