 Open Access Article
Open Access ArticleTailoring Janus In2SeTe monolayers with Al doping: a computational study for NOx sensing applications
Manik Bala,
Md Tawabur Rahman * and
Nilachal Goswami
* and
Nilachal Goswami
Department of Electrical and Electronic Engineering, Khulna University of Engineering & Technology, Khulna-9203, Bangladesh. E-mail: tawabur@eee.kuet.ac.bd
First published on 15th October 2025
Abstract
Anthropogenic emissions of harmful gases from fossil fuel combustion and industrial processes require advanced sensing platforms with exceptional sensitivity and selectivity. Herein, we present a comprehensive first-principles study of a Janus In2SeTe monolayer and its aluminum-doped analogue as multifunctional gas sensors. Using Density Functional Theory (DFT), we examine the adsorption of six common pollutants, including CO, NH3, CO2, CH4, NO, and NO2 on geometrically optimized surfaces, analyzing adsorption energy, distance, charge transfer, Density of States (DOS), Electron Density Difference (EDD), and recovery time. We further evaluate the resultant changes in electronic band structure, conductivity, optical absorption, refractive index, and spin-resolved DOS. Our results reveal that only NO and NO2 chemisorb strongly, inducing the highest adsorption energies and shortest distances, while the other gases physiosorbed weakly. The adsorption of NOx narrows the bandgap by 0.46/0.408 eV (NO/NO2) and 1.068/0.612 eV (NO/NO2) for pristine and Al-doped In2SeTe, respectively, translating into dramatic chemiresistive responses. At the optimized Al-doping level (2.33% Al for NO, 5.5% Al for NO2), the sensitivity was boosted by a factor of 1.26 × 105 (NO) and 51.6 (NO2) compared to the pristine In2SeTe. The optical absorption and refractive index spectra exhibit gas-specific shifts in pristine In2SeTe for NO and Al-doped In2SeTe for NO2, while only NOx adsorption generates a nonzero magnetic moment, enabling concurrent optical, chemiresistive, and magnetic transduction. The obtained faster recovery time supports real-time and reusable detection of NOx. Thus, these findings establish Al-doped In2SeTe as an excellent sensing material for next-generation environmental NOx sensing.
1 Introduction
Toxic gas detection is crucial for safeguarding health, ensuring workplace safety, complying with regulations, protecting the environment, and facilitating prompt emergency actions.1,2 Furthermore, the world's overpopulation and industrialization are constantly increasing emissions, endangering all life on the planet.3 The World Health Organization (WHO) estimates that outdoor air pollution frequently contains harmful gases and causes 4.2 million deaths worldwide. In this industrial era, it is necessary to design stable, ultra-fast, precise, and sensitive gas sensors to detect toxic gases at part-per-billion (ppb) concentrations.4 The sensitivity of conventional Metal Oxide Semiconductor (MOS) devices is restricted at lower concentrations, despite their exceptional sensitivity and selectivity.5 After the discovery of graphene, the area of two-dimensional (2D) materials has expanded enormously.6,7 Their high surface area and excellent electrical and chemical characteristics make 2D materials highly promising for gas substance detection.8 The first-principles investigations are essential for developing gas sensor research because they are crucial in forecasting gas adsorption behavior in emerging 2D materials.9,10In recent years, the layered transition metal dichalcogenides (TMDs) have garnered a lot of interest due to their fascinating physical and chemical properties.11,12 Notable features include topological superconductivity, an indirect-to-direct bandgap crossover, valley-selective optical stimulation, a layer-dependent tunable bandgap, out-of-plane piezoelectric polarization, and a significant Rashba effect due to their out-of-plane asymmetries.4,13 MoSSe, one of the Janus TMDs, has already been developed experimentally using chemical vapor deposition (CVD).14,15 Jin et al. demonstrated the Janus MoSSe, which has shown promise for creating ultrahigh-sensitivity nanoscale sensors with enhanced sensitivity to NH3 and NO2 due to the inner electric field and tunable selectivity resulting from the Janus structure.16 P. Yu et al. employed a Pd-doped Janus HfSeS monolayer as an ultrahigh-sensitive gas sensing material for reversible detection of NO.17 Very recently, the electronic structure, optical, and thermoelectric properties of a new 2D Janus telluride (In2SeTe) monolayer, the Janus In2SeTe monolayer with asymmetric Se/Te surfaces, have been investigated.18 However, the gas adsorption properties of In2SeTe monolayers remain unexplored.
This study utilized DFT calculations to investigate the gas-sensing characteristics of both pristine and aluminum-doped In2SeTe monolayers toward NH3, CO2, CO, NO, NO2, and CH4. Al was chosen as a substitutional dopant due to its similar atomic radius to host atoms, ensuring structural stability, and its trivalent nature, which modulates the electronic structure and enhances charge redistribution. Its higher electropositivity strengthens interactions with electronegative gases. Being abundant, low-cost, lightweight, and non-toxic, Al offers both electronic advantages and practical sustainability for gas-sensing applications. The initial assessment focused on adsorption strength, determined by adsorption energy and height of the gas molecules on the Se-terminated surface of the monolayers. Key structural and electronic properties—including binding energy, charge transfer, sensitivity, recovery time, and EDD—were systematically analyzed. Results indicated significantly stronger interactions between the monolayer and NO/NO2 compared to the other gases. Additionally, DOS analysis provided insight into the role of atomic orbitals in gas adsorption. The pristine In2SeTe monolayer exhibited high sensitivity values of 7.25 × 105 for NO and 2.65 × 105 for NO2. These sensing performances were further enhanced through 2.33% Al doping, which led to substantial sensitivity improvements by factors of 1.26 × 105 for NO and 51.58 for NO2 relative to the undoped system. The effect of aluminum concentration on sensing performance was also evaluated, revealing optimal doping levels of 2.33% for NO and 5.5% for NO2. Importantly, the recovery times for both pristine and Al-doped monolayers remained within feasible limits, supporting real-time, repeatable gas detection. Deviations from these doping levels resulted in a noticeable drop in sensitivity, highlighting the importance of maintaining a balance between structural integrity and electronic responsiveness. The optical analysis further demonstrated selective gas detection capabilities—pristine In2SeTe was responsive to NO, while Al-doped In2SeTe showed specificity toward NO2. Moreover, only NOx gases induced magnetism in both systems, enabling their use as magnetic sensors exclusively for NOx detection. Overall, Al doping significantly improved the electronic, optical, and chemical sensing performance of the In2SeTe monolayer. These enhancements establish Al-doped In2SeTe as a highly effective material for next-generation gas sensors, offering excellent selectivity, sensitivity, and reliability for detecting hazardous environmental gases.
The material's high sensitivity and selectivity toward NOx make it an ideal candidate for urban air quality monitoring, such as in roadside, playground, and smart city nodes, where accurate and reliable NO2 detection is essential for protecting public health. Its robust reaction to NO and NO2 indicates potential for usage in onboard diagnostics and selective catalytic reduction (SCR) systems for vehicle exhaust sensors. Applications in continuous emissions monitoring and fence-line surveillance at industrial sites are supported by exceptional room-temperature performance. Use of tunnel and parking area safety and ventilation systems is made possible by quick reaction and recovery. Its NO sensitivity also indicates the possibility of non-invasive monitoring of exhaled nitric oxide (FeNO), a biomarker for asthma treatment, in medical devices.
2 Computational methods
DFT was employed in this study, and all calculations were performed using the DMol3 modules integrated into the Materials Studio platform.19 Initially, a supercell (3 × 3) of the In2SeTe monolayer was created. Then, the bulk In2SeTe crystal was cleaved, and a 25 Å vacuum zone was included to eliminate spurious interactions with periodic images, ensuring sufficient accuracy for electronic and optical property calculations.20 The DMol3 module optimized the In2SeTe monolayer until the energy converged to precision values of 1.0 × 10−6 Ha. The value of the force limit was 1.0 ×105 Ha per Å. The DSPP (DFT Semi-core Pseudo Potential) approach incorporated relativistic corrections into the core treatment. Smearing was adjusted to 0.005 Ha to expedite convergence. A Monkhorst Pack k-point set of 5 × 5 × 1 was selected for geometry optimization and electronic structure calculations. A Double Numerical Polarized Plus (DNP+) basis set was chosen for the optimization9 The value of the Global Orbital cut-off was 5.0 Å. GGA (Generalized Gradient Approximation) and PBE (Perdew Burke Ernzerhof) functional theory were used for computational efficiency. Using the GGA and GGA + SOC (Spin-Unrestricted) methods, we have calculated the electronic properties of NO and NH3 adsorption, revealing that the interplay between angular momentum and orbital angular momentum causes the splitting of energy bands.21,22 The HSE06 functional, which combines Heyd, Scuseria, and Ernzerhof, is quite close to the experimental band gap values. However, the change in the band gap is more important for sensing applications than the absolute value.22,23 After geometry optimization, various parameters, including bond length, band structure, DOS, and Hirshfeld charge analyses, were calculated. Adsorption energy (Ead) was defined as follows:24,25| Ead = E(monolayer+gas) − E(monolayer) − E(gas) | (1) |
The energy of the monolayer following gas adsorption is denoted by E(monolayer+gas), the energy of the monolayer itself is denoted by E(monolayer), and the total energy of the sole gas layer is denoted by E(gas). Eqn (2) was used to determine the amount of charge transfer during the adsorption phase.
| ΔQ = Qads − Qiso | (2) |
The total charge of the gas molecule before and after adsorption is indicated by Qiso and Qads, respectively. The energy gap can be used to assess the material's change in conductivity. In this instance, EHOMO stands for the energy of the highest occupied molecular orbit, whereas ELUMO indicates the energy of the lowest unoccupied molecular orbit. The structure's energy gap is shown by
| Eg = ELUMO − EHOMO | (3) |
The charge density difference in the adsorption process, Δρ, was defined using eqn (4).
| Δρ = ρ(gas+m) − ρm − ρgas | (4) |
ρtotal, ρm, and ρgas denote the charge densities after gas adsorption, of the pristine/Al-doped structure, and of the isolated gas molecule, respectively. The conductivity and band gap of a material can be found in the following equation:26,27
 | (5) |
The relationship between chemiresistive sensitivity and conductivity is defined by:
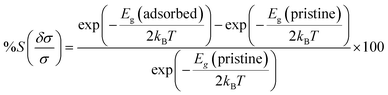 | (6) |
The time required for gas molecules to desorb naturally from the surface of the sensing material is called the recovery time. To determine the recovery time, the Vant Hoff Arrhenius equation was applied, as shown below.4,28
 | (7) |
The attempt frequency, represented as ν0, is commonly assumed to be 1012 s−1 for SO2, as reported in previous research.9 For all other gases, the same value can be applied.
For pristine In2SeTe, In (on top of the In atom), Se (on top of the Se atom), Te (on top of the Te atom), bridge (on top of the In–Se bonds), and bridge (on top of the In–Te bonds) are the five initial adsorption sites that were chosen, as shown in Fig. 1(a). Similarly, for Al-doped In2SeTe, In (on top of the In atom), Se (on top of the Se atom), Te (on top of the Te atom), Al(on top of the Al atom), bridge (on top of the In–Se bonds), and bridge (on top of the In–Te bonds) are the six initial adsorption sites that were chosen, as shown in Fig. 1(b). Lastly, the pristine and Al-doped In2SeTe monolayer's capacity for gas sensing was investigated, considering the sensing parameters of conductivity, refractive index, magnetic moment, and recovery time.
3 Results and discussion
3.1 Parameter optimization of pristine and Al-doped In2SeTe monolayer
This study systematically investigates the structural and electronic properties of pristine and Al-doped In2SeTe monolayers, with a particular focus on their response to gas adsorption. Both configurations exhibit a low-buckled structure, with lattice constants of 4.0381 Å for pristine and 4.054 Å for Al-doped In2SeTe, aligning well with previous reports based on PBE-level calculations.18 A 3 × 3 supercell was employed to model the local atomic interactions with gas molecules. As shown in Fig. 1, the atomic arrangement along the Z-axis follows a Se–In–In–Te sequence, indicating that indium atoms are sandwiched between selenium and tellurium layers. The introduction of Al slightly alters the local geometry. In the pristine structure, the In–In, In–Se, and In–Te bond lengths are 2.768 Å, 2.684 Å, and 2.815 Å, respectively, while in the doped structure, these values shift to 2.767 Å, 2.712 Å, and 2.809 Å. Corresponding changes in bond angles and structural thickness (from 5.40 Å to 5.34 Å) reflect the subtle influence of Al incorporation. Phonon dispersion spectra (Fig. 2(a) and (d)) for both systems show the absence of imaginary frequencies, confirming their dynamic stability. Each unit cell, comprising four atoms, exhibits twelve phonon branches—three acoustic and nine optical—with linear acoustic modes near the Γ-point and a phonon gap separating degenerate and non-degenerate modes, characteristics typical of two-dimensional materials. Electronic band structure analysis (Fig. 2(b) and (e)) reveals that both pristine and Al-doped monolayers are direct bandgap semiconductors with their valence band maximum (VBM) and conduction band minimum (CBM) located at the Γ-point. The calculated band gaps are 1.192 eV for pristine and 1.109 eV for Al-doped In2SeTe, indicating a slight reduction in band gap due to doping while preserving the semiconducting nature of the material. In Fig. 2(c), the projected density of states (PDOS) of the pristine In2SeTe features a pronounced Te-s peak at −15 eV, indicating a localized deep valence state. Near the Fermi level, in p, Se p, and Te p orbitals dominate and strongly hybridize, driving both bonding and electronic behavior, and they also extend modestly into the conduction band. All other s and d orbitals are negligible, underscoring p-orbital interactions as the key determinant of In2SeTe's electronic properties. In the Al-doped In2SeTe monolayer, the PDOS still features a deep, intense Te s peak at −15 eV, indicating that the core valence band structure remains intact, as displayed in Fig. 2(f). Doping introduces new Al-derived features: a small Al-s peak appears around −13 eV, and Al-p states emerge just below and slightly above the Fermi level (0 eV), indicating that Al partially hybridizes with the host p orbitals. The dominant In-p, Se-p, and Te-p contributions near Ef are preserved but become slightly redistributed by the added Al-p density, leading to a modest increase in total DOS at the valence-band edge. Above Ef, faint Al-p weight extends into the conduction band, suggesting that Al doping can enhance carrier availability and subtly narrow the band gap without disrupting the principal p-orbital framework of In2SeTe. Table SI provides the structural properties of pristine and Al-doped In2SeTe, covering bond lengths, bond angles, dynamic stability, band gap values, and the positions of VBM and CBM. Table SII presents a comparison of the computed lattice constant, thickness, bond lengths, and PBE band gap with previously reported results. The strong agreement confirms the reliability of our calculations, while slight deviations are linked to variations in exchange–correlation functionals, computational parameters, and dispersion treatments.18,29–31 | ||
| Fig. 2 (a) Phonon frequency, (b) Band structure, and (c) PDOS of pristine In2SeTe monolayer, (d) phonon dispersion, (e) Band structure, and (f) PDOS of pristine Al-doped In2SeTe monolayer. | ||
3.2 Adsorption configurations of different gases on pristine and Al-doped In2SeTe monolayer
Geometry optimization was performed after placing the gas molecule on the monolayer with a distinct adsorption distance. The same gas molecule can exhibit different characteristics depending on the specific adsorption site, adsorption distance, and orientation of the molecule. Some gas molecules tend to adsorb parallel to the monolayer, while others like to orient vertically or obliquely. Adsorption is generally referred to as chemisorption if the absolute value of the adsorption energy is greater than 0.5 eV.32 The types and combinations of adsorption are represented in detail in the related figures in the following section. | ||
| Fig. 3 Top view and side view of Al-doped In2SeTe monolayer after (a) NH3, (b) CH4, (c) CO2, (d) CO, (e) NO, and (f) NO2 gas adsorption. | ||
3.3 Electronic structures and atomic contributions after gas adsorption
It is anticipated that the electrical structures of Al-doped In2SeTe monolayer and pristine In2SeTe monolayer would change when gas molecules are present. Thus, band structures and orbital- PDOS were calculated. The band structures of every gas-adsorbed system are displayed in Fig. 5 and 6 for both pristine and Al-doped In2SeTe systems. NH3 and CO have a negligible effect on the electronic band structure characteristics of pristine/Al-doped In2SeTe monolayers, as shown in Fig. 5(a) and 6(a). The observed change in the band gap was negligible. As shown in Fig. 7(a) and (d) for pristine In2SeTe and in Fig. 8(a) and (d) for Al-doped In2SeTe, the nitrogen and hydrogen atoms in NH3 molecules and the carbon and oxygen atoms in CO molecules make minimal contributions to the PDOS. As a result, there was no noticeable change in the electronic properties. For pristine In2SeTe, the band gap changed significantly by 0.460 eV and 0.408 eV, respectively, as a result of NO and NO2 being adsorbed onto the In2SeTe monolayer. The PDOS, demonstrated in Fig. 7(e) and (f), shows distinct evidence of hybridization between the nitrogen and oxygen atoms in NOx and the surface atoms of In2SeTe when NOx molecules are adsorbed onto the In2SeTe monolayer. This suggests that these atoms are actively participating in the electronic structure near the edges of the conduction and valence bands. With NOx adsorption, the PDOS displays strong and sharp peaks for the O-p and N-p orbitals of NOx, especially in the energy range close to the Fermi level (from 0 to 1.5 eV). These pronounced features are a clear sign of vigorous mixing between the orbitals of NOx and those of the In2SeTe substrate, particularly with the p orbitals of selenium and tellurium. This hybridization leads to the appearance of entirely new electronic states within the band gap region, near the Fermi level. At the same time, the main features of the In-d and Se-p orbitals in the host material remain mostly unchanged. The strong interaction between NOx and the In2SeTe monolayer, as reflected in both the PDOS and the significant changes in electronic properties, highlights the sensitivity of this material in detecting NOx molecules. Now, for Al-doped In2SeTe, the electronic bandgap drops more rapidly than pristine In2SeTe after NO and NO2 adsorption by a value of 1.068 eV and 0.612 eV, respectively, observed in Fig. 6(e) and (f). From Fig. 8(e) and (f), a stronger hybridization took place among N-p, O-p, Al-p, and Se-p orbitals near the Fermi level than in pristine In2SeTe, resulting in a new state almost near zero energy level (0–0.5 eV). As a result, the band gap shrinks noticeably. | ||
| Fig. 5 Band structure of In2SeTe monolayer after (a) NH3, (b) CH4, (c) CO2, (d) CO, (e) NO, and (f) NO2 gas adsorption. | ||
 | ||
| Fig. 6 Band structure of Al-doped In2SeTe monolayer after (a) NH3, (b) CH4, (c) CO2, (d) CO, (e) NO, and (f) NO2 gas adsorption. | ||
 | ||
| Fig. 7 PDOS of (a) NH3, (b) CH4, (c) CO2, (d) CO, (e) NO, and (f) NO2 gases adsorbed on In2SeTe monolayer. | ||
 | ||
| Fig. 8 PDOS of (a) NH3, (b) CH4, (c) CO2, (d) CO, (e) NO, and (f) NO2 gases adsorbed on Al-doped In2SeTe monolayer. | ||
Additionally, a significant change in the band diagram leads to a corresponding change in the material's conductivity. A significant amount of deflection in the conductivity of the Al-doped In2SeTe monolayer is responsible for the material's sensitivity towards different gases. One of its capabilities is that of a chemiresistive gas sensor. For both CH4 and CO2 adsorption on the pristine/Al-doped In2SeTe monolayer, the PDOS, illustrated in Fig. 7(b, c) and 8(b, c), exhibits that the C, O, and H orbitals from these molecules contribute only minimally near the Fermi level, with no significant new peaks or strong hybridization observed. As a result, the electronic structure of pristine/Al-doped In2SeTe remains unchanged mainly after adsorption, and the band gap (Fig. 5(b, c) and 6(b, c)) shows only a negligible variation. These findings indicate that both CO2 and CH4 interact very weakly with the pristine/Al-doped In2SeTe surface, causing little to no modification in its electronic properties, which highlights the material's selectivity and its limited sensitivity to these gases.
3.4 Effect of Al-doping on In2SeTe for gas sensing
Fig. 9 indicates the effect of Al doping on the electronic and sensing properties of In2SeTe to NO and NO2 detection. After observing Fig. 9(a), which depicts how the change in bandgap deviates with various doping percentages, it can be asserted that at 2.77% percent doping of In2SeTe monolayer with Al atom NO gas, the highest amount of bandgap change (1.068 eV) in comparison to the other doping percentages. Then, eventually, for NO, as the doping percentage is increased, the change in bandgap reduces and reaches a value of 0.722 eV at 11.11% doping. On the contrary, for NO2, the change in bandgap (0.683 eV) is highest at 5.5% of doping. Now, as we move along, Fig. 9(b) demonstrates the change of logarithmic sensitivity with increasing doping percentage. Along with that, with further observation, the study revealed that at 2.77% NO showed the highest amount of logarithmic sensitivity (10.962), and as the doping percentage rose, logarithmic sensitivity reduced, indicating that as the doping percentage increases, the sensing capability reduces. Adding to the study, the logarithmic sensitivity (7.731) of NO2 was highest at 5.5% doping of Al in the In2SeTe monolayer. Furthermore, for 8.33% and 11.11% doping, the logarithmic sensitivity (1.98, 1.99, respectively) was reduced dramatically. From the above explanation, it can be deduced that for NO 2.77% and for NO2 5.5% doping is ideal to have the maximum amount of sensing performance from Al-doped In2SeTe monolayer. All these data have been inserted into Table SIII to identify the optimum doping percentage. | ||
| Fig. 9 (a) Change in bandgap vs. doping percentage (b) logarithmic sensitivity vs. doping percentage. | ||
Table 1 summarizes the adsorption energy (Ead), bandgap (Eg), bandgap change(ΔEg), adsorption distance (D), and Hirshfeld charge transfer (ΔQ) of the most energetically favourable different gas adsorbed structures. From Table 1, it can be clearly seen that among all the gas molecules, NOx exhibits the maximum adsorption energy, maximum change in bandgap, the shortest adsorption distance, and the largest band gap change in both pristine and Al-doped In2SeTe monolayers. Although the adsorption energy and charge transfer increase for NOx after Al doping. Again, the adsorption distance further reduces after Al is introduced. For the pristine In2SeTe monolayer, the adsorption of NO and NO2 resulted in adsorption energies of −0.751 eV and −0.589 eV, adsorption distances of 2.17 Å and 2.75 Å, and charge transfers of −0.074|e| and −0.066|e|, respectively. Upon introducing 2.33% Al doping, all these parameters showed noticeable enhancement, with the adsorption energies increasing to −0.774 eV (NO) and −0.701 eV (NO2), the adsorption distances extending to 2.57 Å and 2.66 Å, and the charge transfers rising to −0.082|e| and −0.080|e|. This suggests that Al doping significantly strengthens the interaction between the monolayer and the NO/NO2 gas molecules. For other gases, there were no such properties that made them distinguishable. Table 1 represents the selective nature of both pristine and Al-doped In2SeTe toward NOx with enhanced sensitivity and selectivity after Al doping.
| Material | Gas molecules | Suitable sites | Adsorption distance D(Å) | Bandgap (Eg) | Change in bandgap (ΔEg) | Adsorption energy (Ead) | Charge transfer(ΔQ) |
|---|---|---|---|---|---|---|---|
| In2SeTe | NH3 | Top of Te | 2.86 | 1.183 | −0.009 | −0.20 | −0.0934 |
| CH4 | Top of the In–Se bridge | 3.27 | 1.168 | −0.024 | −0.11 | −0.064 | |
| CO2 | Top of the In–Te bridge | 3.39 | 1.115 | −0.077 | −0.15 | −0.039 | |
| CO | Top of in | 3.403 | 1.195 | 0.003 | −0.06 | −0.014 | |
| NO | Top of the In–Se bridge | 2.17 | 0.732 | −0.460 | −0.751 | −0.074 | |
| NO2 | Top of the In–Te bridge | 2.75 | 0.784 | −0.408 | −0.589 | −0.066 | |
| Al-doped In2SeTe | NH3 | Top of Te | 2.94 | 1.096 | −0.013 | −0.225 | −0.097 |
| CH4 | Top of the Al–Se bridge | 3.062 | 1.104 | −0.005 | −0.129 | −0.049 | |
| CO2 | Top of Se | 3.31 | 1.113 | −0.004 | −0.196 | −0.041 | |
| CO | Top of Al | 3.8 | 1.103 | −0.006 | −0.135 | −0.028 | |
| NO | Top of the Al–Se bridge | 2.57 | 0.041 | −1.068 | −0.774 | −0.082 | |
| NO2 | Top of the Al–Te bridge | 2.66 | 0.497 | −0.612 | −0.701 | −0.08 |
Table SIV and Table SV provide a detailed summary of the structural parameters for gas adsorption on pristine and Al-doped In2SeTe. Table SIV lists the adsorption heights of gas molecules and the In–N/O bond lengths for NO and NO2, while Table SV presents the In–Se/Te bond lengths and the corresponding bond distortions for various adsorbed gases. Together, these tables offer a comprehensive overview of the adsorption geometries, supporting the analysis of gas adsorption performance.
3.5 Chemiresistive sensitivity and recovery characteristics
From Table SVI, the In2SeTe monolayer with NO and NO2 adsorption exhibits the largest band gap shift of 0.460 eV and 0.408 eV, respectively, and its sensitivity towards NO and NO2 is the highest at 7.249958 × 105 and 2.653623 × 105, respectively, in comparison with other gases, which can be observed in Fig. 10. Consequently, after doping, the sensitivity and change in bandgap for NO and NO2 were 9.17 × 1010, 1.367 × 107, and 1.068 eV, 0.612 eV, respectively. After analyzing Fig. 10(a) and (b), it can be concluded that doping with Al increases the sensitivity and change in bandgap for NO and NO2 exceptionally, demonstrating that Al-doping enhances the gas sensing potential of the In2SeTe monolayer. | ||
| Fig. 10 (a) Comparison of Change in bandgap (ΔEg) (b) bandgap-based logarithmic sensitivity for different gases after adsorption on In2SeTe monolayer and Al-doped In2SeTe monolayer, respectively. | ||
The recovery times for various gases at 300 K and 400 K are listed in Table 2, where 300 K is considered room temperature for the calculations. As the temperature increases, the recovery time decreases significantly. At 300 K, NOx exhibits a relatively long recovery time, indicating strong interaction with both pristine and Al-doped In2SeTe, making it ideal for room-temperature gas sensing. In contrast, the recovery times for other gases are in the picosecond range, suggesting they desorb too quickly to be effectively detected, thereby limiting their sensing viability. A longer recovery time enhances sensing accuracy. Additionally, Al doping further prolongs the recovery time for NOx, improving its detection capability and sensor reusability. Thus, both pristine and Al-doped In2SeTe are promising candidates for NOx sensing at room temperature.
| Gas molecules | NH3 | CH4 | CO2 | CO | NO | NO2 | |
|---|---|---|---|---|---|---|---|
| In2SeTe | Recovery time(s) at 300 k | 2.8557 × 10−9 | 6.8872 × 10−11 | 6.30623 × 10−9 | 1.04426 × 10−11 | 4.027 | 7.7 × 10−3 |
| Al-doped In2SeTe | 6.138 × 10−9 | 1.474 × 10−10 | 2.0223 × 10−9 | 1.8531 × 10−10 | 9.8 | 0.583 | |
A comprehensive comparative analysis, presented in Table 3, was performed to assess the gas-sensing capabilities of pristine and Al-doped In2SeTe monolayers toward NO and NO2 molecules, alongside eight other structurally similar 2D materials reported in the literature. The evaluation includes critical parameters such as adsorption energy (Ead), charge transfer (ΔQ), recovery time (τ), and sensitivity (S). Both pristine and Al-doped In2SeTe exhibit outstanding sensitivity levels, even in their undoped form, with the pristine monolayer achieving 7.25 × 105% sensitivity for NO and 2.65 × 105% sensitivity for NO2. These values already place In2SeTe among the high-performing materials for gas sensing. However, upon Al doping, the sensing performance experiences a dramatic enhancement. The sensitivity rises by an order of magnitude, reaching 9.17× 1010% for NO and 1.367 × 107% for NO2—an exceptional increase that underscores the strong influence of Al dopants in amplifying the surface reactivity and electron interaction of the monolayer. This improvement is further supported by more negative adsorption energies (−0.774 eV for NO and −0.701 eV for NO2) and higher charge transfer values (0.082e and 0.08 e), compared to the pristine values of −0.751 eV and −0.589 eV with 0.074 e and 0.066 e for NO and NO2, respectively. Both pristine and Al-doped In2SeTe have recovery durations that are within a range that is practically feasible, indicating their promise for real-time sensing applications and permitting repeated sensor use. On the other hand, materials such as GaAs have drawbacks. For instance, their incredibly lengthy recovery time (4.45 × 1012 s) makes NO2 detection difficult and prevents sensor reusability following gas adsorption. Similarly, Ga2SSe's ultra-short recovery time (6.78 × 10−8 s) leads to fast desorption before effective detection can take place, making NH3 detection difficult. These materials are not appropriate for accurate gas sensing because of their unfavorable recovery dynamics. The recovery time slightly increases due to stronger molecule binding in the doped system. When benchmarked against eight other 2D gas sensors in Table 3, many of which show lower sensitivity and weaker adsorption interactions, both pristine and especially Al-doped In2SeTe consistently outperform across all metrics. These results clearly demonstrate that Al doping not only enhances the already impressive gas sensing ability of In2SeTe but also establishes it as a leading-edge material for ultra-sensitive NO and NO2 detection at room temperature.
| Materials | Sensing gases | %Sensitivity | Recovery time | Adsorption energy (eV) | Charge transfer (e) | Ref. |
|---|---|---|---|---|---|---|
| In2SeTe | NO2 | 2.653623 × 105 | 7.7 × 10−3 | −0.589 | −0.066 | This work |
| NO | 7.249958 × 105 | 4.027 | −0.751 | −0.074 | ||
| Al doped In2SeTe | NO2 | 1.367 × 107 | 0.583 | −0.701 | −0.08 | This work |
| NO | 9.17 × 1010 | 9.8 | −0.774 | −0.082 | ||
| GaAs | NO2 | 99 | 4.45 × 1012 | −1.48 | 0.2803 | 33 |
| NO | 2.54 × 103 | 3.52 × 10−6 | −0.4476 | −0.18 | ||
| In2SSe | NO2 | 3.48 × 103 | 9.62 × 10−8 | −0.118 | −0.665 | 4 |
| NH3 | 326 | 3.87 × 10−3 | −0.392 | −0.727 | ||
| Ga2SSe | NO2 | 1.13 × 1011 | 6.41 × 10−7 | −0.167 | −1.05 | 4 |
| NH3 | 590 | 6.78 × 10−8 | −0.109 | −1.04 | ||
| MoS2 | NO2 | 20 | 4.32 × 104 | −0.208 | 0.055 | 34 |
| NO | NA | NA | −0.138 | −0.005 | ||
| NbSeTe | NO2 | 45 | 75.74 × 10−6 | −0.59 | −0.284 | 35 |
| NO | 34 | 17.49 × 10−6 | −0.55 | −0.1297 | ||
| Pt-doped HfSSe | H2S | 26.3 | 0.024 | −0.614 | −0.477 | 36 |
| SO2 | 70.1 | 2239.08 | −0.918 | −0.480 | ||
| MoSSe | NO2 | 98 | 2.22 × 102 | −0.252 | 0.137 | 37 |
| SO2 | 89 | 1.30 | −0.623 | 0.056 | ||
| WSSe | NO2 | NA | 4.3 × 10−2 | −0.276 | 0.122 | 38 |
| NO | NA | 3.1 × 10−4 | −0.15 | 0.020 |
3.6 Optical and magnetic properties of pristine and Al-doped In2SeTe monolayer for NOx detection
Recent investigations have demonstrated the significant potential of In2SeTe monolayers for environmental gas sensing through variations in their optical properties. In their pristine form, these monolayers exhibit strong absorption in the ultraviolet (UV) region. Upon the adsorption of gases such as NO, CO, NO2, and NH3, distinct and measurable shifts in optical responses occur, enabling the selective detection of these gases. In Fig. S3(a), for the pristine In2SeTe monolayer, adsorption of NO causes a pronounced shift of the absorption peak toward the near-UV region (∼0 nm), resulting in selective near-UV light absorption and making NO uniquely detectable via absorption coefficient (AC) analysis. In contrast, CO adsorption leads to a notable increase in the absorption coefficient (∼0.58 × 104 cm−1) accompanied by an 18–20 nm blueshift of the absorption peak, indicating a strong optical response. Correspondingly, the refractive index of pristine In2SeTe in Fig. S3(b) increases significantly by approximately 0.47 at 200 nm after CO adsorption. These changes suggest that pristine In2SeTe can selectively detect NO and CO gases through combined absorption and refractive index measurements. Upon aluminum doping (Al-doped In2SeTe monolayer), the optical response shifts, making the material highly selective for NO2 detection, which can be observed in Fig. 11(a) and (b). Specifically, in Fig. 11(a), the absorption coefficient decreases substantially by about 0.55 × 105 at 270 nm when NO2 is adsorbed, while other gases induce negligible changes. In Fig. 11(b), the refractive index concurrently decreases by approximately 0.66 with a large redshift of 80 nm, further confirming the selective sensitivity to NO2. Although CO and NH3 adsorption cause notable blueshifts in the refractive index peak, their effects are less pronounced compared to NO2. Thus, Al doping effectively tunes In2SeTe monolayers for selective NO2 gas sensing. Overall, these findings highlight that nitrogen-based gas detection is enhanced by aluminum doping, as evidenced by more significant absorption peak shifts and refractive index changes. Pristine In2SeTe is optimal for selective NO detection, whereas Al-doped In2SeTe exhibits superior selectivity toward NO2, making these materials promising candidates for optically based gas sensors in environmental monitoring applications. | ||
| Fig. 11 (a) Absorption coefficient, (b) refractive index of Al-doped In2SeTe monolayer, spin resolved PDOS of Al-doped In2SeTe monolayer for (c) NO, (d) NO2. | ||
The potential of the pristine In2SeTe monolayer, as well as Al-doped In2SeTe, as a magnetic gas sensor, has been investigated. The magnetic moment of the gas-adsorbed systems was analyzed using GGA + SOC (spin-unrestricted) computations. When the NOx molecule was adsorbed, a magnetic moment was induced on the In2SeTe and Al-doped In2SeTe monolayer. The separation of the up-spin and down-spin conduction bands in the band structures plot can be observed in Fig. 5(e, f) and 6(e, f). From the figure, it can be inferred that magnetization occurred upon the adsorption of NOx gases only. As demonstrated in Fig. 11(c, d) and S3(c, d), the induced magnetism was further validated by the asymmetry in the up-spin and down-spin density of the states. The emergence of magnetic moments (0.8 μB to 1.2 μB) in the system occurs only when NOx molecules are adsorbed onto the surfaces of pristine In2SeTe or Al-doped In2SeTe monolayers. The analyte systems In2SeTe and Al-doped In2SeTe are non-magnetic in all other gas adsorption situations. The induced magnetic moment is zero μB for these gas adsorbed systems. This unique behavior toward the NOx molecule suggests that the pristine and Al-doped In2SeTe monolayer may have potential applications as a magnetic gas sensor for selectively detecting NOx gases. This enhanced selectivity confirms that the magnetic moment in the In2SeTe-based systems is predominantly influenced by NOx adsorption, unlike earlier studies where multiple gases contributed, thereby establishing the system's superiority in selective gas detection.
4 Conclusions
In conclusion, a theoretical design of a 2D pristine and Al-doped In2SeTe monolayer was developed to investigate potential sensor materials for detecting NH3, CO2, NO2, CO, NO, and CH4 in environmental applications. DFT was employed for geometry optimization to determine the band structure, band gap energy, adsorption distance, charge transfer, lattice constant, DOS, and CDD for NH3, CO2, NO2, CO, NO, and CH4 molecules on the In2SeTe monolayer, evaluating its suitability as a gas sensor material. Eventually, it was doped with Al to observe if its properties and sensing capabilities were enhanced. Results showed that both pristine and Al-doped In2SeTe contain all positive phonon dispersion, making them dynamically stable for gas adsorption. Among all the gases, only the NO and NO2 gases prefer to be chemisorbed, whereas all other gases prefer to be physisorbed. Additionally, the NO and NO2 gas molecules adsorbed on the Al-doped In2SeTe monolayer exhibit the highest adsorption energy and the shortest adsorption distance. The In2SeTe monolayer was highly selective for NO and NO2 gases, with sensitivities of 7.249958 × 105 and 2.653623 × 105, which were approximately a million times higher than those of the other gases. Similarly, with Al doping, the sensitivity increases dramatically by 1.26 × 105 and 51.58 times that of the pristine In2SeTe monolayers, and the sensitivity towards NO and NO2 becomes 9.17 × 1010 and 1.367 × 107, respectively. The recovery time for both pristine and Al-doped In2SeTe monolayers remains within a practically acceptable range, demonstrating their suitability for real-time gas sensing applications and ensuring long-term sensor reusability. In addition to baseline performance evaluation, the sensor response was systematically analyzed as a function of Al doping concentration. It was observed that the sensitivity reached its maximum at a critical doping level of 2.33% for NO and 5.5% for NO2, indicating the existence of optimal doping thresholds for each target gas. Beyond or below these specific doping concentrations, a noticeable decline in sensitivity was recorded, suggesting a delicate balance between structural stability and electronic reactivity introduced by dopant atoms. Optical absorption and refractive–index profiles show that pristine In2SeTe exclusively senses NO, while Al-doping tailors the monolayer for NO2 detection. Furthermore, only NOx adsorption triggers a magnetic moment in both the undoped and Al-doped sheets, enabling their use as selective magnetic NOx sensors. This detailed comparison clearly reveals that Al doping significantly enhances the electrical, optical, and chemical properties of the In2SeTe monolayer. As a result, the Al-doped system exhibits superior selectivity and detection capabilities, positioning it as a highly efficient candidate for advanced gas sensing technologies that aim to detect hazardous environmental gases with high sensitivity and reliability.Conflicts of interest
All authors declare that they have no conflict of interest.Data availability
The data supporting this article have been included as part of the Supplementary Information (SI). Supplementary information: the structural, electronic, and adsorption properties of pristine and Al-doped In2SeTe monolayers, including calculated lattice constants, bond lengths, bond angles, layer thickness, band gaps, VBM/CBM positions, and dynamic stability (Table SI), alongside a comparison with previous theoretical studies validating the computational results (Table SII). It presents optimized adsorption structures and charge-density difference maps for NH3, CH4, CO2, CO, NO, and NO2 molecules on In2SeTe (Fig. S1 and S2), as well as the effect of Al doping on band gap and gas-sensing sensitivity across different concentrations (Table SIII). Gas adsorption parameters such as adsorption heights, In–N/O bond lengths, and In–Se/Te bond distortions for NO and NO2 on pristine and doped surfaces are summarized (Tables SIV and SV), indicating interaction strengths and structural deformation. Finally, optical properties including absorption coefficient, refractive index, and spin-resolved partial density of states of pristine In2SeTe, with and without NO and NO2 adsorption, are reported (Fig. S3). See DOI: https://doi.org/10.1039/d5ra05591a.Acknowledgements
We would like to express our sincere gratitude to the Department of Electrical and Electronic Engineering, Khulna University of Engineering & Technology (KUET), Khulna-9203, Bangladesh, for their continuous support and encouragement throughout this research. This work was supported by the UGC Funded Research Project 2024–2025. We also acknowledge the valuable software and technical support provided by the Central Computer Centre, Khulna University of Engineering & Technology (KUET), Khulna-9203, Bangladesh.References
- D. Ma, et al., C3N monolayers as promising candidates for NO2 sensors, Sens. Actuators, B, 2018, 266, 664–673, DOI:10.1016/j.snb.2018.03.159.
- Q. Wu, L. Cao, Y. S. Ang and L. K. Ang, Superior and tunable gas sensing properties of Janus PtSSe monolayer, Nano Express, 2020, 1(1), 010042, DOI:10.1088/2632-959X/ab95e6.
- L. Wan, D. Chen, W. Zeng, J. Li and S. Xiao, Hazardous gas adsorption of Janus HfSeTe monolayer adjusted by surface vacancy defect: A DFT study, Surf. Interfaces, 2022, 34, 102316, DOI:10.1016/j.surfin.2022.102316.
- K. A. Abdur Nur, M. S. Hasan Khan and M. R. Islam, Superior selectivity for NH3 (NO2) gas molecules in In2SSe (Ga2SSe) Janus materials: a first-principles study, Phys. Scr., 2024, 99(9) DOI:10.1088/1402-4896/ad69d2.
- S. Sucharitakul, et al., Intrinsic Electron Mobility Exceeding 103 cm2/(V s) in Multilayer InSe FETs, Nano Lett., 2015, 15(6), 3815–3819, DOI:10.1021/acs.nanolett.5b00493.
- A. Kandemir and H. Sahin, Janus single layers of In2SSe: A first-principles study, Phys. Rev. B, 2018, 97(15) DOI:10.1103/PhysRevB.97.155410.
- C. Wang, C. Gao, J. Hou and Q. Duan, First-principle investigation of CO, CH4 and CO2 adsorption on Cr-doped graphene-like hexagonal borophene, J. Mol. Model., 2022, 28, 196, DOI:10.21203/rs.3.rs-1456018/v1.
- Selective and sensitive toxic gas-sensing mechanism in a 2D Janus MoSSe monolayer.
- S. Saha, D. I. Sajib and M. K. Alam, Interaction of the III-As monolayer with SARS-CoV-2 biomarkers: implications for biosensor development, Phys. Chem. Chem. Phys., 2024, 26(7), 6242–6255, 10.1039/d3cp05215j.
- H. Cui, M. Ran, X. Peng and G. Zhang, First-principles design of noble metal (Rh and Pd) dispersed Janus WSTe monolayer for toxic gas sensing applications, J. Environ. Chem. Eng., 2024, 12(2), 112047, DOI:10.1016/j.jece.2024.112047.
- A. Y. Lu, et al., Janus monolayers of transition metal dichalcogenides, Nat. Nanotechnol., 2017, 12(8), 744–749, DOI:10.1038/nnano.2017.100.
- Y. Guo, S. Zhou, Y. Bai and J. Zhao, Defects and oxidation of group-III monochalcogenide monolayers, J. Chem. Phys., 2017, 147(10) DOI:10.1063/1.4993639.
- S. Pal Kaur, T. Hussain and T. J. Dhilip Kumar, Substituted 2D Janus WSSe monolayers as efficient nanosensor toward toxic gases, J. Appl. Phys., 2021, 130(1) DOI:10.1063/5.0054319.
- D. Wang, et al., Janus MoSSe monolayer: A highly strain-sensitive gas sensing material to detect SF6 decompositions, Sens. Actuators, A, 2020, 311 DOI:10.1016/j.sna.2020.112049.
- L. Zhang, et al., Recent advances in emerging Janus two-dimensional materials: From fundamental physics to device applications, J. Mater. Chem. A, 2020, 8(18), 8813–8830, 10.1039/d0ta01999b.
- R. Chaurasiya and A. Dixit, Defect engineered MoSSe Janus monolayer as a promising two dimensional material for NO2 and NO gas sensing, Appl. Surf. Sci., 2019, 490, 204–219, DOI:10.1016/j.apsusc.2019.06.049.
- P. Yu, et al., Pd doped Janus HfSeS monolayer: Ultrahigh sensitive gas sensing material for reversible detection of NO, Sens. Actuators, A, 2024, 365, 114864, DOI:10.1016/j.sna.2023.114864.
- A. Marjaoui, M. Zanouni, M. Ait Tamerd, A. El Kasmi and M. Diani, A First-Principles Investigation on Electronic Structure, Optical and Thermoelectric Properties of Janus In2SeTe Monolayer, J. Supercond. Novel Magn., 2021, 34(12), 3279–3290, DOI:10.1007/s10948-021-06028-0.
- R. Shahriar, O. Hassan and M. K. Alam, Adsorption of gas molecules on buckled GaAs monolayer: a first-principles study, RSC Adv., 2022, 12(26), 16732–16744, 10.1039/d2ra02030k.
- H. Chen, et al., Exploring monolayer Janus MoSSe as potential gas sensor for Cl2, H2S and SO2, Comput. Theor. Chem., 2022, 1211, 113665, DOI:10.1016/j.comptc.2022.113665.
- H. T. T. Nguyen, et al., Spin-orbit coupling effect on electronic, optical, and thermoelectric properties of Janus Ga2SSe, RSC Adv., 2020, 10(73), 44785–44792, 10.1039/d0ra08279a.
- M. Bala, M. T. Rahman, R. Al Nahean and M. S. Hasan Khan, NO2 and SO2 adsorption and sensing on Janus B2SeTe: unveiling its electronic, optical, and magnetic properties through DFT and COMSOL, RSC Adv., 2025, 15(31), 25187–25201, 10.1039/D5RA04190B.
- A. Salehi and D. Jamshidi Kalantari, Characteristics of highly sensitive Au/porous-GaAs Schottky junctions as selective CO and NO gas sensors, Sens. Actuators, B, 2007, 122(1), 69–74, DOI:10.1016/j.snb.2006.05.004.
- B. Zhu, et al., Monolayer Janus Te2Se-based gas sensor to detect SO2and NOx: A first-principles study, Phys. Chem. Chem. Phys., 2021, 23(2), 1675–1683, 10.1039/d0cp05750a.
- R. Shahriar, O. Hassan and M. K. Alam, Adsorption of gas molecules on buckled GaAs monolayer: a first-principles study, RSC Adv., 2022, 12(26), 16732–16744, 10.1039/d2ra02030k.
- C. Jin, X. Tang, X. Tan, S. C. Smith, Y. Dai and L. Kou, A Janus MoSSe monolayer: a superior and strain-sensitive gas sensing material, J. Mater. Chem. A, 2019, 7(3), 1099–1106, 10.1039/C8TA08407F.
- P. Panigrahi, D. Jini, H. Bae, H. Lee, R. Ahuja and T. Hussain, Two-dimensional Janus monolayers of MoSSe as promising sensor towards selected adulterants compounds, Appl. Surf. Sci., 2021, 542, 148590, DOI:10.1016/j.apsusc.2020.148590.
- L. Wan, D. Chen, W. Zeng, J. Li and S. Xiao, Hazardous gas adsorption of Janus HfSeTe monolayer adjusted by surface vacancy defect: A DFT study, Surf. Interfaces, 2022, 34, 102316, DOI:10.1016/j.surfin.2022.102316.
- H. Zhao, et al., Janus In2SeTe for photovoltaic device applications from first-principles study, Chem. Phys., 2022, 553 DOI:10.1016/j.chemphys.2021.111384.
- D. Xu, B. Cai, J. Tan and G. Ouyang, Tailoring the anisotropic effect of Janus In2XY (X/Y = S, Se, Te) monolayers toward realizing multifunctional optoelectronic device applications, New J. Phys., 2023, 25(8) DOI:10.1088/1367-2630/ace845.
- T. V. Vu, et al., Electronic, optical, and thermoelectric properties of Janus In-based monochalcogenides, J. Phys. Condens. Matter, 2021, 33(22) DOI:10.1088/1361-648X/abf381.
- M. Bala, M. T. Rahman, R. Al Nahean, B. Sorker, and M. R. Firoz, Adsorption and Gas Sensing Properties of GaAs Monolayer: A DFT Study, in 13th International Conference on Electrical and Computer Engineering, ICECE 2024, Institute of Electrical and Electronics Engineers Inc., 2024, pp. 74–79, DOI:10.1109/ICECE64886.2024.11024722.
- R. Shahriar, O. Hassan and M. K. Alam, Adsorption of gas molecules on buckled GaAs monolayer: a first-principles study, RSC Adv., 2022, 12(26), 16732–16744, 10.1039/d2ra02030k.
- X. Tian, et al., Recent advances in MoS2-based nanomaterial sensors for room-temperature gas detection: A review, Sens. Diagn., 2023, 2(2), 361–381, 10.1039/d2sd00208f.
- D. Singh and R. Ahuja, Highly sensitive gas sensing material for environmentally toxic gases based on Janus NbSeTe monolayer, Nanomaterials, 2020, 10(12), 1–17, DOI:10.3390/nano10122554.
- J. Hu, Q. Zhang, Q. Zhang and H. Cui, Favorable sensing property of Pt-doped Janus HfSSe monolayer upon H2S and SO2: A first-principles theory, J. Mater. Res. Technol., 2022, 20, 763–771, DOI:10.1016/j.jmrt.2022.07.080.
- B. Babariya, D. Raval, S. K. Gupta and P. N. Gajjar, Selective and sensitive toxic gas-sensing mechanism in a 2D Janus MoSSe monolayer, Phys. Chem. Chem. Phys., 2022, 24(25), 15292–15304, 10.1039/d2cp01648f.
- R. Chaurasiya and A. Dixit, Ultrahigh sensitivity with excellent recovery time for NH3and NO2in pristine and defect mediated Janus WSSe monolayers, Phys. Chem. Chem. Phys., 2020, 22(25), 13903–13922, 10.1039/d0cp02063j.
| This journal is © The Royal Society of Chemistry 2025 |


