 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Theoretical study on the generation of Criegee intermediates from the ozonolysis of trifluoropropene (CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) CH2)
CH2)
Yunju Zhang *a,
Meilian Zhaob,
Cen Yao*c,
Zhiguo Wanga and
Yuxi Suna
*a,
Meilian Zhaob,
Cen Yao*c,
Zhiguo Wanga and
Yuxi Suna
aKey Laboratory of Photoinduced Functional Materials, Key Laboratory of Inorganic Materials Preparation and Synthesis, Mianyang Normal University, Mianyang 621000, PR China. E-mail: zhangyj010@nenu.edu.cn; Fax: +86 816 2200819; Tel: +86 816 2200064
bSchool of Public Health, Chengdu University of Traditional Chinese Medicine, ChengDu, PR China
cSchool-enterprise Joint Technology Innovation Laboratory of Novel Molecular Functional Materials of Jilin Province, Institute of Chemical and Industrial Bioengineering, Jilin Engineering Normal University, Changchun 130052, China. E-mail: yaoc773@nenu.edu.cn
First published on 15th December 2025
Abstract
The O3-initiated degradation mechanisms of trifluoropropene (CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) were studied using density functional theory (DFT). Three types of mechanisms were observed for the title reaction, namely, addition/elimination, H-abstraction and substitution. The computations showed that O3 with a C
CH2) were studied using density functional theory (DFT). Three types of mechanisms were observed for the title reaction, namely, addition/elimination, H-abstraction and substitution. The computations showed that O3 with a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond undergoes a 1,3-cycloaddition reaction to generate a primary ozone intermediate (POZ) with a relatively low free energy barrier, which then dissociates to generate an aldehyde group and carbonyl oxide, known as Criegee intermediates (CIs). Detailed analysis was conducted on the subsequent reactions of CIs. It is found that when a new type of CI-containing halogenated alkyl groups reacts with NO, NO2, CH2O, SO2, H2O and O2, its reaction pathway is singularly analogous to that of the general CI. The degradation total rate coefficient and the estimated lifetime are in accordance with the experimental results. The current calculation results are of great significance for the atmospheric chemistry of the ozone oxidation of unsaturated halogenated compounds.
C bond undergoes a 1,3-cycloaddition reaction to generate a primary ozone intermediate (POZ) with a relatively low free energy barrier, which then dissociates to generate an aldehyde group and carbonyl oxide, known as Criegee intermediates (CIs). Detailed analysis was conducted on the subsequent reactions of CIs. It is found that when a new type of CI-containing halogenated alkyl groups reacts with NO, NO2, CH2O, SO2, H2O and O2, its reaction pathway is singularly analogous to that of the general CI. The degradation total rate coefficient and the estimated lifetime are in accordance with the experimental results. The current calculation results are of great significance for the atmospheric chemistry of the ozone oxidation of unsaturated halogenated compounds.
1. Introduction
The international community has realized that chlorofluorocarbons (CFCs) can have adverse effects on stratospheric ozone and induce greenhouse effects, urging researchers to work towards finding environmentally friendly alternatives to these compounds.1,2 Due to the absence of chlorine atoms in hydrofluorocarbons (HFCs), they are not conducive to the establishment of a good chlorine-based catalytic ozone-depletion cycle.3 Therefore, saturated hydrofluorocarbons (HFCs) have proven to be the extensively employed alternatives to CFCs. Unsaturated HFCs are latent alternatives to CFCs and saturated HFCs. The oxidation of the unsaturated HFCs could be induced by reactions with OH,4–6 O3 (ref. 7–10) and NO3 (ref. 9, 11 and 12) radicals in the atmosphere. Ozone decomposition in the atmosphere is one of the common oxidation channels for unsaturated hydrocarbons, which plays a significant role in both the city and the countryside.13 The reaction between O3 and unsaturated HFCs has been proven to involve the addition of double-bond systems to generate an ozonide, which rapidly dissociates to generate carbonyl oxides (Criegee intermediates (CI)) and aldehyde compounds. The reactions involving propylene and halogenated propylenes have recently attracted substantial experimental attention.14–22 Only one research group has investigated the kinetics of the reaction of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 with O3. In 2005, Sulbaek Andersen et al.17 investigated the kinetics and mechanism of the reaction of CF3CH
CH2 with O3. In 2005, Sulbaek Andersen et al.17 investigated the kinetics and mechanism of the reaction of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 with O3 in an N2 atmosphere at 296 K by employing long-path-length FT-IR-smog chamber techniques. The measured rate constant of CF3CH
CH2 with O3 in an N2 atmosphere at 296 K by employing long-path-length FT-IR-smog chamber techniques. The measured rate constant of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 with O3 at 1 atm and 298 K is kCF3CH
CH2 with O3 at 1 atm and 298 K is kCF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2+O3 = (3.50 ± 0.30) × 10−19 cm3 per molecule per s. The atmospheric lifetime of CF3CH
CH2+O3 = (3.50 ± 0.30) × 10−19 cm3 per molecule per s. The atmospheric lifetime of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 is approximately 70 days due to the reaction with O3. Unfortunately, thus far, the reaction between O3 and CF3CH
CH2 is approximately 70 days due to the reaction with O3. Unfortunately, thus far, the reaction between O3 and CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 has not been well established theoretically. In the present work, to assess the oxidation reaction of CF3CH
CH2 has not been well established theoretically. In the present work, to assess the oxidation reaction of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 induced by ozone, the reaction mechanism and the secondary pathways in the presence of NO, NO2, CH2O, H2O, SO2 and O2 are investigated by employing theoretical methods, which have been extensively employed in the environmental area.23–31 The rate constants of primary reaction pathways are predicted using the Rice–Ramsperger–Kassel–Marcus (RRKM) theory32 to forecast the atmospheric lifetime.
CH2 induced by ozone, the reaction mechanism and the secondary pathways in the presence of NO, NO2, CH2O, H2O, SO2 and O2 are investigated by employing theoretical methods, which have been extensively employed in the environmental area.23–31 The rate constants of primary reaction pathways are predicted using the Rice–Ramsperger–Kassel–Marcus (RRKM) theory32 to forecast the atmospheric lifetime.
Up to date, very little is known about the final products and mechanisms of O3 with trifluoropropene (CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2). Moreover, the data on the Criegee intermediates with an aldehyde group and a trifluoromethyl group are scarce. Hence, we investigate the ozonolysis of trifluoropropene using quantum chemical methods. This work provides a comprehensive study on the reaction between trifluoropropene and ozone, which contributes to the literature on the mechanism of the primary step of ozone decomposition and the further reaction of CI. The research data in this work can help us evaluate the impact of these compounds on the atmosphere.
CH2). Moreover, the data on the Criegee intermediates with an aldehyde group and a trifluoromethyl group are scarce. Hence, we investigate the ozonolysis of trifluoropropene using quantum chemical methods. This work provides a comprehensive study on the reaction between trifluoropropene and ozone, which contributes to the literature on the mechanism of the primary step of ozone decomposition and the further reaction of CI. The research data in this work can help us evaluate the impact of these compounds on the atmosphere.
2 Computational methods
All the electronic structure computations for the CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + O3 reaction were performed using the Gaussian 09 program.33 The geometries of all stable points (including reactants, complexes, transition states, intermediates, and products) were optimized using the M06-2X34 method in combination with the 6-311++g(d,p) basis set,35,36 and the vibrational frequency analysis computations were performed at the same level. All the minima have real frequencies, while the transition states have only one imaginary frequency. Intrinsic reaction coordinate (IRC)37,38 computations were carried out to determine the transition states connecting the corresponding reactants and products. The CCSD(T)39//cc-pVTZ method was employed for all the species involved in the CF3CH
CH2 + O3 reaction were performed using the Gaussian 09 program.33 The geometries of all stable points (including reactants, complexes, transition states, intermediates, and products) were optimized using the M06-2X34 method in combination with the 6-311++g(d,p) basis set,35,36 and the vibrational frequency analysis computations were performed at the same level. All the minima have real frequencies, while the transition states have only one imaginary frequency. Intrinsic reaction coordinate (IRC)37,38 computations were carried out to determine the transition states connecting the corresponding reactants and products. The CCSD(T)39//cc-pVTZ method was employed for all the species involved in the CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + O3 reaction to obtain accurate single-point energies.
CH2 + O3 reaction to obtain accurate single-point energies.
3 Results and discussion
Taking the reaction between trifluoropropene and ozone as an example, we describe the generation mechanism of POZ (primary ozone intermediate) and CIs (Criegee intermediates). All the optimized structures of the reactants, products and intermediates and the reaction schematics for the trifluoropropene with ozone reaction are presented in Fig. 1. All the transition states involved in the CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + O3 reaction at the M06-2X/6-311++g(d,p) level of theory are presented in Fig. S1. As can be seen from Fig. 1, the reaction of CF3CH
CH2 + O3 reaction at the M06-2X/6-311++g(d,p) level of theory are presented in Fig. S1. As can be seen from Fig. 1, the reaction of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 with O3 has a total of seven pathways, which mainly include three reaction mechanisms, namely addition/elimination, substitution and hydrogen abstraction. The potential energy surface (PES) of the CF3CH
CH2 with O3 has a total of seven pathways, which mainly include three reaction mechanisms, namely addition/elimination, substitution and hydrogen abstraction. The potential energy surface (PES) of the CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + O3 reaction is presented in Fig. 2, and R in Fig. 2 represents the CF3CH
CH2 + O3 reaction is presented in Fig. 2, and R in Fig. 2 represents the CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + O3. Table 1 summarizes the relative energies, reaction enthalpies and Gibbs free energy of all the species involved in the trifluoropropene with ozone reaction.
CH2 + O3. Table 1 summarizes the relative energies, reaction enthalpies and Gibbs free energy of all the species involved in the trifluoropropene with ozone reaction.
 | ||
Fig. 1 Optimized geometries and reaction schematic of the trifluoropropene (CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) with ozone reaction. CH2) with ozone reaction. | ||
 | ||
Fig. 2 Potential energy surface for the reaction of trifluoropropene (CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) with ozone calculated at the CCSD(T)/cc-pVTZ//M06-2X/6-311++g(d,p) level of theory. CH2) with ozone calculated at the CCSD(T)/cc-pVTZ//M06-2X/6-311++g(d,p) level of theory. | ||
| Species | ΔE | ΔH | ΔG |
|---|---|---|---|
CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + O3 CH2 + O3 |
0.00 | 0.00 | 0.00 |
| CR1 | −28.47 | −28.10 | −18.50 |
| IM1 | −80.50 | −81.84 | −67.29 |
| IM2 | −67.81 | −68.25 | −56.38 |
| IM3 | −68.34 | −68.91 | −56.10 |
| TS1 | −21.35 | −22.17 | −8.89 |
| TS2 | −59.96 | −61.07 | −46.99 |
| TS3 | −52.75 | −53.80 | −39.84 |
| TS4 | −14.69 | −15.58 | −2.20 |
| TS5 | −16.64 | −17.32 | −4.35 |
| TS6 | −62.09 | −63.02 | −49.44 |
| TS7 | −53.09 | −53.38 | −41.90 |
| T-TS1 | 0.75 | 0.61 | 11.02 |
| T-TS2 | 1.83 | 1.67 | 12.43 |
| T-H-TS1 | 9.24 | 9.32 | 19.18 |
| T-H-TS2 | 9.42 | 9.56 | 19.27 |
| P1 | −75.46 | −72.52 | −72.37 |
| P2 | −68.37 | −68.10 | −66.98 |
| P3 | −65.81 | −65.29 | −65.91 |
| P4 | −68.08 | −68.07 | −67.28 |
| P5 | −2.41 | −1.90 | −0.72 |
| P6 | −1.28 | −0.74 | −0.22 |
| H-P1 + HO3 | 9.30 | 4.30 | 2.88 |
| H-P2 + HO3 | 7.05 | 2.12 | 0.55 |
3.1 Reaction mechanism of addition/elimination
As seen from Fig. 1, the two terminal oxygen atoms of the ozone molecule can simultaneously interact with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in CF3CH
C bond in CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 to generate the pre-complex (CR1). The relative energy of CR1 is −28.47 kcal mol−1, and the ΔG is −18.50 kcal mol−1. Subsequently, CR1 surmounts a free energy barrier of 9.61 kcal mol−1 through a transition state (TS1) to generate the primary ozone intermediate, a five-membered ring structure (IM1). The lengths of the two generated C–O bonds in TS1 are 2.20 and 2.22 Å. The two generated C–O bonds in IM1 have equal bond lengths of 1.41 Å. The generation of the intermediate (IM1) released a large amount of heat (81.84 kcal mol−1). The energy-rich intermediate (IM1) (80.50 kcal mol−1) could overcome the free energy barriers of 20.30 and 27.45 kcal mol−1 to generate P1 (CF3CHO + CH2OO (CI1)) and P2 (CF3CHOO (CI2) + CH2O), respectively. The transition states corresponding to the above two direct decomposition processes are TS2 and TS3, respectively. The lengths of the broken C–C and O–O bonds in TS2 and TS3 are 1.952 and 2.032 Å and 1.971 and 2.079 Å, respectively. The energies of P1 and P2 are lower than those of the reactants by 75.46 and 68.37 kcal mol−1, respectively. At the same time, 72.52 and 68.10 kcal mol−1 of heat are released to generate P1 and P2, respectively.
CH2 to generate the pre-complex (CR1). The relative energy of CR1 is −28.47 kcal mol−1, and the ΔG is −18.50 kcal mol−1. Subsequently, CR1 surmounts a free energy barrier of 9.61 kcal mol−1 through a transition state (TS1) to generate the primary ozone intermediate, a five-membered ring structure (IM1). The lengths of the two generated C–O bonds in TS1 are 2.20 and 2.22 Å. The two generated C–O bonds in IM1 have equal bond lengths of 1.41 Å. The generation of the intermediate (IM1) released a large amount of heat (81.84 kcal mol−1). The energy-rich intermediate (IM1) (80.50 kcal mol−1) could overcome the free energy barriers of 20.30 and 27.45 kcal mol−1 to generate P1 (CF3CHO + CH2OO (CI1)) and P2 (CF3CHOO (CI2) + CH2O), respectively. The transition states corresponding to the above two direct decomposition processes are TS2 and TS3, respectively. The lengths of the broken C–C and O–O bonds in TS2 and TS3 are 1.952 and 2.032 Å and 1.971 and 2.079 Å, respectively. The energies of P1 and P2 are lower than those of the reactants by 75.46 and 68.37 kcal mol−1, respectively. At the same time, 72.52 and 68.10 kcal mol−1 of heat are released to generate P1 and P2, respectively.
Starting from CR1, a terminal oxygen atom in the O3 molecule is added to the two carbon atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in CF3CH
C double bond in CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. Thereafter, the H atoms in the –CH2 and –CH– groups shift to the other terminal oxygen atom in the O3 molecule via transition states TS4 and TS5, respectively. The free energy barriers of CR1 → TS4 → IM2 and CR1 → TS5 → IM3 are 16.30 and 14.15 kcal mol−1, respectively. The energies of IM2 and IM3 are −67.81 and −68.34 kcal mol−1, respectively. IM2 and IM3 can generate the final products (P3: CF3CHCHO + HO2 and P4: CF3COCH2 + HO2) through TS6 and TS7, respectively, by breaking the O–O bond. The free energy barriers for IM2 → TS6 → P3 and IM2 → TS7 → P4 are 6.94 and 14.20 kcal mol−1, respectively. However, because of the relatively high energy barriers for the addition process, the contribution of these two addition/elimination channels to the reaction may be relatively small, which will be proven in Section 3.5 (see Fig. 11).
CH2. Thereafter, the H atoms in the –CH2 and –CH– groups shift to the other terminal oxygen atom in the O3 molecule via transition states TS4 and TS5, respectively. The free energy barriers of CR1 → TS4 → IM2 and CR1 → TS5 → IM3 are 16.30 and 14.15 kcal mol−1, respectively. The energies of IM2 and IM3 are −67.81 and −68.34 kcal mol−1, respectively. IM2 and IM3 can generate the final products (P3: CF3CHCHO + HO2 and P4: CF3COCH2 + HO2) through TS6 and TS7, respectively, by breaking the O–O bond. The free energy barriers for IM2 → TS6 → P3 and IM2 → TS7 → P4 are 6.94 and 14.20 kcal mol−1, respectively. However, because of the relatively high energy barriers for the addition process, the contribution of these two addition/elimination channels to the reaction may be relatively small, which will be proven in Section 3.5 (see Fig. 11).
3.2 Reaction mechanism of substitution
One of the end-group oxygen atoms in the O3 molecule is also added to the carbon atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in CF3CH
C double bond in CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, which is associated with the breaking of the O–O bond to generate P5 (CF3CHOCH2 + O2(1Δ)) and P6 (CF3CHCH2O + O2(1Δ)) via triplet transition states T-TS1 and T-TS2, respectively. The corresponding free energy barriers for R → T-TS1 → P5 and R → T-TS2 → P6 are 11.02 and 12.43 kcal mol−1, respectively; hence, the importance of this channel for the reaction may be at higher temperature.
CH2, which is associated with the breaking of the O–O bond to generate P5 (CF3CHOCH2 + O2(1Δ)) and P6 (CF3CHCH2O + O2(1Δ)) via triplet transition states T-TS1 and T-TS2, respectively. The corresponding free energy barriers for R → T-TS1 → P5 and R → T-TS2 → P6 are 11.02 and 12.43 kcal mol−1, respectively; hence, the importance of this channel for the reaction may be at higher temperature.
3.3 Reaction mechanism of hydrogen abstraction
It is worth mentioning that we did not find a transition state for hydrogen extraction on the singlet PES. While on the triplet PES, it was found that ozone can extract the hydrogen atoms on the –CH2 and –CH groups in the CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 molecule to generate products H-P1 + HO3 and H-P2 + HO3 via transition states T-H-TS1 and T-H-TS2, respectively. The two hydrogen abstraction reactions have free energy barriers of 19.18 and 19.27 kcal mol−1, respectively. The lengths of the broken C–H bonds in T-H-TS1 and T-H-TS2 are 1.281 and 1.289 Å, respectively, and the lengths of the generated O–H bonds are 1.212 and 1.202 Å, respectively. The energies of H-P1 + HO3 and H-P2 + HO3 are 9.30 and 7.05 kcal mol−1, respectively. Owing to the relatively high free energy barriers and unstable products, the contributions of the hydrogen extraction reaction channels on the triplet PES to the reaction are negligible.
CH2 molecule to generate products H-P1 + HO3 and H-P2 + HO3 via transition states T-H-TS1 and T-H-TS2, respectively. The two hydrogen abstraction reactions have free energy barriers of 19.18 and 19.27 kcal mol−1, respectively. The lengths of the broken C–H bonds in T-H-TS1 and T-H-TS2 are 1.281 and 1.289 Å, respectively, and the lengths of the generated O–H bonds are 1.212 and 1.202 Å, respectively. The energies of H-P1 + HO3 and H-P2 + HO3 are 9.30 and 7.05 kcal mol−1, respectively. Owing to the relatively high free energy barriers and unstable products, the contributions of the hydrogen extraction reaction channels on the triplet PES to the reaction are negligible.
3.4 Further reactions of the Criegee intermediates
The different Criegee intermediates resulting from the ozonation of trifluoropropene are named CI1 (CH2OO) and CI2 (CF3CHOO). CI1 is commonly found in the reaction of O3 with olefins. The optimized structures of CI1 and CI2 are shown in Fig. 3. The calculated bond lengths of C–O and O–O in CI1 (CH2OO) are consistent with the results observed in the previous investigation.40 CI2 is a new class of CIs with aldehyde groups, and it may have different properties. Therefore, this section presents the reaction mechanisms of Cl2 with NO, NO2, SO2, CH2O, H2O and O2. In CI2, the carbon atom in the –CH– group is the electrophilic reagent. Therefore, the nucleophilic atoms of NO, NO2, SO2, CH2O, H2O and O2 tend to attack the C atom, and the corresponding reaction proceeds, and the PESs are presented in Fig. 4–9 and S2–S7, respectively. All the transition states involved in the reactions of CF3CHOO with NO, NO2, CH2O, H2O, SO2 and O2 at the M06-2X/6-311++g(d,p) level of theory are presented in Fig. S8. These results indicate that the final products from the CI reactions are common atmospheric substances, such as saturated trifluoroacetaldehyde, trifluoroacetic acid and formic acid, as the content of SOA. The present study enriches the literature on the types of CIs and plays a significant role in controlling atmospheric chemical pollution, which will be discussed in detail in the following sections.The O atom in H2O reacts with the C atom in the –CH group, and one of the H atoms in H2O shifts to the terminal-O atom in CI2 at the same time to generate intermediate D-IM1 through transition state D-TS1 crossing a free energy barrier of 13.07 kcal mol−1. The lengths of the new formations (C–O and O–H bonds) and the broken O–H bond are 1.960, 1.734 and 1.008 Å, respectively. D-IM1, with the internal energy of 50.00 kcal mol−1, could decompose to D-P1 (CF3COOH + H2O) through transition state D-TS2. This involves the H atom in the OH group shifting to the middle-O atom in the –COOH group, accompanied by the breaking of the C–O bond through transition state D-TS2, surmounting a free energy barrier of 47.03 kcal mol−1. In addition, D-IM1 can be reacted with monomolecular water via a six-membered ring transition state (D-TS3) to generate D-P1, and this is a two-hydrogen migration process. The free energy barrier of D-IM1 → D-TS3 → D-P1 is 31.93 kcal mol−1, which is 15.10 kcal mol−1 lower than that of D-IM1 → D-TS2 → D-P1, which indicates that the water molecule plays a catalytic role in this process. D-IM1 can also continue to react with the monomolecular water via a six-membered ring (D-TS4) to generate D-P2 (CF3CHO + H2O2). The free energy of D-IM1 → D-TS4 → D-P2 (CF3CHO + H2O2) is 46.58 kcal mol−1, which is 0.45 kcal mol−1 lower than that of D-IM1 → D-TS2 → D-P1 (CF3COOH + H2O). This is also a double hydrogen migration process, in which water plays a catalytic role. Alternatively, CI2 can directly react with the water dimer to form D-IM1 + H2O via transition state D-TS5. In the reaction of CI2 with the water dimer, a water molecule acts as a reactant with CI2, and the other water molecule performs proton exchange. The free energy barrier of this process is 5.69 kcal mol−1, which is 7.38 kcal mol−1 lower than that of CI2 + H2O → D-TS1 → D-IM1. Therefore, the reaction of CI2 with the water dimer is more feasible. Furthermore, it is shown that water autocatalysis will occur in CI with H2O reactions and that the atmospheric resources of H2O2 are realized through these reactions.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. The results of Path F1 and Path F2 supply a resource for the generation of O3, which is crucial for understanding O3 pollution in specific regions. This result provides an important basis for formulating ozone pollution prevention and control measures and then taking corresponding emission reduction measures.
CH2. The results of Path F1 and Path F2 supply a resource for the generation of O3, which is crucial for understanding O3 pollution in specific regions. This result provides an important basis for formulating ozone pollution prevention and control measures and then taking corresponding emission reduction measures.
3.5 Kinetics
In order to understand reaction kinetics and compare the rate constants with experimental values, as well as guide the experimental investigation for the O3 + CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 reaction, the temperature dependence of the total and individual pathways is computed at 200–3000 K using the RRKM theory for the primary pathways (Schemes 1–3), which are considered in the following computations:
CH2 reaction, the temperature dependence of the total and individual pathways is computed at 200–3000 K using the RRKM theory for the primary pathways (Schemes 1–3), which are considered in the following computations:
Where * represents the vibrational excitation of the intermediate (IM1). Steady-state approximation for the energized intermediate (IM1*) leads to the following expressions:
 | (1) |
 | (2) |
 | (3) |
For Scheme 2
 | (4) |
 | (5) |
For Scheme 3
 | (6) |
 | (7) |
The microcanonical rate constant is calculated using the RRKM theory as follows:
| ki(E) = αiCiNi(E≠i)/hρj(Ej) | (8) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 are the total partition functions of O3 and CF3CH
CH2 are the total partition functions of O3 and CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, respectively; Q≠t and Q≠r are the translational and rotational partition functions of the entrance transition states. Na(E≠) is the number of states for the association transition state with excess energy (E≠) above the association barrier. ki(E) is the energy-specific rate constant for the ith channel, and Ci is the ratio of the overall rotational partition function of TSi and IMj; Ni(E≠i) is the number of states at the energy above the barrier height for transition state i; ρj(Ej) is the density of states at energy Ej of the intermediate. The density of states and the number of states are calculated using the extended Beyer–Swinehart algorithm.
CH2, respectively; Q≠t and Q≠r are the translational and rotational partition functions of the entrance transition states. Na(E≠) is the number of states for the association transition state with excess energy (E≠) above the association barrier. ki(E) is the energy-specific rate constant for the ith channel, and Ci is the ratio of the overall rotational partition function of TSi and IMj; Ni(E≠i) is the number of states at the energy above the barrier height for transition state i; ρj(Ej) is the density of states at energy Ej of the intermediate. The density of states and the number of states are calculated using the extended Beyer–Swinehart algorithm.
For the CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 + O3 reaction, the rate constants of the generation of P1, P2, P3, P4, IM1, IM2 and IM3 are denoted as kP1, kP2, kP3, kP4, kIM1, kIM2 and kIM3, respectively, and the total rate constants are denoted as ktot, ktot = kP1 + kP2 + kP3 + kP4 + kIM1 + kIM2 + kIM3, respectively. The temperature dependences of the total rate constants (ktot) and individual rate constants (kP1, kP2, kP3, kP4, kIM1, kIM2 and kIM3) at 760 torr are presented in Fig. 10. The computed ktot at 298 K and 760 torr is 1.50 × 10−19 cm3 per molecule per s, which is overestimated almost two times at 298 K relative to the data reported by Sulbaek Andersen et al.17 in 2005 (ktot = (3.50 ± 0.30) × 10−19 cm3 per molecule per s). The difference in the above result may be due to the bath gas, experimental conditions and measurement technique. The total rate constants and the rate constants for the generation of products P1, P2, P3 and P4, as well as collisional stabilization channels, increase at first but decrease rapidly with increasing temperatures. The branching ratios for the primary products are also displayed in Fig. 11. The primary product is P1 (CF3CHO + CH2OO (CI1)), and the pathway for generating P2 (CF3CHOO (CI2) + CH2O) contributes to the reaction to some extent over the entire temperature range. The contribution of the pathways for generating IM1, IM2, IM3, P3, and P4 to the reaction is negligible.
CH2 + O3 reaction, the rate constants of the generation of P1, P2, P3, P4, IM1, IM2 and IM3 are denoted as kP1, kP2, kP3, kP4, kIM1, kIM2 and kIM3, respectively, and the total rate constants are denoted as ktot, ktot = kP1 + kP2 + kP3 + kP4 + kIM1 + kIM2 + kIM3, respectively. The temperature dependences of the total rate constants (ktot) and individual rate constants (kP1, kP2, kP3, kP4, kIM1, kIM2 and kIM3) at 760 torr are presented in Fig. 10. The computed ktot at 298 K and 760 torr is 1.50 × 10−19 cm3 per molecule per s, which is overestimated almost two times at 298 K relative to the data reported by Sulbaek Andersen et al.17 in 2005 (ktot = (3.50 ± 0.30) × 10−19 cm3 per molecule per s). The difference in the above result may be due to the bath gas, experimental conditions and measurement technique. The total rate constants and the rate constants for the generation of products P1, P2, P3 and P4, as well as collisional stabilization channels, increase at first but decrease rapidly with increasing temperatures. The branching ratios for the primary products are also displayed in Fig. 11. The primary product is P1 (CF3CHO + CH2OO (CI1)), and the pathway for generating P2 (CF3CHOO (CI2) + CH2O) contributes to the reaction to some extent over the entire temperature range. The contribution of the pathways for generating IM1, IM2, IM3, P3, and P4 to the reaction is negligible.
 | ||
| Fig. 10 Temperature dependence of the total and individual rate constants for the trifluoropropene with ozone reaction at 760 torr N2. | ||
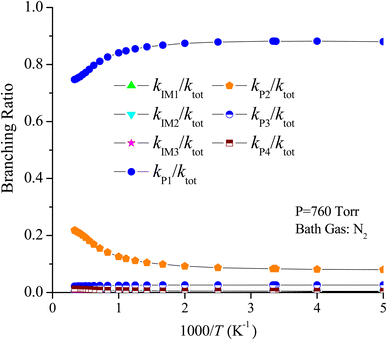 | ||
| Fig. 11 Branching ratios of the important product channels for the trifluoropropene with ozone reaction in the temperature range of 200–3000 K at 760 torr pressure of N2. | ||
We also compute the rate constants for CI2 (CF3CHOO) with a variety of atmospheric species (NO, NO2, CH2O, H2O, SO2 and O2) at 298 K and 1 atm using the RRKM theory. The detailed computation process is similar to the title reaction. The rate constants for CI2 with NO, NO2, CH2O, H2O, SO2 and O2 are 5.21 × 10−16 cm3 per molecule per s, 1.22 × 10−19 cm3 per molecule per s, 7.25 × 10−12 cm3 per molecule per s, 3.14 × 10−18 cm3 per molecule per s, 3.03 × 10−11 cm3 per molecule per s, 3.11 × 10−9 cm3 per molecule per s, respectively. Therefore, the CI2 with O2 reaction is the fastest path, followed by those with SO2 and CH2O. Previous studies on the kinetics of the Criegee intermediate (CF3CHOO, CH2OO and (CH3)2COO) with a variety of atmospheric species (NO, NO2, CH2O, H2O, SO2 and O2)51,52 are provided in the SI (Table S2) and constitute valid evidence for assessing the transport and degradation of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. As shown in Table S2, because the CF3 radical with relatively high electronegativity strongly attracts electrons located at the C
CH2. As shown in Table S2, because the CF3 radical with relatively high electronegativity strongly attracts electrons located at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, the electron density on the C
C double bond, the electron density on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond is reduced. This leads to a decrease in the reactivity of the C atom of the C
C double bond is reduced. This leads to a decrease in the reactivity of the C atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond attack on the O atom of O3; however, the –(CH3)2 group is the donor species, which increases the rate constants. Thus, theoretically, the trend of the reaction rate constant is k((CH3)2COO) > k(CH2OO) > k(CF3CH
C double bond attack on the O atom of O3; however, the –(CH3)2 group is the donor species, which increases the rate constants. Thus, theoretically, the trend of the reaction rate constant is k((CH3)2COO) > k(CH2OO) > k(CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), which is quantitatively in line with the experimental results.
CH2), which is quantitatively in line with the experimental results.
The atmospheric lifetime of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 induced by O3 can be computed using the following formula: τ = (ktot[O3])−1, where ktot is the total rate coefficient for CF3CH
CH2 induced by O3 can be computed using the following formula: τ = (ktot[O3])−1, where ktot is the total rate coefficient for CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 with O3 at 298 K (1.50 × 10−19 cm3 per molecule per s), and [O3] is the atmospheric concentration of the ozone. The average concentration of O3 is 1.00 × 1012 molecule per cm3.53 At a normal temperature and pressure, the atmospheric lifetime of CF3CH
CH2 with O3 at 298 K (1.50 × 10−19 cm3 per molecule per s), and [O3] is the atmospheric concentration of the ozone. The average concentration of O3 is 1.00 × 1012 molecule per cm3.53 At a normal temperature and pressure, the atmospheric lifetime of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 induced by O3 is estimated to be 77.16 days, which is consistent with the experimental data (70 days). It is observed that CF3CH
CH2 induced by O3 is estimated to be 77.16 days, which is consistent with the experimental data (70 days). It is observed that CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 can be degraded to relatively small molecules in the atmosphere.
CH2 can be degraded to relatively small molecules in the atmosphere.
4. Conclusion
In this study, the ozonation reaction mechanism of trifluoropropene was investigated using quantum chemistry methods. Addition/elimination, H-extraction and substitution mechanisms were discovered in the reaction of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 with O3, and the addition/elimination mechanism was dominant. The computed rate constants and atmospheric lifetime at a normal temperature and pressure are 1.50 × 10−19 cm3 per molecule per s and 77.16 days, respectively, which are consistent with the experimental data ((3.50 ± 0.30) × 10−19 cm3 per molecule per s and 70 days).
CH2 with O3, and the addition/elimination mechanism was dominant. The computed rate constants and atmospheric lifetime at a normal temperature and pressure are 1.50 × 10−19 cm3 per molecule per s and 77.16 days, respectively, which are consistent with the experimental data ((3.50 ± 0.30) × 10−19 cm3 per molecule per s and 70 days).
We also studied the reaction mechanism between the Criegee intermediates and atmospheric substances (NO, NO2, CH2O, H2O, SO2 and O2) and summarized the reaction characteristics. The reactions between the Criegee intermediates and NO, NO2, and SO2 are some of the sources of secondary organic aerosols in the atmosphere. Water has a self-catalytic effect in the reaction between H2O and the Criegee intermediate; acid and H2O were discovered in the product. Secondary ozone oxides (SOZ) can also be formed by reacting the Criegee intermediates with CH2O. The addition reaction between O2 and the Criegee intermediate could re-release ozone, achieving the atmospheric circulation reaction of ozone. The computed results manifested that the reaction of CI2 with O2 is the fastest path, followed by those with SO2 and CH2O.
The present investigation supplies many mechanical and kinetic data that could ascertain the degradation channels of CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 by ozonolysis. Some significant conclusions could be obtained from the available data. CF3CH
CH2 by ozonolysis. Some significant conclusions could be obtained from the available data. CF3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 is degraded through the reaction with O3 in the troposphere to generate some products, which would result in the generation of secondary organic aerosols. These computations indicate the formation of Criegee intermediates, peroxynitrates, and peroxysulfates with environmental concerns.
CH2 is degraded through the reaction with O3 in the troposphere to generate some products, which would result in the generation of secondary organic aerosols. These computations indicate the formation of Criegee intermediates, peroxynitrates, and peroxysulfates with environmental concerns.
Author contributions
The authors declared that all the co-authors are aware of and approve of the submission.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data that support the findings of this study are available on request from the corresponding author.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5ra07265d.
Acknowledgements
This work was supported by the Natural Science Foundations of China (No. 21707062), Scientific Research Starting Foundation of Mianyang Normal University (No. QD2016A007). Sichuan Province Science and Technology Project Support Program (No. 2023NSFSC1119).References
- M. J. Molina and F. S. Rowland, Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone, Nature, 1974, 249, 810–812 Search PubMed.
- J. C. Farman, B. G. Gardiner and J. D. Shanklin, Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction, Nature, 1985, 315, 207–210 Search PubMed.
- T. J. Wallington, W. F. Schneider, J. Sehested and O. J. Nielsen, Hydrofluorocarbons and stratospheric ozone, Faraday Discuss., 1995, 100, 55–64 Search PubMed.
- Y. Liu and W. L. Wang, Atmospheric oxidation chemistry of hexafluoroisobutylene initiated by OH radical: Kinetics and mechanism, Comput. Theor. Chem., 2021, 1197, 113137 Search PubMed.
- S. S. Balsini, A. Shiroudi, F. Hatamjafari, E. Zahedi, K. Pourshamsian and A. R. Oliaey, Understanding the kinetics and atmospheric degradation mechanism of chlorotrifluoroethylene (CF2=CFCl) initiated by OH radicals, Phys. Chem. Chem. Phys., 2023, 25, 13630–13644 Search PubMed.
- R. Atkinson, D. L. Baulch, R. A. Cox, R. F. Hampson Jr, J. A. Kerr, M. J. Rossi and J. Troe, Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry, Organic Species: Supplement VII, J. Phys. Chem. Ref. Data, 1999, 28, 191–393 Search PubMed.
- L. Chen, D. Lin, N. T. Tsona, X. Jiang and W. Wang, Atmospheric Chemistry of 2-Methoxypropene and 2-Ethoxypropene: Kinetics and Mechanism Study of Reactions with Ozone, Atmosphere, 2018, 9, 401 Search PubMed.
- R. Atkinson and W. P. L. Carter, Kinetics and mechanisms of the gas-phase reactions of ozone with organic compounds under atmospheric conditions, Chem. Rev., 1984, 84, 437–470 Search PubMed.
- R. Atkinson, Gas-Phase Tropospheric Chemistry of Volatile Organic Compounds: 1. Alkanes and Alkenes, J. Phys. Chem. Ref. Data, 1997, 26, 215–290 Search PubMed.
- D. Johnson and G. Marston, The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere, Chem. Soc. Rev., 2008, 37, 699–716 Search PubMed.
- R. Atkinson, Kinetics and Mechanisms of the Gas-Phase Reactions of the NO3 Radical with Organic Compounds, J. Phys. Chem. Ref. Data, 1991, 20, 459–507 Search PubMed.
- R. P. Wayne, I. Barnes, P. Biggs, J. P. Burrows, C. E. Canosa-Mas, J. Hjorth, G. Le Bras, G. K. Moortgat, D. Perner, G. Poulet, G. Restelli and H. Sidebottom, The nitrate radical: Physics, chemistry, and the atmosphere, Atmos. Environ., 1991, 25A, 1–203 Search PubMed.
- C. C. Womack, M. A. Martin-Drumel, G. G. Brown, R. W. Field and M. C. McCarthy, Observation of the simplest Criegee intermediate CH2OO in the gas-phase ozonolysis of ethylene, Sci. Adv., 2015, 1, l400105–l1400110 Search PubMed.
- R. Quandt, Z. Y. Min, X. B. Wang and R. Bersohn, Reactions of O(3P) with Alkenes: H, CH2CHO, CO, and OH Channels, J. Phys. Chem. A, 1998, 102, 60–64 Search PubMed.
- H. M. Su and R. Bersohn, Mechanisms of Formation of Vinoxy Radicals in the Reaction of O(3P) with Terminal Alkenes, J. Phys. Chem. A, 2001, 105, 9178–9182 Search PubMed.
- V. D. Knyazev, V. S. Arutyunov and V. I. Dedeneev, The mechanism of O(3P) atom reaction with ethylene and other simple olefins, Int. J. Chem. Kinet., 1992, 24, 545–561 Search PubMed.
- M. P. Sulbaek Andersen, O. J. Nielsen, A. Toft, T. Nakayama, Y. Matsumi, R. L. Waterland, R. C. Buck, M. D. Hurley and T. J. Wallington, Atmospheric chemistry of CxF2x+1CH=CH2 (x = 1, 2, 4, 6, and 8): Kinetics of gas-phase reactions with Cl atoms, OH radicals, and O3, J. Photochem. Photobiol., A, 2005, 176, 124–128 Search PubMed.
- T. Nakayama, K. Takahashi, Y. Matsumi, A. Toft, M. P. Sulbaek Andersen, O. J. Nielsen, R. L. Waterland, R. C. Buck, M. D. Hurley and T. J. Wallington, Atmospheric chemistry of CF3CH=CH2 and C4F9CH=CH2: products of the gas-phase reactions with Cl atoms and OH radicals, J. Phys. Chem. A, 2007, 111, 909–915 Search PubMed.
- K. Takahashi, E. Iwasaki, T. Nakayama, Y. Matsumi and T. J. Wallington, Vacuum ultraviolet laser-induced fluorescence kinetic study of the reactions of Cl atoms with fluoroalkenes (CxF2x+1CH=CH2, x=1,2,4,6 and 8) at low pressures, Int. J. Chem. Kinet., 2007, 39, 328–332 Search PubMed.
- S. J. Moss and K. R. Jennings, Relative rate constants for the reaction of ground-state oxygen atoms with partly fluorinated propylenes and butenes, Trans. Faraday Soc., 1969, 65, 415–423 Search PubMed.
- P. M. Cometto, M. A. Teruel, R. A. Taccone and S. I. Lane, Absolute rate determinations and temperature dependences of the gas-phase reactions of O(3P) with halogenated propenes, Chem. Phys. Lett., 2006, 417, 480–485 Search PubMed.
- O. J. Nielsen, M. S. Javadi, M. P. Sulbaek Andersen, M. D. Hurley, T. J. Wallington and R. Singh, Atmospheric chemistry of CF3CF=CH2: Kinetics and mechanisms of gas-phase reactions with Cl atoms, OH radicals, and O3, Chem. Phys. Lett., 2007, 439, 18–22 Search PubMed.
- K. Liu, L. Bai, Y. Shi, Z. Wei and R. Xiao, Simultaneous disinfection of E. faecalis and degradation of carbamazepine by sulfate radicals: An experimental and modelling study, Environ. Pollut., 2020, 263, 114558 Search PubMed.
- Z. Wei, W. Li, D. Zhao, Y. Seo, R. Spinney, D. D. Dionysiou, Y. Wang, W. Zeng and R. Xiao, Electrophilicity index as a critical indicator for the biodegradation of the pharmaceuticals in aerobic activated sludge processes, Water Res., 2019, 160, 10–17 Search PubMed.
- J. Sun, B. Wei, Q. Mei, Z. An and M. He, Theoretical investigation on the degradation of dibutyl phthalate initiated by −OH and SO4− in aqueous solution: mechanism, kinetics and ecotoxicity assessment, Chem. Eng. J., 2019, 382, 122791 Search PubMed.
- W. Kohn and L. J. Sham, Quantum density oscillations in an inhomogeneous electron gas, Phys. Rev. A, 1965, 137, 1697–1705 Search PubMed.
- L. J. Sham, Self-consistent equations including exchange and correlation effects, Phys. Rev. A, 1965, 140, 1133–1138 Search PubMed.
- W. L. Zeng, X. Wang, X. J. Kong and Y. J. Zhang, Synthesis, crystal structures, Hirshfeld surfaces and DFT calculations of two isomers of oxaspirocyclic compound, J. Mol. Struct, 2024, 1298, 137047 Search PubMed.
- W. L. Zeng, D. Y. Yue, X. Wang, H. Y. Li and X. J. Kong, Synthesis, structural, spectroscopic investigation, Hirshfeld surface analysis and DFT calculation of a new spiro compound including 4-nitroaniline moiety, J. Mol. Struct., 2025, 1322, 140371 Search PubMed.
- W. L. Zeng, X. Wang and Y. J. Zhang, Synthesis, Crystal Structures, and Density Functional Theory Studies of Two Salt Cocrystals Containing Meldrum’s Acid Group, ACS Omega, 2022, 7, 25132–25139 Search PubMed.
- W. L. Zeng, X. Wang, T. Zhou and Y. J. Zhang, Crystal Structure, Photophysical Study, Hirshfeld Surface Analysis, and Nonlinear Optical Properties of a New Hydroxyphenylamino Meldrum’s Acid Derivative, Molecules, 2023, 28, 2181–2193 Search PubMed.
- K. A. Holbrook, M. J. Pilling and S. H. Robertson, Unimolecular Reactions, J. Wiley, Chichester, UK, 1996 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc, C.T. Wallingford, 2009 Search PubMed.
- Y. Zhao and D. G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions, J. Chem. Phys., 1980, 72, 650–654 Search PubMed.
- T. Clark, J. Chandrasekhar, G. W. Spitznagel and P. V. R. Schleyer, Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li-F., J. Comput. Chem., 1983, 14, 294–301 Search PubMed.
- C. Gonzalez and H. B. Schlegel, An improved algorithm for reaction path, J. Chem. Phys., 1989, 90, 2154–2161 Search PubMed.
- C. Gonzalez and H. B. Schlegel, Reaction path following in massweighted internal coordinates, J. Phys. Chem., 1990, 94, 5523–5527 Search PubMed.
- K. Raghavachari, G. W. Trucks, J. A. Pople and M. Head-Gordon, A fifth-order perturbation comparison of electron correlation theories, Chem. Phys. Lett., 1989, 157, 479–483 Search PubMed.
- Y. T. Su, Y. H. Huang, H. A. Witek and Y. P. Lee, Infrared absorption spectrum of the simplest Criegee intermediate CH2OO, Science, 2013, 340, 174–176 Search PubMed.
- T. Berndt, J. Voigtländer, F. Stratmann, H. Junninen, R. L. Mauldin III, M. Sipilä, M. Kulmala and H. Herrmann, Competing atmospheric reactions of CH2OO with SO2 and water vapour, Phys. Chem. Chem. Phys., 2014, 16, 19130–19136 Search PubMed.
- Y. Díaz-de-Mera, A. Aranda, E. Martínez, A. A. Rodríguez, D. Rodríguez and A. Rodríguez, Formation of secondary aerosols from the ozonolysis of styrene: effect of SO2 and H2O, Atmos. Environ., 2017, 171, 25–31 Search PubMed.
- K. T. Kuwata, E. J. Guinn, M. R. Hermes, J. A. Fernandez, J. M. Mathison and K. Huang, A computational re-examination of the Criegee intermediate-sulfur dioxide reaction, J. Phys. Chem., 2015, 119, 10316–10335 Search PubMed.
- J. H. Ye, J. P. D. Abbatt and A. W. H. Chan, Novel pathway of SO2 oxidation in the atmosphere: reactions with monoterpene ozonolysis intermediates and secondary organic aerosol, Atmos. Chem. Phys., 2018, 18, 5549–5565 Search PubMed.
- L. Vereecken, H. Harder and A. Novelli, The reaction of Criegee intermediates with NO, RO2, and SO2, and their fate in the atmosphere, Phys. Chem. Chem. Phys., 2012, 14, 14682–14695 Search PubMed.
- L. Chen, W. L. Wang, W. N. Wang, Y. L. Liu, F. Y. Liu, N. Liu and B. Z. Wang, Watercatalyzed decomposition of the simplest Criegee intermediate CH2OO, Theor. Chem. Acc., 2016, 135, 131–143 Search PubMed.
- L. Chen, W. L. Wang, L. T. Zhou, W. N. Wang, F. Y. Liu, C. Y. Li and J. Lü, Role of water clusters in the reaction of the simplest Criegee intermediate CH2OO with water vapour, Theor. Chem. Acc., 2016, 252, 135–252 Search PubMed.
- J. Cai, Y. S. Lu, W. N. Wang, L. Chen, F. Y. Liu and W. L. Wang, Reaction mechanism and kinetics of Criegee intermediate CH2OO with CH2=C(CH3)CHO, Comput. Theor. Chem., 2019, 1170, 112–644 Search PubMed.
- R. Kaipara and B. Rajakumar, Temperature-dependent kinetics of the reaction of a criegee intermediate with propionaldehyde: a computational investigation, J. Phys. Chem., 2018, 122, 8433–8445 Search PubMed.
- W. M. Wei, X. Yang, R. H. Zheng, Y. D. Qin, Y. K. Wu and F. Yang, Theoretical studies on the reactions of the simplest Criegee intermediate CH2OO with CH3CHO, Comput. Theor. Chem., 2015, 1074, 142–149 Search PubMed.
- D. Stone, M. Blitz, L. Daubney, N. U. M. Howes and P. Seakins, Kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO as a function of pressure, Phys. Chem. Chem. Phys., 2014, 16, 1139–1149 Search PubMed.
- R. A. Cox, M. Ammann, J. N. Crowley, H. Herrmann, M. E. Jenkin, V. F. McNeill, A. Mellouki, J. Troe and T. J. Wallington, Evaluated kinetic and photochemical data for atmospheric chemistry: Volume VII - Criegee intermediates, Atmos. Chem. Phys., 2020, 20, 13497–13519 Search PubMed.
- R. Vingarzan, A review of surface ozone background levels and trend, Atmos. Environ., 2004, 38, 3431–3442 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |










