Open Access Article
Open Access ArticleSynthesis of benzylic thioether containing α-substituted thio compounds via carbene insertion into an S–H bond: expeditious access to a new C–S bond
Arumugam Jayarani,
Gruhapriya Jelakam and
Chinnappan Sivasankar
and
Chinnappan Sivasankar *
*
Catalysis and Energy Laboratory, Department of Chemistry, Pondicherry University (A Central University), R. V. Nagar, Kalapet, Puducherry 605014, India. E-mail: siva.che@pondiuni.ac.in; Tel: +91 413 2654709
First published on 25th November 2025
Abstract
Considering the importance of thioethers in biological systems, we developed a strategy to achieve these target molecules. In this regard, an efficient copper(II)-catalyzed synthesis of highly biologically relevant thioethers is realized, employing readily available and inexpensive starting precursors under ambient conditions. The developed new methodology is very useful to selectively synthesize a C–S bond via carbene insertion into an S–H bond. Our strategy also paved the way to produce a wide range of α-thio substituted benzylic esters in a single-pot method in very good yields under short reaction times. All the synthesized compounds have been fully characterized using spectroscopic and analytical techniques. The best features of the developed strategy are S–H insertion, mild reaction conditions, wide substrate scope, and excellent functional group tolerance.
Introduction
Nitrogen, oxygen, and sulphur-containing organic scaffolds are highly useful in the field of pharmaceuticals due to their wide spectrum of biological activity.1 Among the different hetero-atoms, sulfur is one of the most essential elements found in living systems in the form of proteins and amino acids. Particularly, organosulfur compounds play significant roles in the fields of biology and chemistry due to their ubiquitous biological activity.2 Beyond amino acids (methionine and cysteine), the C–S bond is present in several natural products, significant drug molecules and functional materials from the pharmaceutical industry,3 and in products in the food industry.4 These motifs also play a vital role in photoelectric materials5 due to the higher resonance energy of the sulphur atoms. Among the different organosulfur compounds, α-substituted thioethers, in particular, possess a significant role because they act as suitable precursors in the synthesis of biologically active benzothiophenes and Julia reagents.6 Moreover, organosulfur-based structural frameworks are present in various biologically active compounds and synthetic intermediates. Sulphur-containing heterocycles exhibit many pharmacological activities. Particularly, thioethers of benzoxazoles and benzothiazoles have been important targets due to their biological activities. They are extensively useful as antibacterial, antifungal, anti-inflammatory, anticancer, antiviral, antagonizing, and antituberculosis agents and as fungicides7–12 (Fig. 1).Additionally, in recent years, transition metal catalysis has dramatically changed the face of modern organic chemistry by introducing novel synthetic routes. Using these facile catalytic systems, construction of several carbon–heteroatom bond formations, such as C–O, C–N and C–P, as well as other carbon–element bonds based on B, Si, Ge, and Sn, was developed. Until now, the effective construction of C–S bonds has been highly challenging or very rarely explored compared with the formation of other carbon–heteroatom bonds in the field of organic synthesis due to its own limiting factors, such as harsh reaction conditions (higher temperature, toxic and higher boiling solvents, and longer reaction times), deactivation of the catalytic system due to its strong affinity with metals, expensive starting precursors, and usage of high loading metal catalysts. Therefore, we need to develop an alternate, greener and efficient protocol for the construction of organosulphur compounds via C–S bond formation over existing conventional methodologies. In this study, we overcame all these drawbacks by using a new catalytic system to produce biologically relevant organosulfur compounds containing a C–S bond in an effective manner.
Generally, metal-catalysed reactions have been useful synthetic tools to access the target molecules in organic transformations. Particularly, copper-catalysed organic reactions are a promising methodology for synthesizing an important class of organic molecules used in medicinal chemistry and material science.13 For example, diazo compounds are very useful precursors in organic transformations to construct complex organic molecular frameworks using C–C and C–X bond formations via transition metal catalysis. It is well known that reactive intermediates, such as metal carbenes or carbenoids, which are in situ formed from diazo compounds, can undergo various synthetic organic transformations and are sometimes ideal for initiating domino sequences, leading to the generation of structural complexity.14 Moreover, diazo compounds are useful precursors, including C–H and X–H (X= N, O, S, P, and Si) insertion,15 cyclopropanation16 and ylide formation.17 The tandem or cascade construction of biologically relevant organosulphur compounds, such as thioethers/thioesters, via the formation of a new C–S bond by S–H insertion through cross-coupling reactions under metal catalysis via a single pot process is very less due to its synthetic challenges. Particularly, the formation of C–S bonds using heterocyclic compounds containing thiols and diazo compounds is known but has not been explored much in the literature so far. Beller and coworkers have reported S–H insertion into benzoxazole–thiol to afford the product using an exotic Cu-NC/Al2O3 as catalyst.18 Ollevier et al. have reported the synthesis of α-thioesters using α-diazocarbonyl compounds with organo-sulphur compounds via S–H insertion under copper(I) catalysis (Scheme 1a).19 Huo and co-workers have utilized α-diazoesters and thiols to form α-thioethers under ambient temperature in the presence of visible light (Scheme 1b).20 Eosin-Y-catalysed sulfenylation of hydrazones with thiols under visible light to produce the thioether derivatives was developed by the Krishna group (Scheme 1c).21 Chens and coworkers have reported S–H insertion into benzoxazole–thiol to yield the expected product using TfOH as a catalyst (Scheme 1d).22
Inspired by these reports on the significance of thioethers and their biological applications and by our ongoing research program in the field of metal carbenes,23–25 we studied the construction of biologically relevant organic molecular frameworks using a new C–S bond formation via a simple reaction strategy. In this line, we investigated the development of an efficient reaction strategy to construct new C–S bonds, along with chiral centers, using copper-carbenoids with heterocyclic compounds containing thiols at ambient temperature with shorter reaction times.
Results and discussion
In our investigation, initially, we tested the reaction between 2-mercaptobenzoxazole (1a) and methyl 2-diazo-2-phenylacetate (2a) in the presence of 5 mol% of CuI under MeCN solvent at ambient temperature for 12 h. To our delight, we isolated the expected product methyl 2-(benzo[d]oxazol-2-ylthio)-2-phenylacetate (3a) in 76% yield and the desired product was confirmed through spectroscopic techniques such as 1H-NMR, 13C-NMR and mass spectrometry. An interesting feature of this intermolecular organic transformation is generating a new C–S bond, along with one chiral centre, in a single-pot reaction. Encouraged by these interesting results, we attempted to improve the yield of the desired product 3a using optimised reaction conditions. Accordingly, we screened the following metal catalysts, CuCl2·2H2O, CuSO4·5H2O and Cu(OAc)2 of 5 mol%, in MeCN solvent at room temperature for 12 h; the yield of the anticipated product 3a was not improved and the desired product 3a was formed in 66%, 72% and 75%, respectively (Table 1, entries 2–4). Further, the yield of the anticipated product 3a was increased from 71% to 78% when we used 5 mol% of CuBr and Cu(CH3CN)ClO4 as a metal catalyst under the same reaction conditions (Table 1, entries 5 and 6). Further, to improve the yield of product 3a, we used 2 mol% of the metal catalyst Cu(OTf)2; surprisingly, we obtained product 3a with 80% yield (Table 1, entry 7). Further, in order to increase yield, we screened different solvent systems, ranging from MeCN to polar aprotic solvents, such as THF, CHCl3, C2H4Cl2 and CH2Cl2, using 5 mol% of Cu(OTf)2 at ambient temperature with varying reaction times (Table 1, entries 8–12). To our delight, we observed that the yield of 3a was 77% when 5 mol% of Cu(OTf)2 was used in MeCN as a solvent for 12 h (Table 1, entry 13). Further, the reaction was conducted by loading the metal catalyst, 5 mol% Cu(OTf)2, in DCM for 2 h, and the yield of 3a gradually increased to 85% (Table 1, entry 14). Finally, we tested the reaction using 2 mol% Cu(OTf)2 in DCM, and the yield of 3a was 89% (Table 1, entry 15). We tested the reaction in the absence of a metal catalyst in DCM for 5 h, and in this case, the product 3a was not observed (Table 1, entry 16). When we used TfOH as a catalyst, we observed a trace amount of the product (Table 1, entry 17). A control experiment with TfOH confirmed that the reaction is not fully acid-promoted; however, it proceeds with poor selectivity and significant decomposition. Nevertheless, the Cu(OTf)2 effectively accelerated the transformation via the formation of a Cu(II)–carbene intermediate, yielding the required α-thio-substituted benzylic esters in high yield. Therefore, the Lewis acidity of Cu(OTf)2 prevents the excessively acidic conditions associated with TfOH and ensures controlled activation of the diazo molecule. From these optimisation studies, we finally concluded that the selective S–H insertion into the C–S bond is effective in the presence of Cu(OTf)2 (2 mol%) using dichloromethane as the solvent at ambient temperature for 2 h, and TfOH is not suitable for this S–H insertion reaction with α-aryl diazoester.| S. no. | Catalyst (mol%) | Solvent | Time (h) | Isolatedb yield (%) |
|---|---|---|---|---|
| a All reactions were carried out with (1.0 mmol, 1 equiv.) of 2-mercaptobenzoxazole (1a) and (1.0 mmol, 1 equiv.) of α-diazo compound (2a).b Isolated yield of the pure product obtained after column chromatography purification. | ||||
| 1 | CuI (5) | CH3CN | 12 | 76 |
| 2 | CuCl2·2H2O (5) | CH3CN | 12 | 66 |
| 3 | CuSO4·5H2O (5) | CH3CN | 12 | 72 |
| 4 | Cu(OAc)2 (5) | CH3CN | 12 | 75 |
| 5 | CuBr (5) | CH3CN | 12 | 71 |
| 6 | Cu(CH3CN)4ClO4 (5) | CH3CN | 12 | 78 |
| 7 | Cu(OTf)2 (2) | CH3CN | 12 | 80 |
| 8 | Cu(OTf)2 (2) | THF | 12 | 70 |
| 9 | Cu(OTf)2 (2) | Toluene | 10 | 64 |
| 10 | Cu(OTf)2 (2) | CHCl3 | 5 | 82 |
| 11 | Cu(OTf)2 (2) | DCE | 5 | 84 |
| 12 | Cu(OTf)2 (2) | DCM | 5 | 89 |
| 13 | Cu(OTf)2 (5) | MeCN | 12 | 77 |
| 14 | Cu(OTf)2 (5) | DCM | 2 | 85 |
| 15 | Cu(OTf)2(2) | DCM | 2 | 89 |
| 16 | No catalyst | DCM | 5 | nr |
| 17 | TfOH | CH3CN | 5 min | Trace |
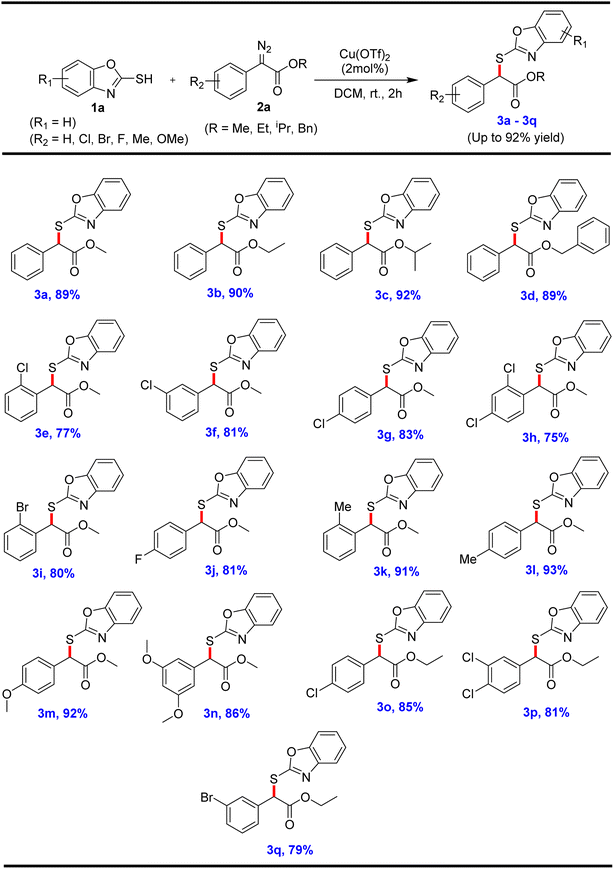
Herein, we report the developed methodology for the synthesis of α-thioether derivatives via carbene insertion into an S–H bond to form a C–S bond, along with one chiral centre. The highlight of this reaction is the high selectivity to produce the C–S bond through S–H insertion. It is important to note that metal carbenoids can be inserted into S–H bond to create thioethers utilising 2-mercaptobenzoxazole as a sulfur source. The scope of the reaction was tested by utilizing different substituted 2-mercaptobenzoxazoles 1a with different α-aryl-α-diazoacetates 2a in the presence of a copper catalyst to form the corresponding α-thioethers (3a–l) in very good to excellent yields, and the results and conditionsa,b are summarized in Scheme 2. The reaction between the substrates 1a and 2a, containing different ester functionalities, viz. methyl 2a, ethyl 2b, isopropyl 2c, and benzyl 2d, produced the corresponding products 3a, 3b, 3c and 3d in 89%, 90%, 92%, and 89% yields, respectively (Scheme 2). The aryl ring of α-diazoester 2a, containing electron-withdrawing groups, such as chloro, bromo and fluoro, furnished the anticipated products 3e–3j in very good to excellent yields. The electron-donating groups, such as methyl and methoxy substituted diazoesters 2a, reacted well with 2-mercaptobenzoxazole derivatives to provide the desired products 3k–3n in excellent yields. The aryl ring of ethyl diazoacetate 2b, having electron-withdrawing groups, such as 4-chloro, 2,4-dichloro and 3-Br substituents, also smoothly reacted with compound 1a to furnish the corresponding products 3o–3q in 85%, 81%, and 79% yield, respectively (Scheme 2).
To further explore the substrate scope of the reaction, we carried out the reaction between substituted 2-mercaptobenzoxazole derivatives and methyl phenyl diazoacetate using the optimized reaction conditions, and the results and conditionsa,b are summarised in Scheme 3. The reaction between methyl phenyl diazoacetate (2) and 2-mercaptobenzoxazole, whose aryl ring has halogen groups (F, Cl, and Br) at the para position, smoothly reacted and afforded the corresponding products 4a–c in 79%, 80%, and 83% yields, respectively (Scheme 3). 2-Mercaptobenzoxazole, containing a strong electron-withdrawing group, such as NO2, also reacted well with compound (2) to furnish the desired product 4d in 78% yield. 2-Mercaptobenzoxazole, bearing electron-donating functionalities like Me and OMe groups at the para position, also reacted smoothly with methyl phenyl diazoacetate (2) to produce the anticipated products 4e and 4f in 81% and 82% yields, respectively (Scheme 3). Both substrates (1) and (2) have aryl rings that contain halogen substituents, such as chloro, bromo and fluoro, at ortho and para positions to afford the respective products 4g–i in very good yields (Scheme 3). 2-Mercaptobenzoxazole containing NO2 substituent at the para position and similarly, compound (2) having OMe group at the para position, smoothly reacted and formed the corresponding product 4j in 88% yield. 4k and 4l are also produced in good yields of 83% and 85%.
Still, to further assess the substrate scope, we tested the reactions of 2-mercapto benzoxazole (1a) and ethyl 2-((6-chlorobenzo[d]thiazol-2-yl) thio) acetate (1a) with ethyl 2-diazoacetate (2b) under standard reaction conditions.a,b Interestingly, the reactions provided the desired products 5a and 5b in 93% and 89% yields, respectively. Additionally, the reaction between ethyl 2-((6-chlorobenzo[d]thiazol-2-yl)thio) acetate (1a) and ethyl 2-diazo-2-phenylacetate, ethyl 2-diazo-2-(3,4-dichlorophenyl)acetate (2b) formed the corresponding desired products 5c and 5d in 81% and 78% yields, respectively (Scheme 4).
To study the scalability of our optimized reaction conditions, we carried out a large scale reaction between 2-mercaptobenzoxazole (1a) (378 mg, 2.5 mmol, 1 equiv.) and isopropyl phenyl diazoacetate (2a) (510 mg, 2.5 mmol, 1 equiv.) in 10 mL DCM (0.33 M) at rt in the presence of the Cu(OTf)2 catalyst (18 mg, 2 mol%). The scale-up reaction afforded the anticipated product 3c in 86% yield (708 mg, 2.1 mmol), confirming the large-scale applicability of the method (Scheme 5).
A plausible mechanism has been proposed below for the formation of α-thio-substituted benzylic esters (III) from I and II, as shown in Fig. 2. Initially, the active catalyst Cu(OTf)2 (A) would react with α-diazoester (I) to generate the intermediate (B) via the formation of a copper-carbenoid with the evolution of nitrogen gas. Simultaneously, 2-mercaptobenzoxazole (II) undergoes nucleophilic addition with the intermediate (B) to generate the intermediate (C). Eventually, the intermediate (C) would undergo hydrogen migration to afford the desired product (III) with the regeneration of the active catalyst Cu(OTf)2.
Conclusion
We successfully developed an efficient protocol to access biologically relevant α-thio substituted benzylic esters from 2-mercaptobenzoxazoles and α-diazoesters in the presence of a copper catalyst. Using this newly developed protocol, we synthesized a variety of α-thioester derivatives at ambient temperature. During this organic transformation, a new C–S bond is formed with a new chiral center. These overall organic transformations proceed via cascade metal-carbenoid formation and S–H insertion, followed by reductive elimination to produce the anticipated product in very good yields. A wide substrate scope, shorter reaction times and excellent functional group tolerance are the best features of this reaction. The synthesized α-thioester derivatives may be useful in the pharmaceutical and chemical industry.Experimental section
General experimental information
Commercial reagents were used without further purification. IR spectra were recorded on a PerkinElmer FTIR spectrometer using solid samples pressed into KBr pellets. For compounds, 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) spectra were recorded in deuterochloroform (CDCl3) on a Bruker 400 MHz spectrometer using tetramethylsilane (TMS, δ = 0) as an internal standard at room temperature. HR-MS was recorded on a UHD Q-TOF mass spectrometer. All the reagents were purchased from Sigma-Aldrich. Reactions were monitored by thin-layer chromatography (TLC) on silica gel 60 F254 aluminium plates. TLC plates were visualized using ultraviolet (UV) light at 254 nm. Column chromatography was performed with silica gel 60 Å (100–200 mesh) from Aldrich using the stated mixture of solvents. Based on the literature procedure, different types of α-aryl diazoesters were synthesized (see SI).Methyl 2-(benzo[d]oxazol-2-ylthio)-2-phenylacetate (3a). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (266 mg, 89% yield). Mp 79–80 °C; 1H NMR (400 MHz, CDCl3) δ 7.53–7.48 (m, 1H), 7.47–7.43 (m, 2H), 7.34–7.31 (m, 1H), 7.30–7.26 (m, 2H), 7.25 (dd, J = 3.9, 1.3 Hz, 1H), 7.21–7.11 (m, 2H), 5.62 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.05, 162.99, 151.97, 141.82, 134.09, 129.24, 129.18, 128.54, 124.48, 124.29, 118.85, 110.12, 53.75, 53.50. IR (KBr): 1738, 1505, 1472, 1453, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H13NO3S 299.0616, found: 300.0693.
5). White solid (266 mg, 89% yield). Mp 79–80 °C; 1H NMR (400 MHz, CDCl3) δ 7.53–7.48 (m, 1H), 7.47–7.43 (m, 2H), 7.34–7.31 (m, 1H), 7.30–7.26 (m, 2H), 7.25 (dd, J = 3.9, 1.3 Hz, 1H), 7.21–7.11 (m, 2H), 5.62 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.05, 162.99, 151.97, 141.82, 134.09, 129.24, 129.18, 128.54, 124.48, 124.29, 118.85, 110.12, 53.75, 53.50. IR (KBr): 1738, 1505, 1472, 1453, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H13NO3S 299.0616, found: 300.0693.
Ethyl 2-(benzo[d]oxazol-2-ylthio)-2-phenylacetate (3b). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (282 mg, 90% yield). 1H NMR (400 MHz, CDCl3) δ 7.47 (m, 3H), 7.32–7.26 (m, 2H), 7.26–7.22 (m, 2H), 7.18–7.09 (m, 2H), 4.13 (m, 2H), 1.13 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.43, 163.00, 151.88, 141.77, 134.14, 129.12, 129.05, 128.48, 124.41, 124.20, 118.72, 110.03, 62.47, 53.87, 14.05. IR (KBr): 1742, 1505, 1452, 1020, 806 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15 NO3S 313.0773, found: 314.0859.
5). White liquid (282 mg, 90% yield). 1H NMR (400 MHz, CDCl3) δ 7.47 (m, 3H), 7.32–7.26 (m, 2H), 7.26–7.22 (m, 2H), 7.18–7.09 (m, 2H), 4.13 (m, 2H), 1.13 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.43, 163.00, 151.88, 141.77, 134.14, 129.12, 129.05, 128.48, 124.41, 124.20, 118.72, 110.03, 62.47, 53.87, 14.05. IR (KBr): 1742, 1505, 1452, 1020, 806 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15 NO3S 313.0773, found: 314.0859.
Isopropyl 2-(benzo[d]oxazol-2-ylthio)-2-phenylacetate (3c). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (301 mg, 92% yield). Mp 63–64 °C; 1H NMR (400 MHz, CDCl3) δ 7.51–7.43 (m, 3H), 7.33 (dd, J = 7.9, 1.4 Hz, 1H), 7.31–7.26 (m, 2H), 7.26–7.11 (m, 3H), 5.55 (s, 1H), 4.99 (p, J = 6.3 Hz, 1H), 1.20 (d, J = 6.2 Hz, 3H), 1.05 (d, J = 6.2 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.00, 163.17, 151.96, 141.87, 134.25, 129.15, 129.06, 128.54, 124.48, 124.24, 118.78, 110.10, 70.29, 54.11, 21.72, 21.51. IR (KBr): 1738, 1505, 1453, 1241, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C18H17NO3S 327.0929, found: 328.1011.
5). White solid (301 mg, 92% yield). Mp 63–64 °C; 1H NMR (400 MHz, CDCl3) δ 7.51–7.43 (m, 3H), 7.33 (dd, J = 7.9, 1.4 Hz, 1H), 7.31–7.26 (m, 2H), 7.26–7.11 (m, 3H), 5.55 (s, 1H), 4.99 (p, J = 6.3 Hz, 1H), 1.20 (d, J = 6.2 Hz, 3H), 1.05 (d, J = 6.2 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.00, 163.17, 151.96, 141.87, 134.25, 129.15, 129.06, 128.54, 124.48, 124.24, 118.78, 110.10, 70.29, 54.11, 21.72, 21.51. IR (KBr): 1738, 1505, 1453, 1241, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C18H17NO3S 327.0929, found: 328.1011.
Benzyl 2-(benzo[d]oxazol-2-ylthio)-2-phenylacetate (3d). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (334 mg, 89% yield). 1H NMR (400 MHz, CDCl3) δ 7.57–7.53 (m, 3H), 7.38–7.26 (m, 8H), 7.26–7.17 (m, 3H), 5.77 (d, J = 1.4 Hz, 1H), 5.31 (d, J = 12.3 Hz, 1H), 5.11 (d, J = 12.4 Hz, 1H). 13C NMR (101 MHz, chloroform-d) δ 169.31, 162.85, 151.86, 141.72, 135.24, 133.74, 129.13, 129.10, 128.52, 128.48, 128.31, 128.07, 124.36, 124.16, 118.72, 110.02, 67.87, 53.81. IR (KBr): 1740, 1510, 1450, 1246, 1134 cm−1. HRMS (ESI) m/z: [M + H] calcd for C22H17NO3S 375.0929, found: 376.0995.
5). White liquid (334 mg, 89% yield). 1H NMR (400 MHz, CDCl3) δ 7.57–7.53 (m, 3H), 7.38–7.26 (m, 8H), 7.26–7.17 (m, 3H), 5.77 (d, J = 1.4 Hz, 1H), 5.31 (d, J = 12.3 Hz, 1H), 5.11 (d, J = 12.4 Hz, 1H). 13C NMR (101 MHz, chloroform-d) δ 169.31, 162.85, 151.86, 141.72, 135.24, 133.74, 129.13, 129.10, 128.52, 128.48, 128.31, 128.07, 124.36, 124.16, 118.72, 110.02, 67.87, 53.81. IR (KBr): 1740, 1510, 1450, 1246, 1134 cm−1. HRMS (ESI) m/z: [M + H] calcd for C22H17NO3S 375.0929, found: 376.0995.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(2-chlorophenyl)acetate (3e). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (257 mg, 77% yield). Mp 80–81 °C; 1H NMR (400 MHz, CDCl3) δ 7.52–7.48 (m, 1H), 7.46–7.42 (m, 2H), 7.35–7.27 (m, 2H), 7.26–7.11 (m, 3H), 5.61 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.07, 163.00, 151.98, 141.82, 134.07, 129.25, 129.20, 128.55, 124.49, 124.30, 118.86, 110.13, 53.74, 53.53. IR (KBr): 1738, 1505, 1453, 1275, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0299.
5). White solid (257 mg, 77% yield). Mp 80–81 °C; 1H NMR (400 MHz, CDCl3) δ 7.52–7.48 (m, 1H), 7.46–7.42 (m, 2H), 7.35–7.27 (m, 2H), 7.26–7.11 (m, 3H), 5.61 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.07, 163.00, 151.98, 141.82, 134.07, 129.25, 129.20, 128.55, 124.49, 124.30, 118.86, 110.13, 53.74, 53.53. IR (KBr): 1738, 1505, 1453, 1275, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0299.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(3-chlorophenyl)acetate (3f). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (270 mg, 81% yield). Mp 97–98 °C; 1H NMR (400 MHz, CDCl3) δ 7.56–7.53 (m, 1H), 7.50 (d, J = 2.1 Hz, 1H), 7.40–7.35 (m, 2H), 7.27–7.16 (m, 4H), 5.63 (s, 1H), 3.74 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.52, 162.49, 152.03, 141.73, 136.27, 135.00, 130.42, 129.41, 128.74, 126.81, 124.58, 124.43, 118.91, 110.19, 53.72, 53.21. IR (KBr): 1742, 1502, 1453, 1128, 1096 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0301.
5). White solid (270 mg, 81% yield). Mp 97–98 °C; 1H NMR (400 MHz, CDCl3) δ 7.56–7.53 (m, 1H), 7.50 (d, J = 2.1 Hz, 1H), 7.40–7.35 (m, 2H), 7.27–7.16 (m, 4H), 5.63 (s, 1H), 3.74 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.52, 162.49, 152.03, 141.73, 136.27, 135.00, 130.42, 129.41, 128.74, 126.81, 124.58, 124.43, 118.91, 110.19, 53.72, 53.21. IR (KBr): 1742, 1502, 1453, 1128, 1096 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0301.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(4-chlorophenyl)acetate (3g). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (277 mg, 83% yield). 1H NMR (400 MHz, CDCl3) δ 7.53–7.49 (m, 1H), 7.44–7.40 (m, 2H), 7.35–7.31 (m, 1H), 7.27–7.23 (m, 2H), 7.21–7.12 (m, 2H), 5.63 (s, 1H), 3.70 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.57, 162.48, 151.94, 141.69, 135.13, 132.94, 129.90, 129.32, 124.49, 124.34, 118.82, 110.09, 53.54, 53.07. IR (KBr): 1743, 1503, 1453, 1132, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0277.
5). White liquid (277 mg, 83% yield). 1H NMR (400 MHz, CDCl3) δ 7.53–7.49 (m, 1H), 7.44–7.40 (m, 2H), 7.35–7.31 (m, 1H), 7.27–7.23 (m, 2H), 7.21–7.12 (m, 2H), 5.63 (s, 1H), 3.70 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.57, 162.48, 151.94, 141.69, 135.13, 132.94, 129.90, 129.32, 124.49, 124.34, 118.82, 110.09, 53.54, 53.07. IR (KBr): 1743, 1503, 1453, 1132, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0277.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(2,4-dichlorophenyl)acetate (3h). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (266 mg, 75% yield). 1H NMR (400 MHz, CDCl3) δ 7.50–7.44 (m, 2H), 7.27 (d, J = 4.7 Hz, 1H), 7.14–7.04 (m, 4H), 6.11 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.58, 162.18, 151.82, 141.43, 135.28, 134.75, 131.27, 130.85, 129.73, 127.57, 124.27, 124.11, 118.61, 109.86, 53.45, 50.29. IR (KBr): 1746, 1587, 1503, 1453, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H11Cl2NO3S 366.9837, found: 367.9939.
5). White liquid (266 mg, 75% yield). 1H NMR (400 MHz, CDCl3) δ 7.50–7.44 (m, 2H), 7.27 (d, J = 4.7 Hz, 1H), 7.14–7.04 (m, 4H), 6.11 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.58, 162.18, 151.82, 141.43, 135.28, 134.75, 131.27, 130.85, 129.73, 127.57, 124.27, 124.11, 118.61, 109.86, 53.45, 50.29. IR (KBr): 1746, 1587, 1503, 1453, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H11Cl2NO3S 366.9837, found: 367.9939.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(2-bromophenyl)acetate (3i). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (302 mg, 80% yield). 1H NMR (400 MHz, CDCl3) δ 7.49–7.44 (m, 3H), 7.29–7.25 (m, 1H), 7.19–7.13 (m, 1H), 7.13–7.01 (m, 3H), 6.10 (s, 1H), 3.65 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.26, 162.62, 151.98, 141.71, 134.02, 133.55, 130.43, 129.98, 128.13, 124.63, 124.40, 124.22, 118.81, 110.03, 53.56, 53.28. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12BrNO3S 376.9721, found: 377.9770.
5). White liquid (302 mg, 80% yield). 1H NMR (400 MHz, CDCl3) δ 7.49–7.44 (m, 3H), 7.29–7.25 (m, 1H), 7.19–7.13 (m, 1H), 7.13–7.01 (m, 3H), 6.10 (s, 1H), 3.65 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.26, 162.62, 151.98, 141.71, 134.02, 133.55, 130.43, 129.98, 128.13, 124.63, 124.40, 124.22, 118.81, 110.03, 53.56, 53.28. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12BrNO3S 376.9721, found: 377.9770.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(4-fluorophenyl)acetate (3j). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (257 mg, 81% yield). Mp 59–60 °C; 1H NMR (400 MHz, CDCl3) δ 7.59–7.52 (m, 3H), 7.37 (dd, J = 8.0, 1.3 Hz, 1H), 7.26–7.17 (m, 2H), 7.05–6.99 (m, 2H), 5.73 (s, 1H), 3.75 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.66, 164.13, 162.51, 161.66, 151.80, 141.59, 130.34, 130.26, 130.11, 130.08, 124.36, 124.20, 118.67, 116.12, 115.91, 109.95, 53.32, 52.86. IR (KBr): 1742, 1603, 1509, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12FNO3S 317.0522, found: 318.0572.
5). White solid (257 mg, 81% yield). Mp 59–60 °C; 1H NMR (400 MHz, CDCl3) δ 7.59–7.52 (m, 3H), 7.37 (dd, J = 8.0, 1.3 Hz, 1H), 7.26–7.17 (m, 2H), 7.05–6.99 (m, 2H), 5.73 (s, 1H), 3.75 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.66, 164.13, 162.51, 161.66, 151.80, 141.59, 130.34, 130.26, 130.11, 130.08, 124.36, 124.20, 118.67, 116.12, 115.91, 109.95, 53.32, 52.86. IR (KBr): 1742, 1603, 1509, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12FNO3S 317.0522, found: 318.0572.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(o-tolyl)acetate (3k). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Pale yellow liquid (285 mg, 91% yield). 1H NMR (400 MHz, CDCl3) δ 7.67–7.62 (m, 1H), 7.60–7.55 (m, 1H), 7.44 (dd, J = 7.7, 1.3 Hz, 1H), 7.31–7.23 (m, 5H), 6.03 (s, 1H), 3.80 (s, 3H), 2.61 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.03, 163.12, 151.79, 141.63, 136.79, 132.07, 130.97, 128.97, 127.80, 127.31, 126.76, 124.25, 124.04, 118.58, 109.88, 77.36, 53.19, 50.29, 38.89, 19.48. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO3S 313.0773, found: 314.0823.
5). Pale yellow liquid (285 mg, 91% yield). 1H NMR (400 MHz, CDCl3) δ 7.67–7.62 (m, 1H), 7.60–7.55 (m, 1H), 7.44 (dd, J = 7.7, 1.3 Hz, 1H), 7.31–7.23 (m, 5H), 6.03 (s, 1H), 3.80 (s, 3H), 2.61 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.03, 163.12, 151.79, 141.63, 136.79, 132.07, 130.97, 128.97, 127.80, 127.31, 126.76, 124.25, 124.04, 118.58, 109.88, 77.36, 53.19, 50.29, 38.89, 19.48. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO3S 313.0773, found: 314.0823.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(p-tolyl)acetate (3l). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (291 mg, 93% yield). Mp 69–70 °C; 1H NMR (400 MHz, CDCl3) δ 7.62–7.58 (m, 1H), 7.44–7.41 (m, 3H), 7.29 (dd, J = 7.5, 1.5 Hz, 1H), 7.25–7.17 (m, 3H), 5.68 (s, 1H), 3.78 (s, 3H), 2.34 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.17, 163.11, 151.96, 141.86, 139.19, 131.03, 129.92, 128.41, 124.45, 124.24, 118.83, 110.09, 53.52, 53.43, 21.30. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO3S 313.0773, found: 314.0842.
5). White solid (291 mg, 93% yield). Mp 69–70 °C; 1H NMR (400 MHz, CDCl3) δ 7.62–7.58 (m, 1H), 7.44–7.41 (m, 3H), 7.29 (dd, J = 7.5, 1.5 Hz, 1H), 7.25–7.17 (m, 3H), 5.68 (s, 1H), 3.78 (s, 3H), 2.34 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.17, 163.11, 151.96, 141.86, 139.19, 131.03, 129.92, 128.41, 124.45, 124.24, 118.83, 110.09, 53.52, 53.43, 21.30. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO3S 313.0773, found: 314.0842.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(4-methoxyphenyl)acetate (3m). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Pale yellow liquid (303 mg, 92% yield). 1H NMR (400 MHz, CDCl3) δ 7.44 (dd, J = 7.6, 1.5 Hz, 1H), 7.33 (d, J = 8.7 Hz, 2H), 7.26–7.21 (m, 1H), 7.07 (m, 2H), 6.72 (d, J = 8.7 Hz, 2H), 5.56 (s, 1H), 3.61 (s, 3H), 3.59 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.01, 162.89, 160.03, 151.71, 141.63, 129.61, 125.67, 124.26, 124.06, 118.59, 114.41, 109.88, 55.14, 53.16, 53.08. IR (KBr): 1738, 1609, 1511, 1481, 1031 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO4S 329.0722, found: 330.0795.
5). Pale yellow liquid (303 mg, 92% yield). 1H NMR (400 MHz, CDCl3) δ 7.44 (dd, J = 7.6, 1.5 Hz, 1H), 7.33 (d, J = 8.7 Hz, 2H), 7.26–7.21 (m, 1H), 7.07 (m, 2H), 6.72 (d, J = 8.7 Hz, 2H), 5.56 (s, 1H), 3.61 (s, 3H), 3.59 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.01, 162.89, 160.03, 151.71, 141.63, 129.61, 125.67, 124.26, 124.06, 118.59, 114.41, 109.88, 55.14, 53.16, 53.08. IR (KBr): 1738, 1609, 1511, 1481, 1031 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO4S 329.0722, found: 330.0795.
Methyl 2-(benzo[d]oxazol-2-ylthio)-2-(3,5-dimethoxyphenyl)acetate (3n). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (309 mg, 86% yield). Mp 101–102 °C; 1H NMR (400 MHz, CDCl3) δ 7.52 (dd, J = 7.2, 1.7 Hz, 1H), 7.35 (dd, J = 7.4, 1.7 Hz, 1H), 7.19 (qt, J = 7.6, 3.7 Hz, 2H), 6.60 (d, J = 2.3 Hz, 2H), 6.36 (s, 1H), 5.56 (s, 1H), 3.71 (s, 9H). 13C NMR (101 MHz, chloroform-d) δ 169.89, 163.06, 161.31, 152.04, 141.89, 136.05, 124.51, 124.30, 118.87, 110.14, 106.60, 101.25, 55.58, 53.95, 53.53. IR (KBr): 1738, 1609, 1511, 1481, 1031 cm−1. HRMS (ESI) m/z: [M + H] calcd for C18H17NO5S 359.0827, found: 360.0894.
5). White solid (309 mg, 86% yield). Mp 101–102 °C; 1H NMR (400 MHz, CDCl3) δ 7.52 (dd, J = 7.2, 1.7 Hz, 1H), 7.35 (dd, J = 7.4, 1.7 Hz, 1H), 7.19 (qt, J = 7.6, 3.7 Hz, 2H), 6.60 (d, J = 2.3 Hz, 2H), 6.36 (s, 1H), 5.56 (s, 1H), 3.71 (s, 9H). 13C NMR (101 MHz, chloroform-d) δ 169.89, 163.06, 161.31, 152.04, 141.89, 136.05, 124.51, 124.30, 118.87, 110.14, 106.60, 101.25, 55.58, 53.95, 53.53. IR (KBr): 1738, 1609, 1511, 1481, 1031 cm−1. HRMS (ESI) m/z: [M + H] calcd for C18H17NO5S 359.0827, found: 360.0894.
Ethyl 2-(benzo[d]oxazol-2-ylthio)-2-(4-chlorophenyl)acetate (3o). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (295 mg, 85% yield). Mp 60–61 °C; 1H NMR (400 MHz, CDCl3) δ 7.52–7.47 (m, 1H), 7.45–7.40 (m, 2H), 7.33 (dd, J = 7.8, 1.4 Hz, 1H), 7.25 (d, J = 8.5 Hz, 2H), 7.21–7.12 (m, 2H), 5.59 (s, 1H), 4.16 (m, 2H), 1.16 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.08, 162.61, 151.97, 141.74, 135.10, 133.10, 129.94, 129.32, 124.52, 124.35, 118.82, 110.12, 62.72, 53.29, 14.09. IR (KBr): 1742, 1504, 1451, 1232, 1015 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14ClNO3S 347.0383, found: 348.0441.
5). White solid (295 mg, 85% yield). Mp 60–61 °C; 1H NMR (400 MHz, CDCl3) δ 7.52–7.47 (m, 1H), 7.45–7.40 (m, 2H), 7.33 (dd, J = 7.8, 1.4 Hz, 1H), 7.25 (d, J = 8.5 Hz, 2H), 7.21–7.12 (m, 2H), 5.59 (s, 1H), 4.16 (m, 2H), 1.16 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.08, 162.61, 151.97, 141.74, 135.10, 133.10, 129.94, 129.32, 124.52, 124.35, 118.82, 110.12, 62.72, 53.29, 14.09. IR (KBr): 1742, 1504, 1451, 1232, 1015 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14ClNO3S 347.0383, found: 348.0441.
Ethyl 2-(benzo[d]oxazol-2-ylthio)-2-(3,4-dichlorophenyl)acetate (3p). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (309 mg, 81% yield). Mp 61–62 °C; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 2.0 Hz, 1H), 7.45–7.41 (m, 1H), 7.30–7.22 (m, 3H), 7.13–7.03 (m, 2H), 5.53 (s, 1H), 4.10 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.47, 162.09, 151.87, 141.53, 134.85, 133.23, 133.03, 130.85, 130.46, 127.84, 124.45, 124.32, 118.72, 110.01, 62.81, 52.77, 13.98. IR (KBr): 1739, 1504, 1452, 1096, 1032 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H13Cl2NO3S 380.9993, found: 382.0070.
5). White solid (309 mg, 81% yield). Mp 61–62 °C; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 2.0 Hz, 1H), 7.45–7.41 (m, 1H), 7.30–7.22 (m, 3H), 7.13–7.03 (m, 2H), 5.53 (s, 1H), 4.10 (m, 2H), 1.10 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.47, 162.09, 151.87, 141.53, 134.85, 133.23, 133.03, 130.85, 130.46, 127.84, 124.45, 124.32, 118.72, 110.01, 62.81, 52.77, 13.98. IR (KBr): 1739, 1504, 1452, 1096, 1032 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H13Cl2NO3S 380.9993, found: 382.0070.
Ethyl 2-(benzo[d]oxazol-2-ylthio)-2-(3-bromophenyl)acetate (3q). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (310 mg, 79% yield). 1H NMR (400 MHz, CDCl3) δ 7.60–7.57 (m, 1H), 7.52–7.48 (m, 3H), 7.46–7.41 (m, 3H), 7.31–7.26 (m, 2H), 5.65 (s, 1H), 4.30–4.18 (m, 2H), 1.25 (t, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.02, 162.59, 151.99, 141.75, 133.65, 132.30, 131.71, 130.24, 129.93, 124.54, 124.38, 123.30, 118.84, 110.14, 62.76, 53.37, 14.11. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14BrNO3S 390.9878, found: 391.9950.
5). White liquid (310 mg, 79% yield). 1H NMR (400 MHz, CDCl3) δ 7.60–7.57 (m, 1H), 7.52–7.48 (m, 3H), 7.46–7.41 (m, 3H), 7.31–7.26 (m, 2H), 5.65 (s, 1H), 4.30–4.18 (m, 2H), 1.25 (t, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.02, 162.59, 151.99, 141.75, 133.65, 132.30, 131.71, 130.24, 129.93, 124.54, 124.38, 123.30, 118.84, 110.14, 62.76, 53.37, 14.11. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14BrNO3S 390.9878, found: 391.9950.
Methyl 2-((4-fluorobenzo[d]oxazol-2-yl)thio)-2-phenylacetate (4a). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (250 mg, 79% yield). Mp 89–90 °C; 1H NMR (400 MHz, CDCl3) δ 7.48–7.42 (m, 2H), 7.30 (td, J = 5.2, 3.2 Hz, 2H), 7.28–7.17 (m, 3H), 6.87 (td, J = 9.1, 2.6 Hz, 1H), 5.61 (s, 1H), 3.70 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.81, 164.95, 161.22, 158.82, 148.21, 142.60, 142.47, 133.84, 129.18, 129.16, 128.45, 111.66, 111.40, 110.24, 110.14, 105.61, 105.35, 53.75, 53.44. IR (KBr): 1742, 1603, 1509, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12FNO3S 317.0522, found: 318.0589.
5). White solid (250 mg, 79% yield). Mp 89–90 °C; 1H NMR (400 MHz, CDCl3) δ 7.48–7.42 (m, 2H), 7.30 (td, J = 5.2, 3.2 Hz, 2H), 7.28–7.17 (m, 3H), 6.87 (td, J = 9.1, 2.6 Hz, 1H), 5.61 (s, 1H), 3.70 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.81, 164.95, 161.22, 158.82, 148.21, 142.60, 142.47, 133.84, 129.18, 129.16, 128.45, 111.66, 111.40, 110.24, 110.14, 105.61, 105.35, 53.75, 53.44. IR (KBr): 1742, 1603, 1509, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12FNO3S 317.0522, found: 318.0589.
Methyl 2-((4-chlorobenzo[d]oxazol-2-yl)thio)-2-phenylacetate (4b). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (267 mg, 80% yield). Mp 100–101 °C; 1H NMR (400 MHz, CDCl3) δ 7.49–7.46 (m, 1H), 7.44–7.40 (m, 2H), 7.28–7.21 (m, 4H), 7.16–7.08 (m, 1H), 5.58 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.87, 164.83, 150.57, 142.92, 133.91, 130.07, 129.29, 128.56, 124.46, 118.92, 110.76, 53.90, 53.57. IR (KBr): 1743, 1503, 1453, 1132, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0299.
5). White solid (267 mg, 80% yield). Mp 100–101 °C; 1H NMR (400 MHz, CDCl3) δ 7.49–7.46 (m, 1H), 7.44–7.40 (m, 2H), 7.28–7.21 (m, 4H), 7.16–7.08 (m, 1H), 5.58 (s, 1H), 3.68 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.87, 164.83, 150.57, 142.92, 133.91, 130.07, 129.29, 128.56, 124.46, 118.92, 110.76, 53.90, 53.57. IR (KBr): 1743, 1503, 1453, 1132, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12ClNO3S 333.0226, found: 334.0299.
Methyl 2-((4-bromobenzo[d]oxazol-2-yl)thio)-2-phenylacetate (4c). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (314 mg, 83% yield). Mp 90–91 °C; 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 2.0 Hz, 1H), 7.48–7.43 (m, 2H), 7.34–7.26 (m, 4H), 7.24–7.18 (m, 1H), 5.61 (s, 1H), 3.71 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.86, 164.70, 151.00, 143.37, 133.90, 129.29, 128.56, 127.20, 121.89, 117.34, 111.28, 53.92, 53.58. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12BrNO3S 376.9721, found: 377.9795.
5). White solid (314 mg, 83% yield). Mp 90–91 °C; 1H NMR (400 MHz, CDCl3) δ 7.66 (d, J = 2.0 Hz, 1H), 7.48–7.43 (m, 2H), 7.34–7.26 (m, 4H), 7.24–7.18 (m, 1H), 5.61 (s, 1H), 3.71 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.86, 164.70, 151.00, 143.37, 133.90, 129.29, 128.56, 127.20, 121.89, 117.34, 111.28, 53.92, 53.58. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12BrNO3S 376.9721, found: 377.9795.
Methyl 2-((4-nitrobenzo[d]oxazol-2-yl)thio)-2-phenylacetate (4d). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (268 mg, 78% yield). 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 2.3 Hz, 1H), 8.13 (dd, J = 8.9, 2.3 Hz, 1H), 7.48–7.41 (m, 3H), 7.33–7.29 (m, 3H), 5.63 (s, 1H), 3.72 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.58, 167.15, 155.34, 145.34, 142.22, 133.53, 129.42, 129.33, 128.89, 128.54, 120.42, 114.89, 110.17, 54.16, 53.65. IR (KBr): 1742, 1510, 1460, 1131, 1085 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12N2O5S 344.0467, found: 345.0477.
5). White liquid (268 mg, 78% yield). 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 2.3 Hz, 1H), 8.13 (dd, J = 8.9, 2.3 Hz, 1H), 7.48–7.41 (m, 3H), 7.33–7.29 (m, 3H), 5.63 (s, 1H), 3.72 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.58, 167.15, 155.34, 145.34, 142.22, 133.53, 129.42, 129.33, 128.89, 128.54, 120.42, 114.89, 110.17, 54.16, 53.65. IR (KBr): 1742, 1510, 1460, 1131, 1085 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H12N2O5S 344.0467, found: 345.0477.
Methyl 2-((4-methylbenzo[d]oxazol-2-yl)thio)-2-phenylacetate (4e). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (253 mg, 81% yield). Mp 75–76 °C; 1H NMR (400 MHz, CDCl3) δ 7.56–7.50 (m, 2H), 7.41–7.33 (m, 4H), 7.29 (d, J = 8.3 Hz, 1H), 7.04 (dd, J = 8.4, 1.7 Hz, 1H), 5.70 (s, 1H), 3.78 (s, 3H), 2.43 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.09, 162.84, 150.28, 142.04, 134.34, 134.24, 129.22, 129.15, 128.57, 125.27, 118.94, 109.48, 53.76, 53.46, 21.55. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO3S 313.0773, found: 314.0839.
5). White solid (253 mg, 81% yield). Mp 75–76 °C; 1H NMR (400 MHz, CDCl3) δ 7.56–7.50 (m, 2H), 7.41–7.33 (m, 4H), 7.29 (d, J = 8.3 Hz, 1H), 7.04 (dd, J = 8.4, 1.7 Hz, 1H), 5.70 (s, 1H), 3.78 (s, 3H), 2.43 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 170.09, 162.84, 150.28, 142.04, 134.34, 134.24, 129.22, 129.15, 128.57, 125.27, 118.94, 109.48, 53.76, 53.46, 21.55. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO3S 313.0773, found: 314.0839.
Methyl 2-((4-methoxybenzo[d]oxazol-2-yl)thio)-2-phenylacetate (4f). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Pale yellow liquid (270 mg, 82% yield). 1H NMR (400 MHz, CDCl3) δ 7.56–7.50 (m, 2H), 7.37–7.30 (m, 3H), 7.26 (d, J = 8.9 Hz, 1H), 7.09 (d, J = 2.5 Hz, 1H), 6.80 (dd, J = 8.9, 2.6 Hz, 1H), 5.70 (s, 1H), 3.78 (s, 3H), 3.75 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.87, 163.33, 157.21, 146.50, 142.54, 134.06, 129.09, 129.02, 128.39, 112.20, 110.01, 102.26, 55.84, 53.66, 53.29. IR (KBr): 1738, 1609, 1511, 1481, 1031 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO4S 329.0722, found: 330.0795.
5). Pale yellow liquid (270 mg, 82% yield). 1H NMR (400 MHz, CDCl3) δ 7.56–7.50 (m, 2H), 7.37–7.30 (m, 3H), 7.26 (d, J = 8.9 Hz, 1H), 7.09 (d, J = 2.5 Hz, 1H), 6.80 (dd, J = 8.9, 2.6 Hz, 1H), 5.70 (s, 1H), 3.78 (s, 3H), 3.75 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.87, 163.33, 157.21, 146.50, 142.54, 134.06, 129.09, 129.02, 128.39, 112.20, 110.01, 102.26, 55.84, 53.66, 53.29. IR (KBr): 1738, 1609, 1511, 1481, 1031 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H15NO4S 329.0722, found: 330.0795.
Ethyl 2-((4-chlorobenzo[d]oxazol-2-yl)thio)-2-(4-chlorophenyl)acetate (4g). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (291 mg, 79% yield). Mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 2.0 Hz, 1H), 7.55–7.51 (m, 2H), 7.40–7.35 (m, 3H), 7.25 (ddd, J = 8.7, 2.1, 0.9 Hz, 1H), 5.68 (s, 1H), 4.35–4.20 (m, 2H), 1.31–1.27 (m, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.94, 164.48, 150.57, 142.83, 135.25, 132.87, 130.13, 129.95, 129.40, 124.54, 118.88, 110.78, 62.84, 53.45, 14.13. IR (KBr): 1743, 1503, 1453, 1132, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H13Cl2NO3S 380.9993, found: 382.0058.
5). White solid (291 mg, 79% yield). Mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 2.0 Hz, 1H), 7.55–7.51 (m, 2H), 7.40–7.35 (m, 3H), 7.25 (ddd, J = 8.7, 2.1, 0.9 Hz, 1H), 5.68 (s, 1H), 4.35–4.20 (m, 2H), 1.31–1.27 (m, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.94, 164.48, 150.57, 142.83, 135.25, 132.87, 130.13, 129.95, 129.40, 124.54, 118.88, 110.78, 62.84, 53.45, 14.13. IR (KBr): 1743, 1503, 1453, 1132, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H13Cl2NO3S 380.9993, found: 382.0058.
Methyl 2-((4-bromobenzo[d]oxazol-2-yl)thio)-2-(2,4-dichlorophenyl)acetate (4h). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Orange liquid (344 mg, 77% yield). 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 2.0 Hz, 1H), 7.50 (d, J = 8.4 Hz, 1H), 7.41 (d, J = 2.2 Hz, 1H), 7.32 (ddd, J = 8.6, 1.9, 0.6 Hz, 1H), 7.25–7.19 (m, 2H), 6.10 (s, 1H), 3.76 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.82, 164.16, 151.15, 143.26, 135.81, 135.11, 131.26, 131.07, 130.17, 127.94, 127.34, 122.00, 117.45, 111.33, 53.86, 50.64. IR (KBr): 1746, 1587, 1503, 1453, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H10BrCl2NO3S 444.8942, found: 445.8997.
5). Orange liquid (344 mg, 77% yield). 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 2.0 Hz, 1H), 7.50 (d, J = 8.4 Hz, 1H), 7.41 (d, J = 2.2 Hz, 1H), 7.32 (ddd, J = 8.6, 1.9, 0.6 Hz, 1H), 7.25–7.19 (m, 2H), 6.10 (s, 1H), 3.76 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.82, 164.16, 151.15, 143.26, 135.81, 135.11, 131.26, 131.07, 130.17, 127.94, 127.34, 122.00, 117.45, 111.33, 53.86, 50.64. IR (KBr): 1746, 1587, 1503, 1453, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H10BrCl2NO3S 444.8942, found: 445.8997.
Methyl 2-((4-chlorobenzo[d]oxazol-2-yl)thio)-2-(4-fluorophenyl)acetate (4i). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (285 mg, 81% yield). Mp 70–71 °C; 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 2.0 Hz, 1H), 7.53 (dd, J = 8.5, 5.2 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.21 (dd, J = 8.6, 2.1 Hz, 1H), 7.06 (t, J = 8.5 Hz, 2H), 5.68 (s, 1H), 3.79 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.68, 164.52, 164.40, 161.92, 150.58, 142.86, 130.50, 130.42, 130.14, 130.02, 129.99, 124.54, 118.93, 116.38, 116.16, 110.77, 53.60, 53.15. IR (KBr): 1742, 1603, 1509, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H11ClFNO3S 351.0132, found: 352.0199.
5). White solid (285 mg, 81% yield). Mp 70–71 °C; 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 2.0 Hz, 1H), 7.53 (dd, J = 8.5, 5.2 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.21 (dd, J = 8.6, 2.1 Hz, 1H), 7.06 (t, J = 8.5 Hz, 2H), 5.68 (s, 1H), 3.79 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.68, 164.52, 164.40, 161.92, 150.58, 142.86, 130.50, 130.42, 130.14, 130.02, 129.99, 124.54, 118.93, 116.38, 116.16, 110.77, 53.60, 53.15. IR (KBr): 1742, 1603, 1509, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C16H11ClFNO3S 351.0132, found: 352.0199.
Methyl 2-((4-methoxybenzo[d]oxazol-2-yl)thio)-2-(4-nitrophenyl)acetate (4j). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (329 mg, 88% yield). Mp 121–122 °C; 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 2.3 Hz, 1H), 8.21 (dd, J = 8.9, 2.4 Hz, 1H), 7.48 (dd, J = 20.7, 8.6 Hz, 3H), 6.90 (d, J = 8.2 Hz, 2H), 5.67 (s, 1H), 3.80 (s, 6H). 13C NMR (101 MHz, chloroform-d) δ 169.80, 167.33, 160.44, 155.36, 145.39, 142.32, 129.87, 125.33, 120.40, 114.89, 114.74, 110.15, 55.46, 53.72, 53.58. IR (KBr): 1742, 1510, 1460, 1131, 1085 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14N2O6S 374.0573, found: 375.0645.
5). White solid (329 mg, 88% yield). Mp 121–122 °C; 1H NMR (400 MHz, CDCl3) δ 8.46 (d, J = 2.3 Hz, 1H), 8.21 (dd, J = 8.9, 2.4 Hz, 1H), 7.48 (dd, J = 20.7, 8.6 Hz, 3H), 6.90 (d, J = 8.2 Hz, 2H), 5.67 (s, 1H), 3.80 (s, 6H). 13C NMR (101 MHz, chloroform-d) δ 169.80, 167.33, 160.44, 155.36, 145.39, 142.32, 129.87, 125.33, 120.40, 114.89, 114.74, 110.15, 55.46, 53.72, 53.58. IR (KBr): 1742, 1510, 1460, 1131, 1085 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14N2O6S 374.0573, found: 375.0645.
Methyl 2-(3,5-dimethoxyphenyl)-2-((4-methylbenzo[d]oxazol-2-yl)thio)acetate (4k). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (310 mg, 83% yield). 1H NMR (400 MHz, CDCl3) δ 7.22 (dt, J = 39.6, 5.6 Hz, 2H), 6.93 (t, J = 7.5 Hz, 1H), 6.62–6.54 (m, 2H), 6.32 (t, J = 7.2 Hz, 1H), 5.53 (s, 1H), 3.68 (s, 10H), 2.32 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.84, 162.78, 161.20, 150.18, 141.94, 136.00, 134.24, 125.18, 118.80, 109.38, 106.49, 101.10, 55.46, 53.82, 53.41, 21.44. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C19H19NO5S 373.0984, found: 374.1046.
5). White liquid (310 mg, 83% yield). 1H NMR (400 MHz, CDCl3) δ 7.22 (dt, J = 39.6, 5.6 Hz, 2H), 6.93 (t, J = 7.5 Hz, 1H), 6.62–6.54 (m, 2H), 6.32 (t, J = 7.2 Hz, 1H), 5.53 (s, 1H), 3.68 (s, 10H), 2.32 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.84, 162.78, 161.20, 150.18, 141.94, 136.00, 134.24, 125.18, 118.80, 109.38, 106.49, 101.10, 55.46, 53.82, 53.41, 21.44. IR (KBr): 1747, 1503, 1453, 1132, 1097 cm−1. HRMS (ESI) m/z: [M + H] calcd for C19H19NO5S 373.0984, found: 374.1046.
Ethyl 2-((4-bromobenzo[d]oxazol-2-yl)thio)-2-(p-tolyl)acetate (4l). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Orange liquid (345 mg, 85% yield). 1H NMR (400 MHz, CDCl3) 7.69 (d, J = 1.9 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 7.31 (dd, J = 8.6, 1.9 Hz, 1H), 7.23 (d, J = 5.9 Hz, 1H), 7.14 (d, J = 7.8 Hz, 2H), 5.59 (s, 1H), 4.20 (m, 2H), 2.31 (s, 3H), 1.22 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.46, 164.94, 151.00, 143.47, 139.25, 130.98, 129.94, 128.45, 127.14, 121.84, 117.32, 111.24, 62.58, 53.89, 21.33, 14.17. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C18H16BrNO3S 405.0034, found: 406.0037.
5). Orange liquid (345 mg, 85% yield). 1H NMR (400 MHz, CDCl3) 7.69 (d, J = 1.9 Hz, 1H), 7.38 (d, J = 8.2 Hz, 2H), 7.31 (dd, J = 8.6, 1.9 Hz, 1H), 7.23 (d, J = 5.9 Hz, 1H), 7.14 (d, J = 7.8 Hz, 2H), 5.59 (s, 1H), 4.20 (m, 2H), 2.31 (s, 3H), 1.22 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.46, 164.94, 151.00, 143.47, 139.25, 130.98, 129.94, 128.45, 127.14, 121.84, 117.32, 111.24, 62.58, 53.89, 21.33, 14.17. IR (KBr): 1747, 1504, 1452, 1096, 1002 cm−1. HRMS (ESI) m/z: [M + H] calcd for C18H16BrNO3S 405.0034, found: 406.0037.
Ethyl 2-(benzo[d]oxazol-2-ylthio)acetate (5a). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (221 mg, 93% yield). 1H NMR (400 MHz, chloroform-d) δ 7.59 (d, J = 7.6 Hz, 1H), 7.46–7.41 (m, 1H), 7.31–7.21 (m, 2H), 4.25 (q, J = 7.1 Hz, 2H), 4.12 (s, 2H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 167.90, 163.32, 152.11, 141.78, 124.42, 124.13, 118.65, 110.01, 62.21, 34.28, 14.11. IR (KBr): 1735, 1502, 1470, 1450, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C11H11NO3S 237.0460, found: 238.0526.
5). White solid (221 mg, 93% yield). 1H NMR (400 MHz, chloroform-d) δ 7.59 (d, J = 7.6 Hz, 1H), 7.46–7.41 (m, 1H), 7.31–7.21 (m, 2H), 4.25 (q, J = 7.1 Hz, 2H), 4.12 (s, 2H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 167.90, 163.32, 152.11, 141.78, 124.42, 124.13, 118.65, 110.01, 62.21, 34.28, 14.11. IR (KBr): 1735, 1502, 1470, 1450, 1130 cm−1. HRMS (ESI) m/z: [M + H] calcd for C11H11NO3S 237.0460, found: 238.0526.
Ethyl 2-((6-chlorobenzo[d]thiazol-2-yl)thio)acetate (5b). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White solid (288 mg, 89% yield). 1H NMR (400 MHz, chloroform-d) δ 7.81 (d, J = 2.1 Hz, 1H), 7.63 (dd, J = 8.5, 1.7 Hz, 1H), 7.25 (dq, J = 8.4, 2.5, 2.0 Hz, 1H), 4.24 (q, J = 7.1 Hz, 2H), 4.15 (s, 2H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.13, 167.13, 153.70, 133.80, 132.25, 124.87, 121.75, 121.61, 62.18, 35.18, 14.21. IR (KBr): 1741, 1501, 1452, 1130, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C11H10ClNO2S2 286.9841, found: 287.9917.
5). White solid (288 mg, 89% yield). 1H NMR (400 MHz, chloroform-d) δ 7.81 (d, J = 2.1 Hz, 1H), 7.63 (dd, J = 8.5, 1.7 Hz, 1H), 7.25 (dq, J = 8.4, 2.5, 2.0 Hz, 1H), 4.24 (q, J = 7.1 Hz, 2H), 4.15 (s, 2H), 1.29 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.13, 167.13, 153.70, 133.80, 132.25, 124.87, 121.75, 121.61, 62.18, 35.18, 14.21. IR (KBr): 1741, 1501, 1452, 1130, 1095 cm−1. HRMS (ESI) m/z: [M + H] calcd for C11H10ClNO2S2 286.9841, found: 287.9917.
Ethyl-2-((6-chlorobenzo[d]thiazol-2-yl)thio)-2-phenylacetate (5c). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (295 mg, 81% yield). 1H NMR (400 MHz, chloroform-d) δ 7.75 (s, 1H), 7.51 (d, J = 8.6 Hz, 1H), 7.45 (dd, J = 7.8, 1.8 Hz, 2H), 7.29 (d, J = 6.1 Hz, 2H), 7.24 (d, J = 1.0 Hz, 1H), 7.16 (dd, J = 8.5, 2.1 Hz, 1H), 5.70 (s, 1H), 4.15 (m, 2H), 1.17 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.46, 166.85, 153.75, 134.18, 133.77, 132.22, 129.15, 129.11, 129.07, 129.00, 128.62, 124.91, 121.78, 121.59, 62.38, 54.76, 14.16. IR (KBr): 1740, 1500, 1450, 1130, 1090 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14ClNO2S2 363.0154, found: 364.0224.
5). White liquid (295 mg, 81% yield). 1H NMR (400 MHz, chloroform-d) δ 7.75 (s, 1H), 7.51 (d, J = 8.6 Hz, 1H), 7.45 (dd, J = 7.8, 1.8 Hz, 2H), 7.29 (d, J = 6.1 Hz, 2H), 7.24 (d, J = 1.0 Hz, 1H), 7.16 (dd, J = 8.5, 2.1 Hz, 1H), 5.70 (s, 1H), 4.15 (m, 2H), 1.17 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 169.46, 166.85, 153.75, 134.18, 133.77, 132.22, 129.15, 129.11, 129.07, 129.00, 128.62, 124.91, 121.78, 121.59, 62.38, 54.76, 14.16. IR (KBr): 1740, 1500, 1450, 1130, 1090 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H14ClNO2S2 363.0154, found: 364.0224.
Ethyl-2-((6-chlorobenzo[d]thiazol-2-yl)thio)-2-(3,4-dichlorophenyl)acetate (5d). Purified by column chromatography (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 95
EtOAc, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). White liquid (377 mg, 78% yield). 1H NMR (400 MHz, chloroform-d) δ 7.83 (s, 1H), 7.67 (s, 1H), 7.63 (d, J = 8.5 Hz, 1H), 7.45–7.37 (m, 2H), 7.27 (d, J = 8.7 Hz, 1H), 5.77 (s, 1H), 4.25 (m, 2H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.70, 165.86, 153.64, 134.90, 133.80, 133.32, 133.19, 132.43, 130.97, 130.67, 128.03, 125.19, 121.87, 121.72, 62.84, 53.54, 14.16. IR (KBr): 1741, 1504, 1455, 1133, 1096 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H12Cl3NO2S2 430.9375, found: 431.9447.
5). White liquid (377 mg, 78% yield). 1H NMR (400 MHz, chloroform-d) δ 7.83 (s, 1H), 7.67 (s, 1H), 7.63 (d, J = 8.5 Hz, 1H), 7.45–7.37 (m, 2H), 7.27 (d, J = 8.7 Hz, 1H), 5.77 (s, 1H), 4.25 (m, 2H), 1.27 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.70, 165.86, 153.64, 134.90, 133.80, 133.32, 133.19, 132.43, 130.97, 130.67, 128.03, 125.19, 121.87, 121.72, 62.84, 53.54, 14.16. IR (KBr): 1741, 1504, 1455, 1133, 1096 cm−1. HRMS (ESI) m/z: [M + H] calcd for C17H12Cl3NO2S2 430.9375, found: 431.9447.
Author contributions
Arumugam Jayarani contributed majorly to this work. The corresponding author developed the methodology, supervised the work, helped in fund generation and analysed the data, and the other co-author contributed to data collection.Conflicts of interest
There are no conflicts to declare.Data availability
The data underlying this study are available in the published article and its supplementary information (SI). Supplementary information: experimental details and spectroscopic data (1H and 13C NMR). See DOI: https://doi.org/10.1039/d5ra06843f.Acknowledgements
C. S. gratefully acknowledges the Anusandhan National Research Foundation (ANRF), New Delhi, India, for the financial support (ANRF/PAIR/2025/000021/PAIR). A. J gratefully acknowledges the University Grants Commission (UGC). We thank the Central Instrumentation Facility for NMR, Pondicherry University and the Department of Science and Technology-Fund for Improvement of Science and Technology (DST-FIST).Notes and references
- (a) D. R. Taylor, M. MacCoss and D. G. A. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef; (b) E. Vitaku, T. D. Smith and T. J. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (c) M. Baumann and R. I. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265–2319 CrossRef; (d) A. Cagir, H. S. Jones, R. GaO, M. B. Eisenhauer and M. S. Hecht, J. Am. Chem. Soc., 2003, 125, 13628–13629 CrossRef CAS; (e) H. D. Boschelli, T. D. Connor, A. D. Bornemeier, D. R. Dyer, A. J. Kennedy, J. P. Kuipers, C. G. Okonkwo, J. D. Schrier and D. C. Wright, J. Med. Chem., 1993, 36, 1802–1810 CrossRef; (f) P. Kohli, D. S. Srivastava and K. S. Srivastava, J. Chin. Chem. Soc., 2007, 54, 1003–1010 CrossRef CAS.
- (a) N. Kharasch and C. Y. Meyers, The chemistry of organic sulfur compound, New york, 1996 Search PubMed; (b) R. J. Cremlyn, An Introduction to Organosulfur Chemistry, John Wiley & Sons, Chichester, U.K., 1996 Search PubMed.
- For selected reviews, see. (a) F. Bernardi, G. I. Csizmadia and A. Mangini, The Chemistry of Organic Selenium and Tellurium Compounds, Elsevier, Amsterdam, the Netherlands, 1985, vol. 19 Search PubMed; (b) C. K. Nicolaou, H. R. C. Hale, C. Nilewski and A. H. Ioannidou, Chem. Soc. Rev., 2012, 41, 5185–5238 RSC; (c) F. Minghao, T. Bingqing, L. H. Steven and J. Xuefeng, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef.
- (a) Y. D. Lin, Z. S. Zhang, E. Block and C. L. Katz, Nature, 2005, 434, 470–477 CrossRef PubMed; (b) E. C. Sansom, S. V. Jones, I. N. Joyce, M. B. Smallfield, B. N. Perry and V. W. J. Klink, J. Agric. Food Chem., 2015, 63, 1833–1838 Search PubMed.
- E. M. Cinar and T. Ozturk, Chem. Rev., 2015, 115, 3036–3140 CrossRef PubMed.
- For application of the Julia olefination: (a) A. Kumar, S. Sharma, D. V. Tripathi and S. Srivastava, Tetrahedron, 2010, 66, 9445–9449 CrossRef CAS; (b) For application of benzothiophene synthesis: J. M. Rospondek, L. Marynowski and M. Gora, Org. Geochem., 2007, 38, 1729–1756 CrossRef.
- (a) S. Aiello, G. Wells, E. L. Stone, H. Kadri, R. Bazzi, D. R. Bell, M. F. G. Stevens, C. S. T. Matthews, D. Bradshaw and A. D. Westwell, J. Med. Chem., 2008, 51, 5135–5139 CrossRef CAS; (b) J. P. Davidson and E. J. Corey, J. Am. Chem. Soc., 2003, 125, 13486–13489 CrossRef CAS PubMed; (c) S. M. Sondhi, N. Singh, A. Kumar, O. Lozach and L. Meijer, Bioorg. Med. Chem., 2006, 14, 3758–3765 CrossRef CAS; (d) C. K. Ryu, R. Y. Lee, N. Y. Kim, Y. H. Kim and A. L. Song, Bioorg. Med. Chem. Lett., 2009, 19, 5924–5926 CrossRef CAS; (e) S. M. Rida, F. A. Ashour, S. A. M. El-Hawash, M. M. ElSemary, M. H. Badr and M. A. Shalaby, Eur. J. Med. Chem., 2005, 40, 949–959 CrossRef CAS PubMed; (f) H. Razavi, S. K. Palaninathan, E. T. Powers, R. L. Wiseman, H. E. Purkey, N. N. Mohamedmohaideen, S. Deechongkit, K. P. Chiang, M. T. A. Dendle, J. C. Sacchettini and J. W. Kelly, Angew. Chem., Int. Ed., 2003, 42, 2758–2761 CrossRef CAS; (g) E. H. Sessions, Y. Yin, T. D. Bannister, A. Weiser, E. Griffin, J. Pocas, M. D. Cameron, C. Ruiz, L. Lin, S. C. Schürer, T. Schröter, P. LoGrasso and Y. Feng, Bioorg. Med. Chem. Lett., 2008, 18, 6390–6393 CrossRef CAS; (h) P. Vianello, P. Cozzi, A. Galvani, M. Meroni, M. Varasi, D. Volpi and T. Vandiera, Bioorg. Med. Chem., 2004, 14, 657–661 CrossRef CAS; (i) A. Halama, J. Jirman, O. Bouskov, P. Gibala and K. Jarrah, Org. Process Res. Dev., 2010, 14, 425–431 CrossRef CAS.
- R. Mitra and G. A. Samuelson, Eur. J. Inorg. Chem., 2014, 11, 3536–3546 CrossRef.
- T. Tankam, J. Srisa, M. Sukwattanasinitt and S. Wacharasindhu, J. Org. Chem., 2018, 83, 11936–11943 CrossRef CAS PubMed.
- C. Safak, R. Simsek, K. Erol and K. Vural, Pharmazie, 1996, 51, 180–182 CAS.
- Y. Xiao, B. Jing, X. Liu, H. Xue and Y. Liu, Beilstein J. Org. Chem., 2019, 15, 279–284 CrossRef CAS PubMed.
- J. Mentado, H. Flores and P. Amador, J. Chem. Thermodyn., 2008, 40, 1106–1109 CrossRef CAS.
- (a) L. Feng, T. Hu, S. Zhang, Y.-H. Xiong and G. Zhang, Org. Lett., 2019, 21, 9487–9492 CrossRef CAS PubMed; (b) P. Liu, M. Yang, Y. Gong, Y. Yu and L.-Y. Zhao, Org. Lett., 2020, 22, 36–40 CrossRef CAS PubMed; (c) Y. Liu and J.-P. Wan, Chem.–Asian J., 2012, 7(7), 1488–1501 CrossRef CAS PubMed; (d) Q. Liao, X. Yang and C. Xi, J. Org. Chem., 2014, 79, 8507–8515 CrossRef CAS.
- (a) D. Gillingham and N. Fei, Chem. Soc. Rev., 2013, 42, 4918–4931 RSC; (b) H. M. L. Davies and J. R. Denton, Chem. Soc. Rev., 2009, 38, 3061–3071 RSC.
- Selected recent reviews for carbene from diazo compound insertion into C–H and X–H bonds, see: (a) H. M. L. Davies and S. J. Hedley, Chem. Soc. Rev., 2007, 36, 1109–1119 RSC; (b) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704–724 CrossRef CAS PubMed; (c) S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365–1377 CrossRef CAS PubMed; (d) J. Wang, Tetrahedron Lett., 2022, 108, 154135 CrossRef CAS; (e) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. Mckervey, Chem. Rev., 2015, 115, 9981–10080 CrossRef CAS PubMed; (f) B. Xu, S.-F. Zhu, Z.-C. Zhang, Z.-X. Yu, Y. Ma and Q.-L. Zhou, Chem. Sci., 2014, 5, 1442–1448 RSC; (g) Y.-Z. Zhang, S.-F. Zhu, Y. Cai, H.-X. Mao and Q.-L. Zhou, Chem. Commun., 2009, 36, 5362–5364 RSC; (h) Z. Zhang, Z. He, Y. Xie, T. He, Y. Fu, Y. Yu and F. Huang, Org. Chem. Front., 2021, 8, 1233–1242 RSC; (i) Z. He, W. Zhao, Y. Li, Y. Yu and F. Huang, Org. Biomol. Chem., 2022, 20, 8078–8082 RSC , ; (j) P. Wang, Y. Gong, X. Wang, Y. Ren, L. Wang, L. Zhai, H. Li and X. She, Chem.–Asian J., 2022, 17, e202200465 CrossRef CAS; (k) Y.-T. Sun, X. Rao, W. Xu and M.-H. Xu, Org. Chem. Front., 2022, 9, 3467–3472 RSC.
- M. Brookhart and B. W. Studabaker, Chem. Rev., 1987, 87, 411–432 CrossRef CAS.
- (a) V. Aggarwal and C. L. Winn, Acc. Chem. Res., 2004, 37, 611–620 CrossRef CAS; (b) Y. Zhang and J. Wang, Coord. Chem. Rev., 2010, 254, 941–953 CrossRef CAS.
- Q. Wang, H. Qi, Y. Ren, Z. Cao, K. Junge, R. V. Jagadeesh and M. Beller, Chem, 2024, 10, 1897–1909 CAS.
- H. Keipour, A. Jalba, L. L. Delage and T. Ollevier, J. Org. Chem., 2017, 82, 3000–3010 CrossRef CAS.
- J. Yang, G. Wang, S. Chen, B. Ma, H. Zhou, M. Song, C. Liu and C. Huo, Org. Biomol. Chem., 2020, 18, 9494–9498 RSC.
- S. Chand, P. A. Kumar, R. Singh, S. Kumar and S. K. Nand, Chem.–Asian J., 2019, 14, 4712–4716 CrossRef CAS PubMed.
- C. Li, R. Wang, L. Huang, J. Chen, J. Jin, Q. Yan, W. Wang, H. Wang and F. Chen, J. Org. Chem., 2023, 88, 4452–4457 CrossRef CAS.
- (a) K. Ramakrishna, M. Murali and C. Sivasankar, Org. Lett., 2015, 17, 3814–3817 CrossRef CAS PubMed; (b) K. Ramakrishna and C. Sivasankar, J. Org. Chem., 2016, 81, 6609–6616 CrossRef CAS PubMed.
- (a) K. Ramakrishna, A. Jayarani, F. F. Koothradan and C. Sivasankar, Appl. Organomet. Chem., 2020, 34, e5748 CrossRef CAS; (b) A. H. Khan, S. Sarkar, M. Shobana and C. Sivasankar, Adv. Synth. Catal., 2023, 365, 4616–4622 CrossRef.
- (a) F. F. Koothradan, A. Jayarani and C. Sivasankar, J. Org. Chem., 2024, 89, 4294–4308 CrossRef CAS; (b) F. F. Koothradan, S. B. Anusree, P. P. Krishnendu, A. Jayarani and C. Sivasankar, J. Org. Chem., 2022, 87, 10564–10575 CrossRef CAS PubMed; (c) G. Jelakam, S. Dey, V. Wotsa and C. Sivasankar, ChemistrySelect, 2025, 10, e202405088 CrossRef CAS; (d) A. Jayarani, M. Deepa, A. H. Khan, F. F. Koothradan, S. Yoganandhini, V. Sreelakshmi and C. Sivasankar, J. Org. Chem., 2023, 88, 15817–15831 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |