Open Access Article
Open Access ArticleEfficient harvesting of triplet excitons via multiple fast TTA up-conversion and high-lying reverse intersystem crossing channels for efficient blue fluorescent organic light-emitting diodes†
Jianwen
Qin
 ,
Xianfeng
Qiao
,
Xianfeng
Qiao
 *,
Shu
Xiao
,
Dezhi
Yang
*,
Shu
Xiao
,
Dezhi
Yang
 ,
Yanfeng
Dai
,
Jiangshan
Chen
,
Yanfeng
Dai
,
Jiangshan
Chen
 ,
Qian
Sun
,
Qian
Sun
 and
Dongge
Ma
and
Dongge
Ma
 *
*
Institute of Polymer Optoelectronic Materials and Devices, Guangdong Basic Research Center of Excellence for Energy & Information Polymer Materials, Guangdong-Hong Kong-Macao Joint Laboratory of Optoelectronic and Magnetic Functional Materials, Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, State Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, China. E-mail: msxqiao@scut.edu.cn; msdgma@scut.edu.cn
First published on 23rd January 2025
Abstract
The efficient harvesting of triplet excitons is key to realizing high efficiency blue fluorescent organic light-emitting diodes (OLEDs). Triplet–triplet annihilation (TTA) up-conversion is one of the effective triplet-harvesting strategies. However, during the TTA up-conversion process, a high current density is necessary due to the competitive non-radiative triplet losses. In this study, we designed blue organic light-emitting diodes with fast TTA up-conversion and high-lying reverse intersystem crossing channels. It can be seen that the utilization of triplet excitons was greatly improved and the non-radiative triplet losses were significantly suppressed due to the availability of multiple efficient channels for triplet exciton utilization. As a result, a high efficiency blue fluorescent OLED was successfully fabricated. The maximum external quantum efficiency (EQE) reached 11.4% with CIE coordinates of (0.13, 0.11). This research provides a new route for the development of high efficiency blue fluorescent OLEDs.
1. Introduction
Organic light emitting diodes (OLEDs) are attracting great interest for display and lighting applications.1 Over the past three decades, high efficiency green and red phosphorescent OLEDs based on heavy metal complexes (generally Ir or Pt) have been applied in commercial products.2,3 However, the phosphorescent OLEDs always exhibit serious efficiency roll-off at high current density caused by triplet–triplet annihilation (TTA) originating from the accumulation of long lifetime triplet excitons.4 Whilst phosphorescent materials, which contain rare metals, for example, Ir, Pt, and Os, have a high cost of commercial production. Moreover, the development of blue phosphorescent OLEDs, especially those with deep-blue emissions, has lagged behind and hit a bottleneck.5 To resolve the above problems, metal-free organic fluorescent emitters that efficiently utilize triplet excitons, for example, via triplet–triplet annihilation (TTA) up-conversion,6,7 hybridized local and charge transfer (HLCT),8 and thermally activated delayed fluorescence (TADF),9,10 have been developed. Among them, TTA up-conversion emitters have shown fairly long operational lifetimes and low efficiency roll-off when used in OLEDs, which is an advantage over the phosphorescent emitters and TADF emitters.11–17 Therefore, TTA up-conversion emitters have become promising candidates for blue fluorescent OLEDs. Polyacenes, such as anthracene, pyrene and tetracene derivatives, exhibit TTA up-conversion properties. Many TTA up-conversion molecules with anthracene and pyrene as the cores have been investigated18–23 because anthracene and pyrene have suitable T1 energy levels, thus making them able to undergo a TTA up-conversion process. However, the theoretical maximum radiative singlet-exciton ratio generated from the TTA process is known to be 15%. If considering the directly generated singlets of 25%, the total singlet excitons available for emission is not greater than 40%. Obviously, the contribution of TTA up-conversion to efficient emission is small. Thus, ways to further improve the contribution of TTA up-conversion to emission has become an important research topic. Friend et al. reported a 26–37% TTA contribution (delayed fluorescence contribution) to the total emission using DPA and perylene as a blue emitter.7 Kim et al. reported a 41–48% TTA contribution to the total emission using a newly developed TTA material and a device structure containing an efficiency enhancement layer (EEL).16,23 Kido et al. synthesized a deep blue TTA emitter with a double anthracene core, however, the devices made from this material had a severe EQE roll-off and no TTA contribution was present.6 On the other hand, the high-lying reverse intersystem crossing (hRISC) process is another rapid triplet exciton conversion channel, which occurs from high-lying triplet states (Tn, n ≥ 2) to singlet states (Sm, m ≥ 1). The internal conversion (IC) process from the Tn to T1 states should be suppressed to guarantee the utilization of triplet excitons. Recently, some HLCT materials with a fast hRISC process have been investigated.24–26 Nevertheless, to date, the reports on HLCT materials discuss few TTA features, which is not beneficial for the utilization of triplet excitons.In this study, we developed a blue organic emitter with a fast TTA up-conversion process and high-lying reverse intersystem crossing features. It can be seen that the availability of multiple efficient channels significantly improved the utilization of the triplet excitons, thus greatly enhancing the efficiency of the resulting blue fluorescent OLEDs. The maximum external quantum efficiency EQEmax reached 11.4% with CIE coordinates of (0.13, 0.11).
2. Results and discussions
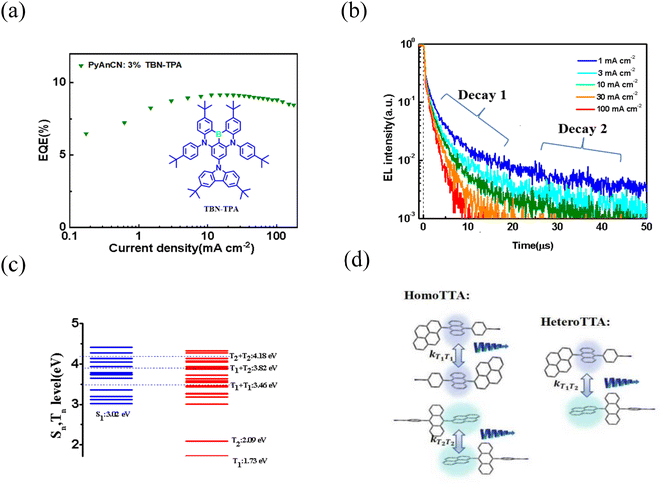
In the case of TTA up-conversion with high-lying reverse intersystem crossing channels, as illustrated in Fig. 1a, the TTA up-conversion process involves two steps: (1) two low-energy triplets collide and form a spin-correlative triplet–triplet (TT) pair, and then (2) the interacting TT pair converts into a high-energy excited state and a ground state. In the first step, the TT pair will consist of nine spin states, which contain one singlet-featuring state 1(TT), three triplet-featuring states 3(TT), and five quintet (Q)-featuring states 5(TT). In the second step, the 1(TT), 3(TT) and 5(TT) states convert into a high-energy singlet state (Sm). Although the internal quantum efficiency (IQE) can theoretically reach 62.5%, it is actually only 40%, corresponding to a maximum EQE of 8%. If there simultaneously exists an efficient high-lying reverse intersystem crossing (hRISC) process from the Tn (n ≥ 2) to singlet state Sm (m ≥ 1) which is fast enough to compete with the TTA up-conversion process, then theoretically the IQE may reach 100%. However, the IQE is greatly reduced due to the existence of internal conversion (IC) from Tn to T1, and thus there is a lack of effective conversion from triplets to singlets, which is non-radiative triplet decay (Fig. 1b). Obviously, to realize an efficient triplet harvesting process, fast rates of hRISC and TTA up-conversion are necessary to restrain the undesired internal conversion and non-radiative triplet decay channels. To trigger a rapid and efficient hRISC process, an ultra-small energy level difference between Sm and Tn (ΔESm→Tn) and a relatively large spin–orbit coupling matrix element (SOCME) between the Tn and Sm states are necessary. Additionally, a sufficiently large energy gap between the Tn and T1 states is favorable to attain a slow rate of internal conversion from Tn to T1. Therefore, the design of molecules with fast rates of TTA and hRISC are desirable.Here, a new highly efficient blue-emitting material, PyAnCN, containing anthracene and pyrene units with fast TTA and HLCT processes was synthesized (Fig. 2a). The synthetic route to obtain PyAnCN is shown in Scheme S1.† The chemical structure of PyAnCN was well characterized using NMR, and mass spectroscopy (MS). The absorption bands of PyAnCN at 377 and 397 nm are characteristics of the π–π* transition of the anthracene moiety. The PL spectrum of PyAnCN shows a deep blue emission, and the emission peak shifts from 443 nm in solution to 454 nm in a neat film (Fig. 2b). As displayed in Fig. S1,† PyAnCN in toluene shows monoexponential decay, and thus the radiative decay constant (kS) of the S1 state can be estimated. A neat PyAnCN film with encapsulation shows multi-exponential decay, and thus may exhibit more up-conversion processes. The photophysical properties of PyAnCN are provided in Table 1. The phosphorescence of PyAnCN was recorded at 77 K in glassy toluene using PtOEP as a sensitizer. As displayed in Fig. S2,† PtOEP is pumped to its excited state, followed by a fast energy transfer to the T1 state of PyAnCN. With a delay of 1 ms, the phosphorescence of PyAnCN can be detected. The first vibrational peak of the phosphorescence is at 704 nm, corresponding to a T1 energy of 1.73 eV. In order to validate the TTA up-conversion characteristics of PyAnCN, we conducted experiments on PyAnCN sensitized by PtOEP. As shown in Fig. S3,† two different linear regimes are obtained when a curve of up-conversion intensity with incident power is plotted. At low excitation power, the TTA up-conversion becomes negligible with respect to spontaneous PyAnCN nonradiative decay, which is the main triplet deactivation channel. In this case, the region can be well fitted with a straight line with a slope of 1.73. At high excitation power, the TTA up-conversion becomes dominant, and the PL intensity becomes proportional to the excitation power density with a slope of 1.18, which is consist with TTA up-conversion kinetics in a multi-component system model under steady-state conditions. To further demonstrate the excited state properties of PyAnCN, the natural transition orbitals (NTOs) were calculated using the TD-B3LYP/6-31G(d) method based on the optimized S1 geometry. As shown in Fig. S4,† the NTOs of the T1 energy state in PyAnCN are as low as 1.8 eV, arising from the long conjugation axis of anthracene, which is in good accordance with the experimental measurements. The T2 energy in PyAnCN is as low as 2.0 eV, which arises from the long conjugation axis of pyrene. To utilize PyAnCN as a blue emitter in OLEDs, we fabricated non-doped OLEDs with a device structure of indium tin oxide (ITO)/HATCN (15 nm)/TAPC (55 nm)/TCTA (10 nm)/PyAnCN (20 nm)/TPBi (40 nm)/LiF (1 nm)/Al (120 nm). HAT-CN and LiF were used as the hole and electron injection layers, respectively. TAPC and TPBi were used as the hole and electron transporting layers, respectively, and TCTA acted as the electron blocking layer. The energy level diagram of the device and the chemical structures of the used materials are also shown in Fig. S5.† The EL spectrum shows an emission peak of 450 nm with CIE coordinates of (0.15, 0.11) at 6 V. The maximum current efficiency reaches 5.9 cd A−1 (Table S1†). The maximum EQE is as high as 5.9% at a high luminescence of 1000 cd m−2. To verify the possibility of using PyAnCN as a host, we further fabricated blue OLEDs based on an emitter made from a multiple resonance (MR) induced material with a narrow full-width at half-maximum (FWHM) in a rigid core structure, as this shows a better color purity.27–32 The investigated device structure was ITO/HAT-CN (15 nm)/TAPC (55 nm)/TCTA (10 nm)/emitter (20 nm)/TPBi (40 nm)/LiF (1 nm)/Al (100 nm), where the emitter was PyAnCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA.33 As shown in Fig. S6,† an effective Förster energy transfer between PyAnCN and TBN-TPA exists due to their large spectral overlap. The optimized device with a PyAnCN host displays an external quantum efficiency (EQE) of 9.1% with a small efficiency roll-off (Fig. 3a). To understand the exciton behavior, the transient EL characteristics of the device were investigated, and the results are presented in Fig. 3b. The transient EL characteristics of the doped device show almost the same current density dependent trend. Given that TTA up-conversion is essentially a bimolecular process, the luminance–current density (L–J) curve of the device was plotted in double-log form, and the slopes of the corresponding fitted lines were calculated. As shown in Fig. S7,† under the excitation intensity conditions of J = 0.5 mA cm−2, the fitted line is almost linearly dependent. The EL decay in the double-log form can be linearly fitted with a slope of −1.9 at a time range of 2–20 μs, which fits well with the TTA up-conversion model expressed as follows:
3% TBN-TPA.33 As shown in Fig. S6,† an effective Förster energy transfer between PyAnCN and TBN-TPA exists due to their large spectral overlap. The optimized device with a PyAnCN host displays an external quantum efficiency (EQE) of 9.1% with a small efficiency roll-off (Fig. 3a). To understand the exciton behavior, the transient EL characteristics of the device were investigated, and the results are presented in Fig. 3b. The transient EL characteristics of the doped device show almost the same current density dependent trend. Given that TTA up-conversion is essentially a bimolecular process, the luminance–current density (L–J) curve of the device was plotted in double-log form, and the slopes of the corresponding fitted lines were calculated. As shown in Fig. S7,† under the excitation intensity conditions of J = 0.5 mA cm−2, the fitted line is almost linearly dependent. The EL decay in the double-log form can be linearly fitted with a slope of −1.9 at a time range of 2–20 μs, which fits well with the TTA up-conversion model expressed as follows:
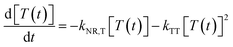 | (1) |
 | ||
| Fig. 2 (a) Molecular structure of PyAnCN. (b) Absorption spectrum of PyAnCN in toluene solution (10−5 M), PL emission spectra of a PyAnCN solution and neat film. | ||
| Compound | Abssola [nm] | PLsola [nm] | PLfilmb [nm] | HOMO/LUMOc [eV] | E g [eV] | Φ PL (%) |
|---|---|---|---|---|---|---|
| a Absorption and fluorescence spectra were measured in toluene solution at 10−5 M. b Measured as a neat film. c HOMO values were determined using the oxidative potentials of the materials with Fc/Fc+ as the reference. LUMO levels were estimated according to the equation, LUMO = HOMO − Eg. d The energy gap (Eg) was estimated from the absorption threshold. e PL quantum yields of the emitters were obtained using an integral sphere with an excitation wavelength of 330 nm. f PLQY in dilute toluene (10−5). g PLQY of the neat film. h PLQY of the doped film (10% in mCBP). | ||||||
| PyAnCN | 341, 377, 396 | 443 | 454 | −5.06/−2.06 | 3.0 | 57f/35g/54h |
Under high exciton density conditions (kTT[T(t)]2 ≫ kNR,T[T(t)]), the solution of eqn (1) becomes
 | (2) |
 | (3) |
 | (4) |
The device with a PyAnCN host shows a small TTA up-conversion saturation current density, and thus the rate constant of TTA up-conversion (kTT) and the rate of non-radiative triplet decay (kNR,T) can be calculated. The device with a PyAnCN host displays a fast rate constant of kTT ∼ 4.1 × 10−14 cm3 s−1 for TTA up-conversion, and a slow rate of kNR,T ∼ 3.4 × 104 s−1 for non-radiative triplet decay. The EL decay curve of the device with a PyAnCN host exhibits obvious two decays, which may contain more than one TTA up-conversion process. Fig. 3c and Table S3† display the energy levels of PyAnCN calculated at the level of B3LYP/6-31G(d) theory. Higher energy can be achieved through collisions and annihilation between the T1 and T1, T1 and T2, T2 and T2 states. Because of the low diffusion rate of the triplet excitons, the triplet excitons form TT pairs on the anthracene and pyrene groups adjacent to the molecule. 3(TT) and 5(TT) can convert quickly into a high-energy singlet state (Sm) through more degenerate channels. So, in this TTA up-conversion process, three TTA up-conversion channels (containing intramolecular TTA and intermolecular TTA) will be formed with spin-correlative triplet–triplet (TT) pairs. These channels may accelerate the TTA up-conversion process and reduce the non-radiative transitions (Fig. 3d). The multiple TTA up-conversion channels present mean that the transient EL decay curve of the device with a PyAnCN host exhibits two decays and more complex up-conversion processes.
Furthermore, the high-lying reverse intersystem crossing (hRISC) channel was investigated in the devices. The transient PL properties of the host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA films were measured to reveal the high-lying reverse intersystem crossing (hRISC) processes, as shown in Fig. 4. It can be seen that the decay curves of the films show two decay components, which can be well fitted by a double exponential decay function. For a more intuitive analysis, the rate constants were calculated according to the photophysical equations and the detailed results are shown in Tables S4 and S5.† It is seen that the PyAnCN
3% TBN-TPA films were measured to reveal the high-lying reverse intersystem crossing (hRISC) processes, as shown in Fig. 4. It can be seen that the decay curves of the films show two decay components, which can be well fitted by a double exponential decay function. For a more intuitive analysis, the rate constants were calculated according to the photophysical equations and the detailed results are shown in Tables S4 and S5.† It is seen that the PyAnCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA film presents two lifetimes, 3.1 and 29.5 ns, corresponding to the prompt and delayed lifetimes, respectively, with a large decay ratio. The MADN
3% TBN-TPA film presents two lifetimes, 3.1 and 29.5 ns, corresponding to the prompt and delayed lifetimes, respectively, with a large decay ratio. The MADN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA film presents also two lifetimes, 3.9 and 51 ns, for the prompt and delayed lifetimes, respectively, with a small decay ratio. Here, the large decay ratio (48.5%) is considered to be related to the fast Tn → Sm hRISC process (5.1 × 107 s−1 for PyAnCN), and the small decay ratio (13.8%) is considered to be related to the slow Tn → Sm hRISC process (0.9 × 107 s−1 for MADN). To gain insight into the hRISC process between the high-lying Tn and Sm states in the hosts, the electronic transition features of the Tn and Sm states were calculated (Fig. S8†). The slow rates of hISC and hRISC in the MADN host indicates that few triplet energy levels are close to the excited singlet energy levels, which is consistent with the calculated results. On the other hand, more triplet energy levels are close to the excited singlet energy levels in the PyAnCN host, and the degenerate triplet energy levels may accelerate the intersystem crossing and reverse intersystem crossing processes. Moreover, relatively large spin–orbit coupling matrix element (SOCME) values between the Tn and Sm states in PyAnCN were calculated (Table S6†). PyAnCN has a higher SOCME value (〈S1|ĤSOC|T5〉 = 0.2616 cm−1) than MADN (〈S1|ĤSOC|T4〉 = 0.10 cm−1),34 and the large value of SOCME in PyAnCN accelerates the hISC and hRISC processes. The temperature-dependent transient PL decay characteristics of PyAnCN are shown in Fig. 4b. The prompt and decay lifetimes show no obvious change as the temperature increases. The delayed component increases monotonically as the temperature increases because hRISC becomes the rate-determining step. The results calculated for khRISC are consist with the activation energy from exp(−ΔEST/kBT), where kB is the Boltzmann constant and T is the temperature (Table S7†). For comparison, the transient PL decay of the PAC
3% TBN-TPA film presents also two lifetimes, 3.9 and 51 ns, for the prompt and delayed lifetimes, respectively, with a small decay ratio. Here, the large decay ratio (48.5%) is considered to be related to the fast Tn → Sm hRISC process (5.1 × 107 s−1 for PyAnCN), and the small decay ratio (13.8%) is considered to be related to the slow Tn → Sm hRISC process (0.9 × 107 s−1 for MADN). To gain insight into the hRISC process between the high-lying Tn and Sm states in the hosts, the electronic transition features of the Tn and Sm states were calculated (Fig. S8†). The slow rates of hISC and hRISC in the MADN host indicates that few triplet energy levels are close to the excited singlet energy levels, which is consistent with the calculated results. On the other hand, more triplet energy levels are close to the excited singlet energy levels in the PyAnCN host, and the degenerate triplet energy levels may accelerate the intersystem crossing and reverse intersystem crossing processes. Moreover, relatively large spin–orbit coupling matrix element (SOCME) values between the Tn and Sm states in PyAnCN were calculated (Table S6†). PyAnCN has a higher SOCME value (〈S1|ĤSOC|T5〉 = 0.2616 cm−1) than MADN (〈S1|ĤSOC|T4〉 = 0.10 cm−1),34 and the large value of SOCME in PyAnCN accelerates the hISC and hRISC processes. The temperature-dependent transient PL decay characteristics of PyAnCN are shown in Fig. 4b. The prompt and decay lifetimes show no obvious change as the temperature increases. The delayed component increases monotonically as the temperature increases because hRISC becomes the rate-determining step. The results calculated for khRISC are consist with the activation energy from exp(−ΔEST/kBT), where kB is the Boltzmann constant and T is the temperature (Table S7†). For comparison, the transient PL decay of the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA film exhibits a moderate khRISC. When the transient PL properties of the PAC
3% TBN-TPA film exhibits a moderate khRISC. When the transient PL properties of the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x% PyAnCN
x% PyAnCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA films were measured, it was found that the khRISC of the PAC
3% TBN-TPA films were measured, it was found that the khRISC of the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20% PyAnCN
20% PyAnCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA film shows a higher decay ratio and faster hRISC rate than the PAC
3% TBN-TPA film shows a higher decay ratio and faster hRISC rate than the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% TBN-TPA films. When the PyAnCN doping concentration increases, the multi-component films show nearly the same decay ratio and PyAnCN dominates the hRISC process (Table S8†). The EL performances of the devices with different hosts are shown in Fig. S9 and Table S9.† High EQE deep blue OLEDs were achieved with small efficiency roll-off. Combined with the fast rate of TTA up-conversion in PyAnCN and the fast rate of hRISC in PAC, the PAC
3% TBN-TPA films. When the PyAnCN doping concentration increases, the multi-component films show nearly the same decay ratio and PyAnCN dominates the hRISC process (Table S8†). The EL performances of the devices with different hosts are shown in Fig. S9 and Table S9.† High EQE deep blue OLEDs were achieved with small efficiency roll-off. Combined with the fast rate of TTA up-conversion in PyAnCN and the fast rate of hRISC in PAC, the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN device displays an EQE of 10.3%, representing a further improvement. The transient EL characteristics were tested to further reveal the rate of TTA up-conversion and triplet non-radiative decay in the hosts (Fig. S10†). It is found that all hosts exhibit TTA up-conversion character with current density-dependent decay times and ratios. By fitting the EL decays, as shown, the host containing PyAnCN shows a large delayed emission ratio (Table S10†). The kTT, kNR,T, khISC and khRISC values of the hRISC and hISC processes were calculated using a previous model (Table S11†). It can be seen that the PAC
50% PyAnCN device displays an EQE of 10.3%, representing a further improvement. The transient EL characteristics were tested to further reveal the rate of TTA up-conversion and triplet non-radiative decay in the hosts (Fig. S10†). It is found that all hosts exhibit TTA up-conversion character with current density-dependent decay times and ratios. By fitting the EL decays, as shown, the host containing PyAnCN shows a large delayed emission ratio (Table S10†). The kTT, kNR,T, khISC and khRISC values of the hRISC and hISC processes were calculated using a previous model (Table S11†). It can be seen that the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN host shows high rates of TTA up-conversion and hRISC due to its TTA–HCLT character (Table 2).
50% PyAnCN host shows high rates of TTA up-conversion and hRISC due to its TTA–HCLT character (Table 2).
| EML | V on (V) | L max (cd m−2) | CEmax/CE1000c (cd A−1) | EQEmax/EQE1000d (%) | ELmaxe (nm) | CIEf (x, y) |
|---|---|---|---|---|---|---|
| a V on: turn-on voltage at a luminescence of 1 cd m−2. b L max: maximum luminescence. c CEmax: maximum current efficiency. d EQEmax/EQE1000: EQE maximum/at 1000 cd m−2. e ELmax: emission peak of EL spectrum at 6 V. f CIE coordinates at 6 V. | ||||||
PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN 50% PyAnCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% M-tDABNA 3% M-tDABNA |
3.6 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
10.3/10.2 | 11.4/11.3 | 462 | (0.13, 0.11) |
The working processes in the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN
50% PyAnCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3%-based OLEDs are presented in Fig. 5a. The multiple TTA up-conversion and high-lying reverse intersystem crossing channels should be dominant in these devices. In the resulting blue OLEDs, electrons and holes encounter and recombine simultaneously on the PAC and PyAnCN hosts during EML. The singlets on the host will convert to singlets of dopant with a fast Förster energy transfer rate. The triplets on the PAC
3%-based OLEDs are presented in Fig. 5a. The multiple TTA up-conversion and high-lying reverse intersystem crossing channels should be dominant in these devices. In the resulting blue OLEDs, electrons and holes encounter and recombine simultaneously on the PAC and PyAnCN hosts during EML. The singlets on the host will convert to singlets of dopant with a fast Förster energy transfer rate. The triplets on the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN host can convert into singlets with a fast high-lying reverse intersystem crossing channel and the more effective TTA up-conversion channel of PyAnCN due to the faster rate of TTA up-conversion. In this triplet harvesting channel, the triplet losses (internal conversion and triplet non-radiative decay) are well suppressed. The PAC
50% PyAnCN host can convert into singlets with a fast high-lying reverse intersystem crossing channel and the more effective TTA up-conversion channel of PyAnCN due to the faster rate of TTA up-conversion. In this triplet harvesting channel, the triplet losses (internal conversion and triplet non-radiative decay) are well suppressed. The PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN-based device combines the excellent TTA up-conversion and hRISC of PyAnCN and the carrier transport characteristics of PAC, thus achieving better EL device performance. Moreover, the deep blue dopant M-tDABNA and low refractive index ETL B3PyMPM were adopted to further optimize the device performance.35 The PAC
50% PyAnCN-based device combines the excellent TTA up-conversion and hRISC of PyAnCN and the carrier transport characteristics of PAC, thus achieving better EL device performance. Moreover, the deep blue dopant M-tDABNA and low refractive index ETL B3PyMPM were adopted to further optimize the device performance.35 The PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN-based device exhibits a deep blue color with CIE coordinates of (0.13, 0.11) and the EQEmax reaches 11.4% with a low efficiency roll-off of 11.3% at a luminance of 1000 cd m−2, which remains 10.7% even at a luminance of 5000 cd m−2. The EQE represents one of the best values among blue fluorescent OLEDs based on TTA/HLCT hosts (Fig. S11†). The higher EQE may also originate from the higher emitting dipole orientation of the emitting dopant. The horizontal dipole orientation ratio of PAC
50% PyAnCN-based device exhibits a deep blue color with CIE coordinates of (0.13, 0.11) and the EQEmax reaches 11.4% with a low efficiency roll-off of 11.3% at a luminance of 1000 cd m−2, which remains 10.7% even at a luminance of 5000 cd m−2. The EQE represents one of the best values among blue fluorescent OLEDs based on TTA/HLCT hosts (Fig. S11†). The higher EQE may also originate from the higher emitting dipole orientation of the emitting dopant. The horizontal dipole orientation ratio of PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN
50% PyAnCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3% M-tDABNA was measured to be as high as 0.90 (Fig. 5c). The fast rates of hRISC and TTA up-conversion restrain the undesired internal conversion and non-radiative triplet decay channels, thus realizing an efficient triplet harvesting process. The transient EL measurement results are presented in Fig. 5d. Benefiting from a large kTTA, the EL decay in the PAC
3% M-tDABNA was measured to be as high as 0.90 (Fig. 5c). The fast rates of hRISC and TTA up-conversion restrain the undesired internal conversion and non-radiative triplet decay channels, thus realizing an efficient triplet harvesting process. The transient EL measurement results are presented in Fig. 5d. Benefiting from a large kTTA, the EL decay in the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN-based device exhibits a high decay ratio at low current density. The ratio of the delayed emission exceeds 40% at a pulse current density of 30 mA cm−2 demonstrating the effective conversion of triplets to singlets by a more effective strategy, thus suppressing harmful triplet–polaron annihilation (TPA) and other exciton annihilation processes. To attain hRISC and TTA contributions, the EQEs of the devices were calculated and the relative contribution of all the relevant mechanisms of the exciton losses are plotted in Fig. S12,† assuming charge balance in the devices. At lower current densities, the dominating deactivation process for triplet states is non-radiative triplet decay, suggesting that a large number of triplets become deactivated during the triplet decay process. With increasing current density, the area corresponding to TTA up-conversion gradually increases and the TTA loss mechanism starts to dominate. The contribution of HLCT decreases from 16% to 9% with increasing current density, and the contribution of TTA increases from 6% to 14% in the PyAnCN-based OLED. It is clear that the HLCT significantly contributes to the fluorescence emission in the PAC
50% PyAnCN-based device exhibits a high decay ratio at low current density. The ratio of the delayed emission exceeds 40% at a pulse current density of 30 mA cm−2 demonstrating the effective conversion of triplets to singlets by a more effective strategy, thus suppressing harmful triplet–polaron annihilation (TPA) and other exciton annihilation processes. To attain hRISC and TTA contributions, the EQEs of the devices were calculated and the relative contribution of all the relevant mechanisms of the exciton losses are plotted in Fig. S12,† assuming charge balance in the devices. At lower current densities, the dominating deactivation process for triplet states is non-radiative triplet decay, suggesting that a large number of triplets become deactivated during the triplet decay process. With increasing current density, the area corresponding to TTA up-conversion gradually increases and the TTA loss mechanism starts to dominate. The contribution of HLCT decreases from 16% to 9% with increasing current density, and the contribution of TTA increases from 6% to 14% in the PyAnCN-based OLED. It is clear that the HLCT significantly contributes to the fluorescence emission in the PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN-based device, and the HLCT ratio rises from 16% to 19% compared with the PyAnCN-based device at 1 mA cm−2. With increasing current density, the area corresponding to HCLT gradually decreases, indicating that TTA up-conversion and HCLT compete with each other and TTA becomes dominant after the triplet exciton population increases. Devices based on the PyAnCN and PAC
50% PyAnCN-based device, and the HLCT ratio rises from 16% to 19% compared with the PyAnCN-based device at 1 mA cm−2. With increasing current density, the area corresponding to HCLT gradually decreases, indicating that TTA up-conversion and HCLT compete with each other and TTA becomes dominant after the triplet exciton population increases. Devices based on the PyAnCN and PAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50% PyAnCN hosts were fabricated for an extended lifetime, and the lifetime of the devices were nearly doubled compared to that of the MADN host device (Fig. S13†). We believe that our work will attract researchers in this field to find more strategies to increase the rate of TTA and hRISC, which will lead to the commercialization of deep-blue OLEDs that break through efficiency limits.
50% PyAnCN hosts were fabricated for an extended lifetime, and the lifetime of the devices were nearly doubled compared to that of the MADN host device (Fig. S13†). We believe that our work will attract researchers in this field to find more strategies to increase the rate of TTA and hRISC, which will lead to the commercialization of deep-blue OLEDs that break through efficiency limits.
3. Conclusion
Deep-blue fluorescent OLEDs based on a newly designed organic emitter with a fast TTA up-conversion and high-lying reverse intersystem crossing channels were developed, and the maximum EQE was improved to 11.4%. It was found that highly efficient triplet harvesting was realized due to the fast TTA up-conversion and HLCT channels. A theoretical model explains the experimental results and clearly demonstrates that the TTA contribution with a fast TTA up-conversion rate reached saturation at low current density. With fast TTA up-conversion and high-lying reverse intersystem crossing channels, the utilization of triplet excitons was greatly improved and the non-radiative triplet losses were significantly suppressed. These findings will create new opportunities for the development of high-efficiency deep-blue OLEDs.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
All authors contributed to the data analysis, discussion and revision of the manuscript. Jianwen Qin designed and synthesized the molecules and contributed to the solution photophysics, electrochemistry and density-functional theory calculations. Jianwen Qin and Shu Xiao fabricated and measured the OLEDs and performed the majority of the solid-state photophysics. Jianwen Qin and Dongge Ma wrote the manuscript. Xianfeng Qiao and Dongge Ma coordinated the project. Dezhi Yang, Yanfeng Dai, Jiangshan Chen and Qian Sun contributed to the data analysis. Dongge Ma supervised and conceived the research.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the support by the National Key R&D Program of China (2022YFA1204404), and the Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, China (2019B030301003).References
-
(a) Y. Im, S. Y. Byun, J. H. Kim, D. R. Lee, C. S. Oh, K. S. Yook and J. Y. Lee, Adv. Funct. Mater., 2017, 27, 1603007 CrossRef
; (b) P. Keerthika and R. K. Konidena, Adv. Opt. Mater., 2023, 11, 2301732 CrossRef CAS
; (c) J. Y. Woo, M. H. Park, S. H. Jeong, Y. H. Kim, B. Kim, T. W. Lee and T. H. Han, Adv. Funct. Mater., 2023, 35, 2207454 CrossRef CAS
.
-
(a) X. Yang, G. Zhou and W. Y. Wong, Chem. Soc. Rev., 2015, 44, 8484–8575 RSC
; (b) C. Poriel and J. Rault-Berthelot, Adv. Funct. Mater., 2021, 31, 2010547 CrossRef
.
-
(a) B. Minaev, G. Baryshnikov and H. Agren, Phys. Chem. Chem. Phys., 2014, 16, 1719–1758 RSC
; (b) M. Idris, S. C. Kapper, A. C. Tadle, T. Batagoda, D. S. Muthiah Ravinson, O. Abimbola, P. I. Djurovich, J. Kim, C. Coburn, S. R. Forrest and M. E. Thompson, Adv. Opt. Mater., 2021, 9, 2001994 CrossRef
.
- Q. Wang, J. Ding, D. Ma, Y. Cheng, L. Wang and F. Wang, Adv. Mater., 2009, 21, 2397–2401 CrossRef
.
-
(a) L.-K. Li, M.-C. Tang, S.-L. Lai, M. Ng, W.-K. Kwok, M.-Y. Chan and V. W.-W. Yam, Nat. Photonics, 2019, 13, 185–191 CrossRef
; (b) J. Song, H. Lee, E. G. Jeong, K. C. Choi and S. Yoo, Adv. Mater., 2020, 32, 1907539 CrossRef PubMed
.
- J.-Y. Hu, Y.-J. Pu, F. Satoh, S. Kawata, H. Katagiri, H. Sasabe and J. Kido, Adv. Funct. Mater., 2014, 24, 2064–2071 CrossRef
.
- D. Di, L. Yang, J. M. Richter, L. Meraldi, R. M. Altamimi, A. Y. Alyamani, D. Credgington, K. P. Musselman, J. L. MacManus-Driscoll and R. H. Friend, Adv. Mater., 2017, 29, 1605987 CrossRef
.
- S. Zhang, L. Yao, Q. Peng, W. Li, Y. Pan, R. Xiao, Y. Gao, C. Gu, Z. Wang, P. Lu, F. Li, S. Su, B. Yang and Y. Ma, Adv. Funct. Mater., 2015, 25, 1755–1762 CrossRef
.
-
(a) H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef
; (b) N. Li, F. Ni, X. Lv, Z. Huang, X. Cao and C. Yang, Adv. Opt. Mater., 2021, 10, 2101343 CrossRef
.
- Z. Yang, Z. Mao, Z. Xie, Y. Zhang, S. Liu, J. Zhao, J. Xu, Z. Chi and M. P. Aldred, Chem. Soc. Rev., 2017, 46, 915–1016 RSC
.
- X. Qiao and D. Ma, Mater. Sci. Eng., R, 2020, 139, 100519 CrossRef
.
- A. Monguzzi, J. Mezyk, F. Scotognella, R. Tubino and F. Meinardi, Phys. Rev. B:Condens. Matter Mater. Phys., 2008, 78, 195112 CrossRef
.
- D. Y. Kondakov, J. Soc. Inf. Disp., 2009, 17, 137 CrossRef CAS
.
- C. Ye, L. Zhou, X. Wang and Z. Liang, Phys. Chem. Chem. Phys., 2016, 18, 10818–10835 RSC
.
- X. Guo, Y. Liu, Q. Chen, D. Zhao and Y. Ma, Adv. Opt. Mater., 2018, 6, 1700981 CrossRef
.
- H. Lim, S. J. Woo, Y. H. Ha, Y. H. Kim and J. J. Kim, Adv. Mater., 2022, 34, e2100161 CrossRef PubMed
.
- S. Xiao, X. Qiao, C. Lin, L. Chen, R. Guo, P. Lu, L. Wang and D. Ma, Adv. Opt. Mater., 2022, 10, 2102333 CrossRef CAS
.
- H. Jung, S. Kang, H. Lee, Y. J. Yu, J. H. Jeong, J. Song, Y. Jeon and J. Park, ACS Appl. Mater. Interfaces, 2018, 10, 30022–30028 CrossRef CAS
.
- W. Liu, S. Ying, R. Guo, X. Qiao, P. Leng, Q. Zhang, Y. Wang, D. Ma and L. Wang, J. Mater. Chem. C, 2019, 7, 1014–1021 RSC
.
- Y. H. Chen, C. C. Lin, M. J. Huang, K. Hung, Y. C. Wu, W. C. Lin, R. W. Chen-Cheng, H. W. Lin and C. H. Cheng, Chem. Sci., 2016, 7, 4044–4051 RSC
.
- M. J. Sung, H. Chubachi, R. Sato, M.-K. Shin, S.-K. Kwon, Y.-J. Pu and Y.-H. Kim, J. Mater. Chem. C, 2017, 5, 1090–1094 RSC
.
- T. Shan, Z. Gao, X. Tang, X. He, Y. Gao, J. Li, X. Sun, Y. Liu, H. Liu, B. Yang, P. Lu and Y. Ma, Dyes Pigm., 2017, 142, 189–197 CrossRef CAS
.
- H. Lim, H. J. Cheon, G. S. Lee, M. Kim, Y. H. Kim and J. J. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 48121–48127 CrossRef CAS
.
- X. Guo, P. Yuan, X. Qiao, D. Yang, Y. Dai, Q. Sun, A. Qin, B. Z. Tang and D. Ma, Adv. Funct. Mater., 2020, 30, 1908704 CrossRef
.
- C. Lin, P. Han, S. Xiao, F. Qu, J. Yao, X. Qiao, D. Yang, Y. Dai, Q. Sun, D. Hu, A. Qin, Y. Ma, B. Z. Tang and D. Ma, Adv. Funct. Mater., 2021, 31, 202106912 Search PubMed
.
- Y. Luo, K. Zhang, Z. Ding, P. Chen, X. Peng, Y. Zhao, K. Chen, C. Li, X. Zheng, Y. Huang, X. Pu, Y. Liu, S.-J. Su, X. Hou and Z. Lu, Nat. Commun., 2022, 13, 6892 CrossRef PubMed
.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef PubMed
.
- J. Lim, J. M. Kim and J. Y. Lee, Adv. Mater., 2024, e2312774 CrossRef PubMed
.
- X. Wang, L. Wang, G. Meng, X. Zeng, D. Zhang and L. Duan, Sci. Adv., 2023, 9, eadh1434 CrossRef PubMed
.
- T. B. Nguyen, H. Nakanotani, C. Y. Chan, S. Kakumachi and C. Adachi, ACS Appl. Mater. Interfaces, 2023, 15, 23557 CrossRef PubMed
.
- C. Kuang, S. Li, I. Murtaza, Z. Meng, H. Li, X. Zhang, C. Wu, K. N. Tong, Y. Shang, Y. He, Y. Zhu, G. Wei and H. Meng, Small, 2023, e2311114 Search PubMed
.
- J. Park, K. J. Kim, J. Lim, T. Kim and J. Y. Lee, Adv. Mater., 2022, 34, 2108581 Search PubMed
.
- X. Liang, Z. P. Yan, H. B. Han, Z. G. Wu, Y. X. Zheng, H. Meng, J. L. Zuo and W. Huang, Angew. Chem., 2018, 57, 11316–11320 CrossRef PubMed
.
- W. Cai, W. Li, X. Song, X. Zheng, H. Guo, C. Lin, D. Yang, D. Ma, M. Ng and M. C. Tang, Adv. Sci., 2024, e2407278 CrossRef PubMed
.
- D. Yokoyama, J. Mater. Chem. C, 2011, 21, 19187 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06232a |
| This journal is © The Royal Society of Chemistry 2025 |