Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An endoplasmic reticulum-targeting hydroxyl radical fluorescent probe for imaging of ferroptosis and screening of natural protectants†
Hongyu
Li
*ac,
Xue
Luo
ac,
Yue
Jian
ac,
Jiajia
Lv
ac,
Xinmin
Li
ac,
Jie
Gao
ac,
Wen
Shi
 bd,
Xiaohua
Li
bd,
Xiaohua
Li
 b,
Zeli
Yuan
*ac and
Huimin
Ma
b,
Zeli
Yuan
*ac and
Huimin
Ma
 *bd
*bd
aCollege of Pharmacy, Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi, Guizhou 563003, China. E-mail: lihongyu@iccas.ac.cn; zlyuan@zmu.edu.cn
bKey Laboratory of Analytical Chemistry for Living Biosystems, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: mahm@iccas.ac.cn
cGuizhou International Science & Technology Cooperation Base of Medical Optical Theranostics Research, Zunyi, Guizhou 563003, China
dUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 28th January 2025
Abstract
The real-time and in situ detection of hydroxyl radicals (˙OH) in the endoplasmic reticulum (ER) is helpful to understand ferroptosis at its very early stage due to the crucial role of ˙OH in the ER in ferroptosis initiation. Herein, an ER-targeting ˙OH fluorescent probe (ER-OH) has been developed, which showed a large fluorescence increase at 645 nm in response to ˙OH. ER-OH was applied to monitor ferroptosis by fluorescence imaging, revealing a significant increase of the ˙OH level in the ER during this process. With the imaging of ER-OH, a high-throughput screening method was developed to evaluate the anti-ferroptosis activity of a series of natural protectants. Through this screening, the natural flavonoid derivative icariside I was found for the first time to be highly effective in inhibiting ferroptosis by direct scavenging of the excess cytotoxic oxides (e.g. ˙OH and lipid peroxides) and restoring the level of GPX4. ER-OH could also be used for in vivo imaging of ˙OH in a mouse tumor model. This work provides not only a new tool for ferroptosis monitoring but also a direct insight into the regulation mechanism of ferroptosis and development of new drugs for ferroptosis-related diseases.
Introduction
Ferroptosis, an iron-dependent regulated cell death, involves a variety of important physiological and pathological processes, such as senility, immunity, tumorigenesis, ischemia/reperfusion injury, and neurodegenerative diseases.1–3 Ferroptosis is driven by lipid peroxidation (LPO) in cellular lipid membranes.4,5 The endoplasmic reticulum (ER) is an organelle containing a large lipid membrane structure, comprising about half of the total lipid membrane content in a cell.6 It has been reported that the ER is the initial site of LPO accumulation during ferroptosis, which then spreads to other membranes.7 Hydroxyl radicals (˙OH) are one of the most important LPO initiators due to their strong oxidation and dehydrogenation ability.8,9 In our previous studies, a significant increase in the ˙OH level has been observed during ferroptosis.10–12 Therefore, the development of real-time and in situ ˙OH assay in the ER may enable monitoring ferroptosis at its very early stage, which is of great significance for the study of ferroptosis and the identification of therapeutic targets for related diseases as well as the screening of new drugs.Fluorescence imaging has promising application prospects in bioimaging research due to its advantages of high sensitivity, non-invasiveness, real-time imaging and great temporal–spatial sampling capability.13–17 A number of fluorescent probes have been proposed for imaging of ˙OH.18–33 However, very few of them, including the commercially available ones, can target the ER (Table S1†). Obviously, it is still a great challenge for real-time and in situ detection of ˙OH in the ER. This may be due to two reasons. One is the nature of ˙OH, such as high reactivity, short lifetime (nanosecond) and low physiological level (nanomolar),34 making it difficult to detect ˙OH in biosystems. The other is that the dense lipid microenvironment of the ER impedes ˙OH capture by the probes. To date, only one ER-targeting ˙OH probe has been reported by our group;35 however, its relatively short analytical wavelength (<500 nm) and low sensitivity limit its biological applications. Therefore, there is an urgent need to develop an ER-targeting ˙OH fluorescent probe with higher sensitivity and longer analytical wavelength (>600 nm), which would be beneficial to not only reducing the biological background fluorescence and photodamage but also enhancing the biological tissue penetration of light signals.
In this work, we report such an ER-targeting ˙OH fluorescent probe ER-OH, which can be used to image ferroptosis. As shown in Scheme 1A, ER-OH is designed based on a stable and bright coumarin fluorophore. In order to increase the sensitivity of the probe, dihydroquinoline, which has specific and sensitive reaction to ˙OH,26,36 was selected as the recognition group. Furthermore, an ER-targetable p-methyl benzenesulfonamide group was introduced to enable the accumulation of the resulting probe in ER.37 After reaction with ˙OH, ER-OH underwent hydrogen abstraction to form the larger π-conjugation and donor–π–acceptor (D–π–A) structure of product 1 (Scheme 1A), which resulted in a large fluorescence increase at 645 nm, thus favoring the sensitive ˙OH detection in the ER. ER-OH has been applied to monitor the ˙OH variations in the ER during ferroptosis. Most importantly, based on the fluorescence imaging of ER-OH and using the initiator of ferroptosis (˙OH in the ER) as a monitoring indicator, a high-throughput screening method has been developed to evaluate the anti-ferroptosis activity of a series of natural protectants (Scheme 1B). Furthermore, the anti-ferroptosis effects of the screened natural protectant icariside I were investigated, revealing its great potential for studying ferroptosis-related diseases.
 | ||
| Scheme 1 (A) Design and fluorescence response mechanism of ER-OH. (B) The application of ER-OH in imaging of ferroptosis and screening of natural protectants for anti-ferroptosis. | ||
Results and discussion
Analytical properties of ER-OH
ER-OH and related intermediates were prepared according to the synthetic procedures in Scheme S1 (ESI†) and characterized by 1H NMR, 13C NMR and HR-ESI-MS (Fig. S1–S13; ESI†). The purity of ER-OH was determined to be 99.1% by HPLC assay (Fig. S14†). The spectroscopic properties of ER-OH in response to ˙OH were investigated in phosphate buffers. ˙OH was generated via the in situ reaction of tetrachloro-1,4-benzoquinone (TCBQ) and H2O2.38 As shown in Fig. S15,† the maximum absorption of ER-OH is at 420 nm; after reaction with ˙OH, an obvious increase at >500 nm is observed, accompanied by a color change from light yellow to purple (inset of Fig. S15†). On the other hand, ER-OH showed a large fluorescence enhancement of about 20-fold at 645 nm with excitation of 510 nm (Fig. 1A) after the reaction with ˙OH. The distinct spectroscopic changes in absorption and fluorescence implied the formation of product 1 with a larger π-conjugation, which was further confirmed by LC-MS (Fig. S16†) and HR-ESI-MS (Fig. S17†) assays.Next, the effects of detection conditions, such as pH and reaction time, were investigated. As shown in Fig. 1B and S18,† regardless of light illumination, the fluorescence intensity of ER-OH in the reaction system with TCBQ/H2O2 reaches a plateau in about 5 min and remains unchanged for at least 60 min; meanwhile, no significant fluorescence change is observed for ER-OH itself. These results suggest the good fluorescence stability of ER-OH. In addition, it was found that ER-OH exhibited significant fluorescence responses to ˙OH under near-physiological pH conditions of 6.0 to 7.4 (Fig. 1C), which may be attributed to the higher ˙OH production efficiency of TCBQ/H2O2 under near-neutral conditions, consistent with our previous work.26 Therefore, a 30 min reaction at physiological pH 7.4 was chosen as the ˙OH detection condition for the subsequent experiments. Furthermore, in the presence of different levels of ˙OH, the fluorescence response of ER-OH showed a linear concentration dependence (Fig. 1D). The linear equation was calculated to be F = 23.6 C (μM) + 34.7 (Fig. 1E), with a detection limit (S/N = 3) of 125 nM, which is much lower than 7 μM reported in the literature.35 A fast and quantitative fluorescence response was also observed in the detection of ˙OH generated by the Fenton reagent (Fe2+-EDTA/H2O2) but with a stronger response under acidic pH conditions due to the acid dependence of the Fenton reaction (Fig. S19†). Moreover, the detection of ER-OH was not affected by other common reactive oxygen species (ROS; Fig. 1F) and physiologically active species (Fig. S20†).
Fluorescence imaging performance of ER-OH
The performance of ER-OH for fluorescence imaging of ˙OH in the ER was evaluated. Before that, the cytotoxicity of ER-OH was tested via standard MTT assays. It was found that ER-OH showed negligible cytotoxicity to both HeLa and HT-1080 cells at a level of 50 μM after a 24 h incubation (Fig. S21†), suggesting that ER-OH is biocompatible. Then, the ER-targeting ability of ER-OH was studied by colocalization imaging with the commercial organelle dyes, including ER-Tracker Green (ETG), Lyso-Tracker Green (LTG) and Rhodamine 123 (R123). As expected, the fluorescence signal of ER-OH showed a high overlap (Pearson's coefficient: 0.86) with that of ER dye ETG (Fig. 2), in contrast to the poor overlap behaviors with the lysosome dye LTG (Pearson's coefficient: 0.52) and mitochondrial dye R123 (Pearson's coefficient: 0.49), which clearly indicated the favorable ER-targeting ability of ER-OH bearing the p-methyl benzenesulfonamide group.The exogenous ˙OH supplied by the Fenton reagent was then imaged in both HeLa and HT-1080 cells. As shown in Fig. S22,† in the probe-loaded group without exogenous ˙OH (Fig. S22b†), the cells show rather weak fluorescence. However, when the cells were incubated with 50 μM or 100 μM Fenton reagent (Fig. S22c and d†), a concentration-dependent fluorescence increase was produced. Next, to monitor the endogenous production of ˙OH, the cells were pretreated with phorbol-12-myristate-13-acetate (PMA), which could trigger the increase in the intracellular ROS level.39 As expected, the PMA treatment (Fig. S23c–e†) could result in significant fluorescence enhancement in both HeLa and HT-1080 cells compared to the untreated cells (Fig. S23b†). In addition, the above increased fluorescence signals produced by exogenous and endogenous ˙OH could be efficiently eliminated upon the addition of the ˙OH scavenger tempol (Fig. S22e and S23f†). These results suggest that ER-OH could be used to monitor the change in the ˙OH level in the ER.
Monitoring of ER ˙OH variation during ferroptosis
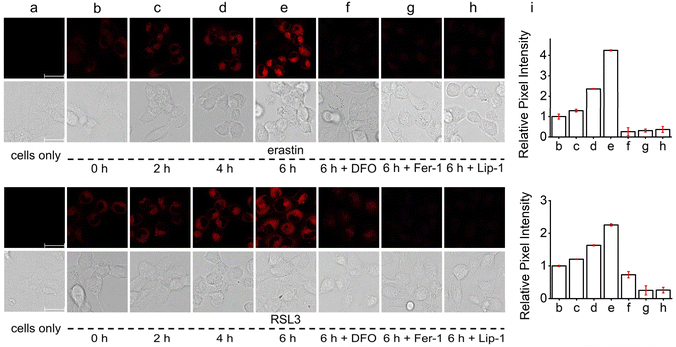
Because of the favorable ˙OH imaging performance and ER-targeting ability of ER-OH, we next applied it for in situ monitoring of ER ˙OH variations during the ferroptosis process. For this, the ferroptosis-susceptible HT-1080 cells were pretreated with two typical ferroptosis initiators with different mechanisms, erastin40 and RSL3,41 to induce ferroptotic cell death and then stained with ER-OH for fluorescence imaging (Fig. 3). Erastin is a potent inhibitor of the cystine/glutamate antiporter system, can inhibit cystine uptake and lead to the depletion of intracellular biothiols, including glutathione (GSH), which can eliminate lipid peroxides with the catalytic action of the antioxidative glutathione peroxidase 4 (GPX4). As a result, cells treated with erastin would undergo LPO accumulation to the lethal level to induce ferroptosis. Therefore, in the first instance, HT-1080 cells were treated with erastin. It was found that a 2, 4 or 6 h incubation of erastin could lead to a 0.29-, 1.36- or 3.25-fold of fluorescence enhancement, respectively (Fig. 3). On the other hand, RSL3 is a covalent inhibitor of GPX4, which can directly block the elimination of LPO by GPX4 to induce ferroptosis. Similarly, treatment with RSL3 for 2, 4 or 6 h also produced a 0.20-, 0.63- or 1.26-fold fluorescence enhancement, respectively (Fig. 3). These results indicated that both erastin- and RSL3-induced ferroptosis exhibited a significant increase in the ER ˙OH level. More importantly, with the co-incubation of ferroptosis inhibitors, deferoxamine (DFO), liproxstatin-1 (Lip-1) and ferrostatin-1 (Fer-1), the intracellular fluorescence signals of both erastin- and RSL3-treated cells were significantly inhibited. This further established the increase of the ER ˙OH level during ferroptosis and also suggested that ER-OH is a usable tool for monitoring the ˙OH variation in ER.High-throughput screening of natural protectants for anti-ferroptosis
During the ferroptotic cell death, the intracellular antioxidants are unable to remove the excess cytotoxic oxides. Therefore, the introduction of exogenous antioxidants is expected to inhibit ferroptosis.42 The commonly used ferroptosis inhibitors (Lip-1 and Fer-1) are typical lipophilic antioxidants.43 Small molecule natural products are an important source of new drug development and modification. Natural products from herbs, such as derivatives of flavonoid, caffeic acid and polyphenol, have shown efficient antioxidant effects.44–47 Herein, a high-throughput screening method by fluorescence imaging was developed for identifying natural antioxidants with ˙OH scavenging ability and investigating their anti-ferroptosis effects. Based on the advantages of fluorescence imaging technology (e.g., high sensitivity, in situ and non-destructive imaging) and taking the initial step of ferroptosis as the monitoring index (that is, ER ˙OH level), it is expected to provide a direct perspective for anti-ferroptosis research. In brief, the cells were co-incubated with the ferroptosis initiator erastin and various natural products for 8 h, respectively, followed by staining with ER-OH for imaging (Fig. 4A). Then the ˙OH scavenging efficiency (E) of the corresponding natural products was calculated using the formula E (%) = (F1 − Fx)/(F1 − F0) × 100%, where F1, F0 and Fx represent the fluorescence intensity of ferroptotic cells, normal cells and ferroptotic cells co-incubated with natural products, respectively. As can be seen from Fig. 4, the two reported natural ferroptosis initiators, artemisinin and artesunate48,49 (images b and c in Fig. 4A), exhibit a synergistic ferroptosis-promoting effect when co-incubated with erastin, resulting in a significant increase in fluorescence (i.e. elevated ER ˙OH level); image d is another control (ferroptosis inhibitor Fer-1). In contrast, all natural antioxidants (images e–u in Fig. 4A) result in a significant decrease in ER ˙OH levels. Among these antioxidants, icariside I, a natural flavonoid derivative from the traditional Chinese herb Herba Epimedii, shows the best ˙OH scavenging efficiency of E = 88.40% (image e in Fig. 4B). On the other hand, a high ˙OH scavenging efficiency of 80.15% was also found in the RSL3-induced ferroptotic cells co-incubated with icariside I (Fig. S24†). Furthermore, icariside I showed concentration- and time-dependent ˙OH scavenging behavior in both erastin- and RSL3-induced ferroptosis processes (Fig. S25 and S26†). In addition, the ˙OH levels in ferroptotic cells treated with two other ferroptosis inducers, FIN56 and FINO2,50,51 were monitored by ER-OH imaging, which also revealed the increase of ˙OH levels in these two different ferroptosis pathways (Fig. S27†). Importantly, in the cells treated with FIN56 or FINO2, icariside I also showed an efficient ˙OH scavenging ability comparable to that of Fer-1. Taken together, these findings suggested that the elevated ˙OH level is a common feature of the four different ferroptosis pathways; icariside I, as a natural antioxidant, could remove excess intracellular ˙OH and effectively inhibit the ferroptosis process of cells. Icariside I was thus chosen for further studies on anti-ferroptosis effects.Studies on anti-ferroptosis effects of icariside I
To study the anti-ferroptosis effects of icariside I, HT-1080 cells were co-treated with the ferroptosis initiator (erastin or RSL3) and icariside I and then subjected to detecting the key molecular events associated with ferroptosis, such as the levels of GSH, LPO and GPX4. First, the intracellular GSH level was monitored by fluorescence imaging of the commercial GSH probe monochlorobimane. As shown in Fig. 5A and C, erastin, by blocking cystine uptake, causes a significant decrease in intracellular GSH levels, whereas co-incubation with icariside I (as well as the ferroptosis inhibitor Fer-1) does not lead to a recovery of GSH levels; in contrast, RSL3 directly inhibits GPX4 and does not affect intracellular GSH levels, whether icariside I is co-incubated or not. This observation indicated that the anti-ferroptosis activity of icariside I was not mediated by regulation of the intracellular GSH level. Then, the level of LPO (ferroptosis executor) was monitored by C11-BODIPY 581/591. It was found that icariside I could effectively reduce the level of intracellular LPO in both erastin- and RSL3-induced ferroptosis pathways, similar to the well-known lipophilic antioxidant Fer-1 (Fig. 5B and D). Further in vitro spectroscopic tests (Fig. S28†) indicated that icariside I is highly effective in scavenging lipid peroxides (produced by lipoxygenase and polyunsaturated fatty acid arachidonic acid), with a half maximal inhibitory concentration (IC50) of 8.19 μM. The intracellular level of free or loosely bound Fe2+ (labile Fe2+) is also a crucial factor of ferroptosis, which was monitored by the commercial Fe2+ probe RhoNox-1. Consistent with the previous reports,52,53 a significant increase in the Fe2+ level was observed in the ferroptotic cells, which could be then suppressed by co-incubation with icariside I or Fer-1 (Fig. S29†). The relative level of the central antioxidative enzyme GPX4 in ferroptosis was also examined (Fig. 5E). After incubation with the GPX4 inhibitor RSL3, the level of GXP4 showed a significant decrease, which, however, was restored with the addition of icariside I. Finally, and most importantly, icariside I demonstrated a potent rescue effect on ferroptotic cell death (Fig. 5F). The direct scavenging effect of icariside I on ˙OH was studied by ER-OH detection (Fig. 5G), revealing an IC50 value of 11.20 μM (Fig. 5H), comparable to that of lipid peroxides. Taken together, these results suggest that icariside I, as a natural antioxidant, can inhibit ferroptosis by direct scavenging of the excess cytotoxic oxides (˙OH and lipid peroxides) and restoring the level of GPX4 during ferroptosis, indicating its great potential in the treatment of ferroptosis-related diseases.In vivo imaging of ˙OH
The feasibility of ER-OH for in vivo fluorescence imaging was further explored in a tumor model, which was reported to be accompanied by a significant increase in the ROS level.54 5 week old SPF BALB/c female mice were selected to establish an ectopic 4T1 tumor model for imaging, and all animal experiments were approved by the Experimental Animal Ethics Committee of Zunyi Medical University (approval number: [2021]2-422). As shown in Fig. S30,† after in situ injection of ER-OH, only very weak fluorescence is observed in the normal tissues (on the left dorsum); however, the tumor tissue (on the right dorsum) shows strong fluorescence, which can be maintained for a long imaging time (more than 48 h). This result also demonstrates the ability of ER-OH for in vivo imaging, enabling its application in a wider range of physiological and pathological conditions.Conclusions
In conclusion, ER-OH as an ER-targeting ˙OH fluorescent probe has been developed for imaging ferroptosis in this work. ER-OH was designed based on a coumarin fluorophore, a dihydroquinoline recognition group, and an ER-targetable p-methyl benzenesulfonamide group. ER-OH showed a significant fluorescence increase at 645 nm in response to ˙OH due to the formation of the larger π-conjugation and D–π–A structure. ER-OH has been applied to monitor ferroptosis, revealing the increase of the ER ˙OH level during this process. Moreover, based on the fluorescence imaging of ER-OH, a high-throughput screening method was developed to evaluate the anti-ferroptosis activity of a series of natural protectants. Through this screening, the natural flavonoid derivative icariside I has been found for the first time to be highly effective in inhibiting ferroptosis by direct scavenging of the excess cytotoxic oxides and restoring the level of GPX4. ER-OH could also be used for in vivo imaging of ˙OH in the mouse tumor model. These studies provide not only a new tool for ferroptosis monitoring but also a direct insight into the regulation mechanism of ferroptosis and development of new drugs for ferroptosis-related diseases.Data availability
Supplementary data for this article, including materials and instruments, experimental methods, synthesis and characterization, and supplementary figures, are provided in the ESI.†Author contributions
Hongyu Li: methodology, validation, investigation, writing – original draft. Xue Luo: investigation, visualization. Yue Jian: investigation. Jiajia Lv: investigation. Xinmin Li: investigation. Jie Gao: investigation. Wen Shi: investigation. Xiaohua Li: investigation. Zeli Yuan: supervision, writing – review & editing. Huimin Ma: supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 82060626, 22004137, 22164022, 22174147, 22374153, 22174148, 22474147, and 22474144), Talents of Guizhou Science and Technology Cooperation Platform ([2020]4104), Excellent Youth Scientific and Technological Talents of Guizhou Province (Qiankehe platform talents [2021]5638), Science and Technology Innovation Team of Higher Education of Guizhou Provincial Education Department (Qianjiaoji [2023]073), Future Science and Technology Elite Talent Cultivation Project of Zunyi Medical University (ZYSE-2021-01), and Zunyi Science and Technology Plan Project (Zunshi Keren Platform [2023]2).References
- B. R. Stockwell, J. P. Friedmann Angeli, H. Bayir, A. I. Bush, M. Conrad, S. J. Dixon, S. Fulda, S. Gascon, S. K. Hatzios, V. E. Kagan, K. Noel, X. Jiang, A. Linkermann, M. E. Murphy, M. Overholtzer, A. Oyagi, G. C. Pagnussat, J. Park, Q. Ran, C. S. Rosenfeld, K. Salnikow, D. Tang, F. M. Torti, S. V. Torti, S. Toyokuni, K. A. Woerpel and D. D. Zhang, Cell, 2017, 171, 273–285 CrossRef CAS PubMed.
- X. Jiang, B. R. Stockwell and M. Conrad, Nat. Rev. Mol. Cell Biol., 2021, 22, 266–282 CrossRef PubMed.
- S. J. Dixon and J. A. Olzmann, Nat. Rev. Mol. Cell Biol., 2024, 25, 424–442 CrossRef CAS.
- V. E. Kagan, G. Mao, F. Qu, J. P. F. Angeli, S. Doll, C. S. Croix, H. H. Dar, B. Liu, V. A. Tyurin, V. B. Ritov, A. A. Kapralov, A. A. Amoscato, J. Jiang, T. Anthonymuthu, D. Mohammadyani, Q. Yang, B. Proneth, J. Klein-Seetharaman, S. Watkins, I. Bahar, J. Greenberger, R. K. Mallampalli, B. R. Stockwell, Y. Y. Tyurina, M. Conrad and H. Bayır, Nat. Chem. Biol., 2017, 13, 81–90 CrossRef CAS.
- W. S. Yang, K. J. Kim, M. M. Gaschler, M. Patel, M. S. Shchepinov and B. R. Stockwell, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E4966–E4975 CAS.
- E. M. Lynes and T. Simmen, Biochim. Biophys. Acta, Mol. Cell Res., 2011, 1813, 1893–1905 CrossRef CAS PubMed.
- A. N. von Krusenstiern, R. N. Robson, N. Qian, B. Qiu, F. Hu, E. Reznik, N. Smith, F. Zandkarimi, V. M. Estes, M. Dupont, T. Hirschhorn, M. S. Shchepinov, W. Min, K. A. Woerpel and B. R. Stockwell, Nat. Chem. Biol., 2023, 19, 719–730 CrossRef CAS.
- M. K. Foret, R. Lincoln, S. Do Carmo, A. C. Cuello and G. Cosa, Chem. Rev., 2020, 120, 12757–12787 CrossRef CAS.
- S. Gligorovski, R. Strekowski, S. Barbati and D. Vione, Chem. Rev., 2015, 115, 13051–13092 CrossRef CAS.
- Y. An, X. Luo, S. Wei, J. Lv, J. Gao, X. Li, M. Yang, J. Luo, Y. Wu, G. Wei, Z. Yuan and H. Li, Sens. Actuators, B, 2023, 397, 134653 CrossRef CAS.
- Y. Chen, Z. Hu, M. Yang, J. Gao, J. Luo, H. Li and Z. Yuan, Sens. Actuators, B, 2022, 362, 131742 CrossRef CAS.
- H. Li, W. Shi, X. Li, Y. Hu, Y. Fang and H. Ma, J. Am. Chem. Soc., 2019, 141, 18301–18307 CrossRef CAS PubMed.
- A. Sharma, P. Verwilst, M. Li, D. Ma, N. Singh, J. Yoo, Y. Kim, Y. Yang, J.-H. Zhu, H. Huang, X.-L. Hu, X.-P. He, L. Zeng, T. D. James, X. Peng, J. L. Sessler and J. S. Kim, Chem. Rev., 2024, 124, 2699–2804 CrossRef CAS.
- X. Wang, Q. Ding, R. R. Groleau, L. Wu, Y. Mao, F. Che, O. Kotova, E. M. Scanlan, S. E. Lewis, P. Li, B. Tang, T. D. James and T. Gunnlaugsson, Chem. Rev., 2024, 124, 7106–7164 CrossRef CAS.
- X. Wu, R. Wang, N. Kwon, H. Ma and J. Yoon, Chem. Soc. Rev., 2022, 51, 450–463 RSC.
- X. Niu, H. Yang, X. Wu, F. Huo, K. Ma and C. Yin, Chem. Sci., 2024, 15, 14924–14930 RSC.
- X. Zhang, S. Shen, D. Liu, X. Li, W. Shi and H. Ma, Chem. Sci., 2023, 14, 2928–2934 RSC.
- X. Bai, Y. Huang, M. Lu and D. Yang, Angew. Chem., Int. Ed., 2017, 56, 12873–12877 CrossRef CAS.
- H. Li, X. Li, W. Shi, Y. Xu and H. Ma, Angew. Chem., Int. Ed., 2018, 57, 12830–12834 CrossRef CAS PubMed.
- X. Wang, P. Li, Q. Ding, C. Wu, W. Zhang and B. Tang, Angew. Chem., Int. Ed., 2019, 58, 4674–4678 CrossRef CAS PubMed.
- Q. Fu, H. Li, D. Duan, C. Wang, S. Shen, H. Ma and Z. Liu, Angew. Chem., Int. Ed., 2020, 59, 21546–21552 CrossRef CAS PubMed.
- Z. Wang, T. D. Cong, W. Zhong, J. W. Lau, G. Kwek, M. B. Chan-Park and B. Xing, Angew. Chem., Int. Ed., 2021, 60, 16900–16905 CrossRef CAS PubMed.
- L. Wu, Y. Ishigaki, W. Zeng, T. Harimoto, B. Yin, Y. Chen, S. Liao, Y. Liu, Y. Sun, X. Zhang, Y. Liu, Y. Liang, P. Sun, T. Suzuki, G. Song, Q. Fan and D. Ye, Nat. Commun., 2021, 12, 6145 CrossRef CAS PubMed.
- Z. Kang, J. Jiang, Q. Tu, S. Liu, Y. Zhang, D.-E. Wang, J. Wang and M.-S. Yuan, J. Am. Chem. Soc., 2022, 145, 507–515 CrossRef PubMed.
- J. Wu, Y. Zhao, K. Li, S. Muhammad, M. Ju, L. Liu, Y. Huang, B. Wang, W. Ding, B. Shen and H. Huang, TrAC, Trends Anal. Chem., 2022, 157, 116734 CrossRef CAS.
- H. Li, Y. An, X. Luo, J. Gao, M. Yang, X. Li, X. Li, W. Shi, Z. Yuan and H. Ma, Chem. Eng. J., 2023, 476, 146749 CrossRef CAS.
- L.-L. Wang, Y.-Z. Mai, M.-H. Zheng, X. Wu and J.-Y. Jin, Sens. Actuators, B, 2022, 373, 132707 CrossRef CAS.
- H. Gao, L. Sun, J. Li, Q. Zhou, H. Xu, X.-N. Ma, R. Li, B.-Y. Yu and J. Tian, Adv. Sci., 2023, 10, 2303926 CrossRef CAS PubMed.
- X. Xie, J. Bian, Y. Song, G. Liu, Y. Zhao, J. Zhang, Y. Li, X. Jiao, X. Wang and B. Tang, Anal. Chem., 2023, 95, 9872–9880 CrossRef CAS.
- Y. Chen, X. Ji, L. Tao, C. Ma, J. Nie, C. Lu, G. Yang, E. Wang, H. Liu, F. Wang and J. Ren, Biosens. Bioelectron., 2024, 246, 115868 CrossRef CAS PubMed.
- Q. Wang, H. Ji, Y. Hao, D. Jia, H. Ma, C. Song, H. Qi, Z. Li and C. Zhang, Anal. Chem., 2024, 96, 20189–20196 CrossRef CAS PubMed.
- Z. Kang, Y. Zhou, W. Wang, S. Wang, S. Wen, H. Yu, X. Liu, J. Wang and M.-S. Yuan, Sens. Actuators, B, 2025, 426, 137121 CrossRef CAS.
- X. Luo, Q. Rao, S. Wei, J. Lv, Y. Wu, M. Yang, J. Luo, J. Gao, X. Li, Z. Yuan and H. Li, Sens. Actuators, B, 2025, 424, 136951 CrossRef CAS.
- J. Fulford, H. Nikjoo, D. T. Goodhead and P. O'Neill, Int. J. Radiat. Biol., 2001, 77, 1053–1066 CrossRef CAS.
- Y. Zhao, H. Li, Z. Chai, W. Shi, X. Li and H. Ma, Chem. Commun., 2020, 56, 6344–6347 RSC.
- Y. Wu, W. Huang, D. Peng, X. A. Huang, J. Gu, S. Wu, T. Deng and F. Liu, Org. Lett., 2021, 23, 135–139 CrossRef CAS.
- H. Xiao, X. Liu, C. Wu, Y. Wu, P. Li, X. Guo and B. Tang, Biosens. Bioelectron., 2017, 91, 449–455 CrossRef CAS PubMed.
- B.-Z. Zhu, B. Kalyanaraman and G.-B. Jiang, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17575–17578 CrossRef CAS PubMed.
- D. Jagnandan, J. E. Church, B. Banfi, D. J. Stuehr, M. B. Marrero and D. J. R. Fulton, J. Biol. Chem., 2007, 282, 6494–6507 CrossRef CAS.
- S. J. Dixon, K. M. Lemberg, M. R. Lamprecht, R. Skouta, E. M. Zaitsev, C. E. Gleason, D. N. Patel, A. J. Bauer, A. M. Cantley, W. S. Yang, B. r. Morrison and B. R. Stockwell, Cell, 2012, 149, 1060–1072 CrossRef CAS PubMed.
- W. S. Yang, R. SriRamaratnam, M. E. Welsch, K. Shimada, R. Skouta, V. S. Viswanathan, J. H. Cheah, P. A. Clemons, A. F. Shamji, C. B. Clish, L. M. Brown, A. W. Girotti, V. W. Cornish, S. L. Schreiber and B. R. Stockwell, Cell, 2014, 156, 317–331 CrossRef CAS PubMed.
- N. Kajarabille and G. O. Latunde-Dada, Int. J. Mol. Sci., 2019, 20, 4968 CrossRef CAS PubMed.
- O. Zilka, R. Shah, B. Li, J. P. Friedmann Angeli, M. Griesser, M. Conrad and D. A. Pratt, ACS Cent. Sci., 2017, 3, 232–243 CrossRef CAS PubMed.
- K. Zheng, Y. Dong, R. Yang, Y. Liang, H. Wu and Z. He, Pharmacol. Res., 2021, 168, 105580 CrossRef CAS PubMed.
- H. A. Alaswad, A. A. Mahbub, C. L. Le Maitre and N. Jordan-Mahy, Int. J. Mol. Sci., 2021, 22, 3085 CrossRef CAS PubMed.
- Z. Xiang and Z. Ning, LWT–Food Sci. Technol., 2008, 41, 1189–1203 CrossRef CAS.
- P. Chen, Q. Wu, J. Feng, L. Yan, Y. Sun, S. Liu, Y. Xiang, M. Zhang, T. Pan, X. Chen, T. Duan, L. Zhai, B. Zhai, W. Wang, R. Zhang, B. Chen, X. Han, Y. Li, L. Chen, Y. Liu, X. Huang, T. Jin, W. Zhang, H. Luo, X. Chen, Y. Li, Q. Li, G. Li, Q. Zhang, L. Zhuo, Z. Yang, H. Tang, T. Xie, X. Ouyang and X. Sui, Signal Transduction Targeted Ther., 2020, 5, 51 CrossRef CAS.
- N. Eling, L. Reuter, J. Hazin, A. Hamacher-Brady and N. R. Brady, Oncoscience, 2015, 2, 517–532 CrossRef PubMed.
- E. Ooko, M. E. M. Saeed, O. Kadioglu, S. Sarvi, M. Colak, K. Elmasaoudi, R. Janah, H. J. Greten and T. Efferth, Phytomedicine, 2015, 22, 1045–1054 CrossRef CAS PubMed.
- L.-L. Wang, Y.-Z. Mai, M.-H. Zheng, G.-H. Yan and J.-Y. Jin, Dev. Cell, 2024, 59, 517–528.e513 CrossRef CAS PubMed.
- B. R. Stockwell, Cell, 2022, 185, 2401–2421 CrossRef CAS PubMed.
- T. Hirayama, A. Miki and H. Nagasawa, Metallomics, 2019, 11, 111–117 CrossRef CAS PubMed.
- W. Xing, H. Xu, H. Ma, S. A. A. Abedi, S. Wang, X. Zhang, X. Liu, H. Xu, W. Wang and K. Lou, Chem. Commun., 2022, 58, 2979–2982 RSC.
- M. Nishikawa, Cancer Lett., 2008, 266, 53–59 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and instruments, experimental methods, synthesis and characterization, and supplementary figures. See DOI: https://doi.org/10.1039/d4sc07953a |
| This journal is © The Royal Society of Chemistry 2025 |