Open Access Article
Open Access ArticleFluorinated and methylated ortho-benzodipyrrole-based acceptors suppressing charge recombination and minimizing energy loss in organic photovoltaics†
Yan-Bo
Wang
a,
Chia-Lin
Tsai
a,
Yung-Jing
Xue
a,
Bing-Huang
Jiang
b,
Han-Cheng
Lu
a,
Jun-Cheng
Hong
a,
Yu-Chi
Huang
a,
Kuo-Hsiu
Huang
a,
Su-Ying
Chien
c,
Chih-Ping
Chen
 b and
Yen-Ju
Cheng
b and
Yen-Ju
Cheng
 *ad
*ad
aDepartment of Applied Chemistry, National Yang Ming Chiao Tung University, 1001 University Road, Hsinchu, 30010, Taiwan
bDepartment of Materials Engineering and Organic Electronics Research Center, Ming Chi University of Technology, New Taipei City 24301, Taiwan
cInstrumentation Center, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 10617, Taiwan
dCenter for Emergent Functional Matter Science, National Yang Ming Chiao Tung University, 1001 University Road, Hsinchu, 30010, Taiwan. E-mail: yjcheng@nycu.edu.tw
First published on 9th January 2025
Abstract
The elimination of the A′ unit from  -type Y6-derivatives has led to the development of a new class of ortho-benzodipyrrole (o-BDP)-based A-DNBND-A-type NFAs. In this work, two new A-DNBND-A-type NFAs, denoted as CFB and CMB, are designed and synthesized, where electron-withdrawing fluorine atoms and electron-donating methyl groups are substituted on the benzene ring of the o-BDP moiety, respectively. CFB exhibits a blue-shifted absorption spectrum, stronger intermolecular interactions, shorter π–π stacking distances, and more ordered 3D intermolecular packing in the neat and blend films, enabling it to effectively suppress charge recombination in the PM6:CFB device showing a higher PCE of 16.55% with an FF of 77.45%. CMB displays a higher HOMO/LUMO energy level, a smaller optical bandgap, and a less ordered 3D packing, which contributes to its superior ability to suppress energy loss in the PM6:CMB device with a high Voc of 0.90 V and a PCE of 16.46%. To leverage the advantages of CFB and CMB, ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices are fabricated. The PM6:Y6-16:CFB device exhibits the highest PCE of 17.83% with an increased Voc of 0.86 V and a Jsc of 27.32 mA cm−2, while the PM6:Y6-16:CMB device displayed an elevated Voc of 0.87 V and an improved FF of 74.71%, leading to a PCE of 17.44%. The high PCE was achieved using the non-halogenated greener solvent o-xylene, highlighting their potential for facilitating more eco-friendly processing procedures. C-shaped disubstituted o-BDP-based A–D–A type acceptors open up new avenues for tailoring electronic properties and molecular self-assembly, achieving higher OPV performance with enhanced charge recombination suppression and reduced energy loss.
-type Y6-derivatives has led to the development of a new class of ortho-benzodipyrrole (o-BDP)-based A-DNBND-A-type NFAs. In this work, two new A-DNBND-A-type NFAs, denoted as CFB and CMB, are designed and synthesized, where electron-withdrawing fluorine atoms and electron-donating methyl groups are substituted on the benzene ring of the o-BDP moiety, respectively. CFB exhibits a blue-shifted absorption spectrum, stronger intermolecular interactions, shorter π–π stacking distances, and more ordered 3D intermolecular packing in the neat and blend films, enabling it to effectively suppress charge recombination in the PM6:CFB device showing a higher PCE of 16.55% with an FF of 77.45%. CMB displays a higher HOMO/LUMO energy level, a smaller optical bandgap, and a less ordered 3D packing, which contributes to its superior ability to suppress energy loss in the PM6:CMB device with a high Voc of 0.90 V and a PCE of 16.46%. To leverage the advantages of CFB and CMB, ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices are fabricated. The PM6:Y6-16:CFB device exhibits the highest PCE of 17.83% with an increased Voc of 0.86 V and a Jsc of 27.32 mA cm−2, while the PM6:Y6-16:CMB device displayed an elevated Voc of 0.87 V and an improved FF of 74.71%, leading to a PCE of 17.44%. The high PCE was achieved using the non-halogenated greener solvent o-xylene, highlighting their potential for facilitating more eco-friendly processing procedures. C-shaped disubstituted o-BDP-based A–D–A type acceptors open up new avenues for tailoring electronic properties and molecular self-assembly, achieving higher OPV performance with enhanced charge recombination suppression and reduced energy loss.
Introduction
Renowned for their flexibility, solution processability and transparency, organic solar cells have received considerable research interest in the realm of photovoltaics. In 2019, Y6 achieved a PCE of 15.7% when blended with donor polymer PM6,1 ushering in a new era for organic non-fullerene acceptors (NFAs). Y6 features a unique structural arrangement characterized as in which the central heptacyclic ladder
in which the central heptacyclic ladder  π-core comprises an ortho-benzodipyrrole core laterally fused with two β-alkylated-thieno[3,2-b]thiophene moieties (TT, denoted as D) and vertically fused with an electron-deficient thiadiazole unit (Tz, denoted as A′). The label A usually corresponds to the 2-(5,6-difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (FIC) end-group acceptor, which is linked to the
π-core comprises an ortho-benzodipyrrole core laterally fused with two β-alkylated-thieno[3,2-b]thiophene moieties (TT, denoted as D) and vertically fused with an electron-deficient thiadiazole unit (Tz, denoted as A′). The label A usually corresponds to the 2-(5,6-difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (FIC) end-group acceptor, which is linked to the  core through olefination. Over the past five years, modifications of the
core through olefination. Over the past five years, modifications of the  architecture to fine-tune the photovoltaic properties have become the central focus of design and synthesis of organic NFA materials (Fig. 1). One strategy involves modifying the aliphatic side chains R1 at nitrogen atoms2–6 and R2 at the β-position of thienothiophene (TT) units.7–11 This approach has been primarily employed to tailor intermolecular interactions and adjust molecular packing within the material. On the other hand, conjugated main-chain engineering of the D units12,13 and the A end-group acceptors14–20 has been utilized to specifically modulate the intrinsic optical and electronic properties. For instance, replacing sulfur with selenium in the D units12 or replacing fluorine with chlorine in the FIC units7,14,20 causes a red-shift in absorption spectra and concomitantly enhances intermolecular interactions due to the presence of heavy atoms with higher polarizability. Another main-chain modification of the
architecture to fine-tune the photovoltaic properties have become the central focus of design and synthesis of organic NFA materials (Fig. 1). One strategy involves modifying the aliphatic side chains R1 at nitrogen atoms2–6 and R2 at the β-position of thienothiophene (TT) units.7–11 This approach has been primarily employed to tailor intermolecular interactions and adjust molecular packing within the material. On the other hand, conjugated main-chain engineering of the D units12,13 and the A end-group acceptors14–20 has been utilized to specifically modulate the intrinsic optical and electronic properties. For instance, replacing sulfur with selenium in the D units12 or replacing fluorine with chlorine in the FIC units7,14,20 causes a red-shift in absorption spectra and concomitantly enhances intermolecular interactions due to the presence of heavy atoms with higher polarizability. Another main-chain modification of the  core involves substituting Tz in the A′ unit with five-membered heterocycles such as triazole,21–24 selenadiazole,25,26 or six-membered pyrazine27–31 and quinoxaline moieties.32–36
core involves substituting Tz in the A′ unit with five-membered heterocycles such as triazole,21–24 selenadiazole,25,26 or six-membered pyrazine27–31 and quinoxaline moieties.32–36
However, synthesis of  where the A′ unit is different from Tz is not straightforward. Cadogan cyclization, a key step in the formation of the benzodipyrrole moiety in
where the A′ unit is different from Tz is not straightforward. Cadogan cyclization, a key step in the formation of the benzodipyrrole moiety in  , cannot be directly carried out in the presence of most heterocyclic A′ moieties. Instead, it usually involves reductive ring-opening of the thiadiazole unit in
, cannot be directly carried out in the presence of most heterocyclic A′ moieties. Instead, it usually involves reductive ring-opening of the thiadiazole unit in  to form a diamino species followed by the respective annulation.37 Therefore, removing the A′ moiety from the
to form a diamino species followed by the respective annulation.37 Therefore, removing the A′ moiety from the  structure can largely reduce synthetic complexity. Additionally, the impact of different heterocycle electron-deficient A′ units on the optical band-gaps associated with HOMO/LUMO energy levels and molecular self-assembly packing has not been well understood. To clarify the merits of the heterocycle-based A′ unit, we recently developed a new NFA, referred to as CB16,38 which exhibits a structure identical to Y6, except for the elimination of the Tz moiety. CB16 can be structurally symbolized as A-DNBND-A where C and B letters stands for C-shaped geometry and the central benzene ring, respectively. It was found that even without the Tz (A′) unit, CB16-based inverted OPVs can achieve comparable efficiencies to Y6 derivatives (Fig. 1). This research has unveiled that the central ortho-benzobipyrrole moiety that leads to a C-shaped geometry of A-DNBND-A in CB16 and
structure can largely reduce synthetic complexity. Additionally, the impact of different heterocycle electron-deficient A′ units on the optical band-gaps associated with HOMO/LUMO energy levels and molecular self-assembly packing has not been well understood. To clarify the merits of the heterocycle-based A′ unit, we recently developed a new NFA, referred to as CB16,38 which exhibits a structure identical to Y6, except for the elimination of the Tz moiety. CB16 can be structurally symbolized as A-DNBND-A where C and B letters stands for C-shaped geometry and the central benzene ring, respectively. It was found that even without the Tz (A′) unit, CB16-based inverted OPVs can achieve comparable efficiencies to Y6 derivatives (Fig. 1). This research has unveiled that the central ortho-benzobipyrrole moiety that leads to a C-shaped geometry of A-DNBND-A in CB16 and  in Y6-based derivatives is pivotal in establishing the 3D molecular packing network for efficient charge transport, ultimately contributing to the superior performance of the devices. The heterocyclic A′ moiety plays a secondary role in finely adjusting intermolecular interactions, both internally within the NFA and externally with the polymer matrix. The further molecular engineering of the A-DNBND-A structure holds significant potential for the development of a new generation of high-performance A–D–A-type CB-based NFAs.39 Removal of the aromatic thiadiazole (Tz) group from
in Y6-based derivatives is pivotal in establishing the 3D molecular packing network for efficient charge transport, ultimately contributing to the superior performance of the devices. The heterocyclic A′ moiety plays a secondary role in finely adjusting intermolecular interactions, both internally within the NFA and externally with the polymer matrix. The further molecular engineering of the A-DNBND-A structure holds significant potential for the development of a new generation of high-performance A–D–A-type CB-based NFAs.39 Removal of the aromatic thiadiazole (Tz) group from  simultaneously exposes two unsubstituted sp2-carbons in the central benzene. Introducing non-aromatic substituents at the two active positions expands the scope of CB-series materials, thereby opening up avenues for new main-chain engineering to tailor their molecular properties. Through the introduction of electron-accepting and electron-releasing substitutions at the o-BDP moiety, it becomes feasible to systematically elucidate the electronic and steric effects of central substitutions on the molecular properties of A-DNBND-A NFAs and their device performances. In this research, we designed and synthesized two new ortho-disubstituted A-DNBND-A-type NFAs, denoted as CFB and CMB, which exhibit a C-shaped backbone similar to Y6-16, while incorporating fluorine and methyl groups onto the central benzene ring of the o-BDP moiety, respectively. Investigating the substitution effects of CMB and CFB helps clarify how the electron-accepting strength of A′ units (thiadiazole, selenadiazole, pyrazine, and triazole) in
simultaneously exposes two unsubstituted sp2-carbons in the central benzene. Introducing non-aromatic substituents at the two active positions expands the scope of CB-series materials, thereby opening up avenues for new main-chain engineering to tailor their molecular properties. Through the introduction of electron-accepting and electron-releasing substitutions at the o-BDP moiety, it becomes feasible to systematically elucidate the electronic and steric effects of central substitutions on the molecular properties of A-DNBND-A NFAs and their device performances. In this research, we designed and synthesized two new ortho-disubstituted A-DNBND-A-type NFAs, denoted as CFB and CMB, which exhibit a C-shaped backbone similar to Y6-16, while incorporating fluorine and methyl groups onto the central benzene ring of the o-BDP moiety, respectively. Investigating the substitution effects of CMB and CFB helps clarify how the electron-accepting strength of A′ units (thiadiazole, selenadiazole, pyrazine, and triazole) in  correlates with their absorption maxima and HOMO energy levels. Compared to the PM6:Y6-16 (the derivative of Y6 which has a 2-hexyldecyl side chain on the nitrogen of pyrrole) device with a PCE of 16.30%, the PM6:CFB device exhibited a higher PCE of 16.55% with an increased Voc of 0.89 V and an improved FF of 77.15%, while the PM6:CMB device displayed an elevated Voc of 0.90 V and an improved Jsc of 26.32 mA cm−2, leading to a comparable PCE of 16.46%. By incorporating CFB and CMB as the second acceptor into the PM6: Y6-16 system, the ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices demonstrated higher performances of 17.83% and 17.44%, respectively. Notably, the devices were fabricated using the greener solvent o-xylene, which addresses the toxicity concerns associated with halogenated solvents.40–43
correlates with their absorption maxima and HOMO energy levels. Compared to the PM6:Y6-16 (the derivative of Y6 which has a 2-hexyldecyl side chain on the nitrogen of pyrrole) device with a PCE of 16.30%, the PM6:CFB device exhibited a higher PCE of 16.55% with an increased Voc of 0.89 V and an improved FF of 77.15%, while the PM6:CMB device displayed an elevated Voc of 0.90 V and an improved Jsc of 26.32 mA cm−2, leading to a comparable PCE of 16.46%. By incorporating CFB and CMB as the second acceptor into the PM6: Y6-16 system, the ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices demonstrated higher performances of 17.83% and 17.44%, respectively. Notably, the devices were fabricated using the greener solvent o-xylene, which addresses the toxicity concerns associated with halogenated solvents.40–43
Results and discussion
Molecular synthesis and thermal properties
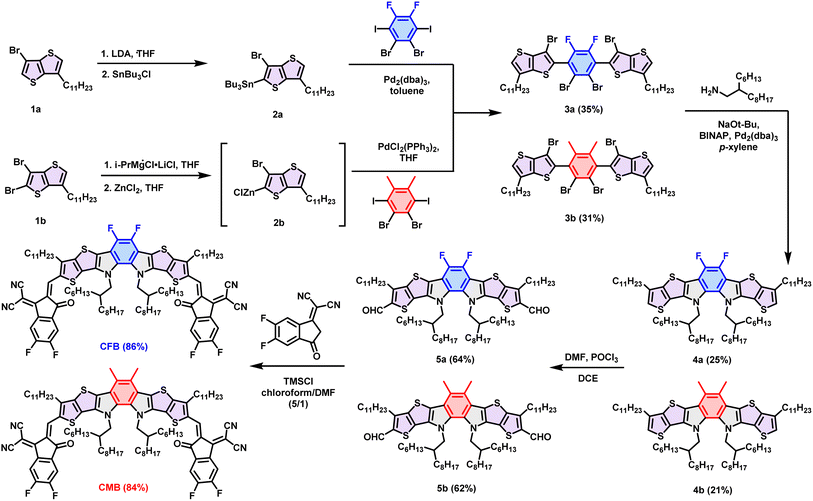
The synthetic routes of o-BDP-based CFB and CMB are depicted in Scheme 1. Compound 1a and 1b were synthesized following procedures described in the literature.38,44 Lithiation of compound 1a by using lithium diisopropylamide (LDA) followed by treating with tributyltin chloride formed compound 2a, which was further reacted with 1,2-dibromo-4,5-difluoro-3,6-diiodobenzene through Stille coupling to yield compound 3a. Grignard metathesis of compound 1b followed by quenching with ZnCl2 resulted in intermediate 2b. The Negishi coupling reaction of the freshly prepared 2b with 1,2-dibromo-3,6-diiodo-4,5-dimethylbenzene afforded compound 3b. Cyclization through multiple C–N bond formation achieved by Buchwald–Hartwig amination of 3a and 3b with 2-hexyldecyl amine led to the formation of 4a and 4b, respectively. Formylation through the Vilsmeier reaction of 4a and 4b yielded compound 5a and 5b, respectively. Knoevenagel condensation of 5a or 5b with FIC unit furnished two desired acceptors, CFB and CMB, with yields of 81% and 84%, respectively.Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were conducted to assess thermal properties of the acceptors (Fig. S1† and 2a). While the decomposition temperature (Td) of CMB resembles that of Y6-16 at 322 °C, the Td of CFB increases to 330 °C, manifesting that the replacement of the Tz group with F atoms enhances thermal stability of the acceptors. In spite of the higher Td, CFB exhibits a lower melting temperature (Tm) of 224 °C compared with Y6-16 (235 °C), indicating the weaker intermolecular interactions resulting from the removal of the central Tz unit. CMB has the smallest Tm of 222 °C, suggesting the least intermolecular interaction of the material.
Electrochemical and optical properties
Cyclic voltammetry was employed to determine the HOMO/LUMO energy levels of CFB and CMB (Fig. S2†), and the resulting energy level diagrams are depicted in Fig. 2b. In comparison with Y6-16, CFB featuring the electron-withdrawing F atoms exhibits a downshifted HOMO energy level of −5.85 eV and a similar LUMO energy level of −4.01 eV, resulting in an enlarged bandgap. Conversely, CMB containing electron-donating methyl groups shows upshifted HOMO/LUMO levels at −5.69/−3.96 eV, which is advantageous for increasing the Voc value.The absorption characteristics of typical A–D–A-type NFAs are primarily governed by push–pull photo-induced intramolecular charge transfer (ICT) from the electron-rich multi-fused D to the electron-withdrawing A moiety. However, the effect of various fused heterocyclic A′ units on the optical properties of  -type Y-series NFAs has not been fully elucidated. The substitution at the central benzene ring of the A-DNBND-A system can provide valuable insight to address this question. The absorption spectra are displayed in Fig. 2c with the features summarized in Table 1. Compared to Y6-16 with an absorption maximum (λmax) at 734 nm in chloroform, CFB with two fluorine atoms exhibited a much blue-shifted λmax at 704 nm, whereas CMB with two methyl groups showed a more red-shifted λmax at 758 nm. The electron-accepting fluorine atoms positioned para to the two nitrogen atoms weaken their electron-donating ability through the inductive effect, which, in turn, reduces the ICT from the DNBND to the A moieties. Conversely, the moderately electron-donating methyl groups enhance the donating ability of the DNBND unit, thereby augmenting the ICT effect.
-type Y-series NFAs has not been fully elucidated. The substitution at the central benzene ring of the A-DNBND-A system can provide valuable insight to address this question. The absorption spectra are displayed in Fig. 2c with the features summarized in Table 1. Compared to Y6-16 with an absorption maximum (λmax) at 734 nm in chloroform, CFB with two fluorine atoms exhibited a much blue-shifted λmax at 704 nm, whereas CMB with two methyl groups showed a more red-shifted λmax at 758 nm. The electron-accepting fluorine atoms positioned para to the two nitrogen atoms weaken their electron-donating ability through the inductive effect, which, in turn, reduces the ICT from the DNBND to the A moieties. Conversely, the moderately electron-donating methyl groups enhance the donating ability of the DNBND unit, thereby augmenting the ICT effect.
| NFA | Extinction coefficienta [×105 cm−1 M−1] | λ max [nm] | Δλ [nm] | λ onset [nm] | E optg [eV] | HOMOd [eV] | LUMOd [eV] | E eleg [eV] | |
|---|---|---|---|---|---|---|---|---|---|
| Solu | Film | ||||||||
| a Calculated at λmax in the solution state. b Calculated in the solid state. c E optg = 1240/λonset. d Determined by cyclic voltammetry. | |||||||||
| Y6-16 | 1.86 | 734 | 819 | 85 | 911 | 1.36 | −5.75 | −4.01 | 1.74 |
| CMB | 1.51 | 758 | 820 | 62 | 902 | 1.37 | −5.69 | −3.96 | 1.73 |
| CFB | 1.80 | 704 | 774 | 70 | 858 | 1.45 | −5.85 | −4.01 | 1.84 |
Combining the A-DNBND-A-type CFB and CMB with four other 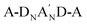 derivatives (Y6-16, AQx-2, Y6-Se, and Y11-M) reported in the literature, the solution λmax values can be used to quantify the electron-accepting ability of their respective A′ units (thiadiazole, pyrazine, selenodiazole, and triazole, depicted in Fig. 2d). The gradually increasing λmax values for CFB (704 nm), Y6-16 (731 nm), AQx-2 (732 nm), Y6-Se (741 nm), Y11-M (753 nm), and CMB (758 nm) suggest that the electron-accepting ability of the A′ units follows this trend: difluoro > thiadiazole > pyrazine > selenodiazole > triazole > dimethyl.
derivatives (Y6-16, AQx-2, Y6-Se, and Y11-M) reported in the literature, the solution λmax values can be used to quantify the electron-accepting ability of their respective A′ units (thiadiazole, pyrazine, selenodiazole, and triazole, depicted in Fig. 2d). The gradually increasing λmax values for CFB (704 nm), Y6-16 (731 nm), AQx-2 (732 nm), Y6-Se (741 nm), Y11-M (753 nm), and CMB (758 nm) suggest that the electron-accepting ability of the A′ units follows this trend: difluoro > thiadiazole > pyrazine > selenodiazole > triazole > dimethyl.
This result provides a guideline for tuning the absorption properties of o-BDP-based NFAs by selecting substitutions on the central benzene ring (Fig. 2e). In addition, the λmax of CFB is red-shifted to 774 nm (Δλmax = 70 nm) from the solution state to the film state. The smaller Δλmax of CFB compared to that of Y6-16 (Δλmax = 85 nm) indicates that the aromatic Tz unit induces stronger intermolecular interactions than F atoms. The λmax of CMB in the film state is almost the same as that of Y6-16, leading to the smallest Δλmax (Δλmax = 62 nm) among the three acceptors. The relatively larger steric hindrance of the methyl groups in CMB might further attenuate the intermolecular interactions.
Computational studies of frontier molecular orbitals
Energy level diagrams and frontier molecular orbitals of CFB and CMB along with Y6-16, AQx-1, Y6-Se, and Y11-M were calculated at the B3LYP/6-311G(d,p) level of theory, as shown in Fig. 2f–h and S3.† Substitution on the benzene ring of the DNBND core has a more pronounced effect on the HOMO energy level than the LUMO energy level. Compared to Y6-16, which has HOMO/LUMO levels at −5.87 eV and −3.84 eV, respectively, CFB exhibits a more downshifted HOMO energy at −5.95 eV while maintaining an unchanged LUMO level at −3.84 eV. This suggests that the fluorine atoms in CFB exert a stronger electron-withdrawing effect than the Tz group in Y6-16. In both Y6-16 and CFB, the electron density in the HOMO is more dispersed into the fluorine atoms and the Tz group, while this effect is less evident in the LUMO. Additionally, the electron-donating dimethyl groups in CMB result in higher-lying HOMO/LUMO energy levels, at −5.75 eV and −3.74 eV, respectively. The theoretical trends in these energy levels are consistent with experimental results obtained from CV measurements. Overall, the calculated HOMO energy levels show a gradual upshift from −5.95 eV (CFB), −5.87 eV (Y6-16), −5.84 eV (AQx-2), −5.83 eV (Y6-Se), and −5.75 eV (Y11-M), to −5.75 eV (CMB), again reflecting the trend in electron-accepting ability: difluoro > thiadiazole > pyrazine > selenodiazole > triazole > dimethyl, as shown in Fig. 2f.The calculated dipole moments (Fig. S4†) of CMB, Y6-16, and CFB were −3.448, 0.136, and 1.299, respectively. The negative dipole of CMB indicates that the dipole is oriented toward the substituent on the central benzene ring, suggesting a relatively positive charge due to its electron-donating ability. The larger positive dipole moment of CFB indicates a stronger electron-withdrawing core compared to Y6-16, which is consistent with the results observed in the absorption spectrum. CFB and CMB display similar molecular electrostatic potential (ESP) distributions, as shown in Fig. 2i. Most regions of the molecular surface exhibit positive ESP values, revealing the electron-accepting characteristics of both NFAs.
Molecular packing in single crystals
To elucidate the effect of substituting the Tz group with smaller substituents in the o-BDP core on molecular packing, single-crystal X-ray diffraction analysis was conducted. Single crystals of CFB and CMB were successfully acquired through vapor diffusion in acetonitrile and chloroform, and the crystallographic parameters for each crystal are provided in Table S1.† Data for single crystals of Y6-16 were extracted from the literature for comparison.38 As depicted in Fig. 3a, CMB displays a relatively twisted central core with a θ value of 9.69° owing to the steric hindrance caused by the methyl groups, and also larger Φ values of 6.37° and 8.52°. In contrast, the torsion angle between two outer thiophene (θ) for CFB is 2.15° (Fig. 3b), which is smaller than that of Y6-16 (5.05°). Likewise, the dihedral angles (Φ) of CFB between the outer thiophene and terminal group for CFB are 1.38° and 4.53°, respectively, also much smaller than those of Y6-16 (4.67° and 8.55°). Overall, CFB exhibits a more planar structure than CMB.Because of the C-shaped molecular geometry, both CMB and CFB molecules form a three-dimensional (3D) grid-like packing mode assembled via multiple π–π interactions (Fig. 3c and d). The π–π stacking distances in each mode are shown in Fig. 3e, f and S5.† CMB demonstrates five distinct dimeric π–π packing configurations (Fig. 3g) in a unit cell: the S-shaped TT-1 mode (TT, terminal to terminal), the U-shaped TT-2 mode, the M-shaped CT-1 mode (CT, core to terminal), the V-shaped CT-2 mode, and the Y-shaped CC–TT mode (CC, core to core). These configurations bear a resemblance to those observed in CB16,38 indicating that the presence of dimethyl groups in CMB does not electronically alter the chemical environment. Consequently, the intermolecular interactions remain in a similar manner. The CC–TT mode, commonly observed in Y6 single crystals45–47 associated with noncovalent S⋯N contacts between the Tz groups, has been theoretically shown to exhibit strong electronic coupling for both hole and electron transport.34,47 Even though the central A′ group is eliminated, CMB also forms the CC–TT mode where the central o-xylene moieties are staggered to avoid the steric hindrance caused by the methyl groups, leading to an increased π–π distance of 3.57 Å between the two central molecules. In comparison to Y6-16, which exhibits 3D grid-like patterns containing a single type of elliptical void, CMB showcases more complex patterns featuring three distinct types of elliptical frames (Fig. 3c). The elliptical frames in CMB, measuring approximately 16.6 × 9.8 Å and 13.4 × 13.8 Å in size, are notably smaller than those observed in Y6-16, which are approximately 13.4 × 17.5 Å. The denser and more interpenetrating molecular arrangement in the CMB crystal provides an advantage for facilitating charge transport.
The unit cell in the crystal structure of CFB comprises four molecules forming only three distinct dimeric modes (Fig. 3f and h) including S-shape TT-1 mode, M-shape CC–TT mode and S-shape CT–CT mode. The core-to-core overlapping in the CC–TT mode within the CFB crystal is restricted to the terminal thiophene region, resulting in a larger π–π distance of 3.51 Å. On the other hand, compared to that of Y6-16, the CT–CT mode of CFB features a closer π–π distance of 3.38 Å and larger π–π overlapping. An additional F–F interaction, with a short contact distance of 3.49 Å, was observed between the ortho-difluorobenzene moiety (C) and the FIC acceptor (T) in the CTCT mode (Fig. 3f).
The CC–TT and CT–CT modes in CFB could offer more efficient charge transport channels.33,34,47–50 Notably, the  -type Y6-16 exhibits four dimeric packing modes. Compared to CMB (5 modes) and Y6-16 (4 modes), the decrease in the number of packing modes found in CFB can be derived from the incorporation of compact difluoro atoms into the π-core structure to electronically manipulate the intermolecular interactions without imposing steric hindrance. The 3D packing framework of CFB reveals a circular void with dimensions of 24.6 × 25.7 Å (Fig. 3d). Viewed from the c-axis, the successive intersection of each circular ring with the other four gives rise to a highly organized kaleidoscope-like 3D structure, a feature previously unseen in any crystal structure within the Y6-series NFAs.45–47 The intersecting regions within the circular frameworks are predominantly composed of FIC end-group acceptors, which form electron-transport channels along the end-group stacking. These results demonstrated that C-shaped A-DNBND-A-type derivatives are capable of forming ordered and 3D interpenetrating packing structures. The two substituents on the central benzene ring could electronically and sterically modulate the packing configurations.
-type Y6-16 exhibits four dimeric packing modes. Compared to CMB (5 modes) and Y6-16 (4 modes), the decrease in the number of packing modes found in CFB can be derived from the incorporation of compact difluoro atoms into the π-core structure to electronically manipulate the intermolecular interactions without imposing steric hindrance. The 3D packing framework of CFB reveals a circular void with dimensions of 24.6 × 25.7 Å (Fig. 3d). Viewed from the c-axis, the successive intersection of each circular ring with the other four gives rise to a highly organized kaleidoscope-like 3D structure, a feature previously unseen in any crystal structure within the Y6-series NFAs.45–47 The intersecting regions within the circular frameworks are predominantly composed of FIC end-group acceptors, which form electron-transport channels along the end-group stacking. These results demonstrated that C-shaped A-DNBND-A-type derivatives are capable of forming ordered and 3D interpenetrating packing structures. The two substituents on the central benzene ring could electronically and sterically modulate the packing configurations.
Device characteristics
OPV devices with an inverted architecture of ITO/ZnO/active layer/MoO3/Ag were fabricated. PM6 was chosen as the p-type polymer to blend with the three acceptors with an optimal weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 in wt%. The active layers were prepared by spin-coating PM6:NFA solutions using o-xylene as the greener solvent without any additive. The standard PM6:Y6-16-based device showed a Jsc of 26.37 mA cm−2, an FF of 74.48% and a Voc of 0.83 V with a PCE of 16.30%. CFB with two electron-withdrawing fluorine atoms shows a significantly blue-shifted absorption compared to Y6-16. Consequently, the PM6:CFB device delivered a relatively lower Jsc of 24.00 mA cm−2. However, it showed a higher FF of 77.45% and an improved Voc of 0.89 V, leading to a better PCE of 16.55%. This suggests a reduction in energy loss for the CFB-based device, given the identical LUMO energy level between CFB and Y6-16. It should also be noted that the integrated Jsc extracted from the EQE spectrum of the PM6:CFB device is larger than that of PM6:Y6-16 before 850 nm, indicating that the former exhibits superior charge dynamics within its own absorption region. The PM6:CFB blend is likely to exhibit a more favorable morphology for efficient charge transport. As evidenced by its single-crystal structure, CFB with fluorine atoms may promote better alignment and packing of the molecules within the active layer, facilitating more effective electron transport pathways. Additionally, the interaction between CFB and the PM6 polymer matrix may lead to improved interfacial properties, enhancing charge extraction efficiency and reducing charge recombination.
1.2 in wt%. The active layers were prepared by spin-coating PM6:NFA solutions using o-xylene as the greener solvent without any additive. The standard PM6:Y6-16-based device showed a Jsc of 26.37 mA cm−2, an FF of 74.48% and a Voc of 0.83 V with a PCE of 16.30%. CFB with two electron-withdrawing fluorine atoms shows a significantly blue-shifted absorption compared to Y6-16. Consequently, the PM6:CFB device delivered a relatively lower Jsc of 24.00 mA cm−2. However, it showed a higher FF of 77.45% and an improved Voc of 0.89 V, leading to a better PCE of 16.55%. This suggests a reduction in energy loss for the CFB-based device, given the identical LUMO energy level between CFB and Y6-16. It should also be noted that the integrated Jsc extracted from the EQE spectrum of the PM6:CFB device is larger than that of PM6:Y6-16 before 850 nm, indicating that the former exhibits superior charge dynamics within its own absorption region. The PM6:CFB blend is likely to exhibit a more favorable morphology for efficient charge transport. As evidenced by its single-crystal structure, CFB with fluorine atoms may promote better alignment and packing of the molecules within the active layer, facilitating more effective electron transport pathways. Additionally, the interaction between CFB and the PM6 polymer matrix may lead to improved interfacial properties, enhancing charge extraction efficiency and reducing charge recombination.
CMB featuring electron-donating methyl groups enhances absorption capability and upshifts the LUMO energy level. Consequently, the PM6:CMB-based device achieved a Jsc of 26.32 mA cm−2 and the highest Voc of 0.90 V, leading to a comparable PCE of 16.46%. However, the FF of 69.50% is only moderate, presumably due to the steric hindrance of the dimethyl groups that cause inferior molecular packing of CMB within the blend. Considering that the PM6:CFB device has a higher Voc, and a higher integrated EQE Jsc before 840 nm, we introduced CFB into the PM6:Y6-16 blend as a second acceptor in an attempt to increase Voc and to complement the absorption range with Y6-16. By combining the absorption/EQE strength of CFB in 500–800 nm and Y6-16 in 800–950 nm, the ternary PM6:Y6-16:CFB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 in wt%) device achieved a largely improved Jsc of 27.32 mA cm−2 along with an improved Voc of 0.86 V, delivering a superior PCE of 17.83% that surpasses those of both the binary PM6:Y6-16 and PM6-CFB devices. By a similar strategy, through the introduction of Y6-16 to modulate the intermolecular packing in the PM6:CMB blend, the ternary PM6:Y6-16:CMB (1
0.6 in wt%) device achieved a largely improved Jsc of 27.32 mA cm−2 along with an improved Voc of 0.86 V, delivering a superior PCE of 17.83% that surpasses those of both the binary PM6:Y6-16 and PM6-CFB devices. By a similar strategy, through the introduction of Y6-16 to modulate the intermolecular packing in the PM6:CMB blend, the ternary PM6:Y6-16:CMB (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 in wt%) device exhibited a much improved FF of 74.71% with a high Voc of 0.87 V, and a slightly enhanced Jsc of 26.76 mA cm−2, achieving a PCE of 17.44% that also outperforms both the binary PM6:Y6-16 and PM6-CMB devices. The J–V curves and EQE spectra of the optimized devices are shown in Fig. 4a–c with device parameters summarized in Tables 2 and S2–S5.†
0.6 in wt%) device exhibited a much improved FF of 74.71% with a high Voc of 0.87 V, and a slightly enhanced Jsc of 26.76 mA cm−2, achieving a PCE of 17.44% that also outperforms both the binary PM6:Y6-16 and PM6-CMB devices. The J–V curves and EQE spectra of the optimized devices are shown in Fig. 4a–c with device parameters summarized in Tables 2 and S2–S5.†
| Active layer | Blend ratio in wt% | V oc [V] | J sc [mA cm−2] | FF [%] | PCE [%] |
|---|---|---|---|---|---|
| PM6:Y6-16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.83 (0.83 ± 0.01) | 26.37 (26.46 ± 0.66) | 74.48 (73.62 ± 1.44) | 16.30 (16.17 ± 0.15) |
| PM6:CFB | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.89 (0.89 ± 0.01) | 24.00 (24.14 ± 0.28) | 77.45 (76.39 ± 0.94) | 16.55 (16.35 ± 0.23) |
| PM6:CMB | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.90 (0.89 ± 0.01) | 26.32 (25.97 ± 0.62) | 69.50 (70.35 ± 1.69) | 16.46 (16.33 ± 0.05) |
| PM6:Y6-16:CFB | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
0.86 (0.867 ± 0.004) | 27.32 (27.00 ± 0.20) | 75.61 (75.01 ± 1.03) | 17.83 (17.57 ± 0.19) |
| PM6:Y6-16:CMB | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 0.6 |
0.87 (0.875 ± 0.002) | 26.76 (26.53 ± 0.16) | 74.71 (74.04 ± 0.56) | 17.44 (17.20 ± 0.14) |
Charge dynamics analysis
To understand the charge recombination behaviors, the dependence of light intensity on Jsc and Voc was investigated and illustrated in Fig. 4d and e. According to the formula Jsc ∝ (Plight)α, the slope of the natural logarithm of light intensity versus Jsc yields the exponential factor α, which would be close to 1 if bimolecular recombination is effectively suppressed.51,52 The relationship of Voc and Plight followed the rule Voc ∝ n(kT/q)ln(Plight) and represented the possibility of trap-assisted recombination.53 The α and n values are close to 1 for all the devices (α = 0.936, 0.971, 0.977; n = 1.14, 1.11, 1.06 for PM6:CMB, PM6:Y6-16 and PM6:CFB, respectively), indicating a relatively low degree of bimolecular recombination and trap-assisted recombination in the devices. Though having similar absorption spectra, Y6-16 based devices showed better suppression of both types of recombination compared to the CMB-based devices, leading to a slightly higher Jsc and FF. The CFB-based devices possessed the lowest degree of recombination, coinciding with the highest FF, again suggesting that the relatively lower Jsc should mainly be attributed to the blue-shifted absorption.To investigate the charge dissociation and charge collection properties, the relationship of photocurrent density (Jph) versus effective voltage (Veff) was evaluated.54 The ratio of Jsc to saturated current density (Jsat) yields exciton dissociation efficiency (Pdiss) while the ratio of Jmax to saturated current density (Jsat) indicates charge collection efficiency (Pcoll). As depicted in Fig. 4f, the CFB-based device possessed the highest value of 88.33% for Pcoll, which may be partially attributed to the better film morphology and higher FF. Although the CMB-based device has a Pdiss value similar to that of the Y6-16 based device, it shows a lower Pcoll, indicating that the slightly lower Jsc is likely due to morphological issues rather than differences in charge generation properties.
Transient photocurrent (TPC) and transient photovoltage (TPV) measurements were conducted to gain insight into the charge carrier dynamics for the OPVs. As shown in Fig. 4g, the shortest charge extraction time of 0.34 μs, extracted by the exponential decay fitting of TPC data,55 was observed for PM6:CFB based devices (compared to 0.39 μs and 0.37 μs for PM6:Y6-16 and PM6:CMB-based devices), suggesting better charge extraction ability. Meanwhile, the longest carrier lifetime of 46.2 μs for the PM6:CFB-based device was extracted from TPV measurements (compared to 44.4 μs and 36.7 μs for PM6:Y6-16 and PM6:CMB-based devices), indicating the lowest charge recombination probability for this blend (Fig. 4h). The combination of more efficient charge extraction and the prolonged carrier lifetime simultaneously contributes to the highest Pcoll and well-suppressed charge recombination behaviors observed in the PM6:CFB-based device. It should be noted that the shorter charge carrier lifetime for PM6:CMB based devices may explain their relatively larger recombination probability.
Moreover, space-charge-limited current (SCLC) measurements were also conducted using both hole-only and electron-only devices to evaluate charge transport properties.56 The calculated hole/electron mobilities are 2.59 × 10−4/3.63 × 10−4, 2.46 × 10−4/3.55 × 10−4 and 2.36 × 10−4/3.52 × 10−4 cm2 V−1 s−1 for PM6:CFB, PM6:Y6-16 and PM6:CMB devices, respectively (Fig. 4i). The CFB device exhibits the highest mobilities and the most balanced electron-to-hole mobility ratio μe/μh of 1.40, which corresponds to the highest PCE value. These findings suggest that incorporating small withdrawing F groups is an effective strategy for reducing charge recombination losses.
For the ternary devices, the charge dynamics analysis is shown in Fig. S6, S7 and Table S6.† Introduction of CFB into the PM6:Y6-16 device resulted in α and n values approaching 1, an extended charge carrier lifetime of over 60 μs, increased Pcoll, and higher mobilities, contributing to a high Jsc above 27 mA cm−2. Likewise, the ternary PM6:Y6-16:CMB device also demonstrated enhanced parameters, suggesting that incorporating CMB into the PM6:Y6-16 device also effectively reduces charge recombination.
Energy loss analysis
To investigate the significant improvement in Voc observed in both CFB- and CMB-based devices compared to Y6-16, the total energy loss (Eloss) of the OPVs, defined by using the equation Eloss = Eg − qVoc, was analyzed. The bandgap (Eg) values were estimated from the cross-point of the normalized FTPS-EQE and electroluminescence (EL) spectra,57,58 yielding values of 1.425, 1.482, and 1.420 for the PM6:Y6-16, PM6:CFB, and PM6:CMB devices, respectively. Total Eloss is determined to be 0.598, 0.593, and 0.522 eV for PM6:Y6-16, PM6:CFB, and PM6:CMB devices, respectively. Although both the CFB and CMB-based devices exhibited lower Eloss compared to the Y6-16 based device, the reasons for the Voc improvement are different. The higher Voc of the CFB-based device can be attributed to both the larger bandgap and reduced Eloss, while the increased Voc of the CMB-based device was solely due to the reduction in Eloss because the Eg values of CMB and Y6-16 are the same. Since Voc is determined by two factors (qVoc = Eg − Eloss), the plot of Eloss/Eg values for the devices, shown in Fig. 5a, intuitively reflects the magnitude of Voc. The smaller Eloss/Eg values of 0.37 and 0.40 for the PM6:CMB and PM6:CFB devices correspond to higher Voc values of 0.90 and 0.89 V, compared to PM6: Y6-16 (Eloss/Eg = 0.40, Voc = 0.83 V). The total Eloss can be further divided into three components (Eloss = ΔECT + ΔEr + ΔEnr, Fig. 5b), as detailed in Table 3 and Fig. 5c: charge generation loss (ΔECT), radiative charge recombination (ΔEr), and non-radiative charge recombination (ΔEnr).58–61 ΔECT is the difference between Eg and ECT (charge transfer state) which can be estimated by fitting normalized FTPS-EQE and EL spectra,55,62,63 as depicted in Fig. 5d–f and S8.† It should be noted that the PM6:CFB and PM6:CMB devices exhibited higher-lying ECT compared to the PM6:Y6-16 device, which resulted in reduced ΔECT. This suggests a potential hybridization of the LE (localized excited) and CT states64,65 and a further suppression of electron coupling between the lowest CT state and the highest vibrational ground state,66 which is one of the contributions to ΔEnr.66,67 ΔEnr was experimentally obtained from EQEEL spectra (Fig. 5g) using the equation ΔEnr = (kT/q)ln(1/EQEEL),68 resulting in values of 0.284 and 0.231 for the PM6:CFB and PM6:CMB-based devices, respectively, which are smaller than the 0.287 observed for the PM6:Y6-16-based device.| Active layer | E g [eV] | V oc [V] | E CT [eV] | ΔECTc [eV] | ΔErd [V] | ΔEnre [V] | EQEEL | E loss [eV] | E u [meV] |
|---|---|---|---|---|---|---|---|---|---|
| a Bandgap was estimated from the cross-point of normalized FTPS-EQE and EL spectra. b E CT was obtained from the fitting curves of normalized FTPS-EQE and EL spectra. c ΔECT was determined from the equation ΔECT = Eg − ECT. d ΔEr was determined from the equation ΔEr = ECT/q-Voc − ΔEnr. e ΔEnr was determined from EQEEL results, which followed the equation ΔEnr = (kT/q)ln(1/EQEEL). f E u was obtained by exponential fitting of the low-energy part of the FTPS-EQE spectra. | |||||||||
| PM6:Y6-16 | 1.425 | 0.827 | 1.378 | 0.047 | 0.261 | 0.287 | 1.46 × 10−5 | 0.598 | 24.39 |
| PM6:CFB | 1.482 | 0.889 | 1.440 | 0.042 | 0.266 | 0.284 | 1.58 × 10−5 | 0.593 | 23.62 |
| PM6:CMB | 1.420 | 0.898 | 1.390 | 0.030 | 0.261 | 0.231 | 1.24 × 10−5 | 0.522 | 24.92 |
| PM6:Y6-16:CFB | 1.428 | 0.867 | 1.391 | 0.037 | 0.265 | 0.259 | 4.19 × 10−5 | 0.561 | 23.94 |
| PM6:Y6-16:CMB | 1.421 | 0.875 | 1.388 | 0.033 | 0.269 | 0.244 | 7.25 × 10−5 | 0.546 | 24.04 |
In the ternary devices, incorporating CFB and CMB into the binary PM6:Y6-16 blend reduces ΔEnr to 0.259 eV and 0.244 eV, and total Eloss to 0.561 eV and 0.546 eV, respectively, with the latter having a more significant effect, leading to much improved Voc values of 0.86 V and 0.87 V compared to the binary PM6:Y6-16 device (Voc = 0.83 V). Fig. 5h displays a radar diagram illustrating the percentage improvements in OPV parameters and the decrease in Eloss for the ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices compared to the reference PM6:Y6-16-based device. The diagram clearly reveals that CMB with electron-donating methyl groups significantly suppress Eloss and thus enhance Voc due to its electronic effects, whereas CFB, with electron-donating fluorine atoms, primarily optimizes intermolecular packing, leading to improved Jsc and FF.
Device film morphology analysis
To elucidate the packing features of the neat NFA films as well as blend films with PM6, grazing incidence wide-angle X-ray scattering (GIWAXS) experiments were carried out at the 25A1 coherent X-ray scattering beamline of the Taiwan Photon Source (TPS). The 2D diffraction patterns as well as their corresponding 1D line-cut profiles in the in-plane (qxy) and out-of-plane (qz) directions are depicted in Fig. 6. As illustrated in Fig. 6a, all the neat films exhibited a strong (010) diffraction peak in the qz direction, suggesting the preference of face-on orientations of the NFAs.Y6-16 exhibited a diffraction at qz = 1.79 Å−1 corresponding to the smallest π–π stacking distance (dπ) of 3.51 Å due to the additional molecular interactions induced by the thiadiazole group. CFB and CMB showed diffractions at qz = 1.77 Å−1 and 1.75 Å−1 corresponding to the larger π–π stacking distance of 3.55 Å and 3.59 Å, respectively, suggesting that molecular interactions in the neat films follow the trend Y6-16 > CFB > CMB, which is consistent with the trend observed in Δλmax in the absorption spectra. Furthermore, the crystalline coherence lengths (CCL, Lc) of π–π stacking (Lc π–π) in the neat films, derived from the full width at half maximum (FWHM) and listed in Table 4, show a gradual increase from 14.50 Å for CMB, to 17.14 Å for CFB, and further to 17.67 Å for Y6-16. The GIWAXS patterns of PM6:Y6-16, PM6:CFB and PM6:CMB blend films exhibited similar face-on oriented π–π stacking reflection centered at 1.77 Å−1, 1.76 Å−1 and 1.74 Å−1, corresponding to a gradually increased dπ of 3.55 Å, 3.57 Å and 3.61 Å, respectively. In comparison to the as-cast neat films, the blend films exhibit a slight increase in the dπ due to the interpenetration of NFAs within the PM6 matrix, and the trend of the π–π stacking distance in the blend films aligns with that observed in the neat films. Besides, all the blend films showed a distinct (100) diffraction peak at qxy ∼0.30 Å−1 corresponding to the lamellar side-chain packing of PM6 (Fig. 6b and c).
| Film | In-plane reflection peak | Out-of-plane reflection peak | ||||||
|---|---|---|---|---|---|---|---|---|
| q xy [Å−1] | d l [Å] | δqxy [Å−1] | L C [Å] | q z [Å−1] | d π [Å] | δq z [Å−1] | L C [Å] | |
| CMB | 0.37 | 16.98 | 0.14 | 40.39 | 1.75 | 3.59 | 0.39 | 14.50 |
| CFB | 0.36 | 17.45 | 0.15 | 37.70 | 1.77 | 3.55 | 0.33 | 17.14 |
| Y6-16 | 0.36 | 17.45 | 0.19 | 29.76 | 1.79 | 3.51 | 0.32 | 17.67 |
| PM6:CMB | 0.30 | 20.94 | 0.08 | 70.69 | 1.74 | 3.61 | 0.30 | 18.85 |
| PM6:CFB | 0.30 | 20.94 | 0.07 | 80.78 | 1.76 | 3.57 | 0.24 | 23.56 |
| PM6:Y6-16 | 0.29 | 21.67 | 0.07 | 80.78 | 1.77 | 3.55 | 0.26 | 21.75 |
| PM6:Y6-16:CMB | 0.30 | 20.94 | 0.05 | 113.10 | 1.75 | 3.59 | 0.255 | 22.17 |
| PM6:Y6-16:CFB | 0.30 | 20.94 | 0.06 | 94.24 | 1.76 | 3.57 | 0.250 | 22.62 |
It is noteworthy that the Lc π–π of the PM6:CFB film reaches the highest value of 23.56 Å attributed to the more ordered packing benefited from the fluorine atoms. We also determined the Urbach energy (Eu) by performing an exponential fit on the low photon energy region of the normalized FTPS-EQE spectra. The results are provided in Table 3 and illustrated in Fig. 5i and S9.† The smallest Eu value of 23.62 meV observed in the PM6:CFB device suggests a higher degree of molecular order, which is consistent with its largest Lc π–π value.25,33,34,69,70
In the ternary devices (Fig. 6d), the introduction of CFB into the PM6:Y6-16 film led to an increase in Lc in both the qxy and qz directions, suggesting that CFB promotes higher crystallinity within the blend, improving film morphology and enhancing charge transport dynamics.11 Similarly, the addition of CMB to the PM6:Y6-16 film demonstrated a similar effect, implying that CMB also interacts well with both PM6 and Y6-16 in the ternary system. However, the increased π–π stacking distance to 3.59 Å indicates that the steric effect of the central dimethyl groups persists which may partly explain the slightly lower FF observed in the PM6:Y6-16:CMB device compared to the PM6:Y6-16:CFB device.
Atomic force microscopy (AFM) was also used to examine the morphology of the neat and blend films. As depicted in Fig. 7, both Y6-16 and CFB neat films showed a very smooth surface and root-meant-square (RMS) roughness of around 1 nm. Despite the similar RMS in neat films, the PM6:CFB film possessed a much smoother surface (RMS = 1.48 nm) with a much clearer interpenetrating fibrillar structure than that of the PM6:Y6-16 film (RMS = 2.19 nm), indicating the better interaction between CFB and PM6. As for CMB, both the neat and blend films have a relatively rougher RMS of 1.61 and 2.68 nm, respectively, compared with the other two acceptors. However, with the largest RMS, the PM6:CMB film still presented a fibrillar structure, which may partially account for the comparable performance with the PM6:Y6-16 device. It should be noted that the introduction of CFB into the PM6:Y6-16 blend led to a dramatic decrease in roughness (RMS = 1.67 nm, Fig. 7d) and the appearance of a much clearer and more delicate fibrillar network structure.28,38 This suggests that CFB may enhance interactions in the PM6:Y6-16:CFB blend, contributing to the high FF and improved performance. A similar phenomenon was observed after introducing CMB into the PM6:Y6-16 blend (Fig. 7d), indicating that CMB also interacts effectively with both PM6 and Y6-16 in this system.
 | ||
| Fig. 7 AFM height images of (a) CFB and PM6:CFB, (b) Y6-16 and PM6:Y6-16, (c) CMB and PM6:CMB and (d) ternary blend films. | ||
Contact angle measurements of the neat films of the NFAs and PM6 were conducted to obtain the Flory–Huggins interaction parameter (χ), which was used to evaluate the miscibility between donor–acceptor and acceptor–acceptor pairs (Fig. S10 and Table S7†). The χ values between PM6 and CFB, Y6-16, and CMB are 0.24, 0.35, and 0.55, respectively. The gradually increasing χ values suggest a decrease in miscibility between the donor and acceptor, which correlates well with the fill factor (FF) results. Moreover, the small χ value between Y6-16 and either CFB or CMB indicates good miscibility between the NFAs and explains the improved FF in the ternary system. Considering all morphological analyses, we can infer that the disubstituted C-shaped o-BDP skeletons of CFB and CMB play a crucial role in their effective interactions with both PM6 and Y6-16 in the ternary system, contributing to the significant improvement in PCE for the ternary devices.
Conclusions
Elimination of the A′ unit in Y6-series type structures enables the introduction of two new non-aromatic substitutions on the central o-benzodipyrrole core, while preserving its C-shaped molecular skeleton. In this work, we designed and synthesized two CB-based A-DNBND-A-type architecture NFAs, CFB and CMB, by introducing two F atoms and methyl groups on the central benzene ring, respectively. The electron-withdrawing F atoms in CFB reduce the electron-donating strength of the o-BDP unit and weaken the ICT effect, leading to a larger bandgap, a blue-shifted absorption spectrum and a downshifted HOMO energy level. Conversely, the electron-donating methyl groups in CMB result in upshifted HOMO/LUMO energy levels, a narrower band gap and a red-shifted absorption spectrum compared to those of Y6-16. The high electronegativity and compactness of F atoms facilitate intermolecular interactions without sterically affecting molecular self-assembly, leading to a highly ordered kaleidoscope-like 3D packing network with shorter π–π stacking distances in the single crystal structure. These characteristics reduce charge recombination, enhance charge mobilities and elongate carrier lifetime in the devices. In contrast, CMB forms a more complicated 3D structure with five dimeric π–π stacking modes due to the steric effect of the dimethyl o-BDP moiety.
type structures enables the introduction of two new non-aromatic substitutions on the central o-benzodipyrrole core, while preserving its C-shaped molecular skeleton. In this work, we designed and synthesized two CB-based A-DNBND-A-type architecture NFAs, CFB and CMB, by introducing two F atoms and methyl groups on the central benzene ring, respectively. The electron-withdrawing F atoms in CFB reduce the electron-donating strength of the o-BDP unit and weaken the ICT effect, leading to a larger bandgap, a blue-shifted absorption spectrum and a downshifted HOMO energy level. Conversely, the electron-donating methyl groups in CMB result in upshifted HOMO/LUMO energy levels, a narrower band gap and a red-shifted absorption spectrum compared to those of Y6-16. The high electronegativity and compactness of F atoms facilitate intermolecular interactions without sterically affecting molecular self-assembly, leading to a highly ordered kaleidoscope-like 3D packing network with shorter π–π stacking distances in the single crystal structure. These characteristics reduce charge recombination, enhance charge mobilities and elongate carrier lifetime in the devices. In contrast, CMB forms a more complicated 3D structure with five dimeric π–π stacking modes due to the steric effect of the dimethyl o-BDP moiety.
Compared to the PM6:Y6-16 device, the binary PM6:CFB device exhibited a higher Voc of 0.89 V, the highest FF of 77.45% but a lower Jsc of 24 mA cm−2, whereas the binary PM6:CMB device exhibited the highest Voc of 0.90 V, a Jsc of 26.32 mA cm−2 but a lower FF of 69.50%. To leverage the advantages of both CFB and CMB, the ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices were fabricated and optimized. With CFB's excellent charge generation, transport, and collection properties, along with its highly ordered packing and well-defined fibrillar structure when blended with PM6, the ternary PM6:Y6-16:CFB device showed a significant increase in Jsc to 27.32 mA cm−2, coupled with a moderate improvement in Voc to 0.86 V, resulting in the highest PCE of 17.83% in comparison with the standard PM6:Y6-16 device. CMB demonstrates a superior ability to suppress Eloss, particularly by reducing ΔEnr. Consequently, the PM6:Y6-16:CMB device effectively improved the Voc to 0.87 V, along with a moderate enhancement in Jsc, yielding a PCE of 17.44%. The enhanced performance of the ternary devices suggests that the disubstituted o-BDP skeleton in both CFB and CMB plays a key role in forming a compatible morphology with both PM6 and Y6-16, thereby maximizing device parameters. Furthermore, the use of a greener nonhalogenated solvent for device fabrication underscores the potential for eco-friendly processing procedures to mitigate toxicity concerns. Substituting the central benzene ring of these C-shaped o-BDP-based A–D–A type NFAs provides valuable insights into the structure–property relationship and offers new avenues for tailoring intrinsic properties and molecular self-assembly that can achieve higher OPV performance with reduced charge recombination and energy loss.
Data availability
The data supporting the findings of this study are available within the article and ESI.† The X-ray crystallographic coordinates for structures of CMB and CFB have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with 2391712 and 2391682.Author contributions
The project was conceived and conceptualized by Yen-Ju Cheng and Yan-Bo Wang. Yan-Bo Wang, Yung-Jing Xue, Jun-Cheng Hong, and Kuo-Hsiu Huang synthesized the compounds and carried out measurements of their optical and electrochemical properties, and GIWAXS analysis. The fabrication and characterization of the OPVs were performed by Yan-Bo Wang, Chia-Lin Tsai, Bing-Huang Jiang, Han-Cheng Lu, Yu-Chi Huang, and Chih-Ping Chen. Su-Ying Chien conducted single-crystal X-ray crystallography. The manuscript was drafted by Yan-Bo Wang and Yen-Ju Cheng.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science and Technology Council, Taiwan (grant no. 112-2221-E-A49-002 and 113-2113-M-A49-015-MY3) and Ministry of Education, Taiwan (SPROUT Project-Center for Emergent Functional Matter Science of National Yang Ming Chiao Tung University). We thank the National Center of High-Performance Computing (NCHC) in Taiwan for computer time and facilities. GIWAXS measurement support from TPS 25 beamlines of the National Synchrotron Radiation Research Center (NSRRC) is acknowledged; special thank goes to Dr Yi-Wei Tsai for help in the GIWAXS data processing.Notes and references
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng and P. A. Johnson, Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- K. Jiang, Q. Wei, J. Y. L. Lai, Z. Peng, H. K. Kim, J. Yuan, L. Ye, H. Ade, Y. Zou and H. Yan, Alkyl chain tuning of small molecule acceptors for efficient organic solar cells, Joule, 2019, 3, 3020–3033 CrossRef CAS.
- Z. Abbas, S. U. Ryu, M. Haris, C. E. Song, H. K. Lee, S. K. Lee, W. S. Shin, T. Park and J.-C. Lee, Optimized vertical phase separation via systematic Y6 inner side-chain modulation for non-halogen solvent processed inverted organic solar cells, Nano Energy, 2022, 101, 107574 CrossRef CAS.
- M. Deng, X. Xu, Y. Duan, L. Yu, R. Li and Q. Peng, Y-type non-fullerene acceptors with outer branched side chains and inner cyclohexane side chains for 19.36% efficiency polymer solar cells, Adv. Mater., 2023, 35, 2210760 CrossRef CAS PubMed.
- C. Kim, J. H. Lee, J. S. Park, S. Lee, T. N.-L. Phan, Y.-H. Kim and B. J. Kim, Impact of the molecular structure of oligo (ethylene glycol)-incorporated Y-series acceptors on the formation of alloy-like acceptors and performance of non-halogenated solvent-processable organic solar cells, ACS Appl. Mater. Interfaces, 2023, 15, 24670–24680 CrossRef CAS PubMed.
- G. U. Kim, C. Sun, D. Lee, G. S. Choi, J. S. Park, S. Seo, S. Lee, D. Y. Choi, S. K. Kwon and S. Cho, Effect of the selective halogenation of small molecule acceptors on the blend morphology and voltage loss of high-performance solar cells, Adv. Funct. Mater., 2022, 32, 2201150 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma and C. An, Single-junction organic photovoltaic cells with approaching 18% efficiency, Adv. Mater., 2020, 32, 1908205 CrossRef CAS PubMed.
- Y. Chang, J. Zhang, Y. Chen, G. Chai, X. Xu, L. Yu, R. Ma, H. Yu, T. Liu and P. Liu, Achieving efficient ternary organic solar cells using structurally similar non-fullerene acceptors with varying flanking side chains, Adv. Energy Mater., 2021, 11, 2100079 CrossRef CAS.
- G. Chai, Y. Chang, J. Zhang, X. Xu, L. Yu, X. Zou, X. Li, Y. Chen, S. Luo and B. Liu, Fine-tuning of side-chain orientations on nonfullerene acceptors enables organic solar cells with 17.7% efficiency, Energy Environ. Sci., 2021, 14, 3469–3479 RSC.
- G. Chai, Y. Chang, Z. Peng, Y. Jia, X. Zou, D. Yu, H. Yu, Y. Chen, P. C. Chow and K. S. Wong, Enhanced hindrance from phenyl outer side chains on nonfullerene acceptor enables unprecedented simultaneous enhancement in organic solar cell performances with 16.7% efficiency, Nano Energy, 2020, 76, 105087 CrossRef CAS.
- D. Jeong, G. U. Kim, D. Lee, S. Seo, S. Lee, D. Han, H. Park, B. Ma, S. Cho and B. J. Kim, Sequentially fluorinated polythiophene donors for high-performance organic solar cells with 16.4% efficiency, Adv. Energy Mater., 2022, 12, 2201603 CrossRef CAS.
- F. Lin, K. Jiang, W. Kaminsky, Z. Zhu and A. K.-Y. Jen, A non-fullerene acceptor with enhanced intermolecular π-core interaction for high-performance organic solar cells, J. Am. Chem. Soc., 2020, 142, 15246–15251 CrossRef CAS PubMed.
- H. Yu, Z. Qi, J. Zhang, Z. Wang, R. Sun, Y. Chang, H. Sun, W. Zhou, J. Min and H. Ade, Tailoring non-fullerene acceptors using selenium-incorporated heterocycles for organic solar cells with over 16% efficiency, J. Mater. Chem. A, 2020, 8, 23756–23765 RSC.
- Y. Cui, H. Yao, L. Hong, T. Zhang, Y. Tang, B. Lin, K. Xian, B. Gao, C. An and P. Bi, Organic photovoltaic cell with 17% efficiency and superior processability, Natl. Sci. Rev., 2020, 7, 1239–1246 CrossRef CAS PubMed.
- G. Li, X. Zhang, L. O. Jones, J. M. Alzola, S. Mukherjee, L.-w. Feng, W. Zhu, C. L. Stern, W. Huang and J. Yu, Systematic merging of nonfullerene acceptor π-extension and tetrafluorination strategies affords polymer solar cells with> 16% efficiency, J. Am. Chem. Soc., 2021, 143, 6123–6139 CrossRef CAS PubMed.
- Y. Shi, J. Pan, J. Yu, J. Zhang, F. Gao, K. Lu and Z. Wei, Optimizing the charge carrier and light management of nonfullerene acceptors for efficient organic solar cells with small nonradiative energy losses, Sol. RRL, 2021, 5, 2100008 CrossRef CAS.
- D. Mo, H. Chen, J. Zhou, L. Han, Y. Zhu, P. Chao, N. Zheng, Z. Xie and F. He, Isomeric effects of chlorinated end groups on efficient solar conversion, J. Mater. Chem. A, 2020, 8, 23955–23964 RSC.
- H. Wang, L. Han, J. Zhou, T. Liu, D. Mo, H. Chen, H. Lai, N. Zheng, Z. Xie and W. Zheng, Isomerism: minor changes in the bromine substituent positioning lead to notable differences in photovoltaic performance, CCS Chem., 2021, 3, 2591–2601 CrossRef CAS.
- Z. Luo, R. Ma, Z. Chen, Y. Xiao, G. Zhang, T. Liu, R. Sun, Q. Zhan, Y. Zou and C. Zhong, Altering the positions of chlorine and bromine substitution on the end group enables high-performance acceptor and efficient organic solar cells, Adv. Energy Mater., 2020, 10, 2002649 CrossRef CAS.
- C. He, Z. Chen, T. Wang, Z. Shen, Y. Li, J. Zhou, J. Yu, H. Fang, Y. Li and S. Li, Asymmetric electron acceptor enables highly luminescent organic solar cells with certified efficiency over 18, Nat. Commun., 2022, 13, 2598 CrossRef CAS PubMed.
- S. Liu, J. Yuan, W. Deng, M. Luo, Y. Xie, Q. Liang, Y. Zou, Z. He, H. Wu and Y. Cao, High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder, Nat. Photonics, 2020, 14, 300–305 CrossRef CAS.
- Z. Li, C. Zhu, J. Yuan, L. Zhou, W. Liu, X. Xia, J. Hong, H. Chen, Q. Wei and X. Lu, Optimizing side chains on different nitrogen aromatic rings achieving 17% efficiency for organic photovoltaics, J. Energy Chem., 2022, 65, 173–178 CrossRef CAS.
- F. Qi, K. Jiang, F. Lin, Z. Wu, H. Zhang, W. Gao, Y. Li, Z. Cai, H. Y. Woo and Z. Zhu, Over 17% efficiency binary organic solar cells with photoresponses reaching 1000 nm enabled by selenophene-fused nonfullerene acceptors, ACS Energy Lett., 2020, 6, 9–15 CrossRef.
- Y. Cho, Z. Sun, K. M. Lee, G. Zeng, S. Jeong, S. Yang, J. E. Lee, B. Lee, S.-H. Kang and Y. Li, CF3-terminated side chain enables efficiencies surpassing 18.2% and 16.1% in small-and large-scale manufacturing of organic solar cells, ACS Energy Lett., 2022, 8, 96–106 CrossRef.
- Z. Zhang, Y. Li, G. Cai, Y. Zhang, X. Lu and Y. Lin, Selenium heterocyclic electron acceptor with small urbach energy for as-cast high-performance organic solar cells, J. Am. Chem. Soc., 2020, 142, 18741–18745 CrossRef CAS PubMed.
- W. Gao, F. Qi, Z. Peng, F. R. Lin, K. Jiang, C. Zhong, W. Kaminsky, Z. Guan, C. S. Lee and T. J. Marks, Achieving 19% power conversion efficiency in planar-mixed heterojunction organic solar cells using a pseudosymmetric electron acceptor, Adv. Mater., 2022, 34, 2202089 CrossRef CAS PubMed.
- Z. Zhou, W. Liu, G. Zhou, M. Zhang, D. Qian, J. Zhang, S. Chen, S. Xu, C. Yang and F. Gao, Subtle molecular tailoring induces significant morphology optimization enabling over 16% efficiency organic solar cells with efficient charge generation, Adv. Mater., 2020, 32, 1906324 CrossRef CAS PubMed.
- M. Xie, Y. Shi, L. Zhu, J. Zhang, Q. Cheng, H. Zhang, Y. Yan, M. Zhu, H. Zhou and K. Lu, Selective halogenation of central and end-units of nonfullerene acceptors enables enhanced molecular packing and photovoltaic performance, Energy Environ. Sci., 2023, 16, 3543–3551 RSC.
- Y. Ding, S. Xiong, M. Li, M. Pu, Y. Zhu, X. Lai, Y. Wang, D. Qiu, H. Lai and F. He, Highly-Efficient 2D Nonfullerene Acceptors Enabled by Subtle Molecular Tailoring Engineering, Small, 2023, 2309169 Search PubMed.
- Z. Chen, J. Ge, Y. Guo, M. Zhao, J. Shi, Y. Qiu, E. Zhou and Z. Ge, Modification on the quinoxaline unit to achieve high open-circuit voltage and morphology optimization for organic solar cells, ACS Energy Lett., 2022, 7, 3432–3438 CrossRef CAS.
- Y. Jiang, Y. Li, F. Liu, W. Wang, W. Su, W. Liu, S. Liu, W. Zhang, J. Hou and S. Xu, Suppressing electron-phonon coupling in organic photovoltaics for high-efficiency power conversion, Nat. Commun., 2023, 14, 5079 CrossRef CAS PubMed.
- H. Chen, H. Liang, Z. Guo, Y. Zhu, Z. Zhang, Z. Li, X. Cao, H. Wang, W. Feng and Y. Zou, Central unit fluorination of non-fullerene acceptors enables highly efficient organic solar cells with over 18% efficiency, Angew. Chem., 2022, 134, e202209580 CrossRef.
- H. Liang, X. Bi, H. Chen, T. He, Y. Lin, Y. Zhang, K. Ma, W. Feng, Z. Ma and G. Long, A rare case of brominated small molecule acceptors for high-efficiency organic solar cells, Nat. Commun., 2023, 14, 4707 CrossRef CAS PubMed.
- Y. Zou, H. Chen, X. Bi, X. Xu, H. Wang, M. Lin, Z. Ma, M. Zhang, C. Li and X. Wan, Peripheral halogenation engineering controls molecular stacking to enable highly efficient organic solar cells, Energy Environ. Sci., 2022, 15, 3519–3533 RSC.
- K. Liu, Y. Jiang, F. Liu, G. Ran, F. Huang, W. Wang, W. Zhang, C. Zhang, J. Hou and X. Zhu, Organic solar cells with over 19% efficiency enabled by a 2D-conjugated non-fullerene acceptor featuring favorable electronic and aggregation structures, Adv. Mater., 2023, 35, 2300363 CrossRef CAS PubMed.
- S. Oh, D. Jeong, K. Bae, G. U. Kim, T. N. L. Phan, J. W. Lee, J. Park, D. Lee, S. Cho and B. J. Kim, Impact of Linker Engineering in Core-Linked Dimeric Acceptors for High-Performance Organic Solar Cells, Adv. Funct. Mater., 2024, 34, 2406501 CrossRef CAS.
- Y.-J. Cheng, C.-H. Chen, Y.-J. Ho, S.-W. Chang, H. A. Witek and C.-S. Hsu, Thieno [3, 2-b] pyrrolo donor fused with benzothiadiazolo, benzoselenadiazolo and quinoxalino acceptors: synthesis, characterization, and molecular properties, Org. Lett., 2011, 13, 5484–5487 CrossRef CAS PubMed.
- Y.-J. Xue, Z.-Y. Lai, H.-C. Lu, J.-C. Hong, C.-L. Tsai, C.-L. Huang, K.-H. Huang, C.-F. Lu, Y.-Y. Lai and C.-S. Hsu, Unraveling the Structure–Property–Performance Relationships of Fused-Ring Nonfullerene Acceptors: Toward a C-Shaped ortho-Benzodipyrrole-Based Acceptor for Highly Efficient Organic Photovoltaics, J. Am. Chem. Soc., 2023, 146, 833–848 CrossRef PubMed.
- H. Lai, Z. Deng and F. He, C-shaped ADA non-fullerene acceptor achieves efficient organic solar cells, Joule, 2024, 8, 572–575 CrossRef.
- M. Kim, S. U. Ryu, S. A. Park, Y.-J. Pu and T. Park, Designs and understanding of small molecule-based non-fullerene acceptors for realizing commercially viable organic photovoltaics, Chem. Sci., 2021, 12, 14004–14023 RSC.
- Y. Yang, S. U. Ryu, F. Wu, H. Lu, K. Jia, C. Zhong, T. Park and L. Zhu, Blending isomers of fluorine-substituted sulfonyldibenzene as hole transport materials to achieve high efficiency beyond 21% in perovskite solar cells, Chem. Eng. J., 2021, 424, 130396 CrossRef CAS.
- C. Kim, S. Chen, J. S. Park, G.-U. Kim, H. Kang, S. Lee, T. N.-L. Phan, S.-K. Kwon, Y.-H. Kim and B. J. Kim, Green solvent-processed, high-performance organic solar cells achieved by outer side-chain selection of selenophene-incorporated Y-series acceptors, J. Mater. Chem. A, 2021, 9, 24622–24630 RSC.
- J. Lee, J. W. Kim, S. A. Park, S. Y. Son, K. Choi, W. Lee, M. Kim, J. Y. Kim and T. Park, Study of burn-in loss in green solvent-processed ternary blended organic photovoltaics derived from UV-crosslinkable semiconducting polymers and nonfullerene acceptors, Adv. Energy Mater., 2019, 9, 1901829 CrossRef.
- J. H. Wan, W. F. Fang, Z. F. Li, X. Q. Xiao, Z. Xu, Y. Deng, L. H. Zhang, J. X. Jiang, H. Y. Qiu and L. B. Wu, Novel Ladder π-Conjugated Materials—Sila-Pentathienoacenes: Synthesis, Structure, and Electronic Properties, Chem. Asian J., 2010, 5, 2290–2296 CrossRef CAS PubMed.
- G. Zhang, X.-K. Chen, J. Xiao, P. C. Chow, M. Ren, G. Kupgan, X. Jiao, C. C. Chan, X. Du and R. Xia, Delocalization of exciton and electron wavefunction in non-fullerene acceptor molecules enables efficient organic solar cells, Nat. Commun., 2020, 11, 3943 CrossRef CAS PubMed.
- W. Zhu, A. P. Spencer, S. Mukherjee, J. M. Alzola, V. K. Sangwan, S. H. Amsterdam, S. M. Swick, L. O. Jones, M. C. Heiber and A. A. Herzing, Crystallography, morphology, electronic structure, and transport in non-fullerene/non-indacenodithienothiophene polymer: Y6 solar cells, J. Am. Chem. Soc., 2020, 142, 14532–14547 CrossRef CAS PubMed.
- G. Kupgan, X. Chen and J.-L. Bredas, Molecular packing of non-fullerene acceptors for organic solar cells: Distinctive local morphology in Y6 vs. ITIC derivatives, Mater. Today Adv., 2021, 11, 100154 CrossRef CAS.
- H. Lai, H. Chen, Z.-Y. Chen, Y. Lang, Y. Zhu, S.-T. Zhang, X. Lai, P. Tan, Y. Zhang and B. Yang, Exploring the significance of packing modes and 3D framework sizes and utilizing three chlorine-mediated acceptors and the “like dissolves like” approach for achieving an efficiency over 19, Energy Environ. Sci., 2023, 16, 5944–5955 RSC.
- D. Yuk, M. H. Jee, C. W. Koh, W. W. Park, H. S. Ryu, D. Lee, S. Cho, S. Rasool, S. Park and O. H. Kwon, Simplified Y6-Based Nonfullerene Acceptors: In-Depth Study on Molecular Structure–Property Relation, Molecular Dynamics Simulation, and Charge Dynamics, Small, 2023, 19, 2206547 CrossRef CAS PubMed.
- S. i. Kato, T. Furuya, M. Nitani, N. Hasebe, Y. Ie, Y. Aso, T. Yoshihara, S. Tobita and Y. Nakamura, A Series of π-Extended Thiadiazoles Fused with Electron-Donating Heteroaromatic Moieties: Synthesis, Properties, and Polymorphic Crystals, Chem.–Eur. J., 2015, 21, 3115–3128 CrossRef CAS PubMed.
- A. K. K. Kyaw, D. H. Wang, C. Luo, Y. Cao, T. Q. Nguyen, G. C. Bazan and A. J. Heeger, Effects of Solvent Additives on Morphology, Charge Generation, Transport, and Recombination in Solution-Processed Small-Molecule Solar Cells, Adv. Energy Mater., 2014, 4, 1301469 CrossRef.
- P. Hartnagel and T. Kirchartz, Understanding the light-intensity dependence of the short-circuit current of organic solar cells, Adv. Theory Simul., 2020, 3, 2000116 CrossRef CAS.
- F. Gao, Z. Li, J. Wang, A. Rao, I. A. Howard, A. Abrusci, S. Massip, C. R. McNeill and N. C. Greenham, Trap-induced losses in hybrid photovoltaics, ACS Nano, 2014, 8, 3213–3221 CrossRef CAS PubMed.
- V. Mihailetchi, J. Wildeman and P. W. Blom, Space-charge limited photocurrent, Phys. Rev. Lett., 2005, 94, 126602 CrossRef CAS PubMed.
- P. Bi, C. An, T. Zhang, Z. Chen, Y. Xu, Y. Cui, J. Wang, J. Li, Y. Wang and J. Ren, Achieving 31% efficiency in organic photovoltaic cells under indoor light using a low energetic disorder polymer donor, J. Mater. Chem. A, 2023, 11, 983–991 RSC.
- P. Murgatroyd, Theory of space-charge-limited current enhanced by Frenkel effect, J. Phys. D: Appl. Phys., 1970, 3, 151 CrossRef.
- J. Wang, M. Zhang, J. Lin, Z. Zheng, L. Zhu, P. Bi, H. Liang, X. Guo, J. Wu and Y. Wang, An asymmetric wide-bandgap acceptor simultaneously enabling highly efficient single-junction and tandem organic solar cells, Energy Environ. Sci., 2022, 15, 1585–1593 RSC.
- H. H. Chiu, B.-H. Jiang, H. C. Wang, X.-M. Su, Y.-H. Kang, Y.-W. Su, H.-S. Shih, C.-P. Chen and Y. J. Chang, Indolocarbazole-based small molecules as guest donors for High-Performance ternary organic photovoltaics, Chem. Eng. J., 2023, 469, 143938 CrossRef.
- O. D. Miller, E. Yablonovitch and S. R. Kurtz, Strong internal and external luminescence as solar cells approach the Shockley–Queisser limit, IEEE J. Photovoltaics, 2012, 2, 303–311 Search PubMed.
- N. An, Y. Cai, H. Wu, A. Tang, K. Zhang, X. Hao, Z. Ma, Q. Guo, H. S. Ryu and H. Y. Woo, Solution-processed organic solar cells with high open-circuit voltage of 1.3 V and low non-radiative voltage loss of 0.16 V, Adv. Mater., 2020, 32, 2002122 CrossRef CAS PubMed.
- Y. Xu, H. Yao, L. Ma, J. Wang and J. Hou, Efficient charge generation at low energy losses in organic solar cells: a key issues review, Rep. Prog. Phys., 2020, 83, 082601 CrossRef CAS PubMed.
- J. Wang, Y. Cui, Y. Xu, K. Xian, P. Bi, Z. Chen, K. Zhou, L. Ma, T. Zhang and Y. Yang, A new polymer donor enables binary all-polymer organic photovoltaic cells with 18% efficiency and excellent mechanical robustness, Adv. Mater., 2022, 34, 2205009 CrossRef CAS PubMed.
- K. Vandewal, K. Tvingstedt, A. Gadisa, O. Inganäs and J. V. Manca, Relating the open-circuit voltage to interface molecular properties of donor: acceptor bulk heterojunction solar cells, Phys. Rev. B:Condens. Matter Mater. Phys., 2010, 81, 125204 CrossRef.
- X.-K. Chen, D. Qian, Y. Wang, T. Kirchartz, W. Tress, H. Yao, J. Yuan, M. Hülsbeck, M. Zhang and Y. Zou, A unified description of non-radiative voltage losses in organic solar cells, Nat. Energy, 2021, 6, 799–806 CrossRef CAS.
- F. D. Eisner, M. Azzouzi, Z. Fei, X. Hou, T. D. Anthopoulos, T. J. S. Dennis, M. Heeney and J. Nelson, Hybridization of local exciton and charge-transfer states reduces nonradiative voltage losses in organic solar cells, J. Am. Chem. Soc., 2019, 141, 6362–6374 CrossRef CAS PubMed.
- J. Benduhn, K. Tvingstedt, F. Piersimoni, S. Ullbrich, Y. Fan, M. Tropiano, K. A. McGarry, O. Zeika, M. K. Riede and C. J. Douglas, Intrinsic non-radiative voltage losses in fullerene-based organic solar cells, Nat. Energy, 2017, 2, 1–6 Search PubMed.
- D. He, F. Zhao, C. Wang and Y. Lin, Non-radiative recombination energy losses in non-fullerene organic solar cells, Adv. Funct. Mater., 2022, 32, 2111855 CrossRef CAS.
- S. Ullbrich, J. Benduhn, X. Jia, V. C. Nikolis, K. Tvingstedt, F. Piersimoni, S. Roland, Y. Liu, J. Wu and A. Fischer, Emissive and charge-generating donor–acceptor interfaces for organic optoelectronics with low voltage losses, Nat. Mater., 2019, 18, 459–464 CrossRef CAS PubMed.
- F. Urbach, The long-wavelength edge of photographic sensitivity and of the electronic absorption of solids, Phys. Rev., 1953, 92, 1324 CrossRef CAS.
- X. Ma, C. Tang, Y. Ma, X. Zhu, J. Wang, J. Gao, C. Xu, Y. Wang, J. Zhang and Q. Zheng, Over 17% efficiency of ternary organic photovoltaics employing two acceptors with an acceptor–donor–acceptor configuration, ACS Appl. Mater. Interfaces, 2021, 13, 57684–57692 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2391682 and 2391712. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07146h |
| This journal is © The Royal Society of Chemistry 2025 |