Open Access Article
Open Access ArticleSpin effects in regulating the adsorption characteristics of metal ions†
Cunyuan
Gao‡
a,
Shiyu
Zhen‡
 b,
Yutong
Wang
a,
Lingwei
Wang
a,
Yang
Cao
a,
Jinhua
Zhan
b,
Yutong
Wang
a,
Lingwei
Wang
a,
Yang
Cao
a,
Jinhua
Zhan
 a,
Liang
Zhang
a,
Liang
Zhang
 *bc and
Bin
Cai
*bc and
Bin
Cai
 *ad
*ad
aSchool of Chemistry and Chemical Engineering, Shandong University, 250100 Jinan, China. E-mail: bin.cai@sdu.edu.cn
bCenter for Combustion Energy, School of Vehicle and Mobility, State Key Laboratory of Intelligent Green Vehicle and Mobility, Tsinghua University, Beijing 100084, China. E-mail: zhangbright@tsinghua.edu.cn
cBeijing Huairou Laboratory, Beijing 101400, China
dShenzhen Research Institute of Shandong University, Shenzhen 518000, China
First published on 8th January 2025
Abstract
Understanding the adsorption behavior of intermediates at interfaces is crucial for various heterogeneous systems, but less attention has been paid to metal species. This study investigates the manipulation of Co3+ spin states in ZnCo2O4 spinel oxides and establishes their impact on metal ion adsorption. Using electrochemical sensing as a metric, we reveal a quasi-linear relationship between the adsorption affinity of metal ions and the high-spin state fraction of Co3+ sites. Increasing the high-spin state of Co3+ shifts its d-band center downward relative to the Fermi level, thereby weakening metal ion adsorption and enhancing sensing performance. These findings demonstrate a spin-state-dependent mechanism for optimizing interactions with various metal species, including Cu2+, Cd2+, and Pb2+. This work provides new insights into the physicochemical determinants of metal ion adsorption, paving the way for advanced sensing technologies and beyond.
Introduction
Adsorption and desorption of intermediates on solid surfaces are fundamental components in various heterogeneous systems, particularly in catalysis,1,2 environmental science,3,4 and materials engineering.5,6 These processes are critical in understanding reaction pathways that occur at solid-involved interfaces and optimizing device efficiencies.7,8 For instance, typical catalysis on solid surfaces consists of the adsorption of reactants, the chemical transformations of intermediates, and the desorption of products.9 Based on these processes, the Sabatier principle demonstrates the importance of optimal catalyst surfaces that bind reactants neither too strongly nor too weakly.10 This balance ensures sufficient interaction for the reaction to occur while allowing products to desorb efficiently, which indicates a primary strategy in catalyst design.11–13 The subsequent development of d-band theory further enriches the catalysis toolbox by explaining how the electronic properties of catalysts affect their adsorption characteristics with adsorbates.14–16 In addition, the impact of geometric properties for these processes has also been well studied, providing further guidance for the optimization of catalyst surfaces.17,18 While understanding these behaviors is essential for the design of solid surfaces to improve heterogeneous systems, current research primarily focuses on non-metallic adsorbates (such as C,19 H,20 and O21), often overlooking their interaction with metal species.Unveiling the physicochemical parameters that govern the adsorption and desorption processes of metal adsorbates at interfaces holds great promise in metallurgy,22 metal-ion batteries,23,24 and sensor technology.25 Electrochemical sensing of metal ions involves adsorption, migration, electron transfer, and desorption processes of metal species at the electrochemical interface, enabling effective investigation of their interaction with solid interfaces.26,27 However, research on the adsorption processes of metallic species, similar to that of the non-metallic adsorbates, is still limited in tuning the electronic structures through doping, morphology, crystal planes, and defects.28,29 Recently, spin, an intrinsic property of electrons, has emerged as another promising degree of electronic freedom to regulate the electronic structure of various catalyst surfaces.30–32 However, the spin state of active centers is influenced by splitting energy and electron pairing energy, making it challenging to directly modulate the spin state.33 Consequently, the correlation between spin properties and adsorption characteristics of metal species remains largely unexplored.
Herein, we address this challenge by correlating the spin state of Co3+ sites with their adsorption characteristics for various metal ions, including Cu2+, Cd2+, and Pb2+ ions. Transition metal oxides are employed due to their strong interdependence between spin, charge, orbital, and lattice degrees of freedom, creating a highly interactive system for manipulating spin states through various pathways.34 Spinel oxide ZnCo2O4 is an ideal choice because Co3+ cations are confined to octahedral sites, allowing exclusive investigation of CoO6 octahedra. The manipulation of the Co3+ spin state is achieved by calcination-induced lattice distortion, which is carefully verified through density functional theory (DFT) calculations, Fourier-transform infrared spectroscopy (FTIR), X-ray absorption spectra (XAS) and Superconducting Quantum Interference Device (SQUID) analysis. Increasing the calcination temperature leads to an increase in the spin state of Co3+ cations, causing the d-band center to shift downward relative to the Fermi level. The lower energy level of the d-band center results in the antibonding orbitals being occupied by more electrons, weakening the adsorption affinity between the Co3+ sites and metal ions. As the desorption process plays a major role in determining the sensing signal, weakened metal ion adsorption leads to enhanced sensing performances. This positive correlation between the spin state and sensing activity applies to various metal ions, including Cu2+, Cd2+, and Pb2+ ions. This work sheds light on the understanding of spin properties in optimizing the adsorption characteristics of metal species and would spur new strategies in optimizing electrochemical sensors by spin engineering.
Results and discussion
In the spinel oxide ZnCo2O4, Zn2+ and Co3+ cations occupy four-coordinated tetrahedral and six-coordinated octahedral sites, respectively (Fig. 1a). Zn2+ cations are considered inert with limited electrochemical activity due to their filled 3d orbital and lower electron affinity compared to other catalytically active 3d-transition metals. Therefore, the Co3+ sites in octahedral units play a dominant role during the electrochemical adsorption and desorption processes of metal ions.27 The spin state of metals is determined by the crystal field splitting energy and electron pairing energy.35 Due to the high crystal field splitting energy, Co3+ typically favors the low-spin state (t62ge0g) in an octahedral geometry, where the t2g orbitals are fully occupied and the eg orbitals are completely empty (Fig. 1b). The empty eg orbitals provide enormous opportunities for spin state engineering. As shown in Fig. 1b, the eg orbitals can accommodate up to two unpaired electrons, resulting in the intermediate-spin state (t52ge1g) and high-spin state (t42ge2g), respectively. Thus, the electronic structure of Co3+ sites can be effectively regulated through spin engineering, resulting in significant changes in electrochemical activity. | ||
Fig. 1 Structural characterization. (a) Crystal structure of spinel ZnCo2O4 with Zn2+ and Co3+ cations occupying tetrahedral and octahedral sites, respectively. (b) d-Electron configurations of the Co3+ cation in different spin states in the spinel ZnCo2O4. (c) AC-HAADF-STEM image of ZCO-600, viewed along the [01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] zone axis. Theoretical models with octahedral and tetrahedral arrangements are overlaid on the image, with blue and pink atoms representing Zn2+ and Co3+ cations, respectively. (d) FFT pattern of the TEM image in (c). (e) XRD patterns of the as-prepared spinel ZnCo2O4 samples, corresponding to the standard pattern PDF# 23-1390. (f)Co L2,3-edge XAS spectra of the as-prepared ZnCo2O4 oxides and Co2O3 reference. The red area indicates the Co3+ high-spin state peak,44 and the blue area indicates the Co3+ low-spin state peak.45 (g) Octahedral distortion (ε) associated with an increase in the spin state. The specific computational details are summarized in the ESI.† The inset shows the model changes caused by lattice distortion. (h) FTIR spectra of the as-prepared spinel ZnCo2O4 oxides. ] zone axis. Theoretical models with octahedral and tetrahedral arrangements are overlaid on the image, with blue and pink atoms representing Zn2+ and Co3+ cations, respectively. (d) FFT pattern of the TEM image in (c). (e) XRD patterns of the as-prepared spinel ZnCo2O4 samples, corresponding to the standard pattern PDF# 23-1390. (f)Co L2,3-edge XAS spectra of the as-prepared ZnCo2O4 oxides and Co2O3 reference. The red area indicates the Co3+ high-spin state peak,44 and the blue area indicates the Co3+ low-spin state peak.45 (g) Octahedral distortion (ε) associated with an increase in the spin state. The specific computational details are summarized in the ESI.† The inset shows the model changes caused by lattice distortion. (h) FTIR spectra of the as-prepared spinel ZnCo2O4 oxides. | ||
A series of ZnCo2O4 spinel oxides with varied Co3+ spin states was obtained by tuning the calcination temperatures to induce lattice distortion during the sol–gel synthesis (i.e. 300, 400, 500, and 600 °C, denoted as ZCO-300, ZCO-400, ZCO-500, and ZCO-600, respectively). Current methods for controlling the spin state include lattice distortion,36,37 defect engineering,38,39 heteroatom doping,40,41 and metal–carrier interaction.42 Among these, the lattice distortion-induced method offers a more straightforward and scalable approach to modulate the volume proportion of high-spin Co3+.33 This facilitates the detailed exploration of the structure–activity relationship, which is critical for advancing spin-state-dependent applications. The as-obtained ZnCo2O4 oxides show irregular morphology according to transmission electron microscopy (TEM) characterization (Fig. S1†). Increasing calcination temperature leads to slight sintering and increased particle sizes. Fig. 1c and d show the aberration-corrected high angular annular dark-field scanning TEM (AC-HAADF-STEM) image and the corresponding fast Fourier transform (FFT) pattern of the ZCO-600 oxide. The atomic arrangement of ZCO-600 matches well the spinel lattice with the space group Fd3m, where Co3+ cations occupy the octahedral sites and Zn2+ cations occupy the tetrahedral sites.43 As shown in Fig. 1e, the X-ray diffraction (XRD) analysis further confirms the successful synthesis of the spinel phase (JCPDS no. 23-1390) at different calcination temperatures. In particular, the main peaks located at about 31.21, 36.81, 59.28 and 65.15° are indexed as 220, 311, 511 and 440 Bragg reflections of the spinel crystal structure, respectively. This aligns with the space group Fd3m, and agrees well with the AC-HAADF-STEM analysis. No obvious diffraction peaks of impurities were observed within the calcination temperature range of 300 to 600 °C, indicating excellent structural robustness of the ZnCo2O4 spinel oxide.
Further analysis of the chemical and spin states of ZnCo2O4 spinel oxide was performed using X-ray photoelectron spectroscopy (XPS) and XAS (Fig. 1f and S2–S4†). The XPS analysis of the Co 2p signal (Fig. S3†) confirms that Co within all ZnCo2O4 spinel oxides exhibits a trivalent oxidation state. The Co L2 and L3 XAS spectra of ZnCo2O4 are presented in Fig. 1f, together with Co2O3 as a Co3+ reference. The center of the L3 spectrum of ZnCo2O4 aligns with that of Co2O3, demonstrating that the Co valence in ZnCo2O4 is indeed trivalent. Previous studies have shown that the presence of a low-energy shoulder (red area) at the Co3+ L3 edge is characteristic of the high-spin state,44 while the high-energy shoulder (blue area) is indicative of the low-spin state.45 This analysis proves that Co3+ cations in ZnCo2O4 exist predominantly in the high-spin state. Hence, the consistent trivalent oxidation state of Co ensures an exclusive examination of the spin-dependent electrochemical activity in ZnCo2O4 spinel oxide.
The emergence of the Co3+ high-spin state is ascribed to lattice distortion induced by high-temperature calcination. We explore the spin-structure correlation using both DFT calculations and FTIR characterization. Fig. 1g shows the relationship between the number of unpaired electrons and the octahedral distortion (ε), which is consistent with previous reports.33 The lattice distortion becomes more pronounced with an increase in the number of unpaired spins in ZnCo2O4. This is because the increased number of unpaired electrons leads to the occupation of eg orbitals, resulting in a degenerate electronic ground state. This electronic state can induce the Jahn–Teller effect, which lowers the energy and symmetry of the system. Furthermore, FTIR analysis was utilized to investigate the local chemical bond changes in the as-prepared ZnCo2O4 (Fig. 1h). The absorption bands at 575 cm−1 and 670 cm−1 correspond to vibrations of the Co–O and Zn–O bond stretching, respectively. As the calcination temperature increases, the peak shapes of the metal–oxygen bonds broaden and shift to higher wavenumbers (Fig. S5†), confirming the emergence of lattice distortion.
To gain insights into the spin-related electronic configuration in spinel ZnCo2O4, the magnetic properties and related spin information were investigated using a SQUID, which consists of a superconducting loop with two parallel Josephson junctions capable of detecting incredibly small changes in magnetic flux.46 As shown in Fig. 2a, the magnetic properties of the ZnCo2O4 oxides were investigated by measuring magnetization as a function of the magnetic field (M–H). All the as-prepared ZnCo2O4 oxides exhibit similar magnetization curves with no hysteresis feature, indicating paramagnetic behavior under ambient conditions. However, the four ZnCo2O4 oxides exhibit distinct magnetic susceptibilities (χ = M/H), confirming variations in their spin structures.
Temperature-dependent magnetization characterization experiments were carried out to further explore the spin-related properties (Fig. 2b). When the temperature is above 150 K, the magnetic susceptibility of magnetic field induction follows the Curie–Weiss law: χ = C/(T − θ), where C is the Curie constant (in emu K mol−1 in centimeter-gram-second units) and θ (in K) is the Curie–Weiss temperature.46 As shown in Fig. 2c, the slope of the fitting curve (susceptibility vs. T) represents C−1. The Curie constant C is directly related to the number of unpaired electrons and, once determined, can be used to calculate the effective magnetic moment (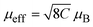 , with units of Bohr magnetons, μB). Based on recent experimental and theoretical studies, we only consider changes between the low-spin state (t62ge0g) and high-spin state (t42ge2g) without introducing the intermediate-spin state (t52ge1g).33,47–51 Therefore, the volume fractions of Co3+ cations in high-spin and low-spin states can be obtained using the following equation:
, with units of Bohr magnetons, μB). Based on recent experimental and theoretical studies, we only consider changes between the low-spin state (t62ge0g) and high-spin state (t42ge2g) without introducing the intermediate-spin state (t52ge1g).33,47–51 Therefore, the volume fractions of Co3+ cations in high-spin and low-spin states can be obtained using the following equation:
 | (1) |
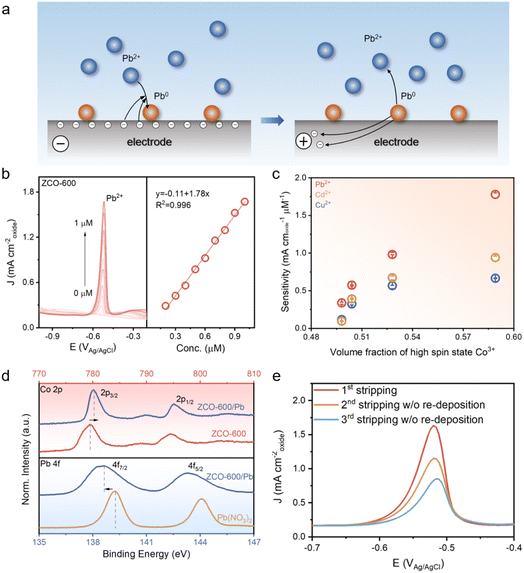
After confirming the increase in the high-spin state of the Co3+ cation, electrochemical sensing of metal ions was used to explore the intrinsic determinants of metal ion adsorption and desorption affinity on the oxide surface (Fig. 3a). First, the electrochemical impedance spectra (EIS) of ZnCo2O4 confirmed the enhanced ion transport at the electrode–electrolyte interface in spin-engineered ZnCo2O4 spinel oxides (Fig. S6†). Then the electrochemical performance of the ZnCo2O4 oxides was studied using square wave anodic stripping voltammetry (SWASV). Specifically, a 0.1 M HAc–NaAc buffer solution (pH = 6) was employed as the electrolyte, and the deposition process was performed at −1.0 V for 150 s. The electrochemical sensing of metal ions is essentially a surface reaction, where the adsorption and desorption of metal ions occur only on the catalyst surface. Considering that changes in the calcination temperature could affect the specific surface area of the oxides, specific activity is employed to evaluate the electrochemical performance by normalizing the current to the catalyst surface area. In this study, the specific surface area of ZnCo2O4 oxides was analyzed by N2 physisorption according to the Brunauer–Emmett–Teller model (Fig. S7†). The electrocatalytic performance of the ZnCo2O4 spinel oxides toward different metal ions (Cu2+, Cd2+, and Pb2+) is shown in Fig. 3b and S8–S10.† The anodic stripping peaks for Pb2+, Cd2+, and Cu2+ ions appear at around −0.55 V, −0.79 V, and −0.05 V respectively, and the peak current increases linearly with the concentration of metal ions. According to the linear equations, the Pb2+ electrochemical detection sensitivities of the ZnCo2O4 oxides are 0.34, 0.575, 0.982 and 1.78 mA cmoxide−2 μM−1 for ZCO-300, ZCO-400, ZCO-500 and ZCO-600, respectively. And the detection sensitivity of Cd2+ and Cu2+ ions also gradually improved with increased calcination temperature (more details are displayed in Table S2†). Notably, we found a quasi-linear relationship between the electrochemical performance and the volume fraction of the high-spin state Co3+. As shown in Fig. 3c, sensitivity increases with the volume fraction of the high-spin state Co3+, indicating the significance of the Co3+ spin state in electrochemical activity. When we normalized the current to the electrochemically active surface area, we obtained the same trend that the sensitivity increased with the volume fraction of high-spin Co3+ (Fig. S11–S13†).
To explore how spin properties affect electrochemical performance, XPS analyses were conducted to reveal the surface chemical interactions between ZnCo2O4 oxide and *Pb species. As shown in Fig. 3d (top), the characteristic peak of Co 2p shifts to higher energies after adsorption of *Pb species, implying a possible charge transfer from Co to O due to the binding with *Pb species. Additionally, the high-resolution XPS spectra of Pb 4f in Pb(NO3)2 and ZCO-600/Pb are shown in Fig. 3d (bottom), where the Pb 4f peak shifts toward lower binding energy after adsorption, indicating that the adsorbed *Pb species accept electrons from the oxide. As the high-spin state increases, the Pb 4f peak shifts less toward lower binding energy, indicating that fewer electrons are obtained and the binding force decreases (Fig. S14†). Since electrochemical sensing involves the adsorption and desorption of metal ions at the electrode surface, previous studies predominantly consider a single stripping as the only sensing signal, often overlooking the potential interactions between the metal ion and the electrode.27 Here, multiple stripping analyses were performed on the adsorbed *Pb species (1 μM). Interestingly, we found that the adsorbed *Pb species cannot be completely stripped in a single cycle (Fig. 3e). As the number of stripping cycles increases, the response current gradually decreases, indicating that the oxide surface still contains unstripped *Pb species after the square wave voltammetry (SWV) process. This confirms that the desorption of metal ions plays a major role in electrochemical sensing performances. These results also suggest that the weaker the binding force between *Pb species and the electrode, the higher the detection performance.
Spin-polarized DFT calculations were further conducted to elucidate the correlation between the Co3+ spin state and electrochemical activity (Fig. 4 and S15–S17†). According to the calculated binding energy diagrams of ZnCo2O4 with varied Co3+ spin states (Fig. 4b), the adsorption affinity of metals on ZnCo2O4 surfaces decreases with the increase of the spin state regardless of the adsorbed metal species (i.e., Pb, Cu and Cd). This is consistent with the experimental electrochemical sensing performances. We further evaluated the projected density of states (PDOS) of the Co 3d orbitals for both low-spin and high-spin states of the ZnCo2O4 surfaces. As shown in Fig. 4c, the high-spin state exhibits a downshift of the d-band center (−2.01 eV) as compared with that of the low-spin state (−0.92 eV). According to the d-band center theory, it is suggested that lowering the d-band center relative to the Fermi level will weaken the surface chemisorption of adsorbed species, thereby enhancing the electrochemical desorption of metal ions and sensing performances (the d-band center discussed here is the average of the spin-up and spin-down d-band centers). The resulting trend indicates that the spin state alters the electronic properties of the Co sites, leading to a moderate affinity toward the intermediate metal species.
Furthermore, Crystal Orbital Hamilton Population (COHP) analysis was performed to evaluate the binding strength of the Co–Pb interaction after adsorption. The integral area of the COHP (ICOHP) is proportional to the bond strength, with higher electron orbital overlap (lower ICOHP) indicating higher bond strength. Compared to the low-spin state, the high-spin state exhibits decreased occupancy of the bonding state, resulting in a decrease in the -ICOHP (as a measure for the bond strength) of the Co–Pb bond from 0.82 to 0.71 (Fig. 4d), which is consistent with the DFT calculated Co–Pb binding trend. In addition, the charge density difference diagram of both the low-spin state and high-spin state of ZnCo2O4 structures shows that the charges accumulate on the *Pb species side (yellow region) and deplete on the ZnCo2O4 side (green region), representing spontaneous electron migration from the Co site to the *Pb species. As illustrated in Fig. 4e, the ZnCo2O4 structure with the low-spin state transfers more electrons and has stronger adsorption energy than that of the structure with the high-spin state. These results demonstrate the electronic effects under the influence of spin states in ZnCo2O4 in optimizing the binding strengths of adsorbed metal species.
Conclusions
In summary, we investigated the correlation between the spin state of Co3+ sites and their adsorption characteristics with various heavy metal adsorbates. The manipulation of the spin state is achieved using ZnCo2O4 spinel oxide as a model based on calcination-induced lattice distortion. By using the electrochemical sensing technique as a measure, the adsorption affinity of metal ions on the ZnCo2O4 surfaces shows a quasi-linear correlation with the volume fraction of high-spin state sites. The high-spin state dominated ZCO-600 shows a Pb2+ electrochemical sensing performance of 1.78 mA cmoxide−2 μM−1, which is approximately 5-fold higher than that of the low-spin state dominated ZCO-300. Similar spin-based correlations were further demonstrated for various metal species, including Cu2+ and Cd2+. DFT calculations show that the d-band center of Co sites gradually shifts downward relative to the Fermi level with the increase of the Co3+ spin state in ZnCo2O4, which weakens the adsorption affinity for metal species. This accounts for the enhanced electrochemical sensing performance toward heavy metal ions. This work unveils the correlation between spin properties and adsorption characteristics of metal species on spinel oxides and points out a potential avenue for the development of next-generation metal-involved technologies, such as sensors, metal batteries and beyond.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
C. G., S. Z. and B. C. designed and carried out the experiments and wrote the manuscript. L. W. collected the TEM data. Y. W. and Y. C. carried out the electrochemical tests. B. C. and L. Z. supervised the whole study and revised the manuscript. C. G. and S. Z. contributed equally to this work. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52201262 and 22373055), the Natural Science Foundation of Shandong Province (ZR2023YQ039 and ZR2022QE001), the Taishan Scholars Program of Shandong Province (tsqn202211042), and the Science and Technology Innovation Committee of Shenzhen Municipality (GJHZ20220913142604008). The authors acknowledge the support from the Tsinghua University Initiative Scientific Research Program, the State Key Laboratory of Intelligent Green Vehicle and Mobility under Project No. ZZ2023-063, and the computation resources from the Tsinghua Center of High-Performance Computing. We acknowledge X-ray magnetic circular dichroism (XMCD) beamline BL12B at the National Synchrotron Radiation Laboratory.References
- E. Roduner, Chem. Soc. Rev., 2014, 43, 8226–8239 RSC.
- C. Vogt and B. M. Weckhuysen, Nat. Rev. Chem., 2022, 6, 89–111 CrossRef PubMed.
- V. I. Parvulescu, F. Epron, H. Garcia and P. Granger, Chem. Rev., 2022, 122, 2981–3121 CrossRef CAS PubMed.
- J. Lin, W. Ye, M. Xie, D. H. Seo, J. Luo, Y. Wan and B. Van der Bruggen, Nat. Rev. Earth Environ., 2023, 4, 785–803 Search PubMed.
- Q. Yun, Y. Ge, Z. Shi, J. Liu, X. Wang, A. Zhang, B. Huang, Y. Yao, Q. Luo, L. Zhai, J. Ge, Y. Peng, C. Gong, M. Zhao, Y. Qin, C. Ma, G. Wang, Q. Wa, X. Zhou, Z. Li, S. Li, W. Zhai, H. Yang, Y. Ren, Y. Wang, L. Li, X. Ruan, Y. Wu, B. Chen, Q. Lu, Z. Lai, Q. He, X. Huang, Y. Chen and H. Zhang, Chem. Rev., 2023, 123, 13489–13692 CrossRef CAS PubMed.
- M. P. Browne, E. Redondo and M. Pumera, Chem. Rev., 2020, 120, 2783–2810 Search PubMed.
- C. Xie, Z. Niu, D. Kim, M. Li and P. Yang, Chem. Rev., 2020, 120, 1184–1249 CrossRef CAS PubMed.
- W. Chang, A. Jain, F. Rezaie and K. Manthiram, Nat. Catal., 2024, 7, 231–241 CrossRef CAS.
- S. Sun, Y. Zhang, X. Shi, W. Sun, C. Felser, W. Li and G. Li, Adv. Mater., 2024, 2312524 Search PubMed.
- A. J. Medford, A. Vojvodic, J. S. Hummelshøj, J. Voss, F. Abild-Pedersen, F. Studt, T. Bligaard, A. Nilsson and J. K. Nørskov, J. Catal., 2015, 328, 36–42 Search PubMed.
- Z. W. Chen, J. Li, P. Ou, J. E. Huang, Z. Wen, L. Chen, X. Yao, G. Cai, C. C. Yang, C. V. Singh and Q. Jiang, Nat. Commun., 2024, 15, 359 CrossRef CAS PubMed.
- X. Chang, Z.-J. Zhao, Z. Lu, S. Chen, R. Luo, S. Zha, L. Li, G. Sun, C. Pei and J. Gong, Nat. Nanotechnol., 2023, 18, 611–616 CrossRef CAS PubMed.
- B. Wang and F. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202111026 Search PubMed.
- B. Hammer and J. K. Norskov, Nature, 1995, 376, 238–240 CrossRef CAS.
- Z.-J. Zhao, S. Liu, S. Zha, D. Cheng, F. Studt, G. Henkelman and J. Gong, Nat. Rev. Mater., 2019, 4, 792–804 CrossRef.
- S. Jiao, X. Fu and H. Huang, Adv. Funct. Mater., 2022, 32, 2107651 CrossRef CAS.
- C. Y. Zhang, X. Lu, X. Han, J. Yu, C. Zhang, C. Huang, L. Balcells, A. G. Manjón, J. Jacas Biendicho, J. Li, J. Arbiol, G. Sun, J. Y. Zhou and A. Cabot, J. Am. Chem. Soc., 2023, 145, 18992–19004 CrossRef CAS PubMed.
- Z. Wang, C. Wang, S. Mao, B. Lu, Y. Chen, X. Zhang, Z. Chen and Y. Wang, Nat. Commun., 2022, 13, 3561 CrossRef CAS PubMed.
- H. Zhang, J. Gao, D. Raciti and A. S. Hall, Nat. Catal., 2023, 6, 807–817 CrossRef CAS.
- Z. Li, Y. Yan, S.-M. Xu, H. Zhou, M. Xu, L. Ma, M. Shao, X. Kong, B. Wang, L. Zheng and H. Duan, Nat. Commun., 2022, 13, 147 CrossRef CAS PubMed.
- S. Yuan, J. Peng, B. Cai, Z. Huang, A. T. Garcia-Esparza, D. Sokaras, Y. Zhang, L. Giordano, K. Akkiraju, Y. G. Zhu, R. Hübner, X. Zou, Y. Román-Leshkov and Y. Shao-Horn, Nat. Mater., 2022, 21, 673–680 Search PubMed.
- T. DebRoy, T. Mukherjee, H. L. Wei, J. W. Elmer and J. O. Milewski, Nat. Rev. Mater., 2021, 6, 48–68 CrossRef CAS.
- Y. Liang, H. Dong, D. Aurbach and Y. Yao, Nat. Energy, 2020, 5, 646–656 CrossRef CAS.
- A. M. Abakumov, S. S. Fedotov, E. V. Antipov and J.-M. Tarascon, Nat. Commun., 2020, 11, 4976 CrossRef CAS PubMed.
- R. Hein, P. D. Beer and J. J. Davis, Chem. Rev., 2020, 120, 1888–1935 CrossRef CAS PubMed.
- L. Jiao, W. Xu, Y. Wu, H. Wang, L. Hu, W. Gu and C. Zhu, Anal. Chem., 2023, 95, 433–443 CrossRef CAS PubMed.
- X.-Y. Xiao, Y.-H. Zhao, Y.-Y. Li, Z.-Y. Song, S.-H. Chen, H.-Q. Huang, M. Yang, P.-H. Li and X.-J. Huang, Anal. Chem., 2022, 94, 13631–13641 Search PubMed.
- S.-M. Lu, Y.-Y. Peng, Y.-L. Ying and Y.-T. Long, Anal. Chem., 2020, 92, 5621–5644 CrossRef CAS PubMed.
- X. Geng, D. Liu, C. C. Hewa-Rahinduwage, S. L. Brock and L. Luo, Acc. Chem. Res., 2023, 56, 1087–1096 Search PubMed.
- P. Huang, M. Meng, G. Zhou, P. Wang, W. Wei, H. Li, R. Huang, F. Liu and L. Liu, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2219661120 Search PubMed.
- C.-C. Lin, T.-R. Liu, S.-R. Lin, K. M. Boopathi, C.-H. Chiang, W.-Y. Tzeng, W.-H. C. Chien, H.-S. Hsu, C.-W. Luo, H.-Y. Tsai, H.-A. Chen, P.-C. Kuo, J. Shiue, J.-W. Chiou, W.-F. Pong, C.-C. Chen and C.-W. Chen, J. Am. Chem. Soc., 2022, 144, 15718–15726 Search PubMed.
- S. Luo, K. Elouarzaki and Z. J. Xu, Angew. Chem., Int. Ed., 2022, 61, e202203564 CrossRef CAS PubMed.
- Y. Sun, X. Ren, S. Sun, Z. Liu, S. Xi and Z. J. Xu, Angew. Chem., Int. Ed., 2021, 60, 14536–14544 Search PubMed.
- D. Yi, N. Lu, X. Chen, S. Shen and P. Yu, J. Phys.: Condens. Matter, 2017, 29, 443004 Search PubMed.
- M. A. Halcrow, Chem. Soc. Rev., 2013, 42, 1784–1795 RSC.
- D. Qian, Y. Hinuma, H. Chen, L.-S. Du, K. J. Carroll, G. Ceder, C. P. Grey and Y. S. Meng, J. Am. Chem. Soc., 2012, 134, 6096–6099 Search PubMed.
- Y. Zhao, X. Jia, G. Chen, L. Shang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung, D. O'Hare and T. Zhang, J. Am. Chem. Soc., 2016, 138, 6517–6524 Search PubMed.
- J. Huang, J. Chen, T. Yao, J. He, S. Jiang, Z. Sun, Q. Liu, W. Cheng, F. Hu, Y. Jiang, Z. Pan and S. Wei, Angew. Chem., Int. Ed., 2015, 54, 8722–8727 CrossRef CAS PubMed.
- S. Zhao, Y. Wang, J. Dong, C.-T. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. M. Khattak, N. A. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao and Z. Tang, Nat. Energy, 2016, 1, 16184 CrossRef CAS.
- G. Yang, J. Zhu, P. Yuan, Y. Hu, G. Qu, B.-A. Lu, X. Xue, H. Yin, W. Cheng, J. Cheng, W. Xu, J. Li, J. Hu, S. Mu and J.-N. Zhang, Nat. Commun., 2021, 12, 1734 CrossRef CAS PubMed.
- J. Li, M. T. Sougrati, A. Zitolo, J. M. Ablett, I. C. Oğuz, T. Mineva, I. Matanovic, P. Atanassov, Y. Huang, I. Zenyuk, A. Di Cicco, K. Kumar, L. Dubau, F. Maillard, G. Dražić and F. Jaouen, Nat. Catal., 2021, 4, 10–19 CrossRef CAS.
- Y. Liu, X. Liu, Z. Lv, R. Liu, L. Li, J. Wang, W. Yang, X. Jiang, X. Feng and B. Wang, Angew. Chem., Int. Ed., 2022, 61, e202117617 CrossRef CAS PubMed.
- T. Maiyalagan, K. A. Jarvis, S. Therese, P. J. Ferreira and A. Manthiram, Nat. Commun., 2014, 5, 3949 Search PubMed.
- J.-M. Chen, Y.-Y. Chin, M. Valldor, Z. Hu, J.-M. Lee, S.-C. Haw, N. Hiraoka, H. Ishii, C.-W. Pao, K.-D. Tsuei, J.-F. Lee, H.-J. Lin, L.-Y. Jang, A. Tanaka, C.-T. Chen and L. H. Tjeng, J. Am. Chem. Soc., 2014, 136, 1514–1519 CrossRef CAS PubMed.
- Z. Hu, H. Wu, M. W. Haverkort, H. H. Hsieh, H. J. Lin, T. Lorenz, J. Baier, A. Reichl, I. Bonn, C. Felser, A. Tanaka, C. T. Chen and L. H. Tjeng, Phys. Rev. Lett., 2004, 92, 207402 Search PubMed.
- S. Mugiraneza and A. M. Hallas, Commun. Phys., 2022, 5, 95 Search PubMed.
- M. J. R. Hoch, S. Nellutla, J. van Tol, E. S. Choi, J. Lu, H. Zheng and J. F. Mitchell, Phys. Rev. B:Condens. Matter Mater. Phys., 2009, 79, 214421 CrossRef.
- V. Křápek, P. Novák, J. Kuneš, D. Novoselov, D. M. Korotin and V. I. Anisimov, Phys. Rev. B:Condens. Matter Mater. Phys., 2012, 86, 195104 Search PubMed.
- M. Karolak, M. Izquierdo, S. L. Molodtsov and A. I. Lichtenstein, Phys. Rev. Lett., 2015, 115, 046401 CrossRef CAS PubMed.
- Y. Duan, S. Sun, S. Xi, X. Ren, Y. Zhou, G. Zhang, H. Yang, Y. Du and Z. J. Xu, Chem. Mater., 2017, 29, 10534–10541 Search PubMed.
- S. Zhou, X. Miao, X. Zhao, C. Ma, Y. Qiu, Z. Hu, J. Zhao, L. Shi and J. Zeng, Nat. Commun., 2016, 7, 11510 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06477a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |