Enhancing students’ agency and coherence in organic chemistry through transformed problem design
Julia
Eckhard
 ,
Rebecca A.
Scheck
,
Rebecca A.
Scheck
 and
Ira
Caspari-Gnann
and
Ira
Caspari-Gnann
 *
*
Tufts University, Medford, MA 02155, USA. E-mail: ira.caspari@tufts.edu
First published on 23rd September 2025
Abstract
Research on mechanistic reasoning in Organic Chemistry has progressed in supporting students’ mechanistic reasoning and understanding how epistemic norms influence students’ personal epistemologies and their mechanistic reasoning practice as individuals. However, not much is known about students’ collaborative knowledge-building as a discursive practice in the moment of their learning in mechanistic reasoning classrooms. Thus, our study focuses on how different problem designs impact students’ knowledge-building in whole class discussions. We use design research comparing the impact of different problem designs, i.e., single-case vs. case-comparison complex mechanisms tasks, in two semesters of a “Mechanistic Reasoning in Organic Chemistry” class for graduate and upper-level undergraduate students. To gain a deeper understanding of how students’ knowledge-building happens in their interactions with the instructor, we draw on sociocultural theory and make use of two specific constructs: (1) “epistemic agency” as power to shape knowledge-building and (2) “coherence” referring to the logical and consistent connection of ideas. Our findings show how transforming complex mechanism tasks from single-case to case-comparison problems provide students with different opportunities to enact epistemic agency and engage in coherent reasoning during discourse. Our findings have implications for developing instructional practices and resources to encourage meaningful, collaborative knowledge-building in mechanistic reasoning classrooms.
Introduction
Mechanistic reasoning in organic chemistry
The practice of mechanistic reasoning is central to problem-solving in Organic Chemistry. Mechanistic reasoning allows students to approach problems in a deeper way, not only uncovering what is happening descriptively, but also how and why it transpires (Machamer et al., 2000; Russ et al., 2008; Cooper, 2015; Cooper et al., 2016). As such, mechanistic reasoning is a powerful knowledge-building tool that supports critical thinking, informed decision making, and clear communication of complex ideas (Krist et al., 2019).In Organic Chemistry, mechanistic reasoning structurally entails breaking down phenomena and processes into entities (e.g., starting materials, intermediates, products, molecules, and atoms), identifying their organization and interactions (e.g., electrophilic and nucleophilic interactions), decoding properties by moving beyond the surface level of structural representations (e.g., electron-rich and electron-poor centers in functional groups), and considering the influences of multiple properties on these phenomena and processes (e.g., ability to stabilize a transition state) (Machamer et al., 2000; Goodwin, 2003; Russ et al., 2008; Caspari et al., 2018a; Moreira et al., 2019). With that, one is enabled to build cause-and-effect relationships that encompass the structural and energetic account explaining, understanding, and predicting phenomena and processes (Goodwin, 2007; Caspari et al., 2018a).
A large body of research shows that in traditional learning environments, students tend not to engage in using mechanistic reasoning in their problem-solving (for reviews see: Graulich, 2015; Dood and Watts, 2022a, b; Graulich, 2025). Instead, common problem-solving approaches include a focus on explicit surface-level features instead of building cause-effect relationships that build on implicit properties (Anderson and Bodner, 2008; Anzovino and Bretz, 2016; Bhattacharyya and Harris, 2017; Popova and Bretz, 2018; Graulich et al., 2019; Rodemer et al., 2020). Further, often students chose to consider only one variable rather than weighing alternatives and engaging in multivariate reasoning (Kraft et al., 2010; Bodé et al., 2019; Deng and Flynn, 2021; Eckhard et al., 2022). Additionally, students’ approaches were characterized as product-oriented instead of process-oriented (Bhattacharyya and Bodner, 2005; Caspari et al., 2018b).
Instructional approaches including problem design
These well-known challenges have inspired a range of instructional approaches that explicitly support students’ mechanistic reasoning. Prior work has focused on curriculum redesign (Cooper and Klymkowsky, 2013; Flynn and Ogilvie, 2015; Galloway et al., 2017; Crandell et al., 2018; Webber and Flynn, 2018; Cooper et al., 2019), the development of “writing-to-learn” activities (Schmidt-McCormack et al., 2019; Gupte et al., 2021; Zaimi et al., 2024), multimedia tools (Bongers et al., 2020; Eckhard et al., 2022; Bernholt et al., 2023), or specially designed problems and scaffolds (Graulich and Schween, 2018; Bodé et al., 2019; Caspari and Graulich, 2019; Lieber and Graulich, 2020; Keiner and Graulich, 2021; Watts et al., 2021; Kranz et al., 2023).With regards to problem design, it is known that traditional problem designs (like predict-the-product or predict-the-mechanism) often lead students to stating correct answers, without engaging in mechanistic reasoning (for reviews see: Graulich, 2015; Dood and Watts, 2022a, b; Graulich, 2025). When solving these problems, students were found to rely on strategies like rote-memorization (Grove and Bretz, 2012; DeFever et al., 2015) or use of heuristics without underlying conceptual understanding (Bhattacharyya and Bodner, 2005; Maeyer and Talanquer, 2010; Graulich et al., 2012; DeFever et al., 2015). With the known challenges students face when solving traditional problems, researchers developed transformed problems with design features that engage students in mechanistic reasoning. Problems with specific design features involve, for example, comparing alternatives, such as case comparisons (Graulich and Schween, 2018; Bodé et al., 2019; Caspari and Graulich, 2019; Lieber and Graulich, 2020; Watts et al., 2021; Kranz et al., 2023) or card-sorting problems (Graulich, 2014; Irby et al., 2016; Krieter et al., 2016; Galloway et al., 2019). These problems encourage students to identify implicit properties, weigh alternatives, and argue based on contrasting features (Irby et al., 2016; Caspari et al., 2018a; Graulich and Schween, 2018; Galloway et al., 2019; Lieber and Graulich, 2020; Rodemer et al., 2020; Watts et al., 2021), fostering a shift from surface-level approaches to deeper reasoning. The use of more complex (or unfamiliar) problems was also found to reduce reliance on rote memorization. Instead, such problems challenge students to adopt more analytical strategies, drawing on conceptual knowledge during problem-solving (Grove et al., 2012; Flynn, 2014; Webber and Flynn, 2018; Lieber and Graulich, 2020; Houchlei et al., 2021; Helix et al., 2022; Blackford et al., 2023). Further, structured or scaffolded prompts that explicitly ask students to provide evidence for their claims encourage students to justify their ideas with increased use of cause-effect relationships (Crandell et al., 2018; Bodé et al., 2019; Flynn, 2021; Petritis et al., 2021; Crowder et al., 2024). The described efforts in instructional approaches make an important contribution to the field in supporting and eliciting students’ mechanistic reasoning. And while these studies involve an in-depth analysis of quality and structure of students’ mechanistic reasoning, other studies have started to focus on how epistemological messages during instruction and in instructional materials impact students’ personal epistemologies and thus their engagement in mechanistic reasoning as a practice.
Research on epistemology in chemistry education
Epistemology is the theory of knowledge and knowing and individuals’ epistemologies determine how and why someone acquires knowledge (Greco, 1999; Kelly et al., 2012). Research on epistemology in chemistry education has ramped up in the last century and focused, for example, on epistemic norms conveyed in instructors’ beliefs and utterances (Gibbons et al., 2018; Popova et al., 2021; DeGlopper et al., 2023), curricular materials (Bowen et al., 2022; Schafer et al., 2023), or assessments (Schwarz et al., 2024). Together, these studies describe that traditional learning environments often convey the epistemic norm that only knowledge products approved by an authority (e.g., by the instructor) are considered valuable. In these environments, students tend to engage in performative practices aimed at pleasing the said authority. The resulting knowledge products are therefore framed to be more “expert-like”, rather than meaningful to the student or reflective of their personal understanding (Schwarz et al., 2024). For example, introductory Organic Chemistry students expressed their personal epistemologies in response to traditional course assessments in interviews as trying to match the instructors expected answer instead of making sense of the questions in a way that is meaningful to them (Schwarz et al., 2024). A related study by Flaherty (2020) evaluated the impact of a redesigned curriculum in Organic Chemistry (OCLUE) (Cooper and Klymkowsky, 2013; Cooper et al., 2019) on students’ beliefs about learning and developing scientific knowledge. They found that the transformed course design shifted the students’ approach from needing to memorize to a building up process that allowed them to authentically piece together different parts of what they already knew (Flaherty, 2020). These findings highlight how instruction influences students’ consideration on how to use and evaluate knowledge, e.g., if they perceive it as valuable to memorize facts and build explanations to please an authority in an expert-like way or whether they perceive it as valuable to engage in mechanistic reasoning as a practice because it is meaningful to them.Positioning this study in the literature
In summary, while research in Organic Chemistry has progressed in analyzing and supporting students’ mechanistic reasoning and understanding how epistemic norms influence students’ personal epistemologies and thus their engagement in mechanistic reasoning as a practice of an individual, Graulich's (2025) recent review on advances in mechanistic reasoning has called for a focus on mechanistic reasoning in classroom interactions. Graulich (2025) argues that the field needs greater understanding of “h[H]ow teaching practices in the classroom and learning are aligned or happen in the actual moment of classroom discourse” (Graulich, 2025, p. 365). Thus, our study focuses on how problem design impacts students’ engagement in knowledge-building as a discursive practice†in classroom interactions between instructors and students, with mechanistic reasoning as part of this knowledge-building practice.Instead of using student written work and think-aloud interviews during which students individually solve mechanism problems (which gave researchers deep insight into individual students’ mechanistic reasoning) or student interviews about their experiences with instruction (which allowed researchers to learn about the influence of student personal epistemologies on their engagement in mechanistic reasoning as a practice), here, we use design research (Sandoval, 2014) that compares the impact of different problem designs in two semesters of a “Mechanistic Reasoning in Organic Chemistry” class on whole class discussions involving the instructors and the students. To gain deep insight into knowledge-building as a discursive practice in these whole class discussions, we draw on sociocultural theory as a framework to understand knowledge-building as a social endeavor in a community. Specifically, we draw on two constructs that distinguish mechanistic reasoning of individual students from knowledge-building as a discursive practice in classroom interactions: (1) We focus on “epistemic agency” as students’ power to shape knowledge-building practice in the classroom community (Scardamalia and Bereiter, 1991; Stroupe, 2014; Miller et al., 2018), (2) we hone in on “coherence” referring to the logical and consistent connection of ideas (BouJaoude, 1991; Taber and Watts, 2000). Note that coherence is in relation to, but not dependent on, the building of cause-effect relationships (Hempel and Oppenheim, 1948; Thagard, 1989), which is a central component of mechanistic reasoning (Cooper et al., 2016; Weinrich and Talanquer, 2016; Caspari et al., 2018a). Thus, while coherence is in relationship to mechanistic reasoning, our study is ultimately not concerned with whether a portion of knowledge-building in classroom discourse is mechanistic or not. Instead the construct of coherence allows us to focus on what makes knowledge-building in a community different from reasoning as an individual because coherence is a way of how members of a knowledge-building community make their individual intellectual work public and collaborate to build deeper shared understanding (Zhang et al., 2009; Damşa et al., 2010; Sandoval et al., 2016). We extend on this theoretical framing in the next section.
Theoretical framing
Knowledge-building and epistemology from a sociocultural perspective
In this study, we draw on sociocultural theory to understand knowledge-building as a social endeavor within a community. According to sociocultural theory, learning is historically and culturally situated, socially mediated through cultural artifacts, tools, and language, and it occurs in interactions within activity systems (Vygotsky, 1978; Engeström, 1999; Roth and Lee, 2007). In these interactions within activity systems, social norms are negotiated through the actors within a community. These norms shape not only which, but also whose, ideas are brought to the fore and accordingly which ideas are positioned as valuable (Engeström, 1999; Nasir and Hand, 2006; Lektorsky, 2009).Within this sociocultural framing, epistemology is understood as a social practice (Lidar et al., 2006; Nasir and Hand, 2006; Kelly et al., 2012). Kelly et al. (2012) note in their work on the relationship between science learning and epistemology that engagement in scientific practices is influenced by disciplinary epistemologies, personal epistemologies, and epistemologies as a social practice (Kelly et al., 2012). Here, we focus on studying epistemologies as a social practice which “entails seeing epistemology as constituted through situated interaction” (Kelly et al., 2012, p. 285). How epistemology is constituted through interactions can be investigated using “Practical Epistemology Analysis (PEA)” (Wickman and Östman, 2002; Wickman, 2004). A practical epistemology “is a description of what the students themselves, in their practice, count as relevant knowledge, and what they count as relevant means of attaining knowledge” (Lidar et al., 2006, p. 149). PEA then studies how actors of a community in an activity, e.g., instructors and students in discourse, build, negotiate, and share meaning from moment to moment (Wickman and Östman, 2002; Wickman, 2004; Hamza and Wickman, 2008; Karch et al., 2024; Maggiore et al., 2024). By using PEA (see Methods) to understand mechanistic reasoning classrooms as knowledge-building communities (Scardamalia and Bereiter, 2003; Zhang et al., 2009; Manz, 2016; Scardamalia and Bereiter, 2021), our study provides insights into how knowledge is built from moment to moment, focusing on the agency students have to engage in these practices and the way they contribute to coherent knowledge-building.
Epistemic agency as key for community knowledge-building
Engagement in knowledge-building practices inherently involves the construct of “epistemic agency” (Scardamalia and Bereiter, 1991; Scardamalia, 2002; Zhang et al., 2009; Damşa et al., 2010; Scardamalia and Bereiter, 2021). Following a sociocultural perspective (Engeström, 1999), “agency” is the ability to act in a goal-directed way towards objects using mediating artifacts, while “epistemic” refers to generation and advancement of ideas (Damşa et al., 2010). With this perspective, we characterize “epistemic agency” as someone's knowledge-driven contributions in an activity towards knowledge-objects, e.g., predicting the most plausible mechanism. Contributions to knowledge-objects necessitate actors in an activity, e.g., students with other students or the instructor, to notice a “gap” or “need” for new relations to make something intelligible to proceed with the activity (Wickman and Östman, 2002; Wickman, 2004; Roth and Lee, 2007). These “gaps” in practical epistemology research are thereby not considered gaps in conceptual knowledge but are socially situated and contextualized (Wickman and Östman, 2002; Wickman, 2004). Students or instructors who notice and fill gaps in classroom discourse make knowledge-driven contributions towards a knowledge-object, and thus, who notices and fills gaps demonstrates who has epistemic agency. We herein characterize students’ epistemic agency, specifically, as “students being positioned with, perceiving, and acting on, opportunities to shape the knowledge building work in their classroom community” (Miller et al., 2018, p. 1058).Analyzing classroom communities and instructors’ facilitation, Stroupe (2014) investigated how beginner teachers and students negotiated epistemic agency. Stroupe (2014) argues that “most science instruction, which I refer to as “conservative,” positions the teacher as the sole instructional, knowledge, and practice authority—the only epistemic agent in a classroom” (Stroupe, 2014, p. 488). In these classrooms, students are rarely positioned with epistemic agency, as underlying power structures assign epistemic agency and authority to instructors rather than to students (Stroupe, 2014; Varelas et al., 2015; Baze and González-Howard, 2025). Stroupe (2014) further argues that in “conservative classrooms” it is likely that students are positioned as individual knowers by the instructor. This leads to “keeping the work of science private” (Stroupe, 2014, p. 494) and hidden from the classroom community. In the actual moment of knowledge-building discourse, which we refer to as “in-the-moment learning” (Walsh et al., 2022; Karch et al., 2024), this privacy of doing science inhibits “the collaborative process of negotiating meanings, understanding, and knowledge as they [students] come into contact with discursive and physical mediating artifacts” (Karch, et al., 2024, p. 1296). Similarly, Damşa et al. (2010) state that engagement in knowledge-building communities “requires combining individual and collective contributions and learners becoming actively involved in the materialization of ideas in order to give conceptual artifacts a concrete shape and to create a tangible representation of what they are making” (Damşa et al., 2010, p. 148). When students have epistemic agency in the classroom “the science work is likely public, since students’ ideas shape the classroom activity” (Stroupe, 2014, p. 494).
In addition to student agency, this publicity of science work and the collaborative negotiation of meaning necessitates that the internal logic of ideas needs to be made explicit in coherent explanations, which we elaborate on in the next section.
Coherence as key for community knowledge-building
Coherence refers to logical and consistent connection of ideas (BouJaoude, 1991; Taber and Watts, 2000). The coherence of an explanation is in relation to but not dependent on causality (e.g., empirically valid cause-effect relations) (Hempel and Oppenheim, 1948; Salmon, 1984; Thagard, 1989); instead it refers to internal consistency, e.g., combining ideas in a logical manner without contradictions. When explanations are coherent, the internal logic of the ideas is evident (Thagard, 1989; Mackonis, 2013). In knowledge-building communities this helps actors to follow, to understand, and to compare these ideas with their own and to expand their knowledge with the ideas, which leads to collaborative knowledge-building. Zhang et al. (2009) argue for complementary contributions that will advance the knowledge-building community and state that this is the “antithesis of much schoolwork in which students are all doing the same thing, with no idea diversity to drive the need for explanatory coherence” (Zhang et al., 2009, p. 11). They claim that in comparison in knowledge-building communities “students build on one another's idea contributions and then rise above to find increasingly high-level accounts, helping to create the coherence that drives them toward deeper understanding” (Zhang et al., 2009, p. 11). Hence, not only are whole class discussions important opportunities for students to take epistemic agency and shape the knowledge-building process, but they are also opportunities to engage in collaborative knowledge-building through explicitly providing ideas that are logical to other members of the community.With this theoretical framing, our study analyzes practical epistemologies in interactions between instructors and students in mechanistic reasoning discourse to characterize engagement in knowledge-building. Specifically, we focus on the constructs of “epistemic agency” and “coherence” as lenses to characterize how meaningful knowledge-building can take place in mechanistic reasoning classrooms.
Research goals and questions
Our goal is to contribute to the field of mechanistic reasoning in Organic Chemistry education research by moving beyond a description of individual student's mechanistic reasoning and how their epistemologies impact that mechanistic reasoning towards a deeper understanding of how students’ knowledge-building as a discursive practice happens in the actual moment of classroom interactions between instructors and students. We do so through design research comparing the impact of different problem designs in a “Mechanistic Reasoning in Organic Chemistry” class on whole class discussions between instructors and students. To shed light on aspects that distinguish student collaborative knowledge-building during classroom interactions from individual student reasoning, we use a sociocultural perspective, and focus on epistemic agency, i.e., the power to position one's own intellectual work as meaningful in classroom interactions, and coherence, i.e., the making explicit of internal logic allowing for collaborative knowledge-building.The following research question guided our investigation: How do different problem designs impact epistemic agency and coherence in student–instructor interactions in whole class discussions?
Methods
Study context
This study is part of a larger design research project (Sandoval, 2014) that seeks to explore ways to engage students in the scientific practice of mechanistic reasoning for creative and independent problem-solving in chemistry classrooms and to support their equitable participation in scientific environments. The project aims to build theory around the connections between dynamics in student–student interactions in small group discussions (SGD) (research in progress) as well as in student–instructor interactions in whole class discussions (WCDs) (this study) and cohesive learning in Organic Chemistry. The focal course in this project is a “Mechanistic Reasoning in Organic Chemistry” course taken by upper-level undergraduate Chemistry majors and Chemistry graduate students at a private, R1, predominantly white university in the Northeast region of the United States. This course takes place each fall semester; and 26 lectures including 7 problem-solving sessions (PSSs) are held throughout the semester. During the PSSs students are asked to solve mechanism problems collaboratively in small group discussions (SGDs) and then bring their ideas to the whole class discussion (WCD) that directly follows. Herein, we focus on the design and the transformation of these problems in two semesters, 2021 and 2023, to explore the impact of these designs on students’ knowledge-building.A design research approach in a mechanistic reasoning class
The course “Mechanistic Reasoning in Organic Chemistry” follows a design research approach with iterative cycles (Sandoval, 2014). The second and third authors of this paper collaborated using their research, content, and pedagogy expertise to transform the course from a traditional lecture, teaching a collection of separate topics, into an interactive course aiming for student-centered, cohesive learning in Organic Chemistry.To structure the design research approach and connect theoretical ideas to specific design elements and observable outcomes, we used conjecture mapping (Sandoval, 2014) (Fig. 1). Conjecture mapping is “a means of specifying theoretically salient features of a learning environment design and mapping out how they are predicted to work together to produce desired outcomes” (Sandoval, 2014, p. 19). When developing the conjecture map for our design research (Fig. 1), we started with high-level conjectures that lay out our “theoretically principled idea of how to support a desired form of learning” (Sandoval, 2014, p. 22).
 | ||
| Fig. 1 Conjecture mapping of 2021 (top) and 2023 (bottom) for our design research project (Sandoval, 2014). | ||
For 2021 (Fig. 1, top), the high-level conjecture that guided the design of the course and problems was based on our goal to engage students in mechanistic reasoning and to provide them opportunities for participating in collaborative knowledge-building as epistemic agents (Fig. 1, top, Box 1). Thus, our conjecture entailed: (a) students use mechanistic reasoning when problems demand it, (b) students act as epistemic agents sharing coherent contributions in discourse if provided opportunities in instruction. Our conjecture became reified in the embodiment (Fig. 1, top, Box 2), in which we (a) purposefully selected and developed complex “predict the mechanisms problems” (see next section and Appendix Fig. 15–19), and (b) ensured opportunities for collaborative problem-solving during class time. As mediating processes (Fig. 1, top, Box 3), we anticipated that students would not be able to match simpler canonical reactions exactly to the complex problems so they would need to engage in mechanistic reasoning as their tool for knowledge-building and that collaborative work would actively engage students as epistemic agents in that practice. As expected outcomes, we hoped for a high degree of student epistemic agency and coherent contributions of how they came to know their proposed predictions of mechanisms were plausible (Fig. 1, top, Box 4).
Our analysis of the data in 2021 did not show us the degree of student agency and coherence that we had hoped for. This was tightly connected to the problem design (see Result and Discussion section), which is why we changed the problem design for 2023 (see next section and Appendix Fig. 20–24)
The conjecture map for 2023 was similar as the one for 2021, with some important changes (Fig. 1, bottom). In 2023, the embodiment also included opportunities for collaborative problem-solving during class time, but the problems were transformed. Specifically, compared to 2021, we changed the prompts, asking students to predict the most likely mechanism with given alternatives for mechanistic pathways, key steps, or intermediates (Fig. 1, bottom, Box 2). With the use of case-comparison problems, we also anticipated a change in mediating processes (Fig. 1, bottom, Box 3). We expected that through comparing and contrasting alternative mechanistic pathways, keys steps, or intermediates, students would need to justify their decisions and weigh different lines of reasoning and thus engage in enhanced mechanistic reasoning compared to the single-case problems in 2021. This engagement was hoped to build coherence for how they came to know. This in turn, was anticipated to lead them to rely more on their own reasoning and share more with others as epistemic agents why their suggestions are plausible. The conjectured outcome was a higher degree of student epistemic agency and coherence compared to 2021 (Fig. 1, bottom, Box 4).
In both years, the class, including the PSSs, was taught by the same main instructor. A secondary instructor observed the PSSs and taught one PSS in 2023, i.e., PSS2. The main instructor of the class had weekly discussions with the research team to reflect on the implementation of the problem designs in both semesters and they also met several times between the two semesters. During these meetings between the two semesters, they developed the 2023 problem design collaboratively.
Problem design features
The use of unfamiliar and complex problems has been shown to engage students to higher extent in mechanistic reasoning as they rely more on analytical strategies compared to problems that can be solved with memorization of simple canonical reactions (Flynn, 2014; Webber and Flynn, 2018; Lieber and Graulich, 2020; Houchlei et al., 2021; Blackford et al., 2023). Therefore, in 2021 we purposefully selected and developed complex “predict-the-mechanisms problems” (seeFig. 2 for an example, and Appendix Fig. 15–19 for all problems with references). Each problem prompted students to predict the mechanism for a given polar or mixed pericyclic/polar transformation. | ||
| Fig. 2 Example problem (top) and one possible key answer in which we show a possible mapping approach and arrow-pushing for the transformation shown in PSS4 in 2021. Problem adapted from Francis's (2004) teaching materials based on Martinet's et al. (1969, 1970, 1971) initial example of a Pinacol-terminated Prins cyclization, adapted with permission from Overman and Pennington (2003). Copyright 2003 American Chemical Society. | ||
The problems were not designed for students to simply match canonical named reactions to the given transformation, but rather to engage students in mechanistic reasoning. For instance, to solve the problems, students had to predict unknown pathways, infer nucleophilic and electrophilic reactivity, consider and combine typical reaction patterns or reaction types (like eliminations, alkyl migrations, or sigmatropic rearrangements), and evaluate multiple plausible steps and alternative pathways with differing energies. While in many cases the solution involved a complex named reaction or a combination of them (e.g., Prins-Pinacol cyclization, Eschenmoser-Claisen rearrangement), students were not expected to be familiar with those named reactions. For example, the problem used in PSS4 in 2021 (Fig. 2) was designed to engage students in mechanistic reasoning involving multiple layers of complexity. First, it required reasoning about the role of acetone, to determine whether it acts as a reagent or only a solvent. Here students were expected to use strategies like mapping atoms of reactants to products, which they were taught in the lecture portion of the class, to confirm the involvement of certain reactants. Additionally, the problem also prompted students to reason about the function of the H+ catalyst (e.g., which reactant or functional group gets protonated, when, and why) and how a potential activation through protonation enables a subsequent nucleophilic attack. Furthermore, the problem integrated multiple reaction patterns, including an electrophilic addition of acetone to an alkene (step F, Prins reaction), followed by an intramolecular rearrangement (step G, Pinacol rearrangement). Also, it required students to consider the orientation of HOMO–LUMO interactions during the rearrangement and how it affects the stereochemistry of the resulting product. Hence, solving this problem requires more than simple recall or memorization of one reaction mechanism; it demanded making use of mechanistic reasoning.
In 2023 the problems were designed in a case-comparison style (see Fig. 3 for an example, and Appendix Fig. 20–24 for all problems), providing students with alternative pathways, key steps, or intermediates. In prior work for simpler mechanistic questions, case comparisons were found to engage students in mechanistic reasoning by supporting them in arguing about contrasting features and weighing alternatives, using causal reasoning to justify their decisions (Caspari et al., 2018a; Rodemer et al., 2020; Deng and Flynn, 2021; Watts et al., 2021; Kranz et al., 2023). Building on this prior work, our design aimed at similar impacts for the much more complex case-comparison problems used in 2023. For example, consider the transformed problem used in PSS4 in 2023 (Fig. 3). Compared to the single-case problem from 2021, the transformed problem in 2023 asked students to combine key steps of pathways A, B, and C to propose a plausible arrow-pushing mechanism for the overall transformation. This necessitated carefully comparing and contrasting the different pathways to determine the changes in bonding associated with product formation and the likely order of reaction patterns based on reactivity. For example, the problem design required students to think about nucleophilic attack on an activated ketone by an alkene (Fig. 3(1B)) vs. an alcohol (Fig. 3(1C)). To decide between the two, students had to consider not only which pathway is more likely to happen first from an energetic or reactivity standpoint, but also which one generates an intermediate that can then undergo further transformation to form the reaction product. As seen in the key answer for 2021 (compare Fig. 2), both reactivities of these options are involved in the complete solution, with the alcohol reacting as a nucleophile first (Fig. 2A and C) and the alkene later (Fig. 2F). The problem therefore allowed students to combine ideas from the multiple pathways given towards the energetically most plausible mechanism. Hence, solving this problem requires making use of mechanistic reasoning for reasoned arguments and decisions, preventing sole recall of a single, known reaction mechanism.
 | ||
| Fig. 3 Example problem of PSS4 in 2023. Problem developed by us into a case comparison based on the original problem from Francis's (2004) teaching materials based on Martinet's et al. (1969, 1970, 1971) initial example of a Pinacol-terminated Prins cyclization, adapted with permission from Overman and Pennington (2003). Copyright 2003 American Chemical Society. | ||
During the semester, the problems were designed to remove scaffolding with alternatives successively: in the beginning of the semester, entire pathways were provided as alternatives (PSS2 & PSS3), then a choice of key steps (PSS4), and towards the end of the semester a choice of intermediates (PSS5 & PSS6).
Research participants
In both semesters, 2021 and 2023, mostly Chemistry graduate students and some upper-level undergraduate Chemistry majors were enrolled in the upper-level Mechanistic Reasoning in Organic Chemistry course in which this design research study took place. In 2021, 22 students were enrolled in the class, 14 of which consented to participate in the research. In 2023, 11 students were enrolled in the class and all of them consented to participate. As mentioned earlier, in both semesters, the class was mainly taught by one instructor, but a second instructor participated in PSSs. Both instructors consented to participate in the study. For contextualization purposes of our participants and findings, the racial and gender demographics for our participating and consented students and the institutional demographics are shown in Table 1. The recruitment of students took place via announcements in lectures and via the course management system. If students chose to participate, they were offered a $150 compensation (via gift card). Participants provided their consent via an online Qualtrics form. Data were de-identified and all participants were given pseudonyms. The institution's institutional review board approved this study.| 2021 participants (n = 14) | Institution total 2021 | 2023 participants (n = 11) | Institution total 2023 | |
|---|---|---|---|---|
| Race/Ethnicity | ||||
| Native American/Alaska Native/American Indian/First Nations | 0% | 0% | 0% | 0.1% |
| Asian | 35.7% | 9.4% | 9.1% | 10.4% |
| Black/African American | 21.4% | 4.1% | 0% | 4.8% |
| Latino/Latinx/Hispanic/Hispanic of any race | 0% | 7.1% | 9.1% | 7.9% |
| Pacific Islander/Native Hawaiian/Other Pacific Islander | 0% | 0% | 0% | 0.1% |
| White | 42.9% | 49.6% | 72.7% | 44.5% |
| Two or more races | 0% | 3.4% | 0% | 3.5% |
| Other/prefer not to answer | 0% | 23.7% (includes international) | 9.1% | 24.8% (includes international) |
| Race/Ethnicity unknown | 0% | 2.7% | 0% | 3.9% |
| Gender | ||||
| Female/Woman | 42.9% | 60% | 72.7% | 59% |
| Male/Man | 57.1% | 40% | 27.3% | 39% |
| Non-binary | 0% | 0% | 0% | 0% |
| Transgender | 0% | 0% | 0% | 0% |
| Other/Unknown | 0% | <1% | 0% | 2% |
| Prefer not to answer | 0% | 0% | 0% | 0% |
Data collection and processing
The data used for this study was collected during whole class discussions (WCDs) of 5 problem solving sessions (PSSs) each semester. For data collection, the instructor recorded the screen of her tablet capturing the discourse during the PSSs, including student and instructor verbal interactions, drawings of mechanisms, or notes and arguments. One of the instructors led the WCD and the other instructor observed and occasionally intersected with an utterance contributing to the discourse. In 2021 the length of the discourse between instructor and students in the PSSs ranged between 14–30 minutes, with a mean of 24 minutes. In 2023 the length of the discourse in the PSSs ranged between 20–82 minutes, with a mean of 30 mins. Since class sessions were 75 minutes long, but the WCD of some PSSs in 2023 lasted longer than that, recordings in these instances spanned two days of class. During each semester 7 PSSs took place, however, the first and last PSS had the same problem design in both semesters and were thus not part of this study. Note that because not all students gave their consent to participate in the study in 2021, we were not allowed to fully transcribe WCDs as non-consented students participated in those discussions. Thus, our data analysis was performed on the raw data (find more information in next section), which had to be deleted after data analysis was finalized. During data analysis, we transcribed relevant parts of the discourse where only consented participants spoke.Data analysis
Fig. 4 shows an overview of the data analysis. | ||
| Fig. 4 Overview of data analysis. Analysis began with watching videos of student–instructor interactions, then Practical Epistemology Analysis (PEA) (Wickman, 2004) was performed. Next, thick descriptions were written and coding was developed and applied simultaneously (Ponterotto, 2006; Saldaña, 2013), followed by statistical analysis of code occurrences and pattern identification. | ||
The writing of the thick descriptions influenced the coding process (Fig. 4, Boxes 3) as writing thick descriptions led to an increased understanding of the epistemic and social dynamics in the WCD. The deeper involvement with theory on practical epistemologies, knowledge-building, epistemic agency, and coherence of in-the-moment-learning for the coding scheme in turn also influenced writing thick descriptions, as our understanding of these concepts was sharpened (Jackson and Mazzei, 2013; Jackson and Mazzei, 2017; Cole, 2023).
We wrote thick descriptions (compare Table 2) for each gap that we identified through PEA, which helped us to describe detailed accounts of the interactions. The thick descriptions characterized the roles of the instructor and the students, e.g., who took epistemic agency. Also, they characterized the behavior of the instructor and the students and the shape of their contributions, e.g., if they were contributing epistemic evidence of how they came to know in a coherent manner. Table 2 shows examples of thick descriptions and how they relate to the different codes we gave, i.e., “Instructor Agency” or “Student Agency”, and “Coherence” or “No Coherence” (elaborated on in the next section).
With regard to the “Coherence” codes, we were not seeking to find causal accounts or evaluate whether utterances are correct, rather, we were interested in how contributions are brought to the fore, i.e., whether logical links of how one knows are made explicit and how that contributes to knowledge-building in a classroom. Fig. 5 shows our 2 × 2 coding table.
Table 2 further shows how we applied the coding categories to our thick descriptions of each gap. The coding allowed us to identify certain patterns that occurred throughout the WCDs, e.g., at which time points in the WCDs students or the instructor were agentic or provided coherent contributions.
To assess whether there is a significant association between the year (2021 vs. 2023) and the code distribution (distribution of “Coherence” and “No Coherence”; and distribution of “Student Agency” and “Instructor Agency”), Chi-Square tests of independence (Pearson, 1900; Yates, 1934; Hedderich and Sachs, 2020) were performed as the initial statistical analysis for both the Coherence distribution (Fig. 6, left) and the Agency distribution (Fig. 6, right). The Chi-Square tests were conducted to evaluate whether the observed changes in code distribution from 2021 to 2023 differ from variation that would be expected by chance. To account for the small numbers of cases, we applied a Yate's continuity correction (Yates, 1934; Hedderich and Sachs, 2020). For both comparisons, the significance was assessed at p < 0.05 and both tests had 1 degree of freedom (df = 1).
To further account for the small number of cases, we performed the more precise Fisher's Exact test (Fisher, 1922; Hedderich and Sachs, 2020) in addition to the initial Chi-Square tests. The Fisher's Exact test is well-suited for the small sample size and allows for more exact probability calculations of the observed distributions. The significance was assessed at p < 0.05 as well.
The null hypothesis (H0) for both statistical tests (Chi-Square test and Fisher's Exact test) states that there is no association between the year and the code distribution, meaning that the distribution of codes is independent of the year. The alternative hypothesis (H1) states that an association between the year and the code distribution exists, indicating a systematic change or shift of the code distribution from 2021 to 2023.
To further assess the strength of any observed association between year and Coherence distribution/Agency distribution, Cramér's V (Cramér, 1999; Hedderich and Sachs, 2020) was calculated, with values ranging from 0 to 1, where higher values indicate a stronger association.
We performed all statistical tests using Python 3.13 with “SciPy” (Virtanen et al., 2020) and “NumPy” (Harris et al., 2020).
Trustworthiness and reliability
To ensure trustworthiness and reliability of our analysis, we used strategies of reflexivity, integration of our theoretical framing, consensus-building procedures, as well an applicable measure for inter-rater reliability (Creswell and Miller, 2000; Macbeth, 2001; Jackson and Mazzei, 2013; Saldaña, 2013; Watts and Finkenstaedt-Quinn, 2021; Cole, 2023). This was achieved through collaboration among project team members and consultation of others not affiliated with this project. As for our positionalities influencing data analysis: Each author engaged in this project brings instruction and research experience that shaped interpretations (individually and shared) but also allowed reflexive engagement with data and the theory guiding our work (Creswell and Miller, 2000; Jackson and Mazzei, 2017). The first author is a postdoctoral researcher, trained in K-12 pedagogy practices, who taught in diverse educational settings, with a strong background in chemistry education research focusing on mechanistic reasoning. The second and corresponding authors are tenured or tenure-track faculty members with experience as instructors in Organic Chemistry and deep content expertise. The second author additionally brought her experience in using mechanistic reasoning in her other courses, including a large upper-level lecture course on Organic Chemistry in living systems, and for her research program in Chemical Biology. The corresponding author is further a trained K-12 teacher and general chemistry instructor who brings expertise in chemistry education research, including research on mechanistic reasoning, facilitation practices, and instructional settings in university contexts to the project. Our multiple perspectives and lived experiences, combined with deep engagement with both the data and theoretical frameworks, in addition to consultations of others not affiliated with the project supported reliability and validity.With regards to the agency code cases that led to disagreements, most were attributed to different interpretations for whose story was centered in the classroom, specifically when contributions of both the instructor and students were shaping discourse. For example, coding agency was challenging for some gaps when the instructor used her facilitation to put students’ ideas to the fore after students had presented them (which would be coded as “Student Agency”), however, then within the same gap, changed to having students fill in gaps, e.g., the instructor asking to tell which named reaction is entailed in the students’ ideas (which would be coded as “Instructor Agency”). Here, we sometimes disagreed, which required us to dive deeper into these specific interactions, revisit the video data, re-engage with the theory guiding our work, and clarify interpretations on who is shaping the story of the interaction with their ideas to a greater extent. Neither the distinction between “Coherence” and “No Coherence” nor the distinction between “Instructor Agency” and “Student Agency” was absolutely binary. Rather, gaps were coded as “Coherence” when they were more coherent than non-coherent and were coded as “Instructor Agency” when there was more instructor agency than student agency. Any disagreements we had with the coherence code cases and the agency code cases were resolved through constructive discussions until we reached 100% consensus.
Results
Quantitative findings: comparison of code occurrences in 2021 and 2023
The cross table for code categories “Coherence” and “No Coherence” (Fig. 6, left) and the cross table for code categories “Student Agency” and “Instructor Agency” (Fig. 6, right) demonstrate differences in distribution of codes between 2021 and 2023. Specifically, the tables show number of gaps where the same code was given in both years (Fig. 6, bold numbers). The tables also show the number of gaps where a different code was given in one year than the other year (Fig. 6, numbers with grey background in the box with bold framing). The cross tables visually show shifts from more “No Coherence” codes in 2021 towards more “Coherence” codes in 2023 and from more “Instructor Agency” codes in 2021 towards more “Student Agency” in 2023 (Fig. 6, numbers with grey background in the box with bold framing).Chi-Square tests with Yate's correction for continuity were performed for coherence (Fig. 6, left) and epistemic agency (Fig. 6, right) separately from each other. The results of the Chi-Square test for coherence (χ2(1) = 4.38; p < 0.05) show a statistically significant association between the years (2021 vs. 2023) and the distribution of “Coherence” and “No Coherence” codes indicating a significant shift from “No Coherence” towards “Coherence” from 2021 to 2023. The results of the Chi-Square test for epistemic agency (χ2(1) = 5.17; p < 0.05) also demonstrate a statistically significant association between the year (2021 vs. 2023) and the distribution of “Student Agency” and “Instructor Agency” codes, indicating a significant shift from “Instructor Agency” towards “Student Agency” from 2021 to 2023. The Fisher's Exact test also showed a statistically significant difference (p < 0.05) for coherence, which confirms the result of the initial Chi-Square test and indicates that the distribution of “Coherence” and “No Coherence” codes differ significantly between 2021 and 2023, which relates to the higher occurrence of “Coherence” codes in 2023. For the “Student Agency” and “Instructor Agency” codes, the value (p < 0.05) of the Fisher's Exact Test indicates the same. This is driven by a higher occurrence of “Student Agency” codes in 2023. To further quantify the strength of association between year and code distributions, Cramér's V was calculated (Cramér, 1999; Hedderich and Sachs, 2020), resulting in a value of V = 0.24, for the Coherence distribution, and resulting in a value of V = 0.26, for the Agency distribution, both values indicate a moderate association.
These results suggest a relevant shift in code distributions from 2021 to 2023, which is unlikely to be due to random variation.
Fig. 7 further shows the distribution of the code category combinations “Instructor Agency & Coherence”, “Instructor Agency & No Coherence”, “Student Agency & Coherence” and “Student Agency & No Coherence” for 2021 and 2023. In 2021, 45% of the gaps were coded as “Coherence” (Fig. 7, solid areas), while 55% were coded as “No Coherence” (Fig. 7, hatched areas). In contrast, in 2023, 70% of the gaps were coded as “Coherence” (Fig. 7, solid areas), while 30% were coded as “No Coherence” (Fig. 7, hatched areas). With regards to epistemic agency, in 2021, approximately 68% of the gaps were coded as “Instructor Agency” (Fig. 7, blue areas) and 32% as “Student Agency” (Fig. 7, yellow areas); whereas in 2023, 41% of the gaps were coded as “Instructor Agency” (Fig. 7, blue areas) and 59% of the gaps were coded as “Student Agency” (Fig. 7, yellow areas). These results also show the change of code distribution towards more coherence (solid areas) and more student agency (yellow) from 2021 to 2023.
Qualitative findings: when and how codes occurred in 2021 and 2023
To further answer our research question on how different problem designs impact epistemic agency and coherence in student–instructor interactions in whole class discussions (WCDs), we supplement our quantitative findings on distribution of codes with qualitative findings that show when and how agency and coherence codes occurred in the two different years. To do so, we report on the progression of the WCDs, i.e., the start, the body, and the end of the discourse, in the different years relating this to the occurrence of codes. We characterize whether and how students were agentic and brought their ideas forward in a coherent manner in relation to the specific problem design, i.e., 2021 “predict complex mechanism” and 2023 “predict the most plausible mechanism with given alternatives” (see Appendix Fig. 15–24 for problems).The start of the whole class discussions
The WCD in both years, 2021 and 2023, often began with the instructor inviting the students to share out. This gave student groups the opportunity to share ideas and approaches they had discussed in their small groups. In both years, students took agency at the start, however, that agency was directed towards different objectives. In 2021, students’ agency was often directed towards establishing a complete mechanism, which involved sharing ideas without establishing coherence. Whereas in 2023, students’ agency was directed towards evaluating the given alternatives, which involved sharing ideas in a coherent manner, with reasoning made explicit.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond attacking carbonyl C of former acetone in intermediate]. This [draws sigma bond migration with an electron pushing arrow to the created carbocation]. Then we had this…. Which gives us the ring [draws intermediate with 5-membered ring] which then flips [draws lone pair of oxygen flipping to the carbon of the primary carbocation] and then to that [draws an arrow to the product].” This quote demonstrates how in 2021 the WCD was driven by the questions “What is the mechanism or what is the next step?”, which often resembled a listing-like telling and drawing of mechanistic steps without sharing how students knew these steps were plausible or might be likely to occur. Their rationale could have entailed, for example, explaining how electronic properties account for these steps, e.g., how they know acetone can react as a nucleophile. As students’ utterances were rather of a descriptive, non-coherent nature without epistemic evidence of how they know, the code “No Coherence” was applied. Since in this part of the discussion, the students’ ideas were positioned as meaningful shaping the knowledge-building process, the code “Student Agency” was applied.
C double bond attacking carbonyl C of former acetone in intermediate]. This [draws sigma bond migration with an electron pushing arrow to the created carbocation]. Then we had this…. Which gives us the ring [draws intermediate with 5-membered ring] which then flips [draws lone pair of oxygen flipping to the carbon of the primary carbocation] and then to that [draws an arrow to the product].” This quote demonstrates how in 2021 the WCD was driven by the questions “What is the mechanism or what is the next step?”, which often resembled a listing-like telling and drawing of mechanistic steps without sharing how students knew these steps were plausible or might be likely to occur. Their rationale could have entailed, for example, explaining how electronic properties account for these steps, e.g., how they know acetone can react as a nucleophile. As students’ utterances were rather of a descriptive, non-coherent nature without epistemic evidence of how they know, the code “No Coherence” was applied. Since in this part of the discussion, the students’ ideas were positioned as meaningful shaping the knowledge-building process, the code “Student Agency” was applied.
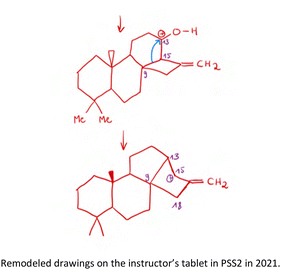
 | ||
| Fig. 8 Student drawing on the instructor's tablet that was projected for the class during the example “Showing What They Got” in PSS4 in 2021. The prompt for the problem was: “Overman and coworkers have used the following tandem reaction in the synthesis of a number of natural products. The original report of this reaction was made by Mousset on the substrate below. Provide a reasonable arrow-pushing mechanism for the following transformation” (also see Appendix for complete problem). Problem adapted from Francis's (2004) teaching materials based on Martinet's et al. (1969, 1970, 1971) initial example of a Pinacol-terminated Prins cyclization, adapted with permission from Overman and Pennington (2003). Copyright 2003 American Chemical Society. | ||
 | ||
| Fig. 9 Instructor writing on her tablet that was shared with the class during the example “Comparing the Alternatives of the Problem” in PSS2 in 2023. The instructor wrote down in a bullet list style what students were sharing, sometimes demarcating arguments for an option with a plus sign and arguments against an option with a minus sign and using parentheses to indicate that students decided against an option. The prompt for the problem was: “Below are four alternative pathways and products that students have proposed for in the past. (a) First, fill in the electron pushing formalism for each pathway. (b) Next, use your mechanistic reasoning skills to predict the most likely product and mechanistic pathway.” See also Appendix Fig. 20 for complete problem. Problem developed by us into a case comparison based on the original problem that was reproduced with permission from SNCSC: Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 147, problem (aa), 2003, Springer Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
The presenting student vocalized their group's thinking, while the instructor wrote down the students’ reasons in yellow next to the mechanism (Fig. 9, yellow). The students’ ideas were positioned as meaningful, thus, the code “Student Agency” was applied. The student stated: “Yeah, we're pretty skeptical of 3, because the rings shift to be from a six-membered ring and four-membered ring to two four-membered rings. So that adds a lot of ring strain. So, it seems pretty unfavorable, especially compared to [option] 2 which starts in a similar way but leads to what looks like a much more stable structure.” The instructor summarized: “Okay. So, sort of a reason against 3, because in 3 we go from a six-membered ring that's still there in P [underlines P] to a four-membered ring in Q [underlines Q] that then stays a four-membered ring, and that, adds ring strain, which is energetically less favorable.” The student shared an argument about ring strain which supports their claim that option 3 is not plausible. The code “Coherence” was applied because the student supported their logic and compared option 3 to the mechanism shown in option 2 in a coherent manner, recognizing that going from a six-membered to a four-membered ring would not be favorable and would lead to a less stable product.
Contrasting the examples of 2021 and 2023, it is apparent that the start of WCDs in 2023 was typically more filled with reasoning and connected ideas were made more explicit in coherent contributions compared to the start of WCDs in 2021 where more of a telling of what a group arrived at in their SGD took place. The examples further demonstrate that this difference between 2021 and 2023 was connected to the problem design: the ultimate goal of the problems in 2021 was to arrive at a correct mechanism, so when students were asked to share out from their SGDs, they shared what they got towards this goal. Similarly, in 2023, the ultimate goal of the problem design was to make decisions about a mechanism using, comparing, and contrasting different alternatives, so when the students were asked to share out from their SGDs, they shared how they used the different alternatives and were thinking about them in contrast to each other.
This qualitative description of the start of the WCD corresponds to the results of the quantitative analysis, supporting when and how an increased appearance of “Coherence” codes in 2023 compared to 2021 occurred.
The body of the whole class discussions
How the body of the discussion typically unfolded also changed from 2021 to 2023. In 2021, the body of the discussion was directed towards establishing correctness of steps by the instructor, adding a validation of that correctness with reasoning afterwards. In 2023 instead, the body of the discussion was more focused on students working with given alternatives and creating a coherent story with connected reasoning immediately. In the following, we first show an example from 2021, in which the instructor takes agency to establish correctness and then validates that correctness with reasoning. Since this reasoning was not always added by the instructor, but the instructor also sometimes facilitated that students added their reasoning after correctness was established, we then add another 2021 example to demonstrate this. Lastly, we show an example from 2023 to highlight how the body of the discussion changed from 2021 to 2023.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the secondary carbocation that the instructor had drawn on the board (Fig. 10, red drawing, center).
C double bond of the secondary carbocation that the instructor had drawn on the board (Fig. 10, red drawing, center).
 | ||
| Fig. 10 Instructor writing on her tablet that was shared with the class during the examples “Let's Generate the Bonds We Need to See” (center) and “Why Do We Like This Step?” (bottom) in PSS2 in 2021. The instructor drawing in the center shows an intermediate that resulted from the second step the class proposed. The instructor drawing at the bottom shows the third mechanistic step the instructor established. See also Appendix Fig. 15 for complete problem. Problem reproduced with permission from SNCSC. Adopted from Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 147, problem (aa), 2003, Springer-Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
The instructor responded “So you totally could do that [form a tertiary carbocation]. That's very plausible in terms of energetic, which carbocation is unstable. But I think in the interest of time I’ll just save us the exercise of doing it and let you know that if you do it, you don’t actually generate the bonds we need to see.” This quote shows how the instructor is the epistemic agent as she is the one who took the cognitive authority of deciding that arriving at a “classically correct” answer by the end of class was more valuable in that moment than exploring the possibility of a reasonable alternative from a reactivity standpoint. The instructor positioned her own thoughts as meaningful, i.e., that this does not lead them where the bonds need to be to get to the product. In doing so, less importance was given to the student's idea of thinking through the alternative of forming a tertiary carbocation. The code “Instructor Agency” was applied. The instructor's response was further coded as “No Coherence”, as the instructor claims that one can form a tertiary carbocation, but that it does not form because this intermediate does not lead to the correct product, i.e., “the bonds we need to see”. By making only this claim, the instructor did not explicitly share on how she knows this. To be coded as “Coherence”, the instructor would have needed to logically connect her claim using reasoning, e.g., how this formation with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond could or could not happen or how this formation does not lead to the bonds of the product on the energetically most favorable pathway.
C double bond could or could not happen or how this formation does not lead to the bonds of the product on the energetically most favorable pathway.
In addition to establishing correctness or incorrectness, as in the example “Let's Generate the Bonds We Need to See”, the body of the discussion in 2021 often served the purpose of adding coherence only after the correct solution was already established, either by the instructor herself or by the students provoked through targeted instructor facilitation, which is shown in the following examples.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond] which generates a carbocation here [circling the double bonded carbon next to carbon 15], and we’ll pull out the protons right here [circling carbon 18] and generate the final product.” This quote shows that after the establishment of the correct steps and arrows and what needs to happen, the instructor often added how she knows a mechanistic step or a product is correct in a coherent manner, i.e., the code “Coherence” was applied. In this case, she explained how the suggestion leads to the reforming of a stable carbonyl and getting rid of the carbocation expressed as “ionization with a proton and a heteroatom happens pretty easily” and how this puts everything in a position that can transform into the product. These kinds of explanations appear as added on, like a “decoration”, after getting to the correct solution. The problem design did not necessarily enforce coherent explanations; it was the instructor who decided to “add it as decoration”.
C double bond] which generates a carbocation here [circling the double bonded carbon next to carbon 15], and we’ll pull out the protons right here [circling carbon 18] and generate the final product.” This quote shows that after the establishment of the correct steps and arrows and what needs to happen, the instructor often added how she knows a mechanistic step or a product is correct in a coherent manner, i.e., the code “Coherence” was applied. In this case, she explained how the suggestion leads to the reforming of a stable carbonyl and getting rid of the carbocation expressed as “ionization with a proton and a heteroatom happens pretty easily” and how this puts everything in a position that can transform into the product. These kinds of explanations appear as added on, like a “decoration”, after getting to the correct solution. The problem design did not necessarily enforce coherent explanations; it was the instructor who decided to “add it as decoration”.
In the aforementioned example “Why do We Like this Step” in 2021, it was the instructor who took the agency and brought the use of reasoning in her coherent explanation to the body of the discussion after the correct solution was already established. In the body of the discussion the establishment of coherence, after the correct solution was on the board, was also sometimes driven by student reasoning and their agency encouraged through instructor facilitation asking students to give a rationale of why. A typical instance occurred in the example “Giving a Rationale of Why” during PSS5 in 2021. Students were asked to provide an arrow-pushing formalism for a shown transformation (Fig. 11).
 | ||
| Fig. 11 Remodeled drawing of a student's solution on the instructor's tablet that was shared with the class during the example “Giving a Rationale of Why” in PSS5 in 2021. Red circles highlight what the student was talking about outlined in the text below. We remodeled the drawing because this student did not give permission for their drawings to be used for publication. See also Appendix Fig. 18 for complete problem. Problem reproduced with permission from SNCSC. Adopted from Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 144, problem (i), 2003, Springer Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
 | ||
| Fig. 12 Instructor drawings of what students were sharing in the example “That Makes Sense” in PSS6 in 2023. Top: Problem prompt for the overall transformation with selection of possible intermediates. Box 1: Students’ approach established before the example discourse starts. Box 2: Student's idea to get the amide group into alpha position of the methyl ester group that would account for stereochemistry but is chemically not possible with the current reactivities. Box 3: Mechanistic steps proposed by student A resulting in needed reactivity. Also see Appendix Fig. 24 for complete problem. Problem developed by us into a case comparison. Mechanism in problem based on initial observations reported by Meerwein et al. (1961). Mechanism adopted with permission from Wick et al. (1964). CLAISEN'sche Umlagerungen bei Allyl- und Benzylalkoholen mit Hilfe von Acetalen des N,N-dimethylacetamids. Vorläufige Mitteilung. Helvetica Chimica Acta. Copyright © 1964 Verlag GmbH & Co. KGaA, Weinheim. | ||
The following excerpts demonstrate details of this interaction, starting with student A sharing: “Like we are thinking that oxygen there [in product 3] could have come from the leaving group [of the bottom substituent of intermediate C, Fig. 12, Box 1]. And then we were trying to find a way to make it intramolecular where as this leaves instead of actually leaving you form a carbon–carbon double bond from the top and that would also explain why it is on top of the ring because that's [the substituent (“leaving group”) of intermediate C in the gamma position relative to the methyl ester substituent] already above of the ring. But we could not find a way to make like that, we labeled it as carbon 3, but that like methyl carbon on the leaving group…”. Another student interrupted and clarified which methyl carbon student A was talking about: “On the structure you [the instructor] drew down below [highlighting the carbon next to the imine carbon of intermediate C, Fig. 12, Box 1]”. Student A went on: “Oh yeah, oh yeah… down there… Yes be able to connect where it [that carbon] is there [highlighting the carbon–carbon bond from the amid group to the ring in product 3]”. The instructor clarified and copied the suggested intermediate C (Box 1 of Fig. 12 to later obtain the structure in Box 2 of Fig. 12) and highlighted that they now, after the explanation of student A can indicate stereochemistry, and asked: “And so what you’re [student A] saying is that, first of all… I can also now indicate the stereochemistry. So you’re saying what you want is to form a bond through an intramolecular process that basically has this carbon [highlights carbon next to the imine carbon [Fig. 12, Box 2]] be the nucleophile, and that would basically form the carbon–carbon bond over here [highlights carbon–carbon bond from amide group to the ring]. That's what you’re saying?”. Student A confirmed: “Yeah and then we would have the same like leaving arrows like the one […] [Fig. 12, Box 1, referring to the approach they have talked about before], breaking the oxygen carbon bond on the ring…” The instructor clarified: “So the arrow would be, like let me just rephrase. The arrows you wanted to draw but couldn’t. So, I’m going to erase these [referring to the yellow arrows she is about to draw, Fig. 12, Box 2], because I’m not going to be comfortable leaving them there, but I will draw them [refers to the yellow arrows she is about to draw], because what you’re saying is you really wanted something like this to happen [Fig. 12, Box 2: draws yellow arrows of the intramolecular reaction and notes down “we wish”]”. Student A confirmed and reasoned further “Yes. And then we were playing around with like possibly getting to a place where we could use [intermediate] B, to where that carbon–carbon double bond is and we could use like a nucleophilic double bond. Then we could not figure out how to get that.” After opening up to the rest of the class for their thoughts, the instructor facilitated: “And maybe while you think, I’m just going to redraw intermediate B. Because [calls student A by their name], one of the things you said is that B, this B situation has the correct pattern of reactivity.” Another student (student B) of the group student A was part of confirmed: “Yeah we [group of student A and student B] have it [referring to intermediate B] on the left.” A student from a different group was wondering what the question was they were trying to make sense of and asked: “The arrow to form the carbon–carbon bond?”. Student B confirmed “Yeah, so this is what I’m saying. Like in ours [Fig. 12, Box 2, idea represented by the drawing with the yellow arrows] – It's illegal. [laughing in the class]”. The other instructor responded: “We’re trying to find a way to make that [Fig. 12, Box 2, intramolecular nucleophilic attack represented by the yellow arrows] actually happen.” Note that this was one of the rare occasions in which the observing instructor intersected with an utterance contributing to the discourse. The main instructor went on and drew intermediate B next to their current drawing (Fig. 12, Box 2) and prompted: “And I think like maybe it would be like a little more leading than I needed to be. I think there [highlighting intermediate B] might be a solution that we’re very close to. Yes – [calling the name of student A]”. Student A then shared out: “Umm… could you like deprotonate that ethyl, flip the double bond back onto the N and then you have a double bond [as shown in intermediate B] …. Like do an elimination and form a double bond there [refers to the bond between the imine carbon and the carbon next to it] and basically… force till we get that… on the bottom one…and then we have a double bond… that was something we thought about…we thought we are forcing it where we want this to be.”. The students of the class nodded their heads and confirmed. Another student from a different group confirmed verbally: “Yeah, that makes sense!” The main instructor erased the ideas of the “illegal” yellow arrows and drew the elimination that student A was suggesting (Fig. 12, Box 3).
This example shows how student A was agentic and contributed how they knew what they were suggesting in a logical manner, engaging in making use of reasoning, i.e., how they thought about getting the functional group in the correct position. Student A shared that through the steps they were suggesting an intramolecular reaction could happen, which would lead to a product with the correct stereochemistry. Further, the student shared how they – with the use of intermediate B – were thinking that the intramolecular nucleophilic attack step might happen through an elimination. The codes “Student Agency” and “Coherence” were applied. This example shows how the problem design made students combine synthetic approaches, with them making use of properties, such as the “nucleophilic bond”, that must be present to arrive at the correct connections in the product.
Contrasting the examples of 2021 and 2023, it is apparent that the body of WCDs in 2023 was typically less driven by the instructor establishing correctness first and gave the students more opportunities to agentically bring in coherent reasoning sharing how they knew from the outset even when correctness or a whole transformation were not yet established. Again, like for the start of WCDs, the examples demonstrate a connection between these differences and the problem design. If the goal of the problems is to establish a correct transformation, like it was the case in 2021, then that correctness gets established first by the instructor who enters class already knowing the correct transformation. In this case, coherent explanations can be sprinkled on “as decoration” by the instructor directly, or through the instructor facilitating students to bring in their reasoning. The correct mechanism and its explanation are the scientific story that is built. However, if the goal of the problems is instead to use and compare different alternatives towards a transformation, like it was in 2023, then correct pieces (like correct intermediates in the example shown here) are already on the page and do not need to be established first, instead student coherent reasoning working with these alternatives becomes necessary because that is what the instructor can work with to build a scientific story. The benefits and drawbacks of different alternatives and their connection to an overall transformation become the scientific story.
This qualitative description of the WCDs corresponds to the results of the quantitative analysis, supporting when and how an increased frequency of the “Student Agency” and “Coherence” codes in 2023 compared to 2021 occurred.
The end of the whole class discussions
At the end of the WCDs, in both years, agency shifted towards the instructor, who established a solution when the time ran out. In 2021, this meant having a correct mechanism established that largely aligned with the canonical solution, whereas in 2023 this meant deciding between alternatives using the students’ arguments. We will present an example from each year in the following. | ||
| Fig. 13 Student drawing on instructor's tablet that was shared with the class during the example “More Plausible Pathway” in PSS4 in 2021. The two colours present two alternative pathways: the blue pathway was already drawn on the tablet by another student before the example “More Plausible Pathway” begins. The red pathway was drawn by a student during the discussion. See also Appendix Fig. 17 for complete problem. Problem adapted from Francis's (2004) teaching materials based on Martinet's et al. (1969, 1970, 1971) initial example of a Pinacol-terminated Prins cyclization, adapted with permission from Overman and Pennington (2003). Copyright 2003 American Chemical Society. | ||
At the end of this WCD, the instructor guided the students to a more plausible transformation than the one initially proposed by the students at the start of this WCD (compare “Showing What They Got” Example, Fig. 8). The instructor directed the conversation to rethinking whether acetone – as suggested by the students in the beginning of the WCD (Fig. 13, blue pathway) – is likely to be a good nucleophile, since the class had never seen the oxygen lone pairs of acetone act as a nucleophile: “Well I think, I mean, I think the mechanism [blue mechanism in Fig. 13, drawn by student in the beginning of the WCD] is plausible in that you get to the correct product. All of the correct bonds are formed. I think that step in particular [referring to acetone attacking], that there is an alternate pathway that would be more plausible than—I mean, the only thing we’ve ever seen those lone pairs [of acetone] do is coordinate to Lewis Acids. We’ve never seen them act as the nucleophiles. I’m seeing some nods going. I feel like that was probably part of some of your discussion. So, is there another way you can actually get to something very similar? By doing a different first step.”
While the fact that the instructor did not correct the blue mechanism to the red mechanism (Fig. 13) earlier in this WCD (compare Fig. 8) demonstrates that absolute correctness was not always of highest priority in all parts of WCDs in 2021, the excerpt here shows that absolute correctness became a higher priority to the instructor, shaping both agency and coherence within this part of the WCD. The students then filled in blanks of the instructor's questions. For instance, when prompted, they agreed that the acetone carbonyl could be protonated in the first step to become the electrophile, that either the alkene part or the hydroxyl group of the reactant could be the nucleophile, and that a proton transfer could allow water to leave as a leaving group. In these interactions, the instructor judged the student's utterances as immediately right or wrong, moving ahead with correct answers while steering to an alternative for wrong ones. The instructor had the cognitive authority, i.e., coded as “Instructor Agency”, while a student drew out what the instructor evaluated as correct in red (Fig. 13, red pathway). The instructor then asked: “So how is that [Fig. 13, referring to the last red intermediate in the red pathway] different than the product you got when you [referring to the students’ suggested pathway in blue] had an acetone being the nucleophile [Fig. 13, blue pathway, referring to the intermediate after acetone was attacking]?” The drawing student responded that it is not different. The instructor confirmed: “It's not, exactly. This is not a trick question. So what comes out—I mean, I think, one of the things here is, you know, kind of the order of events, and which of those pathways is going to be lower energy. Even though it's a little, I think, on these structures, I think the second half of this drawing in red is a little harder to spot, partially because I think we’re so used to looking at the structures given and accepting that hydroxyl to be converted to a leaving group [like suggested in the blue pathway by the students]. But OH's can also be nucleophiles. And I think overall, that red pathway is going to be lower in energy than the things you have to do to accomplish the like first two steps of the [inaudible, referring to blue pathway]. […] We actually thought through the answer before the end of class, which I’m really proud of us for.”
This interaction shows how the instructor used her agency towards getting to the more plausible mechanism. While she shares that this pathway is more plausible due to energetics and that one could recognize the difference in plausibility by pattern recognition of acetone usually acting a as an electrophile and not a nucleophile (“We’ve never seen them act as the nucleophiles”), having the complete correct pathway is ultimately prioritized (“We actually thought through the answer before the end of class, which I’m really proud of us for.”) instead of building coherence that would allow the students to logically decide between the two pathways based on pattern recognition or causality: with pattern recognition one might think that the red pathway is more plausible than the blue pathway (Fig. 13), but to the contrary, one might also think that the blue pathway is more plausible because of the typical pattern of OH getting protonated to leave as a leaving group occurring first (“I think we’re so used to looking at the structures given and accepting that hydroxyl to be converted to a leaving group”). In terms of causality via energetics, the instructor claims that the red pathway is more plausible because of energetics but she does not explain how one knows it is energetically more favorable, i.e., the code “No Coherence” was applied.
 | ||
Fig. 14 Annotated slide of the instructor's tablet that was shared with the class in the example “Using Students’ Arguments to Decide” in PSS2 in 2023. Yellow highlighting shows the difference between the protonation of different functional groups in option 1 and option 2. Circled minus signs indicate contra arguments, circled plus signs indicate pro arguments made by the students and written down by the instructor. Note that in the purple con argument against Option 1 ‘C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vs. C O vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C’ not ‘C C’ not ‘C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vs. C O vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O’ was meant to be written. See also Appendix Fig. 20 for complete problem. Problem developed by us into a case comparison based on the original problem that was reproduced with permission from SNCSC: Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 147, problem (aa), 2003, Springer Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. O’ was meant to be written. See also Appendix Fig. 20 for complete problem. Problem developed by us into a case comparison based on the original problem that was reproduced with permission from SNCSC: Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 147, problem (aa), 2003, Springer Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
With time running out, the instructor again stepped in at the end of the WCD as students could not reach a conclusion and were wondering how to decide. The instructor then used students’ own arguments to do so, adding an argument about the energy pathways: “I like that like you're thinking about, how… how do we decide (…) how do we need to go about this? And in the end, you need to think about the energy landscape overall, they lead to the same products. So, there is really only a difference in pathway… because the end product is the same stable for both of them. And in terms of pathway, I guess I would say I'll make an argument against what we just said here [Fig. 14, bottom, instructor strikes out the reasoning written last in purple about how creating a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond will create more ring strain from compound B to C that then gets relieved from D to E]. Because [referring to pathway 1] we wouldn't go all the way up [in energy as a result of ring strain]. And if we imagine an energy pathway like that [referring to option 1], we wouldn't go all the way up [gesturing the energy pathway and the activation energy] just that we can then fall down [through the relieve of the higher ring strain] [gesturing how the energy pathway would fall down] but rather, we want to stay like here [referring to option 2, gesturing a lower point in the energy pathway] and not go all the way up because that activation energy…basically, not really a helpful reason…[to go with option 1] to want to go all the way up when the ring strain argument still holds true for 2 as well.” This quote shows that the instructor is the one who shaped the interaction and decided which arguments hold true. Hence, the instructor is the epistemic agent of this interaction, i.e., the code “Instructor Agency” was applied. She decided that the students’ argument for option 1, as a plausible option on its own, does not hold true. She therefore explains how the difference in energy pathways is an important discriminating factor to decide between the options. Compared to the quote of 2021, she explains “how we [they] need to go about this”, sharing how one needs to reason about the energy of the pathways, since the end products are the same stable, sharing which approach is useful now, i.e., reasoning about the energy landscape. The instructor built coherence in her explanation and in combining students’ arguments, explaining how in pathway 1, more ring strain would first be created and then relieved, which would not be favored in terms of kinetic aspects. In doing so, the instructor supports her logic by explaining and gesturing that in option 1, the energy would first increase more as the ring strain is added due to the formation of a fused cyclobutene (from compound B to C), and then decrease as the ring strain is released (from compound D to E), in contrast to option 2 where the ring strain release occurs first. In this interaction, the instructor coherently contributes a comprehensive explanation, bringing together multiple perspectives; the code “Coherence” was applied.
C bond will create more ring strain from compound B to C that then gets relieved from D to E]. Because [referring to pathway 1] we wouldn't go all the way up [in energy as a result of ring strain]. And if we imagine an energy pathway like that [referring to option 1], we wouldn't go all the way up [gesturing the energy pathway and the activation energy] just that we can then fall down [through the relieve of the higher ring strain] [gesturing how the energy pathway would fall down] but rather, we want to stay like here [referring to option 2, gesturing a lower point in the energy pathway] and not go all the way up because that activation energy…basically, not really a helpful reason…[to go with option 1] to want to go all the way up when the ring strain argument still holds true for 2 as well.” This quote shows that the instructor is the one who shaped the interaction and decided which arguments hold true. Hence, the instructor is the epistemic agent of this interaction, i.e., the code “Instructor Agency” was applied. She decided that the students’ argument for option 1, as a plausible option on its own, does not hold true. She therefore explains how the difference in energy pathways is an important discriminating factor to decide between the options. Compared to the quote of 2021, she explains “how we [they] need to go about this”, sharing how one needs to reason about the energy of the pathways, since the end products are the same stable, sharing which approach is useful now, i.e., reasoning about the energy landscape. The instructor built coherence in her explanation and in combining students’ arguments, explaining how in pathway 1, more ring strain would first be created and then relieved, which would not be favored in terms of kinetic aspects. In doing so, the instructor supports her logic by explaining and gesturing that in option 1, the energy would first increase more as the ring strain is added due to the formation of a fused cyclobutene (from compound B to C), and then decrease as the ring strain is released (from compound D to E), in contrast to option 2 where the ring strain release occurs first. In this interaction, the instructor coherently contributes a comprehensive explanation, bringing together multiple perspectives; the code “Coherence” was applied.
In both years the instructor was the epistemic agent when the WCD came to an end. Comparing both examples, however, shows how in 2023 the problem design with alternatives leads to the instructor using students’ arguments about the different alternatives and building coherence with their reasoning to decide why one alternative is preferable over the other. In contrast, in 2021, the instructor directed her agency towards the goal of the problems of getting to the more plausible/correct solution, without contributing coherent explanations of how she knows, which might limit students to build coherence between the “correct” solution and what they were thinking about.
This qualitative description of the WCDs corresponds to the results of the quantitative analysis, supporting when and how “Instructor Agency” was present in both years and “Coherence” occurred more often in 2023 than in 2021.
Discussion and implications for practice
In the following, we interpret the results through different bodies of literature and show how our work contributes to this existing research. We also draw implications for practice.Extension of research on problem design
Our work extends research on problem design to foster mechanistic reasoning in Organic Chemistry in several ways. With regards to the use of case comparisons as problem type, like in 2023, our work demonstrates that case comparisons do not only promote a switch from surface-level to deeper reasoning for individual students in interviews or in written work (Caspari et al., 2018a; Graulich and Schween, 2018; Bodé et al., 2019; Rodemer et al., 2020; Deng and Flynn, 2021; Watts et al., 2021; Kranz et al., 2023), but also that students in the actual classroom discourse express their reasoning more coherently making their internal logic more explicit, which in turn allows for more in-depth collaborative knowledge-building. Additionally, we demonstrate that the case-comparison design gives students more opportunities to engage in classroom discourse agentically, indicating that case comparisons cannot only support students’ individual reasoning, but also contribute positively to their ability to communicate their reasoning in classroom discussions.While most research in the past has demonstrated that case comparisons offer students the opportunity to reason deeply (Caspari et al., 2018a; Bodé et al., 2019; Deng and Flynn, 2021; Rodemer et al., 2021; Watts et al., 2021; Kranz et al., 2023), direct comparisons between engagement in reasoning when solving case comparisons to engagement in reasoning when solving analogous single-case tasks have not been made. Instead, researchers have inferred differences in reasoning from previous work on student reasoning (e.g., Bhattacharyya and Bodner, 2005; Kraft et al., 2010; Strickland et al., 2010; Bhattacharyya, 2013; DeFever et al., 2015; Graulich, 2015). Through our design research, we now provide this direct comparison, allowing for an enhanced comparability, and show how students’ engagement in reasoning changes with different problem designs.
There has been a call by many researchers for tasks fostering meaningful engagement in authentic practices in Organic Chemistry (Raker and Towns, 2012a, b; Flynn, 2014; Stowe and Cooper, 2017; Esselman et al., 2023). We contribute to this ongoing effort by expanding the pool of tasks that foster literacy in authentic problem solving in mechanistic reasoning contexts. Together, the open single-case problem design from 2021 and the case-comparison problem design for these complex mechanism problems from 2023 provide those who teach Organic Chemistry with a range of problems they can use to engage their students in mechanistic reasoning (see Appendix Fig. 15–24 for all problems).
Our work brings together the benefits of design of more authentic and complex problems (Flynn, 2011; Raker and Towns, 2012a; Flynn, 2014; Stowe and Cooper, 2017; Webber and Flynn, 2018; Helix et al., 2022) and case comparisons (Graulich and Schween, 2018; Bodé et al., 2019). Since case comparisons in the past have only been designed for one-step or simpler multi-step reactions (Caspari et al., 2018a; Graulich and Schween, 2018; Bodé et al., 2019; Deng and Flynn, 2021; Watts et al., 2021), our case-comparison design can give Organic Chemistry instructors innovative ideas of how to design case comparison for more complex mechanism problems. Depending on the degree of scaffolding that may be beneficial for students in different stages of their learning, entire pathways can be shown as alternatives (as in PSS2 & PSS3 in 2023), or scaffolding can be decreased with a choice of keys steps (as in PSS4 in 2023), or intermediates (as in PSS5 and PSS6 in 2023). These designs can be used to complement traditional problem designs that ask students to predict the entire transformation (as in PSS2 through PSS6 in 2021).
Relationships between problem design and facilitation
Our study advances research on facilitation by showing how problem design and facilitation practices are interconnected. We show how problem designs provide opportunities for facilitation, but also how facilitation provides opportunities for students and the classroom community to bring aspects to the fore that the problem designs do not “enforce”.Several examples in our results demonstrate how the problem design in 2023 provided opportunities for dialogic instructor facilitation that centers students’ perspective and promotes student-centered learning (Mortimer and Scott, 2003; Scott and Mortimer, 2005; Scott et al., 2006; Dini et al., 2020; Carlos et al., 2023; Maggiore et al., 2024). Through the alternatives in the problem design, various student ideas were elicited that then gave the instructor the opportunity to position students’ ideas in dialogue, e.g., by revoicing and repeating (“And so what you’re saying is that…”, “That's what you’re saying?”, see example “That Makes Sense”, Fig. 12) and in written form, e.g., noting down students’ various arguments and drawing their ideas (see example “Comparing the Alternatives of the Problem”, Fig. 9). Through the problem design supporting students to compare, contrast, and weigh different thoughts, the instructor was able to facilitate in a more explorative, responsive way clarifying, repeating, and noticing student needs which was important for shaping the knowledge-building in a meaningful way for students (Hammer, 1997; Stanford et al., 2016; Carlos et al., 2023).
While the problem design in 2021 did not “enforce” clarifying and working with student thoughts about different alternatives, and instructor facilitation was often more authoritative focused on getting to one correct answer, sometimes the instructor still used dialogic facilitation focused on student reasoning to establish coherence in the knowledge-building process. For example, the instructor elicited student reasoning asking them to give a rationale of why they were claiming something (see example “Give a Rationale of Why”, Fig. 11). Here, the instructor as a facilitator took up the role to “enforce” coherence and explore students’ conceptual ideas and thus instructor facilitation brought in the “missing” element of coherence that the problem design was open for but did not provoke directly.
In practice, we need to anticipate and plan for how facilitation can direct the opportunities of the problem design. As we show, facilitation can support bringing elements to the fore that are “missing” after engagement with a problem, e.g., eliciting students’ ideas and different perspectives to build coherence, for example, through asking “how do you know?” (Drageset, 2014; Arnesen and Rø, 2024); and facilitation can also support to identify and productively guide towards building on what is already “present” after engagement with the problem, e.g., through representing and using students’ ideas to allow for meaningful knowledge-building. Since most instructor facilitation in college-level courses is focused on correctness instead of student ideas (Alkhouri et al., 2021; Gehrtz et al., 2022) and in our study thinking about alternatives, coherence, student agency, and dialogic facilitation went hand in hand, we recommend for college- or graduate-level STEM instructors to consider more often facilitating in a way that allows students’ ideas to be represented in dialogue and in writing, even when incorrect, to not only position them as valuable but to engage in exploring students’ needs after engagement with the problem. On the instructor's end this might lead to uncertainty when not facilitating towards a predetermined “correct” outcome of a problem solution. However, this could allow for “discovery” knowledge-building that is meaningful for the whole community, including the instructor, but especially the students (Hammer, 1997).
Extension of research on epistemology
Our research extends findings on epistemology and how it impacts mechanistic reasoning in Organic Chemistry. While past research demonstrates that students in classes that are designed for mechanistic reasoning show personal epistemologies that are more productive for engaging in mechanistic reasoning as a practice to make sense of phenomena instead of memorizing (Flaherty, 2020; Bowen et al., 2022), our study looks at this in the actual moment of interaction, understanding epistemology as a social practice. Our study shows that students’ epistemic agency can be a mechanism in the moment of interactions that enables students to engage in mechanistic reasoning as a practice. This occurred in both years, e.g., in 2023 in the example “Comparing the Alternatives of the Problem” (Fig. 9) and in 2021 in the example “Giving a Rationale of Why” (Fig. 11). Both examples show how when students are positioned as epistemic agents, they can engage in making use of mechanistic reasoning, e.g., drawing on conceptual reasoning about the how and why underpinning their claims when sharing how they came to know.While we did not directly investigate instructor epistemic messaging like others did (Russ, 2018; Popova et al., 2021; DeGlopper et al., 2023), our study of the impact of problem design on epistemic agency and coherence in student–instructor interactions in whole class discussions shows that problem design, agency, and coherence were also tightly connected to epistemic messaging. In both years, the instructor was overall engaged in eliciting students’ approaches, sending epistemic messages of being interested in their approaches. This allowed student agency and contributed to students’ needs and their ideas shaping the discourse. In 2023, this was more prevalent throughout the whole discourse, as the problem design positioned the instructor as a supportive facilitator rather than a cognitive authority, whereas in 2021, this was prevalent at the beginning of the WCDs. In 2021, during the body of the discussion, the instructor often seemed challenged to choose between establishing the entire “correct” mechanism that the problems were asking for vs. granting student agency, eliciting their reasoning that might not lead to the “correct” solution. As seen in our results, in those instances, the instructor often chose to direct agency to arrive at the most plausible mechanism (see example “Let's Generate the Bonds We Need to See”, Fig. 10, and example “More Plausible Mechanism”, Fig. 13), taking the agency herself by either providing her own reasoning or prompting students to fill in blanks. The latter relates to a concept that Miller et al. (2018) refer to as “pseudoagency” (Miller et al., 2018, p. 1065) “where students will be treated as agentive in constructing useful knowledge, only to the extent that they construct expected/canonical knowledge products” (Miller et al., 2018, p. 1065). The instructor having greater agency or granting pseudoagency positioned students rather as passive actors and “blank fillers” and sent the epistemic message that getting to the “correct” answer, as decided by the instructor, is valuable.
Thus, we see two contributions that our work is making with respect to epistemology in Organic Chemistry teaching. Not only does instructor epistemic messaging impact students’ epistemologies (Russ, 2018; Schafer et al., 2023; Schwarz et al., 2024), but the design of problems that students and instructors are working on set the stage for what students and instructors, through their facilitation and epistemic messaging, can and cannot do. In addition to being very intentional about problem design for their teaching, instructors can also take away from our study that paying attention to whether students have the opportunity to take epistemic agency can be a good indicator to assess whether they have opportunities to engage in mechanistic reasoning during the learning process.
Characterizing knowledge-building in organic chemistry
Our work adds to the knowledge-building literature in other areas of STEM (Stroupe, 2014; Manz, 2016; Miller et al., 2018; Ko and Krist, 2019; Krist et al., 2019) by demonstrating how knowledge-building can look like in WCDs in college- or graduate-level Organic Chemistry. Here, we point to two specific examples from our results that highlight moments of in-depth collaborative knowledge-building in which the instructor and “students build on one another's idea contributions and then rise above to find increasingly high-level accounts, helping to create the coherence that drives them toward deeper understanding” (Zhang et al., 2009, p. 11).How students acting as epistemic agents and sharing coherent contributions enabled knowledge-building can be shown in the example “That Makes Sense” in 2023. In the example, a student (student A) built upon another group's idea (Fig. 12, Box 1, center). Student A shared how an intramolecular reaction could account for the correct stereochemistry, making their logic explicit in a coherent explanation. This supported the knowledge-building process of the classroom at that moment, as accounting for the observed stereochemistry was the exact roadblock faced by the other group. The group could therefore use the newly introduced reasoning of student A (e.g., intramolecular reaction), which included explicit explanations for how student A built coherence for themselves. This enabled the group to build coherence in their thinking, which was indicated by one member saying that the suggested ideas of student A made sense to them. Thus, we see how epistemic agency and coherence allowed knowledge-building to happen collaboratively, with students and the facilitating instructor functioning collectively as a knowledge-building community. Importantly, the problem design gave the students and the instructor the opportunity to do this communal knowledge-building work by necessitating reasoning with two different alternative intermediates. Of these, one had the correct connectivity but incorrect reactivity to produce the needed stereochemistry (Fig. 12, top, intermediate C) and the other had the appropriate reactivity but not the correct connectivity for what the students “wished” would happen (Fig. 12, top and Box 1, intermediate B). These purposefully designed features in the alternatives presented allowed the students to build on each other's ideas collaboratively, producing a higher-level understanding for all parties involved.
The examples of PSS2 in 2023 (see example “Comparing the Alternatives of the Problem”, Fig. 9 and example “Using Students Arguments to Decide”, Fig. 14) also show how coherent, moment-to-moment contributions from different students throughout the discourse are an important driver for community knowledge-building. In the WCD of PSS2, students mostly had epistemic agency and shared coherent contributions, contributing arguments underpinned with conceptual reasoning throughout the discussion. This resulted in many different coherent arguments being collected by the instructor who functioned as a clarifying scribe on her tablet (Fig. 9 and 14). Although time ran out at the end of the WCD, causing the instructor taking agency to wrap up the discussion, the end of the discussion still demonstrates how working with multiple coherent student contributions allowed the building of new knowledge. Only through the different student contributions, including one that the instructor had not anticipated (about it being beneficial to first create more ring strain so that then more ring strain could be released), was the instructor prompted to switch from a structural account to an energetic account (Caspari et al., 2018a) and to explicitly discuss different energy pathways to decide between two alternatives. In fact, the instructor admitted that she had not explicitly thought about energy profiles for the problem prior to encountering and working with the student contributions. Again, it was the problem design itself that provided the platform for the knowledge-building community to develop arguments for or against various alternatives, allowing all members of the community to arrive at this, deeper than even planned, understanding at the end of the session.
In practice, our findings can provide Organic Chemistry instructors—including, and perhaps especially, those who do not identify as members of the Chemistry Education research community—with several useful indicators to pay attention to during WCDs to evaluate whether the discussion is going into a “good” direction or is “productive”. Instead of focusing solely on whether students arrive at the correct answer or not, our findings suggest that instructors can pay attention to whether the discussion elicits coherent reasoning from students, which then becomes public and usable for collaborative knowledge-building (Zhang et al., 2009; Stroupe, 2014; Kang et al., 2016). As mentioned in the previous section, instructors may also pay attention to whether multiple students are able to take epistemic agency and thus are able to advance the knowledge-building through diversity of ideas (Zhang et al., 2009; Damşa et al., 2010; Varelas et al., 2015). Often, we as instructors feel “good” when the class arrives at a complete, correct solution including in our study in the example “More Plausible Pathway” (Fig. 13) when the instructor was “so proud” that they “actually thought through the answer before the end of class”. Our study gives instructors an alternative of what to encourage during discussion and “feel good” about: when deeper understanding is being built with reasoning because of the diversity of ideas of different students like in the two examples above. In addition, our examples show how this kind of student-driven knowledge-building often also aligned with the canonically correct solution (compare Fig. 12, Box 3, and Fig. 14). Thus, our study demonstrates how focusing on correctness might not necessarily support student knowledge-building but how student-driven knowledge-building often leads to correctness as well.
Limitations and implications for future research
This study focused on the impact of different problem designs on epistemic agency and coherence. We acknowledge that problem design is not the only factor that impacts epistemic agency and coherence; and that epistemic agency and coherence are not the only factors that are impacted by problem design. Instructor–student interactions in whole class discussion are a complex teaching–learning situation that necessarily makes it impossible to capture all complexities. However, within our study design and our theoretical and analytical bounds, the impact of problem design on epistemic agency and coherence was most salient as described in the Results.Elaborating on the fact that problem design is not the only factor that impacts epistemic agency and coherence, we want to come back to two additional factors that were also put in context in our Discussion section: epistemologies and instructor facilitation. With respect to epistemologies, Kelly et al. (2012) have stated in their work on the relationship between science learning and epistemology that engagement in science practices is not only shaped by social practices but also by personal and disciplinary epistemologies. Though these dimensions are beyond the focus of our study, they certainly influence how knowledge-building takes place in mechanistic reasoning classrooms, as other researchers have already shown (Ko and Krist, 2019; Krist et al., 2019; DeGlopper and Stowe, 2024; Schwarz et al., 2024). While we did not capture it systematically limited through our theoretical and analytical approaches, we noticed how instructor epistemic messaging differed between 2021 and 2023. In the Discussion, we demonstrate how we saw a connection between the different problem designs and how this gave the instructor different opportunities for epistemic messaging. In our design research approach, it was the problem design that we changed intentionally from 2021 to 2023, and epistemic messaging seemed to change “automatically” without the team planning for it. The role of epistemic messaging in the complex relationship between problem design and epistemic agency and coherence needs further systematic investigation.
With respect to instructor facilitation, much is already known about how epistemic agency can be negotiated in facilitation (Stroupe, 2014; Moon et al., 2017; Berland et al., 2020; Cherbow, 2022). The instructor, traditionally positioned as an authority, often has cognitive authority with the power to shape the knowledge-building processes in classrooms. However, our study shows that problem design can redistribute this power to students by enabling them to build coherence and contribute ideas. Similarly, as for epistemic messaging, we noticed how instructor facilitation also differed between 2021 and 2023, while we did not capture it systematically through our analytical approaches. In the Discussion, we demonstrate how we saw a connection between the different problem designs and how this gave the instructor opportunities for different ways of facilitating the discourse. Like for epistemic messaging, it was the problem design that we changed intentionally from 2021 to 2023. While epistemic messaging seemed to change “automatically”, we note that our team has studied facilitation practices in college STEM settings (Carlos et al., 2023; Maggiore et al., 2024). This expertise led to explicit discussions between the instructor and the research team during both the development and implementation of the new problem design about the new problem design also necessitating a different way of facilitating WCDs. In fact, the instructor reflected that she adapted her facilitation practices from year to year, which synergized with the problem design. More systematic research is needed to explore how the combination of facilitation and implemented instructional materials empowers students to take epistemic agency and build coherence.
Similarly to factors other than problem design impacting epistemic agency and coherence, the problem design itself can also impact other factors of collaborative knowledge-building during instructor–student interactions than epistemic agency and coherence. While epistemic agency and coherence are first indicators for conceptualizing mechanistic reasoning as part of knowledge-building in discourse, future work needs to make a more direct connection to their impact on making use of structural features of mechanistic reasoning (Machamer et al., 2000; Goodwin, 2003; Russ et al., 2008; Caspari et al., 2018a). We also did not systematically analyze how the problem design influenced students’ ability to independently solve open single-case complex mechanism problems or how they independently built mechanistic explanations throughout the semester. Anecdotal evidence suggests that the 2021 problem design preferentially supported the most vocal students in the class in developing a mechanism from scratch, while the 2023 problem design preferentially supported the most vocal students more in constructing in-depth mechanistic explanations. Parallel to this missing systematic insight in the mechanistic realm, our data also provides a preliminary indication that the different problem designs impacted additional dimensions in the social realm of classroom dynamics, such as the level of participation for different students and the ways students interacted with each other. Furthermore, our analysis focused on data of public discourse in the classroom. We did not examine how all students, including those who participated less vocally and who did not explicitly take epistemic agency, may have contributed to knowledge-building or constructed coherence in more subtle ways. Instead, our analysis was limited to the students who were vocal in presenting approaches to the entire class. Our future work will include analysis of the small group discussions that occurred before the WCDs. It will also include incorporating additional analytical frameworks, like those on shared epistemic agency, which aims at relating individual and group processes with regards to epistemic agency and mechanistic reasoning (Damşa et al., 2010; Baze and González-Howard, 2025).
Lastly, we want to acknowledge that the interactions in this study took place within a graduate-level classroom in the United States of America. The discourse followed Western scientific norms and took place in the English language. Also, we as researchers are situated within Western-centric academic contexts, which shapes how we interpret and understand data and classroom practices. Furthermore, this study does not explicitly examine sociopolitical dimensions of classroom discourse (Varelas et al., 2015; Suárez et al., 2023). We did not analyze how racialized, gendered, or other structural dynamics (e.g., Carlone et al., 2015; Rivera Maulucci et al., 2015; Varelas et al., 2015; Baze and González-Howard, 2025) may influence whose ideas were positioned as valuable. These are critical aspects influencing classroom dynamics that future research and practice should address to move toward a more equitable understanding of knowledge-building practices, specifically because there has been first indication that complex problems through allowing multiple alternative approaches can help make classrooms more equitable (Nolen et al., 2024). To do so, we suggest combining our analytical framework with other critical frameworks.
Conclusion
Our study contributes to the field of mechanistic reasoning in Organic Chemistry research by giving insights into how single-case vs. case-comparison complex mechanism problems implemented in 2021 and 2023 impact students’ opportunities to take epistemic agency and to bring in coherent reasoning during knowledge-building in the actual moment of classroom discourse. Our quantitative results indicate a change of code distributions from “Instructor Agency” towards “Student Agency” and from “No Coherence” towards “Coherence” from 2021 to 2023. Additionally, our qualitative analysis showed when and how agency and coherence codes occurred, allowing us to characterize how knowledge-building took place when students and instructors discussed the different problems. In 2021, the goal of the single-case complex mechanism problems of proposing steps to arrive at a complete and correct mechanism did not necessitate students to build coherence, as proposed steps were often not accompanied by sharing how students came to know they were valid. This was often reinforced by the instructor taking charge as the epistemic agent guiding students towards said goal. Hence, in 2021, the discourse was largely guided towards building authority-driven “correct” solutions instead of engaging students in collaborative knowledge-building with shared reasoning. However, the case-comparison design in 2023 enabled students to be more agentic and bring more coherent explanations to the fore throughout the discourse, making their use of mechanistic reasoning explicit, e.g., through sharing how they used the different alternatives and were thinking about them in contrast to each other. In 2023, this, in combination with dialogic facilitation, allowed student-centered and collaborative knowledge-building. In most cases, this knowledge-building ultimately aligned with the canonically correct solutions, even as the stepwise development of a mechanism (like in 2021) was deprioritized during discussion. Hence, we show how different problem designs impacted epistemic agency and coherence and with that the nature of the knowledge-building process in the actual moment of classroom discourse.Our study provides a set of problems for which we demonstrated that students were largely able to act as epistemic agents who shared their reasoning coherently. These problems not only support literacy in authentic problem-solving but also enable collaborative knowledge-building. In this meaningful knowledge-building, different students’ ideas and their use of mechanistic reasoning practices were elicited and positioned as meaningful. With our analytical lenses of “agency” and “coherence”, we further open the field for new perspectives on evaluating “productivity” of mechanistic reasoning discourse. Observing whether students have the opportunity to take epistemic agency and whether their contributions are coherent might be a promising avenue to evaluate if and how students actively develop deeper understanding in Organic Chemistry.
Ethical considerations
Ethical approval was received from Tufts University Institutional Review Board (IRB # 00001939). Participants provided electronic consent via an online Qualtrics form.Author contributions
ICG and RAS designed the study. ICG and RAS performed data collection. JE and ICG engaged in data analysis. JE and ICG interpreted and discussed the data. JE and ICG wrote the original draft of this manuscript, RAS reviewed and edited it. All authors read and approved this manuscript.Conflicts of interest
There are no conflicts to declare.List of abbreviations
| PEA | Practical Epistemology Analysis |
| SGD | Small group discussion |
| SGDs | Small group discussions |
| WCD | Whole class discussion |
| WCDs | Whole class discussions |
| PSS | Problem-solving session |
| PSSs | Problem-solving sessions |
| i.e. | id est |
| e.g. | Exempli gratia |
Data availability
The data for this study collected from human participants cannot be made available due to ethical confidentiality requirements.Appendix 1. Complex single-case problems used in 2021
Problem-solving session 2 in 2021
 | ||
| Fig. 15 Problem in PSS2 in 2021. Problem reproduced with permission from SNCSC. Adopted from Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 147, problem (aa), 2003, Springer-Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
Problem-solving session 3 in 2021
 | ||
| Fig. 16 Problem in PSS3 in 2021. Problem reproduced with permission from SNCSC. Adopted from Carey and Sundberg, Advanced Organic chemistry: Part A: Structure and Mechanisms, Fifth Edition, p. 381f, problem 3.11, 2007, Springer Science + Business Media. https://link.springer.com/book/10.1007/978-0-387-44899-2 Problem based on the original mechanism of the Cannizzaro reaction reprinted with permission from Swain et al. (1979). Copyright 1979 American Chemical Society. | ||
Problem-solving session 4 in 2021
 | ||
| Fig. 17 Problem in PSS4 in 2021. Problem adapted from Francis's (2004) teaching materials based on Martinet's et al. (1969, 1970, 1971) initial example of a Pinacol-terminated Prins cyclization, adapted with permission from Overman and Pennington (2003). Copyright 2003 American Chemical Society. | ||
Problem-solving session 5 in 2021
 | ||
| Fig. 18 Problem in PSS5 in 2021. Problem reproduced with permission from SNCSC. Adopted from Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 144, problem (i), 2003, Springer Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
Problem-solving session 6 in 2021
 | ||
| Fig. 19 Problem in PSS6 in 2021. Mechanism in problem based on initial observations reported by Meerwein et al. (1961). Mechanism adopted with permission from Wick et al. (1964). CLAISEN'sche Umlagerungen bei Allyl- und Benzylalkoholen mit Hilfe von Acetalen des N,N-dimethylacetamids. Vorläufige Mitteilung. Helvetica Chimica Acta. Copyright © 1964 Verlag GmbH & Co. KGaA, Weinheim. | ||
Appendix 2. Complex case-comparison problems used in 2023
Problem-solving session 2 in 2023
 | ||
| Fig. 20 Problem in PSS2 in 2023. Problem developed by us into a case comparison based on the original problem that was reproduced with permission from SNCSC: Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 147, problem (aa), 2003, Springer Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
Problem-solving session 3 in 2023
 | ||
| Fig. 21 Problem in PSS3 in 2023. Problem developed by us into a case comparison based on the original problem that was reproduced with permission from SNCSC: Carey and Sundberg, Advanced Organic chemistry: Part A: Structure and Mechanisms, Fifth Edition, p. 381f., problem 3.11, 2007, Springer Science + Business Media. https://link.springer.com/book/10.1007/978-0-387-44899-2. Problem based on the original mechanism of the Cannizzaro reaction reprinted with permission from Swain et al. (1979). Copyright 1979 American Chemical Society. | ||
Problem-solving session 4 in 2023
 | ||
| Fig. 22 Problem in PSS4 in 2023. Problem developed by us into a case comparison based on the original problem from Francis's (2004) teaching materials based on Martinet's et al. (1969, 1970, 1971) initial example of a Pinacol-terminated Prins cyclization, adapted with permission from Overman and Pennington (2003). Copyright 2003 American Chemical Society. | ||
Problem-solving session 5 in 2023
 | ||
| Fig. 23 Problem in PSS5 in 2023. Problem developed by us into a case comparison based on the original problem reproduced with permission from SNCSC: Grossman, The Art of Writing Reasonable Organic Reaction Mechanisms, Second Edition, p. 144, problem (i), 2003, Springer Verlag. https://link.springer.com/book/10.1007/978-3-030-28733-7. | ||
Problem-solving session 6 in 2023
 | ||
| Fig. 24 Problem in PSS6 in 2023. Problem developed by us into a case comparison. Mechanism in problem based on initial observations reported by Meerwein et al. (1961). Mechanism adopted with permission from Wick et al. (1964). CLAISEN'sche Umlagerungen bei Allyl- und Benzylalkoholen mit Hilfe von Acetalen des N,N-dimethylacetamids. Vorläufige Mitteilung. Helvetica Chimica Acta. Copyright © 1964 Verlag GmbH & Co. KGaA, Weinheim. | ||
Acknowledgements
This work was supported by Tufts Springboard and the Tufts Faculty Research Fund. Any opinions, findings, conclusions, and recommendations expressed in this material are those of the authors and do not necessarily reflect the views of Tufts. We thank all research study participants for making this work possible. We would also like to thank all research group members past and present who contributed to data collection and provided helpful feedback on our analysis: Jessica Karch, Anais Nevárez, Lee Price, Nicolette Maggiore, Georges Pichard, Chinwendu Igboekulie, Emmanuella Boateng, and Annabelle Wu. Further we would like to thank Sascha Bernholt for advice on statistical questions.References
- Alkhouri J. S., Donham C., Pusey T. S., Signorini A., Stivers A. H. and Kranzfelder P., (2021), Look Who's Talking: Teaching and Discourse Practices across Discipline, Position, Experience, and Class Size in STEM College Classrooms, Bioscience, 71(10), 1063–1078.
- Anderson T. L. and Bodner G. M., (2008), What can we do about ‘Parker’? A case study of a good student who didn't ‘get’ organic chemistry, Chem. Educ. Res. Pract., 9(2), 93–101.
- Anzovino M. E. and Bretz S. L., (2016), Organic chemistry students' fragmented ideas about the structure and function of nucleophiles and electrophiles: a concept map analysis, Chem. Educ. Res. Pract., 17(4), 1019–1029.
- Arnesen K. K. and Rø K., (2024), The complexity of supporting reasoning in a mathematics classroom of shared authority, Math. Think. Learn., 26(2), 159–184.
- Bakhtin M. M., (1983/1934), Discourse in the novel, in Holquist M. (ed.), The dialogic imagination: Four essays, Austin: University of Texas Press, pp. 259–422.
- Banerjee M., Capozzoli M., McSweeney L. and Sinha D., (1999), Beyond kappa: a review of interrater agreement measures, Can. J. Stat., 27(1), 3–23.
- Baze C. and González-Howard M., (2025), A call to explicitly name and account for power in epistemic agency research, Sci. Educ., 109(5), 1499–1505.
- Berland L. K., Russ R. S. and West C. P., (2020), Supporting the Scientific Practices through Epistemologically Responsive Science Teaching, J. Sci. Teach. Educ., 31(3), 264–290.
- Bernholt S., Eckhard J., Rodemer M., Langner A., Asmussen G. and Graulich N., (2023), Designing Tutorial Videos to Support Students’ Learning of Reaction Mechanisms in Organic Chemistry in Dori Y. J., Ngai C. and Szteinberg G. (ed.), Digital Learning and Teaching in Chemistry: An International and Inclusive Approach, Royal Society of Chemistry Publishing, ch. 9, pp. 234–248.
- Bhattacharyya G., (2013), From Source to Sink: Mechanistic Reasoning Using the Electron-Pushing Formalism, J. Chem. Educ., 90(10), 1282–1289.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Bhattacharyya G. and Harris M. S., (2017), Compromised Structures: Verbal Descriptions of Mechanism Diagrams, J. Chem. Educ., 95(3), 366–375.
- Blackford K. A., Greenbaum J. C., Redkar N. S., Gaillard N. T., Helix M. R. and Baranger A. M., (2023), Metacognitive regulation in organic chemistry students: how and why students use metacognitive strategies when predicting reactivity, Chem. Educ. Res. Pract., 24(3), 828–851.
- Bodé N. E., Deng J. M. and Flynn A. B., (2019), Getting Past the Rules and to the WHY: Causal Mechanistic Arguments When Judging the Plausibility of Organic Reaction Mechanisms, J. Chem. Educ., 96(6), 1068–1082.
- Bongers A., Beauvoir B., Streja N., Northoff G. and Flynn A. B., (2020), Building mental models of a reaction mechanism: the influence of static and animated representations, prior knowledge, and spatial ability, Chem. Educ. Res. Pract., 21(2), 496–512.
- BouJaoude S. B., (1991), A study of the nature of students' understandings about the concept of burning, J. Res. Sci. Teach., 28(8), 689–704.
- Bowen G. A., (2009), Supporting a grounded theory with an audit trail: an illustration, Int. J. Soc. Res. Methodol., 12(4), 305–316.
- Bowen R. S., Flaherty A. A. and Cooper M. M., (2022), Investigating student perceptions of transformational intent and classroom culture in organic chemistry courses, Chem. Educ. Res. Pract., 23(3), 560–581.
- Brennan R. L. and Prediger D. J., (1981), Coefficient Kappa: Some Uses, Misuses, and Alternatives, Educ. Psychol. Meas., 41(3), 687–699.
- Carey F. A. and Sundberg R. J., (2007), Advanced organic chemistry: Part A: structure and mechanisms, 5th edn, New York: Springer Science & Business Media.
- Carlone H. B., Johnson A. and Scott C. M., (2015), Agency amidst formidable structures: How girls perform gender in science class, J. Res. Sci. Teach., 52(4), 474–488.
- Carlos C. M. L., Maggiore N. M., Dini V. and Caspari-Gnann I., (2023), Characterizing facilitation practices of learning assistants: an authoritative-to-dialogic spectrum, Int. J. STEM Educ., 10(1), 38.
- Caspari I. and Graulich N., (2019), Scaffolding the structure of organic chemistry students’ multivariate comparative mechanistic reasoning, Int. J. Phys. Chem. Ed., 11(2), 31–43.
- Caspari I., Kranz D. and Graulich N., (2018a), Resolving the complexity of organic chemistry students' reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19(4), 1117–1141.
- Caspari I., Weinrich M. L., Sevian H. and Graulich N., (2018b), This mechanistic step is “productive”: organic chemistry students' backward-oriented reasoning, Chem. Educ. Res. Pract., 19(1), 42–59.
- Cherbow K., (2022), Responsive instructional design for students' epistemic agency: documenting episodes of principled improvisation in storyline enactment, J. Res. Sci. Teach., 60(4), 807–846.
- Cohen J., (1960), A coefficient of agreement for nominal scales, Educ. Psychol. Meas., 20(1), 37–46.
- Cole R., (2023), Inter-Rater Reliability Methods in Qualitative Case Study Research, Sociol. Methods Res., 53(4), 1944–1975.
- Cooper M. M., (2015), Why ask why? J. Chem. Educ., 92(8), 1273–1279.
- Cooper M. and Klymkowsky M., (2013), Chemistry, Life, the Universe, and Everything: A New Approach to General Chemistry, and a Model for Curriculum Reform, J. Chem. Educ., 90(9), 1116–1122.
- Cooper M. M., Kouyoumdjian H. and Underwood S. M., (2016), Investigating Students’ Reasoning about Acid–Base Reactions, J. Chem. Educ., 93(10), 1703–1712.
- Cooper M. M., Stowe R. L., Crandell O. M. and Klymkowsky M. W., (2019), Organic Chemistry, Life, the Universe and Everything (OCLUE): A Transformed Organic Chemistry Curriculum, J. Chem. Educ., 96(9), 1858–1872.
- Cramér H., (1999), Mathematical methods of statistics, vol. 9, Princeton, NJ: Princeton University Press.
- Crandell O. M., Kouyoumdjian H., Underwood S. M. and Cooper M. M., (2018), Reasoning about Reactions in Organic Chemistry: Starting It in General Chemistry, J. Chem. Educ., 96(2), 213–226.
- Creswell J. W. and Miller D. L., (2000), Determining Validity in Qualitative Inquiry, Theor. Pract., 39(3), 124–130.
- Crowder C. J., Yik B. J., Frost S. J. H., Cruz-Ramírez de Arellano D. and Raker J. R., (2024), Impact of Prompt Cueing on Level of Explanation Sophistication for Organic Reaction Mechanisms, J. Chem. Educ., 101(2), 398–410.
- Damşa C. I., Kirschner P. A., Andriessen J. E. B., Erkens G. and Sins P. H. M., (2010), Shared epistemic agency: an empirical study of an emergent construct, J. Learn. Sci., 19(2), 143–186.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental Rolodexing: Senior Chemistry Majors’ Understanding of Chemical and Physical Properties, J. Chem. Educ., 92(3), 415–426.
- DeGlopper K. S., Russ R. S., Sutar P. K. and Stowe R. L., (2023), Beliefs versus resources: a tale of two models of epistemology, Chem. Educ. Res. Pract., 24(2), 768–784.
- DeGlopper K. S. and Stowe R. L., (2024), Modeling students’ epistemic cognition in undergraduate chemistry courses: a review, Chem. Educ. Res. Pract., 25(3), 594–612.
- Deng J. M. and Flynn A. B., (2021), Reasoning, granularity, and comparisons in students’ arguments on two organic chemistry items, Chem. Educ. Res. Pract., 22(3), 749–771.
- Dini V., Sevian H., Caushi K. and Orduña Picón R., (2020), Characterizing the formative assessment enactment of experienced science teachers, Sci. Educ., 104(2), 290–325.
- Dood A. J. and Watts F. M., (2022a), Students’ strategies, struggles, and successes with mechanism problem solving in organic chemistry: a scoping review of the research literature, J. Chem. Educ., 100(1), 53–68.
- Dood A. J. and Watts F. M., (2022b), Mechanistic Reasoning in Organic Chemistry: A Scoping Review of How Students Describe and Explain Mechanisms in the Chemistry Education Research Literature, J. Chem. Educ., 99(8), 2864–2876.
- Drageset O. G., (2014), Redirecting, progressing, and focusing actions—a framework for describing how teachers use students’ comments to work with mathematics, Educ. Stud. Math., 85, 281–304.
- Eckhard J., Rodemer M., Bernholt S. and Graulich N., (2022), What Do University Students Truly Learn When Watching Tutorial Videos in Organic Chemistry? An Exploratory Study Focusing on Mechanistic Reasoning, J. Chem. Educ., 99(6), 2231–2244.
- Engeström Y., (1999), Activity theory and individual and social transformation, in Engeström Y., Miettinen R. and Punamäki-Gitai R.-L. (ed.), Perspectives on activity theory, Cambridge: Cambridge University Press, vol. 19, pp. 19–30.
- Esselman B. J., Hill N. J., DeGlopper K. S., Ellison A. J., Stowe R. L., Schwarz C. E. and Ellias N. J., (2023), Authenticity-Driven Design of a High-Enrollment Organic Laboratory Course, J. Chem. Educ., 100(12), 4674–4685.
- Fisher R. A., (1922), On the interpretation of χ2 from contingency tables, and the calculation of P, J. R. Stat. Soc., 85(1), 87–94.
- Flaherty A. A., (2020), Investigating perceptions of the structure and development of scientific knowledge in the context of a transformed organic chemistry lecture course, Chem. Educ. Res. Pract., 21(2), 570–581.
- Flynn A. B., (2011), Developing problem-solving skills through retrosynthetic analysis and clickers in organic chemistry, J. Chem. Educ., 88(11), 1496–1500.
- Flynn A. B., (2014), How do students work through organic synthesis learning activities? Chem. Educ. Res. Pract., 15(4), 747–762.
- Flynn A. B., (2021), Scaffolding Synthesis Skills in Organic Chemistry, in Tsaparlis G. (ed.), Problems and Problem Solving in Chemistry Education, The Royal Society of Chemistry, pp. 145–165.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before Reactions: A Mechanistic Approach to the Organic Chemistry Curriculum Based on Patterns of Electron Flow, J. Chem. Educ., 92(5), 803–810.
- Francis M. B., (2004), Task based on Mousset's (1969, 1970, 1971) initial example of a Pinacol-terminated Prins cyclization. Course materials from a graduate level Chemistry course (Chem 200, UC Berkeley).
- Galloway K. R., Leung M. W. and Flynn A. B., (2019), Patterns of reactions: a card sort task to investigate students’ organization of organic chemistry reactions, Chem. Educ. Res. Pract., 20(1), 30–52.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18(2), 353–374.
- Gehrtz J., Brantner M. and Andrews T. C., (2022), How are undergraduate STEM instructors leveraging student thinking? Int. J. STEM Educ., 9(1), 18.
- Gibbons R. E., Villafañe S. M., Stains M., Murphy K. L. and Raker J. R., (2018), Beliefs about learning and enacted instructional practices: an investigation in postsecondary chemistry education, J. Res. Sci. Teach., 55(8), 1111–1133.
- Goodwin W., (2003), Explanation in organic chemistry, Ann. N. Y. Acad. Sci., 988(1), 141–153.
- Goodwin W. M., (2007), Structural formulas and explanation in organic chemistry, Found. Chem., 10(2), 117–127.
- Graulich N., (2014), Intuitive Judgments Govern Students’ Answering Patterns in Multiple-Choice Exercises in Organic Chemistry, J. Chem. Educ., 92(2), 205–211.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16(1), 9–21.
- Graulich N., (2025), The tip of the iceberg in organic chemistry – revisited, Chem. Educ. Res. Pract., 26(2), 359–376.
- Graulich N., Hedtrich S. and Harzenetter R., (2019), Explicit versus implicit similarity – exploring relational conceptual understanding in organic chemistry, Chem. Educ. Res. Pract., 20(4), 924–936.
- Graulich N. and Schween M., (2018), Concept-Oriented Task Design: Making Purposeful Case Comparisons in Organic Chemistry, J. Chem. Educ., 95(3), 376–383.
- Graulich N., Tiemann R. and Schreiner P. R., (2012), Heuristic chemistry—a qualitative study on teaching domain-specific strategies for the six-electron case, Chem. Educ. Res. Pract., 13(3), 337–347.
- Greco J., (1999), Introduction: What is Epistemology? in Greco J. and Sosa E. (ed.), The Blackwell Guide to Epistemology, Oxford: Blackwell Publishing Ltd, pp. 1–31.
- Grossman R. B., (2003), The Art of Writing Reasonable Organic Reaction Mechanisms, 2nd edn, New York: Springer-Verlag.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13(3), 201–208.
- Grove N. P., Cooper M. M. and Cox E. L., (2012), Does Mechanistic Thinking Improve Student Success in Organic Chemistry? J. Chem. Educ., 89(7), 850–853.
- Gupte T., Watts F. M., Schmidt-McCormack J. A., Zaimi I., Gere A. R. and Shultz G. V., (2021), Students’ meaningful learning experiences from participating in organic chemistry writing-to-learn activities, Chem. Educ. Res. Pract., 22(2), 396–414.
- Hammer D., (1997), Discovery Learning and Discovery Teaching, Cogn. Instr., 15(4), 485–529.
- Hamza K. M. and Wickman P. O., (2008), Describing and analyzing learning in action: an empirical study of the importance of misconceptions in learning science, Sci. Educ., 92(1), 141–164.
- Harris C. R., Millman K. J., van der Walt S. J., Gommers R., Virtanen P., Cournapeau D., Wieser E., Taylor J., Berg S., Smith N. J., Kern R., Picus M., Hoyer S., van Kerkwijk M. H., Brett M., Haldane A., Del Rio J. F., Wiebe M., Peterson P., Gerard-Marchant P., Sheppard K., Reddy T., Weckesser W., Abbasi H., Gohlke C. and Oliphant T. E., (2020), Array programming with NumPy, Nature, 585(7825), 357–362.
- Hedderich J. and Sachs L., (2020), Angewandte Statistik – Methodensammlung mit R, Heidelberg: Springer Spektrum Berlin.
- Helix M. R., Blackford K. A., Firestein Z. M., Greenbaum J. C., Gibson K. and Baranger A. M., (2022), Characterization of student problem solving and development of a general workflow for predicting organic reactivity, Chem. Educ. Res. Pract., 23(4), 844–875.
- Hempel C. G. and Oppenheim P., (1948), Studies in the Logic of Explanation, Philos. Sci., 15(2), 135–175.
- Houchlei S. K., Bloch R. R. and Cooper M. M., (2021), Mechanisms, Models, and Explanations: Analyzing the Mechanistic Paths Students Take to Reach a Product for Familiar and Unfamiliar Organic Reactions, J. Chem. Educ., 98(9), 2751–2764.
- Irby S. M., Phu A. L., Borda E. J., Haskell T. R., Steed N. and Meyer Z., (2016), Use of a card sort task to assess students' ability to coordinate three levels of representation in chemistry, Chem. Educ. Res. Pract., 17(2), 337–352.
- Jackson A. Y. and Mazzei L. A., (2013), Plugging One Text Into Another, Qual. Inq., 19(4), 261–271.
- Jackson A. Y. and Mazzei L. A., (2017), Thinking with Theory: A New Analytic for Qualitative Inquiry in Denzin N. K. and Lincoln Y. S. (ed.), The SAGE Handbook of Qualitative Research, 5th edn, Thousand Oaks, California: SAGE Publications, Inc., ch. 32, pp. 717–737.
- Kang H., Windschitl M., Stroupe D. and Thompson J., (2016), Designing, launching, and implementing high quality learning opportunities for students that advance scientific thinking, J. Res. Sci. Teach., 53(9), 1316–1340.
- Karch J. M., Maggiore N. M., Pierre-Louis J. R., Strange D., Dini V. and Caspari-Gnann I., (2024), Making in-the-moment learning visible: a framework to identify and compare various ways of learning through continuity and discourse change, Sci. Educ., 108(5), 1292–1328.
- Keiner L. and Graulich N., (2021), Beyond the beaker: students’ use of a scaffold to connect observations with the particle level in the organic chemistry laboratory, Chem. Educ. Res. Pract., 22(1), 146–163.
- Kelly G. J., McDonald S. and Wickman P.-O., (2012), Science Learning and Epistemology in Fraser B. J., Tobin K. and McRobbie C. J. (ed.), Second International Handbook of Science Education, Dordrecht: Springer Netherlands, pp. 281–291.
- Ko M. L. M. and Krist C., (2019), Opening up curricula to redistribute epistemic agency: a framework for supporting science teaching, Sci. Educ., 103(4), 979–1010.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Kranz D., Schween M. and Graulich N., (2023), Patterns of reasoning – exploring the interplay of students’ work with a scaffold and their conceptual knowledge in organic chemistry, Chem. Educ. Res. Pract., 24(2), 453–477.
- Krieter F. E., Julius R. W., Tanner K. D., Bush S. D. and Scott G. E., (2016), Thinking like a chemist: development of a chemistry card-sorting task to probe conceptual expertise, J. Chem. Educ., 93(5), 811–820.
- Krist C., Schwarz C. V. and Reiser B. J., (2019), Identifying essential epistemic heuristics for guiding mechanistic reasoning in science learning, J. Learn. Sci., 28(2), 160–205.
- Landis J. R. and Koch G. G., (1977), An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers, Biometrics, 33(2), 363–374.
- Lektorsky V. A., (2009), Mediation as a Means of Collective Activity in Sannino A., Daniels H. and Gutiérrez K. D. (ed.), Learning and Expanding with Activity Theory, Cambridge: Cambridge University Press, pp. 75–87.
- Lidar M., Lundqvist E. and Östman L., (2006), Teaching and learning in the science classroom: the interplay between teachers' epistemological moves and students' practical epistemology, Sci. Educ., 90(1), 148–163.
- Lieber L. and Graulich N., (2020), Thinking in Alternatives—A Task Design for Challenging Students’ Problem-Solving Approaches in Organic Chemistry, J. Chem. Educ., 97(10), 3731–3738.
- Macbeth D., (2001), On “reflexivity” in qualitative research: two readings, and a third, Qual. Inq., 7(1), 35–68.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about Mechanisms, Philos. Sci., 67(1), 1–25.
- Mackonis A., (2013), Inference to the best explanation, coherence and other explanatory virtues, Synthese, 190(6), 975–995.
- Maeyer J. and Talanquer V., (2010), The role of intuitive heuristics in students' thinking: ranking chemical substances, Sci. Educ., 94(6), 963–984.
- Maggiore N. M., Powers K. P., Lwanga K. L. and Caspari-Gnann I., (2024), The impact of learning assistant facilitation practices on student in-the-moment learning, Int. J. STEM Educ., 11(1), 1–44.
- Manz E., (2016), Examining evidence construction as the transformation of the material world into community knowledge, J. Res. Sci. Teach., 53(7), 1113–1140.
- Martinet P. and Mousset G., (1970), Isomerisation of cyclic acetals. 1. Stereochemical influences on participation of ethylenic systems, Bull. Soc. Chim. Fr., 3, 1071–1076.
- Martinet P. and Mousset G., (1971), Bull. Soc. Chim. Fr., 4093–4096.
- Martinet P., Mousset G. and Michel M., (1969), C. R. Acad. Sci., 268 1303–1306.
- Meerwein H., Florian W., Schön N. and Stopp G., (1961), Über Säureamidacetale, Harnstoffacetale und Lactamacetale, Justus Liebigs Ann. Chem., 641(1), 1–39.
- Miller E., Manz E., Russ R., Stroupe D. and Berland L., (2018), Addressing the epistemic elephant in the room: epistemic agency and the next generation science standards, J. Res. Sci. Teach., 55(7), 1053–1075.
- Moon A., Stanford C., Cole R. and Towns M., (2017), Decentering: A Characteristic of Effective Student–Student Discourse in Inquiry-Oriented Physical Chemistry Classrooms, J. Chem. Educ., 94(7), 829–836.
- Moreira P., Marzabal A. and Talanquer V., (2019), Using a mechanistic framework to characterise chemistry students' reasoning in written explanations, Chem. Educ. Res. Pract., 20(1), 120–131.
- Mortimer E. F. and Scott P. H., (2003), Meaning making in secondary science classrooms, Philadelphia: Open University Press.
- Nasir N. S. and Hand V. M., (2006), Exploring sociocultural perspectives on race, culture, and learning, Rev. Educ. Res., 76(4), 449–475.
- Nolen S., Michor E. and Koretsky M., (2024), The Benefits of Complex Tasks: moving away from single-answer problems can help make classrooms more equitable, Am. Soc. Eng. Educ., 33(3), 22.
- Overman L. E. and Pennington L. D., (2003), Strategic Use of Pinacol-Terminated Prins Cyclizations in Target-Oriented Total Synthesis, J. Org. Chem., 68(19), 7143–7157.
- Pearson K. X., (1900), On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling, Lond. Edinb. Dubl. Phil. Mag., 50(302), 157–175.
- Petritis S. J., Kelley C. and Talanquer V., (2021), Exploring the impact of the framing of a laboratory experiment on the nature of student argumentation, Chem. Educ. Res. Pract., 22(1), 105–121.
- Ponterotto J. G., (2006), Brief note on the origins, evolution, and meaning of the qualitative research concept thick description, Qual. Rep., 11(3), 538–549.
- Popova M. and Bretz S. L., (2018), Organic chemistry students’ interpretations of the surface features of reaction coordinate diagrams, Chem. Educ. Res. Pract., 19(3), 919–931.
- Popova M., Kraft A., Harshman J. and Stains M., (2021), Changes in teaching beliefs of early-career chemistry faculty: a longitudinal investigation, Chem. Educ. Res. Pract., 22(2), 431–442.
- Raker J. R. and Towns M. H., (2012a), Designing undergraduate-level organic chemistry instructional problems: seven ideas from a problem-solving study of practicing synthetic organic chemists, Chem. Educ. Res. Pract., 13(3), 277–285.
- Raker J. R. and Towns M. H., (2012b), Problem types in synthetic organic chemistry research: implications for the development of curricular problems for second-year level organic chemistry instruction, Chem. Educ. Res. Pract., 13(3), 179–185.
- Rivera Maulucci M. S., Brotman J. S. and Fain S. S., (2015), Fostering structurally transformative teacher agency through science professional development, J. Res. Sci. Teach., 52(4), 545–559.
- Rodemer M., Eckhard J., Graulich N. and Bernholt S., (2020), Decoding case comparisons in organic chemistry: eye-tracking students’ visual behavior, J. Chem. Educ., 3530–3539.
- Rodemer M., Eckhard J., Graulich N. and Bernholt S., (2021), Connecting explanations to representations: benefits of highlighting techniques in tutorial videos on students’ learning in organic chemistry, Int. J. Sci. Educ., 43(17), 2707–2728.
- Roth W.-M. and Lee Y.-J., (2007), “Vygotsky's Neglected Legacy”: Cultural-Historical Activity Theory, Rev. Educ. Res., 77(2), 186–232.
- Russ R. S., (2018), Characterizing teacher attention to student thinking: a role for epistemological messages, J. Res. Sci. Teach., 55(1), 94–120.
- Russ R. S., Scherr R. E., Hammer D. and Mikeska J., (2008), Recognizing mechanistic reasoning in student scientific inquiry: a framework for discourse analysis developed from philosophy of science, Sci. Educ., 92(3), 499–525.
- Saldaña J., (2013), The coding manual for qualitative researchers, London: Sage Publications Limited.
- Salmon W. C., (1984), Scientific explanation and the causal structure of the world, Princeton, NJ: Princeton University Press.
- Sandoval W., (2014), Conjecture Mapping: An Approach to Systematic Educational Design Research, J. Learn. Sci., 23(1), 18–36.
- Sandoval W. A., Greene J. A. and Bråten I., (2016), Understanding and Promoting Thinking About Knowledge, Rev. Educ. Res., 40(1), 457–496.
- Scardamalia M., (2002), Collective cognitive responsibility for the advancement of knowledge, in B. S. (ed.), Liberal education in a knowledge society, Chicago, IL: Open Court, ch. 4, pp. 67–98.
- Scardamalia M. and Bereiter C., (1991), Higher Levels of Agency for Children in Knowledge Building: A Challenge for the Design of New Knowledge Media, J. Learn. Sci., 1(1), 37–68.
- Scardamalia M. and Bereiter C., (2003), Knowledge building environments: extending the limits of the possible in education and knowledge work, in DiStefano A., Rudestam K. E. and Silverman R. (ed.), Encyclopedia of distributed learning, Thousand Oaks, CA: Sage Publications, pp. 269–272.
- Scardamalia M. and Bereiter C., (2021), Knowledge Building: Advancing the State of Community Knowledge, in CressU., Rosé C., Wise A. F. and Oshima J. (ed.), International Handbook of Computer-Supported Collaborative Learning, Cham: Springer International Publishing, pp. 261–279.
- Schafer A. G. L., Kuborn T. M., Schwarz C. E., Deshaye M. Y. and Stowe R. L., (2023), Messages about valued knowledge products and processes embedded within a suite of transformed high school chemistry curricular materials, Chem. Educ. Res. Pract., 24(1), 71–88.
- Schmidt-McCormack J. A., Judge J. A., Spahr K., Yang E., Pugh R., Karlin A., Sattar A., Thompson B. C., Gere A. R. and Shultz G. V., (2019), Analysis of the role of a writing-to-learn assignment in student understanding of organic acid–base concepts, Chem. Educ. Res. Pract., 20(2), 383–398.
- Schwarz C. E., DeGlopper K. S., Greco N. C., Russ R. S. and Stowe R. L., (2024), Modeling Student Negotiation of Assessment-Related Epistemological Messages in a College Science Course, Sci. Educ., 109(2), 429–447.
- Scott P. and Mortimer E., (2005), Meaning Making in High School Science Classrooms: A Framework for Analysing Meaning Making Interactions, in Boersma K., Goedhart M., de Jong O. and Eijkelhof H. (ed.), Research and the Quality of Science Education, Dordrecht: Springer Netherlands, pp. 395–406.
- Scott P. H., Mortimer E. F. and Aguiar O. G., (2006), The tension between authoritative and dialogic discourse: a fundamental characteristic of meaning making interactions in high school science lessons, Sci. Educ., 90(4), 605–631.
- Stanford C., Moon A., Towns M. and Cole R., (2016), Analysis of Instructor Facilitation Strategies and Their influences on student argumentation: A Case Study of a process Oriented Guided inquiry learning physical chemistry classroom, J. Chem. Educ., 93(9), 1501–1513.
- Stowe R. L. and Cooper M. M., (2017), Practicing what we preach: assessing “critical thinking” in organic chemistry, J. Chem. Educ., 94(12), 1852–1859.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students’ mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11(4), 293–301.
- Stroupe D., (2014), Examining classroom science practice communities: How teachers and students negotiate epistemic agency and learn science-as-practice, Sci. Educ., 98(3), 487–516.
- Suárez E., Quan G., Hammer D. and Atkins L., (2023), Learning in Interaction: Interacting Scales of Research, in Taşar M. F. and Heron P. R. L. (ed.), The International Handbook of Physics Education Research: Learning Physics, AIP Publishing LLC, ch. 13.
- Swain C. G., Powell A. L., Sheppard W. A. and Morgan C. R., (1979), Mechanism of the Cannizzaro reaction, J. Am. Chem. Soc., 101(13), 3576–3583.
- Taber K. S. and Watts M., (2000), Learner's explanations for chemical phenomena, Chem. Educ. Res. Pract., 1(3), 329–353.
- Thagard P., (1989), Explanatory coherence, Behav. Brain Sci., 12(3), 435–467.
- Varelas M., Settlage J. and Mensah F. M., (2015), Explorations of the structure-agency dialectic as a tool for framing equity in science education, J. Res. Sci. Teach., 52(4), 439–447.
- Virtanen P., Gommers R., Oliphant T. E., Haberland M., Reddy T., Cournapeau D., Burovski E., Peterson P., Weckesser W. and Bright J., (2020), SciPy 1.0: fundamental algorithms for scientific computing in Python, Nat. Methods, 17(3), 261–272.
- Vygotsky L. S., (1978), Mind in society: Development of higher psychological processes, Harvard: Harvard University Press.
- Walsh K. H., Karch J. M. and Caspari-Gnann I., (2022), In-the-moment Learning of Organic Chemistry During Interactive Lectures Through the Lens of Practical Epistemology Analysis, in Graulich N. and Shultz G. (ed.), Student Reasoning in Organic Chemistry, The Royal Society of Chemistry, ch. 9, pp. 141–158.
- Watts F. M. and Finkenstaedt-Quinn S. A., (2021), The current state of methods for establishing reliability in qualitative chemistry education research articles, Chem. Educ. Res. Pract., 22(3), 565–578.
- Watts F. M., Zaimi I., Kranz D., Graulich N. and Shultz G. V., (2021), Investigating students’ reasoning over time for case comparisons of acyl transfer reaction mechanisms, Chem. Educ. Res. Pract., 22(2), 364–381.
- Webber D. M. and Flynn A. B., (2018), How Are Students Solving Familiar and Unfamiliar Organic Chemistry Mechanism Questions in a New Curriculum? J. Chem. Educ., 95(9), 1451–1467.
- Weinrich M. L. and Talanquer V., (2016), Mapping students' modes of reasoning when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 17(2), 394–406.
- Wick A. E., Felix D., Steen K. and Eschenmoser A., (1964), CLAISEN'sche Umlagerungen bei Allyl- und Benzylalkoholen mit Hilfe von Acetalen des N,N-Dimethylacetamids. Vorläufige Mitteilung, Helv. Chim. Acta, 47(8), 2425–2429.
- Wickman P. O., (2004), The practical epistemologies of the classroom: a study of laboratory work, Sci. Educ., 88(3), 325–344.
- Wickman P. O. and Östman L., (2002), Learning as discourse change: a sociocultural mechanism, Sci. Educ., 86(5), 601–623.
- Yates F., (1934), Contingency tables involving small numbers and the χ2test, Supp. J. R. Stat. Soc., 1(2), 217–235.
- Zaimi I., Dood A. J. and Shultz G. V., (2024), The evolution of an assignment: how a Writing-to-Learn assignment's design shapes organic chemistry students’ elaborations on reaction mechanisms, Chem. Educ. Res. Pract., 25(1), 327–342.
- Zhang J., Scardamalia M., Reeve R. and Messina R., (2009), Designs for Collective Cognitive Responsibility in Knowledge-Building Communities, J. Learn. Sci., 18(1), 7–44.
Footnote |
| † A discursive practice is a practice enacted through discourse where meaning is created through the interaction of multiple voices (Bakhtin, 1983/1934). |
| This journal is © The Royal Society of Chemistry 2026 |