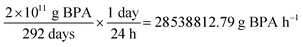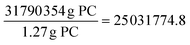DOI:
10.1039/D5RE00047E
(Paper)
React. Chem. Eng., 2025,
10, 1417-1428
A proposed industrial scale-up of circular bisphenol-A (BPA) production
Received
2nd February 2025
, Accepted 13th March 2025
First published on 3rd April 2025
Abstract
To meet the evolving sustainability goals of the modern-day chemical industry, there is a demand for novel chemical processes that minimize environmental impact while also maximizing profitability. This paper proposes a novel commercial solution for producing bisphenol-A (BPA) from the advanced recycling of polycarbonate waste. Current BPA production methods have major environmental and safety concerns from the use of benzene, high temperatures and pressures, and strong acids, and the proposed novel method addresses all these issues. This advanced recycling process utilizes a base-catalyzed methanolysis reaction with a methanol/toluene solvent mixture to produce BPA and dimethyl carbonate (DMC), a sustainable fuel additive both at 99.99 wt% purity. A prototype process was simulated in Aspen Plus®, and a preliminary process flow diagram was developed. With a target production capacity of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of BPA per annum, the major processing equipment is one packed-bed reactor, two crystallizers, and three distillation columns. All required heat exchangers and pumps were integrated into the simulation and can be adjusted based on product specifications and processing capacity. Analysis of green metrics for the novel process demonstrated that the process minimizes waste from a mass standpoint, and a rigorous economic analysis showed that the process is highly profitable in several varied scenarios.
000 metric tons of BPA per annum, the major processing equipment is one packed-bed reactor, two crystallizers, and three distillation columns. All required heat exchangers and pumps were integrated into the simulation and can be adjusted based on product specifications and processing capacity. Analysis of green metrics for the novel process demonstrated that the process minimizes waste from a mass standpoint, and a rigorous economic analysis showed that the process is highly profitable in several varied scenarios.
1. Introduction: overview of traditional BPA manufacturing
There are several pathways to produce bisphenol-A (BPA), most of which use acetone and phenol as raw materials. The predominant method, the Hock process, allows for both phenol and acetone to be produced in a multi-step mechanism that uses propylene and benzene as primary reactants. The production of both raw materials in one process, although complex, is what makes the Hock process convenient in industry. However, it comes with several drawbacks. One of the main intermediates, cumene, is oxidized to cumene hydroperoxide (CHP) at high operating temperatures. CHP poses inherent safety risks as it is an unstable intermediate that undergoes a highly exothermic degradation. In addition to this, the degradation is performed in large quantities of strong acid, which needs to be neutralized and disposed of carefully to avoid harm to operators and the surrounding environment.1,2
The goal of this paper is to evaluate the feasibility of an alternative method of BPA production. The methanolysis of polycarbonate waste has the potential to produce BPA at high yields while also addressing the process safety risks associated with the Hock process. Methanol is a well-known and common industrial reagent, and the solvent mechanism is efficient at significantly lower operating temperatures than the traditional method. At a lab-scale, BPA was synthesized using only methanol and toluene as solvents and relatively low amounts of strong base to catalyze the reaction. If scaled up, this can remedy the need for a complex multi-stage process with extreme operating conditions, thus simultaneously reducing operating costs and improving operational, environmental, and safety concerns. The science behind the methanolysis of plastic waste is still in its infancy, and there are doubts over the feasibility of using waste plastic as a feedstock, but this paper provides a comprehensive review of the scale-up potential of this innovative process.
2. Advanced recycling of waste polycarbonate to BPA
This process chemically recycles polycarbonate waste (PC) to produce bisphenol-A (BPA) and dimethyl carbonate (DMC). PC is reacted with a stoichiometric equivalent of methanol in a solvent mixture of methanol and toluene, catalyzed by sodium hydroxide in a batch reactor for 15 minutes at 60 °C. The reaction is quenched with toluene and then crystallized to produce the products of BPA and DMC. The solvents and catalyst are recovered with distillation.3–5
2.1 Reference data
The reagents, solvents, reaction conditions, and quantities of reagents were scaled up from Hu et al. In the experiment by Hu et al., 1.27 g of polycarbonate was reacted in a solvent mixture of 1 mL of methanol in 1.5 mL toluene.6 The kinetic data for the reaction was obtained from Kim et al.3 The mechanism of the methanolysis reaction was obtained from Piñero et al.4 The solubility of BPA in toluene was obtained from Little.5
2.2 Process assumptions & scale-up methodology
The lab-scale experiment conducted by Hu et al. was scaled up to an industrial-sized process of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of BPA produced per annum at 99.99 wt% purity. With an assumption of 80% on-stream efficiency, the process would stoichiometrically require 31
000 metric tons of BPA produced per annum at 99.99 wt% purity. With an assumption of 80% on-stream efficiency, the process would stoichiometrically require 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 kg h−1 of PC to meet production requirements. The solubility of BPA in the reactor effluent was approximated to be the solubility of BPA in toluene. It is assumed that the catalytic NaOH is regenerated in the reactor and does not leave the reactor. A scale factor between the stoichiometric requirement of PC and the amount of PC used in the lab-scale experiment was calculated, and the amounts of respective solvent were scaled up with this value. These solvent amounts, along with the mathematical determination of the scale factor, are detailed in Appendix A. The scale factor used in this paper serves as a benchmark for initial pilot plant trials. In practice, the exact amounts of feedstock, reagents, and solvents will be determined after these trials are complete. It is assumed that the major contaminants in waste PC feed will be flame retardants and organic materials, as further discussed in section 2.3.1. While there will most likely be other contaminants present in the waste polycarbonate, further lab scale and pilot plant scale trials will need to be conducted to identify all contaminants. Detailed kinetics will also need to be obtained from pilot plant studies. Furthermore, this process design does not consider the production of utilities or disposal of waste products.
790 kg h−1 of PC to meet production requirements. The solubility of BPA in the reactor effluent was approximated to be the solubility of BPA in toluene. It is assumed that the catalytic NaOH is regenerated in the reactor and does not leave the reactor. A scale factor between the stoichiometric requirement of PC and the amount of PC used in the lab-scale experiment was calculated, and the amounts of respective solvent were scaled up with this value. These solvent amounts, along with the mathematical determination of the scale factor, are detailed in Appendix A. The scale factor used in this paper serves as a benchmark for initial pilot plant trials. In practice, the exact amounts of feedstock, reagents, and solvents will be determined after these trials are complete. It is assumed that the major contaminants in waste PC feed will be flame retardants and organic materials, as further discussed in section 2.3.1. While there will most likely be other contaminants present in the waste polycarbonate, further lab scale and pilot plant scale trials will need to be conducted to identify all contaminants. Detailed kinetics will also need to be obtained from pilot plant studies. Furthermore, this process design does not consider the production of utilities or disposal of waste products.
2.3 Process design and description
A potential process flow diagram for the advanced recycling of polycarbonate waste to BPA as simulated in Aspen Plus® is shown below in Fig. 1.
 |
| | Fig. 1 PFD of the methanolysis of PC to BPA and DMC. | |
2.3.1 Polycarbonate washing.
PC waste commonly contains flame-retardant impurities and organic material. This preliminary washing method removes the impurities expected to be associated with PC waste. Data on the exact contaminants in each batch of PC will have to be validated before PC is fed as contaminants can vary based on the source of the PC. Due to the limited availability of contaminant data and the variability in contaminants, this study assumes the PC waste contains flame-retardant impurities and organic material, both of which can be removed via an acetone wash. A more rigorous washing step will be required as more data is available on PC waste contaminants and further pilot plant studies are conducted.7
Waste PC is mixed with a feed of acetone solvent and is continuously agitated in a washer. The solvent, along with the dissolved contaminants, is separated from the PC and sent to a storage tank. It is then recycled back from the storage tank into the cleaning process to reduce the cost and the volume of acetone required. To produce 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of BPA per annum, the washing step is estimated to require 50 tonnes of acetone cycling through the system at a given time.8 As the solvent will eventually become saturated with contaminants, it will have to periodically be replaced with fresh acetone. This washing process is shown below in Fig. 2.
000 tonnes of BPA per annum, the washing step is estimated to require 50 tonnes of acetone cycling through the system at a given time.8 As the solvent will eventually become saturated with contaminants, it will have to periodically be replaced with fresh acetone. This washing process is shown below in Fig. 2.
 |
| | Fig. 2 PFD of the washing step of the process. | |
2.3.2 Reactor.
The reactor section of the process is shown below in Fig. 3. The reactor conditions are 60 °C and 1 bar. A solvent mixture of methanol and toluene is combined with a fresh feed of PC waste that reacts over a fixed bed of catalytic sodium hydroxide to produce BPA and DMC. The quantity of solvent required at startup was calculated using the PC scale factor. As the system reaches steady state with the recycle streams, a solvent makeup feed is required to replace the amount lost through the product effluent.
 |
| | Fig. 3 PFD of the reactor step of the process. | |
To minimize the increased risk of corrosion due to sodium hydroxide, acid neutralization facilities will need to be installed downstream of the reactor to neutralize any sodium hydroxide carry over. A sour water stripper skid will purify the water byproduct and return the water to the process water system. Salt byproducts will be disposed of as process waste. A full detailed description of these systems are outside the scope of this study but allow for further process development.
2.3.3 Crystallization and filtration.
The first post-reaction product processing step of the process involves coalescing and purifying the BPA product. This section of the process flow diagram is shown in Fig. 4. Streams of DMC, BPA, methanol, and toluene flow into a surface-cooled crystallizer that is maintained at 15 °C. The temperature change of the process stream from 60 °C to 15 °C drastically reduces the solubility of BPA in toluene, causing most of the BPA to precipitate out from the solution. This slurry of suspended, solid BPA is then fed to a rotary drum vacuum filter (RDVF). This filter will use a mesh with a 5-micron beta 1000 rating to give filtration efficiencies of 99.99%. The filter cake from the RDVF is comprised of the BPA with the desired product concentration of 99.8 wt%. The RDVF effluent stream contains the solvents and the valuable DMC byproduct.
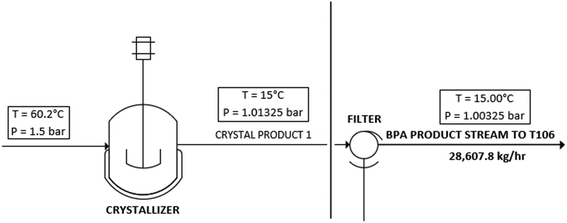 |
| | Fig. 4 PFD of the solid separation step of the process. | |
2.3.4 Distillation.
The solvent recovery and DMC purification component of the process is comprised of three distillation columns, which are detailed below in Fig. 5. The first distillation column separates nearly all the methanol in the distillate stream, which is then cooled with a heat exchanger and recycled back to the reactor inlet. The second and third distillation columns are used to separate the toluene and DMC streams. However, toluene and DMC form an azeotrope at a 78% molar quantity of DMC. Having the azeotrope at this purity also results in an undesirable amount of DMC to be recycled back to the reactor with the toluene bottoms stream, greatly increasing energy costs and required equipment size. To break this azeotrope, a pressure swing distillation method was used.
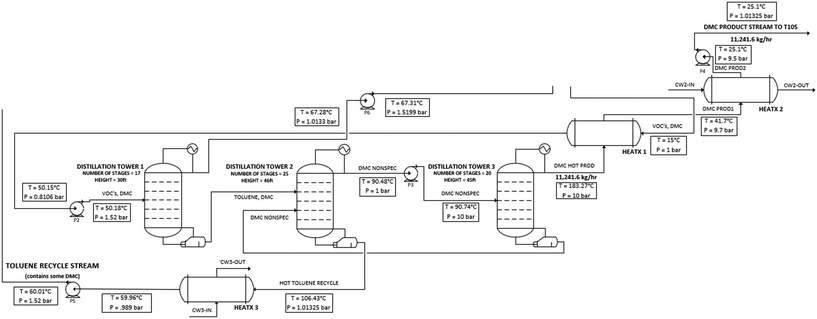 |
| | Fig. 5 PFD of the solvent recovery and DMC purification step of the process. | |
Pressure swing distillation uses two columns running at different pressures. The system of toluene and DMC is a minimum-boiling system, in other words, the boiling point of the combined system is lower than the boiling point of the individual components. The lower-pressure column operates at 1.01325 bar while the high-pressure column is operated at 10 bar. The low-pressure column separates the DMC and toluene to the azeotropic limit at atmospheric pressure. The distillate of this lower-pressure column goes through a pump and becomes the feed of the high-pressure column. Because of the much higher pressures, the azeotropic concentration of DMC decreases, bypassing the azeotropic point in column 3. This allows column 3 to achieve higher toluene–DMC separation. The higher purity DMC in the distillate of column 3 is then combined with the initial feed to the low-pressure column. This recycle stream makes it so that the initial feed to the low-pressure column is a higher concentration than the azeotropic concentration at atmospheric pressure, which then bypasses the azeotrope. Fresh toluene will be injected into the reactor to account for solvent loss with DMC. In an industrial setting, this will most likely be accomplished by an online product analyzer for DMC (and BPA) with a feedback control loop to open a control valve to allow for more toluene to flow into the reactor. Further pilot plant studies will give valuable experimental data on recycle efficiency and solvent makeup streams.
At steady-state operation, the bottoms of the low-pressure column is at 84.9 wt% toluene and is then cooled and subsequently recycled back to the initial reactor inlet. The distillate of the low-pressure column is pressurized and fed into the high-pressure column. The bottoms of the high-pressure column is recycled back into the feed of the low-pressure column. The distillate of the high-pressure column is 99.9 wt% DMC that is throttled to a lower pressure, passed through a heat exchanger to bring it to room temperature, and sent to storage to be sold as a valuable byproduct. DMC is sold as “high purity” with other studies defining high purity as >99.8 wt% DMC.9,10
2.4 Process equipment
2.4.1 Heat exchangers.
The heat exchangers were first optimized using heat integration. The process does not require any extreme pressure or temperature differentials, so traditional BEM TEMA-type exchangers were chosen. The hot fluid is in the shell for exchangers 1 and 3. The tube pattern was selected as a 45-rotated square for all exchangers as they ensure optimal heat transfer and ease of maintenance. Single segmental baffle types were selected as they are the standard design. The heat exchanger specifications are shown below in Table 1.
Table 1 Heat exchanger specifications
| Heat exchanger |
HX1 heater |
HX2 cooler |
HX3 cooler |
| TEMA type |
BEM |
BEM |
BEM |
| Hot fluid location |
Shell side |
Tube side |
Shell side |
| Tube OD/pitch (cm) |
1.91/2.38 |
1.91/2.38 |
1.91/2.38 |
| Tube length (cm) |
540 |
390 |
405 |
| Shell ID/OD (cm) |
20.5/21.9 |
20.5/21.9 |
20.5/21.9 |
| Tube pattern |
45-Rotated square |
45-Rotated square |
45-Rotated square |
| No. of tubes/passes |
40/1 |
40/1 |
39/1 |
| Baffle type |
Single segmental |
Single segmental |
Single segmental |
| No. of baffles |
72 |
18 |
54 |
| Baffle cut orientation |
Vertical |
Horizontal |
Vertical |
| Baffle spacing (cm) |
7 |
19 |
7 |
2.4.2 Distillation columns.
The distillation columns were optimized with sieve trays spaced 2 ft apart with a diameter of 2.65 m. This data is summarized below in Table 2.
Table 2 Distillation tower summaries
| Distillation column |
Distill 1 |
Distill 2 |
Distill 3 |
| No. of stages |
17 |
25 |
20 |
| Tot. height (m) |
9.144 |
14.021 |
13.716 |
| Section diameter (m) |
3.071 |
2.650 |
1.456 |
| Tray type |
Sieve |
Sieve |
Sieve |
| Tray spacing (m) |
0.610 |
0.610 |
0.762 |
| Pressure drop (bar) |
0.139 |
0.226 |
0.177 |
| No. of passes |
1 |
1 |
1 |
3. Advantages of the methanolysis of polycarbonate waste process
3.1 Improved process safety
The methanolysis of polycarbonate has several advantages over the traditional Hock process from a process safety perspective. It has become a trend in the past decade to find replacements for hazardous solvents leading to an inherently safer design of a chemical process. The only solvents used in the process are toluene and methanol, both considered green solvents and less hazardous to humans than benzene and sulfuric acid used in the Hock process.11,12 Furthermore, the methanolysis reaction is run mostly at atmospheric pressure and a mild reaction temperature of 60 °C reducing the risk of overpressure scenarios. There are no explosive intermediates in the methanolysis reaction which is a hazard in the Hock process. Furthermore, by having less hazardous materials at the facility, the risks to the surrounding community are minimized.
3.2 Environmental benefits
The methanolysis of polycarbonate has minimal process wastewater. The Hock process reaction produces water, while the water produced in this process is produced from the neutralization of the catalyst carryover. Furthermore, the emissions from the process are CO2 from the flare and the production of steam. There is minimal waste from the process as both solvents are recycled and the byproduct, DMC, can be sold. The low process temperatures and pressures result in a lower utility demand than the Hock process. The use of a non-metal catalyst eliminates the need for a non-renewable metal catalyst. Finally, the methanolysis of polycarbonate contributes to a circular economy by reducing the need for virgin materials.
3.2.1 Mass intensity analysis.
To compare the mass intensity of the two processes, five standard green metrics were used to analyze the methanolysis of polycarbonate waste and the traditional Hock process. The green metrics evaluated were atom economy (AE), reaction mass efficiency (RME), stoichiometric factor (SF), material recovery parameter (MRP) and yield.13 The equations to calculate each parameter are shown in Appendix C. The ideal value for each parameter is 1. As shown in Fig. 6 below, the methanolysis of polycarbonate has higher metrics than the Hock process indicating that the process has less waste than the Hock process and therefore less environmental impact. The exact metric values are shown below in Table 4.
 |
| | Fig. 6 The comparison of the green metrics for the methanolysis of polycarbonate and the Hock process. | |
3.3 Byproduct: dimethyl carbonate (DMC)
DMC is a valuable product since it is a sustainable gasoline additive for meeting oxygenate specifications as outlined in the Clean Air Act. DMC has a high oxygen content of 53.3% and an octane number of 116. Furthermore, DMC in fuels can reduce particle matter, total hydrocarbon and soot emissions.14 DMC is also used as a nontoxic solvent and an intermediate in pharmaceutical production. In addition, DMC is an electrolyte solvent of lithium-ion batteries mixed with ethylene carbonate. Due to the rising demand for lithium-ion batteries, the demand for DMC is also expected to increase.10 DMC has a low toxicity and biodegrades quickly. However, the current processes to produce DMC are complex and use toxic reagents such as phosgene. Therefore, there is a global demand for DMC produced from safer and more sustainable sources.15,16
4. Challenges with industrial implementation of the methanolysis of polycarbonate waste process
While there are many advantages with the advanced recycling of PC to BPA over the traditional processes, there are still many unknowns about this process that will require a pilot plant and more upfront costs. Further pilot plant scale testing will be required to fully develop this advanced recycling process. One of the biggest challenges with any advanced recycling process is the management of contaminants and balancing the economics of more processing steps to clean the PC feed versus higher feed cost by purchasing PC at a higher purity. First, a characterization method will need to be developed with experimental data to efficiently and accurately determine the contaminant composition of PC waste. Some common characterization methods used in advanced recycling today are Fourier transform, infrared spectroscopy (FT-IR), comprehensive 2D gas chromatography (GC × GC), Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS), and liquid chromatography.17 Furthermore, experimental data will be needed to determine how contaminants affect reaction kinetics, catalyst activity, separation efficiency and product purity. In addition to the further development of contaminant impacts, a full detailed mechanism of all byproduct reactions will need to be developed and their impact on the process fully realized. Catalyst life span will need to be determined based on the contaminant load and reaction byproducts. Further economics will need to be developed to determine if an online catalyst change out (with a spare reactor) is feasible. The distillation and product separation will need to be optimized to ensure product quality and energy efficiency. Process utilities will need to be fully developed and integrated into the process including wastewater treatment from the neutralization of sodium hydroxide with acid.
For the sourcing of PC, it may be difficult to find suppliers of the plastic waste for continuous operation of the process for many years. It is recommended that several reliable suppliers are identified to maintain a constant supply of PC waste to the process. A summary of the advantages and disadvantages of the methanolysis of PC process versus the traditional Hock process are shown below in Table 5.
Table 5 Summary of the advantages and disadvantages of the methanolysis of PC versus the traditional Hock process
| Process parameter |
Methanolysis of polycarbonate waste |
Hock process |
| Solvents and reactants used |
Methanol and toluene |
Benzene and sulfuric acid |
| Reactor temperature |
140 °F |
600 °F |
| Waste streams |
All byproducts are profitable |
Multiple waste streams |
| Catalyst |
Non-metal catalyst |
Precious metal catalysts |
| Waste reduction |
Reducing waste going to landfills |
Requires the use of new materials |
| Financial risk |
Process has not been completed on an industrial scale |
95% of BPA is produced using this method |
| Economics |
All cases are above a 15% hurdle rate |
Some cases fail to meet a 15% hurdle rate |
5. Economics
5.1 Existing PC advanced recycling facilities
In August of 2023, Covestro® announced successful laboratory trials of polycarbonate advanced recycling via chemolysis and are heavily invested into pilot plant trials. While we cannot determine if the Covestro® process is a methanolysis reaction since the company has not released the exact chemical method for the recycling process, Covestro's® investment shows that the advanced recycling of polycarbonate is both possible and profitable.18
5.2 Existing PET advanced recycling facilities
Even though the advanced recycling of polycarbonate (PC) has not yet been completed on an industrial scale, advanced recycling of polyethylene terephthalate (PET) plastic is a very similar process that can be used as a proxy for the risk analysis of the advanced recycling of PC. PET is a thermoplastic polymer and is the most used polyester in the plastic industry. There are various PET advanced recycling methods such as methanolysis, glycolysis, and hydrolysis. Since the process we are proposing utilizes the methanolysis reaction, the focus of this section will cover the similarities between the methanolysis of PET and PC. By looking at various successful PET recycling facilities, a rough estimation of process costs, environmental benefits, and capital investments can be extrapolated and compared to the PC recycling process. There are currently several large-scale PET advanced recycling facilities across the globe.
5.2.1 Northwest England PET methanolysis plant.
Although the PET and polycarbonate methanolysis processes are not identical, they are similar, and therefore the CO2 emissions and economics can be extrapolated to give a rough estimates for the methanolysis of polycarbonate process. A table showing estimations of capital investments for the Northwest England PET methanolysis plant can be seen below in Fig. 7.
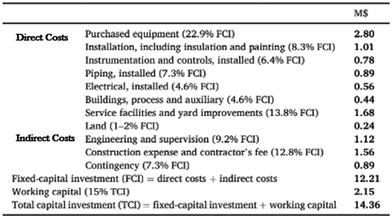 |
| | Fig. 7 Estimation of capital investments for PET facility in Northwest England.19 | |
Although the figure is not directly translatable to the methanolysis of polycarbonate, the process does require similar unit operations and provides a good estimation of capital required.19
5.2.2 Eastman Chemical – Kingsport, Tennessee.
Currently, Eastman Chemical® is constructing a PET advanced recycling facility with a capacity of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons. For the first 6 months of 2023, Eastman spent $413 MM on capital expenditures. Although not all the capital is being used for the plant, they have stated that “a large majority” of it is. In the future, Eastman is planning to pursue another two PET advanced recycling plants, one in France, and another somewhere in the United States.20
000 metric tons. For the first 6 months of 2023, Eastman spent $413 MM on capital expenditures. Although not all the capital is being used for the plant, they have stated that “a large majority” of it is. In the future, Eastman is planning to pursue another two PET advanced recycling plants, one in France, and another somewhere in the United States.20
5.3 OPEX, CAPEX, ROI, IRR
It was assumed that the polycarbonate waste price is $586 per metric ton.21–25 This price was determined by the approximate trend associated between the price of various virgin plastics and the price of the respective waste plastic. This assumption is valid since we scaled the waste plastic price of polycarbonate based on data for other plastic and estimated a conservative price. For the government subsidies, there is billions of dollars allocated by the U.S. government through legislation such as the Inflation Reduction Act to advanced recycling projects. While an exact subsidy amount requires an application to the EPA, DOE and other regulatory bodies, a conservative subsidy amount of $160 MM was assumed since other projects have received similar amounts.26–28
Due to the uncertainty and variability of a novel process, a sensitivity analysis was conducted to determine which scenarios are profitable and above the hurdle rate of 15%. One estimate for the FCI was the capital investment Eastman Chemical publicly shared for their PET advanced recycling facility. The company states that they spent “$413 MM in capital expenditures” during the first half of 2023 “most of which went to the PET advanced recycling facility”. While the exact FCI of Eastman's process is unknown, a conservative estimate of $400 MM for the FCI was used.20 For a second FCI estimate, the $130 MM FCI of the PET advanced recycling facility in Northwest England was taken as a lower bound.19 Since the price of waste polycarbonate is widely varied and the cost of cleaning the waste is unknown, two estimates for the price of polycarbonate waste were used. The $586 per metric ton price was determined by the approximate trend associated between the price of various virgin plastics and the price of their respective waste plastics. The $1000 per metric ton price was a conservative estimate to account for unforeseen costs in cleaning and acquiring the waste polycarbonate. For all economic calculations, a $6.4 MM total shipping cost for the polycarbonate was added, the tax rate was assumed to be 25% (estimated total federal and state tax), and a straight-line depreciation method over 10 years with the final salvage value being 10% of the FCI was used. The OPEX was calculated from the sum of the raw material cost, utilities, and the labor/maintenance cost. The results of the sensitivity analysis are shown below in Tables 6 and 7. The yellow color indicates close to the hurdle rate of 15%, while the green color indicates a ROI significantly above the hurdle rate.
Table 6 ROI values for an FCI (CapEx) of $400 MM
| PC price ($ per MT) |
586 |
1000 |
| ROI with subsidies |
58% |
44% |
| ROI without subsidies |
33% |
18% |
Table 7 ROI values for an FCI (CapEx) of $130 MM
| PC price ($ per MT) |
586 |
1000 |
| ROI with subsidies |
176% |
131% |
| ROI without subsidies |
98% |
53% |
As seen in Tables 6 and 7 above, all scenarios show a ROI above the hurdle rate. In addition to the sensitivity analysis, a working estimate of the economics was determined. From the Aspen Process Economic Analyzer®, the purchase price of the equipment for the process was estimated to be $20 MM (scaled to fall 2023 price using CPI, including cost of storage tanks for 30 days of reactants). No extra material multiplier was required since carbon steel will be used for the entire process. The FCI was estimated from a list of multipliers provided to account for installation, process piping, instrumentation, insulation, electrical, buildings, yard improvement, auxiliary facilities, engineering, construction, contractor's fee, and contingency. For each multiplier, the most conservative value was used. The internal rate of return was calculated by assuming a three-year startup period (with the land cost being $0, FCI for the first and second year being $50 MM and the FCI for the third year being $102 MM) and the after-tax cash flow is a constant $202 MM a year for 10 years. The sensitivity analysis was conducted for the FCI value of $202 MM. The final economic estimations are shown below in Tables 8 and 9 assuming $1000 per metric ton of PC, and government subsidies.
Table 8 ROI values for an FCI (CapEx) of $202 MM
| PC price ($ per MT) |
586 |
1000 |
| ROI with subsidies |
126% |
96% |
| ROI without subsidies |
75% |
46% |
Table 9 Summary of economic parameters
| FCI |
$201 MM |
| TCI |
$237 MM |
| Annual operating cost |
$284 MM |
| Cash flow |
$585 MM |
| Annual after-tax profit |
$230 MM |
| ROI |
96% |
| IRR |
64% |
As seen in Table 9 above and in the sensitivity analysis in Table 8, even with conservative estimates, this process is profitable. Further economic analysis will need to be developed for an industrial scale process since there are more upfront costs with a pilot plant design and further research. Economic values for an industrial plant are expected to be lower but still be profitable. Detailed economic calculations are shown in Appendix D.
6. Conclusion
Due to the environmental benefits, minimal process safety risks, and the high profitability of the process, the development of the methanolysis of polycarbonate waste process into BPA should be further researched for commercial applications. For this phase of the process development, further research needs to be done with suppliers of PC waste to determine exact economics for the process and the impurities that will come in the waste. Once the concentration of impurities has been determined, a more detailed washing step can be developed, or it may be determined that it is more economically feasible for a third-party to clean the waste. The full mechanism and chemistry of the reaction (with the known impurities present in the PC waste) will need to be thoroughly analyzed to make sure there are no unwanted side reactions. Eventually, a pilot plant would need to be developed to determine the unknowns associated with the process.
While this process has not been completed on an industrial scale and therefore has an increased economic risk, there has recently been a shift in the chemical industry to implement circular manufacturing into chemical processing. This is seen with companies such as Eastman Chemical® and Covestro® investing in advanced recycling facilities with very similar methods to the methanolysis of polycarbonate waste. More research should be conducted to ensure the development of more sustainable chemical processes to protect our planet for generations to come.
Data availability
The datasets supporting this article have been uploaded as part of the appendices.
Conflicts of interest
The authors have no conflict of interest to declare.
Appendix A: process scale-up calculations
Amount of PC required
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonne BPA = 2 × 1011 g BPA 000 tonne BPA = 2 × 1011 g BPA |
*Assuming 80% on-stream efficiency, the facility is operating for 292 days:
Scale factor
*From the lab-scale experiment done by Hu et al., the amount of PC used was 1.27 g
Amount of methanol solvent required
*Assuming an equivalent scale factor for all species.
*The experiment in Hu et al. used 1 mL of methanol solvent
0.791 g MeOH × 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031 031![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774.8 = 19 774.8 = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134 g MeOH ≈ 19 134 g MeOH ≈ 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.1 kg MeOH 800.1 kg MeOH |
Amount of toluene solvent required
*Assuming an equivalent scale factor for all species.
*The experiment in Hu et al. used 1.5 mL of toluene solvent
0.866 g C6H5CH3 × 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031 031![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774.8 = 32 774.8 = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 516 516![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276 g C6H5CH3 ≈ 32 276 g C6H5CH3 ≈ 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 516.3 kg C6H5CH3 516.3 kg C6H5CH3 |
Appendix B: detailed stream summaries
Table 10 Detailed stream summaries
| Stream summaries |
Flowrate (kg h−1) |
Temperature/pressure (C bar−1) |
PC (mass frac.) |
Methanol (mass frac.) |
Toluene (mass frac.) |
Acetone (mass frac.) |
BPA (mass frac.) |
DMC (mass frac.) |
| Dirty PC |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
25/1.01325 |
1 |
0 |
0 |
0 |
0 |
0 |
| Acetone |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
25/1.01325 |
0 |
0 |
0 |
1 |
0 |
0 |
| Clean PC |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
25/1.01325 |
1 |
0 |
0 |
0 |
0 |
0 |
| Acetone recycle |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
25/1.10325 |
0 |
0 |
0 |
1 |
0 |
0 |
| Solvent feed |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 184.4 184.4 |
59.9/1.01325 |
0 |
0.4771 |
0.5229 |
0 |
0 |
0 |
| DMC from recycle |
8564 |
60/1.51988 |
0 |
0 |
0 |
0 |
0 |
1 |
| MeOH reactant |
8010 |
60/1.01325 |
0 |
1 |
0 |
0 |
0 |
0 |
| Reactor inlet |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4 607.4 |
59.9/1.01325 |
0.4319 |
0.2723 |
0.1795 |
0 |
0 |
0.1163 |
| Reactor outlet |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4 607.4 |
60/0.8106 |
0 |
0.1635 |
0.1795 |
0 |
0.3877 |
0.2693 |
| High P reactor out. |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4 607.4 |
60/1.51988 |
0 |
0.1635 |
0.1795 |
0 |
0.3877 |
0.2693 |
| Crystallizer outlet |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4 607.4 |
15/1.01325 |
0 |
0.1635 |
0.1795 |
0 |
0.3877 |
0.2693 |
| BPA product |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.8 607.8 |
15/1.00325 |
0 |
0.0006 |
0.0006 |
0 |
0.998
|
0.0008 |
| VOCs |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.6 999.6 |
15/1.00325 |
0 |
0.2670 |
0.2931 |
0 |
0 |
0.4399 |
| Heated VOCs |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.6 999.6 |
50.15/0.8183 |
0 |
0.2670 |
0.2931 |
0 |
0 |
0.4399 |
| High P heated VOCs |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.6 999.6 |
50.19/1.51988 |
0 |
0.2670 |
0.2931 |
0 |
0 |
0.4399 |
| Distill1 tops |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.6 794.6 |
67.28/1.01325 |
0 |
0.6392 |
0.0266 |
0 |
0 |
0.3342 |
| MeOH recycle |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.6 794.6 |
67.31/1.51988 |
0 |
0.6392 |
0.0266 |
0 |
0 |
0.3342 |
| Distill 1 bottoms |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205 205 |
98.28/1.01325 |
0 |
0 |
0.4843 |
0 |
0 |
0.5157 |
| Distill 2 bottoms |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.3 963.3 |
106.43/1.01325 |
0 |
0 |
0.8475 |
0 |
0 |
0.1525 |
| Cooled tol. recycle |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.3 963.3 |
59.97/0.9897 |
0 |
0 |
0.8475 |
0 |
0 |
0.1525 |
| Toluene recycle |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.3 963.3 |
60/1.51988 |
0 |
0 |
0.8475 |
0 |
0 |
0.1525 |
| Distill 2 tops |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492.6 492.6 |
90.5/1.01325 |
0 |
0 |
0.01824 |
0 |
0 |
0.9817 |
| High P distill 3 feed |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492.6 492.6 |
90.75/10 |
0 |
0 |
0.01824 |
0 |
0 |
0.9817 |
| Distill 3 bottoms (PSD cycle) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250.9 250.9 |
184.13/10 |
0 |
0 |
0.0357 |
0 |
0 |
0.9643 |
| Distill 3 tops |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6 241.6 |
183.27/10 |
0 |
0 |
0.0007 |
0 |
0 |
0.9993 |
| Hot DMC prod. 2 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6 241.6 |
41.71/9.74176 |
0 |
0 |
0.0007 |
0 |
0 |
0.9993 |
| High P DMC prod. |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6 241.6 |
25/9.54627 |
0 |
0 |
0.0007 |
0 |
0 |
0.9993 |
| DMC product |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6 241.6 |
25.1/1.01325 |
0 |
0 |
0.0007 |
0 |
0 |
0.9993
|
Appendix C: green metric equations
Atom economy (AE)
Reaction mass efficiency (RME)
Stoichiometric factor
Material recovery parameter
where c is the mass of the catalyst, s is the mass of the solvent, w is the mass of the waste and mp is the mass of the product.
Appendix D: detailed economic calculations
CAPEX calculations
The purchase price for the plant from Aspen Process Economic Analyzer® (Aspen®) was $14 MM (2013 dollars). This price was scaled up to the fall 2023 USD price using a ratio of CPI values. The price of the tanks for storage of reactants was calculated to be $5 MM from Aspen®. The price of the crystallizer was underestimated by Aspen® by approximately $3 MM. These three values were summed up to get the total purchase price, which was then scaled to their 2023 USD price resulting in a purchase price of $20 MM. A cost multiplier was used to calculate the FCI. The most conservative values for each multiplier were used as shown in Table 11 below.
Table 11 Summary of cost multipliers for FCI
| Cost item |
Multiplier |
Total cost (USD) |
| Total plant direct cost (TPDC) |
| Installation |
1.5 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 356![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830.04 830.04 |
| Process piping |
0.6 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 542![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.01 732.01 |
| Instrumentation |
0.6 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 542![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.01 732.01 |
| Insulation |
0.05 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 045![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227.67 227.67 |
| Electrical |
0.2 |
480![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910.67 910.67 |
| Buildings |
3 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713 713![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660.07 660.07 |
| Yard improvement |
0.2 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910.67 910.67 |
| Auxiliary facilities |
1 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904 904![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553.36 553.36 |
| Total plant indirect cost (TPIC) |
| Engineering |
0.3 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271 271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366.00 366.00 |
| Construction |
0.4 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 821.34 821.34 |
| Total plant cost |
|
164![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743.90 743.90 |
| Contractor's fee |
0.08 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 059.51 059.51 |
| Contingency |
0.15 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 615![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111.58 111.58 |
| Direct fixed capital |
|
201![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843 843![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914.90 914.90 |
From these calculations, the FCI was estimated to be $201![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 843
843![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 914.95.
914.95.
WCI was estimated from 15% of the TCI.
OPEX calculations
*Assume $1000 per MT for PC raw material cost. This is already factored into the total raw material cost
| OpEx = Tot. Raw Material Cost + Utilities + Labor/Maintenance |
| OpEx = $271.4 MM + $11.26MM + $2.06 MM |
ROI calculations
Annual Income.
*Government subsidies are considered
| Ann. Income = BPA Product Cost + DMC Product Cost + Subsidies |
| Ann. Income = $346.02 MM + $79.64 MM + $160 MM |
Depreciation.
*Assuming straight-line depreciation, 10 year period, salvage value = 10% of FCI
Annual after-tax profit.
*Assuming tax rate is 25% (state and federal taxes)
| Ann. Net (after tax) Profit = (Ann. Income − OPEX − Depreciation) × (1 − tax rate) + Depreciation |
| ($585.66 MM − $284.7 MM − $18.2 MM) × (1 − 0.25) + $18.2 MM = $230.3 MM |
ROI.
*Assume WCI is 15% of TCI
Acknowledgements
The authors would like to thank Dr. Gary Blizzard, Dr. Ahmad Hilaly, and Mr. Robert Wygle for their support with this research.
References
-
C. Heil, FT-NIR Analysis of the Hock Process for the Production of Phenol and Acetone, Madison, WI, 2008, https://assets.thermofisher.com/TFS-Assets%2FCAD%2FApplication-Notes%2FD14214.pdf, (accessed 2025-02-25) Search PubMed.
- J. Drönner, P. Hausoul, R. Palkovits and M. Eisenacher, Solid Acid Catalysts for the Hock Cleavage of Hydroperoxides, Catalysts, 2022, 12(1) DOI:10.3390/catal12010091.
- D. Kim, B. Kim, Y. Cho, M. Han and B.-S. Kim, Kinetics of Polycarbonate Methanolysis by a Consecutive Reaction Model, Ind. Eng. Chem. Res., 2009, 48(14), 6591–6599, DOI:10.1021/ie801893v.
- R. Piñero, J. García and M. J. Cocero, Chemical Recycling of Polycarbonate in a Semi-Continuous Lab-Plant. A Green Route with Methanol and Methanol–Water Mixtures, Green Chem., 2005, 7(5), 380–387, 10.1039/B500461F.
-
D. J. Little, Recrystallization of Bisphenol A by Azeotropically Drying the Solvent, US4638102A, 1987.
- L.-C. Hu, A. Oku and E. Yamada, Alkali-Catalyzed Methanolysis of Polycarbonate. A Study on Recycling of Bisphenol A and Dimethyl Carbonate, Polymer, 1998, 39(16), 3841–3845, DOI:10.1016/S0032-3861(97)10298-1.
-
P. Wesley Bell, A. Stanislaus, V. Ramanarayana, G. Bhotla and R. Jothi Mahalingam, Method for Alcoholysis of Polycarbonate Compositions Containing Flame Retardant or Acrylonitrile-Butadiene-Styrene, US20140179892A1, 2014.
-
J. W. J. Jackson, K. P. Perry and J. R. Caldwell, Polycarbonate Purification, US3213060A, 1965.
-
I. Patraşcu, C. S. Bîldea and A. A. Kiss, High-Purity DMC Production by Indirect Alcoholysis of Urea: Optimal Design and Control, in 30th European Symposium on Computer Aided Process Engineering, ed. S. Pierucci, F. Manenti, G. L. Bozzano and D. Manca, Computer Aided Chemical Engineering, Elsevier, 2020, vol. 48, pp. 931–936, DOI:10.1016/B978-0-12-823377-1.50156-7.
- S. Kim, S. G. Lee and D. H. Jeong, Direct Synthesis of Dimethyl Carbonate from CO2: From the Perspective of Dimethyl-Carbonate, a Promising Material for the Future, Chem. Eng. Res. Des., 2024, 203, 630–639, DOI:10.1016/j.cherd.2024.02.010.
-
G. Wypych and A. Wypych, Databook of Green Solvents, ChemTec Publishing, 2nd edn, 2019 Search PubMed.
-
G. Wypych and A. Wypych, Databook of Green Solvents, ChemTec Publishing, 2nd edn, 2019 Search PubMed.
- J. Andraos, Unification of Reaction Metrics for Green Chemistry: Applications to Reaction Analysis, Org. Process Res. Dev., 2005, 9(2), 149–163, DOI:10.1021/op049803n.
- H. Huang, R. C. Samsun, R. Peters and D. Stolten, Greener Production of Dimethyl Carbonate by the Power-to-Fuel Concept: A Comparative Techno-Economic Analysis, Green Chem., 2021, 23(4), 1734–1747, 10.1039/D0GC03865B.
- M. A. Pacheco and C. L. Marshall, Review of Dimethyl Carbonate (DMC) Manufacture and Its Characteristics as a Fuel Additive, Energy Fuels, 1997, 11(1), 2–29, DOI:10.1021/ef9600974.
- M. Zhang, Y. Xu, B. L. Williams, M. Xiao, S. Wang, D. Han, L. Sun and Y. Meng, Catalytic Materials for Direct Synthesis of Dimethyl Carbonate (DMC) from CO2, J. Cleaner Prod., 2021, 279, 123344, DOI:10.1016/j.jclepro.2020.123344.
- R. L. Ware, S. M. Rowland, R. P. Rodgers and A. G. Marshall, Advanced Chemical Characterization of Pyrolysis Oils from Landfill Waste, Recycled Plastics, and Forestry Residue, Energy Fuels, 2017, 31(8), 8210–8216, DOI:10.1021/acs.energyfuels.7b00865.
-
S. Emre-Flender, Chemical recycling of polycarbonates reaches a major milestone, https://www.covestro.com/press/chemical-recycling-of-polycarbonates-reaches-a-major-milestone/, (accessed 2025-02-25) Search PubMed.
- W. T. Lang, S. A. Mehta, M. M. Thomas, D. Openshaw, E. Westgate and G. Bagnato, Chemical Recycling of Polyethylene Terephthalate, an Industrial and Sustainable Opportunity for Northwest of England, J. Environ. Chem. Eng., 2023, 11(5), 110585, DOI:10.1016/j.jece.2023.110585.
-
J. Paben, Eastman provides updates on massive pet recycling plant, Plastics Recycling Update, https://resource-recycling.com/plastics/2023/08/01/eastman-provides-updates-on-massive-pet-recycling-plant/, (accessed 2025-02-25) Search PubMed.
-
Citi Research, Price of recycled materials worldwide between 2015 and 2017 (in U.S. dollars per ton) [Graph], https://www.statista.com/statistics/520728/recycled-material-prices-in-the-us/, (accessed 2025-02-25) Search PubMed.
-
JCPRA, Average costs for chemical recycling of plastic packaging per ton in Japan from fiscal year 2013 to 2022 (in 1,000 Japanese yen) [Graph], https://www.statista.com/statistics/1263954/japan-plastic-packaging-chemical-recycling-costs-per-ton/, (accessed 2025-02-25) Search PubMed.
-
China Market Monitor, Acrylonitrile butadiene styrene (ABS) plastic recycling market prices worldwide from2017 to 2022, with a forecast to 2028 (in U.S. dollars per ton) [Graph], https://www.statista.com/statistics/856695/acyrlonitrile-butadiene-styrene-global-market-value-forecast/, (accessed 2025-02-25) Search PubMed.
-
Statista & K. R., Price of polyethylene terephthalate (PET) worldwide from 2017 to 2022 (in U.S. dollars per metric ton) [Graph], https://www.statista.com/statistics/964331/market-value-polyethylene-terephthalate-worldwide/, (accessed 2025-02-25) Search PubMed.
-
Resource Recycling Systems, Average price of recovered commodities in the United States as of October 2018 [Graph], https://www.statista.com/statistics/1007380/average-price-recovered-commodities-us/, (accessed 2025-02-25) Search PubMed.
-
EPA, Business Assistance State Recycling Tax Incentives for Recycling Market Development, https://archive.epa.gov/wastes/conserve/tools/rmd/web/html/rec-tax.html, (accessed 2025-02-25) Search PubMed.
-
DOE, New 48C tax credit will spur historic investments in manufacturing and Critical Materials, https://www.energy.gov/mesc/articles/new-48c-tax-credit-will-spur-historic-investments-manufacturing-and-critical, (accessed 2025-02-25) Search PubMed.
-
K. Sparks, Tax credits: An incentive for recycling?, https://archive.epa.gov/wastes/conserve/tools/rmd/web/pdf/taxcred.pdf, (accessed 2025-02-25) Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ,
Megan
Novak
,
Megan
Novak
 *,
Jonathan
Ochoa
,
Dyron
Powell
,
Josh
Ramsey
and
Logan
Stadtmueller
*,
Jonathan
Ochoa
,
Dyron
Powell
,
Josh
Ramsey
and
Logan
Stadtmueller
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of BPA per annum, the major processing equipment is one packed-bed reactor, two crystallizers, and three distillation columns. All required heat exchangers and pumps were integrated into the simulation and can be adjusted based on product specifications and processing capacity. Analysis of green metrics for the novel process demonstrated that the process minimizes waste from a mass standpoint, and a rigorous economic analysis showed that the process is highly profitable in several varied scenarios.
000 metric tons of BPA per annum, the major processing equipment is one packed-bed reactor, two crystallizers, and three distillation columns. All required heat exchangers and pumps were integrated into the simulation and can be adjusted based on product specifications and processing capacity. Analysis of green metrics for the novel process demonstrated that the process minimizes waste from a mass standpoint, and a rigorous economic analysis showed that the process is highly profitable in several varied scenarios.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of BPA produced per annum at 99.99 wt% purity. With an assumption of 80% on-stream efficiency, the process would stoichiometrically require 31
000 metric tons of BPA produced per annum at 99.99 wt% purity. With an assumption of 80% on-stream efficiency, the process would stoichiometrically require 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 kg h−1 of PC to meet production requirements. The solubility of BPA in the reactor effluent was approximated to be the solubility of BPA in toluene. It is assumed that the catalytic NaOH is regenerated in the reactor and does not leave the reactor. A scale factor between the stoichiometric requirement of PC and the amount of PC used in the lab-scale experiment was calculated, and the amounts of respective solvent were scaled up with this value. These solvent amounts, along with the mathematical determination of the scale factor, are detailed in Appendix A. The scale factor used in this paper serves as a benchmark for initial pilot plant trials. In practice, the exact amounts of feedstock, reagents, and solvents will be determined after these trials are complete. It is assumed that the major contaminants in waste PC feed will be flame retardants and organic materials, as further discussed in section 2.3.1. While there will most likely be other contaminants present in the waste polycarbonate, further lab scale and pilot plant scale trials will need to be conducted to identify all contaminants. Detailed kinetics will also need to be obtained from pilot plant studies. Furthermore, this process design does not consider the production of utilities or disposal of waste products.
790 kg h−1 of PC to meet production requirements. The solubility of BPA in the reactor effluent was approximated to be the solubility of BPA in toluene. It is assumed that the catalytic NaOH is regenerated in the reactor and does not leave the reactor. A scale factor between the stoichiometric requirement of PC and the amount of PC used in the lab-scale experiment was calculated, and the amounts of respective solvent were scaled up with this value. These solvent amounts, along with the mathematical determination of the scale factor, are detailed in Appendix A. The scale factor used in this paper serves as a benchmark for initial pilot plant trials. In practice, the exact amounts of feedstock, reagents, and solvents will be determined after these trials are complete. It is assumed that the major contaminants in waste PC feed will be flame retardants and organic materials, as further discussed in section 2.3.1. While there will most likely be other contaminants present in the waste polycarbonate, further lab scale and pilot plant scale trials will need to be conducted to identify all contaminants. Detailed kinetics will also need to be obtained from pilot plant studies. Furthermore, this process design does not consider the production of utilities or disposal of waste products.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of BPA per annum, the washing step is estimated to require 50 tonnes of acetone cycling through the system at a given time.8 As the solvent will eventually become saturated with contaminants, it will have to periodically be replaced with fresh acetone. This washing process is shown below in Fig. 2.
000 tonnes of BPA per annum, the washing step is estimated to require 50 tonnes of acetone cycling through the system at a given time.8 As the solvent will eventually become saturated with contaminants, it will have to periodically be replaced with fresh acetone. This washing process is shown below in Fig. 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 gal
000 gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 gal
652 gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 gal
000 gal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476
476![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500a
500a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800a
800a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons. For the first 6 months of 2023, Eastman spent $413 MM on capital expenditures. Although not all the capital is being used for the plant, they have stated that “a large majority” of it is. In the future, Eastman is planning to pursue another two PET advanced recycling plants, one in France, and another somewhere in the United States.20
000 metric tons. For the first 6 months of 2023, Eastman spent $413 MM on capital expenditures. Although not all the capital is being used for the plant, they have stated that “a large majority” of it is. In the future, Eastman is planning to pursue another two PET advanced recycling plants, one in France, and another somewhere in the United States.20
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonne BPA = 2 × 1011 g BPA
000 tonne BPA = 2 × 1011 g BPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031
031![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774.8 = 19
774.8 = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134 g MeOH ≈ 19
134 g MeOH ≈ 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.1 kg MeOH
800.1 kg MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031
031![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774.8 = 32
774.8 = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 516
516![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276 g C6H5CH3 ≈ 32
276 g C6H5CH3 ≈ 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 516.3 kg C6H5CH3
516.3 kg C6H5CH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790
790![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790
790![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 184.4
184.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4
607.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4
607.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4
607.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.4
607.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.8
607.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.6
999.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.6
999.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999.6
999.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.6
794.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794.6
794.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205
205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.3
963.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.3
963.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963.3
963.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492.6
492.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492.6
492.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250.9
250.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6
241.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6
241.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6
241.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 241.6
241.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356
356![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830.04
830.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542
542![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.01
732.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542
542![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.01
732.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045
045![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227.67
227.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910.67
910.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713
713![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660.07
660.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180
180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910.67
910.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904
904![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553.36
553.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271
271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366.00
366.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361
361![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 821.34
821.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743.90
743.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 128
128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 059.51
059.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615
615![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111.58
111.58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843
843![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914.90
914.90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 843
843![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 914.95.
914.95.