Kinetically tunable, subzero-active, visual time–temperature indicators based on the permanganate–oxalate reaction†
Jorvani
Cruz Villarreal
 ab,
Emil
Ljungberg
ab,
Nilojan
Jehanathan
c,
Milap
Owens
ab,
Anika
Li
ab and
Chad R.
Borges
ab,
Emil
Ljungberg
ab,
Nilojan
Jehanathan
c,
Milap
Owens
ab,
Anika
Li
ab and
Chad R.
Borges
 *ab
*ab
aSchool of Molecular Sciences, Arizona State University, Tempe, AZ 85287, USA. E-mail: Chad.Borges@asu.edu
bThe Biodesign Institute at Arizona State University, Tempe, AZ 85287, USA
cCryoVeritas, Inc., Tempe, AZ 85287, USA
First published on 16th June 2025
Abstract
Biological products and specimens often require consistent ultracold storage to preserve their integrity. Existing time–temperature indicators (TTIs) are inadequate for monitoring ultracold conditions at the individual aliquot level. We adapted the autocatalytic permanganate–oxalate reaction to create visual TTIs functional below 0 °C. Using eutectic compositions of LiClO4, NaClO4, and Mg(ClO4)2, we depressed the melting points of the reaction mixtures to −18 °C, −37 °C, and −67 °C, respectively. The incorporation of perchlorate salts as antifreeze systems did not derail the kinetic behavior of the permanganate–oxalate reaction and allowed the reactions to pause below their melting points. Here, we developed and characterized eight customized TTIs, running from five minutes at 25 °C to 7 days at −20 °C. Temperature sensitivity was consistent with Arrhenius behavior (i.e., exponential increases in run time with linear decreases in temperature). The TTIs exhibited good accuracy and reproducibility, with within-batch and between-batch run-time precision at the targeted temperatures of ≤4.8% CV and ≤7.5% CV, respectively. The average absorbance vs. time trajectories, expressed as RMSD %CVs, were 4.5% for intra-batch and 10.4% for inter-batch runs. Indicators withstood multiple freeze/thaw cycles or extended pre-freezing periods with minimal impact on reaction kinetics. Once activated and stored below their melting points, TTIs maintained color intensity for at least 12 months. This work establishes the permanganate–oxalate system in eutectic perchlorate-based antifreeze solutions as a simple, inexpensive approach for ultracold-active TTIs, offering customizable kinetics and robust performance. The described TTIs can serve to improve quality monitoring of biologicals and biospecimens during ultracold storage and handling.
1. Introduction
Cold-chain transportation and storage are critical in the management of temperature-sensitive products, such as food products, pharmaceuticals, and biological products. Many biological analytes relevant to clinical and biomedical research, as well as biological elements in medical products (such as vaccines), are prone to instability when the specimens in which they reside are exposed to thawed conditions, compromising the quality of the specimens. Despite the existence of guidelines for proper handling and storage of biological products and biospecimens, improprieties and inconsistencies can occur unwittingly, such as accidental exposure to elevated temperatures.1,2 The use of compromised specimens can lead to costly false leads in research and to biological products with limited efficacy.3To avoid the use of compromised specimens, it is critical to track the biospecimen exposure to thawed conditions. However, there are only a few options that are cost- and time-effective, easily implemented, and that provide evidence-based quality assurance related to thawed exposure for products and/or biospecimens that require storage and handling at ultracold temperatures.
Currently, the techniques used to monitor the proper storage temperature of biospecimens include following the handling and storage guidelines, keeping a detailed record of the specimen's manipulation, and the use of electronic temperature monitors. However, it is complicated to apply these techniques to the aliquot or single product level, and they are often not accurate. Time–temperature indicators (TTIs) can be used as an evidence-based quality assurance tool for tracking the exposure of products and/or biospecimens to thawed conditions. TTIs are devices that track the cumulative influence of time–temperature history and expire whenever the temperature is above a specific range for a certain amount of time. TTIs differ from temperature indicators, or “thermochromic” indicators, as the latter report if the temperature has been below or above a specific critical temperature, usually via an irreversible change of color without any information on the overall exposure time.
According to their working principles, TTIs can be divided into chemical, enzymatic, biological, or mere physical processes.4,5 Detailed reviews of the main systems are available by Wang et al.5 and Liu et al.6 A review on TTI modelling methods and classification of response type is also available elsewhere.7 Other TTIs are described in reviews focused on the cold chain of food products,8,9 as exposure of specific products to warmer temperatures than desired represents a safety risk. However, food products rarely require ultracold transportation or storage. As biospecimens often require ultracold storage and handling,10 we focus our work on the need for TTIs that are active at ultracold temperatures.
A summary of commercially available TTIs that work at subzero temperatures can be found in ESI† Table S1. Additionally, functional TTIs at subzero temperatures have been recently developed for cold chain management of vaccines.11–13 For example, to monitor mRNA vaccine integrity, Hao et al.14 reported TTIs based on a dyed mixture of ethylene glycol and water that melts near the mRNA conservation temperature (−69 °C), facilitating the detection of relatively small changes in temperature within subzero ranges.
We recently described how the classic autocatalytic permanganate–oxalate reaction can be adapted to create visually assessed, TTIs that are “programmed” to run for predetermined times at specific temperatures.15 The net permanganate/oxalate reaction is shown in eqn (1):
| 2MnO4− + 6H3O+ + 5H2C2O4 → 2Mn2+ + 14H2O + 10CO2 | (1) |
The permanganate ion (MnO4−) is the pink/purple reactant that is completely reduced into colorless Mn2+ by the end of the reaction. Control of the reaction kinetics allows for the design of specific time–temperature intervals based on the application(s) of interest. In principle, the extremely low cost of this chemistry facilitates its adaptation into TTIs that, if deployed in creatively engineered devices, can function at the individual-aliquot level—opening up a frontier of granularity in biospecimen integrity monitoring that is both time and cost efficient. While the indicators previously reported by our group are useful at 0 °C and above,15 most biospecimens—particularly those archived in biorepositories—require ultracold storage and/or handling (i.e., at −80 °C or below) and risk damage if stored at nominally “frozen”, but unacceptably warm conditions such as the common laboratory freezer temperature of −20 °C.1,2,16–25 As such, effective TTIs for ultracold storage monitoring should be in an “active” or “running” state when exposed to subzero conditions that are unacceptably warm. Few existing TTIs possess these characteristics, and none of those that do are viable for use at the individual aliquot-level (ESI† Table S1). Therefore, we sought to adapt the permanganate–oxalate reaction chemistry to run with predictable kinetics in aqueous solutions with severely depressed melting points.
To accomplish this goal, antifreeze systems needed to be identified that could facilitate severe melting point depression at a eutectic composition with chemicals that are inert to the permanganate–oxalate reaction. A eutectic composition is necessary in order to prevent disturbances in reaction kinetics under partially frozen/solidified conditions—e.g., due to freeze-acceleration effects.26 Functionally, as will be shown here, eutectic antifreeze compositions keep the reaction system essentially undisturbed by freeze–thaw cycles, maintaining predictable macroscopic behaviour. As described in this work, eutectic compositions of NaClO4, LiClO4 and Mg(ClO4)2 meet these criteria and produce aqueous solutions with melting points of −18 °C, −37 °C, and −67 °C, respectively, that are compatible with customizable and reproducible permanganate–oxalate reaction kinetics at temperatures below 0 °C.
2. Methodology
2.1. Materials
Perchloric acid (HClO4, Cat. No. 311421), sodium oxalate (Na2C2O4, Cat. No. 379735), potassium permanganate (KMnO4, Cat. No. 223468), sodium perchlorate hydrate (NaClO4, Cat. No. 381225), magnesium perchlorate (Mg(ClO4)2, Cat. No. 63095), lithium perchlorate (LiClO4, Cat. No. 431567), and cetyltrimethylammonium bromide (CTAB, Cat. No. H6269) were purchased from Sigma Aldrich (St. Louis, MO). Manganese(II) perchlorate hexahydrate (Mn(ClO4)2·6H2O Cat. No. 44318) was purchased from Alfa Aesar, ThermoFisher Scientific. Nitric acid (HNO3, Cat. No. 87003-261) was purchased from VWR. Ethylene glycol (Cat. No. 324558) was obtained from MilliporeSigma (MA, USA). Perchlorate standard solution and ion strength adjustor (ISA) solution were obtained from perchlorate ISE double junction solution kit (Cat. No. EW-27502-85, Cole-Parmer). A Purelab Flex 3 water purification system (ELGA LabWater, USA) was used to generate the deionized (DI) water, 18.2 MΩ cm type I.All materials involved in this work were rinsed with 2% HNO3 before used, including volumetric flasks, 96-well plates, pipette tips, Falcon tubes, Eppendorf tubes, glassware, cuvettes. For nitric acid rinse, materials were soaked in 2% HNO3 overnight and then air dried overnight in a hood. All solutions were prepared using 18.2 MΩ cm DI water. KMnO4 solutions were prepared daily.
2.2. Antifreeze solution preparation
Perchlorate salts can exist as poorly defined hydrates in the solid state. Therefore, it is impossible to simply weigh out the perchlorate salt and dilute it to the correct volume to create a well-defined perchlorate salt solution. To run the permanganate–oxalate reaction at eutectic compositions of perchlorate salts, it is necessary to quantify the perchlorate stock solutions. This was readily accomplished via titration with CTAB and a perchlorate selective electrode.Perchlorate solutions (NaClO4, LiClO4 and Mg(ClO4)2), referred to here as antifreeze solutions, were prepared in 100 mL volumetric flasks by dissolving the perchlorate salt in DI water close to saturation. The 100 mL perchlorate solution was weighed to determine its density. To determine the concentration of the perchlorate solution, potentiometric titration of the perchlorate was performed. In this procedure, the perchlorate ion was titrated with standardized cetyltrimethylammonium bromide (CTAB) as reported by W. Selig.27 An ion-selective electrode (ISE) for perchlorate (EW-27504-24, Cole Parmer, USA) was used for the titration. First, the CTAB was standardized using a 1000 ppm perchlorate standard (EW-27502-85, Cole-Parmer, USA). Then, 1 mL of the perchlorate solution was diluted to 500 mL with DI water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution). The titration mixture was prepared by mixing 20 mL of the 1
500 dilution). The titration mixture was prepared by mixing 20 mL of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 perchlorate dilution, 30 mL of DI water and 1 mL of ionic strength adjustor (ISA) solution. The potential of the system was monitored with the electrode as CTAB was added in small steps (0.5 mL at the time) to the mixture until the potential reached a plateau. The second derivative of the potential vs. CTAB volume curve was used to determine the end point, which was indicated by the point of inflection at which the value of the second derivative was zero. Based on the estimated end point, the moles of perchlorate in the mixture were calculated as mol ClO4− = VCTABMCTAB(1 mol ClO4−/1 mol CTAB) for NaClO4 and LiClO4, and as mol ClO4− = VCTABMCTAB(1 mol ClO4−/2 mol CTAB) for Mg(ClO4)2.
500 perchlorate dilution, 30 mL of DI water and 1 mL of ionic strength adjustor (ISA) solution. The potential of the system was monitored with the electrode as CTAB was added in small steps (0.5 mL at the time) to the mixture until the potential reached a plateau. The second derivative of the potential vs. CTAB volume curve was used to determine the end point, which was indicated by the point of inflection at which the value of the second derivative was zero. Based on the estimated end point, the moles of perchlorate in the mixture were calculated as mol ClO4− = VCTABMCTAB(1 mol ClO4−/1 mol CTAB) for NaClO4 and LiClO4, and as mol ClO4− = VCTABMCTAB(1 mol ClO4−/2 mol CTAB) for Mg(ClO4)2.
The total perchlorate in the stock solution was calculated based on the dilutions performed. The obtained sample concentration (mol L−1), density and molecular weight of each sample were used to determine the weight percentage the perchlorate solutions (Table 1). The density of the perchlorate solutions at their eutectic compositions were estimated by preparing eutectic compositions of each solution at known volume and weighing them. This information facilitated calculation of the volume of each perchlorate solution needed in the TTIs to achieve the eutectic composition.
2.3. Antifreeze solution preparation
Differential Scanning Calorimetry (DSC) of DI water and the three perchlorate solutions were performed using a DSC Q1000 instrument (TA instruments, DE, USA). The antifreeze solutions were prepared at their eutectic composition (3.11 molal LiClO4, 52% (w/w) NaClO4, and 44% (w/w) Mg(ClO4)2) for the analysis.Water, LiClO4, and NaClO4 were analyzed at Arizona State University with a DSC system with cooling capabilities down to −80 °C. Around 4 mg of each sample was transferred to the Tzero pans for analysis. The analysis was set up to start at 25 °C, cool down to −80 °C at a ramp of 10 °C min−1, then warm up to 25 °C at a ramp of 10 °C min−1. Mg(ClO4)2 was analyzed at the Polymer Characterization and Processing Facility from the University of Minnesota using a DSC system that cooled down to −150 °C. Similarly, around 17 mg of the sample was transferred to the pan for analysis, which was set up to start at 25 °C, cool down to −150 °C at a ramp of 10 °C min−1, then warm up to 25 °C at a ramp of 10 °C min−1. Data were processed using the TA Universal Analysis software and exported as thermographs.
2.4. Time–temperature indicators (TTIs) preparation
Similar to our previously described antifreeze-free systems,15 TTIs were created by mixing all reagents together, except for the KMnO4. Reagents included the antifreeze solution (LiClO4, NaClO4, or Mg(ClO4)2), HClO4, Na2C2O4 and Mn(ClO4)2. The reagent concentrations varied depending upon the targeted reaction times, except for the antifreeze solutions that were fixed to their eutectic composition. An antifreeze-based TTI system contained only one of the perchlorate salts depending on the desired melting point. The prepared mixture was either vortexed if prepared in Eppendorf tubes or mixed by pipetting if prepared in a 96-well plate. Then, mixtures were incubated for 5 min right before activation, which was achieved by adding the KMnO4. The solution of 10 mM KMnO4 was prepared fresh daily. The reaction run time was initiated immediately after adding the KMnO4 to the solution. An “inactive” indicator refers to the clear mixture before the KMnO4 is added. An “active” indicator means that the KMnO4 has been added to the mixture and the reaction is running (should be pink). The reaction was monitored visually or spectrophotometrically at an absorbance of 525 nm over time (see section 2.5). For experiments at 25 °C, all experiments were performed with a total volume of 200 μL, which corresponded to a pathlength of 0.555 cm in the 96-well plates employed for these experiments. For experiments below 25 °C, the same volume was prepared but only 100 μL were transferred to a 1-mm pathlength cuvette for monitoring in a Cary60 spectrophotometer as described in section 2.5. An “expired” indicator refers to a colorless indicator after the reaction has taken place and went from pink to clear.For experiments at 25 °C, the reaction was monitored visually or by absorbance readings at 525 nm. For experiments at subzero temperatures, solutions were pre-chilled at 4 °C (fridge) or −20 °C (freezer) prior to activation depending on the experiment's temperature. Then, TTI solutions were activated and transferred to cuvettes for spectrophotometric monitoring at 525 nm. Flash-freezing of TTI solutions was performed by preparing them in Eppendorf tubes, activating them by adding KMnO4, vortexing and immediately transferring them to a dry ice and ethanol bath. Solutions were kept in the dry ice/ethanol bath for 10 min before their transfer to the freezer. For tight temperature control between 0 °C and −80 °C, a Messenger portable 1 L freezer was used (inTEST Thermal Solutions, MA, USA).
2.5. Data acquisition and processing
A plate reader (SpectraMax iD5, Molecular Devices, USA) was employed to monitor the absorbance at 525 nm for experiments at 25 °C. The samples were prepared in 96-well plates, pipette-mixed and incubated for 5 min before activation. The activation was performed by adding the KMnO4 directly into the wells and pipette mixed. The reaction was considered active as soon as KMnO4 was added and mixed. Therefore, the time interval between KMnO4 addition and the start of measurements in the instrument was tracked for time correction during data processing. The reaction end was defined by an absorbance cutoff of 0.03 for the pathlength of 0.555 in the instrument, as previously described.15 Experiments were performed in triplicates and a blank for baseline subtraction, unless otherwise stated.As previously described,15 experiments at temperatures <25 °C were performed using a Cary 60 UV-vis spectrophotometer (Agilent Technologies, CA, USA) equipped with a qChanger 6 multi-cuvette changer system (Quantum Northwest, WA, USA) operating in tandem with a refrigerated circulating liquid system (MX7LR-20, PolyScience, IL, USA). The absorbance was monitored at 525 nm over time using Quartz cuvettes with a 1 mm light path (Cat No. 6610019600, Agilent Technologies, USA). The coolant in the circulated bath allowed the cold temperature in the system. A 35% ethylene glycol in water solution was used as a coolant for temperatures down to −18 °C, while polycool HC −50 (060330, PolyScience, IL, USA) was used as a coolant for experiments at −20 °C. The temperature was tightly controlled in the cuvette chamber using the qChanger 6 controller, while also being monitored by the system at all times (current temperature shown in the controller screen). The system included a nitrogen gas line to avoid condensation on the cuvettes. Prior to experiments, the cuvettes (up to six) were placed on the qChanger 6 at the preset temperature to allow for temperature stabilization while pressurized nitrogen at 15 psi purged the system to avoid condensation. The TTI solutions were prepared in Eppendorf tubes and pre-chilled at either 4 °C or −20 °C depending on the experiment's temperature. The KMnO4 solution was also pre-cooled at 4 °C. For activation, KMnO4 was added to the TTI mixture in the Eppendorf tubes and vortexed. Then, 100 μL of the activated solutions were transferred to the pre-chilled cuvettes in the system. The time between activation and start of the absorbance run was tracked for correction during data processing. The reaction end was defined by an absorbance cutoff of 0.005 for the instrument's pathlength of 1 mm.15 All experiments were performed in triplicates along with a blank for baseline subtraction unless otherwise indicated.
The data were exported from the instrument's software and processed in Microsoft Excel to carry out baseline subtraction, time-correction and then to determine the reaction run time based on the absorbance cutoff. For trajectory analysis, data processing was carried out as previously described.15
2.6. Impact of initial Na2C2O4, HClO4, and Mn(ClO4)2 concentrations
For each of the three antifreeze systems, the effect of Na2C2O4, HClO4, and Mn(ClO4)2 concentration was tested. The antifreeze salt was used at their respective eutectic composition in all experiments: 52% (w/w) NaClO4, 25% (w/w) LiClO4, and 44% (w/w) Mg(ClO4)2. Using a fixed concentration of 1.38 mM Na2C2O4, HClO4 concentration was varied from 4 mM to 150 mM. Using a fixed concentration of 100 mM HClO4, the concentration of Na2C2O4 was varied from 1.38 mM up to 20.7 mM. Fixing both HClO4 and Na2C2O4 concentrations, the solutions were spiked with different Mn(ClO4)2 concentrations up to 0.5 mM. For Mn(ClO4)2 variation study, each antifreeze system had different fixed concentrations of HClO4 and Na2C2O4. For NaClO4, HClO4 and Na2C2O4 were fixed to 10 mM and 1.38 mM, respectively. For LiClO4, HClO4 and Na2C2O4 were fixed to 5 mM and 1.38 mM, respectively. For Mg(ClO4)2, HClO4 and Na2C2O4 were fixed to 8 mM and 5.0 mM, respectively. For all conditions run, the KMnO4 was fixed to 0.5 mM.2.7. Kinetic behaviour under variable temperature
To evaluate the kinetic behaviour of the antifreeze-containing systems under variable temperature conditions, the 60 minute-at-0 °C composition of each antifreeze system (TTIs #2, #5 and #8 in Table 2) was subjected to multiple different variable-temperature exposure scenarios after activation, while the reaction was running. This was executed on the Cary 60 spectrophotometer (see section 2.5 for details on the system set-up). For temperatures at −20 °C and below, a Messenger portable 1 L freezer was used at the desired temperature. For each condition, triplicates and a blank were run together. The TTI solutions were prepared in Eppendorf tubes according to each antifreeze system composition. The pre-mixed solutions were equilibrated in a refrigerator at 4 °C for the LiClO4 system and in a freezer at −20 °C for the NaClO4 and Mg(ClO4)2 systems. The KMnO4 solution was pre-chilled at 4 °C prior to the experiment. The pre-mixed aliquots and KMnO4 were transferred to ice immediately prior to activation. Once activated, the solutions were immediately transferred to the spectrophotometer, which was pre-set at the desired starting temperature for experiments at −20 °C or higher. For temperatures below −20 °C, the solutions were immediately transferred to the portable freezer pre-set at the desired starting temperature. Once the storage time at <−20 °C temperature had passed, the tubes were quickly transferred to ice and immediately transferred to the spectrophotometer that had been pre-set at the desired temperature. When necessary, the temperature was changed manually via the front panel of the qChanger or of the Messenger freezer. The time between activation and the run start in the spectrophotometer was recorded for time correction during data processing. Absorbance readings at 525 nm were acquired every 30 s. The total experimental time was different for each scenario.| # | Antifreeze system (melting point) | Target time and temperature | Temperature | Avg. run time (min.) | Description of runs | Run time | Trajectory RMSD | |||
|---|---|---|---|---|---|---|---|---|---|---|
| %Accuracy | Intra-batch (%CV) | Inter-batch (%CV) | Intra-batch (%CV) | Inter-batch (%CV) | ||||||
| a A new stock solution of KMnO4 was made for every run. Different runs for the same indicator at a given temperature were often carried out by different analysts. b Using 4 different stock solutions of antifreeze salt. c Using 3 different stock solutions of antifreeze salt. d Using 2 different stock solutions of antifreeze salt. e 4 runs of n = 3 each, 1 run of n = 4 and 1 run of n = 5. f 2 runs of n = 3 each and 1 run of n = 5. | ||||||||||
| 1 | 52% (w/w) NaClO4 (−37 °C) | 5 min at 25 °C | 25 °C | 4.91 | 15 runs of n = 3 each | 98.2 | 3.4 | 5.3 | 4.6 | 11.6 |
| 0 °C | 65 | 6 runs of n = 3 eachc | N/A | 4.3 | 4.7 | 6.0 | 9.2 | |||
| −20 °C | 1218 (20.3 h) | 6 runs of n = 3d | N/A | 4.5 | 9 | 8.6 | 14.3 | |||
| 2 | 52% (w/w) NaClO4 (−37 °C) | 60 min at 0 °C | 25 °C | 4.43 | 9 runs of n = 3 eachb | N/A | 2.1 | 4.9 | 4.2 | 9.8 |
| 0 °C | 57.7 | 9 runs of n = 3 each | 96.2 | 2.9 | 7.5 | 3.3 | 9.4 | |||
| −20 °C | 894 (14.9 h) | 6 runs of n = 3d | N/A | 3.4 | 5.5 | 4.6 | 8.9 | |||
| 3 | 52% (w/w) NaClO4 (−37 °C) | 7 days (168 h) at −20 °C | 25 °C | 44.0 | 6 runs of n = 3 eachd | N/A | 2.4 | 3.4 | 3.4 | 6.1 |
| 0 °C | 656 | 21 runs over 6 batchesd,e | N/A | 3.5 | 5.1 | 6.1 | 11.2 | |||
| −20 °C |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 500 (175 h) 500 (175 h)
|
11 runs over 3 batches , | 103.9 | 4.8 | 7.5 | 6.4 | 14.3 | |||
| 4 | 44% (w/w) Mg(ClO4)2 (−67 °C) | 5 min at 25 °C | 25 °C | 5.0 | 12 runs of n = 3 each | 100.0 | 3.3 | 5.8 | 4.6 | 13.6 |
| 0 °C | 70.8 | 6 runs of n = 3 eachc | N/A | 2.0 | 3.6 | 2.3 | 5.2 | |||
| −20 °C | 21.2 | 4 runs of n = 3 eachb | N/A | 2.5 | 5.1 | 4.8 | 7.4 | |||
| 5 | 44% (w/w) Mg(ClO4)2 (−67 °C) | 60 min at 0 °C | 25 °C | 4.3 | 7 runs of n = 3 eachc | N/A | 2.2 | 3.7 | 3.5 | 9.7 |
| 0 °C | 61.3 | 6 runs of n = 3 each | 102.2 | 2.0 | 3.7 | 2.4 | 7.0 | |||
| −20 °C | 17.2 | 19 runs over 6 batchesd | N/A | 3.3 | 6.1 | 4.7 | 13.0 | |||
| 6 | 44% (w/w) Mg(ClO4)2 (−67 °C) | 7 days (168 h) at −20 °C | 25 °C | 32.2 | 7 runs of n = 3 each | N/A | 1.2 | 6.0 | 1.3 | 9.0 |
| 0 °C | 440 | 18 runs over 5 batches | N/A | 1.5 | 8.7 | 3.7 | 13.7 | |||
| −20 °C |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 560 (176 h) 560 (176 h)
|
11 runs over 3 batches | 104.8 | 2.1 | 2.4 | 3.6 | 4.4 | |||
| 7 | 25% (w/w) LiClO4 (−18 °C) | 5 min at 25 °C | 25 °C | 5.0 | 45 runs over 14 batches (n = 3) | 100.0 | 2.9 | 5.4 | 5.1 | 11.2 |
| 0 °C | 70.4 | 6 runs of n = 3 eachc | N/A | 1.8 | 7 | 4.1 | 11.4 | |||
| 8 | 25% (w/w) LiClO4 (−18 °C) | 60 min at 0 °C | 25 °C | 3.95 | 33 runs over 10 batches (n = 3)c | N/A | 3.6 | 7.4 | 6.9 | 19.3 |
| 0 °C | 55.7 | 8 runs of n = 3 each | 92.8 | 2.1 | 2.5 | 4.9 | 9.5 | |||
2.8. Evaluation of freeze/thaw cycles
A total of seven sets of triplicates, and a blank, were prepared in Eppendorf tubes for each TTI system to be studied: 30 min-at-25 °C formulation for each of the three antifreeze systems. One of the seven sets was run in the plate reader immediately after preparation. For this, the solutions were transferred to a 96-well plate, activated and monitored at 525 nm. The other six sets were activated, flash-frozen and then transferred to the −80 °C freezer (LiClO4 and NaClO4) or to the −140 °C LN2 freezer (Mg(ClO4)2). The other six sets were exposed to different freeze/thaw cycles from one cycle up to six cycles. DI water was used as control for nominal but consistent thawing reference for each freeze/thaw cycle. For sample thawing, the corresponding set of tubes were removed from the freezer and placed on a bench at RT until the water control was completely thawed. Then, the tubes were placed back into the freezer. The removal of tubes from the freezer, thawing and going back to freezer was considered a single cycle. After the corresponding number of cycles (one to six), the thawed solutions were transferred to a 96-well plate for analysis by monitoring the absorbance at 525 nm over time using the plate reader.Similarly, a set of triplicates and a water control were prepared in Eppendorf tubes, activated, flash-frozen and transferred to the freezer. This set was exposed to as many cycles as necessary until the color of the solution turned from pink to colorless. The freeze/thaw cycle was carried out as described above. This study was performed using a 30 min-at-25 °C formulation for each of the three antifreeze systems.
2.9. Long-term stability of frozen TTIs and effect of storage time
The TTI solutions were prepared in Eppendorf tubes, activated, flash-frozen and transferred to the freezer. Before samples were stored in the freezer, a reference picture was taken. TTI solutions were kept in the freezer for at least 12 months, taking pictures every month for comparison.A total of seven sets of triplicates, and blank, of each antifreeze system were prepared in Eppendorf tubes. The prepared solutions were pre-cooled at 0 °C using a water/ice bath for LiClO4, and at −20 °C for NaClO4 and Mg(ClO4)2. One set was immediately run at 0 °C after the pre-cooling using the Cary60 spectrophotometer pre-chilled at 0 °C. The indicators were activated in the Eppendorf tubes by adding the KMnO4, vortexed and then 100 μL of solution was transferred to the cuvettes. The remaining six sets of solutions were also activated but immediately transferred to the −80 °C freezer (for LiClO4 and NaClO4) or to the −140 °C LN2 freezer, vapor phase (for Mg(ClO4)2). Sample sets were stored in the freezer for specific periods of times including 2 weeks, 1 month, 2 months, 4 months, 6 months, and 9 months. After each period, the sample set was removed from the freezer, thawed and transferred to the Cary60 spectrophotometer for absorbance monitoring. The thawing protocol for LiClO4 consisted of transferring the tubes to a water/ice bath for a 4 min incubation, then the samples were vortexed and transferred to the cuvettes. The thawing protocol for NaClO4 consisted of transferring the tubes to an ice bucket placed in the −20 °C freezer for a 2 min incubation. Samples were then vortexed and transferred to the cuvettes. The thawing protocol for Mg(ClO4)2 consisted in transferring the tubes to an ice bucket followed by incubation for 1 min before being transferred to the cuvettes. All runs included a blank for baseline subtraction. Together with each of the sample sets, a fresh control and a recently-briefly-frozen control were prepared and run. The fresh control was a set of the same system prepared, pre-cooled and activated without being frozen beforehand. The recently-briefly-frozen sample was a set of the same system prepared together with the fresh control, pre-cooled, activated and transferred to the freezer to be stored for 24 h. After the 24 h incubation in the corresponding freezer, the samples were thawed according to the corresponding antifreeze protocol. This study was performed using 60 min-at-0 °C TTI formulations.
3. Results
3.1. Use of salt-based antifreeze solutions for melting point depression
Previously, we reported the development of TTIs based on kinetic control of the permanganate–oxalate redox reaction.15 These water-based TTIs are active at temperatures above 0 °C. In this work, we depressed the freezing and melting point of the permanganate–oxalate reaction system using eutectic compositions of the perchlorate salts LiClO4, NaClO4 and Mg(ClO4)2 as antifreeze solutions.Stock perchlorate solutions were prepared as described in section 2.2. The eutectic composition and melting point (m.p.) of the perchlorate salts reported in literature28,29 are shown in Table 1, including their density and the concentration in g of salt per mL solution. The eutectic values of NaClO4 and Mg(ClO4)2 were reported in weight percentage (% w/w),28 while the eutectic composition of LiClO4 was reported in molality and mass fraction (considered as salt mass per solvent mass).29 The eutectic composition of LiClO4 was also calculated in %w/w (this unit will be used throughout this article for consistency) and reported in Table 1.
To confirm the eutectic point of the antifreeze systems, DSC of each perchlorate system at its eutectic composition was performed as described in section 2.3. The phase diagrams of the antifreeze solutions have been reported elsewhere and used as reference.28,30 The thermographs obtained by DSC for water, LiClO4, NaClO4 and Mg(ClO4)2 are shown in ESI† Fig. S1a–d. The thermographs show a cooling step followed by a heating step. The cooling step ranged from 25 °C to −80 °C with 10 °C min−1 increments for water, LiClO4, and NaClO4, and from 25 °C to −150 °C with 10 °C min−1 increments for Mg(ClO4)2.
The peak in the positive direction (while cooling down) represents an exothermic heat flow change, corresponding to solidification. The second step, heating, goes from either −80 °C or −150 °C back to 25 °C with 10 °C min−1 increments. The negative heat flow peaks represent the melting point of the solution. All four solutions exhibited different freezing and melting behaviors. It can be observed that water froze at around −20 °C (ESI† Fig. S1a), while it melted around 0 °C. It is generally considered that water freezes at 0 °C, however, in the absence of particulates, it often undergoes supercooling (also known as undercooling),31,32 represented by the space between the freezing point and the melting point. The supercooling effect refers to the decrease in temperature without freezing, typically due to the lack of seed crystals. For water, this happens normally down to −20 °C, as observed in this work, but it can go down to the homogenous nucleation temperature of water, −38 °C,33–35 depending on the purity and volume of the water. As observed in ESI† Fig. S1b and c, LiClO4 and NaClO4 also exhibit supercooling. The solidification peak of LiClO4 and NaClO4 are shown at around −28 °C and −49 °C, respectively. However, the melting peak matches the expected m.p. at −18 °C and −37 °C for LiClO4 and NaClO4, respectively. Based on these m.p. values, the DSC results confirm that the eutectic composition was achieved for LiClO4 and NaClO4, and the solutions melted at the expected temperature based on published values.
The supercooling effect in LiClO4 and NaClO4 systems does not represent a concern since their use will be suggested for typical storage temperatures below their melting point, such as −40 °C or below for LiClO4 and −80 °C or below for NaClO4.
The thermograph of Mg(ClO4)2 (ESI† Fig. S1d) does not show a clear solidification or melting peak although the temperature went considerably below its reported m.p. of −67 °C. The low magnitude endothermic shift on the heating cycle could correspond to a glass transition temperature; but one would expect an opposing exothermic step-up during the cooling cycle if this were the case (not an endothermic step-down). Given the ambiguous nature of the obtained DSC data, samples were submitted for cold-stage polarized light microscopy analysis (Chemical Microscopy LLC). The resulting data indicated clear formation of crystals at −68 °C – but such crystals only formed in two out of six trials (ESI† Fig. S1e). It is possible that under the right conditions, the Mg(ClO4)2 system supercools to the point of vitrification without always going through a crystallization event like the other two antifreeze solutions.
Notably, the DSC data reported here for a eutectic composition of Mg(ClO4)2 are nearly identical to those recently published by Bravenec and Catling.36 Visually, the appearance of Mg(ClO4)2 does not change immediately when chilled to −80 °C or −140 °C (liquid nitrogen vapor phase) compared to the liquid form, however, it does immediately become solid based on the lack of movement when shaken (see ESI† Fig. S2a–b). After a period of at least one month stored at these temperatures (<−80 °C), the Mg(ClO4)2 systems visually appear solid (more opaque and less translucent) (ESI† Fig. S2c–d). On the other hand, LiClO4 and NaClO4 show an obvious appearance of change to the solid state and a visual opaque appearance when chilled to −80 °C (ESI† Fig. S2f–g).
3.2. Design of TTIs and temperature sensitivity
As previously reported for the antifreeze-free TTIs,15 the formulation of the antifreeze-containing TTIs reported in this work can be manipulated to design indicators for specific time–temperature intervals. It is important to note that the antifreeze composition itself must be fixed to the eutectic composition to maintain the m.p. of the systems. The manipulation of TTI formulations (Fig. 1) shows a similar kinetic behavior to that observed in the antifreeze-free systems.15 The increase of HClO4 in the system accelerates the reaction run time (Fig. 1A), with different behavior for each one of the three antifreeze systems. In the case of Mg(ClO4)2, a brown precipitate can be observed at HClO4 concentrations below 25 mM due to the accumulation of Mn(IV) as MnO2. This behavior in the Mg(ClO4)2 system limits the design of long run times for this system. Notably, the formation of MnO2 is not directly caused by the presence of Mg(ClO4)2. Nor is it unique to Mg(ClO4)2 containing reaction systems. As we have previously observed, the formation of MnO2 occurs when the acid concentration in the reaction system is too low.15 As noted in the caption to Fig. 1, all else held constant, this effect can be induced by increased concentrations of the base Na2C2O4. Nevertheless, the formation of MnO2 occurs more readily in the Mg(ClO4)2 antifreeze system than in the LiClO4 and NaClO4 systems (Fig. 1A and B). Reasons for this are provided in the Discussion. The increase of Na2C2O4 concentration in the systems extends the reaction run time until reaching a plateau (Fig. 1B) which often ends with a limited amount of Na2C2O4 that can be used before there is a build-up of Mn(IV) and brown precipitate starts to appear. The interplay of varying both HClO4 and Na2C2O4 in the antifreeze systems (see ESI† Fig. S3) shows a similar behavior as in the antifreeze-free system;15 where a detailed discussion on this interplay effect can be found. The antifreeze-containing systems also require a minimum HClO4 concentration for the reaction kinetics to work properly, while there is also a maximum concentration of Na2C2O4 that can be used before the “tail” in the absorbance profile starts disturbing the ideal color profile. If the Na2C2O4 concentration is pushed even higher, mostly in a low acid environment, it results in MnO2 formation and precipitation giving a brown color to the solution. As it can be noted in Fig. 1A and B, the range of HClO4 and Na2C2O4 is more limited for the Mg(ClO4)2 system compared to the other two systems. The longest reaction run time possible for each system is ∼135 min for NaClO4, ∼85 min for LiClO4, and ∼35 min for Mg(ClO4)2 (using 1.38 mM Na2C2O4 as shown in Fig. 1C but can go as long as ∼42 min when using 5 mM Na2C2O4 as shown in ESI† Fig. S3c).To study the effect of the Mn(II) concentration in the systems (Fig. 1C), fixed HClO4 and Na2C2O4 were used at the following initial concentrations without Mn(II) for long reaction times: 10 mM HClO4 and 1.38 mM Na2C2O4 for NaClO4, 4 mM HClO4 and 1.38 mM Na2C2O4 for LiClO4, and 10 mM HClO4 and 5 mM Na2C2O4 for Mg(ClO4)2. To increase the initial concentration of Mn(II) in the system, Mn(ClO4)2 was used. As expected, due to Mn(II) being an autocatalyst in the permanganate–oxalate reaction, as the concentration of Mn(ClO4)2 is increased, the reaction time is accelerated (Fig. 1C). Comparisons to predicted rates using the rate laws described in ref. 15 were not made due to the substantial deviations caused by the presence of the high concentrations of inert antifreeze salts.
Considering that we aim to use the TTIs to track biospecimen stability, they should exhibit temperature sensitivity similar to the temperature sensitivity of biomolecules within their native, ex vivo matrices. Thus, to be conservative, we aimed for high sensitivity to temperature—in principle at least as sensitive as the Arrhenius equation-derived “rule of thumb” (close to 25 °C), that an increase in the reaction temperature by 10 °C approximately doubles the reaction rate constant while a decrease in the temperature by 10 °C approximately halves it. Using the reaction run time to stand in for changes in reaction rate constants with temperature, this desired level of temperature sensitivity was verified previously in the antifreeze-free system15 and for the antifreeze-containing systems in this work.
At least for the TTIs that ran for 30 min at 25 °C, the three antifreeze-containing systems consistently exhibited an exponential increase in run time with a linear decrease in temperature (Fig. 2A). The run-time vs. temperature (in K) data for these three curves were fit to single-phase exponential decay equations in which the pre-exponential factor was limited to the run time at the melting point of each antifreeze system. The decay constant was nearly identical for the LiClO4 and NaClO4 systems, but slightly more negative for the Mg(ClO4)2 system. And because the Mg(ClO4)2 system can get the coldest without solidifying, it can run the longest as indicated by its high pre-exponential factor (ESI† Fig. S4). The absorbance vs. time profiles of the antifreeze-containing 30 min-at-25 °C systems are shown in Fig. 2B–D for LiClO4, NaClO4 and Mg(ClO4)2. To visually show that the reaction kinetics trajectories are not altered by the decrease in temperature, the absorbance profiles at lower temperatures were also included in Fig. 2B–D. The absorbance profile of the reaction run at 4 °C is shown for each of the three antifreeze systems, in addition to the reaction run at −12 °C for LiClO4 or at −18 °C for NaClO4 and Mg(ClO4)2. Because of the autocatalytic nature of the reaction, the antifreeze-free TTIs maintained >50% of the original color intensity for at least 75% of the reaction time.15 However, the antifreeze-based TTIs start decreasing in absorbance with an increasing slope as soon as the reaction starts (ESI† Fig. S5). Each system exhibits a unique change in the shape of the absorbance profile over time, with all systems maintaining >50% of the original absorbance once half of the reaction period has elapsed (ESI† Fig. S5).
 | ||
| Fig. 2 Temperature sensitivity of antifreeze-containing TTIs. (A) Run time vs. temperature plot shows the exponential temperature dependence of all three TTI systems. (B–D) Absorbance profiles over log-scale time for the 30 min-at-25 °C TTI systems demonstrate their temperature sensitivities at 4 °C, −12 °C, and/or −18 °C. (B) 25% (w/w) LiClO4 (m.p. −18 °C) at 25 °C, 4 °C, and −12 °C, (C) 52% (w/w) NaClO4 (m.p. −37 °C) at 25 °C, 4 °C, and −18 °C, and (D) 44% (w/w) Mg(ClO4)2 (m.p. −67 °C) at 25 °C, 4 °C, and −18 °C. The individual absorbance profile of each case, over standard time scales, are included in ESI† Fig. S5. | ||
To study the kinetic behavior of the antifreeze-containing TTIs under the influence of variable temperatures within a single reaction run, a series of reactions were exposed to pre-defined temperature variation periods (experimental design for each antifreeze system is shown in Fig. 3A–C). The results (Fig. 3D–F) indicated that temperature changes during a reaction do not substantially disturb it. Additionally, the total run times are consistent with run times observed at constant temperatures. Absorbance profiles over time for each case are provided in ESI† Fig. S6–S8 for the three systems. For experiments performed at temperatures ≤25 °C, a spectrophotometer set-up (see details in section 2.5) that allowed the tight control of temperature down to −20 °C was used, while it also monitored the actual temperature in the system at all times.
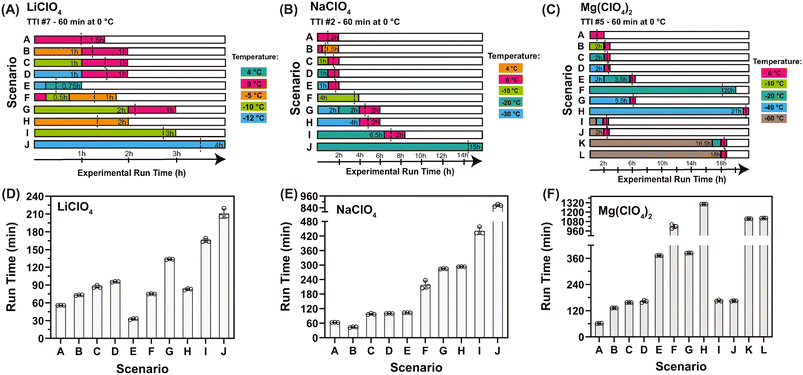 | ||
| Fig. 3 Influence of within-run temperature variability on the reaction run-time of activated TTIs. The experimental design of various temperature-change scenarios on a running reaction: (A) for the 60 min-at-0 °C LiClO4 system (case A, TTI #7), (B) for the 60 min-at-0 °C NaClO4 system (case A, TTI #2), and for the 60 min-at-0 °C Mg(ClO4)2 system (case A, TTI #5). The composition of each system can be found in Fig. 4. Temperatures were set at either 4 °C, 0 °C, −5 °C, −10 °C, or −12 °C for LiClO4 (m.p. −18 °C), at either 4 °C, 0 °C, −10 °C, −20 °C, or −30 °C for NaClO4 (m.p. −37 °C), or at either 0 °C, −10 °C, −20 °C, −40 °C, or −60 °C for Mg(ClO4)2 (m.p. −67 °C) for specific periods of times according to the experimental design. Absorbance data were acquired in the spectrophotometer, which can be set down to −20 °C. In some cases, temperatures of −20 °C and below were set in the Messenger freezer, in which case, no absorbance data were available until the solutions were transferred to the spectrophotometer (at >−20 °C). The total experimental time is indicated in each figure by the colored areas. To facilitate the interpretation of results, a dashed vertical line aligned with to the average observed run time is overlaid on top of each temperature-change case (panels A–C). The results of the scenarios illustrated in the experimental designs (A–C) are shown in (D) for LiClO4, (E) NaClO4, and (F) for Mg(ClO4)2. All experiments were performed in triplicate. Error bars represent SD. Absorbance profiles over time for each scenario are provided in ESI† Fig. S6–S8. | ||
Evaluation of continuous exposure to light and impact of atmospheric composition (considering oxygen, nitrogen, and carbon dioxide environments) was performed in the antifreeze-free permanganate–oxalate reaction, as we have reported elsewhere.15 Similar behavior is expected in the antifreeze-containing indicators. As such, these evaluations were not performed in this work. Briefly, light exposure showed an acceleration of the reaction of about 10%, while a nitrogen environment (or absence of oxygen) causes a ∼5% decrease in the reaction run time. The presence of CO2 was also evaluated with no change observed. Since the TTIs are meant to be run in closed environment (sealed tubes/vessels), humidity and atmospheric pressure are not expected to impact the reaction kinetics significantly.
3.3. Performance characteristics of targeted antifreeze-containing TTIs
 | ||
| Fig. 4 Absorbance profiles of specifically targeted time–temperature indicators (TTIs). Absorbance profile with time at the targeted temperatures of the TTIs developed here (details in Table 2). The targeted run time and temperature for each TTI is listed within its respective panel, along with the specific composition of that TTI. NaClO4-based systems designed for (A) 5 min at 25 °C, (B) 60 min at 0 °C, and (C) 7 days at −20 °C. Mg(ClO4)2-based systems designed for (D) 5 min at 25 °C, (E) 60 min at 0 °C, and (F) 7 days at −20 °C. LiClO4-based systems designed for (G) 5 min at 25 °C, and (H) 60 min at 0 °C. All replicates reported on Table 2 are shown in the plots, with the dotted line representing the mean value. The reaction was deemed completed when the absorbance at 525 nm fell below 0.03. | ||
To study within- and between-day accuracy and reproducibility (precision) of the TTIs, separate batches of at least 3 replicates were run on at least six different days at the targeted run temperature of each TTI. The accuracy was quantified as shown in eqn (2):
 | (2) |
The reaction run time was defined as the blank-subtracted absorbance at 525 nm going below 0.03 at a path length of 0.555 cm for the plate reader instrument or below 0.005 at a path length of 0.1 cm for the Cary60 spectrophotometer employed for experiments below 25 °C. At the target temperature, the within-batch run time precision, expressed as the coefficient of variation (CV), of all eight indicators was ≤4.8% while the inter-batch precision was ≤7.5% (Table 2). Similar run time precision data were observed when running the indicators at a different temperature than the targeted one (Table 2).
 | (3) |
![[y with combining double overline]](https://www.rsc.org/images/entities/i_char_0079_033f.gif) represents the grand average of all absorbance values across all time points of the average run against which individual runs were compared. The average run to which each individual run was compared was calculated by taking the average absorbance value at each time point for all averaged runs. Preparation of data and trajectory precision calculations are detailed in ref. 15.
represents the grand average of all absorbance values across all time points of the average run against which individual runs were compared. The average run to which each individual run was compared was calculated by taking the average absorbance value at each time point for all averaged runs. Preparation of data and trajectory precision calculations are detailed in ref. 15.
The within-batch RMSD was calculated for each replicate run relative to the average trajectory of its batch. The grand average of within-batch RMSDs from all batches was used as the overall within-batch trajectory precision metric, calculated to be ≤6.4% for all eight TTIs at the targeted temperature (Table 2), with an average of 4.5%. For the between-batch trajectory precision, the RMSD for each unique run was calculated relative to the grand average trajectory of all runs across all batches averaged together. The calculated inter-batch reaction trajectory RMSD was ≤14.3% for all eight TTIs at the targeted temperature (Table 2), with an average of 10.4%.
To better understand the effect of new stock solutions, reagent lots and different analysts on TTI run time and trajectory precision, two undergraduate volunteers with minimal prior research experience were recruited. Each created two sets of HClO4, Na2C2O4, LiClO4 and NaClO4 stock solutions (stocks A and B) as well as a third set of these same stock solutions using a different reagent lot number for each chemical (stocks C). Each analyst then used stocks A and B to make 15 replicate runs from each set of stocks for TTIs #1 and #7 (Table 2) on the same day. This was then repeated for stocks A and C. Two replicates of these entire stocks A vs. B and stocks A vs. C experiments were repeated on two additional days. The analysts then swapped stocks A, B, and C and ran 15 replicate runs of TTIs #1 and #7 with each set of Stocks from the other analyst. KMnO4 stocks were created fresh on each day by each analyst.
From the outset, including initial statistical power analysis, the planned approach to statistical analysis of run times was an assessment of how close the data sets were relative to one another. At the n-values employed, findings of statistically significant differences at low magnitudes of difference would have been inevitable—but misleading in the conclusion of there being important differences between the data sets. Thus, the degree of similarity between the data sets was analyzed using a statistical test of equivalence rather than conventional null hypothesis testing. Moreover, since normal distributions could not consistently be achieved through conventional logarithmic transformations of the run time data, the originally planned two one-sided test (TOST) of equivalence could not be deployed due to its reliance on normal data distributions. As such, statistical equivalence was assessed using a Bayesian Bootstrap Region of Practical Equivalence (ROPE) test in which the ROPE defines a range of mean differences between the two groups that is small enough to consider the groups to be practically equivalent.37 A brief description of the test (as markdown text) and the R script employed to run it in JupyterLab are provided as SI Code Package 1.
The average ROPE for the run times of two stock solutions made and run by the same analyst (stocks A vs. B), given as the difference between the means of the two groups expressed as a percentage of the combined mean, was 11% (ESI† Table S2). For stock solutions made from unique reagent lots (stocks A vs. C), the average run time ROPE was 8% (ESI† Table S2). The average run time ROPE for different analysts running the same stock solution was 9% (ESI† Table S2). Univariate plots for all run time comparisons made are provided in ESI† Fig. S9.
Adjacent to each of these plots are overlaid plots of absorbance at 525 nm vs. time for all runs being compared (ESI† Fig. S9). A table that lists the average RMSD (as %CV) of each trajectory from the combined mean of all trajectories under comparison—and does so for all group-wise comparisons described in the preceding paragraph is provided in ESI† Table S3. The average trajectory RMSD value (as %CV) for two stock solutions made and run by the same analyst (stocks A vs. B), was 8%. For stock solutions made from unique reagent lots (stocks A vs. C), the average RMSD (as %CV) was 6%. And the average RMSD (as %CV) for different analysts running the same stock solution was 11% (ESI† Table S3; overlaid trajectories are shown in ESI† Fig. S9).
Neither the creation of new stocks, new stocks from new reagent lots, or runs by different analysts stood out as a statistically significant primary cause of variability in these experiments (p > 0.05; Kruskal-Wallis test). This was true of both run times and trajectory RMSDs (as %CVs) (ESI† Tables S2 and S3). Moreover, the trajectory RMSDs (as %CVs) in these experiments were indistinguishable from those employed to determine inter-batch trajectory precision (cf.Tables 2 and S3†).
3.4. Evaluation of freeze/thaw cycles in the TTI system
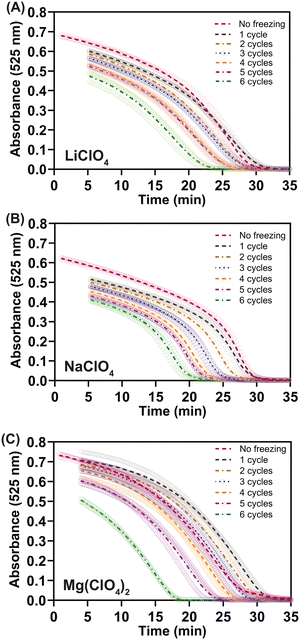
The antifreeze-containing TTIs are intended to be employed at subzero temperatures and follow the temperature exposure of their associated biospecimens by staying physically together with them. Since TTIs may be exposed to freeze/thaw (F/T) cycles, as some biological samples are, we evaluated the impact of F/T cycles on reaction run time and the subsequent run profiles. F/T cycles were performed by warming TTIs up to ∼0 °C in a beaker of stirred room temperature water. F/T cycles were assessed by using a water control sample for the thawing step. Once the water was fully thawed, the TTI tubes were placed back into the freezer. Fig. 5 compares the absorbance profiles of never-frozen TTIs to the same system exposed to up to six F/T cycles. The 30 min-at-25 °C LiClO4, NaClO4, and Mg(ClO4)2 systems were evaluated. As expected, the reaction time is shortened with the increased number of F/T cycles. In general, the TTIs went from a run time of 30 min at 25 °C (when no freezing is performed) to 20–25 min after six F/T cycles. Another set of the three TTIs was used to evaluate how many total F/T cycles can be performed before the reaction eventually turned colorless. The LiClO4, NaClO4, and Mg(ClO4)2 TTI systems took 20, 16 and 7 cycles before turning colorless, respectively. These results are in accord with the descending order of their melting points. Notably, as previously reported,15 the kinetics of antifreeze-free TTIs are rapidly accelerated by F/T cycles, undergoing a total of two F/T cycles before turning colorless for a 1 h reaction at 25 °C.3.5. Impact of preliminary freezing time on activated TTIs
It is envisioned that the TTIs will physically accompany biospecimens through their life span, being exposed to low temperatures for long periods of time and occasionally to freeze/thaw cycles (section 3.4). To evaluate the run-time stability of activated-but-frozen TTIs for different periods of freezing time, sets of samples were prepared and activated prior to their storage at −80 °C (LiClO4 and LiClO4) or −140 °C (Mg(ClO4)2) for up to 9 months. With each sample set, a freshly prepared control was run, as well as a briefly frozen control stored for 24 h at the corresponding temperature of the specimens being tested (i.e., at −80 °C or −140 °C). Results indicated that storage of activated TTIs well below their melting points effectively halts reaction progress until the TTIs are thawed, at which point they proceed for their expected duration considering one freeze/thaw cycle (Fig. 6).3.6. Long-term stability of activated TTIs
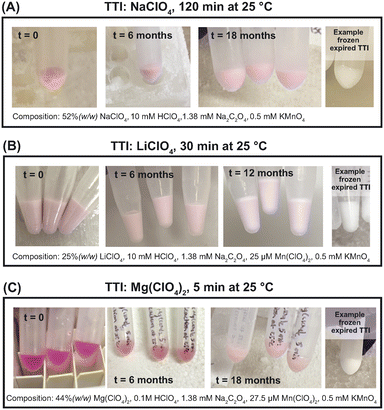
The long-term stability of the TTIs stored at −80 °C was evaluated. For this purpose, the solutions were flash-frozen prior to storage. Fig. 7 shows the 120 min-at-25 °C NaClO4, 30 min-at-25 °C LiClO4, and 5 min-at-25 °C TTIs Mg(ClO4)2 before initially being placed at −80 °C (after flash-freezing), followed by pictures of the frozen TTIs after 6 months and the longest storage time by the time of article submission, with the shortest period of a least 12 months. These three systems are designed to stay pink at −80 °C unless the temperature increases above their melting point.4. Discussion
The selection of antifreeze systems for use with the permanganate–oxalate TTIs was constrained by the reactivity of the antifreeze components themselves with permanganate. Due to the nature of MnO4− as a strong oxidant that will react with almost any organic compound, conventional carbon-based antifreeze systems (such as ethylene or propylene glycol) were deemed incompatible. Moreover, certain salts that contain oxidizable anions (such as chloride) were also contraindicated for use as antifreeze agents with these TTIs. Of the most well-known deep eutectic solvents,28,29 those consisting of a group I or II metal cation and a perchlorate anion provided the best selection of subzero, widely spaced eutectic temperatures combined with reasonable cost and lack of direct chemical interference with the permanganate–oxalate reaction. Notably, however, calcium salts are not generally employable for this purpose because of the low solubility of calcium oxalate.Despite the high solute concentration of melting-point depressed TTIs, supercooling below the nominal eutectic melting point remained possible (ESI† Fig. S1). For example, cooling the NaClO4-based TTI to −40 °C does not always result in solidification. We were able to initiate the permanganate–oxalate reaction in this system and preserve it in the liquid phase for over six months at −40 °C – at which point the reaction, which ran for 5 minutes at 25 °C, was still pink/running. Given this possibility, using an indicator with a melting point that is at least 20–25 °C warmer than the environment in which specimens are stored long term is recommended.
The high concentrations of perchlorate salts employed in the TTIs described here render the kinetics model previously described15 moderately less useful due to decreased predictive accuracy. Moreover, as can be observed in Fig. 4 and S5,† these antifreeze salts modestly perturb the kinetics trajectories—though all remain non-linear and run for extended periods at elevated absorbance values before exhibiting a relatively rapid transition to a colorless solution.
As we have previously described, as acid concentration decreases (pH increases), the permanganate–oxalate reaction eventually derails into production of a brown MnO2 precipitate rather than clear, soluble Mn(II).15 From a thermodynamic perspective (which can be viewed through the lens of the Nernst equation), this occurs because increases in pH lower the potential for the Mn(VII) to Mn(II) reaction faster than for the Mn(VII) to Mn(IV) reaction and eventually, at high enough pH, the latter reaction dominates.
The reason for the lowered tolerance of the Mg(ClO4)2 antifreeze system (relative to the LiClO4 and NaClO4 systems) to limited supplies of acid before the permanganate–oxalate reaction is derailed into generating MnO2 likely has to do with the facts that 1) Mg2+ ions can form a complex with oxalate dianions (C2O42−), and 2) the ionic strength of the Mg(ClO4)2 antifreeze system is substantially greater than that of the LiClO4 and NaClO4 systems.
From a thermodynamic perspective (i.e., the Nernst equation), complexation of Mg2+ by C2O42− anions lowers the free C2O42− concentration. As a reactant in the permanganate–oxalate reaction, this unique decrease of free C2O42− in the Mg(ClO4)2 antifreeze system lowers the potential (driving force) for the Mn(VII) to Mn(II) permanganate–oxalate reaction (where the C2O42− coefficient is 5) more than for the Mn(VII) to Mn(IV) permanganate–oxalate reaction (where the C2O42− coefficient is 3) —and certainly more than it would for either reaction relative to what it would be if no metal–C2O42− complex formed.
Likewise, from this same thermodynamic perspective, the greater ionic strength of the Mg(ClO4)2 antifreeze system results in lower reactant activities in the Mg(ClO4)2 system relative to LiClO4 and NaClO4 systems. This similarly lowers the potential (driving force) for the oxalate-mediated Mn(VII) to Mn(II) reaction more than for the Mn(VII) to Mn(IV) reaction—and, again, more so for both reactions relative to what the reduction potentials would be if LiClO4 or NaClO4 were present rather than Mg(ClO4)2.
As seen from the results in Fig. 1, these effects are clearly not overcome by the fact that Mg2+ ions can form hydroxide and oxalate complexes while Li+ and Na+ ions essentially do not—which slightly lowers solution pH, all else held equal.
While effective at depressing the melting point of aqueous solutions, high-concentration perchlorate salt solutions such as those employed in this work are strong oxidizers and, as such, pose potential risks. For example, if intentionally dried and placed in contact with a combustible organic material, they could potentially cause a fire. When used as described above, however, this risk is negligible. At the compositions used in this work, these materials do not present a serious risk of explosion—even if allowed to dry out in air. Of course, as with any chemical, after use, the TTIs described should be disposed of properly as (liquid) hazardous waste in a glass or suitable plastic container. Metal containers should not be used because some perchlorate salts can corrode metals.
Between the previously reported antifreeze-free TTIs15 and this work, we have presented a total of 14 different TTIs that were designed to run for a specific period of time at a specific temperature—but exhibit run-time sensitivity to changes from this target temperature should they occur. These 14 TTIs represent time–temperature intervals that we felt would likely have the most practical use for biospecimen monitoring. For example, our previously reported antifreeze-free TTIs can be used for tracking the time between blood collection, processing, and/or storage.15 The antifreeze-containing TTIs can be used for applications involving collection and temporary storage of biospecimens at satellite sites (that typically do not have ≤−20 °C storage capabilities) or for long-term storage where no freeze/thaw cycles or storage above the TTI's critical temperature are allowed. Though it is imperfect, the existing kinetics model as well as the example TTIs that have been created to date make it clear that TTIs with run times of as little as a few seconds to as long as 3 hours or more at 25 °C with melting points (kinetic pause points) of 0 °C,15 –18 °C, −37 °C, and −67 °C can be custom-created for any niche application.
As mentioned in the introduction, there is currently a need for TTIs to monitor the cold-chain of vaccines,11,12,14 which has been a particular challenge for the production and distribution of COVID-19 vaccines, as mRNA-based vaccines must be stored at temperatures between −15 and −80 °C.13 The permanganate–oxalate TTIs can be customized for this purpose as they work in this temperature range. Other products such as rAAV vectors for gene therapy applications are required to be stored at ultra-cold temperatures, i.e. ≤−65 °C, with a shelf-life of about 1 year.38 For these applications, Mg(ClO4)2-based TTIs are ideal, since they will be active if the temperature goes above −67 °C (critical temperature point or melting point), which can be designed for a specific period of time or for a short exposure so that any rise in temperature will be indicated by a change in color.
Previously, we have been able to detect the exposure of plasma and serum (P/S) specimens to thawed conditions,2 and therefore identify compromised samples, using the endogenous integrity marker ΔS-Cys-Albumin, which was proposed and validated by our research group.1,2,16,17 Briefly, the ΔS-Cys-Albumin is based on the ex vivo oxidation of albumin in P/S measured by a dilute-and-shoot LC-MS approach.1,39 This assay has been linked to the stability of clinically relevant proteins when stored at different conditions.16 Additionally, a compilation of studies that report on the stability of clinical analytes in blood specimens was reported.2 Although a rapid and accurate approach, the ΔS-Cys-Albumin assay is specific for P/S specimens and required expensive instrumentation. The TTIs developed in this work represent a general tool for any type of biospecimen with an immediate visual response.
However, it is important to note that the time–temperature interval desired for a particular TTI use depends on the application, requiring a check for specific handling and storage guidelines depending on the biospecimen and analyte of interest. For example, most hormones in EDTA plasma and serum show stability when stored at 4 °C for up to 120 h, but this is not true for the adrenocorticotropic hormone (ACTH) which showed instability within 18 h for EDTA plasma and within 3 h for serum.2,40 This can be tracked using antifreeze-free TTIs designed to last either 3 h or 18 h at 4 °C.15 Similarly, a TTI designed to run 18–24 h at 4 °C can be used to monitor analytes such as potassium, bicarbonate, lactate, glucose, magnesium, osteocalcin and C-telopeptide in P/S, as they have been reported to show instability when store at 4 °C for up to 24 h.41,42 In general, P/S specimens are required to be stored at temperatures below −30 °C to ensure they are frozen. Therefore, NaClO4-based indicators are considered a good fit for most P/S applications. However, some analytes might require different considerations. Ascorbic acid has been reported to be rapidly degraded in plasma, requiring plasma specimens to be processed immediately after collection and to be stored at −70 °C to avoid degradation.43 For this case, a short Mg(ClO4)2-based TTI would be ideal since it will be melted and start running right above the critical temperature for ascorbic acid storage and therefore, it will indicate any exposure to ≥−67 °C.
Other biospecimens, not just P/S, have also demonstrated analyte instability when exposed to thawed conditions. For example, amyloid-beta peptides and phosphorylated tau (both biomarkers of Alzheimer's disease) in cerebrospinal fluid have shown degradation when stored at 4 °C for one week or subjected to more than four freeze–thaw cycles.44 Another example is the microbial profile in stool specimens reported to be altered if specimens are not properly stored at ≤−80 °C for transportation or long-term storage.45
While the chemical TTI systems described here and the antifreeze-free ones previously reported15 can be implemented in any common laboratory test tube or vial, their widespread adoption will likely require engineered user-activatable, aliquot-level devices that contain a viewing compartment for the permanganate–oxalate chemistry and a separate compartment for the biospecimen that they are meant to accompany. The cost of the chemicals involved in these reactions is miniscule (a few cents or less per reaction). But implementing the use of these TTIs in a laboratory, biobank, or manufacturing workflow requires either setting them up within a laboratory from scratch or purchasing devices like the ones alluded to above. The former requires more labor than most labs or businesses are willing to invest. The latter depends on whether box-level or individual vial-level devices are used. It is our expectation that, once fully designed and implemented at scale, both options will be far more cost effective than the relative long-term cost of doing research on expired biospecimens or conducting business with expired biological products.
5. Conclusion
The ability to control the kinetics of the permanganate–oxalate redox reaction in the range of 25 °C to −20 °C and keep it running at even colder temperatures—but paused below specific subzero temperature thresholds (−18 °C, −37 °C, and −67 °C)—has facilitated the design of inexpensive, color-changing time–temperature indicators for a diverse range of potential applications. Such applications include but are not limited to those that may require time–temperature monitoring for as little as a few minutes at room temperature to those that permit exposure for as long as two hours or more and, simultaneously, allow for the possibility of a limited number of freeze–thaw cycles. Application fields include those as diverse as biospecimen collection and archiving to food and vaccine transportation and storage. Future work will include the development of user-friendly devices that facilitate indicator activation, viewing and specimen storage in the same device but without physical contact between the indicator chemicals and the specimen of interest.Data availability
Data supporting this article have been included as part of the ESI.† Data corresponding to any data point(s) in the manuscript that are not provided here are available upon request. Code Packages for data analysis are available as supplemental or previously reported.11Author contributions
Jorvani Cruz Villarreal is the first author and contributed to investigation, data curation, validation, formal Analysis and writing of the original draft preparation. Emil Ljungberg and Nilojan Jehanathan contributed to investigation, data curation and validation. Milap Owens and Anika Li contributed to the investigation. Chad R. Borges is the corresponding author and supervisor; he contributed to the conceptualization, funding acquisition, methodology, software, data curation, formal analysis, writing – review and editing.Conflicts of interest
The authors declare the following financial interests and/or personal relationships which may be considered conflicts of interests. Chad R. Borges (corresponding author) is a co-founder and the chief science officer of CryoVeritas, Inc., which was co-founded by General Inception with the intention of commercializing the visual time–temperature indicators described herein. Jorvani Cruz Villarreal and Nilojan Jehanathan are partial shareholders of CryoVeritas, Inc. Nilojan Jehanathan was previously employed by CryoVeritas, Inc.Acknowledgements
This work was funded in part by the National Cancer Institute of the National Institutes of Health, Innovative Molecular Analysis Technologies (IMAT) Program, Grant Number: R21CA250999. We thank Chemical Microscopy LLC for their support characterizing the eutectic Mg(ClO4)2 solution. We acknowledge the support of Professor Matthew Green at the School for Engineering of Matter, Transport and Energy at ASU providing access and training for DSC analysis. The author(s) employed ChatGPT to help write the code package described. The author(s) subsequently reviewed and edited the content as necessary and take responsibility for the content of this publication.References
- J. W. Jeffs, N. Jehanathan, S. M. F. Thibert, S. Ferdosi, L. Pham, Z. T. Wilson, C. Breburda and C. R. Borges, Mol. Cell. Proteomics, 2019, 18, 2121–2137 CrossRef CAS PubMed.
- Y. Hu, C. Mulot, C. Bourreau, D. Martin, P. Laurent-Puig, L. Radoï, P. Guénel and C. R. Borges, Biopreserv. Biobanking, 2020, 18, 376–388 CrossRef CAS PubMed.
- L. P. Freedman, I. M. Cockburn and T. S. Simcoe, PLoS Biol., 2015, 13, e1002165 CrossRef PubMed.
- M. Shetty J, Environ. Conserv. J., 2018, 19, 101–106 CrossRef.
- S. Wang, X. Liu, M. Yang, Y. Zhang, K. Xiang and R. Tang, Packag. Technol. Sci., 2015, 28, 839–867 CrossRef CAS.
- Y. Liu, L. Li, Z. Yu, C. Ye, L. Pan and Y. Song, Packag. Technol. Sci., 2023, 36, 833–853 CrossRef.
- T. Gao, Y. Tian, Z. Zhu and D.-W. Sun, Trends Food Sci. Technol., 2020, 99, 311–322 CrossRef CAS.
- A. T. Pandian, S. Chaturvedi and S. Chakraborty, J. Food Meas. Charact., 2021, 15, 1523–1540 CrossRef.
- H. Yousefi, H.-M. Su, S. M. Imani, K. Alkhaldi, C. D. M. Filipe and T. F. Didar, ACS Sens., 2019, 4, 808–821 CrossRef CAS PubMed.
- M. I. Bravo, S. Grancha and J. I. Jorquera, Pharmeur. Sci. Notes, 2006, 2006, 31–35 CAS.
- C. Huang, Y. Shang, J. Hua, Y. Yin and X. Du, ACS Nano, 2023, 17, 10269–10279 CrossRef CAS PubMed.
- D. Kou, L. Gao, R. Lin, S. Zhang and W. Ma, Adv. Sci., 2024, 11, e2310060 CrossRef PubMed.
- D. J. A. Crommelin, T. J. Anchordoquy, D. B. Volkin, W. Jiskoot and E. Mastrobattista, J. Pharm. Sci., 2021, 110, 997–1001 CrossRef CAS PubMed.
- L. T. Hao, M. Lee, H. Jeon, J. M. Koo, S. Y. Hwang, D. X. Oh and J. Park, ACS Omega, 2021, 6, 8598–8604 CrossRef CAS PubMed.
- J. Cruz Villarreal, E. Ljungberg, A. G. Uy, A. Li, S. Kremer and C. R. Borges, Chem. Eng. J., 2025, 506, 160100 CrossRef CAS PubMed.
- E. P. Kapuruge, N. Jehanathan, S. P. Rogers, S. Williams, Y. Chung and C. R. Borges, Mol. Cell. Proteomics, 2022, 21, 100420 CrossRef CAS PubMed.
- S. Kremer, V. Shakhnovich, A. K. Riffel, L. Harvey and C. R. Borges, Biopreserv. Biobanking, 2024, 22, 578–585 CrossRef CAS PubMed.
- F. Betsou, E. Gunter, J. Clements, Y. DeSouza, K. A. Goddard, F. Guadagni, W. Yan, A. Skubitz, S. Somiari, T. Yeadon and R. Chuaqui, J. Mol. Diagn., 2013, 15, 3–16 CrossRef CAS PubMed.
- A. Hubel, R. Spindler and A. P. Skubitz, Biopreserv. Biobanking, 2014, 12, 165–175 CrossRef PubMed.
- K. Kisand, I. Kerna, J. Kumm, H. Jonsson and A. Tamm, Clin. Chem. Lab. Med., 2011, 49, 229–235 CrossRef CAS PubMed.
- K. G. Kugler, W. O. Hackl, L. A. Mueller, H. Fiegl, A. Graber and R. M. Pfeiffer, J. Clin. Bioinf., 2011, 1, 9 CrossRef PubMed.
- A. E. Barden, E. Mas, K. D. Croft, M. Phillips and T. A. Mori, Anal. Biochem., 2014, 449, 129–131 CrossRef CAS PubMed.
- J. M. Lawrence, M. A. Umekubo, V. Chiu and D. B. Petitti, Clin. Lab., 2000, 46, 483–486 CAS.
- F. Boomsma, G. Alberts, L. van Eijk, A. J. Man in 't Veld and M. A. Schalekamp, Clin. Chem., 1993, 39, 2503–2508 CrossRef CAS.
- I. S. Sourvinou, A. Markou and E. S. Lianidou, J. Mol. Diagn., 2013, 15, 827–834 CrossRef CAS PubMed.
- J. Lv, W. Zuo, C. Tian, M. Wang, Q. Liao and Z. Lin, Cell Rep. Phys. Sci., 2023, 4, 101456 CrossRef CAS.
- W. Selig, Talanta, 1979, 26, 1061–1064 CrossRef CAS PubMed.
- V. F. Chevrier, J. Hanley and T. S. Altheide, Geophys. Res. Lett., 2009, 36, L10202 Search PubMed.
- Y. Marcus, ACS Sustainable Chem. Eng., 2017, 5, 11780–11787 CrossRef CAS.
- A. G. Davidian, A. G. Kudrev, L. A. Myund and M. K. Khripun, J. Near Infrared Spectrosc., 2014, 22, 121–128 CrossRef CAS.
- Z. Zhang and X.-Y. Liu, Chem. Soc. Rev., 2018, 47, 7116–7139 RSC.
- A. Arsiccio and R. Pisano, J. Pharm. Sci., 2020, 109, 2116–2130 CrossRef CAS PubMed.
- E. Reátegui and A. Aksan, J. Phys. Chem. B, 2009, 113, 13048–13060 CrossRef PubMed.
- P. Spichtinger, P. Marschalik and M. Baumgartner, Atmos. Chem. Phys., 2023, 23, 2035–2060 CrossRef CAS.
- M. Baumgartner, C. Rolf, J. U. Grooß, J. Schneider, T. Schorr, O. Möhler, P. Spichtinger and M. Krämer, Atmos. Chem. Phys., 2022, 22, 65–91 CrossRef.
- A. D. Bravenec and D. C. Catling, ACS Earth Space Chem., 2023, 7, 1433–1445 CrossRef CAS PubMed.
- J. K. Kruschke, J. Exp. Psychol., 2013, 142, 573–603 Search PubMed.
- J. Lengler, M. Gavrila, J. Brandis, K. Palavra, F. Dieringer, S. Unterthurner, F. Fuchsberger, B. Kraus and J. A. H. Bort, Sci. Rep., 2024, 14, 27685 CrossRef CAS PubMed.
- C. R. Borges, D. S. Rehder, S. Jensen, M. R. Schaab, N. D. Sherma, H. Yassine, B. Nikolova and C. Breburda, Mol. Cell. Proteomics, 2014, 13, 1890–1899 CrossRef CAS PubMed.
- M. J. Evans, J. H. Livesey, M. J. Ellis and T. G. Yandle, Clin. Biochem., 2001, 34, 107–112 CrossRef CAS PubMed.
- C. Oddoze, E. Lombard and H. Portugal, Clin. Biochem., 2012, 45, 464–469 CrossRef CAS PubMed.
- J. Zhou, A. Fabros, S. J. Lam, A. Coro, R. Selvaratnam, D. Brinc and A. Di Meo, Clin. Chem. Lab. Med., 2024, 62, 1557–1569 CrossRef CAS PubMed.
- A. Karlsen, R. Blomhoff and T. E. Gundersen, Eur. J. Clin. Nutr., 2007, 61, 1233–1236 CrossRef CAS PubMed.
- S. Ho, J. Darrow, F. De Simone, A. Calabro, S. Gannon, R. Esquivel, P. Thakker, K. Khingelova, A. Rao, Y. Zhang and A. Moghekar, J. Appl. Lab. Med., 2024, 9, 789–802 CrossRef PubMed.
- X. M. Li, X. Shi, Y. Yao, Y. C. Shen, X. L. Wu, T. Cai, L. X. Liang and F. Wang, Microbiol. Spectrum, 2023, 11, e0429722 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5re00192g |
| This journal is © The Royal Society of Chemistry 2025 |