Open Access Article
Open Access ArticleDebut of enzyme-responsive anionic cyanine for overlap-free NIR-II-to-I dual-channel tumour imaging†
Feiyi
Chu
a,
Bin
Feng
a,
Yiyang
Zhou
a,
Min
Liu
 ab,
Hailiang
Zhang
a,
Meihui
Liu
a,
Qian
Chen
c,
Shengwang
Zhang
c,
Yeshuo
Ma
c,
Jie
Dong
ab,
Hailiang
Zhang
a,
Meihui
Liu
a,
Qian
Chen
c,
Shengwang
Zhang
c,
Yeshuo
Ma
c,
Jie
Dong
 a,
Fei
Chen
a,
Fei
Chen
 a and
Wenbin
Zeng
a and
Wenbin
Zeng
 *a
*a
aXiangya School of Pharmaceutical Sciences, Central South University, Changsha, 410078, PR China. E-mail: wbzeng@hotmail.com
bDepartment of Pharmacy, Xiangya Hospital, Changsha 410008, PR China
cThird Xiangya Hospital, Central South University, Changsha 410013, PR China
First published on 6th February 2025
Abstract
Bridging the disparity between traditional surgical resection imaging and ex vivo histopathology, fluorescence imaging is considered a promising tool in disease diagnosis and imaging navigation. Nevertheless, its usefulness is undermined by the variability of single-wavelength fluorescence signals and limited penetration of NIR-I (650–900 nm) bioimaging. In this work, we present a novel NIR-II ratiometric fluorescent probe (CFC-GSH) with γ-glutamyl transpeptidase (GGT) sensitivity for multifunctional bioimaging. This probe leverages a GSH-capped anionic cyanine, with advantages of high brightness, excellent photostability, high specificity and favourable biocompatibility. CFC-GSH exhibits an intrinsically stable NIR-II signal prior to triggering, which can be utilized for in vivo systemic circulation vessel outlining and microvascular imaging. At the tumour site with GGT over expression, an intramolecular S,N-rearrangement would initiate the conversion of sulphur-substituted cyanine to amino-substituted cyanine, resulting in a significant emission shift of 270 nm. Using the dual-channel signal changes, CFC-GSH effectively differentiates between subcutaneous hepatocellular carcinoma (HCC) and normal tissue and precisely localizes metastatic HCC tumours in the abdominal cavity. These results reveal that CFC-GSH exhibits promising potential as a multiprospective candidate tool for fluorescence screening and diagnostic imaging in various biological scenarios.
Introduction
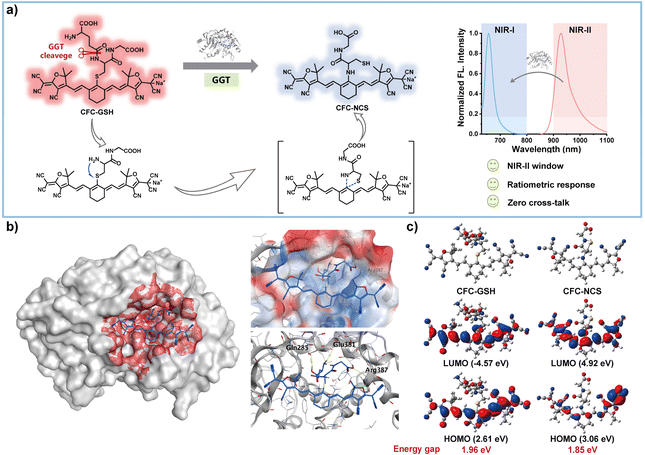
The limitations of conventional in vivo imaging techniques, particularly in spatial resolution and tissue penetration depth, have driven the exploration of novel methods with superior bioimaging performance.1–4 The second near-infrared (NIR-II) window has garnered significant attention due to its superior attributes, including enhanced deep tissue penetration, high resolution, and minimal tissue autofluorescence and photodamage.5–8 However, traditional probes suffer from inherent background signals, hindering accurate target detection.9–11 Activatable probes have emerged as a solution in the visible and NIR-I regions, but designing activatable NIR-II probes remains challenging.12,13 This is primarily due to the lack of generalizable chemical strategies for modulating NIR-II emission, especially in the development of enzyme-activated probes with high catalytic efficiency and substrate selectivity.14,15Gamma (γ)-glutamyl transpeptidase (GGT), a key enzyme in glutathione metabolism primarily found in the liver, is upregulated in a variety of tumours and plays a vital role in their genesis, invasion, and metastasis.16–19 Therefore, GGT is considered an important biomarker of tumours, and quantitatively detecting dynamic GGT activity is clinically significant for early diagnosis, accurate pathologic staging, intraoperative navigation and efficacy assessment of tumours.19–22 Conventional GGT detection techniques, such as p-nitroaniline-based colorimetric analysis, electrochemistry, and high-performance liquid chromatography (HPLC), suffer from inherent drawbacks, such as intricate procedural requirements and complicated sample processing (Table S1†).1,23–26 These factors pose a great challenge in obtaining real-time GGT levels in the organism. In contrast, optical imaging methods, especially probes working in the near-infrared (NIR) window (Table S2†), offer convenient and noninvasive methods for visualizing GGT in organisms due to their ability to provide in situ and real-time information.27–33 Nevertheless, most NIR probes rely on fluorescence signals emitted at a single wavelength, which can be affected by the probe concentration, instrument parameters, and the dynamic biological environment.1,34–37 In addition, the conventional strategy for developing GGT-activatable probes involves linking a γ-glutamyl group to the fluorophore via an amide bond.29–32,38–40 In this context, steric hindrance from the fluorophore near the enzymatic cleavage site may reduce the probe's affinity for the enzyme.27,39 Moreover, enzymatic cleavage of the γ-glutamyl bond releases the fluorophore with an unprotected amine group, making it susceptible to potential oxidation or acylation by other enzymes in the biological system, leading to a complicated fluorescence response.41,42 Thus, developing NIR ratiometric fluorescent probes with optimized kinetic profiles and stable signal output for GGT detection remains a challenge.
In this work, we constructed a novel NIR ratiometric probe, CFC-GSH, by directly conjugating GSH, a natural substrate of GGT, to a tricyanofuran (TCF)-ended anionic cyanine (Scheme 1). Upon enzymatic cleavage of the γ-glutamyl bond, the released amino group in the cysteinyl-glycine residue undergoes intramolecular cascade cyclization, yielding amino-substituted cyanine and resulting in a remarkable emission shift from 930 nm to 660 nm. Additionally, the rearranged amino-substituted product does not contain a free amine group, thus eliminating the possibility of amines being oxidized or acylated by other enzymes. Compared to reported GSH-based probes, polymethine-derived CFC-GSH exhibits an unprecedented shift from NIR-II to NIR-I fluorescence, with zero overlap in the emission spectra. The deep tissue penetration capability of NIR fluorescence enables the diagnosis and monitoring of subcutaneous tumours and metastasized tumours in the abdominal cavity of HCC through dual-channel imaging of GGT activities in a mouse model. To the best of our knowledge, CFC-GSH is the first ratiometric probe using NIR-II (900–1700 nm) signals to track GGT activities in vivo.
Results and discussion
Probe design and synthesis
Fluorescence imaging in the near-infrared II (NIR-II, 900–1700 nm) window provides deeper tissue penetration and higher resolution than NIR-I because it further minimizes tissue autofluorescence, absorption, and scattering.5–7,43 To obtain a NIR-II probe with GGT-triggerable fluorescence, GSH is chosen as the enzymatic substrate and conjugated to an anionic cyanine scaffold featuring tricyanofuran ends to afford the probe CFC-GSH. Anionic cyanine exhibits significantly red-shifted photophysical properties compared to its cationic analogy, and particularly the tricyanofuran-terminated polymethine exhibits NIR-II fluorescence with high photostability.44–47 Prior to GGT incubation, CFC-GSH is expected with inherent NIR-II signals due to the large π-conjugated system in the heptamethine chromophore.44 As shown in Fig. 1, upon enzymatic cleavage of the γ-glutamyl bond, the amino group released in the cysteinyl-glycine residue promotes an intramolecular cascade arrangement of thiolate, which proceeds through a five-membered cyclic transition state to produce amino-substituted CFC-NCS. Given the unique cascade arrangement,38,48 a significant bathochromic shift in absorption and fluorescence would be observed, promising the detection of GGT in a ratiometric mode in the NIR window. Of note, there is no free amine group in CFC-NCS, thus eliminating the interference from oxidation or acylation from other enzymes in the biological environment.The probe CFC-GSH was conveniently synthesized from the nucleophilic substitution of the free thiol group of GSH with chlorine on anionic polymethine scaffold CFC-Cl, as presented in Scheme S1.†CFC-GSH and CFC-Cl were characterized by 1H NMR, 13C NMR and HRMS, respectively (Fig. S1–S5†).
Spectral response to GGT
To evaluate the capability of GGT activation, the UV/vis absorption spectra of CFC-GSH in the absence and presence of GGT were evaluated in phosphate-buffered saline (PBS) solution. As shown in Fig. 1a, upon incubation with GGT, the absorption bands of CFC-GSH at 890 nm decreased gradually, whereas a new absorption peak emerged at 650 nm concomitantly. Along with the bathochromic shift of ca. 240 nm in the absorption spectra, a distinct colour change from green to blue was observed (Fig. 1a, inset). Next, the fluorescence titration experiments of CFC-GSH were carried out. In the absence of GGT, CFC-GSH exhibited fluorescence in the NIR-II region with a peak at 930 nm (λex = 808 nm), which could be attributed to the large π-conjugated system existing in the tricyanofuran-ended heptamethine chromophore. After incubation with GGT, the initial NIR-II fluorescence decreased in a dose-dependent behaviour, whereas new fluorescence increased gradually at 660 nm (λex = 620 nm) (Fig. 1b and c). The remarkable bathochromic shift (ca. 270 nm) in the fluorescent signal from NIR-II to NIR-I confirms the enzymatic cleavage of γ-glutamyl linkage in CFC-GSH. To our delight, zero overlap was found in the two fluorescence spectra, which is beneficial for highly sensitive detection of GGT activity in potent ratiometric mode with self-calibration characteristics.49,50 Harnessing the fluorescence intensity ratio (F660/F930) changes, the NIR ratiometric detection of GGT can be established with CFC-GSH. As shown in Fig. 1d, the fluorescence intensity ratio (F660/F930) exhibited a satisfactory linear relationship with the GGT concentration at 1–100 U per L (R2 = 0.9865). The limit of detection (LOD) was determined to be 0.04 U per L (3σ/k), which meets the requirement for GGT detection in biological samples with the desired sensitivity. In contrast, the fluctuations of fluorescence signals appeared to be blocked when the working system was pretreated with glutamine analogues acivicin, an inhibitor of GGT51 (Fig. 1e). Moreover, the fluorescence intensity ratio (F660/F930) decreased in an acivicin dose-dependent behaviour, demonstrating that GGT is essential for triggering optical changes (Fig. 1f and S6†).Sensing mechanism
To investigate the responsive mechanism, we first explored the binding mode of CFC-GSH to GGT using molecular docking simulations.2,52 As shown in Fig. 2b, the recognition group γ-glutamyl unit can enter the enzymatic pocket successfully and form hydrogen bonds with the surrounding amino acid residues (Gln 285, Glu 381 and Arg 387), suggesting the high affinity of GSH-derived CFC-GSH to GGT. In addition, the fluorophore anionic polymethine was stuck outside the pocket, which may be attributed to the cysteine residue in GSH that could separate the cleavage site away from the cyanine scaffold, thereby diminishing the steric effect from the fluorophore. To validate the subsequent enzymatic activation, high-performance liquid chromatography (HPLC) analysis was carried out, as shown in Fig S7.† After incubation with GGT, the peak corresponding to CFC-GSH (3.11 min) decreased while another peak emerged at 5.17 min, which was further dominated as the incubation time prolonged (10.0 min). The generation of amino-substituted CFC-NCS was identified by the HRMS analysis (Fig. S8†). The incubation of CFC-GSH with GGT manifested a mass peak at 675.2106 identical to that of CFC-NCS (m/z: calc., 675.2143). These results supported that the enzymatic hydrolysis of the γ-glutamyl bond in CFC-GSH underwent a subsequent intramolecular rearrangement cascade reaction to produce amino-substituted polymethine.53,54 To rationalize the spectral change in the presence of GGT, the frontier molecular orbital of probe CFC-GSH and amino-substituted product CFC-NCS were calculated using Gaussian 16.55,56 As shown in Fig. 2c, both the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of CFC-GSH are located mainly in the heptamethine scaffold, while there is partial redistribution of the electronic density on the HOMO and LUMO of CFC-NCS. The energy gap of CFC-GSH (1.96 eV) is larger than that of CFC-GSH (1.86 eV), which could rationalize the bathochromic shift of absorption wavelengths of CFC-GSH after incubation with GGT. Given these results, a possible sensing mechanism for the activation of CFC-GSH with GGT was proposed (Fig. 2a). Upon GGT-induced cleavage of the γ-glutamyl bond on probe CFC-GSH, the released amino group in the cysteinyl-glycine residue promotes an intramolecular cascade arrangement of thiolate, yielding amino-substituted CFC-NCS through a five-membered cyclic transition state. Consequently, the absorption and fluorescence spectra of CFC-GSH exhibited a distinct bathochromic shift upon incubation with GGT.In vitro characterization
After that, the sensing kinetics of CFC-GSH toward GGT was further investigated. As shown in Fig. 1h, the fluorescence ratio signals (F660/F930) of CFC-GSH increased over incubation time and arrived at a plateau in 22 min, indicative of the fast response to GGT. To visualize the fluorescence changes, the response progression of CFC-GSH to GGT was imaged using a full spectrum animal In Vivo Imaging System (IVIS). As shown in Fig. 1g, the NIR-II fluorescence signal of GGT-treated CFC-GSH solution gradually weakened with the extension of incubation time, while the NIR-I fluorescence signal gradually enhanced. The results indicate that CFC-GSH could be an ideal tool for rapid detection and imaging of GGT.The specificity of CFC-GSH was assessed in the presence of potential biological interferents including 10% fetal bovine serum (FBS), enzymes (NTR, APN, ALP, Try, CE, and GOx) and amino acids (Glu and Cys). As shown in Fig. S9a,† no significant fluorescence alteration in both NIR-II and NIR-I channels can be triggered by the biological interferents. Moreover, the presence of these interferents also showed no obvious interference with the GGT-triggered fluorescence changes (Fig. S9b†), demonstrating the potential of CFC-GSH to specifically identify GGT in complex biological samples. Furthermore, the presence of amino acids (Hcy, Iso, Leu, Arg, Lys and Phe), cations (Na+, K+, Mg2+, Ca2+, Al3+, and Fe2+) and anions (H2PO4−, NO3−, and CO32−) also exhibited limited interference in the detection of GGT (Fig. S10†), indicating the excellent selectivity of CFC-GSH. In addition, the photostability of CFC-GSH was examined with the excitation of a 150 W xenon lamp. As shown in Fig. S11,† the fluorescence intensity ratio (F660/F930) of CFC-GSH remained relatively stable during the scanning period of 8000 s, indicative of the desirable resistance to photobleaching. These results suggested that CFC-GSH holds great potential for accurate detection of GGT activity in practical settings.
Fluorescence imaging in living cells
Inspired by the favourable performance in vitro, we subsequently assessed the applicability of CFC-GSH in living cells. Before that, CFC-GSH was invoked to evaluate its biocompatibility with cell lines (HEK293T and HepG2 cells). As shown in Fig. S12,† after incubation with various concentrations of CFC-GSH (0–50 μM) for 24 h, the cell viability of all cells remained at a high level (over 85%), suggesting the good biocompatibility of CFC-GSH. Given the GGT-triggerable NIR-I fluorescence enhancement, the fluorescence imaging of CFC-GSH in different cell lines was carried out using a confocal laser scanning microscope (CLSM). As depicted in Fig. 3a, faint fluorescence signals were observed in human embryonic kidney cells (HEK293T) and mouse embryonic fibroblast cells (NIH-3T3), which suggests that GGT activity remains negligible in normal cells. By contrast, intensive NIR-I fluorescence signals were observed in hepatocellular carcinoma cells (human HepG2 and murine Hepa 1–6), which were 4.63 and 4.40 times higher than those in normal cells, respectively, suggesting higher GGT levels in HCC (Fig. 3d). When the cancer cells were pretreated with GGT inhibitor acivicin (1 mM), the NIR-I fluorescence signals decreased drastically, elucidating the fact that the fluorescence change comes from the GGT-catalysed cleavage of the γ-glutamyl bond in CFC-GSH (Fig. 3a and b). Furthermore, other cancer cells of different origins were also demonstrated with different GGT levels according to the activated NIR-I fluorescence intensity (Fig. 3b). These results verified the capability of CFC-GSH for evaluating the GGT levels in living cells, thus providing the accessibility for differentiating cancer cells from normal cells. Considering the desirable ratiometric response of probe CFC-GSH, the possibility of quantitative detection of endogenous GGT produced by the density gradients of cancer cells was further explored. The Hepa 1–6 cells were pre-transferred into a black 96-well plate with controlled densities, followed by incubation with CFC-GSH for 30 min and the signals were recorded using the IVIS. As shown in Fig. 3c and e, the fluorescence signals in the NIR-II channel exhibited a density-dependent decline process, whereas the signals in the NIR-I channel exhibited a gradual enhancement process. Moreover, a good linear relationship was found between the fluorescence intensity ratio and cell number from 2000 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per well (R2 = 0.9723) with a LOD lower than 320 cells (3σ/k), showing the feasibility of CFC-GSH for quantitatively evaluating GGT activities in cancer cells (Fig. S13†). These results suggest that probe CFC-GSH provided a promising tool for discriminating GGT-overexpressed cancer cells from other cells.
000 per well (R2 = 0.9723) with a LOD lower than 320 cells (3σ/k), showing the feasibility of CFC-GSH for quantitatively evaluating GGT activities in cancer cells (Fig. S13†). These results suggest that probe CFC-GSH provided a promising tool for discriminating GGT-overexpressed cancer cells from other cells.
Dual-channel imaging of subcutaneous tumours
Next, the ratiometric probe CFC-GSH was further employed for real-time in vivo visualization of GGT activity in tumour-bearing mice to monitor HCC. A murine hepatocellular carcinoma xenograft tumour model was established by subcutaneously injecting Hepa 1–6 cells into the assigned site of female BALB/C nude mice.57 After 2 weeks, the tumour grew about 100 mm3 and was then treated with CFC-GSH for NIR-I and NIR-II dual-channel imaging using the IVIS. As exhibited in Fig. 4a and c, distinct NIR-I fluorescence was collected specifically in the tumour region within 1 min and gradually increased to the maximum within 30 min, while, in the NIR-II channel, the signal intensities dropped significantly in the imaging period, indicating the rapid activation of CFC-GSHin situ (Fig. 4a and d). The fluorescence intensity ratio increased in a time-dependent manner and reached a plateau at 30 min (Fig. 4e). To validate the finding that fluorescence changes were indeed triggered by GGT in subcutaneous tumours, the mice were preinjected with inhibitor acivicin for 30 min and then treated orthotopically with CFC-GSH. As shown in Fig. 4a, the enhancement of NIR-I fluorescence and the decline of NIR-II fluorescence in the tumour region were blocked well by acivicin. The fluorescence intensity ratio in the HCC tumour attenuated 19.52-fold compared with that in the absence of acivicin, verifying that the dual-channel fluorescence changes at the tumour site resulted from selective cleavage of CFC-GSH by GGT (Fig. 4a and e). Furthermore, ex vivo fluorescence images of the tumour and main organs (heart, liver, spleen, lung, and kidney) were recorded after sacrificing the mice at 2 h post-injection. As shown in Fig. 4b, faint NIR-II fluorescence signals but intensive NIR-I fluorescence signals were observed only in tumours, suggesting the specific fluorescence changes triggered by tumoral GGT. Consistent with the in vivo results, the fluorescence changes were attenuated as well by the inhibitor, with a decrease in the fluorescence intensity ratio of about 3.48-fold (Fig. 4f). Moreover, the tissue sections were obtained to explore the accessibility of CFC-GSH in tissue imaging. As shown in Fig. S14,† in contrast to the negligible fluorescence in liver slices, stronger fluorescence was observed in tumours with a 12.86-fold signal enhancement, suggesting that CFC-GSH has the potential to discriminate tissues with high GGT expression from those with low expression. These results were in keeping with those in vitro, supporting the superiority of CFC-GSH to reflect the dynamic level changes of GGT in HCC xenograft mice.Dual-channel imaging of metastasis tumours
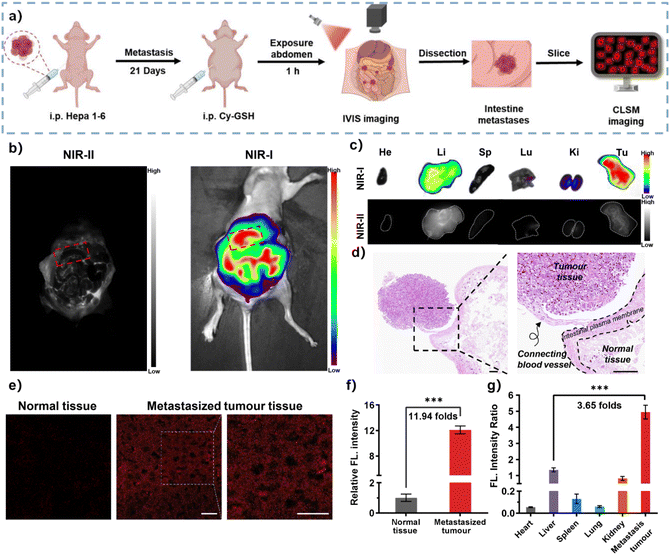
Most malignant cancers, including HCC, tend to metastasize from the primary site to secondary sites within the host.5,58,59 Patients with advanced HCC should be carefully monitored due to the frequent occurrence of extrahepatic metastases, including those in the lungs, lymph nodes, and peritoneal metastases.60 However, metastasized tumours are often difficult to detect and are located early due to their small size and deep location within the body.33,61 To mimic metastasis of HCC to the abdominal cavity, Hepa 1–6 cells were injected into the abdominal cavity of female BALB/C nude mice, and 3 weeks later the probe was administrated via intraperitoneal injection. After 1 h, the abdominal cavity of mice was exposed to dual-channel imaging (Fig. 5a). As shown in Fig. 5b, the peritoneal metastasized tumours in the abdominal cavity could be observed at the intestinal plasma membrane according to the fluorescence signals from two channels, suggesting that HCC tumours predominantly metastasized to the intestines. Haematoxylin–eosin (H&E) stained tissue specimens revealed tumorous regions adjacent to normal intestine regions, connected by a blood vessel, which was distinguishable by the cellular morphology (Fig. 5d). Following the sacrifice of the mice, the intestinal tissue of interest and the main organs were collected for ex vivo fluorescence imaging. The results showed that intensive NIR-I fluorescence and faint NIR-II fluorescence were only found in tumour-containing intestinal tissue (Fig. 5c). Notably, while the liver exhibited significant NIR-I fluorescence, the NIR-II fluorescence was much higher in the liver than in tumour tissues. This discrepancy can be attributed to the excessive uptake of probes by the liver and subsequent enzymatic catalysis of endogenous GGT. The fluorescence ratio allowed for self-calibration of signals in the liver, enabling specific detection and tracking of peritoneal metastasis of HCC in vivo. This result highlights the advantages of the ratiometric response mode provided by the probe CFC-GSH (Fig. 5g). Furthermore, fluorescence imaging of metastatic tumour sections showed that tumour tissues displayed high-contrast fluorescence, with fluorescence intensity 12.1 times that of normal intestinal tissues (Fig. 5e). This indicates the excellent imaging capability of CFC-GSH for in vivo detection of HCC intestinal metastases (Fig. 5f). Collectively, CFC-GSH shows potential as a tool for accurate imaging of metastatic tumours in clinical applications.Fluorescence angiography with CFC-GSH
From the perspective of broader application scenarios, we would like to highlight the innate NIR-II signals of CFC-GSH in fluorescence imaging of systemic circulation vessels. The fluorescence in the NIR-II window offers several advantages, including optimized spatial resolution, improved imaging depth, a high signal-to-background ratio (SBR), and reduced tissue autofluorescence, thereby providing non-invasive, high-resolution imaging at millimetre depths within biological tissues.36,62,63 As demonstrated in Fig. 6a and b, bright NIR-II fluorescence delineated the blood vessels within minutes following intravenous injection of CFC-GSH, clearly visualizing the blood circulatory system in nude mice with high spatial resolution. Impressively, the SBR of vascular signals in the brain, paw, and hindlimb of living mice were quantified as 1.66, 1.32, and 1.35 (Fig. 6c), respectively, indicating that CFC-GSH holds potential for non-invasive imaging of subcutaneous vasculature in live mice. These results demonstrate the superiority of CFC-GSH bioimaging in the NIR-II window, making it a valuable tool for in vivo microangiography and disease monitoring.To investigate its biodistribution, in vivo fluorescence images were recorded from both NIR-I and NIR-II channels using the IVIS at 24 h after intravenous injection of CFC-GSH. As shown in Fig. 6d, strong fluorescence signals were observed in the liver in both channels, indicating that liver metabolism serves as a primary clearance pathway for CFC-GSH. Subsequently, the mice were sacrificed, and their main organs were collected for dual-channel imaging. As shown in Fig. 6e, the intensive NIR-II fluorescence was recorded in the liver, with weaker NIR-II signals also detected in the spleen. In contrast, NIR-I signals were only found in the liver, which has innate expression of GGT, further verifying the optimized specificity of CFC-GSH-supported ratiometric mode. To evaluate the biocompatibility of CFC-GSH, H&E staining of the organ slices was performed. The results showed no obvious pathological damage or lesions (Fig. 6g), suggesting low systematic toxicity of the probe to normal organs and tissues. Consistent with the result, no significant haemolysis occurred at a concentration reaching 512 μM (Fig. 6f). Collectively, these results validated the reliable biocompatibility of CFC-GSH for imaging species of interest in living things.
Conclusions
This work presents a ground-breaking approach for modulating the NIR-II fluorescence of anionic cyanine-based scaffolds using a specific enzymatic trigger. This strategy not only provides a powerful tool for HCC localization but also signifies a valuable approach for developing enzyme-activated NIR-II probes for broader cancer diagnosis and management. Based on the pioneering strategy, CFC-GSH-boosted dual-channel imaging enables real-time monitoring of GGT activity in tumours and precise localization of metastatic HCC lesions within the abdomen. Importantly, the self-calibration properties of the ratiometric probe CFC-GSH diminish interference from innate GGT activities in the liver. Given its inherent NIR-II fluorescence, we also demonstrated the potential application of CFC-GSH in fluorescence microangiography in the NIR-II window. Notably, CFC-GSH exhibits no acute toxicity, suggesting its potential for future clinical application in HCC tumour staging, localization, and treatment decision-making.Experimental section
Synthesis and measurement methods
CFC-GSH was synthesized via three steps, and the specific information of relative compounds is given in the ESI.† The further details of in vitro detection and the cell treatment and imaging are available in the ESI.†Methods of theoretical calculations
Calculations of optimal geometries and electronic structures of CFC-GSH and CFC-NCS were carried out using density functional theory (DFT) at the b3lyp/6-31g(d) level.52 The starting structures with different conformational isomers were considered to ensure that the optimized structure corresponds to a global minimum. All these calculations were carried out with Gaussian 16 software (water was employed in all the calculations). The obtained data were analysed using GaussView 5.0 software.Method of molecular docking simulation
Molecular docking simulations were carried out on MOE2019 software. The crystal structure of human glutaminase in complex with L-glutamine (PDB ID: 3VP0)55 was downloaded from RCSB PDB and underwent a series of optimizations (completing the protein sequence, deleting repeated subunits, adding hydrogen atoms, and removing all water molecules and ligands). Different conformations of CFC-GSH were searched (n = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and the docking cycles and the parameters between the ligand and protein were set according to the defaults.
000) and the docking cycles and the parameters between the ligand and protein were set according to the defaults.
Animal models and fluorescence imaging
BALB/c nude mice (female, 6 weeks old) were obtained from the Animal Centre of Xiangya Medical School, Central South University. All animal procedures complied with the Guide for the Care and Use of Laboratory Animals and were approved (application number: XMXH-2023-1384) by the Laboratory Animal Welfare Ethics Committee, Central South University, China.Cells were pelleted by centrifugation and resuspended in pre-cooled sterile PBS. For subcutaneous tumours, 150 μL PBS containing Hepa 1–6 cells (1 × 106) were implanted subcutaneously into the Balb/c nude mice. After two weeks, the tumour grew about 100 mm3 and the mice were submitted to tumour imaging after being randomly allocated into two groups. In the experimental group, probe CFC-GSH (50 μL, 10 μM in PBS, containing 1% DMSO) was intratumorally injected into the tumour site. In the inhibitor group, the tumour was pretreated with acivicin (1 mM) for 30 min and then treated with CFC-GSH. The in vivo fluorescence images were obtained at different times (1, 5, 10, 20, 30, 40, and 60 min) by using the full spectrum animal In Vivo Imaging System (IVIS) after injection. Then, the mice were sacrificed and dissected after 2 h post-injection to collect major organs including the heart, liver, spleen, lung, and kidney and the tumour for fluorescence imaging.
For metastasis tumours in the abdomen, 200 μL PBS containing Hepa 1–6 cells (5 × 106) were implanted intraperitoneally into the Balb/c nude mice. After three weeks, the hepatocarcinoma ascites formed, and the probe CFC-GSH (100 μL, 20 μM in PBS, containing 1% DMSO) was intraperitoneally in the mice. After one hour, mice were sacrificed and dissected to expose the abdominal cavity for fluorescence imaging using the IVIS, and major organs including the heart, liver, spleen, lung, and kidney and part of the intestine were removed for fluorescence imaging.
IVIS spectral imaging system
For NIR-I fluorescence imaging of the samples, the fluorescence images were acquired upon excitation at 605 nm, and the collected emission was 660 nm. For NIR-II fluorescence imaging of the samples, the fluorescence images were acquired upon excitation at 808 nm, and a 1075 nm long-pass filter was used for collecting the fluorescent signals. The light intensities for NIR-I and NIR-II fluorescence imaging were set to 70% and 80%, respectively.In vivo vascular imaging
Before the experiment, mice were anesthetized under a continuous 2% isoflurane atmosphere. For whole-body vascular imaging, the mice were intravenously injected with CFC-GSH (200 μL, 80 μM in PBS, containing 1% DMSO) and then were imaged immediately by using the full spectrum animal In Vivo Imaging System (IVIS). The excitation laser was at 808 nm and the emission was collected using 1075 nm filters. The light intensity was set to 80%, with an exposure time of 20 ms.Data availability
Additional experimental details and data are provided in the ESI.†Author contributions
Feiyi Chu: conceptualization, investigation, methodology, writing – original draft. Bin Feng: investigation, validation, writing – review & editing. Yiyang Zhou: formal analysis, investigation, validation. Min Liu: resources, formal analysis, writing – review & editing, validation. Hailiang Zhang: formal analysis, resources. Meihui Liu: validation, writing – review & editing, resources. Qian Chen: resources, validation. Shengwang Zhang: formal analysis, methodology. Yeshuo Ma: resources, validation. Jie Dong: formal analysis, software. Fei Chen: validation, resources, writing – review & editing. Wenbin Zeng: supervision, conceptualization, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82272067, 22107123 and M-0696), the Natural Science Foundation of Hunan Province (Grant No. 2022JJ80052), Health Commission of Hunan Province Project (Grant No. D202303017033) and the Innovation Fund for Postgraduate Students of Central South University (Grant No. 2023ZZTS0927).Notes and references
- Y. Zhang, Z. Zhang, M. Wu and R. Zhang, ACS Meas. Sci. Au, 2024, 4, 54–75 CrossRef CAS PubMed.
- B. Feng, F. Chu, Y. Fang, M. Liu, X. Feng, J. Dong, F. Chen and W. Zeng, Chem. Sci., 2024, 15, 7324–7331 RSC.
- C. Chen, X. Zhang, Z. Gao, G. Feng and D. Ding, Nat. Protoc., 2024, 19, 2408–2434 CrossRef CAS PubMed.
- J. Qi, X. Duan, Y. Cai, S. Jia, C. Chen, Z. Zhao, Y. Li, H.-Q. Peng, R. T. K. Kwok, J. W. Y. Lam, D. Ding and B. Z. Tang, Chem. Sci., 2020, 11, 8438–8447 RSC.
- H. Lou, A. Ji, C. Qu, S. Duan, H. Liu, H. Chen and Z. Cheng, Chem. Eng. J., 2022, 449, 137848–137856 CrossRef CAS.
- Y. Yue, T. Zhao, Z. Xu, W. Chi, X. Chai, J. Ai, J. Zhang, F. Huo, R. M. Strongin and C. Yin, Adv. Sci., 2022, 10, 2205080–2205090 CrossRef PubMed.
- A. Ji, H. Lou, J. Li, Y. Hao, X. Wei, Y. Wu, W. Zhao, H. Chen and Z. Cheng, Chem. Sci., 2024, 15, 3339–3348 RSC.
- Y. Lai, Y. Dang, Q. Sun, J. Pan, H. Yu, W. Zhang and Z. Xu, Chem. Sci., 2022, 13, 12511–12518 RSC.
- Y. Zhang, G. Zhang, Z. Zeng and K. Pu, Chem. Soc. Rev., 2022, 51, 566–593 RSC.
- X. Zhang, S. Li, H. Ma, H. Wang, R. Zhang and X.-D. Zhang, Theranostics, 2022, 12, 3345–3371 CrossRef CAS PubMed.
- J. Bao, R. Liu, Z. Yu, Z. Cheng and B. Chang, Adv. Funct. Mater., 2024, 34, 2316646–2316661 CrossRef CAS.
- J. Ouyang, L. Sun, Z. Zeng, C. Zeng, F. Zeng and S. Wu, Angew. Chem., Int. Ed., 2020, 59, 10111–10121 CrossRef CAS PubMed.
- Z. Qin, T. B. Ren, H. Zhou, X. Zhang, L. He, Z. Li, X. B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2022, 61, e202201541–e202201549 CrossRef CAS PubMed.
- L. Shen, J. Li, C. Wen, H. Wang, N. Liu, X. Su, J. Chen and X. Li, Sci. Adv., 2024, 10, eado2037–eado2051 CrossRef CAS PubMed.
- J.-A. Chen, H. Pan, Z. Wang, J. Gao, J. Tan, Z. Ouyang, W. Guo and X. Gu, Chem. Commun., 2020, 56, 2731–2734 RSC.
- I. Castellano and A. Merlino, Cell. Mol. Life Sci., 2012, 69, 3381–3394 CrossRef CAS PubMed.
- Z.-Y. Hu, X.-Y. Chen, Y.-S. Yang, S.-J. Wang, Z.-G. Hu and K. Wang, Coord. Chem. Rev., 2024, 501, 215562–215587 CrossRef CAS.
- Y. Liu, B. Feng, X. Cao, G. Tang, H. Liu, F. Chen, M. Liu, Q. Chen, K. Yuan, Y. Gu, X. Feng and W. Zeng, Analyst, 2019, 144, 5136–5142 RSC.
- D. Yao, D. Jiang, Z. Huang, J. Lu, Q. Tao, Z. Yu and X. Meng, Cancer, 2000, 88, 761–769 CrossRef CAS PubMed.
- X. Wang, S. He, P. Cheng and K. Pu, Adv. Mater., 2023, 35, 2206510–2206518 CrossRef CAS PubMed.
- S.-J. Wu, Y.-X. Lin, H. Ye, X.-Z. Xiong, F.-Y. Li and N.-S. Cheng, Int. J. Surg., 2016, 36, 143–151 CrossRef PubMed.
- K. Wang, X.-Y. Chen, W.-D. Liu, Y. Yue, X.-L. Wen, Y.-S. Yang, A.-G. Zhang and H.-L. Zhu, Anal. Chem., 2023, 95, 14235–14243 CrossRef CAS PubMed.
- M. Orlowski and A. Meister, Biochim. Biophys. Acta, Spec. Sect. Enzymol. Subj., 1963, 73, 679–681 CrossRef CAS.
- S. Ye, S. Wang, D. Gao, K. Li, Q. Liu, B. Feng, L. Qiu and J. Lin, Bioconjugate Chem., 2020, 31, 174–181 CrossRef CAS PubMed.
- K. Kiuchi, K. Kiuchi, T. Nagatsu, A. Togari and H. Kumagai, J. Chromatogr. A, 1986, 357, 191–198 CrossRef CAS PubMed.
- Z. Hai, Y. Ni, D. Saimi, H. Yang, H. Tong, K. Zhong and G. Liang, Nano Lett., 2019, 19, 2428–2433 CrossRef CAS PubMed.
- Q. Liu, J. Yuan, R. Jiang, L. He, X. Yang, L. Yuan and D. Cheng, Anal. Chem., 2023, 95, 2062–2070 CrossRef CAS PubMed.
- K. Wang, X.-Y. Chen, B. Zhang, Y. Yue, X.-L. Wen, Y. Yang, Y.-S. Yang, H.-L. Zhu, H.-J. Liu and A.-G. Zhang, Biosens. Bioelectron., 2023, 241, 115721–115728 CrossRef CAS PubMed.
- K. Li, Y. Lyu, Y. Huang, S. Xu, H.-W. Liu, L. Chen, T.-B. Ren, M. Xiong, S. Huan, L. Yuan, X.-B. Zhang and W. Tan, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2018033118–e2018033127 CrossRef CAS PubMed.
- Y. Liu, J. Tan, Y. Zhang, J. Zhuang, M. Ge, B. Shi, J. Li, G. Xu, S. Xu, C. Fan and C. Zhao, Biomaterials, 2018, 173, 1–10 CrossRef CAS PubMed.
- K. Wang, W. Wang, X.-Y. Chen, Y.-S. Yang and H.-L. Zhu, Biosens. Bioelectron., 2023, 219, 114767–114714 CrossRef CAS PubMed.
- H. Li, Q. Yao, F. Xu, N. Xu, R. Duan, S. Long, J. Fan, J. Du, J. Wang and X. Peng, Biomaterials, 2018, 179, 1–14 CrossRef CAS PubMed.
- J. Ou-Yang, Y. Li, W.-L. Jiang, S.-Y. He, H.-W. Liu and C.-Y. Li, Anal. Chem., 2019, 91, 1056–1063 CrossRef CAS PubMed.
- H. Chen, Z. Cai, J. Gui, Y. Tang, P. Yin, X. Zhu, Y. Zhang, H. Li, M. Liu and S. Yao, J. Mater. Chem. B, 2023, 11, 1279–1287 RSC.
- Y.-L. Qi, Y.-Z. Li, M.-J. Tan, F.-F. Yuan, N. Murthy, Y.-T. Duan, H.-L. Zhu and S.-Y. Yang, Coord. Chem. Rev., 2023, 486, 215130–215167 CrossRef CAS.
- R. Xu, D. Jiao, Q. Long, X. Li, K. Shan, X. Kong, H. Ou, D. Ding and Q. Tang, Biomaterials, 2022, 289, 121780–121792 CrossRef CAS PubMed.
- M. Jiang, Q. Ma, J. Huang, S. Bi and S. Zeng, Chem. Eng. J., 2023, 475, 145977–145984 CrossRef CAS.
- F. Wang, Y. Zhu, L. Zhou, L. Pan, Z. Cui, Q. Fei, S. Luo, D. Pan, Q. Huang, R. Wang, C. Zhao, H. Tian and C. Fan, Angew. Chem., Int. Ed., 2015, 54, 7349–7353 CrossRef CAS PubMed.
- R. Obara, M. Kamiya, Y. Tanaka, A. Abe, R. Kojima, T. Kawaguchi, M. Sugawara, A. Takahashi, T. Noda and Y. Urano, Angew. Chem., Int. Ed., 2020, 60, 2125–2129 CrossRef PubMed.
- S. M. Usama, F. Inagaki, H. Kobayashi and M. J. Schnermann, J. Am. Chem. Soc., 2021, 143, 5674–5679 CrossRef CAS PubMed.
- H. Tong, Y. Zheng, L. Zhou, X. Li, R. Qian, R. Wang, J. Zhao, K. Lou and W. Wang, Anal. Chem., 2016, 88, 10816–10820 CrossRef CAS PubMed.
- R. Huo, X. Zheng, W. Liu, L. Zhang, J. Wu, F. Li, W. Zhang, C.-S. Lee and P. Wang, Chem. Commun., 2020, 56, 10902–10905 RSC.
- B. Feng, F. Chu, A. Bi, X. Huang, Y. Fang, M. Liu, F. Chen, Y. Li and W. Zeng, Biotechnol. Adv., 2023, 68, 108244 CrossRef CAS PubMed.
- Y. Ma, L. Liu, Z. Ye, L. Xu, Y. Li, S. Liu, G. Song and X.-B. Zhang, Sci. Bull., 2023, 68, 2382–2390 CrossRef CAS PubMed.
- P.-A. Bouit, D. Rauh, S. Neugebauer, J. L. Delgado, E. D. Piazza, S. Rigaut, O. Maury, C. Andraud, V. Dyakonov and N. Martin, Org. Lett., 2009, 11, 4806–4809 CrossRef CAS PubMed.
- Z. a. Li, S. Mukhopadhyay, S.-H. Jang, J.-L. Brédas and A. K. Y. Jen, J. Am. Chem. Soc., 2015, 137, 11920–11923 CrossRef CAS PubMed.
- Y. Li, Y. Zhou, X. Yue and Z. Dai, Bioact. Mater., 2021, 6, 794–809 CAS.
- L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928–18931 CrossRef CAS PubMed.
- R. Song, Y. Dong, Z. Zhong, Q. Zhao, Y. Hu, M. Lei, P. Lei, Z. Jiang, K. Qian, C. Shi, Z. He, Y. Qin, J. Wang and H. Chen, J. Med. Chem., 2024, 67, 10275–10292 CrossRef CAS PubMed.
- P. Wu, Z. Qu, J. Zhang, X. Ren, D. Wang, C. Huang, K. Cheng, J. Qi, H. Shi, S. Gan, W. Wei, Y. Zhang, C.-S. Lee, L. Wang and H. Sun, Adv. Funct. Mater., 2024, 2400597–2400607 CrossRef.
- J. B. King, M. B. West, P. F. Cook and M. H. Hanigan, J. Biol. Chem., 2009, 284, 9059–9065 CrossRef CAS PubMed.
- K. Thangavelu, Q. Y. Chong, B. C. Low and J. Sivaraman, Sci. Rep., 2014, 4, 3827–3833 CrossRef CAS PubMed.
- X. Zhou, Y. Liu, Q. Liu, L. Yan, M. Xue, W. Yuan, M. Shi, W. Feng, C. Xu and F. Li, Theranostics, 2019, 9, 4597–4607 CrossRef CAS PubMed.
- B. Feng, Y. Liu, S. Huang, X. Huang, L. Huang, M. Liu, J. Wu, T. Du, S. Wang, X. Feng and W. Zeng, Sens. Actuators, B, 2020, 325, 128786–128795 CrossRef CAS.
- Y. Li, T. Ma, H. Jiang, W. Li, D. Tian, J. Zhu and Z. a. Li, Angew. Chem., Int. Ed., 2022, 61, e202203093–e202203099 CrossRef CAS PubMed.
- F. Chu, B. Feng, M. Liu, M. Liu, F. Chen, J. Dong and W. Zeng, Dyes Pigm., 2024, 222, 111894–111900 CrossRef CAS.
- Y. Wen, N. Jing, M. Zhang, F. Huo, Z. Li and C. Yin, Adv. Sci., 2023, 10, 2206681–2206690 CrossRef CAS PubMed.
- Y.-Y. Zhu, L. Song, Y.-Q. Zhang, W.-L. Liu, W.-L. Chen, W.-L. Gao, L.-X. Zhang, J.-Z. Wang, Z.-H. Ming, Y. Zhang and G.-J. Zhang, Cancer Res., 2023, 83, 3428–3441 CrossRef CAS PubMed.
- J. M. Llovet, R. K. Kelley, A. Villanueva, A. G. Singal, E. Pikarsky, S. Roayaie, R. Lencioni, K. Koike, J. Zucman-Rossi and R. S. Finn, Nat. Rev. Dis. Primers, 2021, 7, 6–34 CrossRef PubMed.
- A. Gupta, R. Sedhom and M. S. Beg, JAMA Oncol., 2020, 6, 308 CrossRef PubMed.
- Y. Liu, L. Teng, C. Xu, T.-B. Ren, S. Xu, X. Lou, L. Yuan and X.-B. Zhang, CCS Chem., 2022, 4, 2153–2164 CrossRef CAS.
- X. Liu, B. Yu, Y. Shen and H. Cong, Coord. Chem. Rev., 2022, 468, 214609–214642 CrossRef CAS.
- S. Liu, C. Chen, Y. Li, H. Zhang, J. Liu, R. Wang, S. T. H. Wong, J. W. Y. Lam, D. Ding and B. Z. Tang, Adv. Funct. Mater., 2019, 30, 1908125–1908134 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06459c |
| This journal is © The Royal Society of Chemistry 2025 |