Open Access Article
Open Access ArticleNi-catalyzed regioselective and site-divergent reductive arylalkylations of allylic amines†
Huan
Meng‡
a,
Jun-Song
Jia‡
b,
Peng-Fei
Yang
a,
Yu-Long
Li
 *b,
Qiong
Yu
*a and
Wei
Shu
*b,
Qiong
Yu
*a and
Wei
Shu
 *a
*a
aShenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Shenzhen Grubbs Institute, Guangming Advanced Research Institute, Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis Southern University of Science and Technology, Shenzhen 518055, Guangdong, P. R. China. E-mail: shuw@sustech.edu.cn; xcyuqiong@163.com
bCollege of Chemistry and Environmental Engineering, Key Laboratory of Green Catalysis of Higher Education Institutes of Sichuan, Sichuan University of Science and Engineering, Zigong, 643000, P. R. China. E-mail: yu_longli@suse.edu.cn
First published on 23rd January 2025
Abstract
Catalytic methods by switching the least parameters for regioselective and site-divergent transformations to construct different architectures from identical and readily available starting materials are among the most ideal catalytic protocols. However, the associated challenge to precisely control both regioselectivity and site diversity renders this strategy appealing yet challenging. Herein, Ni-catalyzed cross-electrophile regioselective and site-divergent 1,2- and 1,3-arylalkylations of N-acyl allylic amines have been developed. This Ni-catalyzed reductive three-component protocol enables 1,2-arylalkylation and 1,3-arylalkylation of allylic amines with aryl halides and alkyl halides with excellent chemo-, regio- and site-selectivity, representing the first example of controlled migratory difunctionalization of alkenes under reductive conditions. A wide range of terminal and internal unactivated allylic amines, aryl halides and alkyl precursors were tolerated, providing straightforward and efficient access to diverse C(sp3)-rich branched aliphatic amines from identical starting materials.
Introduction
Transition-metal-catalyzed cross-coupling reactions are powerful tools for the construction of saturated carbon centres and are widely used in the construction of drugs, natural products, agricultural chemicals, and organic materials.1 Among which, transition-metal-catalyzed reductive coupling reactions have attracted considerable attention due to the sole use of organo-electrophiles, circumventing the stepwise pre-synthesis of organometallic reagents.2 More importantly, reductive cross-coupling reactions are better suited for the construction of saturated carbon centres partially attributed to the low-valent state of metal centres under reducing conditions, which suppresses the unwanted β-hydrogen elimination process thus facilitating the formation of saturated C–C bonds.3 To this end, reductive cross-coupling between two electrophiles (such as aryl halides and alkyl halides) to build saturated C–C bonds has been extensively explored (Fig. 1a).3c,4 However, the construction of C(sp3)-rich scaffolds through multi-component reductive coupling reactions remains challenging due to the difficulties in distinguishing different electrophile coupling partners as well as the associated regio- and site-selectivity issues.5 | ||
| Fig. 1 Reductive cross-coupling enables regioselective and site-divergent arylalkylations of unactivated alkenes. | ||
On the other hand, alkenes have been recognized as a privileged platform for the construction of molecular complexity.6 A notable advantage of alkenes is that they have two potential functionalization sites, offering profound chemical space for rapid buildup of diverse C(sp3)-rich structures.7 Recently, the regioselective incorporation of carbon-based motifs through nickel-catalyzed reductive 1,2-dicarbofunctionalization of conjugated alkenes (such as arylalkene,8 vinyl esters9 and vinyl amides10) has been developed to provide saturated hydrocarbon frameworks.11 Notably, the regioselectivity is controlled by the π-system of the conjugated alkenes to form a thermodynamic stabilized alkyl intermediate.
However, it remains challenging to achieve the formation of double bonds to provide saturated hydrocarbon frameworks. Notably, the regioselectivity is controlled by the late regioselectivity of non-conjugated alkenes for such events.12 Recently, Ni-catalyzed reductive 1,2-alkylarylation and 1,2-diarylation of unactivated alkenes facilitated by using strong coordinating groups (such as 8-aminoquinoline and oxygen atom) have been reported.13 Notably, aryl groups are attached to the proximal carbon of the alkenes to directing groups. Instead, Ni-catalyzed reductive 1,2-arylalkylation of unactivated alkenes with the opposite regioselectivity, installing aryl groups to the distal carbon of the alkenes, remains less developed.5a,14 During the preparation of this paper, MacMillan reported a visible-light-mediated Ni-catalyzed 1,2-arylalkylation of unactivated alkenes with aryl bromides and redox-active ester of aliphatic carboxylic acids.5a The regioselectivity of this method was achieved by bimolecular homolytic substitution (SH2) and only applicable to primary alkyl radical precursors.5a,b However, Ni-catalyzed site-divergent arylalkylation of alkenes remains a formidable challenge due to the difficulties in precise regulation of alkyl–alkyl cross-coupling versus the β-hydrogen elimination process of alkylnickel intermediates (Fig. 1b).15 Herein, we report Ni-catalyzed regioselective and site-divergent arylalkylations of allylic amines under reductive conditions (Fig. 1c). This cross-electrophile coupling protocol enables chemo- and regioselectivity along with site-divergent selectivity of three-component arylalkylation of unactivated alkenes with aryl electrophiles installed on the distal carbon of alkenes. The catalytic conditions regulate 1,2- and 1,3-arylalkylations of allylic amines allowing for rapid access to diverse sp3-rich branched aliphatic amine derivatives from readily available and identical starting materials.
Results and discussion
We started to investigate the feasibility of these regioselective and site-divergent arylalkylations of allylic amines by using N-benzoyl allylic amine 1a, 4-iodoanisole 2a, and 3-phenyl-1-bromopropane 3a as model substrates to evaluate the reaction parameters (Table 1). After extensive preliminary optimization (Tables S1–S5†),16 1,2-arylalkylation product 4aa was formed in 85% yield with rr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in DMA (0.1 M) at room temperature using NiBr2·dme (10 mol%) as precatalyst, bisoxazoline ligand L1 (12 mol%) as ligand, and NaI (2.5 equiv.) as additive in the presence of manganese (Mn, 3.5 equiv.) as sacrificial reductant (conditions A, Table 1, entry 1). The use of NMP as solvent decreased the efficiency of reductive 1,2-arylalkylation, delivering 4aa in 61% with rr > 20
1 in DMA (0.1 M) at room temperature using NiBr2·dme (10 mol%) as precatalyst, bisoxazoline ligand L1 (12 mol%) as ligand, and NaI (2.5 equiv.) as additive in the presence of manganese (Mn, 3.5 equiv.) as sacrificial reductant (conditions A, Table 1, entry 1). The use of NMP as solvent decreased the efficiency of reductive 1,2-arylalkylation, delivering 4aa in 61% with rr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entry 2). Conducting the reaction in protic solvent IPA further decreased the yield of 4aa to 52% with rr > 20
1 (Table 1, entry 2). Conducting the reaction in protic solvent IPA further decreased the yield of 4aa to 52% with rr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entry 3). Next, we further evaluated the ligand effect on this reductive 1,2-arylalkylation reaction (Table 1, entries 4–9). Altering the ligand from L1 to L2–L4 reduced the yields of 4aa to 16–61%, albeit with excellent regioselectivity (rr > 20
1 (Table 1, entry 3). Next, we further evaluated the ligand effect on this reductive 1,2-arylalkylation reaction (Table 1, entries 4–9). Altering the ligand from L1 to L2–L4 reduced the yields of 4aa to 16–61%, albeit with excellent regioselectivity (rr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1, entries 4–6). Phosphine-oxazoline ligand L5 mediated the reaction smoothly, delivering 4aa in 34% yield (Table 1, entry 7). Interestingly, the use of bisoxazoline (L6) as ligand decreased the yield of 4aa to 43% along with the formation of migratory 1,3-arylalkylation product 5aa in 19% yield (Table 1, entry 8). Monophosphine ligand PCy3 delivered 1,2-arylalkylation product 4aa in 34% yield (Table 1, entry 9). The reaction proceeded smoothly in the absence of sodium iodide or ligand, affording 1,2-arylalkylation product 4aa in diminished yields and regioselectivities (Table 1, entries 10 and 11). Inspired by the results of using L6 as ligand, we turned to explore the potential of 1a to undergo migratory 1,3-arylalkylation reaction to give α-branched aliphatic amine 5aa. It was found that reaction temperature was important for the efficient formation of 5aa. When the reaction was conducted at 50 °C, the yield of 5aa was increased to 39% (Table 1, entry 12). Altering the nickel salt from NiBr2·dme to Ni(BF4)2·6H2O in IPA (0.1 M) further improved the yield of 5aa to 49% with rr > 20
1) (Table 1, entries 4–6). Phosphine-oxazoline ligand L5 mediated the reaction smoothly, delivering 4aa in 34% yield (Table 1, entry 7). Interestingly, the use of bisoxazoline (L6) as ligand decreased the yield of 4aa to 43% along with the formation of migratory 1,3-arylalkylation product 5aa in 19% yield (Table 1, entry 8). Monophosphine ligand PCy3 delivered 1,2-arylalkylation product 4aa in 34% yield (Table 1, entry 9). The reaction proceeded smoothly in the absence of sodium iodide or ligand, affording 1,2-arylalkylation product 4aa in diminished yields and regioselectivities (Table 1, entries 10 and 11). Inspired by the results of using L6 as ligand, we turned to explore the potential of 1a to undergo migratory 1,3-arylalkylation reaction to give α-branched aliphatic amine 5aa. It was found that reaction temperature was important for the efficient formation of 5aa. When the reaction was conducted at 50 °C, the yield of 5aa was increased to 39% (Table 1, entry 12). Altering the nickel salt from NiBr2·dme to Ni(BF4)2·6H2O in IPA (0.1 M) further improved the yield of 5aa to 49% with rr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and substantially suppressed the formation of 1,2-arylalkylation product 4aa (Table 1, entry 13). Solvent effect evaluation indicated the use of methanol reduced the formation of 5aa to 28% yield (Table 1, entry 14). To our delight, conducting the reaction in a mixture of alcohols (IPA
1, and substantially suppressed the formation of 1,2-arylalkylation product 4aa (Table 1, entry 13). Solvent effect evaluation indicated the use of methanol reduced the formation of 5aa to 28% yield (Table 1, entry 14). To our delight, conducting the reaction in a mixture of alcohols (IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5
MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) significantly improved the yield of the 1,3-arylalkylation reaction, affording 5aa in 76% yield with 16
1) significantly improved the yield of the 1,3-arylalkylation reaction, affording 5aa in 76% yield with 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr (conditions B, Table 1, entry 15). A mixed solvent of IPA
1 rr (conditions B, Table 1, entry 15). A mixed solvent of IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMA furnished 5aa in only 38% yield (Table 1, entry 16). Other ligands, such as PCy3 and L4, failed to promote this three-component 1,3- and 1,2-arylalkylation reaction under otherwise identical conditions (Table 1, entries 17 and 18). In addition, sodium iodide proved to be essential for the reaction, only 26% of 5aa being formed in the absence of sodium iodide (Table 1, entry 19). A control experiment revealed that ligand was also essential for 1,3-arylalkylation of allylic amines. No desired product 5aa was detected without ligand (Table 1, entry 20).
DMA furnished 5aa in only 38% yield (Table 1, entry 16). Other ligands, such as PCy3 and L4, failed to promote this three-component 1,3- and 1,2-arylalkylation reaction under otherwise identical conditions (Table 1, entries 17 and 18). In addition, sodium iodide proved to be essential for the reaction, only 26% of 5aa being formed in the absence of sodium iodide (Table 1, entry 19). A control experiment revealed that ligand was also essential for 1,3-arylalkylation of allylic amines. No desired product 5aa was detected without ligand (Table 1, entry 20).
| Entry | Solvent | T (°C) | [Ni] | Ligand | Yield of 4aab | Yield of 5aab |
|---|---|---|---|---|---|---|
| a Reactions were conducted using 1a (0.10 mmol), 2a (0.20 mmol), 3a (0.20 mmol), Mn (0.35 mmol), NaI (0.25 mmol), [Ni] (10 mol%), ligand (12 mol%) in indicated solvent (0.1 M) at room temperature for 18 h. ND = not determined. b Yield was determined by GC analysis using n-dodecane as internal standard. Isolated yield is shown in parentheses. c No NaI was used. d No ligand was used. e Reaction conditions: 1a (0.10 mmol), 2a (0.15 mmol), 3a (0.15 mmol), Mn (0.30 mmol), NaI (0.05 mmol), Ni(BF4)2·6H2O (10 mol%), ligand (12 mol%) in solvent (0.1 M) for 18 h. | ||||||
| 1 | DMA | rt | NiBr 2 ·dme | L1 | 85% (82%) | Trace |
| 2 | NMP | rt | NiBr2·dme | L1 | 61% | 2% |
| 3 | IPA | rt | NiBr2·dme | L1 | 52% | 2% |
| 4 | DMA | rt | NiBr2·dme | L2 | 61% | 2% |
| 5 | DMA | rt | NiBr2·dme | L3 | 16% | ND |
| 6 | DMA | rt | NiBr2·dme | L4 | 39% | ND |
| 7 | DMA | rt | NiBr2·dme | L5 | 34% | ND |
| 8 | DMA | rt | NiBr2·dme | L6 | 43% | 19% |
| 9 | DMA | rt | NiBr2·dme | PCy3 | 34% | ND |
| 10c | DMA | rt | NiBr2·dme | L1 | 67% | 14% |
| 11d | DMA | rt | NiBr2·dme | — | 32% | 6% |
| 12 | DMA | 50 | NiBr2·dme | L6 | 28% | 39% |
| 13e | IPA | 50 | Ni(BF4)2·6H2O | L6 | 0% | 49% |
| 14e | MeOH | 50 | Ni(BF4)2·6H2O | L6 | 0% | 28% |
| 15 | IPA/MeOH | 50 | Ni(BF 4 ) 2 ·6H 2 O | L6 | 5% | 76% (72%) |
| 16e | IPA/DMA | 50 | Ni(BF4)2·6H2O | L6 | 12% | 38% |
| 17e | IPA/MeOH | 50 | Ni(BF4)2·6H2O | PCy3 | ND | ND |
| 18e | IPA/MeOH | 50 | Ni(BF4)2·6H2O | L4 | ND | ND |
| 19c,e | IPA/MeOH | 50 | Ni(BF4)2·6H2O | L6 | 3% | 26% |
| 20d,e | IPA/MeOH | 50 | Ni(BF4)2·6H2O | — | ND | ND |
|
||||||
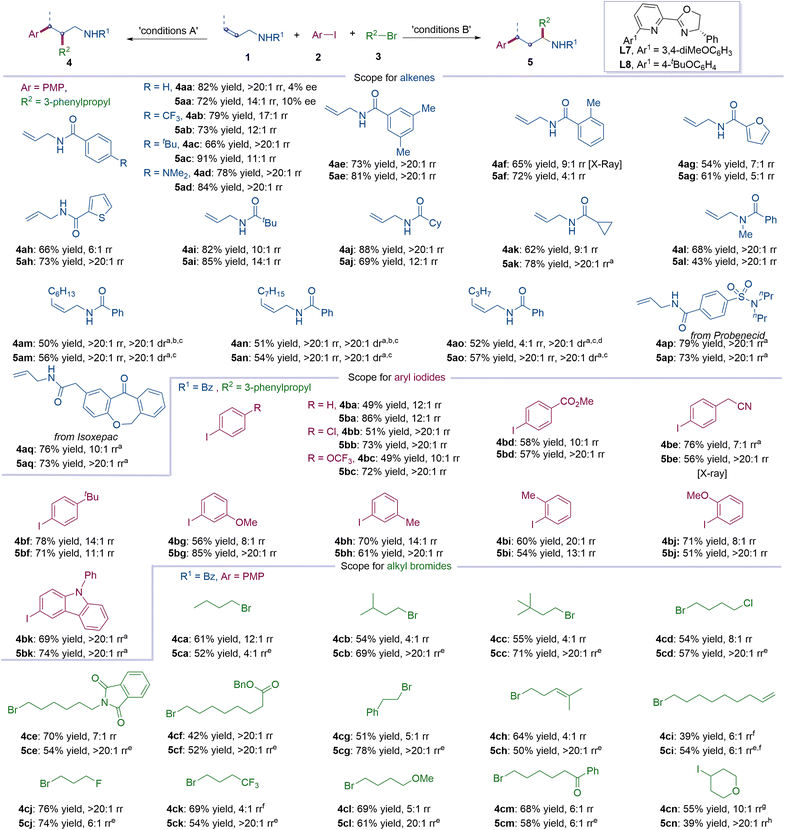
With two sets of optimized reaction conditions in hand, the substrate scope of 1,2- and 1,3-arylalkylation of allylic amines was examined, with the results summarized in Fig. 2. We first investigated the scope of allylic amines. N-Benzoyl allylic amines with electron-withdrawing or electron-donating groups are all good substrates for this reaction, affording corresponding 1,2-arylalkylation products (4aa–4ad) and 1,3-arylalkylation products (5aa–5ad) in good to excellent yields (66–91%) with rr of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 3,5-Dimethyl-substituted N-benzoyl allylic amine yielded products 4ae and 5ae in 73% and 81% yields, with regioselectivity >20
1. 3,5-Dimethyl-substituted N-benzoyl allylic amine yielded products 4ae and 5ae in 73% and 81% yields, with regioselectivity >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ortho-substituted N-benzoyl allylic amine provided 4af and 5af in 65% and 72% yields with 9
1. The ortho-substituted N-benzoyl allylic amine provided 4af and 5af in 65% and 72% yields with 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr. N-Heteroarylacyl-derived allylic amines, such as furans (4ag and 5ag) and thiophenes (4ah and 5ah), were successfully converted to corresponding target products in moderate to good yields (54–73%) and good regioselectivity (rr of 5
1 rr. N-Heteroarylacyl-derived allylic amines, such as furans (4ag and 5ag) and thiophenes (4ah and 5ah), were successfully converted to corresponding target products in moderate to good yields (54–73%) and good regioselectivity (rr of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Acyclic and cyclic aliphatic acyl-derived allylic amines were also compatible under corresponding standard conditions (1,2-arylalkylation: 4ai–4ak (62–88% yields); 1,3-arylalkylation: 5ai–5ak (69–85% yields)). Tertiary allylic amines without free N–H produced desired products in 68% (4al) and 43% (5al) yields with rr > 20
1). Acyclic and cyclic aliphatic acyl-derived allylic amines were also compatible under corresponding standard conditions (1,2-arylalkylation: 4ai–4ak (62–88% yields); 1,3-arylalkylation: 5ai–5ak (69–85% yields)). Tertiary allylic amines without free N–H produced desired products in 68% (4al) and 43% (5al) yields with rr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Notably, internal allylic amines are compatible in the reaction, affording corresponding 1,2-arylalkylation (4am–4ao) and 1,3-arylalkylation (5am–5ao) in moderate yields with good to excellent regioselectivities (rr of 4
1. Notably, internal allylic amines are compatible in the reaction, affording corresponding 1,2-arylalkylation (4am–4ao) and 1,3-arylalkylation (5am–5ao) in moderate yields with good to excellent regioselectivities (rr of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as well as excellent levels of diastereoselectivity (dr > 20
1) as well as excellent levels of diastereoselectivity (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In addition, drug molecule-based N-acyl allylic amines, such as probenecid and isoxepac, were well tolerated, furnishing the desired products in 73–79% yields with rr of 10
1). In addition, drug molecule-based N-acyl allylic amines, such as probenecid and isoxepac, were well tolerated, furnishing the desired products in 73–79% yields with rr of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1,2-arylalkylation: 4ap and 4aq; 1,3-arylalkylation: 5ap and 5aq). Next, the scope of aryl iodides was examined. Aryl iodides with diverse substitution patterns, such as ortho-, meta-, and para-substituents with electron-donating or electron-withdrawing groups, reacted smoothly to deliver 1,2- and 1,3-arylalyations of N-acyl allylic amines in 49–86% yields with rr of 7
1 (1,2-arylalkylation: 4ap and 4aq; 1,3-arylalkylation: 5ap and 5aq). Next, the scope of aryl iodides was examined. Aryl iodides with diverse substitution patterns, such as ortho-, meta-, and para-substituents with electron-donating or electron-withdrawing groups, reacted smoothly to deliver 1,2- and 1,3-arylalyations of N-acyl allylic amines in 49–86% yields with rr of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1,2-arylalkylation: 4ba–4bj (49–78% yields); 1,3-arylalkylation: 5ba–5bj (51–86% yields)). 3-Iodo-N-phenylcarbazole 2l gave the target products (4bk and 5bk) in moderate yields (69% and 74%) with excellent regioselectivity (rr > 20
1 (1,2-arylalkylation: 4ba–4bj (49–78% yields); 1,3-arylalkylation: 5ba–5bj (51–86% yields)). 3-Iodo-N-phenylcarbazole 2l gave the target products (4bk and 5bk) in moderate yields (69% and 74%) with excellent regioselectivity (rr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Finally, we tested the scope of alkyl halides. Linear and branched unactivated alkyl bromides afforded the corresponding selective products of 1,2- and 1,3-arylalkylation of N-acyl allylic amines in 52–71% yields with rr of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1,2-arylalkylation: 4ca–4cc; 1,3-arylalkylation: 5ca–5cc). Alkyl chloride was well tolerated in the reaction, furnishing 1,2-arylalkylation (4cd) and 1,3-arylalkylation (5cd) products with the chloride unattached. Moreover, amide and ester substituents on alkyl bromides were smoothly involved in the two sets of reaction conditions, providing the desired products (4ce and 5ce, 4cf and 5cf) in 42–70% yields with rr of 7
1 (1,2-arylalkylation: 4ca–4cc; 1,3-arylalkylation: 5ca–5cc). Alkyl chloride was well tolerated in the reaction, furnishing 1,2-arylalkylation (4cd) and 1,3-arylalkylation (5cd) products with the chloride unattached. Moreover, amide and ester substituents on alkyl bromides were smoothly involved in the two sets of reaction conditions, providing the desired products (4ce and 5ce, 4cf and 5cf) in 42–70% yields with rr of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 2-Phenylethyl bromide can also undergo 1,2- and 1,3-arylalkylation of N-acyl allylic amines to furnish corresponding products (4cg and 5cg) in 51% and 78% yields with rr of 5
1. 2-Phenylethyl bromide can also undergo 1,2- and 1,3-arylalkylation of N-acyl allylic amines to furnish corresponding products (4cg and 5cg) in 51% and 78% yields with rr of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and >20
1 and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It deserves mentioning that alkene-containing alkyl bromides were well tolerated in the reaction, leaving the alkene intact during the reaction course (1,2-arylalkylation: 4ch and 4ci; 1,3-arylalkylation: 5ch and 5ci). Alkyl bromides with other substituents, such as –F (4cj and 5cj), –CF3 (4ck and 5ck), ethers (4cl and 5cl) and ketones (4cm and 5cm), were all compatible in the reaction, affording 1,2-arylalkylation and 1,3-arylalkylation products of N-acyl allylic amines in 54–76% yields with rr of 4
1. It deserves mentioning that alkene-containing alkyl bromides were well tolerated in the reaction, leaving the alkene intact during the reaction course (1,2-arylalkylation: 4ch and 4ci; 1,3-arylalkylation: 5ch and 5ci). Alkyl bromides with other substituents, such as –F (4cj and 5cj), –CF3 (4ck and 5ck), ethers (4cl and 5cl) and ketones (4cm and 5cm), were all compatible in the reaction, affording 1,2-arylalkylation and 1,3-arylalkylation products of N-acyl allylic amines in 54–76% yields with rr of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to >20
1 to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Impressively, a secondary alkyl iodide was successfully involved in the reaction, affording desired 1,2- and 1,3-arylalkylation products 4cn and 5cn in 55% and 39% yields with rr of 10
1. Impressively, a secondary alkyl iodide was successfully involved in the reaction, affording desired 1,2- and 1,3-arylalkylation products 4cn and 5cn in 55% and 39% yields with rr of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and >20
1 and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Unfortunately, tertiary alkyl iodides/bromides failed to deliver target products under the reaction conditions.
1, respectively. Unfortunately, tertiary alkyl iodides/bromides failed to deliver target products under the reaction conditions.
To further explore the synthetic application of this strategy, gram-scale experiments of 1,2- and 1,3-arylalkylations were conducted. This regioselective and site-divergent, three-component arylalkylation protocol could be easily scaled up to 2.0 mmol scale under the two sets of standard conditions, affording 1,2-arylalkylation product 4aa in 84% yield with rr of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1,3-arylalkylation product 5aa in 78% yield with rr of 10
1 and 1,3-arylalkylation product 5aa in 78% yield with rr of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 3a).
1 (Fig. 3a).
To shed some light on the origin of regio- and site-selectivity of the nickel-catalyzed regiodivergent arylalkylations of allylic amines, a series of mechanistic experiments were conducted (Fig. 3b–f). First, a cross-over reaction was conducted using allylic amine 1a with 2a and 3a under standard conditions for migratory 1,3-arylalkylation reaction in the presence of N-acylpropenylamine 1k′ (Fig. 3b). The reaction proceeded smoothly to afford 1,3-arylalkylation product 5aa from 1a in 38% yield. No cross-over cross-coupling product from 1k′ was detected. These results indicate that nickel migration over the alkyl chain via β-H elimination and insertion to form a nitrogen-stabilized alkyl-Ni intermediate for 1,3-arylalkylation is an intramolecular process. Subsequently, a radical clock experiment of the reaction was conducted using 1a and 2a with cyclopropylmethyl bromide. The ring-opening of cyclopropane occurred under both standard conditions for 1,2-arylalkylation (conditions A) and 1,3-arylalkylation (conditions B), delivering corresponding coupling products 6 and 7 in 50% and 65% yields, respectively (Fig. 3c). These results suggest that oxidative addition of alkyl halides may occur via a single-electron oxidative addition process. When 1,1-deuterated 3-phenyl-1-bromopropane 3a′ was used as alkyl electrophile for both 1,2- and 1,3-arylalkylation reactions, desired cross-coupling products (8 and 9) were obtained in 63% and 59% yields (Fig. 3d). No deuterium scrambling was observed for the reaction. These results exclude the possibility of reversible β-H elimination and insertion of alkyl-Ni intermediate resulting from alkyl electrophiles. Next, the reaction of internal alkenes with different configurations was investigated (Fig. 3e). Under standard conditions for 1,2-arylalkylation (conditions A), 3-phenyl-1-bromopropane (3a) and 4-methyliodobenzene (2b) reacted with Z-configuration of 1o (Z-1o) to deliver 4ao in 57% yield with 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr and >20
1 rr and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. In contrast, the reaction of 2b and 3a with E-configuration 1o (E-1o) yielded the other diastereomer 4ao′, with diminished yield (12%) with 8
1 dr. In contrast, the reaction of 2b and 3a with E-configuration 1o (E-1o) yielded the other diastereomer 4ao′, with diminished yield (12%) with 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rr and >20
1 rr and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Under standard conditions for 1,3-arylalkylation (conditions B), two different diastereomers (10 and 11) were obtained in 52% (10) and 17% (11) yields with rr and >20
1 dr. Under standard conditions for 1,3-arylalkylation (conditions B), two different diastereomers (10 and 11) were obtained in 52% (10) and 17% (11) yields with rr and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr from Z-configuration of 1o (Z-1o) and E-configuration of 1o (E-1o), respectively. The results revealed that the carbometallation of alkenes is a stereospecific process. Furthermore, kinetic studies for each reaction component were conducted to gain insight into the turnover-limiting step of this regioselective and site-divergent process (Fig. 3f). Under standard conditions for 1,2-arylalkylation (conditions A), the reaction was determined as first-order-dependent on Ni catalyst and aryl iodide 2a, and zero-order-dependent on reductant (Mn), allylic amine 1a and alkyl bromide 3a. The results indicate that Ni catalyst and aryl iodides may be involved in the turnover-limiting step, suggesting the oxidative addition of Ni catalyst to aryl iodides may be the rate-determining step for 1,2-arylalkylation. Under standard conditions for 1,3-arylalkylation (conditions B), the reaction was determined as first-order-dependent on Ni catalyst, while zero-order-dependent on reductant (Mn), allylic amine 1a, aryl iodide 2a and alkyl bromide 3a. The results indicate that only the Ni catalyst may be involved in the turnover-limiting step and regeneration of Ni(0) could be the rate-determining step.
1 dr from Z-configuration of 1o (Z-1o) and E-configuration of 1o (E-1o), respectively. The results revealed that the carbometallation of alkenes is a stereospecific process. Furthermore, kinetic studies for each reaction component were conducted to gain insight into the turnover-limiting step of this regioselective and site-divergent process (Fig. 3f). Under standard conditions for 1,2-arylalkylation (conditions A), the reaction was determined as first-order-dependent on Ni catalyst and aryl iodide 2a, and zero-order-dependent on reductant (Mn), allylic amine 1a and alkyl bromide 3a. The results indicate that Ni catalyst and aryl iodides may be involved in the turnover-limiting step, suggesting the oxidative addition of Ni catalyst to aryl iodides may be the rate-determining step for 1,2-arylalkylation. Under standard conditions for 1,3-arylalkylation (conditions B), the reaction was determined as first-order-dependent on Ni catalyst, while zero-order-dependent on reductant (Mn), allylic amine 1a, aryl iodide 2a and alkyl bromide 3a. The results indicate that only the Ni catalyst may be involved in the turnover-limiting step and regeneration of Ni(0) could be the rate-determining step.
Based on the experimental results and previous literature,12b,14,17 possible mechanisms for 1,2- and 1,3-arylalkylations of allylic amines with aryl and alkyl electrophiles are proposed (Fig. 4). First, Ni0L species could be in situ formed from NiII precursors in the presence of L1 and manganese (Mn), which could undergo oxidative addition with aryl electrophiles to give Ar-NiIIL intermediate A. Subsequently, A undergoes regioselective carbometallation into the alkene to provide NiII intermediate B. The presence of N-acyl in the allylic amines enhances the stability of intermediate B by forming a six-membered ring. Intermediate B (NiIIL1) could be reduced to NiIL1 intermediate C by single-electron transfer in the presence of Mn. Subsequently, alkyl bromides 3 are activated by NiIL1 species (C) to give alkyl radicals D and NiIIL1 species B. Trapping of the alkyl radicals D with NiIIL1 (B) leads to the formation of NiIIIL1 species E. E would undergo reductive elimination to give 1,2-arylalkylation products 4 along with NiI species, which could be reduced by Mn to regenerate Ni0L1 to close the catalytic cycle. Using L6 as ligand, the ligated six-membered ring intermediate B undergoes β-H-elimination and insertion to form the five-membered intermediate F.18 Similarly, Mn reduces F (NiIIL6) to G (NiIL6), which interacts with alkyl bromides 3 to furnish alkyl radicals D and NiIIL6 species F. Recombination of F with alkyl radicals D generates NiIIIL6 species H, which undergoes reductive elimination to deliver 1,3-arylalkylation products 5 and NiIL6 species. Further reduction of NiIL6 to Ni0L6 finishes the catalytic cycle.
Conclusions
In summary, a controllable Ni-catalyzed regioselective and site-divergent reductive arylalkylation of allylic amines has been developed for the first time. This work has established a protocol for simultaneous construction of Csp3–Csp3 and Csp3–Csp2 bonds over 1,2- and 1,3-positions of unactivated alkenes. In particular, the aryl group is selectively installed on the distal carbon of the olefin. This reaction proceeds under mild conditions and tolerates a broad range of functional groups as well as terminal and internal alkenes. The catalytically controlled 1,2- and 1,3-arylalkylations of allylic amines provide an ideal way to build diverse C(sp3)-rich α- and β-branched aliphatic amines from identical starting materials.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 4af (CCDC 2361506) and 5be (CCDC 2361509) have been deposited at the Cambridge Crystallographic Data Centre.Author contributions
W. S. conceived and directed the project. H. M. and P. F. Y. discovered and developed the reaction. H. M., Q. Y. and J. S. J. performed the experiments and collected the data. Q. Y. and Y. L. L. co-supervised the project. All authors discussed and analyzed the data. W. S., J. S. J. and H. M. wrote the manuscript with contribution from other authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (22171127, 22371115, 22373056), Sichuan Science and Technology Program (2024ZYD0017), Natural Science Foundation of Sichuan Province(2025ZNSFSC0128), the Pearl River Talent Recruitment Program (2019QN01Y261), Shenzhen Science and Technology Innovation Committee (JCYJ20230807093522044, JCYJ20240813094226034, JCYJ20220530114606013), Guangdong Provincial Key Laboratory of Catalysis (2020B121201002), Scientific Research and Innovation Team Program of Sichuan University of Science and Engineering (no. SUSE652A014) and Sichuan University of Science and Engineering (2023RC10) is gratefully acknowledged. This research is supported by the SUSTech-NUS Joint Research Program. We acknowledge the assistance of the SUSTech Core Research Facilities. We thank Hai-Wu Du (SUSTech) for the X-ray analysis of 4af (CCDC 2361506) and 5be (CCDC 2361509) and Yi-Ming Du (SUSTech) for reproducing the results of 4ag, 4bi, 4cm, 5ag, 5bi, and 5cm.Notes and references
- For selected reviews, see: (a) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed; (b) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651–2710 CrossRef CAS PubMed; (c) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS PubMed; (d) C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS PubMed; (e) A. H. Cherney, N. T. Kadunce and S. E. Reisman, Chem. Rev., 2015, 115, 9587–9652 CrossRef CAS PubMed; (f) W. Xue, X. Jia, X. Wang, X. Tao, Z. Yin and H. Gong, Chem. Soc. Rev., 2021, 50, 4162–4184 RSC; (g) M. C. Kozlowski, Acc. Chem. Res., 2017, 50, 638–643 CrossRef CAS PubMed; (h) Y. Li, D. Wu, H.-G. Cheng and G. Yin, Angew. Chem., Int. Ed., 2020, 59, 7990–8003 CrossRef CAS PubMed; (i) K. M. Korch and D. A. Watson, Chem. Rev., 2019, 119, 8192–8228 CrossRef CAS PubMed; (j) N. Hazari, P. R. Melvin and M. M. Beromi, Nat. Rev. Chem., 2017, 1, 0025 CrossRef CAS PubMed; (k) L.-C. Campeau and N. Hazari, Organometallics, 2018, 38, 3–35 CrossRef PubMed.
- (a) A. Duan, F. Xiao, Y. Lan and L. Niu, Chem. Soc. Rev., 2022, 51, 9986–10015 RSC; (b) R.-D. He, C.-L. Li, Q.-Q. Pan, P. Guo, X.-Y. Liu and X.-Z. Shu, J. Am. Chem. Soc., 2019, 141, 12481–12486 CrossRef CAS PubMed; (c) J. L. Hofstra, A. H. Cherney, C. M. Ordner and S. E. Reisman, J. Am. Chem. Soc., 2018, 140, 139–142 CrossRef CAS PubMed; (d) M. Holmes, L. A. Schwartz and M. J. Krische, Chem. Rev., 2018, 118, 6026–6052 CrossRef CAS PubMed; (e) K. E. Poremba, S. E. Dibrell and S. E. Reisman, ACS Catal., 2020, 10, 8237–8246 CrossRef CAS PubMed; (f) C. C. Meyer, E. Ortiz and M. J. Krische, Chem. Rev., 2020, 120, 3721–3748 CrossRef CAS PubMed; (g) J. Davies, D. Janssen-Müller, D. P. Zimin, C. S. Day, T. Yanagi, J. Elfert and R. Martin, J. Am. Chem. Soc., 2021, 143, 4949–4954 CrossRef CAS PubMed; (h) Y. Wang and Q. Ren, Curr. Org. Chem., 2020, 24, 1367–1383 CrossRef CAS.
- (a) L. M. Wickham and R. Giri, Acc. Chem. Res., 2021, 54, 3415–3437 CrossRef CAS PubMed; (b) J. Diccianni, Q. Lin and T. Diao, Acc. Chem. Res., 2020, 53, 906–919 CrossRef CAS PubMed; (c) J. Gu, X. Wang, W. Xue and H. Gong, Org. Chem. Front., 2015, 2, 1411–1421 RSC.
- (a) T. J. DeLano and S. E. Reisman, ACS Catal., 2019, 9, 6751–6754 CrossRef CAS PubMed; (b) W.-T. Zhao, H. Meng, J.-N. Lin and W. Shu, Angew. Chem., Int. Ed., 2023, 62, e202215779 CrossRef CAS PubMed; (c) D. J. Weix, Acc. Chem. Res., 2015, 48, 1767–1775 CrossRef CAS PubMed; (d) C. E. I. Knappke, S. Grupe, D. Gärtner, M. Corpet, C. Gosmini and A. J. von Wangelin, Chem.–Eur. J., 2014, 20, 6828–6842 CrossRef CAS PubMed; (e) X. Wang, Y. Dai and H. Gong, Top. Curr. Chem., 2016, 374, 43 CrossRef PubMed.
- (a) J. Z. Wang, E. Mao, J. A. Nguyen, W. L. Lyon and D. W. MacMillan, J. Am. Chem. Soc., 2024, 146, 15693–15700 CrossRef CAS PubMed; (b) F. Cong, G.-Q. Sun, S.-H. Ye, R. Hu, W. Rao and M. J. Koh, J. Am. Chem. Soc., 2024, 146, 10274–10280 CrossRef CAS PubMed; (c) B. Shrestha, P. Basnet, R. K. Dhungana, S. Kc, S. Thapa, J. M. Sears and R. Giri, J. Am. Chem. Soc., 2017, 139, 10653–10656 CrossRef CAS PubMed.
- For selected reviews, see: (a) R. I. McDonald, G. Liu and S. S. Stahl, Chem. Rev., 2011, 111, 2981–3019 CrossRef CAS PubMed; (b) R. K. Dhungana, S. KC, P. Basnet and R. Giri, Chem. Rec., 2018, 18, 1314–1340 CrossRef CAS PubMed; (c) G. Yin, X. Mu and G. Liu, Acc. Chem. Res., 2016, 49, 2413–2423 CrossRef CAS PubMed; (d) J. Jose and T. V. Mathew, Adv. Synth. Catal., 2023, 365, 4334–4358 CrossRef CAS.
- For selected reviews, see: (a) T. Koike and M. Akita, Acc. Chem. Res., 2016, 49, 1937–1945 CrossRef CAS PubMed; (b) X. Qi and T. Diao, ACS Catal., 2020, 10, 8542–8556 CrossRef CAS PubMed; (c) J. Derosa, O. Apolinar, T. Kang, V. T. Tran and K. M. Engle, Chem. Sci., 2020, 11, 4287–4296 RSC; (d) J. Heng and A. Studer, Chem. Soc. Rev., 2020, 49, 1790–1811 RSC; (e) B. C. Lee, C. F. Liu, L. Q. H. Lin, K. Z. Yap, N. Song, C. H. M. Ko, P. H. Chan and M. J. Koh, Chem. Soc. Rev., 2023, 52, 2946–2991 RSC; (f) Z.-L. Li, G.-C. Fang, Q.-S. Gu and X.-Y. Liu, Chem. Soc. Rev., 2020, 49, 32–48 RSC.
- (a) J. N. Katzbaer, V. M. Torres, E. Elacqua and R. Giri, J. Am. Chem. Soc., 2023, 145, 14196–14201 CrossRef CAS PubMed; (b) P. Gao, L.-A. Chen and M. K. Brown, J. Am. Chem. Soc., 2018, 140, 10653–10657 CrossRef CAS PubMed; (c) S. KC, R. K. Dhungana, V. Aryal and R. Giri, Org. Process Res. Dev., 2019, 23, 1686–1694 CrossRef CAS.
- (a) A. García-Domínguez, R. Mondal and C. Nevado, Angew. Chem., Int. Ed., 2019, 58, 12286–12290 CrossRef PubMed; (b) X. Hu, I. Cheng-Sánchez, W. Kong, G. A. Molander and C. Nevado, Nat. Catal., 2024, 7, 655–665 CrossRef CAS PubMed; (c) R. S. Mega, V. K. Duong, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2020, 59, 4375–4379 CrossRef CAS PubMed; (d) F. Ye, Y. Yang, W. Wang and W. Yuan, Chem Catal., 2023, 3, 100605 CrossRef CAS; (e) L. Guo, H.-Y. Tu, S. Zhu and L. Chu, Org. Lett., 2021, 21, 4771–4776 CrossRef PubMed.
- (a) Z.-F. Yang, C. Xu, X. Zheng and X. Zhang, Chem. Commun., 2020, 56, 2642–2645 RSC; (b) X. Du, I. Cheng-Sánchez and C. Nevado, J. Am. Chem. Soc., 2023, 145, 12532–12540 CrossRef CAS PubMed; (c) H. Zhao and W. Yuan, Chem. Sci., 2023, 14, 1485–1490 RSC.
- (a) T. Qin, J. Cornella, C. Li, L. R. Malins, J. T. Edwards, S. Kawamura, B. D. Maxwell, M. D. Eastgate and P. S. Baran, Science, 2016, 352, 801–805 CrossRef CAS PubMed; (b) J.-W. Gu, Q.-Q. Min, L.-C. Yu and X. Zhang, Angew. Chem., Int. Ed., 2016, 55, 12270–12274 CrossRef CAS PubMed; (c) S. KC, R. K. Dhungana, B. Shrestha, S. Thapa, N. Khanal, P. Basnet, R. W. Lebrun and R. Giri, J. Am. Chem. Soc., 2018, 140, 9801–9805 CrossRef CAS PubMed; (d) M. Chierchia, P. Xu, G. J. Lovinger and J. P. Morken, Angew. Chem., Int. Ed., 2019, 58, 14245–14249 CrossRef CAS PubMed; (e) S.-Z. Sun, Y. Duan, R. S. Mega, R. J. Somerville and R. Martin, Angew. Chem., Int. Ed., 2020, 59, 4370–4374 CrossRef CAS PubMed; (f) D. Anthony, Q. Lin, J. Baudet and T. Diao, Angew. Chem., Int. Ed., 2019, 58, 3198–3202 CrossRef CAS PubMed; (g) H. Jiang, X. Yu, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2021, 60, 14399–14404 CrossRef CAS PubMed; (h) J. Liu, L.-Q. Lu, Y. Luo, W. Zhao, P.-C. Sun, W. Jin, X. Qi, Y. Cheng and W.-J. Xiao, ACS Catal., 2022, 12, 1879–1885 CrossRef CAS; (i) X. Li, M. Yuan, F. Chen, Z. Huang, F.-L. Qing, O. Gutierrez and L. Chu, Chem, 2023, 9, 154–169 CrossRef CAS PubMed; (j) M.-S. Liu and W. Shu, JACS Au, 2023, 3, 1321–1327 CrossRef CAS PubMed; (k) F. Ye, S. Zheng, Y. Luo, X. Qi and W. Yuan, ACS Catal., 2024, 14, 8505–8517 CrossRef CAS; (l) Y. Koo and S. Hong, Chem. Sci., 2024, 15, 7707–7713 RSC; (m) Y.-C. Luo, C. Xu and X. Zhang, Chin. J. Chem., 2020, 38, 1371–1394 CrossRef CAS; (n) M. Jeganmohan and C.-H. Cheng, Chem.–Eur. J., 2008, 14, 10876–10886 CrossRef CAS PubMed; (o) Y. Ping and W. Kong, Synthesis, 2020, 52, 979–992 CrossRef.
- (a) A. Y. Rulev, I. N. Zubkov, I. A. Ushakov, V. A. Semenov, A. V. Vashchenko and J. Maddaluno, Eur. J. Org. Chem., 2021, 22, 3278–3288 CrossRef; (b) N. Ballav, S. N. Saha, S. Yadava and M. Baidya, Chem. Sci., 2024, 15, 4890–4896 RSC; (c) A. Rentería-Gómez, M. Guerrero, M. Ramirez-Lopez and O. Gutierrez, Org. Lett., 2023, 25, 7440–7445 CrossRef PubMed.
- (a) H. Yang, Z. Zhang, P. Cao and T. Yang, Org. Lett., 2024, 26, 1190–1195 CrossRef CAS PubMed; (b) Z. Dong, Q. Tang, C. Xu, L. Chen, H. Ji, S. Zhou, L. Song and L.-A. Chen, Angew. Chem., Int. Ed., 2023, 62, e202218286 CrossRef CAS PubMed; (c) H.-Y. Tu, F. Wang, L. Huo, Y. Li, S. Zhu, X. Zhao, H. Li, F.-L. Qing and L. Chu, J. Am. Chem. Soc., 2020, 142, 9604–9611 CrossRef CAS PubMed; (d) T. Yang, X. Chen, W. Rao and M. J. Koh, Chem, 2020, 6, 738–751 CrossRef CAS.
- Z. Dong, C. Xu, J. Chang, S. Zhou, P. Sun, Y. Li and L.-A. Chen, ACS Catal., 2024, 14, 4395–4406 CrossRef CAS.
- (a) H. Wang, M. Yang, Y. Wang, X. Man, X. Lu, Z. Mou, Y. Luo and H. Liang, Org. Lett., 2021, 23, 8183–8188 CrossRef CAS PubMed; (b) X. Wei, W. Shu, A. García-Domínguez, E. Merino and C. Nevado, J. Am. Chem. Soc., 2020, 142, 13515–13522 CrossRef CAS PubMed; (c) A. García-Domínguez, Z. Li and C. Nevado, J. Am. Chem. Soc., 2017, 139, 6835–6838 CrossRef PubMed.
- For more details, see ESI.†.
- (a) K. Wang, Z. Ding, Z. Zhou and W. Kong, J. Am. Chem. Soc., 2018, 140, 12364–12368 CrossRef CAS PubMed; (b) B. J. McNicholas, Z. J. Tong, D. Bím, R. F. Turro, N. P. Kazmierczak, J. Chalupský, S. E. Reisman and R. G. Hadt, Inorg. Chem., 2023, 62, 14010–14027 CrossRef CAS PubMed; (c) T. Yang, Y. Jiang, Y. Luo, J. J. H. Lim, Y. Lan and M. J. Koh, J. Am. Chem. Soc., 2020, 142, 21410–21419 CrossRef CAS PubMed; (d) Z.-Q. Li, O. Apolinar, R. Deng and K. M. Engle, Chem. Sci., 2021, 12, 11038–11044 RSC.
- Y. Li and G. Yin, Acc. Chem. Res., 2023, 56, 3246–3259 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2361506 and 2361509. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07728h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |