 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evidence for I2 loss from the perovskite–gas interface upon light-induced halide segregation†
Michael
Lee
 a,
Julian A.
Vigil
a,
Julian A.
Vigil
 ab,
Zhiqiao
Jiang
ab,
Zhiqiao
Jiang
 ac and
Hemamala I.
Karunadasa
ac and
Hemamala I.
Karunadasa
 *acd
*acd
aDepartment of Chemistry, Stanford University, Stanford, California 94305, USA. E-mail: hemamala@stanford.edu
bDepartment of Chemical Engineering, Stanford University, Stanford, California 94305, USA
cDepartment of Materials Science and Engineering, Stanford University, Stanford, California 94305, USA
dStanford Institute for Materials and Energy Sciences, SLAC National Laboratory, Menlo Park CA 94025, USA
First published on 28th April 2025
Abstract
Sunlight-induced halide segregation in (CH3NH3)Pb(BrxI1−x)3 (1 > x > 0.2), which limits obtainable voltages from solar cells with these perovskite absorbers, reverses upon resting in the dark. However, sustained illumination at ca. 1 sun opens a new decomposition pathway, leading to irreversible I2 loss in an open system. We conclusively show I2 off-gassing from halide-segregated (CH3NH3)Pb(Br0.75I0.25)3 by trapping gaseous I2 and tracking the electronic conductivity of the perovskite, which increases from electron-doping as iodides are oxidized to iodine. Importantly, we show that this reaction occurs across the perovskite-air solid–gas interface, without confounding effects from solvent or reactive solid interfaces. This characterization was conducted under a nitrogen atmosphere, avoiding vacuum- and oxygen-driven I2 loss pathways. Consistent observations of I2 loss upon light-soaking CsPb(Br0.75I0.25)3 films show that this reaction is intrinsic to the inorganic framework. We propose that the disruption of iodide-rich domains in the halide-segregated films through I2 loss can masquerade as a light-induced healing or apparent remixing of the segregated film, when in fact it leads to irreversible decomposition. Although I2 off-gassing is less likely in bromide-rich solid solutions, light-induced halide segregation brings the iodides into proximity and forms electronic states that are energetically poised to trap and accumulate holes, providing a driving force for I2 loss. Thus, even bromide-rich mixed-halide perovskite absorbers will benefit from I2-impermeable encapsulation for long-term stability.
Introduction
Lead-halide perovskites have emerged as a leading class of absorber materials for thin-film photovoltaic devices.1–3 At the forefront of applications is the use of perovskites in tandem solar cells,4 where a higher-bandgap perovskite accompanies a lower-bandgap absorber such as silicon,5,6 which have demonstrated impressive power conversion efficiencies well in excess of 30%.7,8 The ideal bandgap for an absorber used in tandem with silicon is 1.7–1.8 eV—9,10higher than the 1.5–1.6 eV bandgap common for lead-iodide perovskites11,12—which demands bandgap tunability from the halide perovskites. Given that the valence and conduction bands of the lead-halide perovskites have dominant halide character,13,14 bandgap control is easily achieved by tuning the halide composition.15 For example, alloying bromide ions into a lead-iodide perovskite increases its bandgap to the ideal range for a tandem solar cell. However, even after tuning the halide composition to reach optimal bandgaps, the expected device-level change—in particular, an increased open-circuit voltage compared to those produced by lead-iodide perovskite absorbers—did not follow.15 The demonstration that photoinduced halide segregation, where iodide- and bromide-rich regions form upon illumination of the mixed-halide perovskite absorber,16 caused the diminished voltage would prove massively consequential in device design. Indeed, the majority of recent high-efficiency perovskite/Si tandem cells are designed with halide segregation in mind.7,8,17,18Although mixed-halide perovskites, such as (MA)Pb(BrxI1−x)3 (MA = CH3NH3+; 1 > x > 0.2), initially show the expected band-edge photoluminescence (PL), upon moderate visible-light soaking (15 mW cm−2 for more than one minute) this PL peak diminishes to be replaced by a lower-energy PL peak at ca. 1.7 eV.16 This observation, along with many others,16,19,20 could be explained through the formation of iodide- and bromide-rich domains, with bandgaps that are smaller and larger, respectively, than those of the unsegregated mixed-halide perovskite film. Therefore, the smaller bandgaps of the iodide-rich domains trap photogenerated carriers that form throughout the perovskite, affording the lower-energy PL.21 As such, a PL peak that redshifts to ca. 1.7 eV has been used as a diagnostic for light-induced halide segregation. Notably, this light-induced halide segregation appeared to be reversible over several cycles of illumination and resting in the dark for several minutes.16
Amidst the surge of research surrounding halide segregation and potential methods to mitigate it22,23 came recent claims of halide re-mixing in halide-segregated perovskites—one in (MA)Pb(Br0.8I0.2)3 micron-sized crystals24 and the other in CsPb(Br0.6I0.4)3 polycrystalline films25—after exposure to higher-intensity illumination. These reports showed that the lower-energy PL (characteristic of the iodide-rich domains in the halide-segregated perovskite) could be replaced by the higher-energy PL (reminiscent of the original mixed-halide perovskite) if the perovskites were exposed to higher-intensity light (1.5 W cm−2 for 30 minutes for CsPb(Br0.6I0.4)3 films; 200 W cm−2 for 20 s for (MA)Pb(Br0.8I0.2)3 crystals), suggesting intriguing possibilities of “healing” or halide re-mixing through additional light-soaking. However, although not mentioned in the report, a close examination of the optical spectra of purportedly self-healed (MA)Pb(Br0.8I0.2)3 crystals24 show a slightly blueshifted PL compared to that of the original perovskite, consistent with the overall loss of iodide and resulting in a larger bandgap.
Several other studies have noted the same loss of low-energy PL in halide-segregated perovskite films under high-intensity illuminations. An older study26 observed this in (MA)Pb(Br2.5I0.5) films and used controlled atmosphere studies to attribute this PL loss to O2-mediated decomposition of the iodide-rich domains by comparison to known decomposition pathways of (MA)PbI3.27 Another group of studies argue for a photothermal halide remixing process that competes with halide segregation, based on PL spectra of polystyrene-encapsulated (MA)Pb(BrxI1−x)3 films (illuminated with a 473 nm continuous-wave laser at 16 W cm−2 for up to 200 s).28,29 This claim was supported by separate temperature-dependent studies indicating the presence of a competing process to halide segregation.30 These contradictory findings on the effects of light-soaking halide-segregated films, and the blueshifted PL of the purportedly “healed” perovskites, motivate further investigation of potential iodide loss from mixed-halide perovskites under high-intensity illumination.
Although I2 loss is most likely to be light-induced, other mechanisms can result in iodide loss from perovskites, especially in organic–inorganic compositions where HI evolution is possible. Iodine loss has particular implications for device stability, with the removal of iodide ions,31 the resulting iodide vacancies,32 and the corrosive I2 vapor33,34 all being connected with photodegradation of perovskite absorber layers. We therefore looked for clear evidence of I2 off-gassing from the perovskite-air interface—without the presence of solvent or oxygen—of mixed-halide perovskites under illumination.
Prior work on light-induced halogen loss
X-site self-exchange is commonly observed in the ABX3 perovskite family. Oxide perovskites maintain a well-studied equilibrium between oxide sites in the crystal and oxygen gas,35 and there is growing evidence of an analogous equilibrium in the halide perovskites through eqn (1),36,37 where XX is an occupied halide site,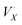 a monocationic halogen vacancy, e′ an electron, and X2 the diatomic halogen gas.
a monocationic halogen vacancy, e′ an electron, and X2 the diatomic halogen gas.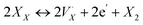 | (1) |
Both directions of the equilibrium in eqn (1) have been shown separately in halide perovskites, even without illumination or heating above ambient temperature. For example, the presence of I2 in the atmosphere was shown to p-dope polycrystalline (MA)PbI3 thin films (reverse reaction) through work-function measurements.38 Both the forward and reverse reactions were inferred from electronic conductivity measurements of a double perovskite single crystal Cs2AgTlBr6 through exposure to a Br2 atmosphere.36 The thermodynamics of the equilibrium was studied in a single crystal of the double perovskite Cs2SnI6; spontaneous I2 off-gassing near room temperature is enabled by a low enthalpy of reaction—breaking metal-iodide bonds and forming I2—and the very large entropy gain for this solid-to-gas reaction.37
Expecting illumination to play a role in halogen off-gassing from halide perovskites can also be justified. The valence-band maximum of most halide perovskites, where photogenerated holes accumulate, is primarily formed by halide p orbitals, leading to halide oxidation and subsequent loss as halogen gas.13 Indeed, halide oxidation under illumination has been studied for centuries, with photodecomposition of silver bromides and iodides to silver metal and halogen vapor serving as the basis of black-and-white photography since the 1830s.39 Time-resolved mass spectrometry and X-ray photoelectron spectroscopy measurements evidence I2 release from (MA)PbI3 under dynamic vacuum in the dark40 and under illumination.40–42 Further, I2 has been detected during illumination of (MA)PbI3 immersed in toluene, by identifying the I2 charge–transfer complex (I2·toluene) using solution-state absorbance measurements.31
Such I2 loss should be less likely in Br-rich mixed-iodide-bromide perovskites. However, light-induced I0/− loss through the solid–liquid interface between these perovskites and organic solvents has been inferred from solution-state measurements of the formation of I3− in dichloromethane (DCM) or I2·toluene in toluene, alongside a steadily increasing bandgap under continuous illumination. Here, we refer to the leaving ligand as I0/−, as the mediating solvent complicates the assignment of the species leaving the perovskite as I− or I2, as described later. For example, films of mixed-halide perovskites (such as (MA)PbI1.5Br1.5 (ref. 43) or Cs0.17FA0.83PbI1.5Br1.5;44 FA = HC(NH2)2+) showed the expected light-induced halide segregation, immersing these films in DCM or toluene and irradiating them led to the formation of I3− or I2·toluene, detected by solution-state absorbance measurements.43,44 The electrochemical injection of holes into mixed iodide-bromide perovskites was also shown to release I0/− through UV-visible spectroscopic detection of I3− in DCM.45,46
Overall, numerous prior studies suggest the oxidation of iodide in the perovskite to iodine, facilitated by light (or the accumulation of holes) and possibly solvent. However, the solvent–perovskite interface complicates the unambiguous identification of the species leaving the perovskite as I2. Although these experiments were performed in DCM or toluene, solvents in which halide perovskites do not visibly dissolve, these solvents can still crystallize halide perovskites and mediate reactions between halide perovskites and reagents, implying slight dissolution and re-precipitation at the perovskite surface.47 If even slight dissolution leads to the loss of I− ions from the perovskite, then light or O2 can oxidize these free I− ions in solution into I2 (or equivalently I2 + I− = I3−), further driving dissolution through Le Chatelier's principle. From UV-visible absorption spectra, we can indeed infer the presence of iodide in DCM sealed with (MA)Pb(Br0.75I0.25)3 powder in the dark over 1 day (Fig. S1†). Further, DCM is also known to produce Cl˙ radicals upon irradiation, a strong oxidant that could react spontaneously with I−.48–50 Such decomposition pathways, involving dissolved iodides, are unlikely to occur in perovskites not immersed in a solvent; more importantly, these reactions are not representative of defect reactions that electronically dope the perovskite. Thus, the solid–air interface is most relevant when studying possible degradation during solar cell operation, particularly since doping of the perovskite can dramatically change the bulk electron transport properties.
Given the importance of identifying degradation pathways in perovskite compositions of great utility in high-efficiency tandem solar cells,7,8 we sought to build on the prior studies to show facile I2 loss through the perovskite-atmosphere interface in the absence of O2. To our knowledge, this work represents the first direct, conclusive evidence of sunlight-driven I2 off-gassing from a mixed iodide-bromide perovskite in realistic operating conditions (ambient pressure). To conclusively demonstrate illumination-driven I2 loss, we:
(1) used a solid chemical trap to detect I2 gas evolving from the perovskite into an O2-free atmosphere under simulated solar illumination, and compared the observations to those from heating the perovskite in the dark, and (2) showed that the electronic conductivity of the perovskite increases upon illumination, evidencing the n-type doping of the perovskite that accompanies I2 loss. Importantly, (2) distinguishes I2 off-gassing from I− loss (as (MA)I or HI), where the latter would not electronically dope the perovskite.
Detection of I2 evolving from the solid–gas interface
To illustrate the effect of illumination on a mixed-halide perovskite, spin-cast thin films of (MA)Pb(BrxI1−x)3 were used as a representative model. An approximate halide composition of x = 0.75, based on the precursor solution (see Methods; ESI†), was chosen to provide sufficient bromide content for halide segregation. Each film was cut into two pieces and half of each film was illuminated under one sun (AM1.5G, 0.1 W cm−2 simulated) for 30 minutes in a glass chamber sealed in a nitrogen atmosphere. For direct comparison, the other half of the film was heated to ca. 42 °C (the highest temperature reached under illumination, Fig. S3†) for 35 minutes in an identical glass chamber sealed under nitrogen. The optical bandgap obtained from fitting absorption spectra show a clear blueshift, from 2.084 eV to 2.182 eV for the illuminated film, consistent with a more bromide-rich average composition (Fig. 1B). X-ray diffraction (XRD) measurements of the illuminated film reveal Bragg peaks systematically shifted to higher angles (Fig. 1C) and a decrease in fitted pseudo-cubic lattice parameter from 6.004 Å to 5.944 Å (Fig. 1D), commensurate with Br enrichment while preserving the overall crystal structure. Both signs of iodide loss seen for the illuminated film were observed in the heated films with smaller magnitudes of change (Fig. 1B and C). | ||
| Fig. 1 Evidence of average composition changes after illumination of a mixed iodide-bromide perovskite | (A) schematic illustration depicting the loss of I2 following illumination and halide segregation; octahedra comprising [PbI6]4− and [PbBr6]4− are shaded in violet and orange, respectively. Figure adapted with permission from ref. 51. (B) Tauc plots from optical transmission measurements of (MA)Pb(Br0.75I0.25)3 thin films following heating at ca. 42 °C (35 minutes; red trace) or illumination at 1 sun (30 minutes; blue trace); optical bandgaps (Eg) obtained from linear-intersection fitting (see ESI†). (C) X-ray diffraction patterns showing the shift in the pseudo-cubic (100) Bragg reflection of the (MA)Pb(Br0.75I0.25)3 thin films following heating or illumination (as in 1B). (D) Pseudo-cubic lattice parameter, a, estimation using the Nelson–Riley method52 (see ESI†); points correspond to the fitted center (in 2θ) of the corresponding Bragg peaks (Fig. S2†). | ||
Similar signs of iodide loss were also observed from CsPb(BrxI1−x)3 thin films of similar halide composition (x ∼0.75) that were subjected to the same heating and illumination conditions, although the effects were less pronounced compared to those of the hybrid perovskite. With light-soaking, the optical bandgap of the fitted UV-visible absorption spectra blueshifted from 2.174 eV to 2.196 eV, whereas the heated film showed a smaller shift in bandgap (Fig. S4†). The fitted pseudo-cubic lattice parameter extracted from XRD patterns also decreased from 5.899 Å to 5.877 Å in the light-soaked perovskite, whereas the heated film showed a smaller decrease (Fig. S5†). Because the same trends are seen in this inorganic analog, which is considered to be more stable,25 we posit that iodide loss is inherent to the metal-halide framework and not directly tied to reactivity with—or volatilization of—an organic cation, although interactions with the A-site cation may influence the reaction rate.
To assess whether these apparent changes in the average composition originate from I2 off-gassing, a bulk powder of (MA)Pb(Br0.75I0.25)3 was sealed in the presence of a chemical trap, but-3-yn-1-aminium (BYA+) chloride. Exposure of the alkyne BYA+ to I2 vapor causes spontaneous, irreversible iodination, yielding (E)-3,4-diiodobut-3-en-1-aminium (BEA-I2+; Fig. 2A). In contrast to detecting I2 dissolved in toluene or DCM, this reaction progresses spontaneously without direct contact between the perovskite and the solid BYA+ chloride salt ((BYA)Cl).
The black trace in Fig. 2B shows the 1H nuclear magnetic resonance (NMR) spectrum of (BYA)Cl in d6-DMSO. Upon exposing solid (BYA)Cl to I2 gas and then dissolving the solid in d6-DMSO, we see the appearance of a vinylic peak shifted to 7.3 ppm (blue trace), indicating the conversion of (BYA)Cl to (BEA-I2)Cl (Fig. S6†). This same peak is present in the NMR spectrum of solid (BYA)Cl that was first illuminated for 16 h in the presence of (MA)Pb(BrxI1−x)3 powder and then dissolved in d6-DMSO, directly showing the release of I2 vapor from the perovskite during illumination (Fig. 2B, inset). Analogous measurements done after heating to ca. 45 °C over the same period do not show a detectable peak at 7.3 ppm, suggesting the release of I2 does not originate from heating during illumination. In an accompanying mass spectrometry measurement of the same sample, an ion matching the m/z value for BEA-I2+ was obtained from the illuminated sample (Fig. 2C). No species with an m/z corresponding to any other addition products of the chemical trap were detected (e.g., BEA-HI+, BEA-IBr+, BEA-Br2+). There was not enough BEA-I2+ to be detected by the same measurement in the sample that was heated in the dark, supporting the conclusions drawn from the NMR spectra (Fig. S7†).
Electronic doping with loss of I2
Because iodide loss through I2 off-gassing as described in eqn (1) dopes the perovskite with excess electrons36,37—assuming the iodine vacancy defect is ionized—electronic conductivity changes are expected following illumination. Although intrinsic defect formation in most lead-halide perovskites is known to be charge-compensated,53,54 the Fermi level has been shown to shift down (becoming more p-type) in (MA)PbI3 films upon I2 exposure38 and to shift up (becoming more n-type) in CsPb(BrxI1−x)3 films upon I2 loss through extended UV illumination under vacuum.55 The change of an illuminated (MA)PbI3 film from p-type to n-type upon apparent iodine loss through a solvent, and the reverse effect upon exposing the film to an I2 atmosphere, has also been confirmed through Hall effect measurements.31Thin films of (MA)Pb(BrxI1−x)3 were spin-cast on interdigitated indium tin oxide electrodes to measure the impedance spectra of the perovskite films. Impedance spectra were collected between 7 MHz and 10 mHz; the bulk electrical resistance was evaluated from the low-frequency limit. The resistance of the film decreases following illumination (from 0.802 GΩ to 0.566 GΩ), consistent with I2 loss increasing the majority carrier (e−) concentration (Fig. 3A). Heating to 42 °C in the dark distinctly increases the resistance (from 1.43 GΩ to 1.92 GΩ), likely due to decomposition, further indicating that n-doping is a light-driven process (Fig. 3B). Subsequent exposure to I2 vapor in a nitrogen atmosphere for 20 minutes shows that the resistance of the illuminated film begins to recover, as expected through the reverse reaction in eqn (1) (Fig. 3A; from 0.566 GΩ to 0.672 GΩ).
Conclusions
We present, to our knowledge, the first evidence for I2 off-gassing, through the solid–air interface, from a mixed-iodide-bromide perovskite following solar illumination. Iodide loss from the bulk perovskite is strongly suggested by a shift to higher optical absorption onset and smaller lattice constant (Fig. 1). By using a solid salt to trap I2 vapor directly, we show that the iodide is lost as I2, eliminating complications inherent to solid–liquid interfaces (Fig. 2). The effects of heating concomitant with illumination were decoupled through control experiments. In contrast to the effects of heating alone, illumination—and the associated I2 off-gassing—substantially increases bulk conductivity through the anticipated n-doping effect (Fig. 3 and eqn (1) forward reaction). Exposing the off-gassed perovskite film to I2 vapor decreases its conductivity (Fig. 3 and eqn (1) reverse reaction), evidencing both sides of the I−/I2 equilibrium.We propose that irreversible I2 loss can masquerade as a “healing” or remixing of a halide-segregated mixed-halide perovskite with illumination. As I2 is lost to the atmosphere and the iodide-rich regions of the halide-segregated films are depleted, photogenerated carriers are no longer trapped in these regions. The n-doping that accompanies I2 loss may prevent photogenerated charge accumulation in the small-gap iodide-rich regions, further quenching PL from these regions. Thus, the lower-energy PL is lost, to be replaced by the higher-energy PL from photogenerated carriers in the bromide-rich regions, which are more stable to halogen loss due to the higher net positive enthalpy for Br2 generation from cleaving Pb–Br bonds. Since the redshifted PL upon illumination has been used as a diagnostic for halide segregation, the loss of this redshifted peak and emergence of the higher-energy PL may only appear as a reversal of halide segregation. Although the prior studies claiming halide-remixing with light24,25 used pulsed and continuous-wave lasers at much higher intensities (e.g., 10–200 W cm−2) where other dynamics may be at play, we expect that light-soaking with high-intensity light will ultimately degrade the perovskite through I2 loss and cannot be used to combat light-induced halide segregation in a device. Our studies were performed at ca. 1 sun (0.1 W cm−2) continuous illumination and I2 loss should be considered as a viable decomposition pathway even at typical solar-cell operating conditions. Although O2 may accelerate film degradation under illumination, as shown previously,26 O2 is not necessary for light-induced I2 loss of the illuminated films.
We thus propose the following modified scheme for light-induced halide segregation: For bromide-rich, mixed-halide compositions, the probability of finding a sufficient concentration of surface iodides, capable of forming I–I bonds, is low. For example, if we assume that the bulk halide stoichiometry is maintained at the surface, the probability of finding a [PbI6]4− coordination sphere in (MA)Pb(Br0.75I0.25)3 is only 0.02% (Fig. S8†), and likewise the probability of finding two iodides in adjacent octahedra in close enough contact to form I2, is also low. However, the local heterogeneity inherent to mixed-halide perovskites56,57 leads to a distribution of bromides and iodides at individual Pb centers, with some probability of having coordination spheres of [PbI6]4−, [PbI5Br]4−, [PbI4Br2]4−, [PbI3Br3]4−, [PbI2Br4]4−, [PbIBr5]4−, and [PbBr6]4−, depending on the overall halide ratio. Thus, we can expect that photogenerated holes will first form at the higher-energy orbitals of iodide-rich Pb centers. Various sophisticated and quantitative models have been proposed to explain the reversible growth of iodide-rich domains under illumination in these materials.20–22,58,59 For the purposes of explaining our observations, we simply propose that positive charges (photoexcited holes), which accumulate at the local iodide-rich lead centers, provide an electrostatic driving force for the negatively charged, mobile iodides31 in the framework to cluster around these regions, growing the iodide-rich domains and stabilizing the holes. Thus, light-induced phase segregation greatly increases the concentration of neighboring iodides at the surface (Fig. 1A). These iodide-rich domains continue to trap holes and are therefore perfectly poised for I2 loss.
Thus, mixed-iodide-bromide perovskites are not protected from I2 loss. As we have noted earlier for iodide perovskites,37 this further motivates the development of I2-impermeable encapsulation materials that can build up the partial pressure of I2 at the perovskite surface and drive reaction (1) in reverse through Le Chatelier's principle.
Note added prior to submission
Just prior to manuscript submission (on 11/28/2024), a study was published online (on 11/25/2024)55 reporting I2 loss (probed by mass loss and X-ray photoelectron spectroscopy) from mixed-iodide-bromide perovskite films under UV illumination (370 nm; 75 mW cm−2) over long durations (24 h) and under vacuum (20 mTorr). Our work shows I2 loss from mixed-iodide-bromide perovskite films and powders under simulated solar illumination (AM1.5G through glass to remove the minor UV component; 100 mW cm−2) over shorter duration (30 minutes) and at ambient pressure, which are more representative of operating conditions in a solar cell with a standard glass top cover that filters out UV light. The two studies are concurrent, complementary, and completely independent.Data availability
Experimental methods, spectra, and the statistical distribution of lead-halide coordination spheres are available in the ESI.†Author contributions
The study was conceived by M. L. , J. A. V. and H. I. K. studies on (CH3NH3)Pb(BrxI1−x)3 were conducted by M. L. and J. A. V. and studies on CsPb(BrxI1−x)3 were conducted by Z. J. the manuscript was written by all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. L. acknowledges a grant received through Stanford University Undergraduate Research and Independent Projects. This work was partially supported by Exxon Mobil through its membership in the Stanford Strategic Energy Alliance. J. A. V. acknowledges fellowship support from the Stanford University Office of the Vice Provost of Graduate Education and the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1656518. Profilometry experiments and thin-film diffuse-reflectance measurements were performed at the Stanford Nano Shared Facilities (SNSF), supported by the National Science Foundation under award ECCS-2026822. Liquid chromatography-mass spectrometry data were collected by the University of Illinois Urbana-Champaign Mass Spectrometry Lab. We thank Profs. Cargnello and Dauskardt at Stanford University for access to equipment and Prof. Alberto Salleo at Stanford University for helpful discussions.References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef.
- H. S. Kim, C. R. Lee, J. H. Im, K. B. Lee, T. Moehl, A. Marchioro, S. J. Moon, R. Humphry-Baker, J. H. Yum, J. E. Moser, M. Grätzel and N. G. Park, Sci. Rep., 2012, 2, 1–7 Search PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef PubMed.
- G. E. Eperon, M. T. Hörantner and H. J. Snaith, Nat. Rev. Chem, 2017, 1, 0095 CrossRef.
- C. D. Bailie, M. G. Christoforo, J. P. Mailoa, A. R. Bowring, E. L. Unger, W. H. Nguyen, J. Burschka, N. Pellet, J. Z. Lee, M. Grätzel, R. Noufi, T. Buonassisi, A. Salleo and M. D. McGehee, Energy Environ. Sci., 2015, 8, 956–963 RSC.
- J. P. Mailoa, C. D. Bailie, E. C. Johlin, E. T. Hoke, A. J. Akey, W. H. Nguyen, M. D. McGehee and T. Buonassisi, Appl. Phys. Lett., 2015, 106, 121105 CrossRef.
- S. Mariotti, E. Köhnen, F. Scheler, K. Sveinbjörnsson, L. Zimmermann, M. Piot, F. Yang, B. Li, J. Warby, A. Musiienko, D. Menzel, F. Lang, S. Keßler, I. Levine, D. Mantione, A. Al-Ashouri, M. S. Härtel, K. Xu, A. Cruz, J. Kurpiers, P. Wagner, H. Köbler, J. Li, A. Magomedov, D. Mecerreyes, E. Unger, A. Abate, M. Stolterfoht, B. Stannowski, R. Schlatmann, L. Korte and S. Albrecht, Science, 2023, 381, 63–69 CrossRef.
- X. Y. Chin, D. Turkay, J. Steele, S. Tabean, S. Eswara, M. Mensi, P. Fiala, C. M. Wolff, A. Paracchino, K. Artuk, D. Jacobs, Q. Guesnay, F. Sahli, G. Andreatta, M. Boccard, Q. Jeangros and C. Ballif, Science, 2023, 381, 59–63 CrossRef.
- T. Leijtens, K. A. Bush, R. Prasanna and M. D. McGehee, Nat. Energy, 2018, 3, 828–838 CrossRef.
- T. J. Coutts, K. A. Emery and J. S. Ward, Prog. Photovolt.: Res. Appl., 2002, 10, 195–203 CrossRef.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef PubMed.
- J. Emara, T. Schnier, N. Pourdavoud, T. Riedl, K. Meerholz and S. Olthof, Adv. Mater., 2016, 28, 553–559 CrossRef PubMed.
- M. G. Goesten and R. Hoffmann, J. Am. Chem. Soc., 2018, 140, 12996–13010 CrossRef CAS PubMed.
- K. P. Lindquist, J. A. Vigil, A. C. Su and H. I. Karunadasa, in Comprehensive Inorganic Chemistry III, Elsevier, 2023, vol. 1, pp. 499–559 Search PubMed.
- J. H. Noh, S. H. Im, J. H. Heo, T. N. Mandal and S. I. Seok, Nano Lett., 2013, 13, 1764–1769 CrossRef CAS.
- E. T. Hoke, D. J. Slotcavage, E. R. Dohner, A. R. Bowring, H. I. Karunadasa and M. D. McGehee, Chem. Sci., 2015, 6, 613–617 RSC.
- F. H. Isikgor, F. Furlan, J. Liu, E. Ugur, M. K. Eswaran, A. S. Subbiah, E. Yengel, M. De Bastiani, G. T. Harrison, S. Zhumagali, C. T. Howells, E. Aydin, M. Wang, N. Gasparini, T. G. Allen, A. ur Rehman, E. Van Kerschaver, D. Baran, I. McCulloch, T. D. Anthopoulos, U. Schwingenschlögl, F. Laquai and S. De Wolf, Joule, 2021, 5, 1566–1586 CrossRef CAS.
- J. Xu, C. C. Boyd, Z. J. Yu, A. F. Palmstrom, D. J. Witter, B. W. Larson, R. M. France, J. Werner, S. P. Harvey, E. J. Wolf, W. Weigand, S. Manzoor, M. F. A. M. Van Hest, J. J. Berry, J. M. Luther, Z. C. Holman and M. D. McGehee, Science, 2020, 367, 1097–1104 CrossRef CAS PubMed.
- S. J. Yoon, S. Draguta, J. S. Manser, O. Sharia, W. F. Schneider, M. Kuno and P. V. Kamat, ACS Energy Lett., 2016, 1, 290–296 CrossRef CAS.
- C. G. Bischak, C. L. Hetherington, H. Wu, S. Aloni, D. F. Ogletree, D. T. Limmer and N. S. Ginsberg, Nano Lett., 2017, 17, 1028–1033 CrossRef.
- S. Peng, Y. Wang, M. Braun, Y. Yin, A. C. Meng, W. Tan, B. Saini, K. Severson, A. F. Marshall, K. Sytwu, J. D. Baniecki, J. Dionne, W. Cai and P. C. McIntyre, Matter, 2023, 6, 2052–2065 CrossRef.
- D. J. Slotcavage, H. I. Karunadasa and M. D. McGehee, ACS Energy Lett., 2016, 1, 1199–1205 CrossRef.
- M. C. Brennan, A. Ruth, P. V. Kamat and M. Kuno, Trends Chem., 2020, 2, 282–301 CrossRef.
- W. Mao, C. R. Hall, S. Bernardi, Y. B. Cheng, A. Widmer-Cooper, T. A. Smith and U. Bach, Nat. Mater., 2021, 20, 55–61 CrossRef PubMed.
- Y. Guo, X. Yin, D. Liu, J. Liu, C. Zhang, H. Xie, Y. Yang and W. Que, ACS Energy Lett., 2021, 6, 2502–2511 CrossRef.
- S. Ruan, M. A. Surmiak, Y. Ruan, D. P. McMeekin, H. Ebendorff-Heidepriem, Y. B. Cheng, J. Lu and C. R. McNeill, J. Mater. Chem. C, 2019, 7, 9326–9334 RSC.
- N. Aristidou, C. Eames, I. Sanchez-Molina, X. Bu, J. Kosco, M. Saiful Islam and S. A. Haque, Nat. Commun., 2017, 8, 1–10 CrossRef.
- J. R. Vicente and J. Chen, J. Phys. Chem. Lett., 2020, 11, 1802–1807 CrossRef PubMed.
- J. R. Vicente, M. E. Kordesch and J. Chen, J. Energy Chem., 2021, 63, 8–11 CrossRef.
- A. D. Wright, J. B. Patel, M. B. Johnston and L. M. Herz, Adv. Mater., 2023, 35, 2210834 CrossRef.
- G. Y. Kim, A. Senocrate, T. Y. Yang, G. Gregori, M. Grätzel and J. Maier, Nat. Mater., 2018, 17, 445–449 CrossRef PubMed.
- A. Senocrate, I. Moudrakovski, G. Y. Kim, T. Y. Yang, G. Gregori, M. Grätzel and J. Maier, Angew. Chem., Int. Ed. Engl., 2017, 56, 7755–7759 CrossRef.
- S. Wang, Y. Jiang, E. J. Juarez-Perez, L. K. Ono and Y. Qi, Nat. Energy, 2017, 2, 16195 CrossRef.
- F. Fu, S. Pisoni, Q. Jeangros, J. Sastre-Pellicer, M. Kawecki, A. Paracchino, T. Moser, J. Werner, C. Andres, L. Duchêne, P. Fiala, M. Rawlence, S. Nicolay, C. Ballif, A. N. Tiwari and S. Buecheler, Energy Environ. Sci., 2019, 12, 3074–3088 RSC.
- D. M. Smyth, Annu. Rev. Mater. Res., 1985, 15, 329–357 CrossRef.
- A. H. Slavney, L. Leppert, A. Saldivar Valdes, D. Bartesaghi, T. J. Savenije, J. B. Neaton and H. I. Karunadasa, Angew. Chem., Int. Ed., 2018, 57, 12765–12770 CrossRef.
- J. A. Vigil, N. R. Wolf, A. H. Slavney, R. Matheu, A. Saldivar Valdes, A. Breidenbach, Y. S. Lee and H. I. Karunadasa, ACS Cent. Sci., 2024, 10, 907–919 Search PubMed.
- A. Zohar, I. Levine, S. Gupta, O. Davidson, D. Azulay, O. Millo, I. Balberg, G. Hodes and D. Cahen, ACS Energy Lett., 2017, 2, 2408–2414 CrossRef.
- L.-J.-M. Daguerre, Historique et description des procédés du daguerréotype et du diorama, Susse frères, Paris, France, 1839 Search PubMed.
- E. J. Juarez-Perez, L. K. Ono, M. Maeda, Y. Jiang, Z. Hawash and Y. Qi, J. Mater. Chem. A, 2018, 6, 9604–9612 RSC.
- Z. Song, C. Wang, A. B. Phillips, C. R. Grice, D. Zhao, Y. Yu, C. Chen, C. Li, X. Yin, R. J. Ellingson, M. J. Heben and Y. Yan, Sustain. Energy Fuels, 2018, 2, 2460–2467 RSC.
- Y. Li, X. Xu, C. Wang, B. Ecker, J. Yang, J. Huang and Y. Gao, J. Phys. Chem. C, 2017, 121, 3904–3910 CrossRef.
- P. S. Mathew, G. F. Samu, C. Janáky and P. V. Kamat, ACS Energy Lett., 2020, 5, 1872–1880 CrossRef.
- Y. Zhou, S. C. W. van Laar, D. Meggiolaro, L. Gregori, S. Martani, J. Heng, K. Datta, J. Jiménez-López, F. Wang, E. L. Wong, I. Poli, A. Treglia, D. Cortecchia, M. Prato, L. Kobera, F. Gao, N. Zhao, R. A. J. Janssen, F. De Angelis and A. Petrozza, Adv. Mater., 2023, 36, 2305567 CrossRef.
- G. F. Samu, Á. Balog, F. De Angelis, D. Meggiolaro, P. V. Kamat and C. Janáky, J. Am. Chem. Soc., 2019, 141, 10812–10820 CrossRef PubMed.
- J. T. DuBose, P. S. Mathew, J. Cho, M. Kuno and P. V. Kamat, J. Phys. Chem. Lett., 2021, 12, 2615–2621 CrossRef PubMed.
- H. Jiang, S. Cui, Y. Chen and H. Zhong, Nano Sel., 2021, 2, 2040–2060 CrossRef.
- O. Cvrčková and M. Ciganek, Polycyclic Aromat. Compd., 2005, 25, 141–156 CrossRef.
- Yu. A. Mikheev, V. P. Pustoshnyi and D. Ya. Toptygin, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1976, 25, 1962–1964 CrossRef.
- I. I. Levina, O. N. Klimovich, D. S. Vinogradov, T. A. Podrugina, D. S. Bormotov, A. S. Kononikhin, O. V. Dement’eva, I. N. Senchikhin, E. N. Nikolaev, V. A. Kuzmin and T. D. Nekipelova, J. Phys. Org. Chem., 2018, 31, e3844 CrossRef.
- A. Jaffe, Y. Lin and H. I. Karunadasa, ACS Energy Lett., 2017, 2, 1549–1555 CrossRef.
- J. B. Nelson and D. P. Riley, Proc. Phys. Soc., 1945, 57, 160–177 Search PubMed.
- A. Walsh, D. O. Scanlon, S. Chen, X. G. Gong and S.-H. Wei, Angew. Chem., Int. Ed., 2015, 54, 1791–1794 CrossRef PubMed.
- J.-H. Yang, W.-J. Yin, J.-S. Park and S.-H. Wei, Sci. Rep., 2015, 5, 16977 CrossRef PubMed.
- Z. Xu, X. Zhong, T. Hu, J. Hu, A. Kahn and B. P. Rand, J. Am. Chem. Soc., 2024, 146, 33368 Search PubMed.
- A. M. Askar, A. Karmakar, G. M. Bernard, M. Ha, V. V. Terskikh, B. D. Wiltshire, S. Patel, J. Fleet, K. Shankar and V. K. Michaelis, J. Phys. Chem. Lett., 2018, 9, 2671–2677 CrossRef PubMed.
- A. Karmakar, A. M. Askar, G. M. Bernard, V. V. Terskikh, M. Ha, S. Patel, K. Shankar and V. K. Michaelis, Chem. Mater., 2018, 30, 2309–2321 CrossRef.
- S. Draguta, O. Sharia, S. J. Yoon, M. C. Brennan, Y. V. Morozov, J. M. Manser, P. V. Kamat, W. F. Schneider and M. Kuno, Nat. Commun., 2017, 8, 200 Search PubMed.
- Z. Chen, G. Brocks, S. Tao and P. A. Bobbert, Nat. Commun., 2021, 12, 2687 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc08092k |
| This journal is © The Royal Society of Chemistry 2025 |


