Open Access Article
Open Access ArticleMultifunctional N-substituted 2-pyridylbenzothiazole derivatives: singlet oxygen generation, protein binding, and photoactivated anticancer and antibacterial activities
Hanan A. Mohamedab,
Ayman A. Abdel-Shafi c,
Hideyuki Miyataked,
Mohamed E. El-Khouly
c,
Hideyuki Miyataked,
Mohamed E. El-Khouly
 *a and
Amr A. Nassrallah*ef
*a and
Amr A. Nassrallah*ef
aNanoscience Program, Faculty of Basic and Applied Sciences, Egypt-Japan University of Science and Technology (E-JUST), New Borg El-Arab City, Alexandria, Egypt. E-mail: mohamed.elkhouly@ejust.edu.eg
bDepartment of Chemistry, Faculty of Science, Kafrelsheikh University, Kafrelsheikh City, Egypt
cDepartment of Chemistry, Faculty of Science, Ain Shams University, 11566 Abbassia, Cairo, Egypt
dNano Medical Engineering Laboratory, RIKEN, 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan
eBiotechnology Program, Faculty of Basic and Applied Sciences, Egypt-Japan University of Science and Technology (E-JUST), New Borg El-Arab City, Alexandria, Egypt. E-mail: amr.nassrallah@ejust.edu.eg
fBiochemistry Department, Faculty of Agriculture, Cairo University, Giza 12613, Egypt
First published on 18th November 2025
Abstract
This study presents the optical characteristics, and biological characteristics of four N-substituted 2-pyridylbenzothiazole derivatives, namely 6-amino-5-(2-benzothiazolyl)-2-oxo-1-phenyl-1,2-dihydro-3-pyridinecarbonitrile (BTZ-Ph), 6-amino-5-(2-benzothiazolyl)-2-oxo-1-(4-chlorophenyl)-1,2-dihydro-3-pyridinecarbonitrile (BTZ-ClPh), 6-amino-5-(2-benzothiazolyl)-2-oxo-1-(4-methylphenyl)-1,2-dihydro-3-pyridinecarbonitrile (BTZ-MePh), and 6-amino-5-(2-benzothiazolyl)-2-oxo-1-(naphthalen-1-yl)-1,2-dihydro-3-pyridinecarbonitrile (BTZ-Nap). The steady-state absorption and emission properties of the examined compounds were assessed in acetonitrile solutions. The fluorescence quantum yields were determined to be 0.27 (BTZ-Ph), 0.24 (BTZ-ClPh), 0.28 (BTZ-MePh), and 0.25 (BTZ-Nap). The biological studies showed a significant interaction of the examined compounds with bovine serum albumin (BSA), where the binding constant (K), Stern–Volmer quenching constant (Ksv), and the number of binding sites were determined. Steady-state fluorescence and time-correlated single photon counting suggest a highly probable energy transfer pathway from the BSA singlet excited state to the examined compounds. The antibacterial activity was evaluated using disc diffusion and minimum inhibitory concentration (MIC) techniques under both dark and light conditions. The compounds exhibited significant antibacterial activity when subjected to blue illumination. The examined N-substituted 2-pyridylbenzothiazole derivatives exhibited markedly enhanced photo-cytotoxicity against human breast cancer MCF-7 (at IC50 values of 69.54, 49.78, 9.86, and 16.54 µg mL−1 for BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap, respectively) and human colorectal carcinoma HCT-116 cell lines (at IC50 values of 47.65, 147.98, 56.98, and 22.32 µg mL−1 for BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap, respectively) when subjected to blue light excitation at 450 nm. Molecular docking was employed to elucidate the interaction mechanism of the compounds with BSA, revealing that BTZ-Ph exhibited a higher binding energy. The determined BSA-binding constants correlated effectively with the antibacterial and cytotoxic outcomes, as well as the molecular docking data. Significantly, the compounds demonstrated encouraging singlet oxygen quantum yields in acetonitrile, ranging from 0.18 to 0.33 (detected via luminescence), suggesting their potential for cancer photodynamic therapy. These findings suggest the potential of these materials as a multifunctional platform for singlet oxygen generation, protein interaction, and photo-enhanced bioactivity.
| Mohamed E. El-Khouly Mohamed E. El-Khouly, an Egyptian-born chemist, earned his PhD in chemistry from Tohoku University, Japan, in 2002. He conducted postdoctoral research in Japan with funding from Venture Business Laboratory (Chiba University, 2003–2004), Center of Excellence (Tohoku University, 2004–2006), and the Japan Society for the Promotion of Science (Tohoku University, 2006–2008). From 2008 to 2012, he served as a specially appointed Associate Professor at the Department of Materials and Life Science, Graduate School of Engineering, Osaka University. Dr El-Khouly's research primarily focuses on artificial photosynthesis, supramolecular light harvesitng complexes, solar fuels, environmental chemsitry, and biomedical applications. |
1. Introduction
Over the years, the investigation of novel pharmaceutical products has established the promising structure–activity correlation as a fruitful and rapidly advancing field of medicinal chemistry. To figure out active components across a broad spectrum of therapeutic domains within a restricted timeframe, the administration of compound derivatives facilitates the utilization of pharmaceuticals containing nitrogen and sulfur heterocycles, which are pivotal in numerous other developed fields, as well as in disciplines associated with specialized areas of pharmaceutical chemistry.1 The heterocyclic substances have exhibited remarkable biological activity, and their research has been a fascinating area of medical chemistry for many years. A literature review shows that heterocyclic compounds and their derivatives with nitrogen and sulfur atoms offer special and adaptable scaffolds for the invention of new drugs.2 Throughout, organic compounds involving pyridine and thiazole platforms have garnered sustained interest due to their diverse biological activities, including antiviral,3 antifungal,4 anticancer,5 antimicrobial,6 anti-hypertensive,7 anti-inflammatory,8 anti-histamine,9 anticonvulsant,10 and analgesic properties,11 along with numerous other significant biological implications.Bovine serum albumin (BSA) binds to heterocyclic compounds, such as pyridothiazole derivatives with nitrogen and sulfur atoms, through complex interactions mainly driven by hydrogen bonds, hydrophobic forces, and the structural bond with the BSA binding sites, which are primarily located around subdomains IIA and IIIA where important tryptophan residues assist in stabilizing the ligand through static quenching mechanisms.12 The interactions described are essential for identifying drug delivery and pharmacokinetics, as BSA functions as a carrier protein with exceptional acceptor properties for several pharmaceutical components, serving as a dynamic resource in producing innovative drug candidates.13–15 BSA is a crucial globular serum albumin utilized as a model protein in many investigations. The molecular weight of BSA is 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and is composed of 582 amino acid residues. It includes 17 disulfide bridges and a free-SH group.16–18 The structure consists of three unique homologous domains: I, II, and III. Each one of these domains is further subdivided into two subdomains, designated A and B. It includes two tryptophan residues, specifically Trp-213 and Trp-134. The Trp-134 is recognized to be situated in a hydrophilic environment near the protein surface (sub-domain-I B). Trp-213 is positioned within the largest hydrophobic cavity, specifically in domain II (sub-domain-II A). The primary binding regions of BSA are located within subdomains IIA and IIIA.19–22 The interactions that occur between BSA and compounds provide significant insights into the drug's intake and distribution. The UV-visible absorbance and fluorescence emission of amino acid residues in BSA are significant in protein–ligand interactions because of their high specificity, exceptional sensitivity, and immediate reaction capabilities. Additionally, UV-visible absorbance, fluorescence emission, and lifetime studies offer critical insights into the binding mechanism, binding method, binding constants, and location.23–27
000 Da and is composed of 582 amino acid residues. It includes 17 disulfide bridges and a free-SH group.16–18 The structure consists of three unique homologous domains: I, II, and III. Each one of these domains is further subdivided into two subdomains, designated A and B. It includes two tryptophan residues, specifically Trp-213 and Trp-134. The Trp-134 is recognized to be situated in a hydrophilic environment near the protein surface (sub-domain-I B). Trp-213 is positioned within the largest hydrophobic cavity, specifically in domain II (sub-domain-II A). The primary binding regions of BSA are located within subdomains IIA and IIIA.19–22 The interactions that occur between BSA and compounds provide significant insights into the drug's intake and distribution. The UV-visible absorbance and fluorescence emission of amino acid residues in BSA are significant in protein–ligand interactions because of their high specificity, exceptional sensitivity, and immediate reaction capabilities. Additionally, UV-visible absorbance, fluorescence emission, and lifetime studies offer critical insights into the binding mechanism, binding method, binding constants, and location.23–27
Photodynamic therapy (PDT) is a low-invasive, targeted therapeutic approach that employs a photosensitizer (a light-sensitive medicine), a particular wavelength of illumination, and oxygen to generate reactive oxygen species (ROS) that eradicate targeted cells. The photosensitizer absorbs light, becomes energized, and transfers energy to oxygen molecules, producing reactive oxygen species (ROS) that trigger cellular death. Photodynamic therapy (PDT) has demonstrated considerable utility in microbial (antimicrobial PDT) and oncological treatments, garnering increasing medical and academic attention.28 The photosensitizer (PS) must produce reactive oxygen species (ROS) when exposed to light and oxygen, and it should exhibit a significant amount of excited electronic triplet states and singlet oxygen.29 Singlet oxygen (1O2), the lowest excited state of the dioxygen molecule, could be produced using photochemical and chemical methods. The predominant photochemical process includes energy transfer from a triplet excited photosensitizer (3PS*) to nearby molecular oxygen.30–32 The features of the photosensitizer significantly influence the efficiency of PDT, and heterocyclic compounds, particularly those incorporating nitrogen and sulfur atoms, are emerging as potential options owing to their adjustable photophysical and biological properties.33 Heterocyclic molecules bearing N and S atoms, including pyridothiazole derivatives, have attracted considerable attention as effective photosensitizers in photodynamic therapy (PDT) against cancer and bacterial infections. When these compounds are exposed to light, they produce reactive oxygen species that damage important parts of the cells, leading to their death. Among these, phenothiazinium dyes such as methylene blue and toluidine blue O are widely used for antimicrobial PDT, showing potent bactericidal effects against Gram-positive and Gram-negative bacteria under red-light exposure.34 Similarly, benzophenothiazinium derivatives like EtNBS demonstrate significant phototoxicity toward cancer cells and are effective even under hypoxic conditions.35 Recent studies have also highlighted thionated heterocycles, such as thieno[3,4-d]pyrimidin-4(3H)-thione (ThiathioHX), as promising non-porphyrinic PDT agents with oxygen-independent activity in tumor environments.36
Motivated by the interesting photophysical and biological properties of pyridine and benzothiazole substitutes, we examined four new N-substituted 2-pyridylbenzothiazole derivatives to investigate their biomedical activities. These compounds are: 6-amino-5-(2-benzothiazolyl)-2-oxo-1-phenyl-1,2-dihydro-3-pyridinecarbonitrile (BTZ-Ph), 6-amino-5-(2-benzothiazolyl)-2-oxo-1-(4-chlorophenyl)-1,2-dihydro-3-pyridinecarbonitrile (BTZ-ClPh), 6-amino-5-(2-benzothiazolyl)-2-oxo-1-(4-methylphenyl)-1,2-dihydro-3-pyridinecarbonitrile (BTZ-MePh), and 6-amino-5-(2-benzothiazolyl)-2-oxo-1-(naphthalen-1-yl)-1,2-dihydro-3-pyridinecarbonitrile (BTZ-Nap) (Fig. 1). This study focuses on their photophysical properties, their interaction with bovine serum albumin (BSA), and their potential as photosensitizers for photodynamic therapy (PDT) against cancer and bacterial infections.
2. Experimental
2.1. Synthesis of the four N-substituted 2-pyridylbenzothiazole derivatives
The pyridine-benzothiazole chemical group under investigation was synthesized through the reaction of 2-(benzo[d]thiazol-2-yl)-3-(dimethylamino)acrylonitrile (10 mmol) with N-aryl-2-cyanoacetamides (10 mmol) and potassium hydroxide in dry 1,4-dioxane (30 mL) under reflux at 101 °C for 1 h, then cooled and poured onto iced water; the resulting precipitate was filtered off and recrystallized from ethanol. The reaction yield of the four compounds was around 80% and they were characterized by FT-IR and HNMR (Fig. S1 and S2) according to established literature, Scheme 1.37 | ||
| Scheme 1 A schematic diagram of the synthesis of four N-substituted 2-pyridylbenzothiazole derivatives. | ||
2.2. Instruments and measurements
The optical absorption spectra of the four N-substituted 2-pyridylbenzothiazole derivatives were recorded using a UV-2600 spectrophotometer (Shimadzu, Japan) equipped with 1 cm quartz cuvettes at room temperature. To evaluate the interaction between BSA and the compounds, absorption titration experiments were performed by recording the absorption spectra of BSA solutions in the absence and presence of the four N-substituted 2-pyridylbenzothiazole derivatives. Stock solutions of the thiazole derivatives were gradually added to 2.0 mL of BSA solution (18 µM), and the corresponding changes in the BSA absorption spectra were determined after each addition.The fluorescence measurements were examined the four N-substituted 2-pyridylbenzothiazole derivatives were recorded using utilizing RF6000 spectrofluorometer (Shimadzu, Japan). The fluorescence quenching experiment employed a fixed concentration of BSA solution (18 µM) while incrementally adding varying amounts of four N-substituted 2-pyridylbenzothiazoles. The fluorescence spectra were obtained at an excitation wavelength of 278 nm, with emission measured between 300 and 600 nm following each quencher addition, and the findings were then evaluated.
The fluorescence quantum yields (Φf) of the examined compounds were calculated utilizing coumarin 460 as a reference (Φref = 0.73 in ethanol)38,39 according to eqn (1).
 | (1) |
Fluorescence lifetimes were measured utilizing the Time-Correlated Single Photon Counting (TCSPC) apparatus (HORIBA Scientific), employing NanoLED pulsed diode light source (at 280 nm) as the excitation wavelength and 340 nm as the emission wavelength. Upon excitation, the fluorescence decay profiles of BSA (18 µM) were measured both in the absence and presence of the examined compounds and were analyzed with the 64-decay analysis software to estimate the fluorescent lifetimes.40
Singlet oxygen quantum yields (ΦΔ) are detected directly by measurement of singlet oxygen luminescence emission at 1272 nm following photoexcitation of the examined compounds at ambient temperature in acetonitrile and compared it with the reference [Ru(bpy)3]2+ at zero time in acetonitrile solution.41 The compounds are excited using the third harmonic of a Q-S Nd:YAG laser (λ = 355 nm, ∼8 ns pulse length, pulse energy ≤20 mJ) incorporated with LP980 from Edinburgh instruments laser flash photolysis system in emission mode. The luminescent signal of singlet oxygen (1O2) at a wavelength of 1275 nm was measured using the Hamamatsu H10330-45 near-infrared (NIR) detector. The optical densities of the substance under study and the standard were equated at a wavelength of 355 nm.
2.3. Antibacterial activity
The antibacterial activity of the benzothiazolyl-pyridinecarbonitrile derivatives was evaluated against three pathogenic bacterial strains: Salmonella enterica (ATCC 14028), Staphylococcus aureus (ATCC 25923), and Escherichia coli (ATCC 25922). Stock solutions of each compound were prepared in dimethyl sulfoxide (DMSO) and diluted with sterile double-distilled water to obtain final concentrations of 25 µg mL−1 and 50 µg mL−1.Bacterial cultures were grown overnight in Luria–Bertani (LB) medium at 37 °C, and cell suspensions were adjusted to a 0.5 McFarland standard (≈1.5 × 108 CFU mL−1). For photodynamic treatment, samples were irradiated for 20 min at 5.2 mW cm−2 (dose ≈ 6.24 J cm−2) using a blue LED light source (440–490 nm) at a fixed distance of 5 cm, while corresponding control samples were maintained in the dark.42
2.4. In vitro cytotoxic activity
The anticancer potential of the benzothiazolyl-pyridinecarbonitrile derivatives was evaluated against two human cancer cell lines—MCF-7 (breast adenocarcinoma, ATCC® HTB-22™) and HCT-116 (colorectal carcinoma, ATCC® CCL-247™)—along with BJ-1 normal human skin fibroblasts (ATCC® CRL-2522™) as a non-cancerous control.Stock solutions (10 mg mL−1) of each compound were prepared in dimethyl sulfoxide (DMSO) and diluted in culture medium to final working concentrations of 0.5, 1, 5, 10, 50, and 100 µg mL−1 immediately before treatment. Experiments were performed under two conditions: in the dark and following blue light irradiation.
Cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin, and 1% L-glutamine, and maintained at 37 °C in a humidified incubator with 5% CO2. Cells were seeded in 24-well plates at a density of 5 × 106 cells per well and allowed to adhere for 24 h before compound exposure. For the light-treated groups, cells were incubated with test compounds for 6 h, then irradiated with a blue LED source (440–490 nm, 5.2 mW cm−2 (dose ≈ 6.24 J cm−2)) for 20 min at a fixed distance of 5 cm. Dark control groups were completely shielded from light. After treatment, cells were incubated for an additional 24 h at 37 °C.
Cell viability was assessed by the MTT assay.46 Briefly, 20 µL of MTT solution (5 mg mL−1 in PBS) was added to each well and incubated for 4 h. The resulting formazan crystals were dissolved in 100 µL of DMSO, and absorbance was measured at 570 nm using a microplate reader. Cell viability was expressed as a percentage relative to untreated controls.44
All experiments were conducted in triplicate, and results are presented as mean ± standard deviation. IC50 values (compound concentration causing 50% reduction in viability) were calculated using nonlinear regression analysis. Statistical comparisons between light-treated and dark groups were performed using one-way ANOVA followed by Tukey's post hoc test, with p < 0.05 considered statistically significant.
2.5. Computational molecular docking studies
Molecular docking investigations were conducted to identify the binding sites and modes of the developed compounds on bovine serum albumin (BSA), distinguished by its several binding pockets. The simulations employed the crystal structure of BSA complexed with 3,5-diiodosalicylic acid (PDB ID: 4jk4).45 All computations were performed utilizing ICM-Pro software.46 Two separate methodologies were used to determine the most potential binding locations. Initially, docking was directed towards three established ligand binding sites on BSA: Drug Site 1 (DS1), Drug Site 2 (DS2), and Drug Site 3 (DS3), the latter occupying the top region of the FA1 fatty acid pocket.45 Docking for these targeted simulations was conducted employing the co-crystallized ligand, 3,5-diiodosalicylic acid, as a template. Simultaneously, non-template docking experiments were performed for the same sites to facilitate unbiased pose prediction. A comprehensive search for all possible binding pockets on the BSA surface was carried out with the icmPocketFinder algorithm.47 Pockets exhibiting a Drug-Like Density (DLID) score exceeding 0.6 were identified as promising binding sites for subsequent docking simulations. The DLID score is an indicator that quantifies the “druggability” of a binding site, with values exceeding 0.5 often regarded as druggable. All simulations employed the 4D SCARE (Scanning and Refinement) docking protocol.48 This method explicitly considers the adaptability of receptor sidechains within the binding pocket during ligand localization and optimization, therefore offering a more accurate representation of the binding interaction.3. Results and discussion
3.1. Optical properties and lifetime of the N-substituted 2-pyridylbenzothiazole derivatives
The UV-vis absorption and emission spectra of the benzothiazolyl-pyridinecarbonitrile derivatives in acetonitrile were obtained to elucidate their optical properties. Fig. 2a of the UV-vis analysis demonstrates that the absorption spectra of all examined substances display a prominent absorption band at 372 nm, accompanied by two weaker absorption bands at 329 nm and 476 nm. The absorption bands at 326 and 379 nm could originate from π–π* transitions, whereas the absorption band at the longer wavelength of 476 nm comes from the n–π* transition. The fluorescence characteristics of the investigated compounds in acetonitrile displayed prominent emission bands in the 410–424 nm region when the samples received an excitation of 372 nm (Fig. 2b). Based on earlier studies, the fluorescence quantum yields of the synthesized compounds in ethanol determined to be 0.28, 0.24, 0.27, and 0.25 for compounds BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap, respectively.37 These optical absorption and emission features agree well with the literature.37 Furthermore, compounds BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap demonstrated monoexponentially fluorescence decay curves (Fig. 2c), with lifetimes recorded at 0.81, 0.59, 1.18, and 0.84 ns, respectively. The fluorescence lifetimes are within the ideal range for facilitating intersystem crossing (ISC) to the triplet excited state, promoting singlet oxygen generation (1O2).49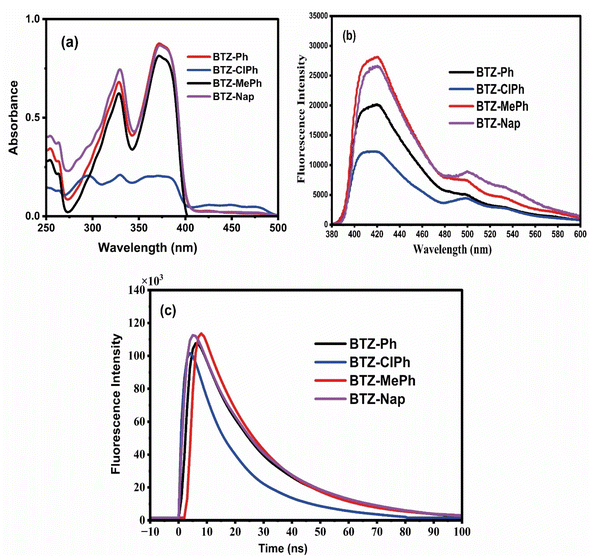 | ||
| Fig. 2 Absorption (a) and emission (b) spectra of the indicated compounds in acetonitrile; λex = 372 nm. (c) Fluorescence lifetime decay profiles in acetonitrile; λex = 372 nm. | ||
3.2. BSA binding studies
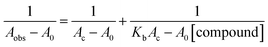 | (2) |
| Compounds | Kb (104 M−1) | ΔG (kcal M−1) | N | Kq (1013 M−1 s−1) | KSV (105 M−1) | fa |
|---|---|---|---|---|---|---|
| BTZ-Ph | 0.64 | −5.18 | 1.32 | 2.08 | 1.19 | 0.9 ± 0.3 |
| BTZ-ClPh | 1.80 | −5.80 | 1.70 | 6.89 | 3.93 | 0.97 ± 0.4 |
| BTZ-MePh | 1.00 | −5.45 | 1.08 | 2.45 | 1.40 | 0.99 ± 0.01 |
| BTZ-Nap | 0.90 | −5.39 | 1.18 | 1.78 | 1.02 | 0.95 ± 0.2 |
The spontaneity of the drug–BSA binding for BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap is indicated by their negative change in Gibbs free energy (ΔG°). Calculated using eqn (3), the values are −5.18, −5.80, −5.45, and −5.39 kcal M−1, respectively. These negative values confirm the spontaneous formation of all compound-BSA complexes.55
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kb Kb
| (3) |
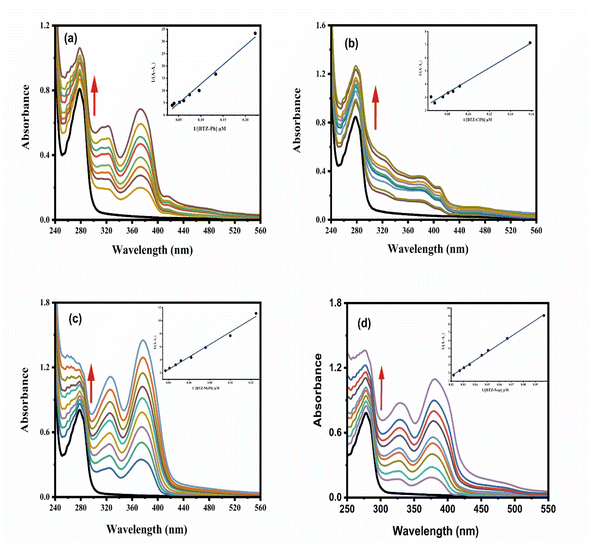
The binding affinity of the four N-substituted 2-pyridylbenzothiazoles for BSA was determined by examining their influence on BSA's fluorescence. As illustrated in Fig. 4, all four compounds caused a substantial quenching of the BSA emission band at 339 nm. The decrease in initial fluorescence intensity was 76.7%, 84.1%, 84.5%, and 80.4% for BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap, respectively. Subsequent analysis using the inset Stern–Volmer plots and eqn (S1)63 yielded the following values: BTZ-Ph (1.4 ×105 M−1), BTZ-ClPh (1.19 ×105 M−1), BTZ-MePh (3.93 ×105 M−1), and BTZ-Nap (1.02 ×105 M−1). Furthermore, the values of n (the number of binding sites), and fa (the fraction of the initial fluorescence of BSA that is accessible to the N-substituted 2-pyridylbenzothiazole) were determined using Fig. S3, S4, eqn (S1) and (S4), while the quenching rate constant kq was calculated by eqn (S3) (Table 1).
As seen from Fig. 4, the emission intensity of BSA was significantly quenched accompanied by formation of the singlet states of the examined four N-substituted 2-pyridylbenzothiazoles derivatives. This suggests the efficient quenching of the singlet state of BSA (3.76 eV) through the energy transfer process to populate the singlet state of the examined N-substituted 2-pyridylbenzothiazole derivatives (∼2.88 eV). The strong overlap between the emission spectrum of BSA and absorption spectra of N-substituted 2-pyridylbenzothiazole derivatives provide further evidence about the occurrence of Förster resonance energy transfer (FRET).64
From Fig. 4, it's clear the emission intensity of BSA significantly quenched as the four examined N-substituted 2-pyridylbenzothiazoles derivatives formed singlet states. This suggests efficient singlet-state quenching of BSA (3.76 eV) occurred via energy transfer to populate the singlet state of the examined pyridylbenzothiazole derivatives (∼2.88 eV). Further evidence for Förster resonance energy transfer (FRET) is provided by the strong overlap between the BSA emission spectrum and the absorption spectra of the N-substituted 2-pyridylbenzothiazole derivatives (Fig. S5).
As is known, the rates of FRET theory depend on the distance between the donor and acceptor, the orientation of their dipoles, and the extent of overlap between the donor's emission spectrum and the acceptor's absorption spectrum. The energy transfer efficiency (E) can be assessed by the distance (r) between the donor (BSA) and the examined N-substituted 2-pyridylbenzothiazole derivatives in the protein using eqn (4).
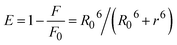 | (4) |
| R06 = 8.8 × 10−25 K2N−4ΦJ | (5) |
In the present situation, the spatial orientation factor of the dipole is K2 = 2/3, the medium (water) refractive index is N = 1.333, and the fluorescence quantum yield of the donor BSA is Φ = 0.15.65 The spectral overlap integral (J), derived from the overlap of the acceptor UV absorption and donor fluorescence emission spectra, is expressed using eqn (6).
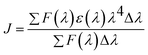 | (6) |
| Compound | J (10−16 L mol−1 cm3) | R0 (nm) | r (nm) | E (%) |
|---|---|---|---|---|
| BTZ-Ph | 1.17 | 1.22 | 1.26 | 0.45 |
| BTZ-ClPh | 1.71 | 1.30 | 1.45 | 0.34 |
| BTZ-MePh | 1.23 | 1.23 | 1.28 | 0.44 |
| BTZ-Nap | 1.56 | 1.28 | 1.40 | 0.36 |
The time-resolved fluorescence spectral features of the BSA/N-substituted 2-pyridylbenzothiazoles interaction confirmed the steady-state emission measurements. Upon excitation at 280 nm, the fluorescence decay profile of the unbound BSA single state, monitored at 340 nm exhibited a mono-exponential decay with a fluorescence lifetime of 5.70 ns (Fig. 5). In contrast, the interaction with N-substituted 2-pyridylbenzothiazoles caused significant quenching of the BSA singlet-state fluorescence lifetime. The observed short lifetime component for the complex confirms that dynamic quenching occurred through Förster resonance energy transfer (FRET).
3.3. Antibacterial activity
The antibacterial activity of the benzothiazolyl-pyridinecarbonitrile derivatives were examined against three representative bacterial strains: Escherichia coli (ATCC 25922), Salmonella enterica (ATCC 14028), and Staphylococcus aureus (ATCC 25923). These strains were chosen to assess efficacy against both Gram-negative and Gram-positive bacteria. The compounds were tested at concentrations of 25 µg mL−1 and 50 µg mL−1 under both dark and light conditions to assess the impact of photoactivation on antibacterial efficacy. Under dark conditions, all four derivatives exhibited no antibacterial activity at both tested concentrations across all three bacterial strains. The minimal zones of inhibition observed in the dark suggest that the compounds are largely inert without light activation, which is a favorable feature for reducing off-target effects during non-irradiated states. However, upon exposure to light, a clear enhancement in antibacterial activity was observed, indicating the photosensitive nature of these molecules and their potential role as photoactivated antimicrobial agents. Among the derivatives, BTZ-Nap showed the most pronounced antibacterial activity under light conditions. At a concentration of 50 µg mL−1, BTZ-Nap generated substantial inhibition zones against E. coli, Salmonella enterica, and Staphylococcus aureus, highlighting its broad-spectrum efficacy. The enhanced activity of BTZ-Nap likely arises from its high ability to generate singlet oxygen (as we will discuss in Section 3.6) that damage bacterial cells. Its planar structure also facilitates membrane binding, positioning ROS close to microbes and amplifying oxidative damage for effective antimicrobial action in PDT.67,68 Similarly, BTZ-MePh showed strong light-activated antibacterial activity, particularly against S. aureus, indicating that the electron-donating methyl group enhances energy transfer to oxygen, and ROS generation. Methyl substitution also improves photostability and membrane affinity, promoting cellular uptake and accumulation, thereby strengthening overall antimicrobial efficacy.69 The quantitative data from Fig. 6 further supports these findings. The antibacterial activity of the compounds increased significantly under light, with BTZ-Nap achieving more than 80% inhibition in some cases, particularly against S. aureus and Salmonella enterica. In contrast, BTZ-Ph and BTZ-ClPh exhibited only moderate enhancement in antibacterial activity upon light exposure, indicating that both the presence and nature of the substituent groups play a critical role in determining photodynamic antibacterial efficacy.Interestingly, Staphylococcus aureus, a Gram-positive organism, appeared more susceptible to the compounds than the Gram-negative strains. This difference in susceptibility may be attributed to the structural differences in bacterial cell walls; the thick but porous peptidoglycan layer in S. aureus may allow better diffusion or interaction with the compounds compared to the outer membrane of Gram-negative bacteria, which can act as a permeability barrier. These results clearly demonstrate that the synthesized benzothiazolyl-pyridinecarbonitrile derivatives possess light-dependent antibacterial activity, with BTZ-Nap and BTZ-MePh being the most effective candidates. Their enhanced activity under light exposure, combined with low dark toxicity, underscores their potential as promising agents for photodynamic antimicrobial applications.
3.4. Cytotoxic activity
The cytotoxic effects of the benzothiazolyl-pyridinecarbonitrile derivatives were evaluated against MCF-7 (breast adenocarcinoma), HCT-116 (colorectal carcinoma), and BJ-1 (normal fibroblasts) using the MTT assay under both dark and light irradiation conditions (Fig. 7 A–H). A range of concentrations (0.5–100 µg mL−1) was tested to assess dose-dependent effects.In the absence of light, all four derivatives exhibited negligible cytotoxicity toward both cancer and normal cell lines. IC50 values exceeded 500 µg mL−1 in MCF-7 (535.87, 527.76, 519.09, and 639.65 µg mL−1 for BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap, respectively) and 700 µg mL−1 in HCT-116 (698.23, 732.12, 964.87, and 1104.76 µg mL−1 for the respective compounds). This minimal dark toxicity is advantageous for phototherapeutic applications, as it suggests low systemic risk in the absence of photoactivation.
Upon light activation, a marked increase in cytotoxicity was observed in both cancer cell lines. In MCF-7 cells, IC50 values decreased dramatically to 69.54, 49.78, 9.86, and 16.54 µg mL−1 for BTZ-Ph, BTZ-ClPh, BTZ-MePh, and BTZ-Nap, respectively. In HCT-116 cells, corresponding IC50 values were 47.65, 147.98, 56.98, and 22.32 µg mL−1. Among the derivatives, BTZ-Nap displayed the strongest activity, inducing >85% cell death at 100 µg mL−1 in both cancer lines. Its higher efficacy is also related to its high singlet oxygen quantum yield that we will discuss in Section 3.6, which helps anticancer photodynamic treatment (PDT) work better by increasing the number of cancer cells that are selectively destroyed when exposed to light.70 Both BTZ-MePh and BTZ-ClPh demonstrated significant levels of phototoxicity; however, BTZ-MePh was slightly more active because of its methyl groups, which improved electron density, and photostability, resulting in increased oxidative damage and ROS generation. Additionally, alkylation improves lipophilicity, which enhances cancer cell uptake and accumulation.71 In contrast, BTZ-Ph exhibited only moderate activity, highlighting the influence of aryl substitution on photodynamic efficiency.
Microscopic analysis further supported these findings (Fig. 8). In normal BJ-1 fibroblasts, BTZ-ClPh and BTZ-MePh preserved healthy morphology, while BTZ-Nap and BTZ-Ph induced only a mild reduction in cell density, consistent with limited toxicity. In contrast, MCF-7 and HCT-116 cells displayed pronounced cytotoxic responses following light activation, including reduced cell density, rounding, and membrane rupture—hallmarks of apoptotic and/or necrotic cell death. Notably, cytotoxicity in BJ-1 cells remained below 30% across all concentrations and derivatives, indicating preferential activity toward malignant cells.
HCT-116 cells appeared slightly more sensitive to the derivatives than MCF-7, potentially reflecting differences in cellular uptake, metabolic activity, or intrinsic vulnerability to oxidative stress. Overall, the benzothiazolyl-pyridinecarbonitrile derivatives exhibited strong light-dependent anticancer activity, with BTZ-Nap emerging as the most potent and selective. The combination of minimal dark toxicity and pronounced photoactivated cytotoxicity underscores their promise as candidates for further development in photodynamic cancer therapy.
3.5. Molecular docking studies
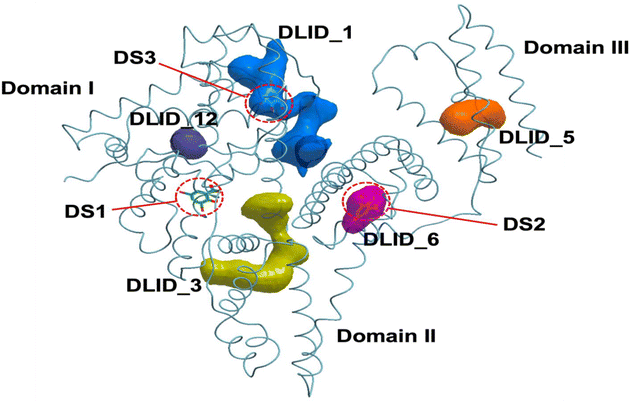
Molecular docking studies were conducted to better understand the structural basis for the binding of the synthesized compounds to BSA. The simulations identified two overlapping high-affinity regions on BSA emerged from docking—DS3 (overlapping with DLID_1) and the adjacent DLID_1 cavity. BTZ-ClPh is anchored in DS3 by a specific H-bond from its pyridone oxygen to Arg458, whereas BTZ-Ph attains high predicted affinity in DLID_1 via π–π stacking with His145 and a putative H-bond to the Tyr147 main-chain amide (Fig. 9, 10 and 11) (Table S1). Our analysis focused on comparing the binding modes of the experimentally most active ligand, BTZ-ClPh, and the computationally highest-scoring ligand, BTZ-Ph. As illustrated in (Fig. 10), the binding of BTZ-ClPh in the DS3 pocket is primarily anchored by a strong and specific hydrogen bond formed between its pyridone oxygen and the side chain of Arg458. This specific interaction, complemented by numerous hydrophobic contacts, provides a clear structural basis for its high experimental affinity. In contrast, BTZ-Ph, which achieved the best docking score of −28.51 in the adjacent DLID_1 pocket, displays a different binding mode (Fig. 11). It lacks the direct hydrogen bond to Arg458. Instead, its high predicted affinity appears to be derived from a combination of other interactions: a significant π–π stacking interaction with the imidazole ring of His145, and a potential hydrogen bond between its nitrile group and the main-chain amide of Tyr147. This comparative analysis offers a potential explanation for the discrepancy between the experimental affinity order (BTZ-ClPh > BTZ-MePh > BTZ-Ph > BTZ-Nap) and the calculated scores.The higher docking score of BTZ-Ph likely reflects predicted hydrogen-bonding in a static pose, whereas experimental results show stronger binding for BTZ-ClPh. This discrepancy highlights the greater impact of cumulative noncovalent forces in the dynamic protein environment. The chlorine substituent enhances polarizability, hydrophobic contacts, and potential halogen bonding, collectively stabilizing the complex more effectively than isolated hydrogen bonds, which docking may overemphasize. The docking study, therefore, not only identifies the key binding region on BSA but also elucidates the nuanced differences in molecular interactions that likely govern the compounds' observed activities.
3.6. Singlet oxygen quantum yields for photodynamic therapy
The singlet oxygen quantum yield (ΦΔ) serves as a key indicator of a material's effectiveness as a photosensitizer in photodynamic therapy (PDT) for cancer treatment. Singlet oxygen (1O2) is generated through a photosensitized process that requires oxygen, light of an appropriate wavelength, and a photosensitizer to absorb that light.72,73 Upon photoexcitation, the photosensitizer is first elevated to its singlet excited state, which then undergoes intersystem crossing (ISC) to its longer-lived triplet excited state (T1), typically on the microsecond timescale. Singlet oxygen is subsequently generated via energy transfer from this excited triplet state (3P*) of the photosensitizer to ground-state triplet oxygen (3O2) (Fig. 12). The produced singlet oxygen quantum yield (ΦΔ) is quantified by directly measuring the weak singlet oxygen phosphorescence signal in the near-infrared (NIR) region at approximately 1275 nm in acetonitrile, following a modified McKenzie et al. method.74–77 In this study, ΦΔ values were obtained under low-energy conditions where singlet oxygen emission decay exhibited a mono-exponential pattern. The kinetic trace obtained was analyzed by a monoexponentially decay using OriginPro ExpDecay1 fitting function (eqn (7)):| y = A1e−(−x−x0)/t1 | (7) |
The quantum yield of singlet oxygen generation (ΦΔ) for the compounds was determined by comparing their extrapolated emission intensity at time zero with that of the standard, [Ru(bpy)3]Cl2. This standard has a reported singlet oxygen quantum yield of ΦΔ = 57% in acetonitrile.78–81 The photosensitized singlet oxygen quantum yields of the examined materials were found to be: BTZ-Ph (0.20), BTZ-ClPh (0.21), BTZ-MePh (0.18), and BTZ-Nap (0.31). These values were in good agreement with those reported in the literature for similar compounds.33 The high ΦΔ value of BTZ-Nap is attributed to the existence of a naphthyl group, which encourages intersystem crossing to the triplet state and stabilizes relevant excited states, leading to higher singlet oxygen quantum yields and improved photosensitizer performance in biomedical and PDT applications.82
4. Conclusion
In summary, the interactions between bovine serum albumin (BSA) and four benzothiazolyl-pyridinecarbonitrile derivatives—1-BTZ-Ph, 2-BTZ-ClPh, 3-BTZ-MePh, and 4-BTZ-Nap have been well characterized using a multi-spectroscopic approach. The results showed a considerable quenching and lifetime reduction of BSA fluorescence emission upon the addition of BTZ derivatives, and strong spectral overlap between the BSA emission spectrum and the absorption spectra of the N-substituted 2-pyridylbenzothiazole derivatives, suggesting that the interaction occurs through dynamic quenching mechanism and energy transfer process (FRET). Importantly, all derivatives exhibited relatively moderate singlet oxygen (1O2) quantum yields (ΦΔ); BTZ-Ph (0.20), BTZ-ClPh (0.21), BTZ-MePh (0.18), and BTZ-Nap (0.31), rendering them as good photosensitizers for photodynamic therapy applications. Upon blue light irradiation, the compounds exhibited pronounced antibacterial effects against Salmonella enterica, Staphylococcus aureus, and E. coli. Likewise, under the same light conditions, they showed strong cytotoxic activity toward human breast cancer (MCF-7) and colorectal carcinoma (HCT-116) cell lines, while exhibiting minimal toxicity toward normal skin fibroblast cells (Bj-1). Collectively, these findings highlight the potential of these benzothiazole derivatives as promising candidates for both photodynamic therapy (PDT), antimicrobial, and anticancer photodynamic inactivation, warranting further investigation into their in vivo efficacy and therapeutic applications.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
Data will be made available on request.Supplementary information is available. See DOI: https://doi.org/10.1039/d5ra06375b.
Acknowledgements
This work supported from the Science, Technology & Innovation Funding Authority (STDF) under grant number 46207. M. E. El-Khouly Acknowledges Prof. Galal H. Elgemeie for providing the examined samples. Hanan Ali gratefully acknowledges the financial support provided by the Egyptian Ministry of Higher Education (MoHE) scholarship at Egypt-Japan University of Science and Technology (EJUST), which also supported the research by providing the facilities and labs required to conduct this study.References
- K. P. Yadav, M. A. Rahman, S. Nishad, S. K. Maurya, M. Anas and M. Mujahid, Synthesis and Biological Activities of Benzothiazole Derivatives: A review, Intell. Pharm., 2023, 1, 122–132 CAS.
- B. C. Yallur, U. Katrahalli, P. M. Krishna and M. D. Hadagali, BSA binding and antibacterial studies of newly synthesized 5, 6-Dihydroimidazo [2, 1-b] thiazole-2-carbaldehyde, Spectrochim. Acta, Part A, 2019, 222, 117192 CrossRef CAS.
- N. H. Metwally, G. H. Elgemeie and F. G. Fahmy, Synthesis and biological evaluation of benzothiazolyl-pyridine hybrids as new antiviral agents against H5N1 bird flu and SARS-COV-2 viruses, ACS Omega, 2023, 8, 36636–36654 CrossRef CAS.
- G. Sharma and R. Sharma, Novel spiro [indoline-3, 2′ thiazolo [5, 4-e] pyrimido [1, 2-a] pyrimidine] derivatives as possible anti-dermatophytic and anti-candidiasis agent, Biomed. Khim., 2024, 70(3), 180–186 CAS.
- A. Drakontaeidi, I. Papanotas and E. Pontiki, Multitarget Pharmacology of Sulfur–Nitrogen Heterocycles: Anticancer and Antioxidant Perspectives, Antioxidants, 2024, 13, 898 CrossRef CAS.
- A. A. Al-Mutairi, H. N. Hafez, A. R. B. El-Gazzar and M. Y. Mohamed, Synthesis and antimicrobial, anticancer and anti-oxidant activities of novel 2, 3-dihydropyrido [2,3-d] pyrimidine-4-one and pyrrolo [2,1-b][1, 3] benzothiazole derivatives via microwave-assisted synthesis, Molecules, 2022, 27, 1246 CrossRef CAS.
- L. M. Aroua, F. M. Alminderej, H. R. Almuhaylan, A. H. Alosaimi, F. Medini, H. A. Mohammed and N. H. Mekni, Benzimidazole(s): synthons, bioactive lead structures, total synthesis, and the profiling of major bioactive categories, RSC Adv., 2025, 15, 7571–7608 RSC.
- R. Bora, D. P. Kemisetti, F. Alam, A. Ghosh, A. Dutta, S. Roy and M. I. Judder, Synthesis, in silico and Pharmacological Activity of 1, 3-Benzothiazol Derivatives, J. Young Pharm., 2025, 17, 138–148 CrossRef CAS.
- M. S. Vasava, M. N. Bhoi, S. K. Rathwa, D. J. Jethava, P. T. Acharya, D. B. Patel and H. D. Patel, Benzimidazole: a milestone in the field of medicinal chemistry, Mini-Rev. Med. Chem., 2020, 20, 532–565 CrossRef CAS.
- J. B. Shaik, M. K. M. Pinjari, D. A. Gangaiah and C. G. R. Nallagondu, Synthetic strategies of functionalized pyridines and their therapeutic potential as multifunctional anti-Alzheimer's agents, in Recent Developments in the Synthesis and Applications of Pyridines, Elsevier, 2023, pp. 69–126 Search PubMed.
- S. Jaiswal and N. K. Verma, Benzothiazole moiety with sulphonamide as anti-inflammatory and analgesic activity: a review, South Asian Res. J. Pharm. Sci., 2021, 3, 90–1012 Search PubMed.
- R. C. Duarte, R. Cercená, B. B. de Araujo, O. A. Chaves, P. F. Gonçalves, E. Zapp, F. S. Santos, F. S. Rodembusch and A. G. DaI-Bó, Synthesis, Characterization, and BSA Binding Properties of Carboxylated Merocyanine-Based Fluorophores, ACS Omega, 2024, 9(49), 48697–48710 CrossRef CAS PubMed.
- T. Ghosh and K. Bhadra, A mini review on human serum albumin-natural alkaloids interaction and its role as drug carrier, J. Biomol. Struct. Dyn., 2024, 1–18 Search PubMed.
- O. Adamczyk, M. Szota, K. Rakowski, M. Prochownik, D. Doveiko, Y. Chen and B. Jachimska, Bovine serum albumin as a platform for designing biologically active nanocarriers—experimental and computational studies, Int. J. Mol. Sci., 2023, 25, 37 CrossRef PubMed.
- S. Tayyab and S. R. Feroz, Serum albumin: clinical significance of drug binding and development as drug delivery vehicle, Adv. Protein Chem. Struct. Biol., 2021, 123, 193–218 CAS.
- S. Behera, P. Mohanty, P. P. Dash, P. Mohapatra, L. Shubhadarshinee, R. Behura, A. K. Barick, P. Mohapatra and B. R. Jali, Selective binding of bovine serum albumin (BSA): a Comprehensive Review, Biointerface Res. Appl. Chem., 2023, 13, 555 CAS.
- S. Sengottiyan, K. Malakar, A. Kathiravan, M. Velusamy, A. Mikolajczyk and T. Puzyn, Integrated approach to interaction studies of pyrene derivatives with bovine serum albumin: insights from theory and experiment, J. Phys. Chem. B, 2022, 126, 3831–3843 CrossRef CAS.
- A. R. Pathania, Chemistry behind serum albumin: a review, in E3S Web Conf., 2021, vol. 309, p. 01086 Search PubMed.
- Z. Y. Hu, W. J. Wang, L. Hu, J. H. Shi and S. L. Jiang, Comprehending the intermolecular interaction of dacomitinib with bovine serum albumin: experimental and theoretical approaches, J. Biomol. Struct. Dyn., 2024, 42, 3579–3592 CrossRef CAS.
- B. Mandal, N. Chowdhury, N. Baildya, R. P. Mandal, A. Bagchi and S. De, Fluorescence probing and molecular docking analysis of the binding interaction of bovine serum albumin (BSA) with the polarity probe AICCN, Phys. Chem. Chem. Phys., 2023, 25, 18197–18214 RSC.
- V. Patil, S. Labade, C. Khilare and S. Sawant, A molecular bridge-like binding mode of buspirone to BSA: multispectroscopic and molecular docking investigation, Chem. Data Collect., 2022, 40, 100892 CrossRef.
- S. Behera, R. Behura, P. Mohanty, M. Sahoo and R. Duggirala, Study of interaction between bovine serum albumin and dolutegravir intermediate: fluorescence and molecular docking analysis, Biointerface Res. Appl. Chem., 2021, 11, 13102–13110 Search PubMed.
- C. Erkmen and M. Z. Kabir, Current analytical methods and applications used in the insight of serum proteins interactions with various food additives, pesticides, and contaminants, Explor. Foods Foodomics, 2024, 2, 195–222 Search PubMed.
- L. Wang, W. Zhang, Y. Shao, D. Zhang, G. Guo and X. Wang, Analytical methods for obtaining binding parameters of drug–protein interactions: a review, Anal. Chim. Acta, 2022, 1219, 340012 CrossRef PubMed.
- U. Katrahalli, G. Shanker, D. Pal and M. D. Hadagali, Molecular spectroscopic and docking analysis of the interaction of fluorescent thiadicarbocyanine dye with biomolecule bovine serum albumin, J. Biomol. Struct. Dyn., 2023, 41, 10702–10712 CrossRef.
- M. L. Kabir, F. Wang and A. H. Clayton, Intrinsically fluorescent anti-cancer drugs, Biology, 2022, 11, 1135 CrossRef PubMed.
- A. Saini and P. Bansal, Quenching studies as important toolkit for exploring binding propensity of metal complexes with serum albumin and DNA (a review), Pharm. Chem. J., 2022, 56(4), 545–558 CrossRef.
- E. Serag, E. M. El-Fakharany, S. F. Hammad and M. E. El-Khouly, Metal-organic frawork MIL-101(Fe) functionalized with folic acid as a multifunctional nanocarrier for targeted chemotherapy-photodynamic therapy, Biomater. Sci., 2025, 13, 2351 RSC.
- Y. Yue, B. Li, D. Wang, C. Wu, Z. Li and B. Liu, Optimizing Photosensitizers with Type I and Type II ROS Generation Through Modulating Triplet Lifetime and Intersystem Crossing Efficiency, Adv. Funct. Mater., 2025, 35, 2414542 CrossRef.
- N. Singh, R. S. Gupta and S. Bose, A comprehensive review on singlet oxygen generation in nanomaterials and conjugated polymers for photodynamic therapy in the treatment of cancer, Nanoscale, 2024, 16, 3243–3268 RSC.
- P. S. Maharjan and H. K. Bhattarai, Singlet oxygen, photodynamic therapy, and mechanisms of cancer cell death, J. Oncol., 2022, 2022, 7211485 Search PubMed.
- R. R. Cheruku, J. Cacaccio, F. Durrani, W. A. Tabaczynski, R. Watson, A. J. Marko, R. Kumar, M. E. El-Khouly, S. Fukuzumi, J. R. Missert, R. Yao, M. Sajjad, D. Chandra, K. Guru and R. K. Pandey, Epidermal Growth Factor Receptor-Targeted Multifunctional Photosensitizers for Bladder Cancer Imaging and Photodynamic Therapy, J. Med. Chem., 2019, 62(5), 2598–2617 CrossRef PubMed.
- S. K. Seth, C. Acquah, L. Levi, S. Jockusch and C. E. Crespo-Hernández, Harnessing the Excited States of 5-(5-Phenylthiophen-2-yl)-6-Azauridine as a Three-Pronged Agent for Skin Cancer Therapy: Photodynamic Action, Cell Imaging, and Cancer Cell Inhibition, ACS Appl. Bio Mater., 2025, 8(8), 7357–7369 CrossRef PubMed.
- C. Wince, C. T. Kassa, J. Insper, D. Amzallag, W. Consonlandich, A. C. A. Tortamano and R. A. Prates, Antimicrobial photodynamic therapy effects mediated by methylene blue in surfactant medium as an adjuvant treatment of teeth with apical periodontitis and presence of fistula–Protocol for randomized, controlled, double-blind clinical trial, PLoS One, 2024, 19(12), e0315169 CrossRef PubMed.
- S. Verma, U. W. Sallum, H. Athar, L. Rosenblum, J. W. Foley and T. Hasan, Antimicrobial photodynamic efficacy of side-chain functionalized benzo [a] phenothiazinium dyes, Photochem. Photobiol., 2009, 85(1), 111–118 CrossRef PubMed.
- L. A. Ortiz-Rodríguez, Y. G. Fang, G. Niogret, K. Hadidi, S. J. Hoehn, H. J. Folkwein and C. E. Crespo-Hernández, Thieno [3, 4-d] pyrimidin-4 (3 H)-thione: an effective, oxygenation independent, heavy-atom-free photosensitizer for cancer cells, Chem. Sci., 2023, 14(33), 8831–8841 RSC.
- R. A. Azzam, G. H. Elgemeie and R. R. Osman, Synthesis of novel pyrido [2,1-b] benzothiazole and N-substituted 2-pyridylbenzothiazole derivatives showing remarkable fluorescence and biological activities, J. Mol. Struct., 2020, 1201, 127194 CrossRef CAS.
- K. Mousa, A. Abd El-Moneim, S. F. El-Mashtoly, M. M. Mohamed and M. E. El-Khouly, Laser-induced graphene functionalized cationic porphyrin: fabrication, characterization, and intra-supramolecular electron transfer process, RSC Adv., 2025, 15, 289–300 RSC.
- A. Ansary, A. Osman and M. E. El-Khouly, Doxorubicin-loaded pH-responsive porphyrin-derived carbon dots as a promising biocompatible drug delivery system for effective chemotherapy of breast cancer, RSC Adv., 2025, 15, 6457–6473 RSC.
- E. Serag, M. Helal and A. El Nemr, Curcumin loaded onto folic acid carbon dots as a potent drug delivery system for antibacterial and anticancer applications, J. Cluster Sci., 2024, 35, 519–532 CrossRef CAS.
- H. N. Akl, D. Salah, H. S. Abdel-Samad, A. A. Abdel Aziz and A. A. Abdel-Shafi, Fractional dependence of the free energy of activation on the driving force of charge transfer in the quenching of the excited states of substituted phenanthroline homoleptic ruthenium (ii) complexes in aqueous medium, RSC Adv., 2023, 13, 13314–13323 RSC.
- M. Pourhajibagher, L. R. Omrani, M. Noroozian, Z. Ghorbanzadeh and A. Bahador, In vitro antibacterial activity and durability of a nano-curcumin-containing pulp capping agent combined with antimicrobial photodynamic therapy, Photodiagn. Photodyn. Ther., 2021, 33, 102150 CrossRef CAS.
- T. M. S. F. de Aguiar Coletti, L. M. De Freitas, A. M. F. Almeida and C. R. Fontana, Optimization of antimicrobial photodynamic therapy in biofilms by inhibiting efflux pump, Photomed. Laser Surg., 2017, 35, 378–385 CrossRef PubMed.
- T. H. Wang, L. F. Chou, Y. W. Shen, N. C. Lin, Y. H. Shih and T. M. Shieh, Mechanistic insights into temoporfin-based photodynamic therapy: ferroptosis as a critical regulator under normoxic and hypoxic conditions in head and neck cancer, J. Photochem. Photobiol., B, 2025, 113165 CrossRef CAS.
- B. Sekula, M. Bilska, B. Wiatrak and A. Bujacz, The crystal structure of bovine serum albumin in the complex with 3,5-diiodosalicylic acid, Int. J. Biol. Macromol., 2013, 60, 316–324 CrossRef CAS PubMed.
- R. A. Abagyan, M. M. Totrov and D. N. Kuznetsov, ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation, J. Comput. Chem., 1994, 15, 488–506 CrossRef CAS.
- J. An, M. Totrov and R. Abagyan, Pocketome via comprehensive identification and classification of ligand binding envelopes, Mol. Cell. Proteomics, 2005, 4, 752–761 CrossRef CAS PubMed.
- G. Bottegoni, I. Kufareva, M. Totrov and R. Abagyan, A new method for ligand docking to flexible receptors by dual alanine scanning and refinement (SCARE), J. Comput.-Aided Mol. Des., 2008, 22, 311–325 CrossRef CAS PubMed.
- S. C. A. Yeh, M. S. Patterson, J. E. Hayward and Q. Fang, Time-resolved fluorescence in photodynamic therapy, Photonics, 2014, 1, 530–564 CrossRef.
- S. Y. Bi, D. Q. Song, Y. Tian, X. Zhou, Z. Y. Liu and H. Q. Zhang, Molecular spectroscopic study on the interaction of tetracyclines with serum albumins, Spectrochim. Acta, Part A, 2005, 61, 629–636 CrossRef.
- X.-W. Li, X.-J. Li, Y.-T. Li, Z.-Y. Wu and C.-W. Yan, Syntheses and structures of new trimetallic complexes bridged by N-(5-chloro-2-hydroxyphenyl)-N′-[3-(dimethylamino)propyl] oxamide: cytotoxic activities, and reactivities towards DNA and protein, J. Photochem. Photobiol., B, 2013, 118, 22 CrossRef CAS PubMed.
- A. W. Tanveer, H. B. Ahmed, A. A.-M. Abdul-Rahman, A. B. Mashooq and Z. Seema, Study of the interactions of bovine serum albumin with the new anti-inflammatory agent 4-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-N0-[(4-ethoxy-phenyl) methylidene] benzohydrazide using a multi-spectroscopic approach and molecular docking, Molecules, 2017, 22, 1258 CrossRef.
- M. L. Verteramo, M. M. Ignjatović, R. Kumar, S. Wernersson, V. Ekberg, J. Wallerstein, G. Carstrom, V. Chadimova, H. Leffler, F. Zetterberg, D. T. Logan, U. Ryde, M. Akke and U. J. Nilsson, Interplay of halogen bonding and solvation in protein–ligand binding, iScience, 2024, 4(19), 109636 CrossRef PubMed.
- C. S. Leung, S. S. Leung, J. Tirado-Rives and W. L. Jorgensen, Methyl effects on protein–ligand binding, J. Med. Chem., 2012, 55(9), 4489–4500 CrossRef CAS.
- K. Karami, Z. Mehri Lighvan, H. Farrokhpour, M. Dehdashti Jahromi and A. A. Momtazi-Borojeni, Synthesis and spectroscopic characterization study of new palladium complexes containing bioactive O,O-chelated ligands: evaluation of the DNA/protein BSA interaction, in vitro antitumoural activity and molecular docking, J. Biomol. Struct. Dyn., 2018, 36, 3324 CrossRef CAS.
- P. Joshi, S. Chakraborty, S. Dey, V. Shanker, Z. A. Ansari and S. P. Singh, Binding of chloroquine–conjugated gold nanoparticles with bovine serum albumin, J. Colloid Interface Sci., 2011, 355, 402–409 CrossRef CAS PubMed.
- B.-L. Wang, D.-Q. Pan, K.-L. Zhou, Y.-Y. Lou and J.-H. Shi, Multi-spectroscopic approaches and molecular simulation research of the intermolecular interaction between the angiotensin-converting enzyme inhibitor (ACE inhibitor) benazepril and bovine serum albumin (BSA), Spectrochim. Acta, Part A, 2019, 212, 15–24 CrossRef CAS PubMed.
- E. Ramachandran, S. P. Thomas, P. Poornima, P. Kalaivani, R. Prabhakaran, V. V. Padma and K. Natarajan, Evaluation of DNA binding, antioxidant and cytotoxic activity of mononuclear Co(III) complexes of 2-oxo-1,2-dihydrobenzo[h]quinoline-3-carbaldehyde thiosemicarbazones, Eur. J. Med. Chem., 2012, 50, 405–415 CrossRef CAS PubMed.
- D. S. Raja, N. S. P. Bhuvanesh and K. Natarajan, A novel water soluble ligand bridged cobalt(ii) coordination polymer of 2-oxo-1,2-dihydroquinoline-3-carbaldehyde (isonicotinic) hydrazone: evaluation of the DNA binding, protein interaction, radical scavenging and anticancer activity, Dalton Trans., 2012, 41, 4365–4377 RSC.
- D. S. Raja, E. Ramachandran, N. S. P. Bhuvanesh and K. Natarajan, Synthesis, structure and in vitro pharmacological evaluation of a novel 2-oxo-1, 2-dihydroquinoline-3-carbaldehyde (2′-methylbenzoyl) hydrazine bridged copper (II) coordination polymer, Eur. J. Med. Chem., 2013, 64, 148–159 CrossRef.
- J. N. Miller, Recent advances in molecular luminescence analysis, Proc. Anal. Div. Chem. Soc., 1979, 16, 203–208 CAS.
- P. Sathyadevi, P. Krishnamoorthy, N. S. P. Bhuvanesh, P. Kalaiselvi, V. V. Padma and N. Dharmaraj, Organometallic ruthenium(II) complexes: synthesis, structure and influence of substitution at azomethine carbon towards DNA/BSA binding, radical scavenging and cytotoxicity, Eur. J. Med. Chem., 2012, 55, 420–431 CrossRef CAS.
- J. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, US, 2006 Search PubMed.
- T. Förster and O. Sinanoglu, Modern Quantum Chemistry, Academic Press, New York, 1965, vol. 3, pp. 93–137 Search PubMed.
- U. Kragh-Hansen, Molecular aspects of ligand binding to serum albumin, Pharmacol. Rev., 1981, 33, 17 CrossRef CAS.
- P. B. Kandagal, S. Ashoka, J. Seetharamappa, S. M. T. Shaikh, Y. Jadegoud and O. B. Ijare, Study of the interaction of an anticancer drug with human and bovine serum albumin: spectroscopic approach, J. Pharm. Biomed. Anal., 2006, 41, 393–399 CrossRef CAS PubMed.
- L. He, S. Yang, W. Xuan, X. Zhen, Q. Qi, Y. Qi, Q. Li, m. Du, M. R. Hamblin and L. Huang, Phenylalanine-Arginine-β-Naphthylamide Enhances the Photobactericidal Effect of Methylene Blue on Pseudomonas aeruginosa, Photobiomodulation, Photomed., Laser Surg., 2023, 41(10), 569–575 CrossRef.
- D. Staneva, A. I. Said, E. Vasileva-Tonkova and I. Grabchev, Enhanced photodynamic efficacy using 1, 8-naphthalimides: potential application in antibacterial photodynamic therapy, Molecules, 2022, 27(18), 5743 CrossRef CAS.
- J. Ghorbani, D. Rahban, S. Aghamiri, A. Teymouri and A. Bahador, Photosensitizers in antibacterial photodynamic therapy: an overview, Laser Therapy, 2018, 27(4), 293–302 CrossRef PubMed.
- Y. Yuan, Z. Wang, R. Yang, T. Qian and Q. Zhou, Naphthyl quinoxaline thymidine conjugate is a potent anticancer agent post UVA activation and elicits marked inhibition of tumor growth through vaccination, Eur. J. Med. Chem., 2019, 171, 255–264 CrossRef CAS.
- G. Varvuolytė, E. Řezníčková, S. Krikštolė, R. Tamulienė, A. Bieliauskas, L. Malina, V. Vojackava, Z. Duben, H. Kolarova, N. Kleiziene, E. Arbaciauskiene, A. Zukausskaite, V. Krystof, A. Sackus and A. Šačkus, Synthesis and photo-induced anticancer activity of new 2-phenylethenyl-1H-benzo [e] indole dyes, Eur. J. Med. Chem., 2024, 277, 116777 CrossRef PubMed.
- S. Cui, X. Guo, S. Wang, Z. Wei, D. Huang, X. Zhang and Z. Huang, Singlet oxygen in photodynamic therapy, Pharmaceuticals, 2024, 17(10), 1274 CrossRef CAS PubMed.
- K. K. H. Wang, J. C. Finlay, T. M. Busch, S. M. Hahn and T. C. Zhu, Explicit dosimetry for photodynamic therapy: macroscopic singlet oxygen modeling, J. Biophotonics, 2010, 3(5-6), 304–318 CrossRef CAS.
- L. K. McKenzie, I. V. Sazanovich, E. Baggaley, M. Bonneau, V. Guerchals, J. A. G. Williams, J. A. Weinstein and H. E. Baryant, Metal Complexes for Two-Photon Photodynamic Therapy: A Cyclometallated Iridium Complex Induces Two-Photon Photosensitization of Cancer Cells under Near-IR Light, Chem.–Eur. J., 2017, 23, 234–238 CrossRef CAS.
- N. M. Shavaleev, H. Adams, J. Best, R. Edge, S. Navaratnam and J. Weinstein, Deep-red luminescence and efficient singlet oxygen generation by cyclometalated platinum(II) complexes with 8-hydroxyquinolines and quinoline-8-thiol, Inorg. Chem., 2006, 45, 9410–9415 CrossRef CAS PubMed.
- P. R. Ogilby and C. S. Foote, Chemistry of singlet oxygen, Singlet molecular oxygen luminescence in solution following pulsed laser excitation. Solvent deuterium isotope effects on the lifetime of singlet oxygen, J. Am. Chem. Soc., 1982, 104, 2069–2070 CrossRef CAS.
- J. R. Hurst, J. McDonald and G. B. Schuster, Lifetime of singlet oxygen in solution directly determined by laser spectroscopy, J. Am. Chem. Soc., 1982, 104, 2065–2067 CrossRef CAS.
- J. Parker and W. Stanbro, Optical determination of the collisional lifetime of singlet molecular oxygen in acetone and deuterated acetone, J. Am. Chem. Soc., 1982, 104, 2067–2069 CrossRef CAS.
- A. A. Abdel-Shafi, M. D. Ward and R. Schmidt, Mechanism of quenching by oxygen of the excited states of ruthenium(II) complexes in aqueous media. Solvent isotope effect and photosensitized generation of singlet oxygen, O2(1Δg), by [Ru(diimine)(CN)4]2−complex ions, Dalton Trans., 2007, 24, 2517–2527 RSC.
- R. Schmidt, C. Taniellian, R. Dunsbach and C. Wolff, Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2 (1Δg) sensitization, J. Photochem. Photobiol., A, 1994, 79, 11–17 CrossRef CAS.
- A. A. Abdel-Shafi, P. D. Beer, R. J. Mortimer and F. Wilkinson, Photosensitized generation of singlet oxygen from ruthenium (II)-substituted benzoaza-crown-bipyridine complexes, Phys. Chem. Chem. Phys., 2000, 2, 3137–3144 RSC.
- S. Mishra, S. B. Shelar, S. Rout, P. A. Hassan, K. C. Barick and N. Agarwal, Enhamced singlet oxygen generation in aggregates of naphthalene-fused BODIPY and its application in photodynamic therapy, ACS Appl. Bio Mater., 2024, 11, 7207–7218 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |