Open Access Article
Open Access ArticleBiosynthesis of silver and gold nanoparticles using cyanobacteria, their pharmaceutical and industrial applications
Sanjana Sabat
a,
Sagar Patra
a,
Surendra Swain
a,
Shuvasree Bej
a,
Ajit Kumar Bishoyi
a,
Goutam Sabatb and
Rabindra Nath Padhy
 *a
*a
aCentral Research Laboratory, Institute of Medical Sciences & Sum Hospital, Siksha ‘O’ Anusandhan Deemed to be University, Bhubaneswar 751003, Odisha, India
bDepartment of Botany and Biotechnology, Khallikote Unitary University, Berhampur 760001, Odisha, India. E-mail: rabindranathpadhy@soa.ac.in; rnpadhy54@gmail.com
First published on 17th December 2025
Abstract
Nanotechnology has become a fast-growing field, and to prepare metallic nanoparticles for clinics or chemical establishments, it is mandatory that a cost-effective, eco-friendly technique be developed, using all nontoxic and renewable resources. This review presents biogenesis of silver and gold nanoparticles based on the utilization of natural metabolic routes leading to the synthesis of these nanoparticles mediated by cyanobacteria. These photosynthetic microorganisms become highly efficient ‘bio-factories’, for metal ion reduction and stabilization on the resulting biosynthesis of nanoparticles in a one-step manner. Significant attention has been directed towards the potent antimicrobial potential of these biogenic nanoparticles against numerous pathogenic multidrug resistant bacteria and fungi, as well. Furthermore, this review also provides a summary of recent understanding on mechanisms of their activities, as well as their huge potentialities for use in a variety of industrial/medical sectors, such as cleansing uses, wastewater remediation and industrial utilities as advanced materials. Indeed, the laboratory-scale biosynthesis with an oxygen-evolving photosynthetic prokaryote, the cyanobacterium, is user-friendly, but it could be scaled up for several industrial processes.
1 Introduction
Cyanobacteria (blue–green algae) are a ubiquitous set of Gram-negative prokaryotes which are able to perform oxygenic photosynthesis. Those provide clear benefits compared to eukaryotic algae as biotechnology models; the high surface-volume ratio allows such algae to absorb nutrients efficiently, and certain strains have specialized heterocysts used to fix nitrogen.1,2 Importantly to nanotechnology, cyanobacteria contain bioactive metabolites such as phycocyanin, phycoerythrin, proteins and several secondary metabolites namely, alkaloids, flavonoids, phenols, ethers; and such compounds are important in reducing and stabilizing metal ions, during the formation of nanoparticles (NPs).3,4 Blithely, their adaptation to different environmental factors and adaptability to pigment change by chromatic adaptation renders them strong bio-factories of producing bioactive compounds.5–7 Significant bioactivity including antifungal, antiviral and anticancer properties had been demonstrated by species of Lyngbya,8 Oscillitoria,9 Nostoc,10 Anabaena,11 and Spirulina,12 making cyanobacteria useful for the synthesis of NPs as newer medicinal molecules.Although metal nanoparticles have unique physicochemical characteristics, including high surface area and increased reactivity, their synthesis had historically been based on physical and chemical processes.13 Those traditional methods tend to be energy expensive and use hazardous reducing agents that release toxic effects on the environment.14 Eventually, this creates an urgent need of green chemistry alternatives. Microorganism-based biosynthesis of NPs provides a long-term solution, as the cellular machinery can reduce metal precursors to nanoparticles without the use of external sources of energy or toxic solvents.15 By the by, cyanobacteria are photo-autotrophic, facilitating the product gets synthesized directly through the use of atmospheric CO2, light and water, unlike heterotrophic bacteria, in which the synthesis of the product is metabolically costly, because it requires the usage of expensive organic carbon sources.16 Thus, cyanobacteria stand out to be the superior source to other biological materials, hence should be promoted for biosynthesis.
AgNPs (silver nanoparticles) and AuNPs (gold nanoparticles) are among the examples of nanoparticles that have attracted a lot of attention. AgNPs are famously known because those have a wide spectrum of antimicrobial efficacy especially against multidrug resistant (MDR) pathogens, which make them useful in wound management and in medical devices.17,18 On the other hand, AuNPs are highly valued due to their surface plasmon resonance (SPR), which is unparalleled in biocompatibility, enabling their use in diagnostics, imaging and photothermal therapy.19 Cyanobacteria such as Spirulina sp., Lyngbya sp. and Oscillatoria sp. Had been used for biosynthesis of AgNPs that exhibit antibacterial activity against human pathogens like, Staphylococcus aureus, Streptococcus pyogenes, Acinetobacter baumannii and Escherichia coli.9,12,20 AgNPs, green-synthesized from Nostoc sp. exhibited antibacterial, antifungal and anticancer properties, by efficiently inhibiting fungal strains such as Aspergillus niger, Trichoderma harzianum, Ralstonia solanacearum and Xanthomonas campestris and cancer cell lines were too documented.21 Similarly, AuNPs prepared using green alga Chlorella sp. showed antifungal activity against human fungal pathogens, Candida tropicalis, C. glabrata and C. albicans. AuNPs synthesized from Anabaena sp. exerted antibacterial activity against Klebsiella oxytoca, methicillin-resistant Staphylococcus aureus (MRSA), and Streptococcus pyogenes.22 Additionally, nanoparticles from Oscillatoria sp. and Spirulina plantensis showed impressive antiviral effects, especially on Herpes Simplex Virus (HSV-1), with approximately 90% reduction in cytopathic effects by inhibiting viral replication.23 Although bacteria and fungi had been studied in terms of extracellular and intracellular synthesis pathways, cyanobacteria provide a clean synthesis route with nanoparticles being crowned by secondary metabolites, proteins and fatty acids for stability of these products.24 Borophycin from the cyanobacterium Nostoc sp. was seen having good activity against human cancer cell lines in vitro, while extracts of the filamentous cyanobacterium Calothrix sp. had reduced the growth of HeLa cells in vitro and chloroquine-resistant strains of, the malaria parasite Plasmodium falciparum. Extracts from Lyngbya lagerhaimanni had anti-HIV properties, while lyngbyatoxin A isolated from the cyanobacterium Lyngbya majuscula was known for its strong inflammatory effects.25 Additionally, intracellularly produced polyhydroxyalkanoates (PHA) of cyanobacteria are currently engineered to be biodegradable polymeric equivalents of polyethylene/polypropylene. Carotenoids such as canthaxanthin, beta-carotene, zeaxanthin and nostoxanthin, from cyanobacteria are widely used as food additives, dietary supplements and colourants in livestock diets. Astaxanthin extracted from the green alga Haematococcus pluvialis had shown a strong antioxidant and has confirmed therapeutic value in managing AIDS related conditions resulting from protease inhibition. Additionally, AgNPs produced by cyanobacteria are embedded in food packaging materials to enhance microbiological security and extend product shelf life.26 Health products, obtained from cyanobacteria, are currently commercially available in pill, powdered and tablet forms, making those an affordable resource for food and health benefits. Unlike heterotrophic bacteria, which require expensive carbon sources for fermentation, cyanobacteria perform photosynthesis and can grow using atmospheric CO2, light, water, and minimal nutrients. This makes economic and carbon-neutral production of biofuels, fine chemicals and bioplastics feasible, avoiding land consumption and environmental side effects.16 Additionally, the ability of the bacterial strains to oxidize hydrocarbons and degrade organic pollutants made them interesting tools for environmental remediation.
Numerous studies have reported green synthesis of nanomaterials, a consolidated analysis focusing specifically on the efficiency of cyanobacterial platforms remains necessary. Thus, the main aim of this review could be considered to evaluate critically the current situation of cyanobacteria-mediated biosynthesis of AgNPs and AuNPs. This methodically assesses mechanistic pathways of reduction; differentiating between intracellular and extracellular processes and examines the decisive role of parameters of optimization that include light intensity, pH and concentration ratio of precursor on morphology of the cyanobacterial synthesized nanoparticle. Furthermore, to bridge the gap between laboratory production and practice with the strict analysis of antimicrobial, anticancer, antioxidant properties and industrial implementation of these biogenic nanoparticles attempts were taken. Lastly, this review deals with the existing barriers to toxicity, reproducibility and scalability and provides an outlook on how these challenges will be circumvented. The review is meant to be a general reference to nanobiotechnology, pharmaceutical chemistry and environmental science researchers to enable the transfer of cyanobacterial nanotechnology laboratory technology to commercial use.
2 Methodology
A systematic literature review was conducted following established protocols to ensure a comprehensive and transparent evaluation of the biosynthesis of AgNPs and AuNPs utilizing cyanobacteria. Through large scientific databases such as Google Scholar, Scopus, PubMed and Web of Science articles were searched; mostly at books and articles published between 2000 to October 2025 were seen. Several keywords such as, cyanobacteria, blue–green algae, silver nanoparticles, AgNPs, gold nanoparticles, AuNPs, NP-biosynthesis and industrial applications of NPs were used. The screening process had used some ‘inclusion and exclusion criteria’ to make sure that the data was useful. Downloads included original research and review articles, brief communications published in peer-reviewed SCOPUS indexed journals; and studies specifically utilizing cyanobacterial strains for the synthesis of AgNPs or AuNPs and papers presenting characterization data such as, UV-vis, TEM/SEM, XRD and discussing pharmaceutical, antimicrobial, anticancer, antioxidant or industrial applications were included for the study. Studies concentrating exclusively on eukaryotic algae, bacteria, or plant-mediated synthesis without a cyanobacterial counterpart; conference abstracts, and editorials; articles deficient in methodological specifics concerning synthesis conditions or characterization were excluded. Firstly, titles and abstracts were screened to find studies that were not needed or were the same as others. After that, the full-text articles were checked to see if they met the standards for inclusion. Analysis of the cyanobacterial species, the amounts of precursor salts, the best conditions for the nanoparticle synthesis such as, pH, temperature and light intensity, their size and shape, and the quantitative bioactivity metrics like MIC, IC50, and zone of Inhibition were done. This methodical approach led to the selection of about 300 studies, which were the basis for the literature, tables and critical analysis embodied in this review.3 Botanical descriptions
Cyanobacteria belong to the empire prokaryote, kingdom eubacteria, phylum cyanobacteria and the cyanophycean class. According to the morphological characteristics and 16s rRNA analysis, 5185 sp. have been identified and placed under 7 orders, namely, Chroococcales, Gloeobacterales, Nostocales, Oscillatoriales, Pleurocapsales, Spirulinales, and Synechococcales.27 Chroococcales, comprising the famous freshwater blooming unicellular cyanobacterium Microcystis aeruginosa is an order of unicellular and colonial cyanobacteria embedded in a mucilaginous sheath.28 Members of the order Gloeobacterales lack thylakoids and are unicellular or irregularly rod-shaped.29 Nostocales are prominent due to their excellent nitrogen-fixing capabilities and the filamentous shape of their species, including of Nostoc and Anabaena.30,31 The highest number of benthic linear filamentous species are associated with Oscillatoriales, consisting of species like Oscillatoria, Phormidium and Pleurocapsales, including the genus Pleurocapsa sp., which can be coccoid cells or resemble filaments or pseudo-filaments, forming complex colonies characterized by their ability to divide by binary fission. Members of the order Spirulinales have open helix screw like coiled filaments, while species of the order Synechococcales, comprising Synechocystis sp. contain both unicellular and filamentous forms.214 Morphology
Cyanobacteria showcase a diversity of morphological traits, essential for their ecological succession and identification. They exist as unicellular, colonial, or multicellular filamentous forms, with specific characteristics such as cell shape, size, and the arrangement of specialized cells like heterocysts and akinetes, crucial for taxonomy. Some taxa exhibit polymorphism with cells of different shapes occurring within the same species. Unicellular forms typically range in size from 0.2 µm to around 40 µm in diameter, with some filamentous forms reaching up to 100 µm in cell diameter. The small cyanobacteria, often termed picocyanobacteria, measure between 0.2 and 2 µm and are significant components of the picoplankton in both marine and freshwater ecosystems. Cyanobacteria grow in different habitats, such as aquatic, planktonic, benthic and periphytic, attached to pteridophyte-plant like Azolla and other submerged objects; in polar regions and deserts.31The cell wall of cyanobacteria is Gram-negative type, which consists of an outer membrane made of a lipopolysaccharide layer and a peptidoglycan layer that is superior, and these can help in maintaining structural integrity and motility.32 Studies published have shown differences in the thickness of the cell wall in terms of different filamentous cyanobacteria, such as Aphanizomenon gracile, depending on consideration of its filament's density, which is correlated with resistance to grazing from planktonic protozoan crustacean Daphnia sp. because of greater stiffness.33 Cell walls of cyanobacteria are also important about the ability to become symbiotic with other organisms. Specialized structures of cyanobacteria, including heterocysts and hormogonia, help in nitrogen-fixing ability and survival of the bacteria in their host environment.34,35 Their cell walls contain large amounts of extracellular polymeric substances, which give them resistance against several environmental stresses and help in bioremediation by capturing the pollutants.36
However, the peptidoglycan layer in cyanobacteria is thicker, 10–700 nm than that in most of Gram-negative bacteria which is 2–6 nm. Unicellular strains such as Synechococcus had been demonstrated to have a thin 15 nm layer of peptidoglycan,37 whereas filamentous strains have been reported to have a layer thickness of more than 700 nm for O. princeps. The cell membrane primarily transfers nutrients and metabolites to and from the cell and prevents environmental stress.38 Certain cyanobacteria, like Gram-negative bacteria, contain external non-flagellar appendages termed pili or fimbriae that are involved in adhesion, movement, DNA absorption, and biofilm formation.39,40
5 Secondary metabolites
Cyanobacteria produce a wide range of secondary metabolites, including alkaloids, flavonoids, phenols, peptides, trans-fatty acids, amino acids, vitamins, carotenes, chlorophylls, phycocyanin and minerals, providing antibacterial, antifungal, antioxidant, and anti-cancerous properties.40 Additionally, those were seen to produce various kinds of cyanotoxins like microcystin, saxitoxin, and anatoxin, which display hepatotoxicity and neurotoxicity.41,42 The methanolic extract of Microcystis sp. demonstrated strong antialgal action against the green alga Bracteacoccus and anticyanobacterial activity against Anabaena BT2 and Nostoc pbr01.43 Furthermore, humans, fish, birds, mammals, and invertebrates are all poisoned by some of the secondary metabolites.44 Therefore, various nations, including Europe, the USA, Canada, Brazil, Australia, South Africa, China, and Japan, have set guidelines and values to protect the population from exposure to cyanotoxins45 (Table 1).| Sl no. | Phycocompound | PubChem ID | Chemical formula | Chemical structure | Activity | Ref. |
|---|---|---|---|---|---|---|
| 1 | Lutein | 5281243 | C40H56O2 | 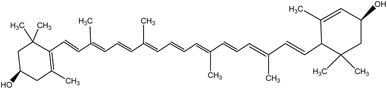 |
Antioxidant, protects eye tissues from sunlight damage | 46 |
| 2 | Alpha-linoelic acid | 5280934 | C18H30O2 | 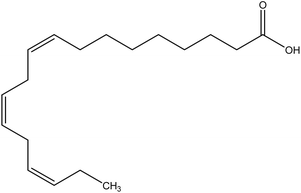 |
Essential omega-3 fatty acid, anti-inflammatory properties | 47 |
| 3 | Aspartic acid | 594 | C4H7NO4 | 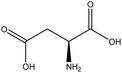 |
Building block for proteins, neurotransmitter | 48 |
| 4 | Asterina-330 | 102293881 | C15H24N2O7 |  |
Mycosporine-like amino acid (MAA) that acts as a natural UV sunscreen | 49 |
| 5 | Capric acid | 2969 | C10H20O2 | 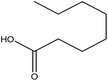 |
Saturated fatty acid with antimicrobial and anti-inflammatory properties | 47 |
| 6 | Caprylic acid | 379 | C8H16O2 | 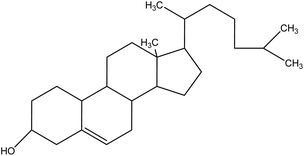 |
Saturated fatty acid with antifungal properties | 47 |
| 7 | Cholesterol | 5997 | C27H46O | 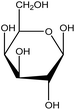 |
Essential component of animal cell membranes; precursor for steroid hormones | 48 |
| 8 | Galactose | 6036 | C6H12O6 |  |
Monosaccharide used as an energy source; component of glycolipids | 48 |
| 9 | Galacturonic acid | 5460395 | C6H10O7 | 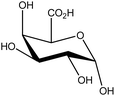 |
Main component of pectin in plant and algal cell walls | 50 |
| 10 | Glucose | 5793 | C6H12O6 | 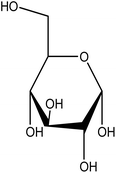 |
Primary source of energy for most living organisms | 48 |
| 11 | Glucouronic acid | 959 | C6H10O7 | 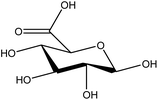 |
Involved in detoxification processes by conjugating with other molecules | 52 |
| 12 | Glutamic acid | 33032 | C5H9NO4 |  |
Building block for proteins, important excitatory neurotransmitter | 48 |
| 13 | Lauric acid | 3893 | C12H24O2 | 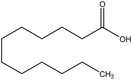 |
Saturated fatty acid with strong antiviral and antibacterial properties | 47 |
| 14 | Mycrosprine amino acids | Variable | 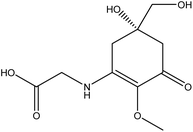 |
A class of compounds that absorb UV radiation, acting as natural sunscreens | 49 | |
| 15 | Oleic acids | 445639 | C18H34O2 | 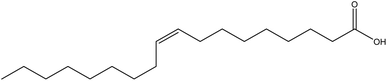 |
Monounsaturated fatty acid; a primary component of olive oil | 47 |
| 16 | Palmitic acid | 985 | C16H32O2 | 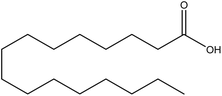 |
The most common saturated fatty acid in animals and plants | 47 |
| 17 | Palmitoleic acid | 445638 | C16H30O2 | 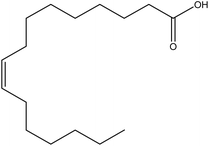 |
Omega-7 monounsaturated fatty acid with potential metabolic benefits | 47 |
| 18 | Phycocyanin | 53837743 | C33H38N4O6 | 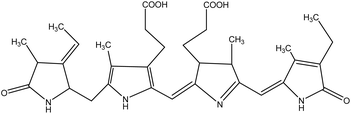 |
Pigment-protein complex with antioxidant and anti-inflammatory activity | 51 |
| 19 | Porphyra 334 | 10255390 | C19H28N2O8 | 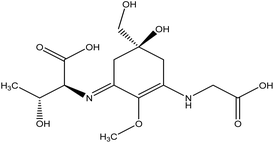 |
A potent UV-absorbing MAA found in red algae | 49 |
| 20 | Radiosumin B | 10812638 | C25H30O7 | 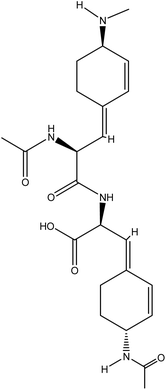 |
Diarylheptanoid with antioxidant properties | 53 |
| 21 | Serine | 5951 | C3H7NO3 | 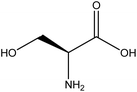 |
Building block for proteins; involved in the biosynthesis of other metabolites | 48 |
| 22 | Glycine | 750 | C2H5NO2 | 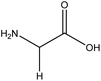 |
Simplest amino acid; building block for proteins and an inhibitory neurotransmitter | 48 |
| 23 | Lysine | 5962 | C6H14N2O2 | 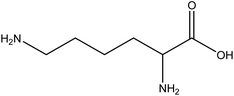 |
Essential amino acid required for protein synthesis and calcium absorption | 48 |
| 24 | Arginine | 6322 | C6H14N4O2 | 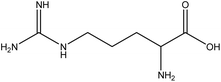 |
Semi-essential amino acid; precursor for nitric oxide synthesis | 48 |
| 25 | Tyrosine | 6057 | C9H11NO3 | 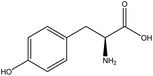 |
Amino acid precursor to key neurotransmitters (dopamine) and hormones | 48 |
| 26 | Zeaxanthin | 5280899 | C40H56O2 |  |
Carotenoid pigment important for eye health; protects against light-induced damage | 46 |
5.1 Alkaloids from cyanobacteria
Cyanobacterial alkaloids are a chemically heterogeneous group of nitrogenous secondary metabolite, with indole, indoline or guanidines remnants that are produced through complex NRPS and PKS pathways.54,55 Anatoxins are type of neurotoxins produced by some species of cyanobacteria, such as, Oscillatoria and Anabaena. These compounds are potent inhibitors of both pre- and post-synaptic neuromuscular transmission and are highly toxic to mammals. Anatoxin-a and homoanatoxin-a are produced via a PKS pathway initiated by L-proline.56 Saxitoxins are also, a class of neurotoxins that are found in species of Anabaena and Aphanizomenon. These are sodium channel blockers and cause paralytic shellfish poisoning. Saxitoxins possess a tetrodotoxin structure and are tricyclic.54 Nostocarboline produced by Nostoc sp. is an acetylcholinesterase inhibitor, which is a promising lead in the treatment of Alzheimer disease therapy.57 In infectious disease contexts, the aerucyclamides of Microcystis aeruginosa are antiplasmodial active with an IC50 of less than 1 µg mL−1 against P. falciparum,58 whereas ambiguine isonitrile displays very strong antifungal activity with an MIC of 0.312 µg mL−1 and 0.39 µg mL−1 against S. cerevisiae and C. albicans respectively.59,60 Moreover, Fischerella sp. and Hapalosiphon sp. hapalindoles possess cytotoxic and antiviral effects55,61 and drugs, such as dolastatin 10 and lyngbyastatins, are crucial structurals leading in antiviral and cancer treatments as approved antibody-drug conjugates.8,62,635.2 Peptides from cyanobacteria
Its peptide profile, which is also linear, cyclic, and depsipeptides, provides a further pharmaceutical potential through ribosomal and non-ribosomal biosynthetic machineries.64 Lipopeptides (hassallidins A and B) of Hassallia sp. are antifungal against candida sp. with MIC of 4.8 µg mL−1,65 but hassallidin D of Anabaena sp. is active at a concentration of 2.8 µg mL−1 or below.66 Inhibitors of particular enzymes have also been discovered; brunsvicamides B and C of Tychonema sp. like M. tuberculosis inositol monophosphatase with IC50 of 7.3 and 8.0 mM, respectively, and scyptolin A of Scytonema hofmanni of bacterial transpeptidases67 have also been identified. Cryptophycin-1 is a cyclic depsipeptide produced by Nostoc sp. and is reported to cause disruption of the assembly of the microtubules in multidrug-resistant cancer cell lines42,68 in the field of oncology. Although promising, the clinical translation of these peptides is hampered by poor yields of purification and complicated scale up needs.695.3 Terpenoids cyclophanes, aromatics and phenols from cyanobacteria
In addition to alkaloids and peptides, cyanobacteria also synthesize bioactive terpenoids, phenolics and fatty acids, which destroy microbial cell membranes. Terpenoids like comnostins and scytoscalarol cause depolarization of the membrane of the pathogenic bacteria.70 Noscomin, which is isolated from Nostoc commune is active against Gram-positive bacteria such as Bacillus cereus and Streptomyces epidermidis with a MIC concentration within 8 and 128 mg mL−1,71 and tetraterpene terscytoscalarol is presented by Scytotema sp. against S. aureus with a MIC of 1.7 mM.72 Out of the exracted lipid products, majusculic acid of Lyngbya majuscula exhibits antifungal effects using C. albicans at an MIC of 8 mM.73 There are also aromatic compounds containing 4,4′-dihydroxybiphenyl by Nostoc insulare and polychlorinated phenolic ethers like ambigols which are a part of the broad-spectrum antimicrobial defense mechanism of these organisms.746 Cyanobacteria-mediated synthesis
6.1 AgNP synthesis
Even though biosynthesis of AgNPs with cyanobacteria is mediated through various mechanism, but mainly bioactive compounds such as polysaccharides, proteins, or other biological molecules were involved in the reduction process, acting as reducing and stabilizing agents to convert Ag+ to Ag0.75 The biosynthesis mechanism was based on the reduction of the Ag+ ions by electron transfer, O–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups that was confirmed using the Fourier transform infrared spectroscopy.76 Nanoparticles, especially AgNPs, have been synthesized in either top-down method or bottom-up method. Such methods have been extensively studied in biological systems for the product of nanoparticles with desired properties and functions. In the top-down approach, lithography, for instance, is used to pinch off larger materials into nanoparticles. This results in the particles being broken down in size to a small nano size material. Furthermore, this technique is employed at high temperature tube furnaces to produce nanoparticles of materials, such as silver, gold or lead, scouting the limitation and growth processes. The surface structures and physical properties of the nanoparticles obtained by these means are much more sensitive to room temperature and surroundings. On the other hand, bottom-top strategies are more common in nanoparticle production, specifically when using biological systems. Microbes accomplish such a bio reduction by a construction inside-out manner, where the cells are self-reducing bioreactors. Three crucial processes form the basis of the synthesis process: activation, growth, and termination. To create larger particles, metal ions are first reduced in the activation step, and then they are nucleated and grown. Proteins and polysaccharides are utilized for capping, stabilizing, and other purposes involving metal nanoparticles. The protein aqueous solution acts as a capping agent and stabilizer for the metal nanoparticles through the action of amino groups, particularly cysteine. There is a growing interest in sustainable nanotechnology where toxic by-product can be reduced to a minimum and bottom to top approach is a green, sustainable and cost-effective strategy. In this method, the pH, temperature, incubation time and the concentration of the substrate significantly influence the size, shape and properties of the synthesized nanoparticles.77 The biosynthesis process of AgNPs mediated by cyanobacteria is mainly subdivided into the biosorption on the EPS molecules and the complex formation mechanism of Ag particle through the nucleation/aggregation–reduction processes. EPS adsorbs Ag+ from stock solution initially and the main process is not dependent on light. Then under light, EPS gives electrons to reduce Ag+ to Ag0, and the better the light, the more efficient this reaction is. Furthermore, the end equilibrated EPS concentration is directly proportional to the size and stability of the produced AgNPs, illustrating that EPS plays a role as a protection matrix of the AgNPs against agglomeration.78 Furthermore, the huge diversity in EPS composition, particularly proteins and polysaccharides, have a strong influence on both reduction and stabilization in the biosynthesis of NPs, thus giving them such a multi-functional character.79
O functional groups that was confirmed using the Fourier transform infrared spectroscopy.76 Nanoparticles, especially AgNPs, have been synthesized in either top-down method or bottom-up method. Such methods have been extensively studied in biological systems for the product of nanoparticles with desired properties and functions. In the top-down approach, lithography, for instance, is used to pinch off larger materials into nanoparticles. This results in the particles being broken down in size to a small nano size material. Furthermore, this technique is employed at high temperature tube furnaces to produce nanoparticles of materials, such as silver, gold or lead, scouting the limitation and growth processes. The surface structures and physical properties of the nanoparticles obtained by these means are much more sensitive to room temperature and surroundings. On the other hand, bottom-top strategies are more common in nanoparticle production, specifically when using biological systems. Microbes accomplish such a bio reduction by a construction inside-out manner, where the cells are self-reducing bioreactors. Three crucial processes form the basis of the synthesis process: activation, growth, and termination. To create larger particles, metal ions are first reduced in the activation step, and then they are nucleated and grown. Proteins and polysaccharides are utilized for capping, stabilizing, and other purposes involving metal nanoparticles. The protein aqueous solution acts as a capping agent and stabilizer for the metal nanoparticles through the action of amino groups, particularly cysteine. There is a growing interest in sustainable nanotechnology where toxic by-product can be reduced to a minimum and bottom to top approach is a green, sustainable and cost-effective strategy. In this method, the pH, temperature, incubation time and the concentration of the substrate significantly influence the size, shape and properties of the synthesized nanoparticles.77 The biosynthesis process of AgNPs mediated by cyanobacteria is mainly subdivided into the biosorption on the EPS molecules and the complex formation mechanism of Ag particle through the nucleation/aggregation–reduction processes. EPS adsorbs Ag+ from stock solution initially and the main process is not dependent on light. Then under light, EPS gives electrons to reduce Ag+ to Ag0, and the better the light, the more efficient this reaction is. Furthermore, the end equilibrated EPS concentration is directly proportional to the size and stability of the produced AgNPs, illustrating that EPS plays a role as a protection matrix of the AgNPs against agglomeration.78 Furthermore, the huge diversity in EPS composition, particularly proteins and polysaccharides, have a strong influence on both reduction and stabilization in the biosynthesis of NPs, thus giving them such a multi-functional character.79
Cyanobacteria, such as Oscillatoria sp., Spirulina platensis, and Nostoc sp., have been used effectively in AgNPs biosynthesis. They have a tremendous capacity to yield bioactive compounds with antibacterial, antioxidant, and anticancer activity. For example, AgNPs generated with Oscillatoria limnetica not only demonstrated high anticancer activity against breast MCF-7 and colon HCT-116 cancer cell lines but also had significant antibacterial activity. Likewise, the nanoparticles synthesized using Spirulina platensis were also found to possess a range of 7–16 nm in size, exhibiting a high ability to reduce biologically and effective antimicrobial potential. For example, AgNPs, mainly 12–15.3 nm in size and with significant cytotoxicity against cancer cells, were produced with the use of polysaccharides obtained from S. platensis to reduce silver ions.80
6.2 AuNP synthesis
Cyanobacterial AuNPs are mainly formed through bottom-up approach. In extracellular synthesis method, the enzymes, proteins and pigments entrapped in the cell membrane display ability to reduce metal ions on the available sites on the cell surface of cyanobacterial cells. The reduction of Au+ to Au0 nucleates and stabilizes AuNPs and is mediated by a series of enzymes, such as NADH-dependent reductase. For instance, oval-shaped AuNPs secreted by Plectonema boryanum are said to facilitate purification. This demonstrates the advantage of extracellular synthesis in minimizing downstream processes.81In intracellular biosynthesis nitrate reductase and nitrogenase are a few of the intracellular enzymes that are being exploited by cyanobacterial cells to synthesize AuNPs. These enzymes reduce gold ions entering the cells. Thylakoid membranes, critical for photosynthesis-1 and the electron transfer chains are involved in the reduction process in these membranes. Here, synthesized NPs were often entrapped in an organic-matrix system and additional extraction procedures were needed, which might be viewed as a drawback. Examples of reagents that serve as capping and reducing agents include cyanobacterial proteins, peptides and polysaccharides. These biomolecules contain some functional groups like, amino (–NH2), hydroxyl (–OH), and carboxyl (–COOH) groups, which may act as stabilizing agents and prevent the aggregation of AuNPs.60
7 Challenges in synthesis process reproducibility and scalability
7.1 Interplay of strain specificity and culture variability
Cyanobacteria exhibit substantial metabolic and genetic diversity across taxa, leading to significant interspecific variability in nanoparticle synthesis mechanisms and efficiency (Table 2). For example, unicellular strains like Synechocystis sp. primarily utilize photo-catalytic reduction via photosynthetic electron transport, while filamentous, nitrogen-fixing strains such as Anabaena sp. may also employ exopolysaccharides and nitrogenase enzymes for metal reduction. This strain-dependent variability is further compounded by high sensitivity to culture conditions—light intensity, temperature, pH, and nutrient availability all critically influence metabolic pathways and nanoparticle synthesis outcomes. Even within the same strain, shifts from exponential to stationary growth phases can drastically alter secreted metabolite profiles and redox states, resulting in inconsistent nanoparticle characteristics when identical protocols are applied under slightly different conditions.94–96| Organism/species | Category | Metal NP | pH | Temp (°C) | Light/cond. | Size (nm) | Morphology | Ref. |
|---|---|---|---|---|---|---|---|---|
| Comparision between different cyanobacterial species | ||||||||
| Khargia iranica KH.T.2 (biomass) | Cyanobacteria | Ag | 7 | 25 | Light | 11–13 | Spherical | 82 |
| Khargia iranica KH.T.2 (supernatant) | Ag | 7 | 25 | Light | 11–13 | Spherical | ||
| Khargia iranica KH.T.2 (biomass) | Au | 7 | 80 | Light | — | Spherical | ||
| Khargia iranica KH.T.2 (supernatant) | Au | 7 | 100 | Light | — | Spherical | ||
| Microchaete NCCU-342 | Ag | 5.5 | 60 | UV (60 min) | 60–80 | Spherical (polydisp.) | 83 | |
| Oscillatoria limnetica | Ag | 6.7 | — | — | 3.3–18 | Quasi-spherical | 81 | |
| Phormidium ambiguum | Ag | — | — | Light | 6.5–12.2 | Spherical (fcc) | 84 | |
| Desertifilum tharense | Ag | — | — | Light | 6.2–11.4 | Spherical (fcc) | 84 | |
| Nostoc linckia (phycocyanin) | Ag | 10 | — | — | 9.4–25.9 | Spherical | 85 | |
| Spirulina platensis | Ag | — | — | — | ∼28.7 | Spherical | 86 | |
| Synechocystis sp. | Ag | — | — | — | 10–35 | Spherical | 87 | |
| Nostoc muscorum 2/91 | Ag | 7.4 | 25 | Light | 11.8 ± 0.5 | Cubic to oval | 82 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Comparison with different organisms | ||||||||
| Noctiluca scintillans | Algae | Ag | 7 | 80 | — | 4.1–4.5 | Spherical | 88 |
| Aconitum violaceum | Plant | Ag | 8 | 60 | — | <100 | Spherical/triangular | 89 |
| Aconitum violaceum | Plant | Au | 8 | 60 | — | <100 | Spherical/triangular | |
| Bacillus cereus | Bacteria | Ag | 9 | 48.5 | — | 5–7.1 | Spherical | 90 |
| Leclercia adecarboxylata | Bacteria | Ag | 7 | 40 | Light | 17.4 | Spherical | 91 |
| Antigonon leptopus | Plant | Ag | 10 | — | — | 93.5 ± 1.9 | Spherical | 92 |
| Hibiscus leaves | Plant | Ag | 6 | 70 | — | 12–17 | Spherical | 93 |
7.2 Batch-to-batch variability and polydispersity
Cyanobacterial metabolism is dynamic and growth-phase dependent, causing fluctuations in the types and concentrations of reducing agents such as proteins, pigments, enzymes available for nanoparticle synthesis. Minor deviations in environmental parameters such as illumination, temperature, or pH can lead to highly polydisperse nanoparticle samples, with significant variability in size and morphology. This heterogeneity complicates downstream applications, especially in clinical contexts where uniformity is essential for safety and efficacy.96,977.3 Maintenance of axenic cultures and contamination
Large-scale cultivation of axenic (pure) cyanobacterial cultures is challenging. Open-pond or bioreactor systems are susceptible to contamination by heterotrophic bacteria or fungi, which can introduce foreign metabolites, compete for metal ions, or alter nanoparticle capping mechanisms. Such contamination increases batch variability and operational complexity, raising costs compared to abiotic chemical synthesis.94,967.4 Scalability and the “self-shading” effect
Scaling up from flask-level to industrial photobioreactors introduces unique engineering challenges. As biomass density increases, self-shading limits light penetration, causing non-uniform illumination and uneven reduction rates throughout the reactor. Since many synthesis pathways are photo-activated, this effect leads to inconsistent nanoparticle yields and quality, impeding reliable industrial-scale production.95,97,988 Characterization of AgNPs and AuNPs synthesized by cyanobacteria
8.1 Ultraviolet-visible spectrophotometry
The UV-visible spectrophotometry approach is a quick and cheap way to be sure that metal NPs have been made. The surface plasmon resonance (SPR) peaks of gold and silver nanoparticles are found in the visible range and have distinct values. This is because free conduction band electrons move about on their surfaces. For gold nanoparticles, the absorption would usually be in the high wavelength range of 510–560 nm, depending on the size, shape, and location of the localized SPR peak. For AgNPs, it would be about 400–450 nm. Characterization tests indicated that the produced S. platensis-derived AuNPs had a λ max at 530 nm, hence validating the synthesis. The produced AuNPs from Lyngbya majuscula exhibited a particle with peak absorbance at a wavelength of 540 nm, thereby validating the stability and monodispersity of the nanoparticles.99 The SPR peak for the AgNPs of Nostoc muscorum was found at 420 nm.100 A further surface plasmon resonance band at 528 nm was identified for gold nanoparticles of Oscillatoria limnetica. The plasmonic nature of the SPR shift may also help us understand how nanosomes stick together, how they look, and how their surfaces can be changed101,102.8.2 Fourier transform infrared spectroscopy (FTIR) and XRD
Functional groups on the surface of the nanoparticle, namely reduction and capping groups, are traced by FTIR. This is important for understanding how cyanobacterial biomolecules can interact with metal ions. Amide (–NH), hydroxyl (–OH), and carboxylic (–COOH) groups in the Spirulina-mediated AuNPs, indicated the role of proteins and polysaccharides in stabilization of nanoparticles.103 The phenolic and carbonyl groups like leads 12–15 on the AgNPs synthesized from Phormidium sp. was observed.104 Notable absorption bands at 3400 cm−1 (–OH), 1650 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1540 cm−1 (amide II) provided strong evidence for the presence of cyanobacterial proteins and flavonoids on the surface of AuNPs synthesized using Oscillatoria limnetica.105 The FTIR analysis of Os-AgNPs showed a strong and broad peak at 3352 cm−1 corresponding to the –OH stretching vibration of alcohol due to intermolecular bonding106 (Fig. 1).
O), and 1540 cm−1 (amide II) provided strong evidence for the presence of cyanobacterial proteins and flavonoids on the surface of AuNPs synthesized using Oscillatoria limnetica.105 The FTIR analysis of Os-AgNPs showed a strong and broad peak at 3352 cm−1 corresponding to the –OH stretching vibration of alcohol due to intermolecular bonding106 (Fig. 1).
 | ||
| Fig. 1 The biosynthesized Os-AgNPs UV-vis (A), FTIR (B), and XRD (C) spectra analysis adapted/reproduced from (Bishoyi et al.,2025)106 with permission from Wiley, copyright 2025. | ||
XRD is commonly used to determine the crystal size and phase purity of nanoparticles. It is well known that the typical AuNPs and AgNPs are face-centered cubic (FCC), and characteristic peaks appearing at 38.1°, 44.3°, 64.5° and 77.4° can be assigned to the (111), (200), (220), and (311) lattice planes, respectively.107 AuNPs synthesized with Anabaena flos-aquae had characteristic FCC structure and significant peaks indicating high degree of crystallinity.108 Significant diffraction peaks of Spirulina-mediated AuNPs were reported, thus confirming crystallinity of gold.103 XRD confirms crystallinity of the AgNPs obtained from Phormidium sp. which estimated the crystallite size to about 18 nm by Debye–Scherrer formula.104
8.3 FESEM (field emission scanning electron microscopy) and EDS (energy dispersive spectrometry)
FESEM offers detailed surface images that are especially useful to reveal face structure and to monitor aggregation on hard surfaces. When combined with energy-dispersive X-ray (EDS), it is used to analyze the chemical composition of a sample containing a nanoparticle. FESEM microstructural analysis of Nostoc muscorum mediated AgNPs, observed that the particles were spherical in shape with a slight aggregation.100 EDS analysis showed a strong peak of silver at 3 keV thus confirming the elemental composition. Furthermore, AuNPs are distributed uniformly on exposed surfaces of Oscillatoria-prepared samples by FESEM, and elemental gold was confirmed by EDS.101 FESEM analysis of Anabaena flos-aquae-induced AuNPs had spherical shaped particles arranged in group of nanoclusters. EDS analysis showed elemental peaks at 2.1 keV and 9.7 keV confirming the presence of metallic gold109 (Fig. 2). | ||
| Fig. 2 The biosynthesized Os-AgNPs FESEM image (A), EDS spectrum (B), and surface colour image (C). The presence of carbon spotted crimson red colour (D), oxygen spotted neon green colour (E), and silver spotted navy-blue colour (F) of Os-AgNPs. adapted/reproduced from (Bishoyi et al.,2025)106 with permission from Wiley, copyright 2025. | ||
8.4 Dynamic light scattering (DLS) and zeta potential assessment
Nanoparticles in colloidal dispersions are characterized by DLS which provides their hydrodynamic size and polydispersity Index (PDI). It often shows a slightly bigger size than TEM on account of solvation layers or surface-bound biomolecules. The hydrodynamic size for the AuNPs synthesized is around approximately 45 nm with PDI< 0.3 indicating a narrow size distribution.99 AgNPs from Phormidium sp. showed an average hydrodynamic diameter of 38 nm and high stability in solution.104 Additionally, average diameters of 35–50 nm and a width of AgNP size distributions are shown on DLS analysis for Anabaena variabilis-synthesized silver nanoparticles.109 Further, doxorubicin functionalized AuNPs for drug delivery showed low polydispersity index, PDI is ∼0.2 which is clinically relevant, AuNPs can also be characterized for their stability and homogeneity using DLS.110Zeta potential measures surface charge and predicts colloidal stability. Values above ±30 mV indicate strong electrostatic repulsion and stable dispersions. A zeta potential of −32 mV for Oscillatoriasynthesized AuNPs, indicated high colloidal stability.105 Zeta potential of −35 mV was observed in their green-synthesized Lyngbya AuNPs, suggesting that it remained stable with no sign of aggregation99 (Fig. 3).
 | ||
| Fig. 3 Intensity distribution (A) and zeta potential (B) of biosynthesized Os-AgNPs by DLS. adapted/reproduced from (Bishoyi et al.,2025)106 with permission from Wiley, copyright 2025. | ||
8.5 Transmission electron microscopy
TEM is a strong imaging method that lets you see the form and size distribution of the nanoparticle directly. It can tell the difference between spherical, rod-shaped, filler-triangle, and unispheric particles, as well as give an idea of the size of the degree of dispersion or agitation level. The AuNPs made by O. limnetica are mostly round and have a diameter of 5 to 25 nm.105 The synthesized AuNPs exhibited a nearly perfect spherical morphology, as indicated by prior findings on Spirulina-mediated nanoparticles with an average dimension ranging from 10 to 30 nm.103 TEM showed that the AgNPs made by Anabaena variabilis were crystalline and had lattices and fringes at the atomic level.109 AuNPs obtained from Phormidium valderianum were synthesized by HR-TEM, revealing a crystalline structure with a spherical morphology, predominantly consisting of particles measuring less than 20 nm110 (Fig. 4). | ||
| Fig. 4 TEM image (A) and the selected area electron diffraction (SAED) pattern (B) of synthesized Os-AgNPs, adapted/reproduced from (Bishoyi et al.,2025)106 with permission from Wiley, copyright 2025. | ||
8.5.1.1 Antibacterial activities of AgNPs. AgNPs produced from Synechococcus showed strong antibacterial activity with maximum inhibition zones of 24 nm and 11 nm for the S. aureus and E. coli. Both Gram positive as well as Gram negative bacteria were significantly inhibited.111,112 Mechanistically, these AgNPs directly interfere with metabolic energy and membrane functions, making them a viable substitute for current antibiotics. Moreover, compared to chemically synthesized AgNPs, Spirulina platensis-AgNPs display better antibacterial capacity and stability. They show good bactericidal effects on Gram positive bacteria such as Enterococcus hirae, E. faecalis and S. aureus, as well as synthetic stability against Gram negative bacteria, P. aeruginosa and Staphylococcus typhimurium.113 AgNPs generated with the cyanobacterium Pseudanabaena/Limnothrix sp. showed strong inhibitory effects on both Gram positive and negative bacteria, such as Corynebacterium glutamicum and E. coli.114 Additionally, Chroococcus sp. Ag NPs as spherical shaped, 11 nm to 13 nm particles displayed significant antibacterial activity against the pathogenic bacteria Micococcus luteus, Bacillus subtilis and Pseudomonas aeruginosa.115
(a) Factors affecting antibacterial activity: the biosynthetic process can be optimized by varying parameters such as the concentration of silver nitrate/gold, pH, temperature, and incubation period. Ag NP synthesis by Chroococcus sp., reported that the extract ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v corresponds to maximum AgNO3 reduction to silver nitrate (10 mM) at pH 7, 60 °C, at 30 minutes.115 Similarly, Synechococcus sp. was used to synthesize AgNPs using different biological approaches like the direct strain powder method, the ethanolic extract of pellet method and the ethanol extract method, producing a higher inhibition zone of 24 mm against Gram-positive bacteria S. aureus.112 AgNPs exhibit antibacterial activity, resulting from their interaction with bacterial cell membranes, which in turn causes membrane disruption and DNA damage, leading to bacterial cell death.87 Moreover, multiple parameters including overall shape, size, concentration, and surface charge of Ag NPs, play a role in their antibacterial effects. Nanoparticles with smaller sizes have demonstrated enhanced antibacterial activity due to their higher surface area to volume ratio that facilitates increased interaction with bacterial cells.84 AgNPs synthesized with the cyanobacterium Desertifilum sp. exhibited a larger range of antibacterial activity against MDR bacteria, with inhibition zones between 9–25 mm.116 The antibacterial activity of AgNPs is also significantly dependent on their concentration. AgNPs show a stronger antibacterial activity at higher concentrations; however, an over-concentration of Ag NPs can lead to agglomeration, which can compromise their action.117
1 v/v corresponds to maximum AgNO3 reduction to silver nitrate (10 mM) at pH 7, 60 °C, at 30 minutes.115 Similarly, Synechococcus sp. was used to synthesize AgNPs using different biological approaches like the direct strain powder method, the ethanolic extract of pellet method and the ethanol extract method, producing a higher inhibition zone of 24 mm against Gram-positive bacteria S. aureus.112 AgNPs exhibit antibacterial activity, resulting from their interaction with bacterial cell membranes, which in turn causes membrane disruption and DNA damage, leading to bacterial cell death.87 Moreover, multiple parameters including overall shape, size, concentration, and surface charge of Ag NPs, play a role in their antibacterial effects. Nanoparticles with smaller sizes have demonstrated enhanced antibacterial activity due to their higher surface area to volume ratio that facilitates increased interaction with bacterial cells.84 AgNPs synthesized with the cyanobacterium Desertifilum sp. exhibited a larger range of antibacterial activity against MDR bacteria, with inhibition zones between 9–25 mm.116 The antibacterial activity of AgNPs is also significantly dependent on their concentration. AgNPs show a stronger antibacterial activity at higher concentrations; however, an over-concentration of Ag NPs can lead to agglomeration, which can compromise their action.117
AgNPs synthesized from cyanobacteria exhibit antifungal activity through the generation of reactive oxygen species, damaging plasma membranes, and disturbing the metabolic process of the cell by adsorption on the cell membrane. Additionally, nanoparticles disrupt the cell wall and pierce the fungal cell wall, which leads to lysis of the cells and death due to the leakage of cell contents. These mechanisms also are evidenced in two studies where inhibition of critical expression of enzymes such as lactate dehydrogenase and glutathione peroxidase in fungal cell after exposure to AgNPs were found.119 Chitosan-stabilized AgNPs were more stable and demonstrated antifungal activity against pathogenic fungi, such as Candida sp., Aspergillus fumigatus and Cladosporium sp., than other AgNPs.120 For example, endophytic fungus with ability of producing chitosan capped AgNPs from Curvularia kusanoi exhibited highly potential in antifungal action toward Aspergillus fumigatus along with 83% thread growth inhibition at doses of 50 mg L−1.106 Ag NPs produced using Oscillatoria salina were found to be effective against Trichophyton rubrum and Candida tropicalis pathogenic fungi, with inhibition zones ranging 20–30 mm.121 Spirulina platensis and Nostoc linckia have been characterized as aggressive reducers of AgNPs. These organisms biosynthesize and produce phycobiliproteins, which can act as a reducing and stabilizing agent. The TEM results revealed that AgNPs produced from S. platensis had a particle size distribution between 15.1 and 27.4 nm in size with a mean size of 21.211 nm. The AgNPs produced by N. linckia have average particle sizes ranging from 16.3 to 25.8 nm and an average particle size of 21.052 nm. Moreover, the antifungal activity of SPI-AgNPs has been demonstrated to be potent against Candida albicans.122 Anabaena variabilis-mediated AgNPs has prevented growth of biofilm formation of C. albicans (62.5% inhibition of biofilm after 25 µg mL−1 concentrations).123 Similarly, AgNPs synthesized with Synechocystis sp. have wound healing effect in diabetic animal model that manifests as the potential biomedical applications.84 (Table 3).
| Nanoparticle type & source | Synthesis mechanism | Key metabolites/reducing agents | Characterization methods | Pathogen/cancer target | Antimicrobial efficacy (MIC/ZOI) | Anticancer/cytotoxic potency (IC50) | Ref. |
|---|---|---|---|---|---|---|---|
| AgNPs (Nostoc linckia) | Extracellular | C-phycocyanin (pigment) | UV-vis, TEM, XRD, FTIR, DLS | S. aureus, P. aeruginosa, E. coli, K. pneumoniae, MCF-7 | ZOI: significant | 27.79 µg mL−1 (MCF-7) | 85 |
| AgNPs (Oscillatoria limnetica) | Enzymatic/metabolic | Proteins (amine/carboxyl groups) | UV-vis, FTIR, TEM, SEM, XRD | E. coli, B. cereus, MCF-7, HCT-116 | Not specified | 6.15 µg mL−1 (MCF-7); 5.37 µg mL−1 (HCT-116) | 81 |
| ZnO-NPs (Arthrospira platensis) | Extracellular | Phycobiliproteins & polysaccharides | UV-vis, XRD, TXRF, TEM | B. subtilis, S. aureus, P. aeruginosa, C. albicans, Caco-2 | MIC: 12.5–50 ppm; ZOI: 19.1–24.1 mm | 9.95 µg mL−1 (Caco-2); 53.34 µg mL−1 (WI38) | 124 |
| AgNPs (Desertifilum sp.) | Intracellular | Phycoerythrin & phycocyanin | UV-vis, TEM, XRD, FTIR | 5 pathogenic bacteria, MCF-7, HepG2, Caco-2 | Not specified | 58 µg mL−1 (MCF-7); 32 µg mL−1 (HepG2); 90 µg mL−1 (Caco-2) | 117 |
| AuNPs (Nostoc calcicola) | Extracellular | Phenolic compounds & proteins | UV-vis, FTIR, TEM, EDX, XRD | 5 bacteria, 2 fungi, breast/cervical cancer | MIC: 11–18 µg mL; ZOI: 11–18 mm | 37.3 µg mL−1 (breast); 44.5 µg mL−1 (cervical) | 125 |
| AgNPs (Anabaena variabilis) | Intracellular | Exopolysaccharides (EPS) | UV-vis, FTIR, TEM, SEM, XRD | HeLa, SiHa, HepG2, HEK-293 | Not specified | 23.76 µg mL−1 (HeLa); 11.21 µg mL−1 (SiHa) | 126 |
| AgNPs (Oscillatoria princeps) | Biomimetic | Nitrate reductase (enzyme) | UV-vis, FESEM, EDX | Not specified | ZOI: 10.6–14.6 mm | Not specified | 127 |
| AgNPs (Chroococcus/Characium) | Extracellular | Extracellular proteins | UV-vis, XRD, TEM, TGA | MCF-7, HepG2 | ZOI: 2–6.9 mm | 40.9 µg mL−1 (MCF-7); 20.8 µg mL−1 (HepG2) | 128 |
| SeNPs (gamma irradiation) | Enzymatic | (Gamma ray induced reduction) | UV-vis, FTIR, SEM, EDX | HepG2, others | MIC: 0.313 µg mL; ZOI: 36.33 mm | 8.87 µg mL−1 (HepG2) | 125 |
| CuO-Se BNPs (L. siceraria) | Extracellular | Flavonoids & phenolics | UV-vis, XRD, FTIR, SEM | MCF-7, Hep-G2, Wi-38 | MIC: 7.8–250 µg mL; ZOI: 10–21 mm | 31.1 µg mL−1 (MCF-7); 83.4 µg mL−1 (HepG2) | 129 |
| CMC-AuNPs (carboxymethyl cellulose) | Photo-catalytic | CMC polymer (stabilizer) | UV-vis, TEM, FTIR, DLS | S. aureus, B. cereus, K. oxytoca, MCF-7 | MIC: 25–100 µg mL; ZOI: 13–26 mm | 2.56 µg mL−1 (MCF-7) | 130 |
| GA@Ag-CuO Nanocomposite | Intracellular | Gum Arabic (polysaccharide) | UV-vis, TEM, FTIR, EDX | S. epidermis, S. aureus, L. plantrum, MCF-7 | MIC: 15.6–125 µg mL−1 | 26.11 µg mL−1 (MCF-7); 59.5 µg mL−1 (HepG2) | 131 |
| AgNPs (Pseudanabaena/Limnothrix) | Biomimetic | C-phycocyanin & C-phycoerythrin | UV-vis, XRD, TEM, SEM | E. coli, C. glutamicum | Not specified | Not specified | 132 |
| MgO-NPs (Cystoseira crinita) | Nanocomposite | Fucoidan (polysaccharide) | UV-vis, FTIR, XRD, DLS | Bacteria, Caco-2, Vero cell | MIC: 12.5–50 µg mL−1 | 113.4 µg mL−1 (Caco-2); 141.2 µg mL−1 (Vero) | 133 |
| AgNPs (actinobacteria) | Polymer-stabilized | Extracellular enzymes | UV-vis, FTIR, TEM, zeta | E. coli, K. pneumoniae, P. aeruginosa, MCF-7 | MIC: 8–128 µg mL−1 | 16.3 µg mL−1 (MCF-7) | 134 |
| AgNPs (Streptomyces/Bacillus) | Extracellular | Microbial proteins | UV-vis, FTIR, TEM, XPS | MCF-7, DU-145 | Not specified | <3.5 µg mL−1 (MCF-7); <2.5 µg mL−1 (DU-145) | 129 |
8.7 Antiviral activity
AgNPs prepared from Spirulina platensis demonstrated that AgNPs have an inhibition rate of 48.334% of hepatitis C virus (HCV) compared with the standard drug of hepatitis, ribavirin.135 The antiviral activities of Ag NPs derived from cyanobacteria were assigned to their interaction with viral proteins, which disrupt key steps in the virus life cycle. It has been reported that the viral entry of host cells can be inhibited by Ag NPs, which affects viral glycoproteins from binding to receptors of host cells. This mechanism was evident in an experiment where Ag NPs prevented IBV from entering the centrosome by inhibiting the formation of viral RNA genome.136,137 Ag NPs also has the ability to act against both tropic forms of HIV-1 by preventing the protease activity of this virus (having wider spectrum of antiviral action) may classify this as a broad-spectrum antiviral.13 In the case of SARS-CoV-2, the cyanobacterial metabolites involved in the Ag NPs synthesis inhibit viral replication by interacting with the key proteases, that are required for viral polyprotein cleavage, such as, Mpro and PLpro.138 Likewise, the Ag-NPs of Phormidium ambiguum exhibited the highest scavenging activity of 48.7% comparing with that of the cyanobacterium Desertifilum tharense, which displayed 43.753%116 (Fig. 5).8.8 Anticancer activity
Silver Nanoparticles synthesized using O. salina show immense lethality towards human derived cancer cell lines, HeLa, the cervical adenocarcinoma and MDAMB-231 the breast adenocarcinoma, which reflects their possible employment as anticancer capability.121 Similarly, AgNPs prepared from Desertifilum sp. showed cytotoxic effects on MCF-7, HepG2, and Caco-2 cancer cell lines, with IC50 values of 58, 32, and 90 µg mL−1, respectively.118 Studies demonstrated an unprecedented approach for the bio synthesis of AgNPs, using the polysaccharide of Spirulina platensis as reducing and capping agents, with superior anticancer activity against a hepatocellular carcinoma cell line. The IC50 for polysaccharides isolated from Spirulina platensis (PSP) and Ag-NPs were 65.4 and 24.5 µg mL−1, respectively. Moreover, cell apoptosis assays for PSP and Ag-NPs against the growth of Hep-G2 cells revealed superior growth inhibitory effects of the bio synthesized Ag-NPs that encouraged tracing the apoptotic signaling pathway.80 Silver nanoparticles produced by Nostoc sp. Bahar M induce oxidative stress and apoptosis leading to significant inhibition of growth of ehrlich ascites carcinoma tumour in the organs of tumour-bearing mice.118 Similarly, in breast cancer cells (T47D) cell line, treated by Anabaena flos-aquae-synthesized Ag NPs, apoptosis was observed due to the induction of ROS and DNA fragmentation.138 Silver nanoparticles (AgNPs) prepared by Desertifilum sp. displayed substantial cytotoxicity against MCF-7, HepG2 and Caco-2 cancer cell lines with IC50 values of 58, 32 and 90 µg mL−1, respectively.118,1198.9 Antioxidant property of AgNPs
The antioxidant property of AgNPs is related to its potential in scavenging free radicals and reducing oxidative stress and enhancing that of antioxidant enzymes as catalase, superoxide dismutase and glutathione peroxidase. Silver NPs prepared using cyanobacterial extracts of Spirulina platensis and Nostoc linckia, showed notable DPPH radical scavenging and total antioxidant capacity tests showing IC50 values of 45.2 µg mL−1 and 38.5 µg mL−1, respectively.135 The antioxidant potential is correlated with the content of phenolic compounds and other metabolites, which could be involved in free radicals scavenging and thus in the cellular protection against oxidative damage.139,140 The scavenging activity was noted to be highly significant in the case of AgNPs synthesized by O. salina which showed IC50 32.4 µg mL−1.121 Size, shape and surface charge of AgNPs also affect their antioxidant performance. Smaller NPs with higher surface area are often more efficient in being reactive and passing through cellular barriers in their potential role as antioxidant agents. Chroococcus sp. produces AgNPs ranging in size from 5–50 nm, and Bacillus cereus generates AgNPs in a smaller size range of 1–5 nm.1159 Gold nanoparticles
9.1 Antibacterial activity
Over the past decade, cyanobacteria biofabricated AuNPs have been synthesized that are nontoxic, green and show potential antibacterial activity against several bacterial pathogens. Biosynthesized nanoparticles are being used as antibacterial mediated agents based on their geometrically designed extremely large surface area that originates from their nanoscale. It can attach to the bacterial peptidoglycan cell wall by electrostatic forces to degrade it. Membrane potential is altered, and ATPase activity is inhibited, which leads to a decrease in ATP content in the bacterial cell. Additionally, AuNP exposures cause a pore formation on the cell walls, which results in the leaking of the cell contents and interferes with tRNA binding to the ribosomal subunit inhibiting thus the transcription process. The accumulation of AuNPs in biofilms induces a higher cell wall tension, leading into nano-toxicity and metabolic interruption, subsequently leading to bacterial cell death. The deformation of the cell membrane due to nanoparticle clustering could then shorten treatment duration and reduce the side-effects of the nanomedicine.22 Studies demonstrated AuNPs biosynthesized using Oscillatoria sp. and S. platensis showed antibacterial activity against Gram-positive bacteria, B. subtilis ATCC 19,659, and S. aureus ATCC 25,923, MRSA ATCC MP-3 and Gram-negative bacteria, Salmonella typhi ATCC 14,028, Klebsiella pneumoniae ATCC 70,063 and Pseudomonas aeruginosa ATCC 9027.140 Spirulina subsalsa synthesized spherical AuNPs exhibited excellent activity against Staphylococcus aureus, S. pyogenes, Escherichia coli and A. baumannii.141 Anabaena spiroides synthesized AuNPs exhibited antibacterial activity against K. oxytoca, MRSA, and S. pyogenes22 (Fig. 6).9.2 Antifungal activity
The cyanobacterium, Hassallia sp. produced a variety of cyclic lipopeptides, such as, hassallidins and puwainaphycins, which target cell membranes of pathogenic fungi such as, Aspergillus fumigatus and Candida albicans, by binding to cholesterol and ergosterol, and ultimately inducing cell death.142 Some phytochemicals found to have a positive relationship with antifungal effectiveness against infections by Fusarium solani and Botryodiplodia theobromae include phenolics, flavonoids and enzymes such as xylanase and glucanase.143 The antifungal properties of AuNPs are size-dependent. In the case of Candida sp., fungal inhibition by 25 nm particles was higher as compared to 30 nm particles.144 AuNPs may possibly interact with fungal proteins. Interaction of AuNPs with biologically active fungal H+-ATPase enzyme in cells leads to deviation from the natural orientation of enzyme, hence loss of the fungal function. This disturbance leads to the detriment of metabolism, with reduced nutrition adsorption, eventually leading to death of the fungal cell. Additionally, the possible interaction between the shapes and sizes of the AuNPs and some components of the plasma membrane like sulfur containing protein or phosphorus of the DNA base can result in improved antifungal activity.145 Oscillatoria sp. was used in bio synthesis of gold nanoparticles (AuNPs), which exhibited anti-fungal activities against C. albicans ATCC 24,423, A. favus ATCC 9643 and C. tropicalis ATCC 13,80 with ith inhibition zones ranging from 10 to 15 mm. Polysaccharides from S. maxima cyanobacterium were successfully employed for synthesis of AuNPs that further showed anticandidal activity toward C. albicans.1409.3 Antiviral activity
The antiviral activity of gold nanoparticles (AuNPs) against several viruses such as, H3N2, H1N1, the herpes simplex virus, and the foot-and-mouth disease virus have been reported. The absorbance of nanoparticles on the virus surface was investigated using microscopic methods which induced modifications that further restrains viral entry into cells. Phosphates or thiols from amino acids and nucleic acids are the electron donor groups. The electron donor groups thus formed complexes with Au ions, inhibiting proviral transcription or reverse transcription by attaching directly to DNA or RNA molecules. It can also be assumed that AuNPs denatured inherent disulfide bonds to modify the viral protein. In vitro anti-HIV activity was shown by C-60-based AuNP and AgNPs. Thus, nanotechnology is highly anticipated to bring excessive benefits as an aid for the HIV/AIDS patients for years to come. Proteins from blue–green algae, namely scytovirin (SVN) and grifthsin (GRFT) contain unique structural organizations that facilitate simultaneous attachment to multiple carbohydrate moieties as well as flexible loop regions in their structures that harbour anti-HCV activity. SVN and GRFT both efficiently inhibit HCV envelope glycoproteins.229.4 Anticancer activity of AuNPs
Gold nanoparticles because of their ability to be shaped, sized, and surface chemistry are extensively used in anticancer drug delivery for targeting, internalization, and improved cytotoxicity of drugs. They are integrated into different types of delivery systems, including light-responsive, pH-sensitive and glutathione-responsive systems. For instance, gum karaya-stabilized AuNPs loaded with gemcitabine hydrochloride exhibited 19.2% of drug-loading efficiency and remarkably conferred the cytotoxicity upon A549 lung cancer cells to 10% more than that with the free drug, with marked suppression of colony formation along with intracellular ROS generation. The size and shape of AuNPs have a substantial impact on the efficiency of delivery of drugs. The study showed that the nano stars had the most systemic cytotoxicity compared with the nanospheres and nanorods; the results also demonstrated anti-osogenesis of the bone cancer cells. Gelatin coated AuNPs which are spherical 50/100 nm; nanorods 20/50/100 nm applied in methotrexate delivery in the treatment of breast cancer were also used to study the influence of size on methotrexate release, in which the smaller NPs were barreling faster at acidic pH (5.4), and the efficiency at drug delivery was higher in the case of nanorods, which has also showed higher intrinsic toxicity. AuNPs also have a promising potential for PTT, which irradiates the tumor with a laser to trigger release or uptake of drugs by the cells. The doxorubicin-loaded AuNP vesicles killed 50% of HeLa cells upon laser irradiation compared to non-irradiated groups. Similarly, fabricated paclitaxel-loaded PEGylated aptamer-conjugated AuNPs that featured 86% encapsulation efficiency and the maximum release of drug at pH5.5 upon near-infrared (NIR) irradiation (160 mW cm−2), almost 10% greater release in response to NIR exposure than under dark conditions. These NPs considerably reduced cell viability and increased apoptosis from 12.8% to 41.49% specifically targeting MUC-1-positive cells, accumulating almost twice as much as in MUC-1-negative cells.18AuNPs derived from cyanobacteria have recently gained momentum in cancer therapeutics due to their exceptional physiochemical features and eco-friendly preparation. Cyanobacterial species like S. platensis, A. variabilis, O. limnetica and N. muscorum served as biological factories to generate AuNPs using cellular reducing and stabilizing metabolites such as proteins, flavonoids and polysaccharides. These biomolecules not only promote the reduction of gold ions into particles, but also cap and stabilize the particles, giving biocompatible and functional surface groups. This bio synthetic method is free from toxic reagents and the AuNPs produced are safer for biomedical applications and in oncology.
The anticancer action of cyanobacteria-mediated AuNPs is largely due to selective induction of cytotoxicity in cancer cells without causing damage to normal healthy cells. These NPs are taken up into cancer cells via endocytosis and accumulate into organelles, such as mitochondria, to produce reactive oxygen species (ROS). Increase in ROS levels caused oxidative stress, loss of mitochondrial membrane potential and release of pro-apoptotic factors such as cytochrome c leading to activation of caspase-3 and caspase-9 and ensuing apoptosis. Further, AuNPs can disrupt the cell cycle, resulting in arrest at the G2/M phase that suppresses cellular growth. They also influence the major regulatory pathways such as PI3K/Akt, p53 in tumor cells to actively promote the apoptotic events.146
The anticancer effectiveness of these NPs has been proved through many recent studies. For example, AuNPs successfully synthesized with Lyngbya majuscula showed higher cytotoxicity with A549 lung carcinoma cells, although normal fibroblasts were not influenced.99
N. muscorum generated AuNPs showed cytotoxicity in-7 breast and liver HepG2 cancer cells; apoptosis was mediated by increased ROS formation and mitochondrial depolarization by nanoparticles.101 Studies reported that O. limnetica-induced AuNPs were effective at inhibitingthe growth of the HeLa cervical cancer cells and they caused fragmentation of the DNA.147 The AuNPs from S. platensis showed anticancer activity against, HepG-2 and A549 cell lines which were successfully killed by apoptosis, as identified by nuclear condensation and caspase activation.148 Some decapeptides such as cryptophycins have shown potential antitumor properties. For example, cryptophycin A and B are reported active against KB cells which are effective against drug-resistant and drug-sensitive tumor cells. It has been well documented that several species of Lyngbya, Nostoc, Oscillatoria, and Phormidium produce bioactive compounds, which were proved to be having effective anticancer effect. A cyanobacterium Gloeocapsa sp. acted as a reducing and capping agent, in a successfully synthesized AuNPs of spherical and triangular shapes intracellularly; and its anticancer activity was seen against human cervical cancer cell line. AuNPs bioreduced by cyanobacterial extracts of Oscillatoria sp. and S. platensis showed control activity against human colon CaCo-2 and cervical HeLa cancer cells with IC50 value of 311.00, and 382.90 µg mL−1 (Fig. 7).149
9.5 Antioxidant activity AuNPs
AuNPs synthesized by cyanobacteria are employed for biomedical applications in various fields including antioxidants therapy, and diseases associated with oxidative stress. For instance, AuNPs prepared from Cyanothece sp. have previously been used as drugs in a model of reversal of cardiomyopathy caused by systemic administration of isoproterenol in the experimental rat,150 that may have clinical applications for cardiac therapy. As natural antioxidant AuNPs originated from cyanobacteria have also been reported for treating neurodegenerative diseases because oxidative stress is one of the major factors of these diseases.15010 Applications for cyanobacteria AgNPs
Cyanobacteria-produced AgNPs have also been investigated in the context of wound healing, notably in diabetic wounds. Blithely, due to their antioxidant and anti-inflammatory properties, these nanoparticles can promote wound closure, collagen synthesis, and angiogenesis, making them suitable candidates for therapeutic applications as well.150 Besides, AgNPs also appear to possess an antioxidant activity that would offer protection against heavy metal induced oxidative stress, as shown in its efficacy in sequestering and minimizing the effect of mercury ions151 (Table 4).| Sl. no. | Nanoparticle type | Application area | Specific use | Mechanism/function | Examples | Benefits | Challenges | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Silver (ag NPs) | Textiles | Antimicrobial fabrics | Releases Ag+ ions to inhibit microbial growth | Sportswear, socks, hospital linens | Durable, odour-resistant | Leaching during washing | 152 |
| 2 | Water treatment | Pathogen removal | Adsorption and inactivation of bacteria on nanoparticle surfaces | Nanocomposite filters (e.g., LG NanoH2O) | High efficiency, low energy use | Environmental accumulation | 153 | |
| 3 | Medical implants | Antimicrobial coatings | Prevents biofilm formation on implants by releasing Ag+ ions | Silver-coated catheters, orthopedic implants, bone cement | Reduces risk of post-operative infections | Long-term cytotoxicity, ion leaching | 154 | |
| 4 | Cosmetics | Preservative in creams | Prevents microbial spoilage, extending product life | AgNP-infused creams provide better skin permeabilit y | Long shelf life, non-irritating | Regulatory restrictions in some regions | 155 | |
| 5 | Air purification | Antibacterial air filters | Captures and inactivates airborne pathogens through silver ion release | HEPA filters with silver coating | Improved air quality, potential health benefits | Long-term effectiveness, ion release rate | 156 | |
| 6 | Wound dressing | Infection prevention & healing | Releases Ag+ ions to kill bacteria and reduce inflammation | Acticoat™, Aquacel® Ag dressings | Reduces infection risk, promotes faster healing | Potential cytotoxicity, bacterial resistance | 157 | |
| 7 | Agriculture | Crop protection | Acts as a broad-spectrum fungicide and pesticide | AgNP-coated seeds, foliar sprays | Increased yield, disease resistance | Soil and water ecotoxicity | 158 | |
| 8 | Electronics | Conductive inks | Sintered nanoparticles form conductive traces for circuits | Printed flexible electronics, RFID antennas | Low-cost fabrication, flexibility | Oxidation (tarnishing), electromigration | 159 | |
| 9 | Food packagin g | Antimicrobial packaging | Inhibits the growth of spoilage microbes on the packaging surface | Food packaging films and wrappers | Prolonged shelf life, improved food safety | Risk of nanoparticles leaching into food | 152 | |
| 10 | Gold (au NPs) | Medicine & theranostics | Cancer therapy & imaging | Generates localized heat (photothermal therapy) when exposed to NIR light to kill tumor cells | AuroLase® therapy, targeted contrast agents | Targeted, non-invasive treatment; combines diagnosis with therapy | Precise targeting, long-term clearance | 160 |
| 11 | Sensors | Pathogen & biomolecule detection | Surface plasmon resonance (SPR) shifts colorimetrically upon binding to a target molecule | COVID-19 lateral flow tests, glucose biosensors | High sensitivity, rapid results, visual detection | Requires precise surface functionalization | 161 | |
| 12 | Pharmaceuticals | Targeted drug delivery | Act as carriers to transport drugs directly to diseased cells, enhancing absorption | AuNP-doxorubicin conjugates for cancer treatment | Targeted therapy, improved drug stability, controlled release | Biocompatibility, high cost | 162 | |
| 13 | Electronics | Conductive inks & transparent films | Provides high electrical conductivity and stability for printing circuits on various substrates | Printed flexible circuits, components in OLEDs | Miniaturization, flexibility, enhanced performance | Cost, long-term stability of printed components | 163 | |
| 14 | Catalysis | Industrial synthesis & hydrogen production | High surface area and unique electronic properties accelerate chemical reactions | Catalytic converters, catalysts for producing hydrogen fuel | High efficiency, selectivity, works under mild condition s | Cost, deactivation over time | 164 | |
| 15 | Data storage | High-density optical storage | Encodes data in 5 dimensions (3D position + orientation + wavelength) using lasers on nanorods in glass | “Superman memory crystal” prototypes | Extremely high data density and longevity | Slow writing/reading speeds, complex tech | 165 | |
| 16 | Solar energy | Photovoltaic cells | Plasmonic effects enhance light absorption across the solar spectrum | AuNP-enhanced thin-film or dye-sensitized solar cells | Increased light-harvesting efficienc y | High production cost, stability issues | 166 | |
| 17 | Cosmetics | Anti-aging & skin products | Claimed antioxidant properties; reflect light to give skin a radiant glow | Snail slime based cosmetics, luxury anti-wrinkle creams | Immediate glow effect, perceived luxury | Scientific evidence is debated, high cost | 167 | |
| 18 | Anti-counterfeiting | Security labels | Creates a unique, unclonable optical signature (“plasmonic fingerprint”) | Security tags for luxury goods, pharmaceuticals | Extremely high level of security | Cost of implementation, specialized readers | 168 | |
| 19 | Environmental remediation | Water purification | Catalyze the degradation of persistent organic pollutants (e.g., dyes, pesticides) | Lab-scale systems for degrading industrial wastewater | High catalytic efficiency for breaking down pollutants | Catalyst recovery, potential for leaching | 169 | |
| 20 | Biotechnology | DNA/RNA detection | Binds to nucleic acids, enabling detection through electrochemical or optical signals | AuNP-based biosensors for genetic analysis | High specificity and sensitivity for diagnostics | Sample preparation complexity | 170 |
10.1 Agriculture
Cyanobacterial AgNPs have been also employed in the agricultural sector for increasing crop yield and plant pathogen control. The potential of these nano nutrients and pesticides is mediated either through enhancement of nutrients uptake by the plants and their growth, or as nano-pesticides controlling the plant pathogens and pests.171 Additionally, AgNPs have been documented to stimulate the production of EPS in cyanobacteria, an important biopolymer used in food, pharmaceuticals, and cosmetics industries.172 Additionally, silver nanoparticles from cyanobacterium are sustainable to different sectors of agriculture like plant protection, promotion of plant growth and post-harvest management of plants. Cyanobacterial like species of Nostoc, Anabaena and Oscillatoria mediated cyanogenic AgNPs were found to have strong antifungal activity against Alternaria alternata, Pseudomonas syringae and F. oxysporum. They exert their mode of action by attacking the microbe membrane, leaking cell contents and oxidative stress causes cell proliferation inhibition of the pathogen.173 Additionally, cyanobacteria-assisted AgNPs improve the seed germination, root length, and overall vigour of the plant. These NPs, if applied at low doses, enable phytohormone signaling and nutrient uptake thus enhancing biomass and chlorophyll content of the treated plants. Enhanced yield and disease control of rice, tomato, and wheat have been reported when treated with biosynthesized AgNPs. Furthermore, these nanoparticles can form part of antimicrobial coverings for fruit and vegetables, thereby prolonging their duration and contentment during refrigerated storage systems and transportation.19From an industrial standpoint, agrochemical companies are beginning to adopt cyanobacteria-derived AgNPs for use in nano-fertilizers, nano-pesticides, and bio-formulated sprays. These formulations offer prolonged effectiveness and some reduced environmental impact compared to conventional agrochemicals. However, while the benefits are substantial, ongoing research is addressing concerns related to nanoparticle accumulation in soil and crops, as well as potential toxicity to beneficial soil microbiota. Regulatory frameworks are being developed to guide the safe use of nanomaterials in agriculture, with biosynthesized nanoparticles receiving particular interest due to their natural origins and lower ecological footprint.174
10.2 Environmental applications
These nanoparticles have been employed for the degradation of organic contaminants like methylene blue by photocatalysis, which is practical in wastewater treatment, as microbial disinfections. These have shown a strong bactericidal and virucidal efficacy towards a broad range of pathogens, like, E. coli, Salmonella sp. and Vibrio cholerae found in the contaminated water systems. They act by disrupting bacterial membranes, causing oxidative stress by ROS and disrupt enzymatic systems, finally causing the lysis of pathogenic cells. Thus, cyanobacteria-fabricated AgNPs are promising to use in water treatment equipment, filter coatings, and sterilization spray.175 Additionally, AgNPs based cyanobacteria have been used for heavy metal biosorption from industrial effluents, effectively participating in a circular economy process, including recovery and reuse of metals.176 The cyanobacterial AgNPs have also been used for producing bioplastics, since polyhydroxyalkanoates (PHA) is biodegradable and therefore any application in producing PHA would pave way for more sustainable alternatives to conventional plastics.177 Silver nanoparticles synthesized using cyanobacteria offer considerable promise in environmental remediation, due to their potent antimicrobial activity, catalytic potential, and sustainable synthesis makes those popular. As concerns about pollution, waterborne pathogens, and industrial waste intensify, the use of biologically synthesized nanomaterials is emerging as a sustainable solution.173Heavy metal removal is just one of the important applications of these AgNPs. Cyanobacteria synthesized AgNPs have been found to reduce and sequester toxic heavy metal pollutants such as, cadmium, lead, mercury that are involving in redox reactions as well as, nanoparticle adsorption. Biomolecules on nanoparticle surfaces, originated from the cyanobacteria extract increase the binding affinity towards metal ions and therefore effectively remove them from alkali solutions.178 These characteristics render them appropriate for nano-adsorbents, bioreactors and industrial effluent treatment devices. Those have been demonstrated for the degradation of many organic pollutants including dyes, e.g., methylene blue, rhodamine B, and pesticides via photo or catalytic oxidation. Due to their high surface area and electron exchange ability, those rapidly transform toxic substances to less toxic intermediates, thus contributing to environmental detoxification.99 This renders those useful for applications like textiles, tanning and agrochemicals that often generate dye-laden or pesticide-contaminated wastewater. AgNPs prepared through cyanobacteria have been included in antimicrobial paints, coatings and packages that allow inhibiting of biofouling and microbial adhesion on surfaces. This would be especially beneficial in lowering microbiological contamination in public places, health care centers and food industries. Biosynthesized nanoparticles are less toxic and biodegradable in the environment compared with chemically synthesized AgNPs, meeting the global objectives of sustainable environmental technologies.174 However, despite these benefits, environmental implications of cyanogenic AgNPs should be strictly controlled in terms of their toxicological consequences. Issues concerning nanoparticle accumulation in aquatic biospheres as well as, disruption in microbe diversity and long-term presence in soil and water are all currently being explored. However, available evidence indicates that biosynthesized AgNPs from cyanobacteria with biocompatible coatings and a smaller synthesis footprint have a safer and cleaner status compared with those of traditional nanomaterials in pollution control technology applications.
10.3 Food packaging and preservation
Cyanobacteria mediated silver nanoparticles is incorporated into biodegradable polymers or cellulose films, which function as antimicrobial agents to improve the shelf-life of perishable food products by inhibiting microorganism contamination and spoilage, imparting antioxidant properties to the packaging films. These cyanobacteria AgNP-containing film packaging had been reported to be effective against the food borne pathogens such as Listeria monocytogenes, Salmonella typhimuriam, Aspergillus niger on meat, dairy and fruit surfaces.179 These bifunctionalities inhibit lipid oxidation or retards discoloration and thus preserving the organoleptic and nutritional quality of food stuffs.172 Meanwhile, for commercial food companies the prospect of using nano-packaging is attractive as a means of meeting clean-label and eco-friendly packaging trends and responding to the demands of expanded exports of fresh produce, seafood and dairy. Use of green-synthesized nanoparticles lessens the possibility of regulatory rebuttal for synthetic chemical residues and are advantageous for international food safety regulations.10.4 Cosmetics and personal care
Biosynthesized silver nanoparticles are now widely used in cosmetics and personal care applications, because of their antimicrobial, anti-inflammatory, and anti-aging effects. Cyanobacteria mediated synthesis of AgNPs show better skin-compatibility properties attributable to the organic capping agents derived from the metabolites of cyanobacteria such as polysaccharides, flavonoids and peptides. There are also nanoparticles in creams, lotions, face masks, deodorants and sunscreens that inhibit microbial growth, calm inflamed skin and protect from oxidative damage. In contrast to their synthetic counterparts, cyanogenic AgNPs represent a clean-label and environmentally friendly alternative to what current consumers are seeking for: natural and sustainable products. Furthermore, their diminutive size is suited for cutaneous penetration which increases the efficacy of the active agents without irritating the skin. AgNPs are also used in some of the formulations for the whitening and anti-pigmentation, as it inhibits synthesis of the melanin, leading to a decrease in the oxidative stress. Consequently, cosmetic industries are now getting inclined toward cyanobacterial nanoparticles to fulfil the demand for green and efficacious multifunctional ingredients.18010.5 Ammonia sensing
Recently, cyanobacterium Haloleptolyngbya alkalis KR2005/106 had been reported for the photo-biochemical synthesis of silver nanoparticles (approx. 50 nm). This cyanobacterium was isolated from a saline-alkaline habitat, a soda lake. The considerable decrease in the blue absorption observed is attributed to the interaction between silver nanoparticles and ammonia. The increased sensitivity of this transition was previously observed between 50 and 500 ppm ammonia.18110.6 Degradation of carcinogenic dyes
Silver nanoparticles were bio synthesized with a cyanobacterial extract, and the photocatalytic degradation of methylene blue dye was studied. Silver nanoparticles can act as catalysts for the decomposition of methylene blue dye under UV-H2O2 (20 mg L−1) to less than 4 h, with dye removal of 18% in this time span.175 A. variabilis and S. platensis were used for the synthesis of silver nanoparticles and as bio-sorbents to removal of malachite green dye. The dye concentration was reduced when the removal efficiency increased to 93% and 82% for S. platensis and A. variabilis-AgNPs, respectively. Results indicate that when compared with A. variabilis, larger specific surface area and lower particle size of S. platensis-AgNPs led to its superior catalytic activity in dye degradation. After dye treatment, Ag NPs were re-inoculated in growth of Triticum aestivum L., Giza 171 seedlings to compare the apparent nontoxicity of Ag NPs toward environmental system with its safety for agricultural purposes.122 AgNPs with cell free aqueous cyanobacterial extract Microchaete NCCU 342 were prepared and used for the degradation of azo-dye, Methyl Red (MR).182 MR dye (50 mg L−1) was decolorized by 84.60% at 2 h and the extract alone without nanoparticles could remove 49.80% of the dye.18310.7 Biosensing and diagnostics
Cyanobacteria-mediated silver nanoparticles are being widely employed in sensors and diagnostic devices due to their efficient surface plasmon resonance (SPR), electronic conductivity and high surface area-to-volume ratio. These nanoparticles can be used to increase the sensitivity and selectivity of detection platforms for pathogens, toxins, heavy metals and disease markers.184 As examples, AgNPs prepared with species of non-nitrogen-fixing Oscillatoria or nitrogen-fixing Nostoc have been functionalized with bio-probes to diagnose glucose, DNA fragments or cancer bio-markers at some ultra trace level. The biocompatible, non-toxic surface of cyanobacterial AgNPs enhances the immobilization of probes, signal transduction, and minimizes background noise in electrochemical and colorimetric assays. These AgNPs are being incorporated into lateral flow test strips, electrochemical biosensors and lab-on-chip devices for medical, environmental and agricultural diagnostics. Thus, the use of bio synthesis approach drives these biosensing platforms to be earth-conscious.17910.8 Dentistry and oral care
In dental sciences, these have applications on oral hygiene products, restorative materials, and endodontics. Additionally, these are ideal candidates for toothpastes, mouth rinses, composites and implants etc., having their antimicrobial activity against dental and oral pathogens such as, Streptococcus mutans, Lactobacillus acidophilus and Candida albicans. Cyanobacteria mediated synthesized AgNPs are potential candidates, as they offer nontoxic and mucosal compatibility especially those synthesized from A. cylindrica. Those also are being explored for biofilm control on dental prosthetics, and root canal disinfection in a manner that would obviate the use of harsh chemical irritants such as sodium hypochlorite. The slow liberation of ionic silver from the naturally derived capping protein matrix leads to long-term anti-microbial activity without staining or irritation. Dental products businesses are starting to spend on these types of green nano materials to satisfy elevated requirements from clients who need less risky and natural oral care products19.10.9 Textile industry
The use of silver nanoparticles is growing in the textile industry, as those are natural antimicrobial and are applied to produce antimicrobial textiles in paper and polymer materials, where a good standard of hygiene and long lifetime are critical. Notably, AgNPs have been incorporated into medical textiles to provide antimicrobial protection, which is an essential aspect in health care, that is able to prevent infections, and the spreading of nosocomial infections. Products for an actual industrial application of textiles with cyanobacteria made AgNPs are still in part pending.11811 Applications of gold nanoparticles
Synthesis of AuNPs, particularly with cyanobacteria, has attracted considerable attention for its eco-compatibility, cost-effectiveness, and amenability from energy saving, for mass production. By secretion of extracellular molecules such as peptides, polysaccharides, and proteins, cyanobacteria are effective biological synthesizing agents of nanoparticles. Due to the biologically capped layer, these AuNPs show improved biocompatibility and stability; and therefore, are suitable for several applications in industry.11.1 Pharmaceutical and biomedical applications
Cyanobacteria-fabricated AuNPs have great potential in biomedicine, because its non-toxic synthesis and the natural capping agents on the surface improve biological interaction. Their drug delivery applications have been reported, where the biomolecules immobilized on AuNPs can be employed to assist desired delivery and release of anticancer drugs. Additionally, they are promising in the field of photothermal therapy of cancer being able to absorb near-infrared (NIR) light effectively and convert it into heat and kill cancer cells selectively without affecting the adjacent tissues. These nanoparticles are also highly antimicrobial that is suiting as wound healing dressings and surgical instruments coating too. The growth suppressive abilities are due to the cooperative effects of gold along with bioactive cyanobacterial compounds on pathogenic bacteria such as, E. coli and S. aureus.1811.2 Targeted drug delivery
Naturally coated with biomolecules like peptides and polysaccharides, these nanoparticles serve as perfect surfaces for the conjugation of ligands, such as antibodies, nucleic acids, or chemotherapeutics. These nanoparticles can passively accumulate in tumor tissues exploiting the enhanced permeability and retention (EPR) effect. In addition, the delivery of drugs can be only induced by certain stimuli like, pH, enzymes, or light, which enable a target therapy and any systemic side effect is avoided.18511.3 Diagnostic imaging
Gold nanoparticles synthesized by cyanobacteria are also useful contrast agents for CT, as well as photoacoustic imaging. They are appealing because of their large atomic numbers that enhance X-ray absorption and SPR properties leading to enhanced photoacoustic signals. Cyanobacteria-capped AuNPs show higher biocompatibility in comparison with iodine-based agents and may be tailored for actively targeting specific disease indicators, which makes diagnoses more accurate.186,18711.4 Self-cleaning and antimicrobial coatings
Coatings generated with cyanobacteria-assembled AuNPs were found to be effective in self-cleaning, anti-fouling and antimicrobial applications. These coatings are useful in marine applications, electronic platforms, and biomedical surfaces by preventing the colonization of microorganisms or the collecting of dirt. The biocompatibility and chemical inertness of such AuNPs provide long-term stability, and their optical behaviour provides aesthetic or functional features.188,18911.5 Diagnostic devices
Biosynthesized AuNPs have been utilized in diagnostic devices, for detecting biomolecules or pathogens. AuNPs prepared from cyanobacteria maintain the surface plasmon resonance features that are critical for use in colorimetric and lateral flow assays. Functionalization with antibodies or nucleic acids can offer further specificity for detection of disease biomarkers in point-of-care diagnostic kits Their own naturally occurring capping agents provide for an excellent bioconjugation and stability in biological media.19011.6 Catalysis in the environment
Cyanobacteria-mediated gold nanoparticles have great potential in environmental catalysis, since they are green-synthesized and high surface-area-to-volume nanostructures. These AuNPs can also be used to catalyze different oxidation reactions of interest such as degradation of VOCs and CO, even at low temperatures. Their efficiency in decomposing harmful industrial gases renders them suitable for incorporation in catalytic converters and gas treatment systems. These particles are stabilized by biological capping agents derived from cyanobacteria, thereby enhancing catalytic turnover as well as being sustainable for the environment. Furthermore, these biogenic NPs avoid the use of toxic chemical stabilizing and reducing agents that are used in traditional synthesis, thus, following the principles of green chemistry.19111.7 Chemical synthesis catalysis
Moreover, biogenic AuNPs are highly active catalysts in a wide range of redox reactions, which are applied in fine chemical and pharmaceutical industries. AuNPs made by cyanobacteria were reported to catalyze the reduction of nitroarenes to amines and the oxidation of alcohols to aldehydes or ketones at room temperature. These reactions are important for the synthesis of dyes, agrochemicals and pharmaceuticals. These biosynthesized AuNPs are, therefore, considered ideal for green chemical synthesis, as the reaction is carried out under mild conditions and with high selectivity. Moreover, these nanoparticles are also recyclable and can be utilized repeatedly for several cycles of MCNPs toward their economic and environmental efficiency.19211.8 Electronics and sensor technology
The high conductivity and biocompatibility of cyanobacteria AuNPs make those interesting candidates for electronic and biosensor systems. When used in electrochemical sensors, those serve as a signal enhancer to sense pollutants or biomolecules with high sensitivity. Through bio-reduction, unique shapes and surface functionalities are typically added that can act as a fingerprint resulting in improved sensor performance. These attributes make them attractive building blocks for flexible electronics, wearable sensors, and nano-circuit construction as well.19311.9 Environmental applications
Cyanobacteria synthesized-AuNPs are being actively used for environmental monitoring as well as remediation. Their surface can be modified for heavy metal detection of lead, arsenic, and cadmium in water. Besides that, those are used as catalysts in degradation reactions to eliminate organic pollutants as dyes, pesticides, and pharmaceutical residues from industrial effluents.19411.10 Agricultural and veterinary applications
In agriculture, biocomponent AuNPs–fertilizer–pesticide combination can be used as a smart delivery system. Their bio-functional nature enhances their ability to interact with plant tissues, which can help increase nutrient uptake and utilization. They play a role in lowering the misuse of agrochemicals, which leads to avoid the pollution of the environment.194 Cyanobacteria-mediated AuNPs also appeared to enhance seed germination and promote growth of the plant, because of potential modulation of stress responsive genes. In veterinary medicine, these NPs are employed in lateral flow assays to enable the rapid diagnosis of diseases in farm animals. Due to their bio-safety profile, they have a great potential for food-producing animal applications.11.11 Photonics and electronics
Cyanobacteria-mediated AuNPs exhibit distinct plasmonic characteristics, which are crucial for emerging next-generation photonic and nano-optoelectronic architectures. These nanoparticles display what is known as surface plasmon resonance (SPR) in which light is absorbed and scattered very strongly at certain wavelengths. It has been widely used in the design of optical switches, waveguides and photonic crystals. As biosynthesized pathways frequently give shape to monodisperse, spherical, anisotropic NP with stable capping of cyanobacterial biomolecular networks, the optical responses of these can be custom tuned for a specific device need.17912 Barriers to clinical and industrial translation
It would not be out of place to cite, an old work of this laboratory, monitoring cyto-toxicity of AgNPs biosynthesized with the higher timber-yielding plant, Anogeisus acuminata against lymphocytes culturing in vitro from human umbilical cord blood, as an offal.195 Such work of monitoring cytotoxicity and nuclear toxicity of cyanobacterial nanoparticles with human lymphocytes are in progress and gaining much more attention now a days. Despite the promising in vitro antimicrobial and anticancer properties of silver and gold nanoparticles synthesized by cyanobacteria reported in recent literature, several significant barriers hinder their clinical and industrial translation.12.1 Standardization and reproducibility
A major challenge is the lack of standardized protocols for nanoparticle synthesis. The physicochemical properties of nanoparticles—such as size, shape, and surface charge—are highly sensitive to variations in cyanobacterial strains, culture conditions, and synthesis parameters. This leads to batch-to-batch variability and polydispersity, complicating reproducibility and making it difficult to ensure consistent efficacy and safety in clinical or industrial settings.82,196,19712.2 Scalability and process control
Scaling up biosynthesis from laboratory to industrial levels introduces further complexity. Factors such as light distribution, nutrient supply, and contamination risk become more difficult to control in large-scale photobioreactors, often resulting in inconsistent nanoparticle yields and quality. Maintaining axenic (pure) cultures at scale is also challenging and increases operational costs.19612.3 Regulatory and safety concerns
There is a lack of comprehensive toxicological data for cyanobacteria-derived nanoparticles. While in vitro studies show low toxicity to healthy cells at certain concentrations, in vivo biosafety, long-term effects, and environmental impacts remain underexplored. Regulatory agencies require robust, standardized data on nanoparticle characterization, safety, and efficacy, which is currently insufficient for clinical approval196,19712.4 Quality control and good manufacturing practice (GMP)
Industrial translation demands strict quality control and compliance with GMP standards. The biological variability inherent in cyanobacterial systems makes it difficult to meet these requirements, especially regarding nanoparticle uniformity, purity, and stability.196,19713 Conclusion and future perspectives
Biosynthesis of AgNPs and AuNPs using cyanobacteria appears to be attractive and sustainable, in comparison to the traditional physicochemical methods. This clean technology is at the same time convenient, inexpensive and meeting the demand of green technology around the world without using any hazardous reagents. Current situation has rendered it quite obvious that the outcome of laboratory findings and the clinical preparedness differ significantly. It has already been demonstrated that these nanoparticles possess a powerful antimicrobial activity; numerous studies have consistently revealed a high bactericidal efficacy with regard to the MDR pathogens which is a pointer that in the days of age it could substitute the conventional antibiotics. On the other hand, application of nanocrystals as far as clinical cancer therapy and targeted drug delivery is seriously a conjecture. Despite the perceived presence of reported cytotoxicity on specific cell lines, in vitro observations are yet to be converted into clinical efficacy since adequate in vivo pharmacokinetic data of nanotoxicity of its prospective off targets have not been investigated in detail. Not only that, the existing impediment in bench-top synthesis to manufacturing is the economies of scale as a technological aspect in the form of scalability and repeatability. This means that biological synthesis may be dynamic and hence change in size and shape of nanoparticles batch to batch, making it quite challenging to standardize it in case it is utilized as a pharmaceutical. However, biosynthesis of cyanobacterials is field of genomics is currently developing. The futures of synthetic biology and genetic engineering development give hope to enable the control of the morphology of a nanoparticle at a molecular level because of manipulation of biosynthetic pathways. To sum up, despite the fact that the chemical variety of cyanobacteria offers a great channel towards the new therapeutic options, the potential has to be brought into reality by turning simple record of synthesis of various compounds into tangible form of standardization. The future work should put into consideration the mechanisms of fine formation, biosafety profile and scalable bioreactors. Only once these and other types of engineering and regulatory-level bottlenecks are overcome, the massive therapeutic promise of cyanobacteria be translated into practical solutions to problems of world health.Author contributions
S Sabat: writing – original draft, writing review & editing, visualization. S Patra: formatting and editing. S Swain: formatting and editing., S Bej: formatting and editing. AK Bishoyi: writing – original draft & editing, visualization. G Sabat: formal analysis. RN Padhy: writing – review & editing, supervision & fund acquisition.Conflicts of interest
The authors declare no competing interests.Data availability
No primary research results and no new data were generated or analysed as part of this review.Acknowledgements
No. extramural fund was available; the only support is for S. Sabat and the work as the PhD research fellow (No. 24P119A02) of SOA University, Bhubaneswar. We are grateful to Prof. Dr PK Nanda, Honourable Vice-Chancellor, Shikha O Anusandhan deemed to be University, Bhubaneswar-3, Odisha, for facilities and encouragements. S. Sabat was supported by SOA-PhD fellowships, Regn. No. 24P119A02. We are grateful to Honourable Prof. Dr MR Nayak, Honourable President, SOA deemed to be University, Bhubaneswar-3, for extended facilities.References
- J. P. Zehr and P. J. Turner, Methods Microbiol., 2001, 30, 271–286 CrossRef CAS.
- F. R. Tabita, J. L. Gibson, Y. Jouanneau, D. L. Falcone and A. M. Rainey, in Microbial Growth on C1 Compounds: Proceedings of the 5th International Symposium, Springer, Netherlands, Dordrecht, The Netherlands, 1987, pp. 238–245 Search PubMed.
- M. Kurahashi and A. Naka, J. Appl. Biosci., 2025, 4, 2 CrossRef.
- P. N. Leao, N. Engene, A. Antunes, W. H. Gerwick and V. Vasconcelos, Nat. Prod. Rep., 2012, 29, 372–391 RSC.
- C. S. Ting, G. Rocap, J. King and S. W. Chisholm, Trends Microbiol., 2002, 10, 134–142 CrossRef CAS PubMed.
- S. Sabat, S. Bej, S. Swain, A. K. Bishoyi, C. R. Sahoo, G. Sabat and R. N. Padhy, Bioresour. Bioprocess., 2025, 12, 27 CrossRef PubMed.
- H. U. Dahms, X. Ying and C. Pfeiffer, Biofouling, 2006, 22, 317–327 CrossRef CAS PubMed.
- S. S. Swain, R. N. Padhy and P. K. Singh, Antonie Van Leeuwenhoek, 2015, 108, 223–265 CrossRef CAS PubMed.
- K. Bishoyi, C. P. Mandhata, C. R. Sahoo, S. K. Paidesetty and R. N. Padhy, Naunyn Schmiedeberg's Arch. Pharmacol., 2024, 397, 1347–1375 CrossRef PubMed.
- M. M. El-Sheekh and H. Y. El-Kassas, J. Genet. Eng. Biotechnol., 2016, 14, 299–310 CrossRef PubMed.
- N. A. Ahmad, A. Zaki and T. Fatma, J. Appl. Phycol., 2021, 33, 829–841 CrossRef.
- S. Bej, S. Swain, A. K. Bishoyi, C. R. Sahoo, B. R. Jali, M. S. Khan and R. N. Padhy, J. King Saud Univ. Sci., 2024, 36, 103336 CrossRef.
- F. S. Hussain, N. Q. Abro, N. Ahmed, S. Q. Memon and N. Memon, Front. Nanotechnol., 2022, 4, 1064615 CrossRef.
- V. Kumar and S. K. Yadav, J. Chem. Technol. Biotechnol., 2009, 84, 151–157 CrossRef CAS.
- P. Jegadeeswaran, R. Shivaraj and R. Venckatesh, Dig. J. Nanomater. Biostruct., 2012, 7, 991–998 Search PubMed.
- V. Rani, N. K. Aye, R. Saksena, K. C. Dabi, M. A. Mannan and R. Gaind, Eur. J. Clin. Microbiol. Infect. Dis., 2024, 43, 767–775 CrossRef CAS PubMed.
- Z. H. Ali, L. Abdulazeem, W. A. Kadhim, M. H. Kzar and O. J. Al-Sareji, Sci. Rep., 2024, 14, 31593 CrossRef CAS PubMed.
- F. Eker, H. Duman, E. Akdaşçi, A. M. Witkowska, M. Bechelany and S. Karav, Nanomaterials, 2024, 14, 1618 CrossRef CAS PubMed.
- U. Khan, M. Khan, N. Malik, M. H. Cho and M. M. Khan, Bioprocess Biosyst. Eng., 2019, 42, 1–5 CrossRef PubMed.
- S. Swain, S. Bej, A. K. Bishoyi, B. R. Jali and R. N. Padhy, Naunyn-Schmiedeberg's Arch. Pharmacol., 2024, 397, 9123–9133 CrossRef CAS PubMed.
- S. Sonker, J. Pathak, V. K. Kannaujiya and R. P. Sinha, Can. J. Biotech., 2017, 1, 26 CrossRef.
- P. Mandhata, C. R. Sahoo, C. S. Mahanta and R. N. Padhy, Bioprocess Biosyst. Eng., 2021, 44, 1617–1626 CrossRef PubMed.
- M. M. El-Sheekh, M. T. Shabaan, L. Hassan and H. H. Morsi, Int. J. Environ. Health Res., 2022, 32, 616–627 CrossRef CAS PubMed.
- K. S. Siddiqi, A. Husen and R. A. K. Rao, J. Nanopart. Res., 2018, 20, 1–19 CrossRef.
- A. A. Amadu, K. A. deGraft-Johnson and G. K. Ameka, in Cyanobacteria-Recent Advances in Taxonomy and Applications, 2021, p. 25 Search PubMed.
- S. Dubey, S. Lohakar, S. J. Kulkarni and A. K. Goswami, in Exploring Nanomaterial Synthesis, Characterization, and Applications, IGI Global, Hershey, PA, USA, 2025, pp. 461–488 Search PubMed.
- J. Komárek, in Freshwater Algae of North America, ed. J. D. Wehr, R. G. Sheath and J. P. Kociolek, Academic Press, San Diego, CA, USA, 2nd edn, 2003, pp. 59–116 Search PubMed.
- M. D. Guiry and G. M. Guiry, in AlgaeBase. World-wide Electronic Publication, National University of Ireland, Galway, 2017 Search PubMed.
- A. M. Dolman, J. Rücker, F. R. Pick, J. Fastner, T. Rohrlack, U. Mischke and C. Wiedner, PLoS One, 2012, 7, e38757 CrossRef CAS PubMed.
- L. Vidal, A. Ballot, S. M. Azevedo, J. Padisák and M. Welker, in Toxic Cyanobacteria in Water, eds. I. Chorus and M. Welker, WHO/IWA, London, UK, 2nd edn, 2021, pp. 163– 211 Search PubMed.
- P. Durai, M. Batool and S. Choi, Mar. Drugs, 2015, 13, 4217–4230 CrossRef CAS PubMed.
- L. Wejnerowski, S. Cerbin and M. K. Dziuba, Zool. Stud., 2015, 54, 2 CrossRef PubMed.
- D. G. Adams and P. S. Duggan, J. Exp. Bot., 2008, 59, 1047–1058 CrossRef CAS PubMed.
- M. Devaprakash, R. Thirumalaivasan, N. Sivakumar and R. Shyamkumar, in Cyanobacteria, Academic Press, New York, NY, USA, 2024, pp. 425–489 Search PubMed.
- R. Chug and S. Mathur, Int. J. Eng. Res. Sci. Technol., 2013, 3, 49–53 Search PubMed.
- A. D. Samuel, J. D. Petersen and T. S. Reese, BMC Microbiol., 2001, 1, 1 CrossRef PubMed.
- E. Hoiczyk and A. Hansel, J. Bacteriol., 2000, 182, 1191–1199 CrossRef CAS PubMed.
- N. Schuergers and A. Wilde, Life, 2015, 5, 700–715 Search PubMed.
- V. Mimouni, L. Ulmann, V. Pasquet, M. Mathieu, L. Picot, G. Bougaran, J. P. Cadoret, A. Morant-Manceau and B. Schoefs, Curr. Pharm. Biotechnol., 2012, 13, 2733–2750 CAS.
- S. Sabat, S. Patra, S. Swain, S. Bej, A. K. Bishoyi, C. R. Sahoo and R. N. Padhy, ACS Omega, 2025, 10(23), 23957–23980 CrossRef PubMed.
- K. Gademann and C. Portmann, Curr. Org. Chem., 2008, 12, 326–341 CrossRef CAS.
- M. Mehdizadeh Allaf and H. Peerhossaini, Microorganisms, 2022, 10, 696 CrossRef CAS PubMed.
- D. B. Van de Waal, V. H. Smith, S. A. Declerck, E. C. Stam and J. J. Elser, Soil Ecol. Lett., 2014, 17, 736–742 CrossRef PubMed.
- M. Picardo, D. Filatova, O. Nunez and M. Farré, TrAC, Trends Anal. Chem., 2019, 112, 75–86 CrossRef CAS.
- B. Eisenhauer, S. Natoli, G. Liew and V. M. Flood, Nutrients, 2017, 9, 120 CrossRef PubMed.
- T. M. Mata, A. A. Martins and N. S. Caetano, Renewable Sustainable Energy Rev., 2010, 14, 217–232 CrossRef CAS.
- D. L. Nelson, A. L. Lehninger and M. M. Cox, in. Lehninger Principles of Biochemistry, Macmillan, New York, 2008 Search PubMed.
- J. M. Shick and W. C. Dunlap, Annu. Rev. Physiol., 2002, 64, 223–262 CrossRef CAS PubMed.
- Z. A. Popper, G. Michel, C. Hervé, D. S. Domozych, W. G. Willats, M. G. Tuohy, B. Kloareg and D. B. Stengel, Annu. Rev. Plant Biol., 2011, 62, 567–590 CrossRef CAS PubMed.
- C. Romay, R. Gonzalez, N. Ledon, D. Remirez and V. Rimbau, Curr. Protein Pept. Sci., 2003, 4, 207–216 CrossRef CAS PubMed.
- R. H. Tukey and C. P. Strassburg, Annu. Rev. Pharmacol. Toxicol., 2000, 40, 581–616 CrossRef CAS PubMed.
- A. A. Elzaawely, T. D. Xuan, H. Koyama and S. Tawata, Food Chem., 2007, 104, 1648–1653 Search PubMed.
- G. Vasas, G. Borbely, P. Nanasi and P. P. Nanasi, Mini-Rev. Med. Chem., 2010, 10, 946–955 CrossRef CAS PubMed.
- K. Walton and J. P. Berry, Mar. Drugs, 2016, 14, 73 CrossRef PubMed.
- C. Portmann, J. F. Blom, M. Kaiser, R. Brun, F. Jüttner and K. Gademann, J. Nat. Prod., 2008, 71, 1928–1932 Search PubMed.
- P. G. Becher, H. I. Baumann, K. Gademann and F. Jüttner, J. Appl. Phycol., 2009, 21, 103–110 CrossRef CAS.
- Y. Khatri, R. M. Hohlman, J. Mendoza, S. Li, A. N. Lowell, H. Asahara and D. H. Sherman, ACS Synth. Biol., 2020, 9, 1349–1360 CrossRef CAS PubMed.
- C. J. Knoot, R. M. Hohlman, D. H. Sherman and H. B. Pakrasi, ACS Synth. Biol., 2019, 8, 1941–1951 CrossRef CAS PubMed.
- S. Mo, A. Krunic, G. Chlipala and J. Orjala, J. Nat. Prod., 2009, 72, 894–899 Search PubMed.
- R. Nagarajan, in Nanoparticles: Building Blocks for Nanotechnology, ed. V. M. Rotello, Springer US, Boston, MA, 2004, pp. 27–45 Search PubMed.
- D. Dhakal, S. Kokkaliari, G. M. Rubin, V. J. Paul and Y. Ding, J. Nat. Prod., 2022, 86, 85–93 Search PubMed.
- D. Kallifidas, D. Dhakal, M. Chen, Q. Y. Chen, S. Kokkaliari, N. A. Colon Rosa, R. Ratnayake, S. D. Bruner, V. J. Paul, Y. Ding and H. Luesch, Org. Lett., 2024, 26, 1321–1325 Search PubMed.
- N. J. Al-Mousawi, I. J. Al-Assadi and M. J. Al-Aarajy, Biomed. Chem. Sci., 2023, 2, 119–124 Search PubMed.
- S. S. Swain, S. K. Paidesetty and R. N. Padhy, Biomed. Pharmacother., 2017, 90, 760–776 Search PubMed.
- J. Vestola, T. K. Shishido, J. Jokela, D. P. Fewer, O. Aitio, P. Permi, M. Wahlsten, H. Wang, L. Rouhiainen and K. Sivonen, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E1909–E1917 Search PubMed.
- G. Trimurtulu, I. Ohtani, G. M. Patterson, R. E. Moore, T. H. Corbett, F. A. Valeriote and L. Demchik, J. Am. Chem. Soc., 1994, 116, 4729–4737 CrossRef CAS.
- A. Montaser, M. Sivonen, H. Leinonen, J. Hepojoki, L. Ma, V.-P. Lehto and W. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 58434–58447 CrossRef PubMed.
- R. Pathak, in Bioactive Peptides, CRC Press, 2021, pp. 447–467 Search PubMed.
- R. E. Moore, J. Org. Chem., 1996, 61, 3125–3129 Search PubMed.
- J. H. Jung, R. E. Moore and G. M. Patterson, J. Org. Chem., 1991, 56, 6701–6703 Search PubMed.
- V. Mimouni, L. Ulmann, V. Pasquet, M. Mathieu, L. Picot, G. Bougaran, J. P. Cadoret, A. Morant-Manceau and B. Schoefs, Curr. Pharm. Biotechnol., 2012, 13, 2733–2750 Search PubMed.
- H. T. Bui, R. Jansen, H. T. Pham and S. Mundt, J. Nat. Prod., 2007, 70, 499–503 Search PubMed.
- V. Singh, in Green Chemistry Approaches to Environmental Sustainability, Elsevier, Amsterdam, The Netherlands, 2024, pp. 239–259 Search PubMed.
- J. B. MacMillan and T. F. Molinski, J. Nat. Prod., 2005, 68, 604–606 CrossRef CAS PubMed.
- D. Borah, N. Das, P. Sarmah, K. Ghosh, M. Chandel, J. Rout, P. Pandey, N. N. Ghosh and C. R. Bhattacharjee, Mater. Today Commun., 2023, 34, 105110 CrossRef CAS.
- S. Tripathy, J. Rodrigues and N. G. Shimpi, in Nanobiomaterials: Perspectives in Medical Applications, Diagnosis and Treatment of Diseases, 2023, 145, 92–130 Search PubMed.
- A. Rahman, S. Kumar, A. Bafana, J. Lin, S. A. Dahoumane and C. Jeffryes, Molecules, 2019, 24, 3506 CrossRef CAS PubMed.
- F. Kang, P. J. Alvarez and D. Zhu, Environ. Sci. Technol., 2014, 48, 316–322 CrossRef CAS PubMed.
- A. H. Al-Badwy, A. M. Khalil, A. H. Bashal and R. Kebeish, Microb. Cell Fact., 2023, 22, 247 CrossRef CAS PubMed.
- M. F. Lengke, B. Ravel, M. E. Fleet, G. Wanger, R. A. Gordon and G. Southam, Environ. Sci. Technol., 2006, 40, 6304–6309 CrossRef CAS PubMed.
- R. A. Hamouda, M. H. Hussein, R. A. Abo-Elmagd and S. S. Bawazir, Sci. Rep., 2019, 9, 13071 CrossRef PubMed.
- R. S. Hamida, M. A. Momenah, M. Alkhateeb, H. Alfassam and M. M. Bin-Meferij, ACS Omega, 2025, 10, 34123–34141 CrossRef CAS PubMed.
- S. Husain, S. Afreen, H. Yasin, D. Yasin, B. Afzal and T. Fatma, J. Microbiol. Methods, 2019, 162, 77–82 CrossRef CAS PubMed.
- A. L. Hanna, H. Hamouda, H. Goda, T. Elsayed and M. Sadik, Bioinorg. Chem. Appl., 2022, 2022, 9072508 CrossRef PubMed.
- N. E. El-Naggar, M. H. Hussein and A. A. El-Sawah, Sci. Rep., 2017, 7, 13348 CrossRef PubMed.
- A. Harutyunyan, L. Gabrielyan, A. Aghajanyan, S. Gevorgyan, R. Schubert, C. Betzel, W. Kujawski and L. Gabrielyan, ACS Omega, 2024, 9, 29410–29421 CrossRef CAS PubMed.
- N. S. Younis, M. E. Mohamed and E. Semary, Mar. Drugs, 2022, 20, 56 CrossRef CAS PubMed.
- A. Elgamouz, H. Idriss, C. Nassab, A. Bihi, K. Bajou, K. Hasan, M. A. Haija and S. P. Patole, Nanomaterials, 2020, 10, 1861 CrossRef CAS PubMed.
- S. Ahmad, S. Ahmad, Q. Xu, I. Khan, X. Cao, R. Yang and H. Yan, Front. Bioeng. Biotechnol., 2024, 11, 1320739 CrossRef PubMed.
- S. Ibrahim, Z. Ahmad, M. Manzoor, M. Mujahid, Z. Faheem and A. Adnan, Sci. Rep., 2021, 11, 1941 CrossRef PubMed.
- H. E. Abdelmoneim, T. H. Taha, M. S. Elnouby and H. M. Abushady, Microb. Cell Fact., 2022, 21, 122 CrossRef PubMed.
- M. Gastelum-Cabrera, P. Méndez-Pfeiffer, M. Ballesteros-Monrreal, B. Velasco-Rodríguez, P. Martinez-Flores, S. Silva-Bea, V. Domínguez-Arca, G. Prieto, S. Barbosa, A. Otero, P. Taboada and J. Juárez, Pharmaceutics, 2025, 17, 5672 CrossRef PubMed.
- A. Singh, B. Gaud and S. Jaybhaye, Mater. Sci. Energy Technol., 2020, 3, 288–293 Search PubMed.
- M. Ataeian, A. Vadlamani, M. Haines, D. Mosier, X. Dong, M. Kleiner, M. Strous and A. Hawley, iScience, 2021, 24, 103405 CrossRef CAS PubMed.
- R. Schuurmans, P. Van Alphen, J. Schuurmans, H. Matthijs and K. Hellingwerf, PLoS One, 2015, 10, e0139061 CrossRef PubMed.
- M. Madsen, G. Hamilton, P. Herzyk and A. Amtmann, Front. Bioeng. Biotechnol., 2021, 8, 619055 CrossRef PubMed.
- C. Grettenberger, R. Abou-Shanab and T. Hamilton, Microb. Biotechnol., 2024, 17, e14519 CrossRef CAS PubMed.
- H. Grimm, P. Erlsbacher, H. Medipally, L. Malihan-Yap, L. Sovic, J. Zoehrer, S. Kosourov, Y. Allahverdiyeva, C. Paul and R. Kourist, Green Chem., 2025, 27, 2907–2920 RSC.
- N. E.-A. El-Naggar, M. H. Hussein and A. A. El-Sawah, Sci. Rep., 2018, 8, 8925 CrossRef PubMed.
- N. Abdel-Raouf, E. N. Sholkamy, N. Bukhari, N. M. Al-Enazi, K. I. Alsamhary, S. H. Al-Khiat and I. B. Ibraheem, Environ. Res., 2022, 204, 111630 Search PubMed.
- D. M. Ali, M. Sasikala, M. Gunasekaran and N. Thajuddin, Dig. J. Nanomater. Biostruct., 2011, 6, 385–390 Search PubMed.
- P. Rauwel, S. Küünal, S. Ferdov and E. Rauwel, Adv. Mater. Sci. Eng., 2015, 2015, 682749 Search PubMed.
- I. Ahamad, N. Aziz, A. Zaki and T. Fatma, J. Appl. Phycol., 2021, 33, 829–841 CrossRef CAS.
- A. Goldstein, Y. Soroka, M. Frušić-Zlotkin, I. Popov and R. Kohen, J. Microsc., 2014, 256, 237–247 CrossRef CAS PubMed.
- M. E. El-Naggar, A. M. Abdelgawad, D. A. Elsherbiny, W. A. El-shazly, S. Ghazanfari, M. S. Abdel-Aziz and Y. K. Abd-Elmoneam, J. Cluster Sci., 2020, 31, 1349–1362 CrossRef CAS.
- A. K. Bishoyi, C. P. Mandhata, C. R. Sahoo, P. Samal, D. Dubey, B. R. Jali, A. M. Alamri, M. S. Khan and R. N. Padhy, Cell Biochem. Funct., 2025, 43, e70043 Search PubMed.
- A. Defaei, M. Shahrian and J. Karimi, S. Afr. J. Chem. Eng., 2025 Search PubMed.
- B. Nowruzi, H. Beyranvand and J. Shahid, Sadoughi Univ. Med. Sci., 2024 Search PubMed.
- A. K. Bhardwaj and R. Naraian, 3 Biotech, 2021, 11, 445 CrossRef PubMed.
- M. P. Patil and G. D. Kim, Colloids Surf., B, 2018, 172, 487–495 CrossRef CAS PubMed.
- R. M. Alharbi, N. Abdel-Raouf, R. Omar, S. Hassan, K. N. Elsayed and I. B. Ibraheem, Int. J. Nanomed., 2023, 18, 649–663 Search PubMed.
- S. Giri and A. Mukherjee, J. Environ. Chem. Eng., 2021, 9, 105978 CrossRef CAS.
- H. A. Hussein, D. F. Syamsumir, S. A. Radzi, J. Y. Siong, N. A. Zin and M. A. Abdullah, Bioresour. Bioprocess., 2020, 7, 39 CrossRef.
- U. Çiğdem, B. Yılmaz Öztürk and İ. Dağ, ChemistrySelect, 2024, 9, e202304895 CrossRef.
- N. A. Semary and H. M. Bakir, J. Adv. Res., 2022, 39, 277–290 Search PubMed.
- S. Gevorgyan, R. Schubert, S. Falke, K. Lorenzen, K. Trchounian and C. Betzel, Sci. Rep., 2022, 12, 14077 CrossRef CAS PubMed.
- R. S. Hamida, N. E. Abdelmeguid, M. A. Ali, M. M. Bin-Meferij and M. I. Khalil, Int. J. Nanomed., 2020, 15, 49–63 CrossRef CAS PubMed.
- Y. N. Slavin and H. Bach, Nanomaterials, 2022, 12, 4470 CrossRef CAS PubMed.
- S. Vijayan, K. Divya, S. Varghese and M. S. Jisha, J. Bionanosci., 2020, 10, 974–982 CrossRef.
- S. S. Al-Zahrani and S. M. Al-Garni, Biocatal. Agric. Biotechnol., 2023, 51, 102769 CrossRef CAS.
- G. A. Ismail, M. M. El-Sheekh, R. M. Samy and S. F. Gheda, Bionanoscience, 2021, 11, 355–370 CrossRef.
- I. Ahmad, N. Aziz, A. Zaki and T. Fatma, J. Appl. Phycol., 2021, 33, 829–841 CrossRef.
- D. Naidoo, A. Roy, P. Kar, T. Mutanda and A. Anandraj, J. Biomol. Struct. Dyn., 2021, 39, 6218–6230 CrossRef CAS PubMed.
- E. F. El-Belely, M. M. S. Farag, H. A. Said, A. S. Amin, E. Azab, A. A. Gobouri and A. Fouda, Nanomaterials, 2021, 11, 95 CrossRef CAS PubMed.
- C. P. Mandhata, A. K. Bishoyi, C. R. Sahoo, S. K. Swain, S. Bej, B. R. Jali, R. K. Meher, D. Dubey and R. N. Padhy, Bioprocess Biosyst. Eng., 2023, 46, 1341–1350 Search PubMed.
- I. Ahamad, M. Nadeem, M. Rizvi and T. Fatma, Discover Nano, 2025, 20, 12 CrossRef PubMed.
- M. Ovais, A. T. Khalil, M. Ayaz, I. Ahmad, S. K. Nethi and S. Mukherjee, Int. J. Mol. Sci., 2018, 19, 4100 CrossRef PubMed.
- S. Ghosh, R. Ahmad, K. Banerjee, M. Alajmi and S. Rahman, Front. Microbiol., 2021, 12, 638068 Search PubMed.
- F. Mohamed and H. Chenia, Int. J. Mol. Sci., 2025, 26, 3306 Search PubMed.
- A. Doghish, A. Hashem, A. Shehabeldine, A. Sallam, G. El-Sayyad and S. Salem, J. Drug Delivery Sci. Technol., 2022, 76, 103874 CrossRef.
- A. Hashem, M. A. Abdel-Maksoud, S. Fatima, S. Almutairi, M. Ghorab, A. El-Batal and G. El-Sayyad, Sci. Rep., 2025, 15, 12345 CrossRef PubMed.
- D. Karageorgou, P. Zygouri, T. Tsakiridis, M. Hammami, N. Chalmpes, M. Subrati, I. Sainis, K. Spyrou, P. Katapodis, D. Gournis and H. Stamatis, Nanomaterials, 2022, 12, 2296 Search PubMed.
- A. Fouda, A. M. Eid, M. A. Abdel-Rahman, E. F. El-Belely, M. A. Awad, S. E. Hassan, Z. E. Al-Faifi and M. F. Hamza, Front. Bioeng. Biotechnol., 2022, 10, 849921 CrossRef PubMed.
- M. Wypij, T. Jędrzejewski, J. Trzcińska-Wencel, M. Ostrowski, M. Rai and P. Golińska, Front. Microbiol., 2021, 12, 632505 Search PubMed.
- I. Ahmad, N. Aziz, A. Zaki and T. Fatma, J. Appl. Phycol., 2021, 33, 829–841 Search PubMed.
- D. Naidoo, A. Roy, P. Kar, T. Mutanda and A. Anandraj, J. Biomol. Struct. Dyn., 2021, 39, 6218–6230 Search PubMed.
- Z. Ebrahimzadeh, A. Salehzadeh, A. S. Naeemi and A. Jalali, Bull. Mater. Sci., 2020, 43, 1–7 CrossRef.
- M. Huang, A. A. Keller, X. Wang, L. Tian, B. Wu, R. Ji and L. Zhao, Environ. Sci. Technol., 2020, 54, 15996–16005 CrossRef CAS PubMed.
- M. M. El-Sheekh, L. H. Hassan and H. H. Morsi, Rend. Lincei Sci. Fis. Nat., 2021, 32, 747–759 CrossRef.
- S. Bej, S. Swain, A. K. Bishoyi, C. R. Sahoo, B. R. Jali and R. N. Padhy, Naunyn Schmiedeberg’s Arch. Pharmacol., 2025, 398, 15363–15375 CrossRef CAS PubMed.
- T. Neuhof, P. Schmieder, K. Preussel, R. Dieckmann, H. Pham, F. Bartl and H. Von Döhren, J. Nat. Prod., 2005, 68, 695–700 CrossRef CAS PubMed.
- H. H. Senousy, S. Abd Ellatif and S. Ali, Environ. Sci. Pollut. Res., 2020, 27, 18463–18474 CrossRef CAS PubMed.
- M. Mmola, M. L. Roes-Hill, K. Durrell, J. J. Bolton, N. Sibuyi, M. E. Meyer, D. R. Beukes and E. Antunes, Molecules, 2016, 21, 1633 CrossRef CAS PubMed.
- Y. N. Tan, K. H. Lee and X. Su, Anal. Chem., 2011, 83, 4251–4257 CrossRef CAS PubMed.
- S. Nisha, R. S. Sachan, A. Singh, A. Karnwal, A. Shidiki and G. Kumar, Front. Nanotechnol., 2024, 6, 1490980 CrossRef.
- M. Aamir, S. Hassan, A. H. Khan, M. Ibrar, S. Sarwar, K. Mahmood, N. Khan, D. A. Aljumaiah, A. H. Aldiaram, A. K. Alameer and A. J. Alsalman, J. Sol-Gel Sci. Technol., 2025, 1–3 Search PubMed.
- J. Singh, T. Dutta, K. H. Kim, M. Rawat, P. Samddar and P. Kumar, J. Nanobiotechnol., 2018, 16, 84 CrossRef CAS PubMed.
- C. P. Mandhata, C. R. Sahoo and R. N. Padhy, Biol. Trace Elem. Res., 2022, 200, 5307–5327 CrossRef CAS PubMed.
- A. Guerreiro, M. A. Andrade, C. Menezes, F. Vilarinho and E. Dias, Toxins, 2020, 12, 548 CrossRef CAS PubMed.
- P. Aletayeb, P. Ghadam and P. Mohammadi, IET Nanobiotechnol., 2020, 14, 707–713 CrossRef PubMed.
- A. Haider and I. K. Kang, Adv. Mater. Sci. Eng., 2015, 2015, 165257 Search PubMed.
- L. Rizzello, R. Cingolani and P. P. Pompa, Nanomedicine, 2013, 8, 807–821 CrossRef CAS PubMed.
- S. Kokura, O. Handa, T. Takagi, T. Ishikawa, Y. Naito and T. Yoshikawa, Nanomed.: Nanotechnol. Biol. Med., 2010, 6, 570–574 CrossRef CAS PubMed.
- T. S. Le, T. H. Dao, D. C. Nguyen, H. C. Nguyen and I. L. Balikhin, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2015, 6, 015016 CAS.
- V. P. Giri, P. Shukla, A. Tripathi, P. Verma, N. Kumar, S. Pandey, C. O. Dimkpa and A. Mishra, Plants, 2023, 12, 815 CrossRef CAS PubMed.
- A. Kamyshny and S. Magdassi, Chem. Soc. Rev., 2019, 48, 1712–1740 RSC.
- M. D'Acunto, P. Cioni, E. Gabellieri and G. Presciuttini, Nanotechnology, 2021, 32, 192001 CrossRef PubMed.
- F. W. Fendi, W. M. Mukhtar and M. Abdullah, Sens. Actuators, A, 2023, 362, 114617 CrossRef CAS.
- K. Siddique, S. Hussain, M. Shahid, T. Shahzad, F. Mahmood, O. Sadak, S. Gunasekaran, T. Kamal and I. Ahmad, in Catalytic Degradation of Methylene Blue and 4- Nitrophenol Dyes, 2023 Search PubMed.
- S. Kaliamurthi, G. Selvaraj, Z. E. Çakmak and T. Çakmak, Phycologia, 2016, 55, 568–576 CrossRef CAS.
- G. Sharma, N. D. Jasuja, M. Kumar and M. I. Ali, J. Nanotechnol., 2015, 2015, 132675 Search PubMed.
- S. Rashad, G. A. El-Chaghaby and M. A. Elchaghaby, Egypt. J. Aquat. Biol. Fish., 2019, 23, 261–266 CrossRef.
- D. Ye, Y. Ding, Y. Duan, J. Su, Z. Yin and Y. A. Huang, Small, 2018, 14, 1703521 CrossRef PubMed.
- S. A. Carabineiro, in Catalysis for a Sustainable Environment: Reactions, Processes and Applied Technologies, 2024, pp. 481–514 Search PubMed.
- R. Sardar, A. M. Funston, P. Mulvaney and R. W. Murray, Langmuir, 2009, 25, 13840–13851 CrossRef CAS PubMed.
- X. Chen, L. Zuo, W. Fu, Q. Yan, C. Fan and H. Chen, Sol. Energy Mater. Sol. Cells, 2013, 111, 1–8 CrossRef CAS.
- V. Rizzi, J. Gubitosa, P. Fini, S. Nuzzo, A. Agostiano and P. Cosma, J. Photochem. Photobiol., B, 2021, 224, 112309 CrossRef CAS PubMed.
- D. J. de Aberasturi, A. B. Serrano-Montes and L. M. Liz-Marzán, Adv. Opt. Mater., 2015, 3, 602–617 CrossRef.
- Y. He, S. Xiao, T. Dong, P. Nie and J. Su, Int. J. Mol. Sci., 2019, 20, 2817 CrossRef CAS PubMed.
- M. Cordeiro, F. F. Carlos, P. Pedrosa, A. Lopez and P. V. Baptista, Diagnostics, 2016, 6, 43 CrossRef PubMed.
- V. Gupta, N. K. Verma and K. K. Choudhary, in Cyanobacterial Lifestyle and its Applications in Biotechnology, Academic Press, New York, NY, USA, 2022, pp. 23–40 Search PubMed.
- S. Yadav, S. Chandra, A. Yadav, A. Kumar and M. Awasthi, in Cyanobacteria- Recent Advances and New Perspectives, IntechOpen, London, UK, 2022 Search PubMed.
- A. Lateef, M. A. Akande, I. A. Adelere, E. B. Gueguim-Kana and L. S. Beukes, Biotechnol. Biotechnol. Equip., 2016, 30, 1–8 CrossRef.
- C. Parisi, M. Vigani and E. Rodríguez-Cerezo, Nano Today, 2015, 10, 124–127 CrossRef CAS.
- N. O. San Keskin, N. K. Kılıç, G. Dönmez and T. Tekinay, J. Nano Res., 2016, 40, 120–127 Search PubMed.
- M. Ciani and A. Adessi, Front. Microbiol., 2023, 14, 1166612 CrossRef PubMed.
- P. Agarwal, R. Soni, P. Kaur, A. Madan, R. Mishra, J. Pandey, S. Singh and G. Singh, Front. Microbiol., 2022, 13, 939347 CrossRef PubMed.
- A. U. Khan, M. Khan, N. Malik, M. H. Cho and M. M. Khan, Bioprocess Biosyst. Eng., 2019, 42, 1–5 Search PubMed.
- S. Kumar, D. Kang, V. H. Nguyen, N. Nasir, H. Hong, M. Kim, D. C. Nguyen, Y. Lee, J. Lee, N. Lee and Y. Seo, ACS Appl. Mater. Interfaces, 2021, 13, 40976–40985 CrossRef CAS PubMed.
- S. Singh, S. K. Pandey and N. Vishwakarma, in Handbook of Functionalized Nanomaterials for Industrial Applications, Elsevier, 2020, pp. 717–730 Search PubMed.
- A. K. Tomer, T. Rahi, D. K. Neelam and P. K. Dadheech, Int. Microbiol., 2019, 22, 49–58 CrossRef CAS PubMed.
- S. Husain, S. Afreen, D. Yasin, B. Afzal and T. Fatma, J. Microbiol. Methods, 2019, 162, 77–82 Search PubMed.
- M. H. Sayadi, N. Salmani, A. Heidari and M. R. Rezaei, Surf. Interfaces, 2018, 10, 136–143 CrossRef CAS.
- S. Iravani, H. Korbekandi, S. V. Mirmohammadi and B. Zolfaghari, Res. Pharm. Sci., 2014, 9, 385–406 CAS.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759–1782 Search PubMed.
- A. Jain and S. K. Jain, Eur. J. Pharm. Sci., 2008, 35, 404–416 Search PubMed.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS PubMed.
- J. Liang, J. Wang, X. Shen, B. Lu, G. Li, H. Wang, H. Wang and L. Yuan, J. Mater. Chem. B, 2022, 10, 4203–4215 RSC.
- S. Das, A. Tripathi and M. M. Ghangrekar, Chemosphere, 2024, 352, 141392 CrossRef CAS PubMed.
- R. R. Patel, S. K. Singh and M. Singh, Mater. Adv., 2023, 4, 1831–1849 RSC.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 Search PubMed.
- A. Roy, A. Sharma, S. Yadav, L. T. Jule and R. Krishnaraj, Bioinorg. Chem. Appl., 2021, 2021, 1764647 Search PubMed.
- H. Sarma, S. Deka and S. K. Panda, Environ. Nanotechnol., Monit. Manage., 2020, 14, 100333 Search PubMed.
- M. P. Mishra and R. N. Padhy, J. Appl. Biomed., 2018, 16, 120–125 CrossRef.
- R. Aguilar-Garay, L. Lara-Ortiz, M. Campos-López, D. González-Rodriguez, M. Gamboa-Lugo, J. Mendoza-Pérez, Á. Anzueto-Ríos and D. Nicolás-Álvarez, Pharmaceuticals, 2024, 17, 1134 CrossRef CAS PubMed.
- X. Jiang, S. Khan, A. Dykes, E. Stulz and X. Zhang, Molecules, 2025, 30, 3104 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |