Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Coordination-driven assembly of a ferrocene-functionalized lead iodide framework with enhanced stability and charge transfer for photocatalytic CO2-to-CH3OH conversion†
Jinlin
Yin‡
,
Yani
He‡
,
Chen
Sun
,
Yilin
Jiang
and
Honghan
Fei
 *
*
Shanghai Key Laboratory of Chemical Assessment and Sustainability, School of Chemical Science and Engineering, Tongji University, 1239 Siping Rd., Shanghai 200092, China. E-mail: fei@tongji.edu.cn
First published on 31st March 2025
Abstract
Hybrid lead halides are promising photocatalysts due to their high structural tunability and excellent photophysical properties, but their ionic structures suffer from instability in polar environments and suppressed charge transfer between lead halide units and organic components. Herein, we successfully incorporated a ferrocene-based light-harvesting antenna into a lead iodide framework by coordination-driven assembly. The π-conjugated Pb2+-carboxylate linkage affords synergistic interactions between [Pb2I2]2+ chains and ferrocene linkers, achieving broad visible absorption up to 612.7 nm and efficient ligand-to-metal charge transfer for spatial charge separation. This ultrastable framework combines strong visible-light absorption of ferrocene centers with excellent charge transport of lead halide units, achieving 6e− CO2 photoreduction to CH3OH coupled with ethanol oxidation. Mechanistic studies reveal that ferrocene photoexcitation followed by linker-to-metal charge transfer significantly enhances carrier accumulation, accelerating CH3O* intermediate formation as indicated by in situ spectroscopy and theoretical calculations.
Introduction
Chemical reduction of CO2 to methanol (CH3OH) is considered as the basis of the “methanol economy”, which is thermodynamically challenging and requires high energy input under industrially relevant conditions (e.g. high temperature and pressure).1–3 Mimicking the natural photosynthesis process, photocatalytic CO2 reduction is one of the promising approaches for realizing a carbon neutral methanol economy.4–6 However, selectivity control in CO2 photoreduction is extremely challenging, because the kinetically favorable 2e− reduction products (i.e. CO and HCOOH) often have high energy requirements for further protonation.7–12 Compared to other C1 products, CH3OH is more demanding owing to its liquid state for easy storage and transportation as well as its role as a precursor to many commodity chemicals and biodiesel.13–15 Photocatalytic reduction of CO2 to CH3OH requires the simultaneous transfer of 6e− and 6H+ to the semiconductor surface, suppressing the undesired electron–hole annihilation.5,16 Therefore, it is of key importance to improve carrier accumulation on the semiconductor to realize CO2-to-CH3OH transformation, and only a few studies have reported high selectivity using environmentally friendly reductants.5,17,18 For example, carbon nitride (CN) decorated with carbon dots (mCD) produces stoichiometric oxygen and CH3OH from water and CO2, which is largely ascribed to mCD extracting holes from CN and preventing the surface adsorption of CH3OH.16Lead halide hybrids are an emerging class of photocatalysts, spanning a variety of reactions including hydrogen production, CO2 reduction and organic transformation.19–26 This class of semiconductors exhibits intriguing photophysical properties, such as tunable bandgaps, high light-absorption coefficients and excellent carrier transport characteristics.27–29 However, the vast majority of them are susceptible to degradation upon exposure to high-polarity protic molecules, ascribed to their soft ionically bound lattices.30,31 Up to now, their photocatalytic applications in CO2 reduction are largely confined to low-polarity chemical conditions and limited to non-protic reducing products (e.g. CO and CH4).32
Our group focuses on the synthesis of lead halide frameworks based on carboxylate ligands as the organic component.33 Unlike conventional hybrid halides featuring ionic structures, our lead halide frameworks exhibit remarkable structural stability with excellent photophysical properties.33–39 For example, adipate-intercalated layered lead halide frameworks ([Pb2X2]2+[−O2C(CH2)4CO2−], X = F−/Cl−/Br−/I−) exhibit high chemical stability in aqueous solutions across a wide range of pH levels, as well as under aqueous boiling conditions.33 This synthetic strategy enables lead halide frameworks to achieve CO2 photoreduction to CO using water vapor as well as photocatalytic overall water splitting without any sacrificial agent.40,41 However, their photocatalytic efficiency remains limited due to the spatial separation of lead halide units by photochemically “inert” organocarboxylates, which often restricts visible-light absorption.40,41 Although unexplored in the literature, the integration of light-harvesting ferrocene-based linkers into lead iodide frameworks will be a promising photocatalysis platform, offering both strong photon absorption and enhanced carrier accumulation.
Ferrocene (Fc)-functionalized moieties exhibit outstanding visible-light absorption and charge transfer characteristics, making them excellent organometallic components in optoelectronic applications.42,43 However, there have been very few reports on Fc-functionalized lead halide hybrids, and the ionic bonding between lead halide units and Fc ligands hinders intrinsic electron transfer and effective charge separation for photocatalysis.44,45 Recently, Dong and colleagues demonstrated that grafting ferrocenecarboxylic acid (FCA) onto CsPbBr3 quantum dots substantially enhances photocatalytic efficiency for CO2-to-CO conversion, facilitating hot electron transfer from the FCA to the quantum dots.46 This improvement is attributed to the coordination bonds formed between these two components.46 However, the coordination-driven assembly of Fc-functionalized moieties and lead halide sublattices to afford an intrinsic framework-type structure remains an outstanding challenge, representing a significant advance for improving both light absorption and charge transfer in lead halide photocatalysis.
Herein, we report a robust lead iodide framework possessing 1D linear [Pb2I2]2+ chains, which are bridged by [Pb2O2] clusters into inorganic lamellar units. The inorganic sheets are coordinatively decorated with interlamellar 1,1′-ferrocene-dicarboxylates (Fcdc2−) as light-harvesting chromophores, stacked by van der Waals interactions. In contrast to Fc-based perovskite ((FMTMA)PbI3) with non-covalent interactions between the Fc moieties and lead halide building blocks, the formation of π-conjugated Pb2+-carboxylate coordination in our framework results in enhanced carrier delocalization and carrier transport. The material exhibits a broad visible-light wavelength coverage with an indirect semiconductive bandgap of ∼1.92 eV and efficient photoinduced charge transfer from Fcdc2− to the 1D [Pb2I2]2+ chains. The favorable ligand-to-metal charge transfer (LMCT) process greatly improves photogenerated charge separation, as thoroughly investigated by surface photovoltage spectroscopy, Hall effect measurement and density functional theory (DFT) calculations. Combining the ultrastable nature and the intriguing carrier dynamics, the lead iodide framework achieves photoreduction of CO2 to CH3OH with exceptionally high selectivity, by using ethanol (EtOH) as an environmentally benign electron donor and solvent.
Results and discussion
Synthesis and crystal structures
Conventional Fc-functionalized lead iodide perovskite, (FMTMA)PbI3 (FMTMA = ferrocenylmethyltrimethylammonium), was synthesized following a previously established procedure.45 This compound exhibits a typical ABX3-type perovskite structure, consisting of a hexagonal arrangement of infinite linear chains of face-sharing PbI6 octahedra along the c axis, with the FMTMA cations separating the chains through non-covalent electrostatic interactions (Fig. 1 top). In contrast, our ferrocene-functionalized lead iodide framework was synthesized by solvothermal reactions between PbI2 and 1,1′-ferrocenedicarboxylic acid (H2Fcdc) in DMF/EtOH, forming reddish-brown platelike crystals of [Pb2I2]2+[PbO][Fcdc2−], (TJU-26, TJU = Tongji University) (Fig. 1 bottom and S1†). X-ray crystallography reveals that the framework consists of an array of unidimensional linear [Pb2I2]2+ chains propagating along the b-axis (Fig. S2†). All of the iodide species are μ3-bridged among Pb2+ centers with Pb–I bond lengths between 3.287(1) and 3.590(1) Å. The iodo-plumbate chains are connected by square [Pb2O2] units, forming lead oxoiodide inorganic layers along the ab plane (Fig. S2†). Moreover, the Fcdc2− ligands coordinatively decorate the Pb2+ centers to form a layered hybrid architecture (Fig. S3†). Moreover, the strong coordination bonding between the Pb2+ centers in the [Pb2I2]2+ chains and the carboxylate groups in the Fcdc2− ligands is evidenced by the short Pb–O bond distances of 2.276–2.430 Å, which are significantly lower than the Pb–O distances of 2.753–2.872 Å observed between neutral [Pb2O2] units and Fcdc2− ligands (Fig. S4†). Using carboxylate as the coordinating ligand, rather than conventional Fc-based organoammonium, facilitates an essential transition from an ionically bound lead halide perovskite to a lead halide framework, affording high structural stability and efficient charge-transfer behavior between the [Pb2I2]2+ chains and the Fcdc2− ligands, as discussed in detail later.A good match between experimental powder X-ray diffraction (PXRD) and the simulated pattern from the single-crystal data confirms the high yield and good phase purity of TJU-26 and (FMTMA)PbI3 (Fig. 2a and S5†). Fourier transform infrared (FT-IR) spectra suggest the presence and deprotonation of Fcdc2− in TJU-26 (Fig. S6†). Stability measurements show that the as-synthesized (FMTMA)PbI3 crystals rapidly dissolve in polar organic solvents (e.g. DMF and DMSO). However, the as-synthesized TJU-26 is stable in these solvents for at least 24 h with no obvious loss in both crystallinity and mass balance, evidencing the high chemical stability of the coordination network (Fig. 2a). Thermogravimetric analysis (TGA) and ex situ thermodiffraction show high thermal stability up to 220 °C for TJU-26, higher than 200 °C for (FMTMA)PbI3 (Fig. S7 and S8†). Moreover, the photostability of TJU-26 was examined by continuous irradiation with AM 1.5G simulated sunlight (42 mW cm−2) for 48 h at room temperature. The overall stability of TJU-26 remained largely unaffected as confirmed by PXRD, while the slight discrepancy in absorption spectra is likely due to the partial surface modification during prolonged light irradiation (Fig. 2b and S9†). In contrast, a new diffraction peak (28.5°) in PXRD was clearly observed for (FMTMA)PbI3 after continuous irradiation for 24 h, suggesting the decomposition of the perovskite structure (Fig. 2b). Overall, the stability tests suggest that TJU-26 is a robust compound to perform photocatalysis in high-polarity protic solvents.
Electronic band structure
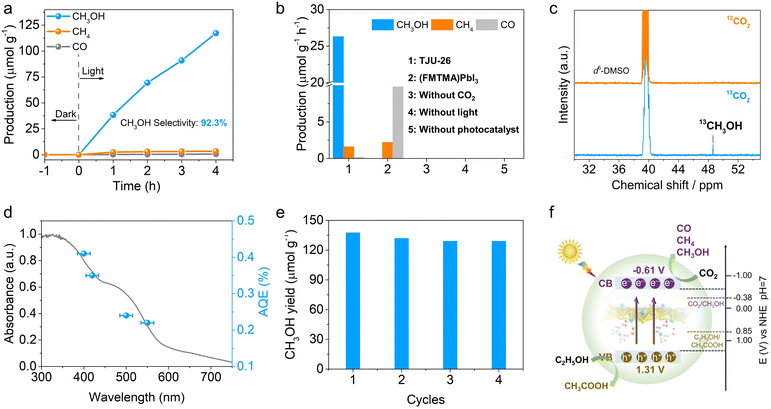
Ultraviolet-visible (UV-vis) diffuse reflectance spectroscopy shows that TJU-26 has a broad visible absorption range with the absorption edge extending to 612.7 nm, superior to all of our previously reported lead halide frameworks (Fig. 2c).33 The band gap energy (Eg) of TJU-26 was estimated to be 1.92 eV using the Tauc plot method (Fig. 2d). This enhancement in visible light absorption is attributed to the incorporation of the light-harvesting Fcdc2− ligand, which substantially improves phonon absorption across the solar spectrum. The ferrocene-functionalized perovskite, (FMTMA)PbI3, exhibits a narrower visible absorption range with an absorption edge of 571.5 nm and a larger Eg of 2.10 eV (Fig. 2c and d). The reduced Eg of TJU-26 suggests that the formation of Pb2+-carboxylate coordination bonds between the Fcdc2− and the lead iodide chains effectively enhances the conjugation of the framework.47 In addition, ultraviolet photoelectron spectroscopy (UPS) revealed that the valence band (VB) potentials (vs. the vacuum level) of TJU-26 and (FMTMA)PbI3 were −5.81 eV (+1.31 V vs. NHE pH 7) and −5.91 eV (+1.41 V vs. NHE pH 7), respectively (Fig. S10 and S11†). Their corresponding conduction band (CB) potentials (vs. the vacuum level) were −3.89 eV (−0.61 V vs. NHE pH 7) and −3.81 eV (−0.69 V vs. NHE pH 7), respectively. These values indicate that both TJU-26 and (FMTMA)PbI3 are thermodynamically capable of photocatalytic CO2 reduction coupled with EtOH oxidation (Fig. 2e).DFT calculations suggest the calculated bandgap of TJU-26 to be 1.98 eV, close to the experimental value of 1.92 eV and again confirming its semiconductive nature (Fig. 2f). The total density of states (DOS) and the projected density of states (pDOS) on the Pb, I and Fcdc2− ligands indicate that the valence band maximum (VBM) is dominated by I 5p orbitals, while Pb 6p orbitals largely contribute to the conduction band minimum (CBM) (Fig. 2f). The significant roles of Pb and I in frontier orbitals are analogous to the conventional hybrid lead halides, evidencing their contributions to carrier formation for photocatalysis.48,49 Meanwhile, owing to the Pb2+-carboxylate coordination motifs, the Fcdc2− linkers are involved in frontier orbitals as well, which agrees with the potential ligand-to-metal charge transfer (Fig. 2f).50
Charge transport dynamics
Since the incorporation of the light-harvesting chromophore largely extended the visible-light absorption of TJU-26, it is important to investigate the LMCT process after the photoexcitation of the Fcdc2− linkers and the e−–h+ separation. First, from the structural point of view, the π-conjugation between the cyclopentadiene ring and the carboxylate in the Fcdc2− ligands allows for the transfer of delocalized electrons from Fc moieties to the soft [Pb2I2] chains, unlike ionic Fc-based perovskites (Fig. 1).45 Following this, the LMCT is evidenced by the substantial photoluminescence decrease of Fcdc2− after incorporation into TJU-26, which exhibits a strong emission band at 379 nm for the free Fcdc2− ligands (Fig. S12 and S13†). Meanwhile, TJU-26 shows largely quenched but broad photoluminescence ranging from 380 to 700 nm, with an average lifetime of 150.8 ns based on time-resolved photoluminescence (tr-PL) decay (Fig. 3a). The best fit of the decay provides a bi-exponential function with time constants of a long carrier lifetime of 601.7 ns and a short lifetime of 46.7 ns. Meanwhile, the tr-PL spectra of H2Fcdc and (FMTMA)PbI3 show that the average lifetimes are lower than that of TJU-26, with 98.4 ns for H2Fcdc and 109.3 ns for (FMTMA)PbI3, respectively (Fig. 3a). The higher PL lifetime of TJU-26 again supports the claim that the Pb2+-carboxylate coordination motifs between the conjugated Fcdc2− ligand and the Pb2+ center are more favorable for the LMCT process. Indeed, DOS calculations show the contribution of the Fcdc2− ligands and the Pb 6p orbitals to the VBM and CBM, respectively, agreeing with the claim of the LMCT process (Fig. 2f). In addition, the presence of the LMCT process in TJU-26 was confirmed by X-ray photoelectron spectroscopy (XPS). In the high-resolution XPS spectra of Fe 2p, the two peaks located at 710.6 eV and 723.1 eV are ascribed to Fe 2p3/2 and Fe 2p1/2, respectively.51 Meanwhile, a series of XPS characteristic peaks of Fe3+ (714.7 eV for Fe3+ 2p3/2 and 728.1 eV for Fe3+ 2p1/2) were also observed upon irradiation, which confirms the charge transfer process from the Fcdc2− ligands to the Pb2+ sites (Fig. S14†).52Importantly, LMCT is known to facilitate the separation and transport of photogenerated carriers, and surface photovoltage (SPV) spectroscopy is an important approach for investigating charge separation.53 The photovoltage (PV) onset of TJU-26 occurs at 2.86 eV (433 nm), and the maximum voltage of 819 mV is observed at 3.27 eV (379 nm) (Fig. 3b). Based on the band structure of TJU-26, the PV response is determined to be the charge separation of the band states. Given the SPV measurement of the TJU-26 film on FTO glass (see the Experimental section in the ESI†), the maximum theoretical photovoltage for the FTO/TJU-26 contact is 990 mV. Therefore, the experimental photovoltage of TJU-26 reaches ∼83% of this limit.54,55 However, the ionically bound Fc-based perovskite, (FMTMA)PbI3, exhibits a decreased maximum PV of 194 mV, corresponding to approximately 20% of the theoretical maximum PC (Fig. 3b). Despite the resemblance in SPV spectra, the nearly fourfold higher PV of TJU-26 indicates a significantly greater charge separation efficiency that is attributed to the efficient LMCT in TJU-26. Enhanced charge separation in TJU-26 is further supported by (photo)electrochemical studies. The photocurrent signal of TJU-26 is substantially higher than that of (FMTMA)PbI3, reflecting improved electron–hole separation efficiency (Fig. S16†).56 In addition, the smaller radius observed in the electrochemical impedance spectral (EIS) plots for TJU-26 implies a lower electron transfer resistance (Fig. S17†). The interfacial charge transfer resistance (Rct) for TJU-26 is 3.6 kΩ, significantly lower than the 24.9 kΩ measured for (FMTMA)PbI3, suggesting superior electron mobility in TJU-26.57
Hall effect measurements at room temperature reveal that TJU-26 exhibits n-type semiconductor behavior, with a carrier concentration of 9.8 × 1013 cm−3 and an estimated carrier mobility of ∼0.114 cm2 V−1 s−1. These values are comparable to those of many benchmark 3D lead halide perovskites and several orders of magnitude higher than those observed for 1D (FMTMA)PbI3 (4.1 × 1010 cm−3 and 0.032 cm2 V−1 s−1).28,29 This enhancement suggests that the incorporation of light-harvesting Fcdc2− ligands improves visible-light photon absorption in TJU-26, followed by an LMCT process that further enhances charge separation. In addition, the average carrier diffusion lengths of TJU-26 and (FMTMA)PbI3 were determined to be 0.21 μm and 0.09 μm, respectively, based on the equation
 | (1) |
 and 0.51 m0 for holes
and 0.51 m0 for holes  , respectively. It is well established that small carrier effective masses contribute to high carrier mobility and long carrier diffusion lengths.58
, respectively. It is well established that small carrier effective masses contribute to high carrier mobility and long carrier diffusion lengths.58
To obtain detailed insights into the charge carrier dynamics of TJU-26 under light irradiation, femtosecond transient absorption (fs-TA) spectroscopy was performed upon 320 nm excitation. The pseudo-color fs-TA spectra of TJU-26 exhibit a positive signal across the 450–800 nm range, with a maximum at 515 nm (Fig. 3c). This signal is attributed to the absorption from photoinduced electron transfer and directly reveals the process of charge separation within 1D lead iodide chains under light exposure.59 This signal reaches its maximum value at ∼10 ps at 515 nm, suggesting a high rate of charge separation (Fig. S18†). However, the peak at 515 nm rapidly weakens around 50 ps, with a concomitant appearance of a new peak at 667 nm, indicating a change in the interactions between photogenerated electrons and holes (Fig. S18†).60 This new observation is attributed to electron transfer from the Fcdc2− ligand to the [Pb2I2]2+ SBUs via the Pb2+-carboxylate linkage under photoexcitation, which facilitates spatial charge separation and transport. Analysis of the recovery kinetics at 515 nm in the TA spectra shows that the decay of TJU-26 follows a biexponential function with two time constants, τ1 = 25.6 ps and τ2 = 250.4 ps (Fig. 3d), resulting in a long average lifetime of 122.6 ps. These findings indicate that the charge transfer between the Fcdc2− ligand and the lead halide structural unit probably delays TA decay kinetics, thereby suppressing electron–hole recombination.61
To further investigate the exciton dissociation behaviors of TJU-26 and (FMTMA)PbI3, temperature-dependent photoluminescence (PL) measurements were conducted to determine their exciton binding energies (Eb) (Fig. 3e and f). The PL intensities of both materials decreased as the temperature increased, primarily due to thermally activated nonradiative recombination processes.62 The Eb was estimated by combining integrated PL intensity with temperature, using the following equation:
 | (2) |
Photocatalytic CO2-to-CH3OH conversion
Given the suitable band positions and excellent carrier separation/transport of TJU-26, CO2 photoreduction was studied by introducing 20 mg as-synthesized TJU-26 into a sealed reaction system containing 10 mL EtOH and 1 atm CO2. No additional photosensitizer, metal co-catalyst or sacrificial reducing agent was necessary for this photocatalytic system. EtOH functions not only as a donor scavenger, accepting photogenerated holes, but also as an environmentally benign solvent. Both 1H nuclear magnetic resonance (NMR) spectroscopy and gas chromatography (GC) have been employed to quantitatively identify the reduction products. Upon AM 1.5G simulated illumination, TJU-26 steadily catalyzed CO2 reduction to produce CH3OH as the major product over a time span of 4 h (Fig. 4a and S19†). A linear increment in the CH3OH yields was observed and the average CH3OH evolution rate was determined to be 26.31 μmol h−1 g−1, while trace amounts of CO (0.14 μmol h−1 g−1) and CH4 (1.60 μmol h−1 g−1) were detected as well (Fig. 4a). These values correspond to photocatalytic selectivities of 92.3% (electron basis) and 93.7% (product basis) for CO2-to-CH3OH transformation, which are among the highest reported values for hybrid lead halides (Table S2†). Moreover, the CO2 photoreduction performance of TJU-26 is comparable to that of benchmark CO2-to-CH3OH photocatalysts, such as carbon dot-based photocatalysts (mCD) (13.9 μmol h−1 g−1) and Bi19Br3S27 (0.62 μmol h−1 g−1) (Table S3†).7,16Control experiments conducted in the absence of TJU-26, light, or CO2 yielded no methanol, confirming that the methanol formation results from the CO2 reduction reaction catalyzed by TJU-26 (Fig. 4b). To further confirm the origin of the carbon source, a control photocatalytic experiment was carried out using 13C isotopically labelled CO2. The chemical shift signal peak at 48.6 ppm (13CH3OH) was clearly observed by 13C NMR spectroscopy. Meanwhile, no peak was observed at 48.6 ppm using 12CO2 under identical reaction conditions, demonstrating that the CH3OH product arises from the reduction of CO2 instead of other carbon sources (Fig. 4c). In order to confirm the multi-electron CO2 reduction process, the kinetically favorable 2e− reduced product (i.e. formic acid, HCOOH) is employed as the precursor. Photocatalysis in a closed reaction system containing 40 mg TJU-26 and 20 μL formic acid in 8 mL EtOH without CO2 produces CH3OH as the major product at an evolution rate of 29.54 μmol h−1 g−1 (Fig. S20†). This set of experiments confirm that the populated carrier accumulation nature of TJU-26 enables the further reduction of 2e− intermediates to afford the final product of CH3OH. Moreover, we have performed control photocatalysis experiments to exclude the presence of ethanol, using sodium sulfite as the hole scavenger in aqueous solution instead. In this system, the evolution rates of CH3OH, CH4, and CO were measured to be 10.53 μmol h−1 g−1, 0.67 μmol h−1 g−1, and 6.34 μmol h−1 g−1, respectively (Fig. S21†). These results provide further evidence that methanol is derived from the photoreduction of CO2, rather than from the decomposition of ethanol.
The wavelength-dependent apparent quantum efficiencies (AQEs) were measured to be 0.41% at 400 nm (±15 nm), 0.35% at 420 nm (±15 nm), 0.24% at 500 nm (±15 nm) and 0.22% at 550 nm (±15 nm) (Fig. 4d). The trend is consistent with the UV-vis absorption of TJU-26, implying an intrinsic photo-driven catalytic process. In control experiments, (FMTMA)PbI3 was measured under identical photocatalytic conditions, resulting in CO2 reduction products of CO and CH4 with evolution rates of 9.83 μmol h−1 g−1 and 2.21 μmol h−1 g−1, respectively (Fig. 4b). The significantly enhanced photocatalytic CO2 reduction performance of TJU-26 again confirms the essential role of the π-conjugated coordination linkage between Fcdc2− ligands and [Pb2I2]2+ chains in facilitating charge separation and transport (Fig. 3g).
The long-term photocatalytic stability of TJU-26 was studied by performing photocatalysis under continuous AM 1.5G illumination for a total of 16 h, with evacuation and CO2 refilling every 4 h (Fig. 4e). The photocatalyst retained over 93% of the initial CO2 photoreduction activity for the next three runs, showing the excellent durability of TJU-26 under the photocatalytic conditions. Moreover, PXRD, UV-vis and FTIR spectroscopy synergistically indicate that TJU-26 largely retains its structural integrity after photocatalysis (Fig. S22–S24†). Indeed, a negligible amount of Pb2+ leaching was noticed in the solution after the photocatalytic reaction (1.12 ppm by inductively-coupled plasma optical emission spectroscopy, ICP-OES). Meanwhile, the role of EtOH as an electron donor was confirmed by the presence of acetic acid (CH3COOH) as the oxidized product by 1H NMR, which was performed by a continuous photocatalytic reaction for 12 h (Fig. 4f). The quantitative characterization of acetic acid was performed by using DMSO as the internal standard, corresponding to an evolution rate of 32.2 μmol h−1 g−1 (Fig. S25†). This value accounts for ∼83% of the theoretical yield for the oxidation product, demonstrating that CH3CH2OH-to-CH3COOH oxidation is the major coupling reaction in CO2 photoreduction. Notably, coupling CO2 photoreduction with alcohol oxidation has emerged as an appealing approach for synergistic utilization of photogenerated electrons and holes, offering an advantage over the conventional use of hole scavengers such as triethylamine (TEA).64
Photocatalytic mechanism
To understand the photoreaction mechanism on TJU-26, the surface species evolved in the reaction were monitored by in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) (Fig. 5a). First, during the DRIFT studies, no intermediate was observed in N2 or in the dark under identical conditions, suggesting the CO2 photoreduction process. When performing CO2 catalysis with light irradiation, a prominent band at 1620 cm−1 for carboxyl stretching is gradually observed with the irradiation time increasing from 0 to 60 min. This peak suggests the presence of *COOH species as one of the intermediates.65,66 The *CHO intermediate with a characteristic band at 1070 cm−1 is noticed as well, implying the possible protonation of COOH* to afford *CO and the follow-up proton transfer to form *CHO.67 More importantly, the concomitant absorption bands at 1040 cm−1 and 1160 cm−1 are clearly observed, suggesting the presence of H3CO*, which is one of the key intermediates during the CO2-to-CH3OH transformation.7,68 According to the above results, it is speculated that the most likely reduction pathway could be as follows: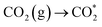 | (3) |
 | (4) |
| COOH* + H+ + e− → *CO + H2O | (5) |
| *CO + H+ + e− → *CHO | (6) |
| *CHO + H+ + e− → H2CO* | (7) |
| H2CO* + H+ + e− → H3CO* | (8) |
| H3CO* + H+ + e− → CH3OH | (9) |
Following this, we have explored the active sites for photocatalytic CO2 reduction on TJU-26 by quasi in situ XPS spectroscopy. Under light irradiation, the I 3d peak shifted to higher binding energy, indicating a decrease in the electron density around the I site (Fig. S26†).69 Meanwhile, we have observed characteristic peaks of both Fe2+ and Fe3+ in the high-resolution Fe 2p spectra, and the percentage of Fe3+ increased substantially under light irradiation, which was attributed to the charge transfer from the Fcdc2− ligands to the Pb2+ sites through the Pb2+-carboxylate coordination bridge (Fig. S14 and S15†).52 Indeed, the Pb 4f peak shifted to lower binding energy, indicating that the electron density around the Pb2+ site in TJU-26 increased after light irradiation (Fig. S27†).70 Overall, the increase in the electron density of the Pb2+ sites in TJU-26 facilitates CO2 adsorption and activation.
To further understand the reaction mechanism, we have performed Gibbs free energy calculations using the (001) facet as the representative surface of TJU-26 to model the possible reaction pathways involved in the CO2-to-CH3OH process. First, the CO2 adsorption calculations indicate that the CO2 adsorption and activation on TJU-26 are thermodynamically favorable (Fig. 5b). Importantly, the formation of the COOH* intermediate (eqn (4)) is found to be a spontaneous process on TJU-26, which is often the rate-determining step in many previous studies.7 In this exothermic step, the oxygens of the COOH* species preferentially bind with two adjacent Pb2+ sites in a bidentate manner, resulting from the suitable Pb⋯Pb distance of 3.691 Å in the lead oxyiodide layers. To note, both Pb2+ centers originate from the [Pb2I2]2+ chains instead of [Pb2O2] clusters, verifying that the iodoplumbate species indeed serve as photocatalytically active sites. The protonation of *COOH to produce *CO by TJU-26 has a low free-energy barrier of 0.81 eV to form the C-anchored *CO intermediate. This reaction pathway is further confirmed by a control experiment using formic acid instead of CO2 as the precursor to produce CH3OH at an evolution rate of 29.54 μmol h−1 g−1. Since the carbon of *CO has a contact distance of 2.175 Å with one Pb2+ site, the moderate adsorption strength of *CO tends to accept a proton–electron pair to form *CHO (eqn (6)) instead of CO desorption as the by-product (evolution rate of 0.14 μmol g−1 h−1). The thermodynamically favorable process for the conversion of *CO to *CHO is supported by both DFT calculations and the observed *CHO species (1070 cm−1) in the aforementioned in situ DRIFT studies (Fig. 5a). Following this, DFT calculations suggest that the reaction undergoes the formyl pathway, where the addition of another electron–hole pair leads to the formation of H2CO* through an exothermic process (eqn (7)), thanks to the bidentate binding motif with dual Pb2+ centers. This suggests the important transition from C-anchored *CHO to O-anchored H2CO* on TJU-26 (Fig. 5b). Subsequently, H2CO* accepts one more electron–hole pair to form CH3O*, which agrees with the in situ DRIFT observations. Although the hydrogenation of *OCH3 to CH3OH is an uphill process, the free-energy change for this step is much smaller than that for the conversion of *OCH3 to *CH3via the acceptance of two e−/H+ pairs (Fig. 5b). This effectively rules out the possibility of CH4 being the major product on TJU-26 (Fig. 5b). Overall, the reaction mechanism for the photocatalytic conversion of CO2 to CH3OH has been thoroughly investigated through a combination of in situ spectroscopic experiments and theoretical calculations.
Since the major oxidation half-reaction is experimentally determined to be EtOH oxidation, the competitive absorption behaviors of TJU-26 towards CH3OH and EtOH are studied using vapor adsorption isotherms and contact angle measurements. First, the hydrophobic nature of TJU-26 is confirmed by an average contact angle of 110.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) , suggesting that TJU-26 has a higher affinity towards EtOH rather than CH3OH (Fig. 5c). The vapor adsorption isotherms of TJU-26 indicate the uptake amounts of 5.12 cm3 g−1 for EtOH and 1.86 cm3 g−1 for CH3OH at 298 K under 1 bar (Fig. 5d). The substantially higher amount of EtOH adsorption suggests strong interactions between EtOH and TJU-26. Since the layered material has no apparent open pore window, the vapor adsorption probably occurs on the crystal surfaces of TJU-26. Given the substantially higher EtOH adsorption over CH3OH adsorption, TJU-26 favors the desorption of CH3OH as the product that agrees with the low desorption barrier of 0.17 eV based on DFT calculations (Fig. 5b).
, suggesting that TJU-26 has a higher affinity towards EtOH rather than CH3OH (Fig. 5c). The vapor adsorption isotherms of TJU-26 indicate the uptake amounts of 5.12 cm3 g−1 for EtOH and 1.86 cm3 g−1 for CH3OH at 298 K under 1 bar (Fig. 5d). The substantially higher amount of EtOH adsorption suggests strong interactions between EtOH and TJU-26. Since the layered material has no apparent open pore window, the vapor adsorption probably occurs on the crystal surfaces of TJU-26. Given the substantially higher EtOH adsorption over CH3OH adsorption, TJU-26 favors the desorption of CH3OH as the product that agrees with the low desorption barrier of 0.17 eV based on DFT calculations (Fig. 5b).
Conclusions
In this study, we present the first example of a hybrid lead halide that can achieve photocatalytic CO2-to-CH3OH transformation. By incorporating an Fc-based ligand as a light-harvesting antenna into the ultrastable lead iodide framework, its visible-light absorption edge is extended to over 600 nm, followed by an efficient LMCT process that triggers carrier transport along the linear lead iodide chains. Compared to the control ionic Fc-based perovskite ((FMTMA)PbI3), our lead iodide framework exhibits synergistic interactions between inorganic and organic photoactive components for enhanced carrier transport by multiple orders of magnitude. Notably, TJU-26 demonstrates high structural robustness in high-polar protic environments and achieves photocatalytic CO2 reduction to high value-added CH3OH under AM 1.5G irradiation, whereas most hybrid metal halides produce CO as the primary product. The AQE of TJU-26 for CH3OH production reaches 0.41% at 400 nm, comparable to those of many benchmark photocatalysts (e.g., mCD/CN). Importantly, the oxidative half-reaction coupled with CO2 photoreduction involves environmentally benign EtOH oxidation, resulting in the overall photocatalysis reaction in a sustainable manner. In situ DRIFT experiments and DFT calculations clearly indicate that the photocatalysis undergoes the formyl pathway to afford the key intermediate CH3O*, which occurs on the dual Pb2 active sites residing in [Pb2I2]2+ chains. Given the numerous examples of light-harvesting antenna-functionalized organocarboxylate linkers, we believe that this study opens up many possibilities for chemical functionalization of ultrastable lead halide frameworks for a variety of photoactive applications.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
J. Y., Y. H. and H. F. conceived the project. H. F. supervised the project. J. Y. and Y. H. performed the majority of experimental studies. C. S. assisted in the crystallographic analysis. Y. J. assisted in the synthesis and photophysical studies. J. Y., Y. H. and H. F. wrote and revised the manuscript. All authors contributed to discussion and revisions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (22471196 and 22171214), the Natural Science Foundation of Shanghai (22ZR1463200), the Recruitment of Global Youth Experts by China, the Xiaomi Young Talents Program and the Science & Technology Commission of Shanghai Municipality (19DZ2271500).Notes and references
- J. Graciani, K. Mudiyanselage, F. Xu, A. E. Baber, J. Evans, S. D. Senanayake, D. J. Stacchiola, P. Liu, J. Hrbek, J. F. Sanz and J. A. Rodriguez, Science, 2014, 345, 546–550 Search PubMed.
- S. Kar, R. Sen, A. Goeppert and G. K. S. Prakash, J. Am. Chem. Soc., 2018, 140, 1580–1583 CrossRef CAS.
- C. Liu, B. Yang, E. Tyo, S. Seifert, J. DeBartolo, B. von Issendorff, P. Zapol, S. Vajda and L. A. Curtiss, J. Am. Chem. Soc., 2015, 137, 8676–8679 CrossRef CAS.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Angew. Chem., Int. Ed., 2013, 52, 7372–7408 CrossRef CAS.
- S. Navarro-Jaén, M. Virginie, J. Bonin, M. Robert, R. Wojcieszak and A. Y. Khodakov, Nat. Rev. Chem, 2021, 5, 564–579 CrossRef.
- S. C. Roy, O. K. Varghese, M. Paulose and C. A. Grimes, ACS Nano, 2010, 4, 1259–1278 CrossRef CAS PubMed.
- J. Li, W. Pan, Q. Liu, Z. Chen, Z. Chen, X. Feng and H. Chen, J. Am. Chem. Soc., 2021, 143, 6551–6559 CrossRef CAS PubMed.
- K.-Q. Lu, Y.-H. Li, F. Zhang, M.-Y. Qi, X. Chen, Z.-R. Tang, Y. M. A. Yamada, M. Anpo, M. Conte and Y.-J. Xu, Nat. Commun., 2020, 11, 5181 CrossRef CAS PubMed.
- Y. Li, B. Li, D. Zhang, L. Cheng and Q. Xiang, ACS Nano, 2020, 14, 10552–10561 CrossRef CAS PubMed.
- Z. Jiang, H. Sun, T. Wang, B. Wang, W. Wei, H. Li, S. Yuan, T. An, H. Zhao, J. Yu and P. K. Wong, Energy Environ. Sci., 2018, 11, 2382–2389 RSC.
- D. Liu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Angew. Chem., Int. Ed., 2020, 59, 4519–4524 CrossRef CAS.
- E. Gong, S. Ali, C. B. Hiragond, H. S. Kim, N. S. Powar, D. Kim, H. Kim and S.-I. In, Energy Environ. Sci., 2022, 15, 880–937 RSC.
- G. A. Olah, Angew. Chem., Int. Ed., 2005, 44, 2636–2639 CrossRef CAS PubMed.
- K. Natte, H. Neumann, M. Beller and R. V. Jagadeesh, Angew. Chem., Int. Ed., 2017, 56, 6384–6394 CrossRef CAS PubMed.
- A. Goeppert, M. Czaun, J.-P. Jones, G. K. Surya Prakash and G. A. Olah, Chem. Soc. Rev., 2014, 43, 7995–8048 RSC.
- Y. Wang, X. Liu, X. Han, R. Godin, J. Chen, W. Zhou, C. Jiang, J. F. Thompson, K. B. Mustafa, S. A. Shevlin, J. R. Durrant, Z. Guo and J. Tang, Nat. Commun., 2020, 11, 2531 CrossRef CAS.
- W. Tu, Y. Zhou and Z. Zou, Adv. Mater., 2014, 26, 4607–4626 CrossRef CAS PubMed.
- A. Lais, M. A. Gondal and M. A. Dastageer, Environ. Chem. Lett., 2018, 16, 183–210 CrossRef CAS.
- S. Park, W. J. Chang, C. W. Lee, S. Park, H.-Y. Ahn and K. T. Nam, Nat. Energy, 2016, 2, 16185 CrossRef.
- Y. Wu, P. Wang, X. Zhu, Q. Zhang, Z. Wang, Y. Liu, G. Zou, Y. Dai, M.-H. Whangbo and B. Huang, Adv. Mater., 2018, 30, 1704342 CrossRef PubMed.
- Y. Wu, P. Wang, Z. Guan, J. Liu, Z. Wang, Z. Zheng, S. Jin, Y. Dai, M.-H. Whangbo and B. Huang, ACS Catal., 2018, 8, 10349–10357 CrossRef CAS.
- Y.-F. Xu, M.-Z. Yang, B.-X. Chen, X.-D. Wang, H.-Y. Chen, D.-B. Kuang and C.-Y. Su, J. Am. Chem. Soc., 2017, 139, 5660–5663 CrossRef CAS PubMed.
- Z. Hong, W. K. Chong, A. Y. R. Ng, M. Li, R. Ganguly, T. C. Sum and H. S. Soo, Angew. Chem., Int. Ed., 2019, 58, 3456–3460 CrossRef CAS PubMed.
- C. Tang, C. Chen, W. Xu and L. Xu, J. Mater. Chem. A, 2019, 7, 6911–6919 RSC.
- X. Zhu, Y. Lin, Y. Sun, M. C. Beard and Y. Yan, J. Am. Chem. Soc., 2019, 141, 733–738 CrossRef CAS PubMed.
- K. Chen, X. Deng, G. Dodekatos and H. Tüysüz, J. Am. Chem. Soc., 2017, 139, 12267–12273 CrossRef CAS PubMed.
- T. M. Brenner, D. A. Egger, L. Kronik, G. Hodes and D. Cahen, Nat. Rev. Mater., 2016, 1, 15007 CrossRef CAS.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344–347 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- M. Jung, S.-G. Ji, G. Kim and S. I. Seok, Chem. Soc. Rev., 2019, 48, 2011–2038 RSC.
- H. Huang, B. Pradhan, J. Hofkens, M. B. J. Roeffaers and J. A. Steele, ACS Energy Lett., 2020, 5, 1107–1123 CrossRef CAS.
- C. Sun, R. Xi and H. Fei, Acc. Chem. Res., 2023, 56, 452–461 CrossRef CAS PubMed.
- Z. Zhuang, C. Peng, G. Zhang, H. Yang, J. Yin and H. Fei, Angew. Chem., Int. Ed., 2017, 56, 14411–14416 CrossRef CAS PubMed.
- J. Yin, H. Yang and H. Fei, Chem. Mater., 2019, 31, 3909–3916 CrossRef CAS.
- C. Peng, X. Song, J. Yin, G. Zhang and H. Fei, Angew. Chem., Int. Ed., 2019, 58, 7818–7822 CrossRef CAS PubMed.
- J. Yin, Y. Yu, X. Song, Y. Jiang and H. Fei, CCS Chem., 2021, 4, 540–547 CrossRef.
- C. Peng, Z. Zhuang, H. Yang, G. Zhang and H. Fei, Chem. Sci., 2018, 9, 1627–1633 RSC.
- Y. Jiang, J. Yin, R. Xi and H. Fei, Chem. Sci., 2024, 15, 2848–2856 RSC.
- X. Song, G. Wei, J. Sun, C. Peng, J. Yin, X. Zhang, Y. Jiang and H. Fei, Nat. Catal., 2020, 3, 1027–1033 CrossRef CAS.
- X. Chen, C. Peng, W. Dan, L. Yu, Y. Wu and H. Fei, Nat. Commun., 2022, 13, 4592 CrossRef CAS PubMed.
- S. K. Singh, R. Chauhan, B. Singh, K. Diwan, G. Kociok-Köhn, L. Bahadur and N. Singh, Dalton Trans., 2012, 41, 1373–1380 RSC.
- S.-J. Yao, N. Li, J. Liu, L.-Z. Dong, J.-J. Liu, Z.-F. Xin, D.-S. Li, S.-L. Li and Y.-Q. Lan, Inorg. Chem., 2022, 61, 2167–2173 CrossRef CAS PubMed.
- N. Fillafer, H. Kuper, A. Schaate, S. Locmelis, J. A. Becker, Y. Krysiak and S. Polarz, Adv. Funct. Mater., 2022, 32, 2201126 CrossRef CAS.
- Z.-X. Zhang, H.-Y. Zhang, W. Zhang, X.-G. Chen, H. Wang and R.-G. Xiong, J. Am. Chem. Soc., 2020, 142, 17787–17794 CrossRef CAS PubMed.
- C. Du, J. Sheng, F. Zhong, Y. He, H. Liu, Y. Sun and F. Dong, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2315956121 CrossRef CAS PubMed.
- H. Sun, Z. Zhao, Y. Xing, K. Wei, X. Zhao, Y. Zhao, X. Wang, Z. Kang, Y. Li and H. Tan, Adv. Funct. Mater., 2024, 34, 2311591 CrossRef CAS.
- C. Zhou, H. Lin, H. Shi, Y. Tian, C. Pak, M. Shatruk, Y. Zhou, P. Djurovich, M.-H. Du and B. Ma, Angew. Chem., Int. Ed., 2018, 57, 1021–1024 CrossRef CAS PubMed.
- M. D. Smith, B. A. Connor and H. I. Karunadasa, Chem. Rev., 2019, 119, 3104–3139 CrossRef CAS PubMed.
- S. Li, Z. Li, J. Yue, H. Wang, Y. Wang, W. Su, G. I. N. Waterhouse, L. Liu, W. Zhang and Y. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202407638 CrossRef CAS PubMed.
- H. Zhang, S. Si, G. Zhai, Y. Li, Y. Liu, H. Cheng, Z. Wang, P. Wang, Z. Zheng, Y. Dai, T. X. Liu and B. Huang, Appl. Catal., B, 2023, 337, 122909 CrossRef CAS.
- L. Xia, W. Zhou, Y. Xu, Z. Xia, X. Wang, Q. Yang, G. Xie, S. Chen and S. Gao, Chem. Eng. J., 2023, 451, 138747 CrossRef CAS.
- X.-P. Wu, L. Gagliardi and D. G. Truhlar, J. Am. Chem. Soc., 2018, 140, 7904–7912 CrossRef CAS PubMed.
- M. A. Melo Jr, Z. Wu, B. A. Nail, A. T. De Denko, A. F. Nogueira and F. E. Osterloh, Nano Lett., 2018, 18, 805–810 CrossRef PubMed.
- J. Wang, J. Zhao and F. E. Osterloh, Energy Environ. Sci., 2015, 8, 2970–2976 RSC.
- Y. Shi, G. Zhan, H. Li, X. Wang, X. Liu, L. Shi, K. Wei, C. Ling, Z. Li, H. Wang, C. Mao, X. Liu and L. Zhang, Adv. Mater., 2021, 33, 2100143 CrossRef CAS PubMed.
- H. Zhang, S. Liu, A. Zheng, P. Wang, Z. Zheng, Z. Wang, H. Cheng, Y. Dai, B. Huang and Y. Liu, Angew. Chem., Int. Ed., 2024, 63, e202400965 CrossRef CAS PubMed.
- X. Wang, T. Li, B. Xing, M. Faizan, K. Biswas and L. Zhang, J. Phys. Chem. Lett., 2021, 12, 10532–10550 CrossRef CAS PubMed.
- S. Li, W. Wei, K. Chi, C. T. J. Ferguson, Y. Zhao and K. A. I. Zhang, J. Am. Chem. Soc., 2024, 146, 12386–12394 CrossRef CAS PubMed.
- Y.-Z. Cheng, W. Ji, X. Wu, X. Ding, X.-F. Liu and B.-H. Han, Appl. Catal., B, 2022, 306, 121110 CrossRef CAS.
- Z. Liu, H. Yin, J. Sun, L. Bai, Z. Li, X. Zhao, X. Yan, M. Zhao and L. Jing, Adv. Energy Mater., 2024, 14, 2401713 CrossRef CAS.
- G. Fu, D. Yang, S. Xu, S. Li, Y. Zhao, H. Yang, D. Wu, P. S. Petkov, Z.-A. Lan, X. Wang and T. Zhang, J. Am. Chem. Soc., 2024, 146, 1318–1325 CrossRef CAS PubMed.
- C. Cheng, S. Zhang, J. Zhang, L. Guan, M. E. El-Khouly and S. Jin, Angew. Chem., Int. Ed., 2024, 63, e202411359 CAS.
- W. Zhang, C.-C. Song, J.-W. Wang, S.-T. Cai, M.-Y. Gao, Y.-X. Feng and T.-B. Lu, Chin. J. Catal., 2023, 52, 176–186 CrossRef CAS.
- X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu and Y. Xie, Nat. Energy, 2019, 4, 690–699 CrossRef CAS.
- P. Chen, B. Lei, X. a. Dong, H. Wang, J. Sheng, W. Cui, J. Li, Y. Sun, Z. Wang and F. Dong, ACS Nano, 2020, 14, 15841–15852 CrossRef CAS PubMed.
- J. Xu, Z. Ju, W. Zhang, Y. Pan, J. Zhu, J. Mao, X. Zheng, H. Fu, M. Yuan, H. Chen and R. Li, Angew. Chem., Int. Ed., 2021, 60, 8705–8709 CrossRef CAS PubMed.
- Y. Wang, E. Chen and J. Tang, ACS Catal., 2022, 12, 7300–7316 CrossRef CAS PubMed.
- C. Ban, Y. Wang, Y. Feng, Z. Zhu, Y. Duan, J. Ma, X. Zhang, X. Liu, K. Zhou, H. Zou, D. Yu, X. Tao, L. Gan, G. Han and X. Zhou, Energy Environ. Sci., 2024, 17, 518–530 RSC.
- L. Zhao, J. Bian, X. Zhang, L. Bai, L. Xu, Y. Qu, Z. Li, Y. Li and L. Jing, Adv. Mater., 2022, 34, 2205303 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details of synthesis methods, additional XRD patterns, UV-vis absorption spectra, TGA spectra, UPS spectra, photocatalytic experiments, steady and transient state PL spectra, and computational methods for DFT. CCDC 2218953. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08216h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |