 Open Access Article
Open Access ArticlePlasmonic effect-driven strategy for boosting photoelectrocatalytic degradation of rhodamine B using a SnS2/Au self-supported photoelectrode
Yuanyuan Tian *,
Qianqian Zhang,
Jianjun Zhang,
Chao Ren and
Xixin Liu
*,
Qianqian Zhang,
Jianjun Zhang,
Chao Ren and
Xixin Liu
Shanxi College of Technology, Shuozhou, Shanxi Province 036000, China. E-mail: tyy20220418@163.com
First published on 31st October 2025
Abstract
Photoelectrocatalytic degradation of pollutants is regarded as one of the green disposal schemes for water pollutants under the “dual carbon” strategy. In this paper, a gold-based tin disulfide self-supporting photoelectrode (SnS2–Au/Ti foil) was successfully prepared by a two-step method and applied to the photoelectrocatalytic degradation of rhodamine B (RhB) pollutants. The phase structure of the self-supported photoelectrode was characterized by scanning electron microscopy, X-ray diffraction, ultraviolet-visible spectrophotometry, and fluorescence spectrophotometry. The test results for photoelectrocatalytic degradation performance demonstrated that the degradation performance of the pollutant RhB was significantly enhanced via the plasmonic effect of gold in synergy with the intrinsic photocatalytic characteristics of the tin sulfide semiconductor. The photoelectrocatalytic degradation efficiency of the self-supported photoelectrode reached 81.25% within 120 minutes. The composite electrode SnS2–Au/Ti foil demonstrated an 38.18% and 8.45% increase in photoelectrocatalytic degradation of RhB efficiency over the single Au and SnS2 electrodes, respectively. The plasmonic effect-driven strategy for boosting photoelectrocatalytic efficiency opens new perspectives for RhB degradation.
1 Introduction
Driven by rapid industrialization and urbanization, the surging global energy demand has exacerbated environmental degradation, particularly through escalating pollutant emissions. Among these pollutants, Rhodamine B (RhB), a synthetic cationic dye widely used in the textile, paper, and leather industries, has garnered significant attention owing to its resistance to natural degradation and its potent biotoxicity.1,2 It is classified by the World Health Organization as a Group 3 carcinogen.3 Currently, the degradation treatment of RhB primarily relies on four main methodologies: photocatalytic oxidation, Fenton and Fenton-like methods,4,5 combined degradation agents, and biological synergy techniques.6 However, the presence of secondary pollution, high costs, and low degradation efficiency, among other issues, presents substantial challenges in establishing a green and efficient pathway for rhodamine B degradation. Photoelectrocatalytic degradation technology represents a green and highly efficient degradation approach that leverages the synergistic effects of solar energy and electrical energy. This technology has been extensively applied to degrade methylene blue7–9 and various other toxic and hazardous substances. The design and fabrication of photoelectrodes are critical factors in determining the efficiency and effectiveness of photoelectrocatalytic degradation processes.Tin disulfide (SnS2) semiconductor material (Eg ≈ 2.2 eV) exhibits effective visible-light absorption and is widely utilized for photocatalysts.10 Shuai et al. successfully synthesized Mn-doped SnS2 photocatalysts, which significantly enhanced the performance of photocatalytic degradation of RhB.11 Chen et al. prepared SnS2/CPAN nanocomposites by compounding cyclized polyacrylonitrile (CPAN), effectively improving the photocatalytic degradation efficiency and stability.12 The plasmonic effect refers to a range of optical, thermal, and electronic phenomena that arise from the coupling of photons with collective oscillations of free electrons in metals or materials with high free electron density. It has been widely utilized to significantly enhance photocatalytic performance by leveraging three key advantages: a large extinction cross-section, tunable resonant energy, and ultrasensitive sensing capabilities.13,14 The Au@TiO2 core–shell nanostructure catalyst developed by Chen et al.15 demonstrates enhanced adsorption capacity and photocatalytic degradation efficiency through synergistic integration of the plasmonic effect from Au cores and interfacial charge transfer engineering in the heterostructure. The strategic integration of the plasmonic effect in Au/SnS2 heterojunction photoelectrodes is expected to achieve remarkable enhancement in photodegradation efficiency.
In this paper, a gold-based tin sulfide self-supporting photo-electrode (SnS2/Au) was fabricated on a titanium foil substrate through a combination of ion sputtering and solvothermal methods. The surface morphology and optical behavior of the photoelectrode were comprehensively characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), ultraviolet-visible spectrophotometry (UV-vis), and fluorescence spectrophotometry (FL), respectively. The experimental results reveal that the SnS2/Au heterostructure not only synergistically extends photon harvesting in the visible light region but also suppresses photoinduced electron–hole recombination, compared with monocomponent SnS2 or Au catalysts. The photoelectrochemical degradation rate of RhB on the SnS2–Au/Ti foil composite self-supporting photoelectrode reached 81.25% within 120 min, which was superior to that of the single SnS2/Ti foil (72.80%) and the single Au/Ti foil electrode (43.07%). This work presents a novel approach to enhancing the photoelectric degradation of RhB through the utilization of the plasmon effect.
2 Experimental
2.1 Experimental reagents
Tin(IV) chloride pentahydrate (SnCl4·5H2O), thioacetamide (C2H5NS), anhydrous sodium sulfite (Na2SO3), rhodamine B (C28H31ClN2O3), potassium chloride (KCl), and absolute ethanol (C2H6O) were all purchased from Shanghai Macklin Biochemical Technology Co., Ltd. The titanium foil and gold target (99.999%) were purchased from Zhongnuo New Materials (Beijing) Technology Co., Ltd. The deionized water used in the experiment was self-prepared by a pure water machine (TTL-6B). The reagents involved in the experiment were all directly purchased and used without further processing.2.2 The preparation of SnS2–Au/Ti foil self-supporting photoelectrode
The self-supported SnS2–Au/Ti foil photoelectrode was successfully fabricated on the metal titanium substrate using the ion sputtering method and the solvothermal method, as illustrated in Scheme 1.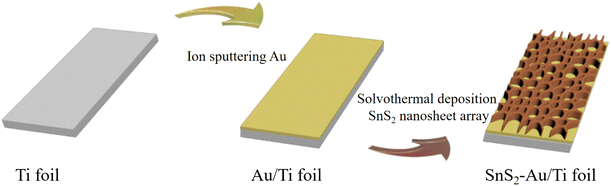 | ||
| Scheme 1 Schematic illustration of the synthesis process of SnS2–Au/Ti foil self-supporting photoelectrode. | ||
2.3 Characterization
The crystal structure of the catalyst on the photoelectrode surface was analyzed by X-ray diffraction (XRD, MiniFlex600). The scanning range was set from 20° to 80°, and the scanning speed was set at 10° min−1. Microscopic morphology and structure were analyzed by using a scanning electron microscope (SEM, JSM-7800F). The elemental composition and valence state were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250). The optical behavior of the self-supporting photoelectrode was analyzed using an ultraviolet-visible spectrophotometer (UV-vis, UV-690) and a fluorescence spectrophotometer (FL, F-7000). The excitation light wavelength of FL spectrophotometer was set to 410 nm.2.4 Photoelectrochemical degradation of RhB measurements
The standard three-electrode system was employed as the photoelectrochemical degradation cell. The CHI 760E instrument served as the electrochemical workstation, and the degradation bias was set at 0.3 V vs. SCE. Specifically, the as-prepared photoelectrode, the saturated calomel electrode, and the platinum sheet were used as the working electrode, the reference electrode, and the counter electrode, respectively. A mixed solution of 20 mM Na2SO3, 0.5 M KCl, and 4 mg L−1 RhB was used as the degradation solution. Visible light irradiation was provided by xenon lamps (CEL-HXF300), and the concentration of RhB during the degradation process was monitored using the ultraviolet-visible spectrophotometer (752G, Shanghai Yidian).3 Results and discussion
3.1 Structure characterization
Fig. 1a–f presents the SEM images of the Ti foil, Au/Ti foil, SnS2/Ti foil, and SnS2–Au/Ti foil electrodes, respectively. The SEM image of Ti foil depicts a surface with relatively low roughness (shown in Fig. 1a). Fig. 1b corresponds to the Au/Ti foil electrode, demonstrating that gold nanoparticles are uniformly distributed on the titanium metal substrate. Fig. 1c and d is associated with the SnS2/Ti foil electrode, where the as-prepared SnS2 nanosheet arrays are evenly deposited on the titanium metal substrate, exhibiting a pore size of approximately 200 nanometers. The SEM images of the SnS2–Au/Ti foil (shown in Fig. 1e and f) are similar to those of the SnS2/Ti foil, suggesting that the incorporation of an intermediate Au nanoparticle layer does not affect the morphology of SnS2.Elemental mapping images of SnS2–Au/Ti foil are illustrated in Fig. 1g1–3, showing uniform distributions of S, Au, and Sn elements on the titanium substrate. Furthermore, the energy-dispersive X-ray spectroscopy (EDX) analysis was employed to analyze the atomic content of corresponding elements (Fig. 1h). The results indicate that the SnS2–Au/Ti foil electrode is predominantly composed of Sn, S, and Au elements, with an atomic ratio of Sn to S approximately equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, confirming the formation of SnS2. Notably, Au accounts for only 1.63% of the total elemental composition of the SnS2–Au/Ti foil electrode. Besides, the EDX spectra of Ti foil, Au120/Ti foil and Au200/Ti foil were displayed in Fig. S1. It is evident that the gold content on the electrode surface increases with prolonged ion sputtering duration.
2, confirming the formation of SnS2. Notably, Au accounts for only 1.63% of the total elemental composition of the SnS2–Au/Ti foil electrode. Besides, the EDX spectra of Ti foil, Au120/Ti foil and Au200/Ti foil were displayed in Fig. S1. It is evident that the gold content on the electrode surface increases with prolonged ion sputtering duration.
The crystal structure of the prepared electrode has been characterized, as depicted in Fig. 2. The diffraction peaks at 44.37° and 64.56° are indexed to (200) and (220) planes of Au, respectively (JCPDS card no. 89-3697). Obviously, the XRD diffraction peaks of Au nanoparticles for the AuX/Ti foil electrode become sharper with an extended sputtering duration, as shown in Fig. 2a. Besides, the diffraction peaks at 15.05°, 30.38°, 50.11°, and 58.55° correspond to the (001), (002), (110), and (200) planes of SnS2, respectively (JCPDS card no. 83-1705). Additionally, the remaining diffraction peaks can be attributed to the titanium metal substrate. A comparison of the XRD diffraction patterns for the SnS2–Au/Ti foil, Au/Ti foil, SnS2/Ti foil, and Ti foil electrodes (Fig. 2b) clearly demonstrates that SnS2 can be effectively synthesized on both Au/Ti foil and Ti foil substrates via the solvothermal method.
 | ||
| Fig. 2 XRD patterns of (a) the AuX/Ti foil electrodes prepared with different sputtering durations, (b) SnS2–Au/Ti foil, Au/Ti foil, SnS2/Ti foil and Ti foil electrodes. | ||
The XPS analysis was performed on the SnS2–Au/Ti foil electrode to characterize the elemental composition and valence state, as shown in Fig. 3. It is obvious that the SnS2–Au/Ti foil electrode contains the primary elements of Sn, S, and Au (as shown in Fig. 3a). In Fig. 3b, the peaks at 161.7 and 162.9 eV are attributed to S2− 2p3/2 and S2− 2p1/2, respectively. The peaks at 486.8 and 495.2 eV correspond to Sn4+ 3d5/2 and Sn4+ 3d3/2, respectively (Fig. 3c).16–18 Furthermore, the weak peaks of Au 4f7/2 and Au 4f5/2 are located at 84.2 and 87.6 eV, respectively (Fig. 3d).19,20
 | ||
| Fig. 3 XPS spectrum of the SnS2–Au/Ti foil, (a) survey spectra and high-resolution XPS (HRXPS) spectrum of (b) S 2p, (c) Sn 3d, and (d) Au 4f. | ||
3.2 Optical property
The optical behavior of self-supported photoelectrodes serves as a critical factor influencing the photoelectrocatalytic degradation performance. The UV-vis absorption spectra and FL emission spectra of SnS2–Au/Ti foil, Au/Ti foil, and SnS2/Ti foil electrodes were illustrated in Fig. 4. Obviously, the as-prepared Au/Ti foil displays a noteworthy absorption peak near 560 nm, which is indicative of the localized surface plasmon resonance (LSPR) effect (Fig. 4a).21,22 For the SnS2/Ti foil electrode, the absorption peak observed at 580 nm can be attributed to the intrinsic bandgap (2.2 eV) of the SnS2 semiconductor.23 Furthermore, the light absorption of the as-prepared SnS2–Au/Ti composite self-supported photoelectrode exhibits a red shift, indicating that the plasmonic effect of the Au layer can effectively synergize with the inherent light absorption properties of the SnS2 semiconductor, leading to an enhancement in the overall light absorption capacity of the self-supported SnS2–Au/Ti composite photoelectrode within the visible light spectrum. | ||
| Fig. 4 (a) UV-vis absorption spectra and (b) FL emission spectra of SnS2–Au/Ti foil, Au/Ti foil, and SnS2/Ti foil electrodes. | ||
As shown in Fig. 4b, the FL emission spectra of the as-synthesized electrodes are illustrated, with the peak intensity at 600 nm being proportional to the recombination probability of photogenerated carriers.24 The Au/Ti foil electrode exhibits a substantially higher FL emission peak in comparison to the SnS2–Au/Ti foil and SnS2/Ti foil electrodes. This phenomenon can be attributed to the plasmonic effect of Au, which generates photogenerated carriers in significantly higher magnitudes and causes much faster recombination than those in semiconductor materials.25 Most importantly, the SnS2–Au/Ti foil electrode exhibits much lower emission intensity, revealing that the synergistic effect of SnS2 and Au significantly reduces the recombination of photogenerated carriers.
3.3 Photoelectrochemical degradation of RhB measurements
Fig. 5a presents the photoelectrocatalytic degradation performance test results of SnS2–Au/Ti composite electrodes, which were fabricated by in situ growing SnS2 nanosheet arrays on Au40/Ti foil, Au80/Ti foil, Au120/Ti foil, Au160/Ti foil, and Au200/Ti foils, respectively. It can be observed that the photoelectrocatalytic degradation performance of the composite electrode initially increases with the prolongation of the sputtering time of Au nanoparticles and subsequently decreases. The SnS2–Au120/Ti foil composite electrode exhibits the highest photoelectric degradation efficiency for RhB within 120 min, achieving a degradation rate of 81.25%. The photo response current density serves as a key indicator for evaluating the photoelectrocatalytic performance of the photoelectrode. As illustrated in Fig. 5b, the J–t curves depict the behavior under a constant bias of 0.3 V vs. SCE in the same photoelectrochemical degradation cell, measured under intermittent illuminated and dark conditions. The Au/Ti foil electrode shows a weak photoelectric response due to the plasmonic effect of Au nanoparticles, but exhibits the lowest photoelectric response among the samples because of limited active reaction sites. When the tin disulfide semiconductor was synergistically integrated with the Au nanoparticles, the photo response current density reaches its maximum, indicating optimal photoelectrocatalytic performance of the SnS2–Au/Ti foil electrode.Fig. 5c illustrates the photoelectrocatalytic, photocatalytic, and electrocatalytic degradation performance of the SnS2–Au/Ti foil composite electrode under identical testing conditions. Within 120 min, the pure electrocatalytic degradation and photocatalytic degradation efficiencies of the SnS2–Au120/Ti foil composite electrode are 45.59% and 68.99%, respectively, both of which are lower than its photoelectrocatalytic degradation efficiency. Furthermore, the repeated photoelectrocatalytic degradation performance was also tested to evaluate the stability of the composite electrode, as displayed in Fig. 5d. It can be clearly observed that the degradation rate of the electrode reached approximately 85% within 120 minutes in five repeated experiments, and then stabilized in the third test cycle. This stabilization might be due to the excessive catalyst shedding from the surface of the composite electrode during the first two degradation cycles.
To further investigate the origin of the photoelectrocatalytic degradation performance of RhB using the SnS2–Au/Ti foil composite electrode, the Ti foil, SnS2/Ti foil, and Au/Ti foil electrodes were individually evaluated under the same testing conditions, as illustrated in Fig. 6a. The degradation rate of the bare Ti foil substrate was about 7.74% within 120 min, which might be attributed to the adsorption effect of the metal substrate on RhB.26 Besides, the SnS2–Au/Ti foil composite electrode exhibits a degradation rate of 81.25%, demonstrating an enhancement of 88.87% and 11.59% compared to the pure Au/Ti foil electrode (43.02%) and the SnS2/Ti foil electrode (72.81%), respectively. Besides, the pseudo-first-order kinetic model was employed to analyze the degradation kinetics of RhB on various photoelectrodes, as illustrated in Fig. 6b. Among them, the degradation rate of SnS2–Au/Ti foil composite electrode was the highest, reaching 0.0137 min−1, which was higher than that of Ti foil (0.0006 min−1), SnS2/Ti foil (0.0113 min−1), and Au/Ti foil (0.0044 min−1) electrodes. It is evident that the SnS2–Au/Ti foil composite electrode has the strongest photoelectrocatalytic degradation activity. Thus, the experimental results demonstrate that the SnS2–Au/Ti foil composite electrode, fabricated by loading SnS2 nanosheet arrays onto a bottom layer of Au nanoparticles, can achieve both the plasmonic effect of Au and the semiconductor photocatalytic effect of SnS2. This effectively enhances the photoelectric degradation process, thereby promoting the degradation of RhB.
 | ||
| Fig. 6 (a) Photoelectrocatalytic degradation performance and (b) corresponding kinetic curves of Ti foil, SnS2/Ti foil, Au/Ti foil, and SnS2–Au/Ti foil electrodes, respectively. | ||
4. Conclusions
In summary, we successfully fabricated the SnS2–Au/Ti foil composite self-supporting photoelectrode on a titanium metal substrate through the combination of ion sputtering and solvothermal methods, and demonstrated its application in the photoelectrocatalytic degradation of RhB. The experimental results demonstrate that the synergistic integration of the plasmonic effect in Au with the semiconductor inherent characteristics of SnS2 enables the composite electrode to exhibit enhanced performance in photoelectrocatalytic degradation of RhB. Under a constant potential of 0.3 V, the system achieves a degradation efficiency of 81.25% within 120 minutes. This strategy of enhancing the semiconductor photodegradation performance via the synergy of the plasmonic effect offers a novel perspective for the investigation of pollutant photodegradation.Conflicts of interest
There are no conflicts to declare.Data availability
All relevant data are within the manuscript and its additional files.Supplementary information: the EDS spectrum (EDS) of SnS2–Au/Ti foil electrode. See DOI: https://doi.org/10.1039/d5ra04205d.
Acknowledgements
This study has been financially supported by Fundamental Research Program of Shanxi Province (Grant No. 202203021222328) and Research Initiation Fund Programs of Shanxi College of Technology (Grant No. 200101).References
- D. Xu and H. L. Ma, Degradation of rhodamine B in water by ultrasound-assisted TiO2 photocatalysis, J. Cleaner Prod., 2021, 313, 127758–127764 CrossRef CAS.
- X. C. Du, J. H. Zhu and Z. J. Quan, et al., Adsorption of rhodamine B by organic porous materials rich in nitrogen, oxygen, and sulfur heteroatoms, New J. Chem., 2021, 45, 3448–3453 RSC.
- P. S. Priya, P. P. Nandhini and S. Vaishnavi, et al., Rhodamine B, an organic environmental pollutant induces reproductive toxicity in parental and teratogenicity in F1 generation in vivo, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2024, 280, 109898–109910 CAS.
- Q. W. Zheng, X. Liu and L. T. Tian, et al., Mechanism of Fenton catalytic degradation of Rhodamine B induced by microwave and Fe3O4, Chin. Chem. Lett., 2025, 36, 109771–109780 CrossRef CAS.
- L. X. Wang, X. L. Liu and R. Zhang, et al., Synthesis of Zeolite-Based Cu/Fe-X from Coal Gangue for Fenton-Like Catalytic Degradation of Rhodamine B, J. Inorg. Organomet. Polym. Mater., 2024, 34, 722–734 CrossRef CAS.
- D. P. Wu, F. F. Li and Q. Chen, et al., Mediation of rhodamine B photodegradation by biochar, Chemosphere, 2020, 256, 127082–127088 CrossRef CAS PubMed.
- O. N. Nkwachukwu, C. Muzenda and K. D. Jayeola, et al., Photoelectrocatalytic Degradation of Methylene Blue on Electrodeposited Bismuth Ferrite Perovskite Films, Materials, 2023, 16, 2769–2790 CrossRef CAS PubMed.
- Z. H. Guo, G. Q. Wang and H. Q. Fu, et al., Photocatalytic degradation of methylene blue by a cocatalytic PDA/TiO2 electrode produced by photoelectric polymerization, RSC Adv., 2020, 10, 26133–26141 RSC.
- C. B. Tang, P. Huang and Z. L. Liu, et al., Improved photoelectrocatalytic degradation of methylene blue by Ti3C2Tx/Bi12TiO20 composite anodes, Ceram. Int., 2022, 48, 24943–24952 CrossRef CAS.
- G. Matyszczak, P. Jozwik and E. Polesiak, et al., Sonochemical preparation of SnS and SnS2 nano- and micropowders and their characterization, Ultrason. Sonochem., 2021, 75, 105594–105603 CrossRef CAS PubMed.
- X. F. Shuai, Y. Wang and J. Zhang, et al., Mn-Dependent Morphology and Structure of SnS2 Nanosheets and the Photocatalytic Performance, ChemistrySelect, 2022, 7, 202201068–202201076 CrossRef.
- J. Chen, B. X. Wang and J. C. Wang, et al., Development of a new high-performance visible-light photocatalyst by modifying tin disulfide with cyclized polyacrylonitrile, Mater. Lett., 2023, 330, 133353–133357 CrossRef CAS.
- K. A. Dahan, Y. Li and J. Xu, et al., Recent progress of gold nanostructures and their applications, Phys. Chem. Chem. Phys., 2023, 25, 18545–18576 RSC.
- M. L. Xue, W. Mao and J. S. Chen, et al., Application of Au or Ag nanomaterials for colorimetric detection of glucose, Analyst, 2021, 146, 5726–5740 Search PubMed.
- J. Q. Chen, Y. J. Chen and J. T. Shuai, et al., Enhancement of visible light adsorption and photocatalytic degradation activity of organic dye by coating semiconducting TiO2 shell on plasmonic Au particles, J. Alloys Compd., 2024, 1009, 176938–176945 CrossRef CAS.
- J. Sakthivel and A. R. Warrier, Ultrasound assisted reductive degradation of toxic textile dye effluents using Sn nanoparticles, Mater. Chem. Phys., 2022, 278, 125610–125619 CrossRef.
- D. Kim, J. Park and J. Choi, et al., Compositional ratio effect on the physicochemical properties of SnSe thin films, Phys. B, 2021, 612, 412890–412896 CrossRef CAS.
- P. Parameswari and A. Sakthivelu, Microwave-Assisted Green Process of Cobalt Ferrous Codoped Tin Oxide Nanoparticles: Antibacterial, Anticancer, and Toxicity Performance, BioNanoScience, 2022, 12, 1211–1219 CrossRef.
- Z. Wang, X. H. Wang and M. Z. Yun, et al., Polyethyleneimine modified Au core Rh shell nanodendrites for light-promoted nitrite reduction reaction at low concentration, J. Energy Chem., 2025, 103, 400–407 CrossRef CAS.
- A. Mellor, A. Wilson and C. L. Pang, et al., Photoemission core level binding energies from multiple sized nanoparticles on the same support: TiO2(110)/Au, J. Chem. Phys., 2020, 152, 024709–024715 CrossRef CAS PubMed.
- Y. Y. Gao, Q. H. Zhu and J. F. Zhao, et al., Regulating Charge Separation Via Periodic Array Nanostructures for Plasmon-Enhanced Water Oxidation, Adv. Mater., 2025, 37, 2414959–2414964 CrossRef CAS PubMed.
- Y. Y. Zhou, J. Y. Wu and W. B. Cui, et al., Molecular sieve redefines SPR sensible range for “win-win” dual functions to enhance the sensitization and anti-fouling, Nano Today, 2025, 61, 102670–102680 CrossRef CAS.
- X. W. Guo, F. Zhang and Y. C. Zhang, et al., Review on the Advancement of SnS2 in the Photocatalysis, J. Mater. Chem. A, 2023, 11, 7331–7343 RSC.
- Y. Y. Tian, Y. Song and J. J. Liu, et al., MoSx Coated Copper Nanowire on Copper Foam as A Highly Stable Photoelectrode for Enhanced Photoelectrocatalytic Hydrogen Evolution Reaction via. Plasmon-Induced Hot Carriers, Chem. Eng. J., 2020, 398, 125554–125564 CrossRef CAS.
- R. S. Haider, S. Y. Wang and J. F. Zhao, et al., Prominence of copper in AuCu alloy towards enhance surface plasmon resonance-driven water oxidation, Nano Energy, 2025, 133, 110499–110510 CrossRef CAS.
- Y. H. Chen, D. Zhao and T. Q. Sun, et al., The preparation of MoS2/δ-FeOOH and degradation of RhB under visible light, J. Environ. Chem. Eng., 2023, 11, 110353–110364 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |


