DOI:
10.1039/D5RA04410C
(Review Article)
RSC Adv., 2025,
15, 32155-32171
Exploring 4D printing of smart materials for regenerative medicine applications
Received
20th June 2025
, Accepted 22nd August 2025
First published on 5th September 2025
Abstract
The field of biomaterials has evolved rapidly with the introduction of time as a transformative factor, giving rise to four-dimensional (4D) materials that can dynamically change their structure or function in response to external stimuli. This review presents a comprehensive comparison between traditional three-dimensional (3D) and emerging 4D biomaterials, highlighting the key distinctions in design, adaptability, and functionality. We explore the development of smart biomaterials at the core of 4D systems, including stimuli-responsive polymers, shape-memory materials, and programmable hydrogels. The ability of these materials to undergo controlled transformations under physiological or engineered stimuli offers promising avenues in tissue engineering, drug delivery, regenerative medicine, and soft robotics. By integrating responsiveness and temporal control, 4D biomaterials represent a paradigm shift in biomedical engineering, with the potential to revolutionize patient-specific therapies and next-generation implants. Future challenges and opportunities for clinical translation are also discussed.
1. Introduction
Four-dimensional (4D) printing has emerged as ground breaking advancement in the field of regenerative medicine, building upon the foundational principles of three-dimensional (3D) bioprinting by introducing the dimension of time into material design and function. Conventional 3D printing has significantly advanced the fabrication of architecturally intricate, yet static biomedical constructs such as scaffolds and implants. However, it lacks the ability to impart dynamic responsiveness. In contrast, 4D printing integrates time as a functional dimension by utilizing smart biomaterials that can actively respond to external stimuli—such as temperature, pH, light, or humidity—after fabrication. This enables the production of constructs capable of real-time adaptation, offering behaviors that more closely resemble the dynamic nature of native biological tissues.1–3 The core enabler of 4D printing is the development of smart biomaterials, including shape-memory polymers (SMPs), hydrogels, and bioactive composites. These materials are designed to undergo reversible or irreversible changes in geometry, stiffness, or porosity through processes such as swelling, contraction, folding, or self-assembly in response to physiological cues.4 For instance, shape-memory hydrogels that respond to hydration levels or temperature fluctuations are being explored for applications in soft tissue engineering, where dynamic mechanical behavior is critical. Similarly, tunable scaffolds with controlled degradation kinetics and stiffness modulation can influence cell fate decisions, enhance extracellular matrix (ECM) remodeling, and guide tissue maturation in a biologically relevant manner.5,6 One particularly promising application of 4D printing is the fabrication of biomimetic ECM scaffolds that more accurately replicate the spatiotemporal dynamics of native tissue microenvironments. In natural systems, the ECM is not a static entity; it undergoes constant remodeling influenced by mechanical forces and cellular signaling. Traditional 3D-printed scaffolds fail to capture this dynamic adaptability. In contrast, 4D-printed materials can be engineered to gradually stiffen, degrade, or release bioactive molecules in a temporally controlled manner, facilitating processes such as angiogenesis, cell migration, and tissue integration.7 Furthermore, the self-healing capacity of certain 4D-printed biomaterials represents another frontier in regenerative medicine. These materials often incorporate stem cells or growth factors that can be activated by biomechanical or biochemical cues to initiate endogenous repair mechanisms. For example, self-healing hydrogels have shown promise in cartilage repair, responding to microfractures by autonomously restoring structural integrity.8 The field of biomaterials has experienced significant advancement with the introduction of the fourth dimension-time-leading to the development of 4D materials capable of dynamic structural and functional transformations in response to external stimuli. This review provides an in-depth examination of the differences between traditional three-dimensional (3D) biomaterials and the emerging 4D counterparts, with a focus on their distinct design principles, adaptability, and functional capabilities. Central to 4D systems are smart biomaterials, including shape-memory polymers, stimuli-responsive materials, and hydrogels with programmable behaviors. These materials can undergo precise modifications in response to various physiological or engineered cues, offering exciting possibilities for applications in tissue engineering, drug delivery, regenerative medicine, and even soft robotics.9–14 The integration of time-sensitive and responsive characteristics in these materials heralds a transformative shift in biomedical engineering, enabling innovations in personalized treatments and advanced implants. This review also discusses the challenges and opportunities related to the clinical application and development of 4D biomaterials.15,16
2. Distinction between 3D and 4D bioprinting in biomedical contexts
Three-dimensional (3D) bioprinting and four-dimensional (4D) bioprinting are advanced fabrication technologies employed in biomedical applications, particularly in tissue engineering, regenerative medicine, and drug delivery.9,17–19 While both methods involve the layer-by-layer deposition of biomaterials, cells, and bioactive molecules to create functional biological structures, they differ significantly in terms of their dynamic capabilities and responsiveness to external stimuli. 3D bioprinting focuses on constructing static, predefined structures that replicate the architecture of native tissues or organs. It utilizes biomaterials known as bioinks, which are typically composed of hydrogels, cells, and growth factors, to fabricate complex tissue constructs with high spatial precision. Once printed, these constructs retain a fixed shape and functionality, making them suitable for static tissue replacements or in vitro disease models.1,20 In contrast, 4D bioprinting introduces the element of time as the fourth dimension. 4D bioprinting extends the capabilities of 3D bioprinting by integrating smart, stimuli-responsive materials that can alter their shape, structure, or function over time. These transformations are programmed to occur in response to environmental cues such as temperature, pH, humidity, or biochemical signals, enabling dynamic interactions with biological systems and enhancing the relevance of printed constructs in regenerative medicine.21 This dynamic behavior allows 4D-printed constructs to mimic the adaptive nature of living tissues more closely, enabling applications in self-assembling tissue scaffolds, stimuli-responsive implants, and shape-morphing biomedical devices.22 A key distinction lies in the material properties and design philosophy. 3D bioprinting emphasizes structural fidelity and biocompatibility, whereas 4D bioprinting prioritizes responsiveness and adaptability, leveraging stimuli-responsive polymers and shape-memory materials.23,24 Additionally, 4D bioprinting demands more advanced computational modeling and predictive algorithms to control the temporal evolution of printed structures.25 While 4D printing utilizes the same fabrication methods as traditional 3D printing, it distinguishes itself through the use of stimuli-responsive materials. These materials enable the printed constructs to alter their shape and/or functionality over time when exposed to external stimuli as shown in Fig. 1.
 |
| | Fig. 1 4D printing of smart biomaterials enables stimulus-driven shape and function changes for applications in regenerative medicine. | |
3. Smart biomaterials for 4D bioprinting
pH-Responsive materials are particularly advantageous for targeted drug delivery in pathological environments characterized by abnormal acidity. For instance, the tumor microenvironment and intracellular compartments such as endosomes and lysosomes typically exhibit a lower pH than normal physiological tissues. Materials designed to undergo structural changes or degrade in response to acidic conditions can enable site-specific release of therapeutic agents, enhancing efficacy while minimizing systemic toxicity. This makes pH-sensitive polymers and hydrogels highly suitable for cancer therapy and intracellular delivery systems. The foundation of 4D bioprinting lies in the strategic use of smart biomaterials engineered substances capable of responding to specific environmental cues. These materials enable printed constructs to undergo controlled, time-dependent changes in shape, mechanical properties, or biofunctionality. In the context of regenerative pathology, such adaptability is critical for achieving seamless integration with dynamic biological systems.26–28 Stimuli-responsive polymers (SRPs) are a unique class of soft materials that can change their shape or behavior when exposed to specific environmental cues—such as light, heat, pH, moisture, electric or magnetic fields, or even solvents. Because of this remarkable adaptability, they've become central to the development of flexible actuators, soft robotics, wearable technology, sensors, and biomedical devices. In recent years, the integration of SRPs with 3D printing—commonly referred to as 4D printing—has opened up exciting possibilities. This technology allows researchers to create smart, personalized structures that don't just sit passively but actively transform over time by bending, expanding, twisting, or reshaping in response to their surroundings. As a result, 4D printing with SRPs is drawing growing interest across engineering, healthcare, and materials science.29
3.1. pH-Sensitive polymers in 4D printing
pH-Sensitive polymers are integral to 4D printing, enabling constructs to undergo controlled transformations in response to the pH variations within biological environments. These polymers contain functional groups that ionize or deionize at specific pH levels, leading to changes in their physical properties such as swelling, solubility, or mechanical strength.30,31 This responsiveness is particularly valuable in biomedical applications where pH gradients are prevalent, such as in the gastrointestinal tract, tumor microenvironments, and sites of inflammation. pH-Sensitive polymers can be broadly classified into two categories—anionic and cationic-based on the nature of their ionizable functional groups. Anionic polymers contain acidic groups, such as carboxylic acids, which undergo deprotonation at elevated pH levels.32–35 This results in an increased negative charge density, leading to polymer chain expansion and swelling. Common examples include poly(acrylic acid) (PAA), poly(methacrylic acid) (PMAA), and alginate. These materials are particularly advantageous for drug delivery systems designed to release therapeutic agents in alkaline environments, such as the intestines. In contrast, cationic polymers possess basic groups, like amines, which become protonated under acidic conditions. This protonation imparts a positive charge and promotes polymer swelling. Chitosan, a naturally derived polysaccharide, exemplifies this class and demonstrates solubility and swelling behavior in acidic media, making it well-suited for targeted delivery in acidic environments such as the stomach or tumor microenvironments. Representative chemical structures of several pH-responsive polymers utilized in 4D printing—such as alginate, poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA), chitosan, poly(acrylic acid), and poly(methacrylic acid)—are illustrated in Fig. 2.4,36
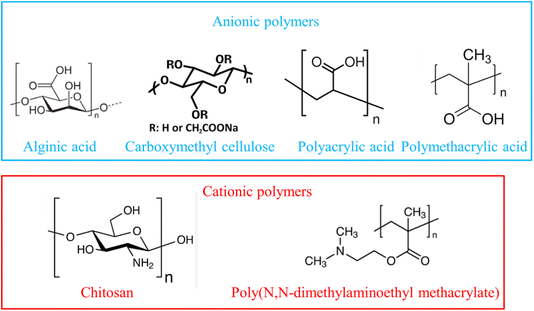 |
| | Fig. 2 Examples of pH-responsive polymers frequently utilized in 4D printing for biomedical applications. | |
3.2. Light-sensitive polymers for 4D printing
Light-responsive materials offer precise spatial and temporal control over activation, making them ideal for externally triggered therapies. Upon exposure to specific wavelengths—such as ultraviolet, visible, or near-infrared light—these systems can undergo conformational changes, bond cleavage, or generate heat or reactive species. Such responses enable controlled drug release, photothermal therapy, or photodynamic action, particularly useful in localized cancer treatments and wound healing. Their non-invasive trigger mechanism adds versatility, especially when minimal disturbance to surrounding tissues is desired. A wide range of light-sensitive (photosensitive) functional groups have been reported in the literature, each exhibiting specific photoresponsive behaviors such as photoisomerization, photocleavage, or photodimerization. In the context of 4D printing, particular attention has been given to photosensitive moieties that enable spatiotemporal control over material properties in response to light stimuli.37–40 This section focuses on the photosensitive groups that have already been successfully integrated into 4D printing systems. These include, but are not limited to, azobenzene derivatives (photoisomerization), spiropyrans (reversible switching between hydrophobic and hydrophilic states), coumarins (photodimerization and photocleavage), and o-nitrobenzyl esters (photodegradation). Their incorporation allows for light-triggered changes in shape, mechanical properties, or chemical functionalities, enabling dynamic behavior in smart materials tailored for biomedical, soft robotics, and tissue engineering applications. Chemical structures of photosensitive groups are illustrated in Fig. 3.
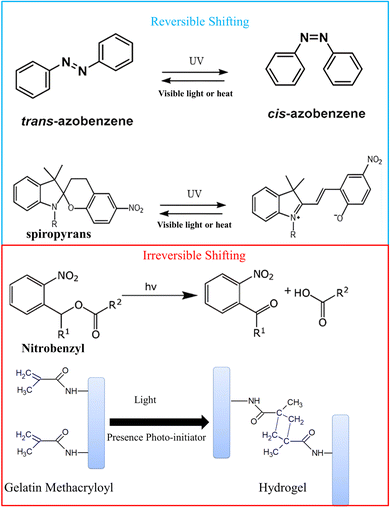 |
| | Fig. 3 Representative light sensitive polymers commonly employed in 4D printing applications. | |
3.2.1. Azobenzene-based polymers. Azobenzene groups undergo reversible photoisomerization upon exposure to UV or visible light, leading to changes in polymer conformation and properties.41 Azobenzene moieties undergo isomerization from the trans to cis state upon UV irradiation, causing an expansion of the polymer matrix. Used in shape-memory polymers (SMPs) for soft robotics, actuators, and smart sensing applications.
3.2.2. Spiropyran-containing polymers. Spiropyran groups undergo reversible photochemical transformations upon UV light exposure, altering the polymer's structure and properties.42 They are utilized in stimuli-responsive systems, particularly in applications involving sensors and actuators.
3.2.3. Nitrobenzyl-functionalized polymers. Nitrobenzyl groups undergo photochemical cleavage upon UV light exposure, leading to changes in the polymer network and properties.43 Utilized in controlled drug release systems44 and tissue engineering scaffolds.45
3.2.4. Gelatin methacryloyl (GelMA). GelMA (gelatin methacryloyl) is a photopolymerizable hydrogel that undergoes crosslinking when exposed to UV light in the presence of a photoinitiator,46 resulting in the formation of a stable network suitable for both 3D and 4D printing.47 This material is extensively utilized in biomedical applications, particularly in tissue engineering and regenerative medicine. The unique properties of GelMA, as well as its wide range of applications, have been thoroughly investigated in the literature, with numerous studies exploring its potential in 3D and 4D bioprinting.
3.3. Thermo-responsive polymers in 4D printing
Thermo-responsive materials exploit temperature fluctuations to trigger functional changes such as sol–gel transitions, swelling, or deswelling. These systems are designed to respond near physiological or slightly elevated temperatures, enabling applications in controlled drug release, injectable hydrogels, and tissue engineering. In the context of 4D printing, these polymers play a pivotal role by enabling printed structures to transform their shape, functionality, or mechanical properties over time when subjected to specific thermal stimuli.48–50 The integration of thermo-responsive polymers into 4D printing not only enhances the adaptability of printed structures but also expands their applicability in fields like tissue engineering, wearable electronics, and responsive implants. Fig. 4 presents selected examples of thermo-sensitive polymers.
 |
| | Fig. 4 Representative thermo-sensitive polymers commonly employed in 4D printing applications. | |
3.3.1. Polycaprolactone (PCL) and poly(ethylene-co-octene) (POE) blends. Zhu et al. (2023) investigated the development of thermo-responsive shape-memory polymers through the blending of polycaprolactone (PCL) and polyolefin elastomer (POE). The optimal blend exhibited a transition temperature range of 55–60 °C, with shape fixation and recovery efficiencies exceeding 90%, maintaining stable performance over multiple cycles. Enhanced shape memory behavior was observed, attributed to improved phase compatibility and the formation of a co-continuous structure within the blend.51
3.3.2. Poly(acrylic acid)-based hydrogels. Abdullah and Okay (2023) developed 4D-printed hydrogels based on poly(acrylic acid) (PAA) that exhibit both shape-memory and self-healing capabilities. These hydrogels undergo reversible transitions around human body temperature (approximately 37 °C), making them ideal for biomedical applications. The mechanical properties, such as Young's modulus and toughness, can be finely tuned by modifying the monomer composition.52
3.3.3. Poly(N-isopropylacrylamide) (PNIPAM) hydrogels. Abdullah and Okay (2023) designed 4D-printed hydrogels derived from poly(acrylic acid) (PAA) that demonstrate both shape-memory and self-healing properties. These hydrogels undergo reversible transitions near human body temperature (around 37 °C), which makes them well-suited for various biomedical applications. Additionally, the mechanical characteristics, including Young's modulus and toughness, can be precisely adjusted by altering the monomer composition.53 A smart hydrogel capsule was fabricated through an extrusion-based 4D printing technique, utilizing UV-induced crosslinking of poly(N-isopropylacrylamide) (PNIPAM) to form the capsule shell. The resulting PNIPAM hydrogel exhibited a lower critical solution temperature (LCST) of around 34.9 °C. To generate a macroporous structure, high molecular weight polyethylene glycols (PEGs) were employed as porogens during the preparation process.54 Thermo-responsive smart hydrogels have been explored for additive manufacturing applications through the development of two novel hydrogel systems. These systems are based on copolymers combining poly(oxazoline) and poly(acrylamide) segments. In both designs, thermal sensitivity is provided by either poly(2-isopropyl-2-oxazoline) (PiPrOx) or poly(N-isopropylacrylamide) (PNIPAM), while the poly(oxazoline) components serve as the crosslinkable domains. During the stereolithography (SLA) printing process, photo-induced crosslinking results in the formation of a stable resin network.55
3.3.4. Biodegradable shape-memory elastomers. Biodegradable shape-memory elastomers were 4D printed using poly(D,L-lactide-co-trimethylene carbonate) methacrylates. These materials offer customizable transition temperatures within physiological ranges and show effective shape recovery at 37 °C. The resulting printed devices are cytocompatible and degrade in physiological environments, making them promising candidates for medical applications.56
3.3.5. Bio-based thermoplastic polyurethane (SMTPU). Park et al. (2025) characterized a bio-based thermoplastic polyurethane (SMTPU) synthesized from renewable resources, specifically bio-based polypropylene succinate (polyester polyol) and 1,3-propanediol (chain extender), for 4D printing applications. The SMTPU demonstrates remarkable shape recoverability and favorable mechanical properties, such as tensile strength and elongation, making it a promising material for applications that require both sustainability and high performance.57
4. Multiresponsive
Multi-responsive materials in 4D printing represent a significant advancement in smart manufacturing, enabling printed structures to dynamically adapt to multiple environmental stimuli. By integrating materials that respond to various triggers—such as temperature, pH, light, magnetic fields, or reactive oxygen species—researchers have developed constructs capable of complex, programmable transformations. For instance, a study introduced a nanocomposite combining polypyrrole-coated iron oxide nanoparticles within a shape-memory polymer matrix, resulting in structures that can be remotely actuated using near-infrared light and magnetic fields. Another research effort focused on protein-based hydrogels that respond to temperature, pH, and enzymatic activity, showcasing autonomous shape changes suitable for biomedical applications. These innovations highlight the potential of multi-responsive 4D-printed materials in creating adaptive systems for fields ranging from soft robotics to personalized medicine.58–60 Multiresponsive hydrogels hold great promise as advanced biomaterials because they can respond to multiple physiological cues—such as changes in temperature, pH, and levels of reactive oxygen species (ROS)—often encountered simultaneously in the body.61–63 In a recent study, researchers developed a triple-responsive hydrogel using UV light-initiated polymerization, combining three key components: N-isopropylacrylamide (NIPAM) for temperature sensitivity, methacrylic acid (MAA) for pH responsiveness, and a specially designed diacrylate thioether monomer (EG3SA) that reacts to ROS. The resulting hydrogel, P[NIPAMx-co-MAAy-co-(EG3SA)z], is compatible with digital light processing (DLP) 4D printing, enabling the fabrication of dynamic, stimuli-sensitive structures. The team evaluated the hydrogel's responsiveness through swelling and rheological tests under various temperatures (25–37 °C), pH levels (3–11), and oxidative conditions. It also served as a matrix for loading ketoprofen, showing controllable drug release depending on the surrounding environment. Importantly, cytotoxicity tests using fibroblasts and macrophages confirmed the hydrogel's biocompatibility. Interestingly, even without any drug, one particular formulation—P[NIPAM80-co-MAA15-co-(EG3SA)5]—significantly reduced inflammation markers in activated immune cells, suggesting intrinsic anti-inflammatory potential. These findings pave the way for customizable, 4D-printable hydrogel scaffolds that could be tailored for treating inflammatory diseases.63,64
5. Applications of 4D printing in regenerative medicine
The advent of 3D printing has revolutionized the fabrication of complex biomedical structures, particularly in tissue engineering. However, it falls short in replicating the dynamic, adaptive nature of native tissues. One key limitation is the insufficient vascularization of large constructs, which restricts the effective delivery of oxygen and nutrients. 4D (bio)printing addresses these shortcomings by enabling the creation of responsive, living tissues capable of evolving over time.65–67 These constructs can incorporate vascular or stem cells, promoting maturation and functional integration. Their adaptive behavior makes them particularly relevant for organ-specific applications.
5.1. Bone tissue engineering
4D printing has been utilized to create biodegradable scaffolds that can adapt to bone defects, promoting regeneration. Materials like polylactic acid (PLA) and poly(glycolic acid) (PGA) are commonly used due to their biocompatibility and ability to degrade into non-toxic byproducts. These scaffolds can be designed to change shape in response to physiological conditions, ensuring a better fit and integration with the host tissue.68–70 Porous shape memory cryogel microspheres (CMS) made from GelMA have been fabricated using an emulsion technique combined with gradient cooling. These CMS can be loaded with human bone marrow-derived mesenchymal stem cells (hBMSCs) and human umbilical vein endothelial cells (HUVECs), promoting vascularized bone tissue regeneration upon subcutaneous injection in animal models.71,72 Hybrid scaffolds combining PCLDA and PLLA have been designed to enhance osteogenic differentiation of human mesenchymal stem cells (hMSCs). These scaffolds exhibit shape memory behavior and support bone tissue regeneration without the need for osteogenic inducers.73 Blending PLA with PCL results in composites with improved shape memory behavior and mechanical properties suitable for bone fixation applications. These composites can restore their original shape upon heating, mimicking the structure of cancellous bone.74 Poly(butylene fumarate) based scaffolds demonstrate humidity-responsive shape memory properties, supporting osteoblast attachment and proliferation. These scaffolds can be functionalized with bone morphogenetic protein-2 (BMP-2) for sustained release, enhancing bone regeneration.75 Researchers have developed biodegradable SMPU scaffolds that exhibit excellent shape memory properties, making them suitable for minimally invasive surgical procedures. These scaffolds can recover their original shape at body temperature, promoting bone regeneration. Incorporating nanohydroxyapatite into the SMPU matrix enhances mechanical strength and biocompatibility.76
5.2. Cartilage regeneration
Cartilage repair has benefited from 4D bioprinting through the development of constructs that mimic the natural curvature and mechanical properties of cartilage. For instance, hydrogels embedded with human mesenchymal stromal cells have been printed to form structures that swell and conform to cartilage defects, promoting regeneration.77,78 Researchers have developed 4D-printed hydrogel scaffolds that exhibit swelling–stiffening behavior, enabling them to mimic the mechanical properties of native cartilage. These scaffolds can undergo programmable deformations in response to specific stimuli, offering potential for minimally invasive cartilage repair.79 Magneto-responsive 4D-bioprinting has emerged as a powerful strategy for dynamic cartilage tissue engineering. A recent study demonstrated the integration of anisotropic magnetic nanoparticles (MNPs) into a silk fibroin–gelatin bioink containing human bone marrow-derived mesenchymal stromal cells to fabricate 4D-bioprinted constructs designed for articular cartilage regeneration (Fig. 5). The constructs exhibited actuation and thermal responsiveness under an external magnetic field. Mechanical actuation was applied cyclically every other day for either 5 or 30 minutes over a 21-day period. Constructs actuated for 30 minutes displayed superior chondrogenic differentiation.80
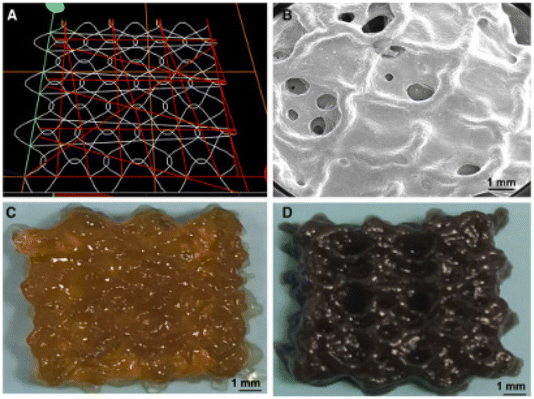 |
| | Fig. 5 Morphological evaluation of acellular constructs. (A) Simulated printing path via G-code. (B) Field-emission scanning electron microscopy image showing sinusoidal morphology. (C and D) Optical images of 4D-bioprinted constructs: (C) without MNPs and (D) with 5 mg mL−1 MNPs. (Reproduced under Creative Commons license, B. Liu et al., 2024 (ref. 80).) | |
To address the limitations of conventional scaffolds in auricular cartilage repair, magnetoresponsive hydrogel scaffolds fabricated via 4D printing have been developed. Unlike traditional 3D-printed structures, these 4D-printed constructs respond dynamically to external magnetic fields, enabling enhanced biological and mechanical performance. The surface modification of magnetic nanoparticles (MNPs) with chitosan, which imparted anti-inflammatory and antibacterial functions. This modification facilitated the polarization of macrophages toward the pro-regenerative M2 phenotype, effectively reducing inflammatory responses and bacterial contamination at the graft site.81 A shape memory composite scaffold for cartilage repair has been engineered by incorporating nano-hydroxyapatite (nHA) into a polyurethane (PU) matrix. The strong hydrogen bonding interactions between nHA and PU enhance both the mechanical integrity and biocompatibility of the composite material. Drawing inspiration from the structural adaptability of mangrove root systems, a biomimetic 4D-printed cartilage scaffold was developed to mimic natural dynamic environments. This scaffold demonstrates excellent shape memory behavior, capable of reverting from a deformed (temporary) configuration back to its original geometry within 60 seconds when exposed to temperatures approximating physiological conditions (∼37 °C). Such rapid and reversible transformation makes this system highly promising for minimally invasive cartilage repair and regeneration applications.76 In a significant advancement for tracheal tissue engineering, a self-folding bilayer scaffold was fabricated via 4D bioprinting using gelatin crosslinked with (3-glycidoxypropyl)trimethoxysilane (GPTMS). Designed to undergo shape transformation upon hydration, the scaffold transitions from a flat 2D structure into a tubular configuration with a controllable final diameter. Moreover, the potential of 4D bioprinted millimeter-scale scaffolds to guide cell behavior and tissue maturation through dynamic, stimulus-responsive structural changes (Fig. 6).82
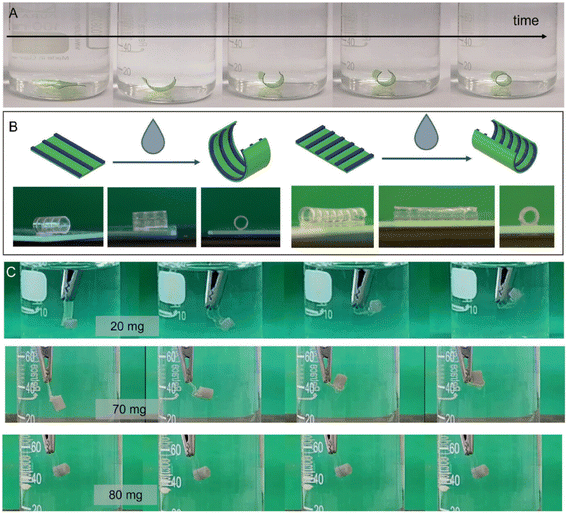 |
| | Fig. 6 (A) Time-dependent self-folding of scaffolds (10 × 5 mm) with bioprinted lines aligned longitudinally; food dyes added for visual contrast. (B) Line orientation governs the folding behavior of the 4D-printed scaffold. (C) Force analysis during self-folding: folding occurs under loads up to 70 mg, while 80 mg prevents actuation. (Reproduced under Creative Commons license CC BY-NC 4.0 Carmelo De Maria, et al., 2024 (ref. 82).) | |
5.3. Cardiac tissue repair
In cardiac applications, 4D-printed patches have been designed to conform to the heart's surface and accommodate its contractions. These patches, often composed of hydrogels and seeded with cardiac cells, aim to repair myocardial infarctions by integrating seamlessly with the heart tissue. Researchers developed a 4D-printed cardiac construct utilizing thermo-responsive SMPs designed to transform from a compact shape into a cardiac patch at body temperature (∼36 °C). This construct supports the delivery of human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and integrates seamlessly with myocardial tissue. The design allows for minimally invasive delivery and conforms to the heart's curvature, promoting cell proliferation and maturation without compromising contractile function.83 4D-printed cardiac constructs fabricated using a digital light processing (DLP) technique with a composite of shape memory polymers and graphene. These constructs exhibit adjustable curvature and can be remotely actuated using NIR light to match the heart's topology. The aligned microgroove structures promote uniform cell distribution and maturation of hiPSC-CMs, mesenchymal stem cells (MSCs), and endothelial cells (ECs), enhancing myocardial regeneration.84 Utilizing a beam-scanning stereolithography approach, researchers fabricated a 4D cardiac patch composed of gelatin methacryloyl (GelMA) and polyethylene glycol diacrylate (PEGDA). The patch transitions from a flat to a curved configuration, mimicking the heart's natural curvature (Fig. 7). In vivo studies in a murine model of chronic myocardial infarction demonstrated enhanced cellular engraftment, vascularization, and a reduction in infarct size, indicating the patch's regenerative potential.85 A 4D printing approach employed soybean oil epoxidized acrylate (SOEA) to create thin films capable of bending or rolling upon exposure to body temperature. These films are designed to conform to the heart's surface, facilitating integration with damaged myocardial tissue. The shape transformation is achieved through UV crosslinking, resulting in a crosslink density gradient that induces self-folding behavior.86 Miao et al. developed a 4D cardiac patch using a PSTS approach with smart soybean oil epoxidized acrylate (SOEA) inks. The flat sheet scaffold autonomously bends or self-assembles upon immersion in ethanol, allowing precise control over curvature by adjusting sheet thickness. This method supports the culture and cardiomyogenic differentiation of human bone marrow MSCs, offering potential for substituting damaged cardiac tissues.87
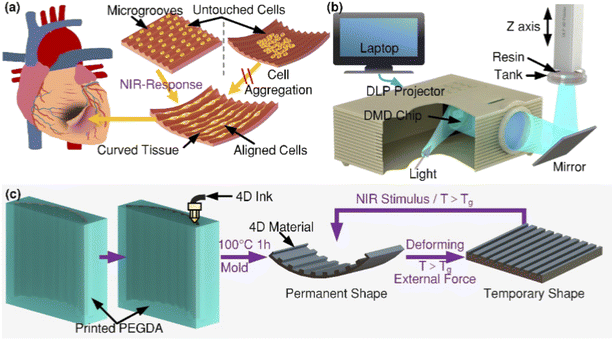 |
| | Fig. 7 Schematic of a 4D-printed cardiac patch and its fabrication. (a) A cell-laden construct transforms from a flat to a curved configuration, enabling implantation onto the infarcted myocardium to aid tissue repair. In contrast, precurved scaffolds lead to uneven cell distribution due to gravitational settling. (b) Diagram of the DLP-based printing setup featuring a digital micromirror device (DMD), lens system, and motion controller. (c) Illustration of the printing process using a PEGDA mold for 4D extrusion. The curved structure flattens upon heating above the SMP's glass transition temperature (Tg), with reversible shape change triggered by heat or NIR light (this figure has been adapted/reproduced from ref. 84 with permission from American Chemical Society, copyright 2021). | |
5.4. Vascular tissue engineering
In vascular applications, 4D printing enables the fabrication of dynamic structures that can form functional blood vessels. By using bio-inks containing endothelial cells, researchers have developed constructs that undergo shape transformations to create vascular networks, essential for tissue viability and integration.2 Researchers have developed a coaxial 4D printing technique utilizing poly(N-isopropylacrylamide) (PNIPAAm)-based hydrogels to fabricate vein-inspired actuators. These constructs exhibit temperature-responsive behavior, allowing for controlled lumen diameter changes (∼30%) and reversible shape transformations. The hydrogels demonstrate biocompatibility with endothelial cells, making them suitable for perfusable vascular applications.88 A novel bio-ink combining sodium alginate (SA) and collagen peptides (COP) has been used to 4D print small-diameter vascular grafts. The SA–COP hybrid enhances mechanical strength and cell adhesion. Post-printing, the grafts undergo shape transformations in response to calcium ion crosslinking and are matured in bioreactors, resulting in functional vascular constructs (Fig. 8).89
 |
| | Fig. 8 Fabrication and 4D bioprinting strategy for vascular grafts. (a) EPCs and HUVECs are incorporated into collagen peptide-modified sodium alginate (SA–COP) to prepare the bio-ink. (b) Instant crosslinking of SA–COP is initiated by CaCl2. (c) Extrusion is performed using a custom co-axial, dual-chamber nozzle. (d) Printing occurs within a CaCl2-based support medium using a six-axis robotic system for structural fidelity. (e) Post-printing, grafts are transferred to a bioreactor. (f) Final grafts exhibit a lumen diameter of 3–3.5 mm and (g) a total length of 30–40 cm. (Reproduced under Creative Commons license CC BY-NC 4.0, Rouven Berndt et al., 2024 (ref. 89).) | |
A dual-component hydrogel system incorporating carbonized alginate (CAlg) and methylcellulose (MC) has been utilized to fabricate programmable, bifurcated vascular channels. These hydrogels exhibit moisture-responsive shape deformations, enabling the creation of complex, perfusable structures that support endothelial and fibroblast cell viability.90 Researchers have employed methacrylated alginate (AA-MA) and hyaluronic acid (HA-MA) to create self-folding hydrogel tubes via 4D bioprinting. These structures, crosslinked using green light to maintain cell viability, can form hollow tubes suitable for vascular tissue engineering. The approach allows for the fabrication of complex architectures with potential for vascular network formation.91
5.5. Skin and wound healing
4D printing has been applied to create skin grafts capable of adapting to wound environments. These dynamic grafts can change shape to cover wounds effectively and release therapeutic agents in response to environmental cues, enhancing the healing process.92 Researchers developed a 4D-printed hydrogel dressing utilizing N-isopropylacrylamide (NIPAm) combined with curcumin-loaded Pluronic F127 micelles and a degradable crosslinker, poly(ethylene glycol) diacrylate-dopamine (PEGDA575-Do). This hydrogel exhibits temperature-responsive contraction, strong tissue adhesion, and antibacterial properties. In diabetic rat models with MRSA-infected wounds, the dressing accelerated healing by promoting wound closure and tissue regeneration.93 A self-healing hydrogel composed of biodegradable polyurethane nanoparticles and photo-/thermo-responsive gelatin-based biomaterials was developed for 4D bioprinting applications. This hydrogel exhibits excellent printability, structural stability, and shape memory properties. It supports the proliferation and differentiation of neural stem cells, indicating its potential for tissue engineering and wound healing applications.94 Chitosan, known for its biocompatibility and antibacterial properties, has been utilized in 3D and 4D printing to create customized wound dressings. These scaffolds can be tailored to patient-specific needs, enhancing tissue regeneration and improving healing outcomes. The integration of chitosan with advanced printing techniques offers promising avenues for sophisticated wound care solutions.95 By incorporating intelligent materials and CAD systems, 4D bioprinting enhances traditional 3D techniques, enabling printed structures to respond to various stimuli through changes in form and functional properties. This adaptability, driven by bioinks with shape-memory, self-repair, and responsive behaviors, is particularly valuable in skin repair and supports real-time control during in situ applications, facilitating clinical advancement (Fig. 9).96
 |
| | Fig. 9 Overview of 4D skin bioprinting: patient-derived skin cells are cultured and combined with smart biomaterials and growth factors to create bioinks. CAD-integrated bioprinting systems use wound imaging to design customized skin constructs, which are then printed and transplanted back onto the patient. (Reproduced under Creative Commons license CC BY-NC 4.0, Damiati et al., 2025 (ref. 96).) | |
5.6. Self-healing implants
Advancements in 4D printing have led to the development of self-healing implants. These constructs can repair minor damages autonomously, reducing the need for additional surgeries. Materials like biodegradable polyurethane-based hydrogels have demonstrated self-healing properties while supporting cell proliferation and differentiation.97–99 Researchers developed a 4D-printed structure using a double-network SH–SMP system composed of polycaprolactone (PCL) integrated into a methacrylate-based shape memory polymer. This combination enables high-resolution printing (up to 30 μm) and imparts self-healing capabilities, with mechanical properties recovering over 90% after damage. The material is compatible with digital light processing (DLP) 3D printing technology, making it suitable for complex biomedical applications.100 A study introduced 4D-printed thermally activated self-healing shape memory polyurethanes (SMPUs). These materials exhibit both shape memory and self-healing properties upon thermal stimulation. The SMPUs demonstrated excellent mechanical performance and healing efficiency, making them promising candidates for biomedical implants that require durability and adaptability.101 4D printing has emerged as a powerful tool for creating dynamic, shape-shifting structures, with shape memory polymers (SMPs) offering high responsiveness and stiffness. However, traditional SMPs lack self-repair capabilities due to their permanent cross-linked networks. A recent advancement integrates Ultraviolet Curable polycaprolactone (PCL) into a methacrylate-based SMP matrix, enabling both self-healing and high-resolution 4D printing. This double-network system restores over 90% of mechanical strength after damage and is compatible with digital light processing. Such innovations pave the way for durable, adaptable implants with long-term functionality.100 Researchers developed a 4D-printed shape memory polymer using polycaprolactone (PCL) chains and 2-ureido-4[1H]-pyrimidinone (UPy) units. This material exhibits thermally induced shape memory and self-healing functionalities. The printed objects showed high elongation at break and maintained shape memory properties after healing, indicating their suitability for applications in soft robotics and biomedical devices (Fig. 10).101
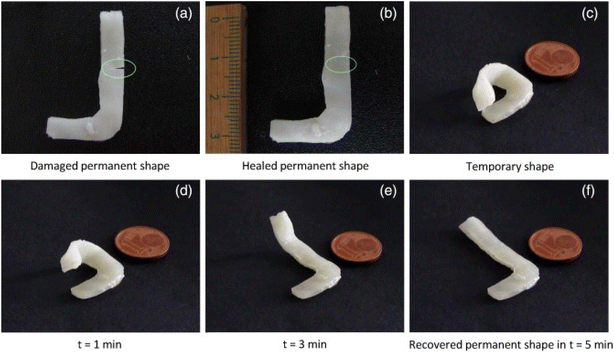 |
| | Fig. 10 Shape memory behavior of PCLDMA–UPyMA samples: after being cut (a), the specimen was thermally healed at 80 °C for 1 h (b). Upon deformation (c), heating at 70 °C initiated and completed shape recovery (d–f) (this figure has been adapted/reproduced from ref. 101 with permission from Elsevier, copyright 2018). | |
The advancement of 4D printing has enabled the design of deployable medical devices (DMDs) that can be implanted in a compact form and later recover their functional shape within the body. A photocrosslinked polycaprolactone (PCL)-based material, created via a rapid thiol–acrylate click reaction, demonstrates excellent biocompatibility, shape memory, and suitability for 3D printing. In vivo evaluations confirm favorable tissue integration and reduced inflammatory response. Furthermore, incorporating everolimus through β-cyclodextrin complexes allows for sustained drug release, enhancing therapeutic outcomes and supporting the potential of 4D-printed DMDs in minimally invasive treatments.102
6. Challenges and limitations of 4D printing in regenerative medicine
Four-dimensional (4D) printing has emerged as a transformative approach in regenerative medicine, enabling the creation of dynamic, stimuli-responsive structures that can adapt over time. Despite its promising applications, several challenges hinder its widespread clinical adoption.
6.1. Material limitations
The development of suitable materials is a primary challenge in 4D printing. Smart materials, such as shape-memory polymers and hydrogels, must exhibit not only responsiveness to external stimuli but also appropriate mechanical properties and biocompatibility. Many existing materials lack the necessary strength, flexibility, or degradation rates required for specific biomedical applications. Moreover, integrating multiple smart materials into a single construct remains complex, limiting the functionality of 4D-printed devices.16
6.2. Biocompatibility and cell viability
Ensuring biocompatibility is critical for any biomedical application. Some stimuli-responsive materials may release residual chemicals or possess properties that adversely affect cell viability. Additionally, the dynamic nature of 4D-printed structures can influence cellular responses, potentially impacting tissue integration and function.78
6.3. Fabrication complexities
The fabrication process of 4D-printed structures involves intricate design and precise control. Achieving high-resolution, multi-material printing is technically demanding. Furthermore, scalability remains a significant hurdle; current 4D printing techniques are often time-consuming and may not be suitable for mass production, which is essential for clinical applications.103
6.4. Limited stimuli responsiveness
Most current 4D-printed materials respond to a single type of stimulus, such as temperature or pH. However, the human body presents a complex environment where multiple stimuli may be present simultaneously. Developing materials that can respond to multiple stimuli in a controlled manner is essential for creating more sophisticated and functional biomedical devices.78
6.5. Integration with living tissues
Achieving seamless integration of 4D-printed structures with native tissues is challenging. The dynamic behavior of these constructs must be synchronized with the biological environment to ensure proper function and avoid adverse reactions. Further research is needed to understand and optimize the interactions between 4D-printed materials and living tissues.104
7. Future prospects in 4D bioprinting for regenerative medicine
7.1. AI integration in 4D bioprinting
Artificial intelligence (AI) and machine learning are poised to enhance 4D bioprinting by enabling predictive modeling of bioink behaviors in response to various stimuli, such as pH, temperature, and mechanical forces. These technologies facilitate the optimization of stimuli-responsive biomaterials, improving construct fidelity and functionality. Moreover, AI can tailor scaffold properties to patient-specific needs, ensuring personalized therapeutic solutions.105
7.2. Functional organ bioprinting
The development of fully functional, dynamic organs remains a central goal in 4D bioprinting. Current research focuses on creating bioengineered organs that can adapt to physiological changes, such as 4D-printed kidneys capable of modulating filtration rates in response to hydration levels. This adaptability is achieved by embedding shape-memory properties into bioinks, allowing scaffolds to alter their configuration in response to external stimuli. A significant challenge in organ bioprinting is achieving adequate vascularization to ensure cell viability and function. Advancements in co-printing techniques that incorporate endothelial and parenchymal cells, along with pro-angiogenic factors, are facilitating the development of functional microvascular networks. These networks are essential for efficient nutrient and oxygen delivery, waste removal, and enhanced long-term survival of bioprinted constructs.106
7.3. Clinical translation and commercialization
The clinical implementation of 4D bioprinting faces challenges related to scalability, cost-effectiveness, and regulatory approval. High material costs and specialized printing infrastructure currently limit widespread adoption. However, innovations in material synthesis and automated bioprinting platforms are expected to reduce production costs and enhance accessibility. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are evolving guidelines to evaluate the safety and efficacy of dynamic biomaterials. Establishing standardized validation protocols is essential for ensuring successful clinical adoption of 4D bioprinted constructs. Beyond technical and regulatory aspects, ethical considerations must be addressed to ensure responsible implementation. Issues such as patient consent, long-term safety, and equitable access to advanced bioprinting therapies require careful deliberation.
7.4. Advanced biomaterials and tissue applications
Recent advancements in smart polymers, including polyanisidine-based photostabilizers and nanocomposite hydrogels, provide robust platforms for engineering bioresponsive scaffolds with enhanced durability and tunable degradation kinetics. Biofunctionalized polymeric networks inspired by extramural venous invasion-targeting materials may facilitate the development of vascularized 4D constructs with improved physiological relevance. Advances in pediatric bone repair have demonstrated the potential of integrating stem cells with calcium hydroxyapatite in 3D-printed scaffolds, a concept that can be expanded into 4D bioprinting to create dynamically adaptable bone grafts for complex reconstructive procedures. A significant milestone in 4D bioprinting will be the realization of fully functional, bioadaptive organs with integrated vascular networks capable of responding dynamically to physiological changes. This progress hinges on the advancement of bioink formulations that mimic the extracellular matrix while supporting cell viability and tissue maturation. Research on grafted sodium alginate copolymers and montmorillonite-reinforced biomaterials suggests promising avenues for enhancing the mechanical stability and bioactivity of printed constructs. Furthermore, interdisciplinary collaboration between biomedical engineers, oncologists, and material scientists will be crucial in overcoming regulatory, ethical, and commercial challenges associated with 4D bioprinting. Regulatory frameworks, such as those established by the FDA and EMA, must evolve to accommodate the unique characteristics of dynamic implants and ensure their safe clinical deployment.
8. Conclusion
The integration of 4D printing with smart biomaterials marks a transformative advancement in regenerative medicine, offering dynamic, stimuli-responsive platforms capable of mimicking the complexity of native tissue behavior. Unlike conventional static scaffolds, 4D-printed constructs adapt over time in response to physiological cues, thereby enhancing cell–material interactions, tissue maturation, and overall healing outcomes. Applications in bone, cartilage, skin, vascular and cardiac tissue regeneration have already demonstrated promising results both in vitro and in vivo. However, challenges remain, including limited biocompatibility of some smart polymers, precise control of shape transformation, and full integration with living tissues. Continued interdisciplinary research in material science, biomedical engineering, and cellular biology will be essential to overcome these limitations and fully realize the clinical potential of 4D printing in personalized regenerative therapies.
Author contributions
All authors were responsible for the conceptualization, drafting, and revision of the manuscript, contributing equally to its development.
Conflicts of interest
The authors declare that they have no financial or personal conflicts of interest that could influence the integrity or outcomes of this study.
Data availability
The information and data referenced in this review are derived from publicly accessible scientific publications, such as peer-reviewed journal articles. Proper attribution has been provided for all cited sources within the manuscript.
References
- S. V Murphy and A. Atala, 3D bioprinting of tissues and organs, Nat. Biotechnol., 2014, 32(8), 773–785, DOI:10.1038/nbt.2958.
- M. Ramezani and Z. Mohd Ripin, 4D Printing in Biomedical Engineering: Advancements, Challenges, and Future Directions, J. Funct. Biomater., 2023, 14(7), 347, DOI:10.3390/jfb14070347.
- J. Kim, G. D A, P. Debnath and P. Saha, Smart Multi-Responsive Biomaterials and Their Applications for 4D Bioprinting, Biomimetics, 2024, 9(8) DOI:10.3390/biomimetics9080484.
- T. S. Tran, R. Balu, S. Mettu, N. Roy Choudhury and N. K. Dutta, 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery, Pharmaceuticals, 2022, 15(10) DOI:10.3390/ph15101282.
- N. A. A. Ebrahim and S. M. A. Soliman, Innovations in Functional Materials and Advanced Imaging Techniques for Targeting Extramural Venous Invasion (EMVI) in Colorectal Cancer: A Comprehensive Review, Biomed. Mater. & Devices, 2025 DOI:10.1007/s44174-025-00284-7.
- M. E. Furth, A. Atala and M. E. Van Dyke, Smart biomaterials design for tissue engineering and regenerative medicine, Biomaterials, 2007, 28(34), 5068–5073, DOI:10.1016/J.BIOMATERIALS.2007.07.042.
- A. Mandal and K. Chatterjee, 4D printing for biomedical applications, J. Mater. Chem. B, 2024, 12(12), 2985–3005, 10.1039/D4TB00006D.
- S. Talebian, et al., Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering?, Adv. Sci., 2019, 6(16), 1801664, DOI:10.1002/advs.201801664.
- A. Solanki, M. S. Ranganath and A. K. S. Singholi, Review on advancements in 3D/4D printing for enhancing efficiency, cost-effectiveness, and quality, Int. J. Interact. Des. Manuf., 2025, 19(5), 3153–3169, DOI:10.1007/s12008-024-02029-0.
- A. Haleem, M. Javaid, R. P. Singh and R. Suman, Significant roles of 4D printing using smart materials in the field of manufacturing, Adv. Ind. Eng. Polym. Res., 2021, 4(4), 301–311, DOI:10.1016/J.AIEPR.2021.05.001.
- B. Farham and L. Baltazar, A Review of Smart Materials in 4D Printing for Hygrothermal Rehabilitation: Innovative Insights for Sustainable Building Stock Management, Sustainability, 2024, 16(10), 4067, DOI:10.3390/su16104067.
- S. Y. Hann, H. Cui, M. Nowicki and L. G. Zhang, 4D printing soft robotics for biomedical applications, Addit. Manuf., 2020, 36, 101567, DOI:10.1016/J.ADDMA.2020.101567.
- X. Huang, et al., 4D Printing Hybrid Soft Robots Enabled by Shape-Transformable Liquid Metal Nanoparticles, Adv. Mater., 2024, 36(46) DOI:10.1002/adma.202409789.
- S. Bharani Kumar, S. D. Sekar, G. Sivakumar, J. Srinivas, R. Lavanya and G. Suresh, Modern concepts and application of soft robotics in 4D printing, J. Phys.: Conf. Ser., 2021, 2054(1), 012056, DOI:10.1088/1742-6596/2054/1/012056.
- A. Sheikh, M. A. S. Abourehab and P. Kesharwani, The clinical significance of 4D printing, Drug Discovery Today, 2023, 28(1), 103391, DOI:10.1016/j.drudis.2022.103391.
- M. Ramezani and Z. Mohd Ripin, 4D Printing in Biomedical Engineering: Advancements, Challenges, and Future Directions, J. Funct. Biomater., 2023, 14(7), 347, DOI:10.3390/jfb14070347.
- S. Mallakpour, F. Tabesh and C. M. Hussain, 3D and 4D printing: From innovation to evolution, Adv. Colloid Interface Sci., 2021, 294, 102482, DOI:10.1016/J.CIS.2021.102482.
- D. Khorsandi, et al., 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications, Acta Biomater., 2021, 122, 26–49, DOI:10.1016/j.actbio.2020.12.044.
- T. Makki, et al., 3D and 4D printing: A review of virgin polymers used in fused deposition modeling, Compos., Part C: Open Access, 2024, 14, 100472, DOI:10.1016/J.JCOMC.2024.100472.
- W. Zhou, et al., 4D-Printed Dynamic Materials in Biomedical Applications: Chemistry, Challenges, and Their Future Perspectives in the Clinical Sector, J. Med. Chem., 2020, 63(15), 8003–8024, DOI:10.1021/acs.jmedchem.9b02115.
- Z. Wan, P. Zhang, Y. Liu, L. Lv and Y. Zhou, Four-dimensional bioprinting: Current developments and applications in bone tissue engineering, Acta Biomater., 2020, 101, 26–42, DOI:10.1016/J.ACTBIO.2019.10.038.
- N. Ashammakhi, et al., Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs, Mater. Today Bio, 2019, 1, 100008, DOI:10.1016/J.MTBIO.2019.100008.
- A. Kirillova, R. Maxson, G. Stoychev, C. T. Gomillion and L. Ionov, 4D Biofabrication Using Shape-Morphing Hydrogels, Adv. Mater., 2017, 29(46), 1703443, DOI:10.1002/adma.201703443.
- D. B. Mahmoud and M. Schulz-Siegmund, Utilizing 4D Printing to Design Smart Gastroretentive, Esophageal, and Intravesical Drug Delivery Systems, Adv. Healthcare Mater., 2023, 12(10) DOI:10.1002/adhm.202202631.
- S. Tibbits, 4D Printing: Multi-Material Shape Change, Archit. Des., 2014, 84(1), 116–121, DOI:10.1002/ad.1710.
- H. Chu, et al., 4D Printing: A Review on Recent Progresses, Micromachines, 2020, 11(9), 796, DOI:10.3390/mi11090796.
- A. Ding, F. Tang and E. Alsberg, 4D Printing: A Comprehensive Review of Technologies, Materials, Stimuli, Design, and Emerging Applications, Chem. Rev., 2025, 125(7), 3663–3771, DOI:10.1021/acs.chemrev.4c00070.
- J. Chen, C. Virrueta, S. Zhang, C. Mao and J. Wang, 4D printing: The spotlight for 3D printed smart materials, Mater. Today, 2024, 77, 66–91, DOI:10.1016/J.MATTOD.2024.06.004.
- A. Tariq, et al., Recent Advances in the Additive Manufacturing of Stimuli-Responsive Soft Polymers, Adv. Eng. Mater., 2023, 25(21) DOI:10.1002/adem.202301074.
- M. W. Sabaa, A. M. Ali and S. M. A. Soliman, Physical hydrogel based on alginate and poly(2-hydroxyethyl methacrylate) for water treatment, Desalin. Water Treat., 2020, 174, 152–160, DOI:10.5004/DWT.2020.24841.
- M. Sabaa, R. Mohamed, Y. El-Gabry and S. Soliman, Synthesis of nanoparticles based on pH-sensitive alginate-g-polyacrylonitrile copolymer and its application in drug loading, Egypt. J. Chem., 2019 DOI:10.21608/ejchem.2019.15257.1928.
- T. S. Tran, R. Balu, S. Mettu, N. Roy Choudhury and N. K. Dutta, 4D Printing of Hydrogels: Innovation in Material Design and Emerging Smart Systems for Drug Delivery, Pharmaceuticals, 2022, 15(10), 1282, DOI:10.3390/ph15101282.
- M. Nadgorny, Z. Xiao, C. Chen and L. A. Connal, Three-Dimensional Printing of pH-Responsive and Functional Polymers on an Affordable Desktop Printer, ACS Appl. Mater. Interfaces, 2016, 8(42), 28946–28954, DOI:10.1021/acsami.6b07388.
- A. Sadraei, S. M. Naghib and N. Rabiee, 4D printing chemical stimuli-responsive hydrogels for tissue engineering and localized drug delivery applications – part 2, Expert Opin. Drug Delivery, 2025, 22(4), 491–510, DOI:10.1080/17425247.2025.2466768.
- N. A. A. Ebrahim, M. O. Othman, R. A. Salama, D. Abdelfatah and N. S. Tahoun, Diagnostic insights into solid pseudopapillary neoplasms of the pancreas: a decade of experience with pediatric representation, Diagn. Pathol., 2025, 20(1), 57, DOI:10.1186/s13000-025-01648-9.
- N. Ashammakhi, et al., Advances and Future Perspectives in 4D Bioprinting, Biotechnol. J., 2018, 13(12), 1800148, DOI:10.1002/biot.201800148.
- H. Cui, et al., A novel near-infrared light responsive 4D printed nanoarchitecture with dynamically and remotely controllable transformation, Nano Res., 2019, 12(6), 1381–1388, DOI:10.1007/s12274-019-2340-9.
- M.-Y. Shie, et al., Review of Polymeric Materials in 4D Printing Biomedical Applications, Polymers, 2019, 11(11), 1864, DOI:10.3390/polym11111864.
- B. Zhang, et al., Mechanically Robust and UV-Curable Shape-Memory Polymers for Digital Light Processing Based 4D Printing, Adv. Mater., 2021, 33(27) DOI:10.1002/adma.202101298.
- F. Adnane, S. M. A. Soliman, E. ElZayat, E. M. Abdelsalam and H. M. Fahmy, Evaluation of chlorophyll-loaded mesoporous silica nanoparticles for photodynamic therapy on cancer cell lines, Laser Med. Sci., 2024, 39(1), 45, DOI:10.1007/s10103-024-03988-2.
- A. Mahmood, F. Perveen, S. Chen, T. Akram and A. Irfan, Polymer Composites in 3D/4D Printing: Materials, Advances, and Prospects, Molecules, 2024, 29(2) DOI:10.3390/molecules29020319.
- M. Hisham, A. E. Salih, M. Shebeeb C, M. K. Catacutan, S. Lee and H. Butt, 4D-printed photochromic contact lenses for ultraviolet monitoring and protection, Cell Rep. Phys. Sci., 2024, 5(10), 102244, DOI:10.1016/J.XCRP.2024.102244.
- S. M. A. Soliman, C. Nouvel, J. Babin and J.-L. Six, o-nitrobenzyl acrylate is polymerizable by single electron transfer-living radical polymerization, J. Polym. Sci., Part A: Polym. Chem., 2014, 52(15), 2192–2201, DOI:10.1002/pola.27232.
- S. M. A. Soliman, et al., Light-sensitive dextran-covered PNBA nanoparticles to continuously or discontinuously improve the drug release, Colloids Surf., B, 2019, 182, 110393, DOI:10.1016/J.COLSURFB.2019.110393.
- P. E. Antezana, et al., 4D Printing: The Development of Responsive Materials Using 3D-Printing Technology, Pharmaceutics, 2023, 15(12) DOI:10.3390/pharmaceutics15122743.
- Z. Dong, Q. Yuan, K. Huang, W. Xu, G. Liu and Z. Gu, Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration, RSC Adv., 2019, 9(31), 17737–17744, 10.1039/C9RA02695A.
- S. Das, J. T. Jegadeesan and B. Basu, Gelatin Methacryloyl (GelMA)-Based Biomaterial Inks: Process Science for 3D/4D Printing and Current Status, Biomacromolecules, 2024, 25(4), 2156–2221, DOI:10.1021/acs.biomac.3c01271.
- V. Thakur, R. Singh, R. Kumar and A. Gehlot, 4D printing of thermoresponsive materials: a state-of-the-art review and prospective applications, Int. J. Interact. Des. Manuf., 2023, 17(5), 2075–2094, DOI:10.1007/s12008-022-01018-5.
- F. Alam, J. Ubaid, H. Butt and N. El-Atab, Swift 4D printing of thermoresponsive shape-memory polymers using vat photopolymerization, NPG Asia Mater., 2023, 15(1), 65, DOI:10.1038/s41427-023-00511-x.
- F. Cerbe, M. Sinapius and M. Böl, Methodology for FDM 4D printing with thermo-responsive SMPs, Mater. Today: Proc., 2024, 101, 1–6, DOI:10.1016/J.MATPR.2022.11.440.
- L. Zhu, D. Liu, X. Luo, J. Zhou, J. Zheng and C. Lai, Preparation and characterization of thermo-responsive shape memory polycaprolactone/poly(ethylene-co-octene) blends for 4D printing, Polym. Eng. Sci., 2023, 63(9), 2828–2840, DOI:10.1002/pen.26408.
- T. Abdullah and O. Okay, 4D Printing of Body Temperature-Responsive Hydrogels Based on Poly(acrylic acid) with Shape-Memory and Self-Healing Abilities, ACS Appl. Bio Mater., 2023, 6(2), 703–711, DOI:10.1021/acsabm.2c00939.
- S. Naficy, R. Gately, R. Gorkin III, H. Xin and G. M. Spinks, 4D Printing of Reversible Shape Morphing Hydrogel Structures, Macromol. Mater. Eng., 2017, 302(1), 1600212, DOI:10.1002/mame.201600212.
- S. Zu, et al., A bioinspired 4D printed hydrogel capsule for smart controlled drug release, Mater. Today Chem., 2022, 24, 100789, DOI:10.1016/J.MTCHEM.2022.100789.
- T. Brossier, M. Habib, B. T. Benkhaled, G. Volpi, V. Lapinte and S. Blanquer, 4D printing of hydrogels based on poly(oxazoline) and poly(acrylamide) copolymers by stereolithography, Mater. Adv., 2024, 5(7), 2750–2758, 10.1039/D3MA00665D.
- N. Paunović, D. Meyer, A. Krivitsky, A. R. Studart, Y. Bao and J. C. Leroux, 4D printing of biodegradable elastomers with tailorable thermal response at physiological temperature, J. Controlled Release, 2023, 361, 417–426, DOI:10.1016/J.JCONREL.2023.07.053.
- Y. S. Jung, J. Park, S. Lee and E. J. Shin, Characterization of thermo-responsive shape memory bio-based thermoplastic polyurethane (SMTPU) for 3D/4D printing applications, Fash. Text., 2025, 12(1), 3, DOI:10.1186/s40691-025-00412-3.
- S. Guo, T. Agarwal, S. Song, K. Sarkar and L. G. Zhang, Development of novel multi-responsive 4D printed smart nanocomposites with polypyrrole coated iron oxides for remote and adaptive transformation, Mater. Horiz., 2025 10.1039/D4MH01804D.
- B. Narupai, P. T. Smith and A. Nelson, 4D Printing of Multi-Stimuli Responsive Protein-Based Hydrogels for Autonomous Shape Transformations, Adv. Funct. Mater., 2021, 31(23) DOI:10.1002/adfm.202011012.
- N. A. A. Ebrahim, S. M. A. Soliman, M. O. Othman, R. A. Salama and N. S. Tahoun, Nanotechnology in Neuro-Oncology: Evaluating the Potential of Graphene and Boron Nitride Nanostructures, Biomed. Mater. & Devices, 2025, 3(2), 691–701, DOI:10.1007/s44174-025-00352-y.
- Y. Wang, Y. Wang, M. Liu, Q. Wei and B. Du, 4D printing light-/thermo-responsive shape memory composites based on thermoplastic polyurethane/polylactic acid/polyaniline blends, High Perform. Polym., 2023, 35(4), 366–378, DOI:10.1177/09540083221135499.
- I. Choi, et al., A dual stimuli-responsive smart soft carrier using multi-material 4D printing, Mater. Horiz., 2023, 10(9), 3668–3679, 10.1039/D3MH00521F.
- M. Regato-Herbella, et al., Multiresponsive 4D Printable Hydrogels with Anti-Inflammatory Properties, ACS Macro Lett., 2024, 13(9), 1119–1126, DOI:10.1021/acsmacrolett.4c00404.
- N. A. A. Ebrahim and S. M. A. Soliman, Advanced Biomaterials and Biomedical Devices for Studying Tumor-Associated Fibroblasts: Current Trends, Innovations, and Future Prospects, Biomed. Mater. & Devices, 2025 DOI:10.1007/s44174-025-00287-4.
- S. Amukarimi and M. Mozafari, 4D bioprinting of tissues and organs, Bioprinting, 2021, 23, e00161, DOI:10.1016/J.BPRINT.2021.E00161.
- M. Kalogeropoulou, P. J. Díaz-Payno, M. J. Mirzaali, G. J. V. M. van Osch, L. E. Fratila-Apachitei and A. A. Zadpoor, 4D printed shape-shifting biomaterials for tissue engineering and regenerative medicine applications, Biofabrication, 2024, 16(2), 022002, DOI:10.1088/1758-5090/ad1e6f.
- A. Mandal and K. Chatterjee, 4D printing for biomedical applications, J. Mater. Chem. B, 2024, 12(12), 2985–3005, 10.1039/D4TB00006D.
- A. Kantaros, F. I. T. Petrescu and T. Ganetsos, From Stents to Smart Implants Employing Biomimetic Materials: The Impact of 4D Printing on Modern Healthcare, Biomimetics, 2025, 10(2) DOI:10.3390/biomimetics10020125.
- A. Prakash, R. Malviya, S. B. Sridhar and J. Shareef, 4D printing in dynamic and adaptive bone implants: Progress in bone tissue engineering, Bioprinting, 2024, 44, e00373, DOI:10.1016/J.BPRINT.2024.E00373.
- N. A. A. Ebrahim and S. M. A. Soliman, Advances in Stem Cell Integration and Calcium Hydroxyapatite Utilization in 3D-Printed Scaffolds for Pediatric Bone Repair Following Conservative Surgery, Biomed. Mater. & Devices, 2025 DOI:10.1007/s44174-025-00317-1.
- Y.-J. Wang, U.-S. Jeng and S. Hsu, Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering, ACS Biomater. Sci. Eng., 2018, 4(4), 1397–1406, DOI:10.1021/acsbiomaterials.8b00091.
- Z. Yuan, et al., Injectable GelMA Cryogel Microspheres for Modularized Cell Delivery and Potential Vascularized Bone Regeneration, Small, 2021, 17(11), 2006596, DOI:10.1002/smll.202006596.
- A. S. Arabiyat, M. R. Pfau, M. A. Grunlan and M. S. Hahn, Intrinsic osteoinductivity of PCL-DA/PLLA semi-IPN shape memory polymer scaffolds, J. Biomed. Mater. Res., Part A, 2021, 109(11), 2334–2345, DOI:10.1002/jbm.a.37216.
- X. Zhang, et al., Four-Dimensional Printing and Shape Memory Materials in Bone Tissue Engineering, Int. J. Mol. Sci., 2023, 24(1) DOI:10.3390/ijms24010814.
- Y. Guo, et al., A biodegradable functional water-responsive shape memory polymer for biomedical applications, J. Mater. Chem. B, 2019, 7(1), 123–132, 10.1039/C8TB02462F.
- Z. Wang, et al., 4D printing polymeric biomaterials for adaptive tissue regeneration, Bioact. Mater., 2025, 48, 370–399, DOI:10.1016/J.BIOACTMAT.2025.01.033.
- P. Morouço, W. Lattanzi and N. Alves, Four-Dimensional Bioprinting As a New Era for Tissue Engineering and Regenerative Medicine, Front. Bioeng. Biotechnol., 2017, 5 DOI:10.3389/fbioe.2017.00061.
- L. Faber, A. Yau and Y. Chen, Translational biomaterials of four-dimensional bioprinting for tissue regeneration, Biofabrication, 2024, 16(1), 012001, DOI:10.1088/1758-5090/acfdd0.
- B. Liu, et al., 4D printed hydrogel scaffold with swelling-stiffening properties and programmable deformation for minimally invasive implantation, Nat. Commun., 2024, 15(1), 1587, DOI:10.1038/s41467-024-45938-0.
- J. Chakraborty, et al., Development of 4D-bioprinted shape-morphing magnetic constructs for cartilage regeneration using a silk fibroin-gelatin bioink, Cell Rep. Phys. Sci., 2024, 5(3), 101819, DOI:10.1016/J.XCRP.2024.101819.
- H. Zhang, et al., Application of 4D-Printed Magnetoresponsive FOGS Hydrogel Scaffolds in Auricular Cartilage Regeneration, Adv. Healthcare Mater., 2025, 14(9), 2404488, DOI:10.1002/adhm.202404488.
- I. Chiesa, A. Esposito, G. Vozzi, R. Gottardi and C. De Maria, 4D Bioprinted Self-Folding Scaffolds Enhance Cartilage Formation in the Engineering of Trachea, Adv. Mater. Technol., 2025, 10(6), 2401210, DOI:10.1002/admt.202401210.
- S. Y. Hann, H. Cui, T. Esworthy and L. G. Zhang, 4D Thermo-Responsive Smart hiPSC-CM Cardiac Construct for Myocardial Cell Therapy, Int. J. Nanomed., 2023, 18, 1809–1821, DOI:10.2147/IJN.S402855.
- Y. Wang, et al., 4D Printed Cardiac Construct with Aligned Myofibers and Adjustable Curvature for Myocardial Regeneration, ACS Appl. Mater. Interfaces, 2021, 13(11), 12746–12758, DOI:10.1021/acsami.0c17610.
- T. Agarwal, et al., Recent advances in bioprinting technologies for engineering cardiac tissue, Mater. Sci. Eng., C, 2021, 124, 112057, DOI:10.1016/J.MSEC.2021.112057.
- F. Kabirian, P. Mela and R. Heying, 4D Printing Applications in the Development of Smart Cardiovascular Implants, Front. Bioeng. Biotechnol., 2022, 10 DOI:10.3389/fbioe.2022.873453.
- S. Miao, et al., Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation, Biofabrication, 2018, 10(3), 035007, DOI:10.1088/1758-5090/aabe0b.
- D. Podstawczyk, M. Nizioł, P. Śledzik, J. Simińska-Stanny, A. Dawiec-Liśniewska and A. Shavandi, Coaxial 4D Printing of Vein-Inspired Thermoresponsive Channel Hydrogel Actuators, Adv. Funct. Mater., 2024, 34(10), 2310514, DOI:10.1002/adfm.202310514.
- J. Pfarr, et al., 4D Printing of Bioartificial, Small-Diameter Vascular Grafts with Human-Scale Characteristics and Functional Integrity, Adv. Mater. Technol., 2024, 9(12), 2301785, DOI:10.1002/admt.202301785.
- A. Nain, et al., A 4D printed nanoengineered super bioactive hydrogel scaffold with programmable deformation for potential bifurcated vascular channel construction, J. Mater. Chem. B, 2024, 12(31), 7604–7617, 10.1039/D4TB00498A.
- L. Zeenat, A. Zolfagharian, Y. Sriya, S. Sasikumar, M. Bodaghi and F. Pati, 4D Printing for Vascular Tissue Engineering: Progress and Challenges, Adv. Mater. Technol., 2023, 8(23), 2300200, DOI:10.1002/admt.202300200.
- J. Halper, Narrative Review and Guide: State of the Art and Emerging Opportunities of Bioprinting in Tissue Regeneration and Medical Instrumentation, Bioengineering, 2025, 12(1) DOI:10.3390/bioengineering12010071.
- Z. Lu, et al., A 4D Printed Adhesive, Thermo-Contractile, and Degradable Hydrogel for Diabetic Wound Healing, Adv. Healthcare Mater., 2024, 13(10), 2303499, DOI:10.1002/adhm.202303499.
- S.-D. Wu and S. Hsu, 4D bioprintable self-healing hydrogel with shape memory and cryopreserving properties, Biofabrication, 2021, 13(4), 045029, DOI:10.1088/1758-5090/ac2789.
- S. M. Naghib, S. N. Hosseini and A. Beigi, 3D/4D printing of chitosan-based scaffolds for wound dressing applications, Carbohydr. Polym. Technol. Appl., 2024, 8, 100594, DOI:10.1016/J.CARPTA.2024.100594.
- L. A. Damiati, et al., 4D printing in skin tissue engineering: A revolutionary approach to enhance wound healing and combat infections, Bioprinting, 2025, 45, e00386, DOI:10.1016/J.BPRINT.2025.E00386.
- N. A. A. Ebrahim, S. M. A. Soliman, M. O. Othman and N. S. Tahoun, Molecular mechanisms and clinical significance of perineural invasion in malignancies: the pivotal role of tumor-associated Schwann cells in cancer progression and metastasis, Med. Oncol., 2025, 42(5), 171, DOI:10.1007/s12032-025-02729-x.
- N. A. A. Ebrahim, A. A. Abou-Bakr, H. N. Tawfik, H. R. Nassar and I. Adel, Decoding β-catenin expression patterns in ovarian serous carcinoma with clinicopathological implications: insights from National Cancer Institute, Clin. Transl. Oncol., 2024 DOI:10.1007/s12094-024-03770-4.
- S.-D. Wu and S. Hsu, 4D bioprintable self-healing hydrogel with shape memory and cryopreserving properties, Biofabrication, 2021, 13(4), 045029, DOI:10.1088/1758-5090/ac2789.
- B. Zhang, et al., Self-Healing Four-Dimensional Printing with an Ultraviolet Curable Double-Network Shape Memory Polymer System, ACS Appl. Mater. Interfaces, 2019, 11(10), 10328–10336, DOI:10.1021/acsami.9b00359.
- M. Invernizzi, S. Turri, M. Levi and R. Suriano, 4D printed thermally activated self-healing and shape memory polycaprolactone-based polymers, Eur. Polym. J., 2018, 101, 169–176, DOI:10.1016/j.eurpolymj.2018.02.023.
- W. He, et al., A Biocompatible 4D Printing Shape Memory Polymer as Emerging Strategy for Fabrication of Deployable Medical Devices, Macromol. Rapid Commun., 2023, 44(2) DOI:10.1002/marc.202200553.
- J. Lai and M. Wang, Developments of additive manufacturing and 5D printing in tissue engineering, J. Mater. Res., 2023, 38(21), 4692–4725, DOI:10.1557/s43578-023-01193-5.
- L. Bonetti and G. Scalet, 4D fabrication of shape-changing systems for tissue engineering: state of the art and perspectives, Prog. Addit. Manuf., 2025, 10(4), 1913–1943, DOI:10.1007/s40964-024-00743-5.
- Z. Zhang, X. Zhou, Y. Fang, Z. Xiong and T. Zhang, AI-driven 3D bioprinting for regenerative medicine: From bench to bedside, Bioact. Mater., 2025, 45, 201–230, DOI:10.1016/J.BIOACTMAT.2024.11.021.
- K. Zheng, et al., Recent progress of 3D printed vascularized tissues and organs, Smart Mater. Med., 2024, 5(2), 183–195, DOI:10.1016/J.SMAIM.2024.01.001.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *d and
Soliman M. A. Soliman
*d and
Soliman M. A. Soliman *e
*e










