 Open Access Article
Open Access ArticleEffect of xanthan gum on the interfacial and foam properties of an acidic sophorolipid solution: experimental and theoretical studies
Jinlu Zhong ,
Yanan Weng,
Sensen Xie,
Shuang Li and
Dinghua Yu
,
Yanan Weng,
Sensen Xie,
Shuang Li and
Dinghua Yu *
*
College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211816, PR China. E-mail: yudh@njtech.edu.cn; Tel: +86-25-58139386
First published on 15th October 2025
Abstract
The interaction mechanism between surfactants and polymers has important guiding significance in the field of high-performance fluid design. However, research on the interactions between biological surfactants and microbial polysaccharides remains limited. In this paper, the effect of xanthan gum on the interfacial properties of an acidic sophorolipid solution, such as surface tension, viscosity, rheology and foam properties, was systematically studied by means of experimental and theoretical methods. The results showed that the addition of 0.1 wt% xanthan gum had little effect on the critical micelle concentration of acidic sophorolipid, and increased the contact angles of the aqueous solution with PTFE and PP. At the same time, the interaction between acidic sophorolipid and xanthan gum reduced the bulk viscosity of the xanthan gum solution. Furthermore, the shear-thinning characteristics of the mixed system did not change, but the yield value gradually decreased with increasing acidic sophorolipid concentration. Significantly, the addition of xanthan gum slightly reduced the foaming property of acidic sophorolipid but significantly improved the stability of the foam. Foam counting and image analysis showed that xanthan gum not only made the foam small and evenly distributed, but also inhibited the aggregation of foam effectively as acidic sophorolipid concentration increased. Finally, in order to reveal the molecular mechanism underlying the interaction between xanthan gum and acidic sophorolipid, DFT calculations were used to simulate their interaction. The results indicated that robust hydrogen bonding interactions involving carboxyl and hydroxyl groups exist between acidic sophorolipids and xanthan gum, thereby contributing to the enhancement of foam stability. These findings would provide useful references for biosurfactant applications in complicated formulations across diverse fields.
1 Introduction
Biosurfactants are surface-active compounds synthesized by microorganisms during metabolic processes. They are distinguished by their exceptional interfacial activity, biodegradability, and environmental compatibility, which render them extensively applicable in various domains, including petroleum extraction, environmental remediation, food production, pharmaceuticals, and cosmetics.1 Compared with conventional synthetic surfactants, biosurfactants present notable advantages in terms of their natural origin, sustainability, and environmental compatibility.2–4 Sophorolipids (SLs), a representative class of glycolipid biosurfactants, are composed of a hydrophilic sophorose head and a hydrophobic fatty acid tail. These compounds typically exist in two structural forms: lactonic and acidic. The molecular structure of SLs is influenced by factors such as chain length, degree of unsaturation, and acetylation, which vary according to the fermentation substrate utilized.5 Among these, acidic sophorolipids, characterized by the presence of a free carboxyl group at the molecular terminus, demonstrate enhanced solubility and foaming properties,6 thereby facilitating their widespread application in foam cleaning, oil recovery, and wetting systems.7However, foam systems generally encounter stability challenges, including drainage, film rupture, and coalescence, which are particularly pronounced at low concentrations. Although surfactants can facilitate foam formation by reducing interfacial tension, their capacity to stabilize the liquid film is inherently limited. Consequently, enhancing the foam stability of acidic sophorolipid has emerged as a pivotal area of research.8
In recent years, the integration of biopolymers and surfactants has been acknowledged as an effective strategy to enhance foam stability. Polysaccharides such as xanthan gum, carrageenan, pectin, alginates, gelatin, and gum arabic are extensively utilized in the food and cosmetic industries due to their non-toxicity, biodegradability, and favorable rheological properties. These polymers can effectively inhibit droplet aggregation and membrane rupture through electrostatic interactions and steric hindrance, thereby significantly enhancing foam stability.9–12 Among these, xanthan gum (XG), an anionic polysaccharide derived from microorganisms, is recognized for its excellent solubility and high viscosity. It is commonly employed to thicken and stabilize foam systems.13 By increasing system viscosity, enhancing liquid film strength, and regulating interfacial adsorption behavior, xanthan gum collaborates with surfactant molecules to improve the overall stability of foam systems.
Consequently, the combination of acidic sophorolipid and xanthan gum not only possesses the potential to enhance foam performance but also broadens its applicability in fields such as oil recovery, environmental remediation, and foam materials. This study aims to systematically examine the effects of the combination of acidic sophorolipid and xanthan gum on interfacial behavior, foam performance, and rheological properties. Through molecular simulations and electrostatic potential analysis, the potential synergistic mechanisms are elucidated. Comparative studies across various concentration systems provide a theoretical foundation for the development of biosurfactant–biopolymer formulations, thereby facilitating their practical application in detergents, cosmetics, and environmental engineering.
2 Experimental methods
2.1 Reagents and materials
Sodium hydroxide, sulfuric acid (98%), and dichloromethane were procured from Aladdin Regent Co., Ltd (Shanghai, China). Sophorolipids were provided by Shandong Lierkang Medical Technology Co., Ltd (Shandong, China). Xanthan gum was obtained from Sigma-Aldrich Corp (St. Louis, MO, USA). All standard solutions and samples were prepared using ultrapure water (resistivity = 18.2 MΩ cm) purified through a laboratory water purification system (Nanjing Yioud).2.2 Methods
Foam generation was conducted utilizing the double-syringe method,15 which involves two 30 mL syringes connected by a three-way valve. One syringe contained 5 mL of foam solution, while the other contained 5 mL of air, resulting in a liquid volume fraction of 50%. The piston was oscillated back and forth 30 times to ensure thorough mixing of the air and liquid, thereby generating uniform foam. Subsequently, the foam was transferred to a 15 mL glass tube for further analysis.
The performance of the foam was evaluated based on two parameters: foam capacity (FC) and foam stability (FS). Experimental observations revealed that both pre- and post-combination foams exhibited a rapid drainage rate within the initial 5 minutes. Consequently, foam height after 5 minutes was selected as the time point for measuring foam stability. Furthermore, to provide a precise quantification of the liquid retention capacity of the foam, the liquid drainage percentage (LDP) was introduced as an additional evaluative parameter. Its calculation is as follows:
 | (1) |
The formulas for calculating FC and FS are as follows:16
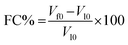 | (2) |
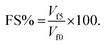 | (3) |
Prior to measurement, all samples were diluted to the target concentration using deionized water. Measurements were performed at room temperature utilizing a Zetasizer instrument (model EEN3690, Malvern Instruments Ltd, UK). This instrument operates based on the principle of electrophoretic light scattering, which facilitates the accurate determination of the electrophoretic mobility of charged particles under an applied electric field, from which the zeta potential was subsequently calculated.
2.3 Statistical analysis
Error bars indicate the standard deviations (SD), and the data are presented as the arithmetic means ± SD of three independent replicates. To evaluate statistical significance among different treatment groups, one-way analysis of variance (ANOVA) was performed, followed by the least significant difference (LSD) test for multiple comparisons at a significance level of p < 0.05. All statistical analyses were conducted using Origin 2021 software.3 Results and discussion
3.1 Screening of XG concentration
To clarify the effect of XG concentration on the SL-COOH foam system, this study conducted compounding experiments with different XG concentrations of 0–0.5 wt% and 0.4 mM SL-COOH.The results are shown in Fig. 1. The experimental findings indicate that as the XG concentration increases, foam stability exhibits a significant upward trend, especially at concentrations of 0.1 wt%, 0.3 wt%, and 0.5 wt%, where FS values reached 62.6%, 74.1%, and 82.4% respectively. By contrast, a low concentration of 0.05 wt% XG only brought a limited improvement in foam stability (FS = 35.7%), indicating that the effect of trace amounts of XG is limited. At a concentration of 0.1 wt%, FC and FS reached a relative balance, which is favorable for both foam formation and stability. Although further increasing the XG concentration can continue to enhance foam stability, the significantly increased viscosity of the system limits foam generation, with foaming ability decreasing by more than 10%.
Considering both FC and FS, 0.1 wt% XG exhibited a good synergistic stabilizing effect while exerting minimal adverse impact on foamability and was therefore selected as the representative concentration for subsequent experiments.
3.2 Surface tension
One of the key parameters of surfactant solutions is the critical micelle concentration (CMC), defined as the concentration at which the solution begins to form a substantial number of micelles.23 The surface tension of SL-COOH and its blend with 0.1 wt% XG was measured, and a linear plot of surface tension versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C was constructed, as shown in Fig. 2A. With increasing surfactant concentration, surface tension decreased due to enhanced adsorption of surfactant molecules at the air–water interface.24 The minimum surface tension of the SL-COOH solution was 38.2 mN m−1. Upon addition of XG, the surface tension remained constant at 38.2 mN m−1, indicating no change in the minimum value. Further linear fitting, as illustrated in Fig. 2B, yielded a CMC of 0.42 mM for SL-COOH, while the presence of XG slightly increased the CMC to 0.556 mM. This marginal variation suggests that under the experimental conditions, XG exerted no significant influence on the micellization behavior of SL-COOH. This observation aligns with findings reported in previous studies.25,26
C was constructed, as shown in Fig. 2A. With increasing surfactant concentration, surface tension decreased due to enhanced adsorption of surfactant molecules at the air–water interface.24 The minimum surface tension of the SL-COOH solution was 38.2 mN m−1. Upon addition of XG, the surface tension remained constant at 38.2 mN m−1, indicating no change in the minimum value. Further linear fitting, as illustrated in Fig. 2B, yielded a CMC of 0.42 mM for SL-COOH, while the presence of XG slightly increased the CMC to 0.556 mM. This marginal variation suggests that under the experimental conditions, XG exerted no significant influence on the micellization behavior of SL-COOH. This observation aligns with findings reported in previous studies.25,26
 | ||
| Fig. 2 Surface tension of SL-COOH and SL-COOH-0.1 wt% XG solutions. (A) Surface tension profiles; (B) linear correlation between surface tension and −ln(C). | ||
This phenomenon may be attributed to XG, as a polysaccharide polymer, augmenting the number of hydrophilic sugar rings in the solution upon combination, which consequently diminishes the coverage of the hydrophobic groups at the oil–water interface. Therefore, a higher concentration is necessitated to achieve conditions requisite for micelle formation.
To further investigate the adsorption performance of SL-COOH, we calculated the surface excess concentration (Γ) and the cross-sectional area occupied by each SL-COOH molecule at the micelle (A) using the following formula:
 | (4) |
The molecular area (A), measured in nm,2 was calculated as follows:
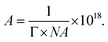 | (5) |
Based on the calculations presented in Table 1, the excess concentration (Γ) values were determined as follows: SL-COOH: 1.163 × 10−6 mol m−2 and SL-COOH (XG): 1.098 × 10−6 mol m−2. The results for the molecular area (A) were as follows: SL-COOH: 1.427 nm2, SL-COOH (XG): 1.513 nm2.
| System | CMC (mM) | Γ (μmol m−2) | A (nm2) | Minimum surface tension (γ, mN m−1) |
|---|---|---|---|---|
| SL-COOH | 0.42 | 1.163 | 1.427 | 38.2 |
| SL-COOH(XG) | 0.556 | 1.098 | 1.513 | 38.2 |
The addition of XG significantly enhanced the hydrophilicity of the system, thereby increasing the likelihood of molecular dispersion in the aqueous phase and reducing adsorption at the oil–water interface. This phenomenon resulted in a decrease in the surface excess concentration. Furthermore, the presence of XG slightly augmented the molecular spacing at the interface. Additionally, the arrangement of molecules at the interface became more loosely packed, which contributed to an increase in the area occupied by the molecules at the interface. These experimental findings indicate that XG can modify the interfacial adsorption behavior of SL-COOH through weak intermolecular interactions. Such alterations may encompass changes in adsorption density, molecular arrangement, and structural properties of the interfacial layer, which in turn can significantly influence the surface and foam characteristics of the system.
3.3 Foam characteristics
Fig. 3 illustrates that the foam capacity and foam stability of acidic sophorolipid display staged behavior in response to variations in concentration. Utilizing the critical micelle concentration (CMC) value of SL-COOH as a reference point, the incorporation of XG resulted in a reduction in foaming volume by less than 10%, with negligible effects on overall foam capacity. Nevertheless, at concentrations below the CMC, foam stability experienced an increase of 370.8%. When the SL-COOH concentration surpassed the CMC, the enhancement in foam stability plateaued, yet remained above 270%. This indicates that low concentrations of XG can substantially improve foam stability in SL-COOH systems, albeit with a slight reduction in foam capacity. Petkova et al.29 experimentally investigated the effect of polymer–surfactant systems on foam properties. They found that, compared with pure surfactant solutions, mixtures of oppositely charged polymers and surfactants enhanced foam stability while reducing foaming capacity.
The adsorption data presented in Table 1 indicate that, following the addition of XG, the interfacial adsorption (Γ) of SL-COOH decreased, while the molecular area increased, resulting in a loosely packed arrangement of molecules at the interface.
The molecular migration rate at the interface has a direct impact on foam formation. The incorporation of polysaccharide polymers typically enhances the viscosity of the system, thereby improving the viscoelasticity of the foam liquid film, which in turn reduces the interfacial migration rate and inhibits foam formation.30–33
This finding aligns with observations reported in the literature,34 which suggest that a decrease in the instantaneous adsorption of surfactants may lead to diminished interface coverage, consequently inhibiting foam formation. A critical mechanism underlying foam stability is foam drainage, which is intricately linked to the viscosity of the foam solution. XG enhances foam stability by increasing the viscosity and strength of the liquid film, thereby preventing bubble coalescence and improving both bubble number and distribution.35,36
When the concentration exceeds the critical micelle concentration (CMC), the interfacial adsorption of the system approaches saturation. At this point, additional surfactant molecules are more likely to form micelles rather than continue adsorbing at the gas–liquid interface. Meanwhile, the enhancing effect of XG on the viscoelasticity of the liquid film also reaches a plateau, resulting in no further significant increase in foam stability, although it remains at a high level. This phenomenon has already been reported in similar literature.37
Therefore, when incorporating XG, it is essential to carefully consider its dual effects on foamability and foam stability.
Fig. 4 further shows liquid drainage behavior, underscoring significant disparities in foam stability across the various systems. Overall, foams generated from SL-COOH solutions exhibited rapid liquid drainage within the initial 5 minutes, with nearly all liquid expelled by the 15-minute mark. This phenomenon was particularly pronounced at low concentrations, where foam films thinned rapidly due to water loss, resulting in an increased likelihood of film rupture and a swift decline in foam volume.
 | ||
| Fig. 4 Liquid drainage percentage of SL-COOH foams with and without XG at different concentrations over time. Error bars indicate the standard deviation (n = 15). | ||
By contrast, the incorporation of XG significantly enhanced the liquid retention capacity of the foams. This phenomenon was particularly pronounced in the 12 mM SL-COOH system, which indicates that low foam liquid drainage volume corresponds to enhanced foam stability.38
This observation is consistent with the prior analysis regarding the enhanced foam stability conferred by XG. The improvement can be attributed to the substantial increase in solution viscosity, which augments the viscoelastic properties of the foam films, thereby suppressing gravitational drainage both within Plateau borders and across films. This stabilization mechanism aligns with the three-stage foam drainage theory delineated in the literature:39 (i) an initial stage of forced drainage driven by foam injection, (ii) a gravity-controlled intermediate stage of free drainage, and (iii) a final stage of accelerated drainage induced by bubble coarsening and film rupture. In the present system, the addition of XG effectively postponed the onset of both free drainage and coarsening-induced drainage, enabling the foam to retain a high liquid content for an extended duration and thereby enhancing overall foam stability.
3.4 Foam visualization
To effectively characterize the coarsening behavior of the foam, optical microscopy was employed to monitor temporal changes in foam structure. The following characteristics were systematically analyzed: (1) variations in the number of bubbles within the field of view and (2) changes in the average bubble diameter. | ||
| Fig. 5 Microscopic foam morphology of SL-COOH (0.4, 3, and 12 mM) recorded at 5 and 20 min. (A) SL-COOH solutions and (B) SL-COOH-0.1 wt% XG. | ||
Foam coarsening results from the combined effects of Ostwald ripening and bubble coalescence.40–43 On one hand, a Laplace pressure differential exists between bubbles of varying sizes (with small bubbles exhibiting high internal pressure), which induces the diffusion of gas from smaller to larger bubbles through the intervening liquid film (i.e., Ostwald ripening). The corresponding ripening rate is expressed by eqn (6):44
 | (6) |
Conversely, liquid film drainage results in the thinning and subsequent rupture of the liquid film, thereby promoting bubble coalescence. The thermal activation energy barrier for coalescence, which reflects the resistance of the liquid film to coalescence, can be expressed via eqn (7):44
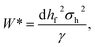 | (7) |
As shown in Fig. 5A, the SL-COOH foam system drains liquid relatively quickly over time, especially at the low concentration of 0.4 mM. At 5 minutes, large bubbles appear, and by 20 minutes, the bubble size increases further and becomes unevenly distributed, indicating poor system stability. With the addition of XG, the foam at the initial 5 minutes becomes uniform and fine, with an even bubble distribution and improved stability.
It is hypothesized that the incorporation of XG suppresses both primary coarsening mechanisms. The polymer chains of XG significantly increase the viscosity of the system, slow the diffusion rate of gas through the liquid film, and markedly reduce the average foam diameter, thereby lowering the Ostwald ripening rate. Simultaneously, interactions between XG and the surfactant augment the liquid film thickness and interfacial viscoelasticity, thereby elevating the coalescence energy barrier W* and rendering the liquid film resistant to rupture. Therefore, the introduction of XG reduces foam coarsening in SL-COOH alone and improves foam stability.
Fig. 6A shows the initial bubble size distribution for SL-COOH alone, revealing that the bubbles formed were large. As anticipated, bubble size increased over time as a consequence of liquid drainage and coalescence. For example, in the 0.4 mM SL-COOH solution, the mean foam diameter expanded from 117.2 μm to 208.5 μm. When the concentration was elevated to 3 mM and 12 mM, the growth in bubble diameter was less pronounced, and the size distribution became narrower, implying that higher SL-COOH concentrations enhanced foam stability to a certain degree.
 | ||
| Fig. 6 Time-dependent bubble size distribution: (A) SL-COOH solutions at different concentrations (0.4, 3, and 12 mM) at 5, 10, and 20 min and (B) SL-COOH-0.1 wt% XG under the same conditions. | ||
Following the incorporation of XG, as illustrated in Fig. 6B, the mean bubble size exhibits a marked reduction over time, accompanied by a narrowly distributed size range. This observation suggests that the presence of XG leads to an increase in system viscosity, a diminished migration rate of surfactant molecules, a reduced liquid drainage rate, and a suppression of bubble coalescence, thereby retarding the coarsening process.45–47 These observations further corroborate the analysis of the coarsening phenomenon shown in Fig. 5.
3.5 Wettability
Surfactants are widely employed to enhance the wettability of aqueous solutions on hydrophobic surfaces.48 Fig. 7 depicts the influence of XG incorporation on the wettability of SL-COOH solutions at varying concentrations on two hydrophobic substrates: polytetrafluoroethylene (PTFE) and polypropylene (PP).As shown in Fig. 7A and B: the contact angles of SL-COOH solutions all decrease gradually over time; moreover, as the concentration increases, the change in contact angle tends to flatten, indicating that the substrate surface is approaching its inherent hydrophobic limit. A further comparison between different substrates reveals that the wettability of the PP plate is superior to that of the PTFE plate. This difference stems from the material properties of PTFE.49–51 As a polymer polymerized from tetrafluoroethylene, PTFE not only possesses high thermal stability and excellent chemical stability but also has extremely low surface energy. The contact angle of water on its surface is 107°, thus resulting in inferior wettability compared with the PP plate.
Following the incorporation of XG, the contact angle of the solution exhibits a slight increase. Although this observation appears to contrast with the pronounced foam stability-enhancing effect of XG, it can be rationalized in terms of its intrinsic physicochemical properties and the nature of the intermolecular interactions involved.
On one hand, the high molecular weight and extended chain conformation of XG give rise to a steric hindrance effect upon interaction with the SL-COOH surface, thereby impeding the approach of water molecules to the substrate and directly resulting in a modest increase in the contact angle.
On the other hand, both SL-COOH and XG possess hydroxyl and carboxyl functional groups within their molecular frameworks. Upon complexation, extensive intermolecular hydrogen bonding occurs, which reduces the availability of free hydroxyl and carboxyl groups in the system, thereby slightly diminishing overall hydrophilicity. This mechanism has been substantiated in the sodium alginate-krill protein system, where the introduction of graphene oxide facilitates hydrogen bond formation, similarly leading to reduced hydrophilicity and an increased contact angle.52 Furthermore, Liang et al.53 reported in their investigation of polymer–surfactant composite systems that, at elevated concentrations, van der Waals forces promote molecular aggregation, resulting in the formation of a saturated interfacial film structure. This structural arrangement inhibits further reduction of the contact angle, thereby providing an additional explanation for the aforementioned phenomenon.
In conclusion, it can be inferred that the synergistic effects of hydrogen bonding and van der Waals interactions constitute the principal factors underlying the slight increase in the contact angle observed in the SL-COOH(XG) composite solution.
3.6 Quantum chemistry and interaction mechanism analysis
In solution, the formation of hydrogen bonds is believed to significantly enhance foam stability. Generally, a shorter hydrogen bond length correlates with greater bond energy, indicating a more stable hydrogen bond interaction. Previous studies have demonstrated that the formation of short-range hydrogen bonds can strengthen intermolecular interactions at the interface, thereby improving the structural stability of the foam membrane and enhancing the durability of the foam and its resistance to rupture.54 Table 2 presents the electronic energies and binding energies of SL-COOH, XG, and SL-COOH(XG). The binding energy (ΔE, in kJ mol−1) was calculated using the following equation:
| ΔE = −2625.5 × (Efc − Ef − Ec), |
| Electronic energy (Hartree) | Electronic energy (kJ mol−1) | Binding energy (kJ mol−1) | |
|---|---|---|---|
| SL-COOH | −2154.49 | −5.65 × 106 | — |
| XG | −4020.83 | −10.55 × 106 | — |
| SL-COOH(XG) | −6175.32 | −16.21 × 106 | −97.2 |
The interactions between SL-COOH and XG were visualized utilizing the independent gradient model (IGMH) based on Hirshfeld partitioning,55 as illustrated in Fig. 8B. The IGMH analysis distinctly elucidates the interactions within the SL-COOH (XG) system, with blue, green, and red denoting strong attraction, van der Waals forces, and strong repulsion, respectively. The findings indicate that two predominant types of interactions govern the combined system. The blue regions, indicated by arrows in the figure, are concentrated between the carboxyl group of SL-COOH and the hydroxyl group of XG, corresponding to the sign(λ2)ρ < −0.02 range and accompanied by multiple high δginter peaks, suggesting robust hydrogen bond attraction between the two. This interaction contributes to the enhancement of the mechanical strength of the liquid film, thereby improving the resistance of the foam to rupture.56 This observation is consistent with the hydrogen bond interaction sites indicated in the previous ball-and-stick model, further corroborating the existence of stable non-covalent interactions between the two.
Furthermore, the green region, corresponding to sign(λ2)ρ ≈ −0.01, signifies the presence of hydrophobic van der Waals interactions within the system. As a short-range attractive force, van der Waals forces influence the aggregation and arrangement of interfacial molecules, resulting in a more loosely packed structure, which subsequently affects the wettability of the interface. This mechanism provides a plausible explanation for the observed phenomenon where the contact angle increased rather than decreased during the experiment. Overall, the IGMH analysis elucidates the non-covalent interaction characteristics of hydrogen bonds and van der Waals forces within the system, thereby aiding in the interpretation of experimental observations regarding foam stability and contact angle variations following the combination.
Further rheological analysis, as illustrated in Fig. 9B and C, was conducted to investigate the flow behavior of the mixed systems at varying SL-COOH concentrations. As the concentration of SL-COOH increased, the apparent viscosity of the 0.1 wt% XG solution exhibited a decrease with rising shear rate, indicating that all samples displayed non-Newtonian, shear-thinning behavior (i.e., pseudoplasticity). This shear-thinning phenomenon is likely attributable to hydrogen bonding or other non-covalent interactions between SL-COOH and XG molecules, which disrupt the original entangled network structure of XG. Under shear conditions, these competitive interactions result in the breakdown of the polymer network, thereby enhancing fluidity and significantly reducing the shear stress required for flow, thus demonstrating typical shear-thinning characteristics.
Changes in zeta potential serve as an indicator of the evolution of surface charge states on colloidal particles within the system.57 As illustrated in Fig. 9D, the absolute value of the zeta potential exhibits a gradual decrease with increasing concentrations of SL-COOH, which signifies a reduction in surface charge density.
In conditions where both SL-COOH and XG are anionic, the observed charge-shielding effect is likely attributable to non-covalent interactions, such as hydrogen bonding, between SL-COOH and XG. These interactions may transpire through hydrophilic groups (e.g., carboxyl and hydroxyl groups on the sugar ring), thereby facilitating the aggregation or adsorption of SL-COOH molecules around the XG polymer chains. Consequently, this leads to the shielding or attenuation of the surface charges originally present on XG, ultimately compromising the integrity of the three-dimensional network structure and resulting in a decrease in solution viscosity.
Molecular electrostatic potential (MESP) serves as an indicator of relative electron density within a molecule, thereby offering valuable insights into non-bonding interactions between molecules.58 As illustrated in Fig. 9E and F, following the interaction between SL-COOH and XG, distinct negatively charged regions emerge on the molecular surface, constituting 58.72% of the total surface area. These regions are predominantly situated near functional groups such as carboxyl and hydroxyl, thereby confirming the overall negatively charged character of the system. The uneven distribution of these highly negative potential areas generates a polarity gradient on the molecular surface, facilitating interactions between SL-COOH and XG at multiple binding sites through hydrogen bonding and other polar interactions. This type of interaction may diminish the entanglement of XG molecular chains, disrupt their ordered arrangement, and undermine the stability of the XG network structure.
4 Conclusions
This study systematically investigates the synergistic mechanism of foam stabilization between acidic sophorolipids (SL-COOH) and xanthan gum (XG). Interestingly, their combination results in a significant decrease in viscosity, which is contrary to the commonly observed viscosity enhancement in conventional polymer–surfactant systems. Zeta potential and electrostatic potential analyses suggest that the interactions between SL-COOH and XG affect the intermolecular entanglements of XG molecular chains, inducing polymer relaxation and thereby resulting in a decrease in bulk viscosity.Although the viscosity of the high-concentration SL-COOH system decreases to some extent, the viscosity of the SL-COOH (XG) compound system still maintains a certain level compared with that of the pure SL-COOH system, and this compound system exhibits significantly improved foam stability. Mechanistically, combined with molecular simulation results, it is found that multiple hydrogen bonds formed between SL-COOH and XG significantly enhance the interfacial viscoelasticity. This enhanced interfacial viscoelasticity can effectively inhibit liquid drainage and bubble coalescence. It is precisely this dominant enhancement effect of interfacial viscoelasticity that ultimately improves the foam stability of the SL-COOH (XG) system.
In summary, this work demonstrates that a well-structured interfacial network formed through weak intermolecular interactions can yield excellent foam stability even under low-viscosity conditions. These findings challenge the conventional view that high viscosity is a prerequisite for foam stability and provide a theoretical basis for the functional optimization of sustainable, bio-based foaming systems.
Author contributions
Jinlu Zhong: data curation, methodology, writing, review and editing. Yanan Weng: data curation, investigation. Sensen Xie: conceptualization, methodology Shuang Li: data curation, review Dinghua Yu: supervision, data curation, project administration and funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Acknowledgements
This work was supported by the National Key Research and Development Plan (No. 2021YFC2103800).References
- M. Krzan, A. Drabczyk, S. Kudłacik-Kramarczyk and M. Jamroży, Foams based on biosurfactants solutions. Part I. Influence of biosurfactant origin on foaming properties, Curr. Opin. Colloid Interface Sci., 2024, 72, 101821 CrossRef CAS.
- P. Vandana and D. Singh, Review on Biosurfactant Production and its Application, Int. J. Curr. Microbiol. Appl. Sci., 2018, 7(8), 4228–4241 CrossRef CAS.
- S. Vijayakumar and V. Saravanan, Biosurfactants-Types, Sources and Applications, Res. J. Microbiol., 2015, 10, 181–192 CrossRef.
- M. Fakruddin, Biosurfactant: Production and Application, J. Petrol Environ. Biotechnol., 2012, 3(4), 1–4 Search PubMed.
- M. Borsanyiova, A. Patil, R. Mukherji, A. Prabhune and S. Bopegamege, Biological activity of sophorolipids and their possible use as antiviral agents, Folia Microbiol., 2015, 61, 85–89 CrossRef PubMed.
- I. N. A. Van Bogaert, K. Saerens, C. De Muynck, D. Develter, W. Soetaert and E. J. Vandamme, Microbial production and application of sophorolipids, Appl. Microbiol. Biotechnol., 2007, 76, 23–34 CrossRef CAS PubMed.
- V. Shah, G. F. Doncel, T. Seyoum, K. M. Eaton, I. Zalenskaya, R. Hagver, A. Azim and R. Gross, Sophorolipids, Microbial Glycolipids with Anti-Human Immunodeficiency Virus and Sperm-Immobilizing Activities, Antimicrob. Agents Chemother., 2005, 49(10), 4093–4100 CrossRef CAS PubMed.
- S. Pal, N. Chatterjee, A. K. Das, D. J. McClements and P. Dhar, Sophorolipids: A comprehensive review on properties and applications, Adv. Colloid Interface Sci., 2023, 313, 102856 CrossRef CAS PubMed.
- M.-P. Wang, X.-W. Chen, J. Guo, J. Yang, J.-M. Wang and X.-Q. Yang, Stabilization of foam and emulsion by subcritical water-treated soy protein: Effect of aggregation state, Food Hydrocoll., 2019, 87, 619–628 CrossRef CAS.
- X. Zang, J. Wang, G. Yu and J. Cheng, Addition of anionic polysaccharides to improve the stability of rice bran protein hydrolysate-stabilized emulsions, LWT–Food Sci. Technol., 2019, 111, 573–581 CrossRef CAS.
- M. Manzoor, J. Singh, J. D. Bandral, A. Gani and R. Shams, Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds, Int. J. Biol. Macromol., 2020, 165, 554–567 CrossRef CAS PubMed.
- A. Yemenicioğlu, S. Farris, M. Turkyilmaz and S. Gulec, A review of current and future food applications of natural hydrocolloids, Int. J. Food Sci. Technol., 2019, 54, 1445–1456 Search PubMed.
- H. Zhu, L. Chen, J. Xu and Q. Han, Experimental study on performance improvement of anionic surfactant foaming agent by xanthan gum, Constr. Build. Mater., 2020, 230, 116993 CrossRef CAS.
- X. Gu, D. Li, H. Yuan, C. Li, D. Yu, G. Wang and S. Li, Tertiary/quaternary amine derivatives of sophorolipid for baicalin solubility and antioxidating performance improvement: solubilization or hydrotropy?, J. Mol. Liq., 2024, 376, 124122 CrossRef.
- Z. Mitrinova, S. Tcholakova, N. Denkov and K. P. Ananthapadmanabhan, Role of interactions between cationic polymers and surfactants for foam properties, Colloids Surf., A, 2016, 489, 378–391 CrossRef CAS.
- W. Li, L. Zhu, W. Zhang, C. Han, P. Li and J. Jiang, Foam and fluid properties of purified saponins and non-purified water extracts from Camellia oleifera cake (by-product), Food Chem., 2024, 440, 138313 CrossRef CAS PubMed.
- S. P. Tiwari, J. A. Steckel, M. Sarma, J. Bryant, C. A. Lippert, L. R. Widger, J. Thompson, K. Liu, N. Siefert and W. Shi, Foaming Dependence on the Interface Affinities of Surfactant-like Molecules, Ind. Eng. Chem. Res., 2019, 58(43), 19877–19889 CrossRef CAS.
- F. Hu, Y. Liu, J. Lin, W. Wang, D. H. Yu and S. Li, Acetoin modulates conformational change of surfactin: Interfacial assembly and crude oil-washing performance, Colloids Surf., B, 2021, 200, 111602 CrossRef CAS PubMed.
- Z. Mitrinova, S. Tcholakova, N. Denkov and K. P. Ananthapadmanabhan, Role of interactions between cationic polymers and surfactants for foam properties, Colloids Surf. A: Physicochem. Eng. Aspects., 2016, 489, 378–391 CrossRef CAS.
- T. Lu and F. Chen, Multiwfn: A multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu and Q. Chen Q, Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems, J. Comput. Chem., 2022, 43(8), 539–555 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graphics, 1996, 14(1), 33–38 CrossRef CAS PubMed.
- J. Bergfreund, S. Siegenthaler, V. Lutz-Bueno, P. Bertsch and P. Fischer, Surfactant Adsorption to Different Fluid Interfaces, Langmuir, 2021, 37, 6722–6727 CrossRef CAS PubMed.
- M. B. M. Monte and J. F. Oliveira, Flotation of sylvite with dodecylamine and the effect of added long chain alcohols, Miner. Eng., 2004, 17(3), 425–430 CrossRef CAS.
- B. Kolaric, W. Jaeger, G. Hedicke and R. v. Klitzing, Tuning of Foam Film Thickness by Different (Poly)electrolyte/Surfactant Combinations, J. Phys. Chem. B, 2003, 107, 8152–8157 CrossRef CAS.
- R. Petkova, S. Tcholakova and N. D. Denkov, Role of polymer-surfactant interactions in foams: Effects of pH and surfactant head group for cationic polyvinylamine and anionic surfactants, Colloids Surf., A, 2013, 438, 174–185 CrossRef CAS.
- A. Bureiko, A. Trybala, N. Kovalchuk and V. Starov, Current applications of foams formed from mixed surfactant–polymer solutions, Adv. Colloid Interface Sci., 2015, 222, 670–677 CrossRef CAS PubMed.
- W. Li, L. Zhu, F. Zhang, C. Han, P. Li and J. Jiang, Synergistic effect and performance characterization of an efficient environmental-friendly Camellia oleifera saponins based foamed cleaning agents, J. Clean. Prod., 2022, 376, 134217 CrossRef.
- R. Petkova, S. Tcholakova and N. D. Denkov, Foaming and Foam Stability for Mixed Polymer-Surfactant Solutions: Effects of Surfactant Type and Polymer Charge, Langmuir, 2012, 28(11), 5000–5009 CrossRef PubMed.
- B. Jean and L.-T. Lee, Noninteracting versus interacting Poly(N-isopropylacrylamide)-surfactant mixtures at the air-water interface, J. Phys. Chem. B, 2005, 109(11), 5162–5167 CrossRef CAS PubMed.
- N. Politova, S. Tcholakova, Z. Valkova, K. Golemanov and N. D. Denkov, Self-regulation of foam volume and bubble size during foaming via shear mixing, Colloids Surf., A, 2018, 539, 18–28 CrossRef CAS.
- B. Petkova, S. Tcholakova, M. Chenkova, K. Golemanov, N. Denkov, D. Thorley and S. Stoyanov, Foamability of aqueous solutions: Role of surfactant type and concentration, Adv. Colloid Interface Sci., 2020, 276, 102084 CrossRef CAS PubMed.
- W. Yang, T. Wang, Z. Fan, Q. Miao, Z. Deng and Y. Zhu, Foams Stabilized by In Situ-Modified Nanoparticles and Anionic Surfactants for Enhanced Oil Recovery, Energy Fuels, 2017, 31(5), 4721–4730 CrossRef CAS.
- B. Petkova, S. Tcholakova, M. Chenkova, K. Golemanov, N. Denkov, D. Thorley and S. Stoyanov, Foamability of aqueous solutions: Role of surfactant type and concentration, Adv. Colloid Interface Sci., 2020, 276, 102084 CrossRef CAS PubMed.
- Z. Zhang, X. Chen, C. Li, H. Feng, H. Yu, R. Zhu and T. Wang, Foam sclerotherapy during shunt surgery for portal hypertension and varices, Open Med., 2017, 12(1), 384–390 CrossRef PubMed.
- W. Kang, L. Yan, F. Ding and Z. Xu, Effect of polysaccharide polymers on the surface and foam properties of aqueous film-forming foam, Colloid Interface Sci., 2021, 45, 100540 CrossRef CAS.
- H. Emami, A. Ayatizadeh Tanha, A. Khaksar Manshad and A. H. Mohammadi, Experimental Investigation of Foam Flooding Using Anionic and Nonionic Surfactants: A Screening Scenario to Assess the Effects of Salinity and pH on Foam Stability and Foam Height, ACS Omega, 2022, 7, 14832–14847 CrossRef CAS PubMed.
- Y. Xiong, B. Li, C. Chen and Y. Zhang, Properties of foamed concrete with Ca(OH)2 as foam stabilizer, Cem. Concr. Compos., 2021, 118, 103985 CrossRef CAS.
- Y. Sheng, S. Lu, N. Jiang, X. Wu and C. Li, Drainage of aqueous film-forming foam stabilized by different foam stabilizers, J. Dispersion Sci. Technol., 2017, 39(9), 1266–1273 CrossRef.
- W. Kang, Y. He, Z. Li, H. Yang, Z. Ye, W. Li, H. Jiang, D. Liu, H. Ding and S. Turtabayev, Stability mechanisms of viscoelastic zwitterionic-anionic surfactants enhanced foam system for low-permeability reservoirs, J. Mol. Liq., 2023, 369, 120883 CrossRef CAS.
- N. Hu, Y. Li, Z. Wu, K. Lu, D. Huang and W. Liu, Foams stabilization by silica nanoparticle with cationic and anionic surfactants in column flotation: Effects of particle size, J. Taiwan Inst. Chem. Eng., 2018, 88, 62–69 CrossRef CAS.
- D. Joshi, D. N. Ramesh, S. Prakash, R. K. Saw, N. K. Maurya, K. B. Rathi, S. Mitra, O. P. Sinha, P. K. Bikkina and A. Mandal, Formulation and characterisation of polymer and nanoparticle-stabilized anionic surfactant foam for application in enhanced oil recovery, Surf. Interfaces, 2025, 56, 105615 CrossRef CAS.
- Z. Briceño-Ahumada and D. Langevin, On the influence of surfactant on the coarsening of aqueous foams, Adv. Colloid Interface Sci., 2017, 244, 124–131 CrossRef PubMed.
- Z. Xue, A. Worthen, A. Qajar, I. Robert, S. L. Bryant, C. Huh, M. Prodanović and K. P. Johnston, Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes, J. Colloid Interface Sci., 2016, 461, 383–395 CrossRef CAS PubMed.
- X. Dong, H. Liu, C. Wang, C. Lu and F. Yan, The polymer-enhanced foam injection process: An improved-oil-recovery technique for light oil reservoirs after polymer flooding, Energy Sources, Part A, 2016, 38, 354–361 CrossRef CAS.
- D. Joshi, D. N. Ramesh, S. Prakash, R. K. Saw, N. K. Maurya, K. B. Rathi, S. Mitra, O. P. Sinha, P. K. Bikkina and A. Mandal, Formulation and characterisation of polymer and nanoparticle-stabilized anionic surfactant foam for application in enhanced oil recovery, Surf. Interfaces, 2025, 56, 105615 CrossRef CAS.
- H. Zhu, L. Chen, J. Xu and Q. Han, Experimental study on performance improvement of anionic surfactant foaming agent by xanthan gum, Constr. Build. Mater., 2020, 230, 116993 CrossRef CAS.
- B. Bera, O. Carrier, E. H. G. Backus, M. Bonn, N. Shahidzadeh and D. Bonn, Counteracting Interfacial Energetics for Wetting of Hydrophobic Surfaces in the Presence of Surfactants, Langmuir, 2018, 34, 41 Search PubMed.
- D. Mańko, A. Zdziennicka and B. Jańczuk, Surface tension of polytetrafluoroethylene and its wetting by aqueous solution of some surfactants and their mixtures, Appl. Surf. Sci., 2017, 392, 117–125 CrossRef.
- Y. Huang, Q.-L. Huang, H. Liu, C.-X. Zhang, Y.-W. You, N.-N. Li and C.-F. Xiao, Preparation, characterization, and applications of electrospun ultrafine fibrous PTFE porous membranes, J. Membr. Sci., 2017, 523, 317–326 CrossRef CAS.
- Y.-Y. Peng, F. Lu and Q.-X. Tong, One-step synthesis, wettability and foaming properties of high-performance non-ionic hydro-fluorocarbon hybrid surfactants, Appl. Surf. Sci., 2018, 433, 264–270 CrossRef CAS.
- R. Zhang, J. Guo, M. Zhao, J. Wu, S. Zhang and Y. Yu, Effect of graphene oxide on the molecules of a sodium alginate-krill protein composite system and characterization of the system's composite fiber morphology and mechanical properties, J. Appl. Polym. Sci., 2018, 135(22), 46642 CrossRef.
- M. Liang, X. Zhao, J. Wang and Y. Feng, A Comparative Study on CO2-Switchable Foams Stabilized by C22- or C18-Tailed Tertiary Amines, Molecules, 2023, 28(6), 2567 CrossRef CAS PubMed.
- C. Stubenrauch, M. Hamann, N. Preisig, V. Chauhan and R. Bordes, On how hydrogen bonds affect foam stability, Adv. Colloid Interface Sci., 2017, 247, 435–443 CrossRef CAS PubMed.
- T. Lu and Q. Chen, Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems, J. Comput. Chem., 2022, 43(8), 539–555 CrossRef CAS PubMed.
- M. Kumar, A. Thakur, U. K. Mandal, A. Thakur and A. Bhatia, Foam-Based Drug Delivery: A Newer Approach for Pharmaceutical Dosage Form, AAPS PharmSciTech, 2022, 23, 244 CrossRef PubMed.
- R. Prathapan, R. Thapa, G. Garnier and R. F. Tabor, Modulating the zeta potential of cellulose nanocrystals using salts and surfactants, Colloids Surf., A, 2016, 509, 11–18 CrossRef CAS.
- K. R. Raghi, D. R. Sherin, M. J. Saumya, P. S. Arun, V. N. Sobha and T. K. Manojkumar, Computational study of molecular electrostatic potential, docking and dynamics simulations of gallic acid derivatives as ABL inhibitors, Comput. Biol. Chem., 2018, 74, 239–246 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





