Open Access Article
Open Access ArticleUnified approach to synthesize diverse heterocyclics: a metal-free visible-light-promoted cyclization reaction to acquire sulfonylated spiro-trienones, coumarins and their derivatives†
Xin Sun *a,
Si-Yu Li‡
a,
Su-Yue Chen‡a,
Cheng-Cheng Zhang‡a,
Jia Lia,
Bin Zhanga,
Xiang-Fei Zhanga,
Jianghong Dong
*a,
Si-Yu Li‡
a,
Su-Yue Chen‡a,
Cheng-Cheng Zhang‡a,
Jia Lia,
Bin Zhanga,
Xiang-Fei Zhanga,
Jianghong Dong a,
Wen-Ke Baic,
Xin-Qi Hao
a,
Wen-Ke Baic,
Xin-Qi Hao d,
Qi-Jie Xu*a,
Bin Wu
d,
Qi-Jie Xu*a,
Bin Wu *b and
Miao Yu*a
*b and
Miao Yu*a
aSchool of Chemistry and Pharmaceutical Engineering, Huanghuai University, Zhumadian, 463000, China. E-mail: sunxin@bjmu.edu.cn; miaoy050666@126.com; qijie001@163.com
bSchool of Pharmaceutical Sciences, South-Central Minzu University, Wuhan 430074, China. E-mail: 2015084@mail.scuec.edu.cn
cHenan Wei Nuo Biotechnology Co., Ltd, Zhumadian 463000, China
dGreen Catalysis Center and College of Chemistry, Zhengzhou University, Zhengzhou 450001, China
First published on 8th July 2025
Abstract
Herein, a visible-light-promoted 9-thioxanthone-catalyzed cascade cyclization reaction to synthesize sulfonylated spiro-trienones, coumarins and their derivatives in yields of up to 98% under mild irradiation reaction conditions is reported. Furthermore, extensive studies, including gram-scale, radical capture, isotope and DFT experiments, were performed to gain insights into the possible reaction mechanism.
Spiro[4.5]trienones and coumarins are recognized as important heterocyclic skeletons of biologically active molecules,1 and they widely exist in many natural products (1 and 2, Fig. 1) and pharmaceutical agents (3 and 4, Fig. 1). Therefore, the synthesis of spiro[4.5]trienone compounds with strong structural diversities and potential bioactivities has always been desired in the field of organic synthesis and has drawn much attention from scientists. Consequently, some protocols have been devoted to the development of novel and efficient ways for the preparation of spiro[4.5]trienones.2 Generally, spiro[4.5]trienone structures are constructed via the oxidative spiro-cyclization of phenol derivatives,3 electrophilic ipso-cyclization,4 transition-metal-mediated intramolecular nucleophilic ipso-cyclization,5 and radical-coupling ipso-cyclization.6 Notably, various substituent groups have been successfully introduced into spiro[4.5]trienone compounds via alkylation,7 alkenylation,8 amination,9 halogenation,10 siliconization,11 phosphonylation,12 nitrification,13 acylation,14 sulfuration,15 selenization,16 telurination,17 and germylation.18 In parallel, sulfone compounds are a class of important organic molecules, many of which have been found to exhibit unique pharmacological activities.19 Most importantly, they also serve as the key building blocks in many organic transformations.20 Furthermore, the introduction of sulfonyl groups into drug molecules may significantly enhance their biological activities.21 As a result, it is of great significance to develop methods to introduce a sulfonyl group into spiro[4.5]trienone skeletons. Electrophilic cyclization of heteroatom-containing alkynes with a neighboring aromatic or heteroaromatic ring, such as N-arylalkynamides, provides a useful strategy to develop annulated heterocycles. Several sulfonyl radical precursors, such as sulfonyl chloride,22 sulfonyl hydrazide,23 sulfonic acid,24 DABSO25 and metabisulfite salt,26 have been investigated to synthesise spiro[4.5]trienones. In 2018, Zhou and Liu22 reported the visible-light-induced radical sulfonylation and ipso-cyclization of N-substituted propiolamides with sulfonyl chloride using 2 mol% eosin Y as the photocatalyst and Na2CO3 as the base in a mixture of CH3CN and H2O. Wang and Wei23 described the I2O5-mediated direct oxidative spirocyclization of N-arylpropiolamides with sulfonylhydrazides, leading to 3-sulfonylated azaspiro[4.5]trienones. Wang and Wei24 also established a method for the synthesis of various 3-sulfonyl and 3-sulfenyl azaspiro[4.5]trienones from N-(p-methoxyaryl)-propiolamides and sulfinic acids using Na2-eosin Y in CH3CN/H2O. Tang25a in 2019 and Volla25b in 2020 independently developed a visible-light-promoted one-pot synthesis of sulfonylated spiro[4.5]trienones from anilines and diaryliodonium salts via SO2 insertion under transition-metal-free conditions. In 2023, Zhao26 developed a protocol to access sulfonylated spiro[4.5]trienones via SO2 insertion by the visible-light-induced cyanoalkylsulfonylation/ipso-cyclization of N-arylpropiolamide with cyclobutanone oxime esters and Na2S2O5 in the presence of the eosin Y disodium salt in CH3CN. As part of our continued interest in the synthesis of sulfonylated 2-oxindole frameworks,27 herein, we report a visible-light-promoted cascade cyclization reaction to synthesize sulfonylated spiro-trienones, coumarins and their derivatives.
 | ||
| Fig. 1 Representative natural products and biologically active pharmaceuticals containing spiro-trienone frameworks. | ||
Herein, a metal-free visible-light-promoted dearomatization ipso-cyclization reaction to synthesize spiro[5.5]trienones is reported. The study was initiated with the screening of the reaction solvent. As shown in Table 1, when the reaction was performed in CH3CN, DMF, and CH3OH, spiro[4.5]trienone product 6a could be isolated in 31–40% yields in the presence of 9-thioxanthone derivative PC-1 (0.2 equiv.) and K2S2O8 (2.0 equiv.) under the irradiation of white light (Entries 1–3, Table 1). The yield increased up to 65% in CH3CN/H2O (Entry 4, Table 1). The situation changed under different systems of mixed solvents (Entries 5–8, Table 1). Next, the investigation of photocatalysts was carried out. Several thioxanthone derivatives photocatalyst were subjected to the reaction conditions, and the results showed that PC-4 could afford the desired spiro[4.5]trienone product 6a in 84% yield (Entries 9–12, Table 1). Besides K2S2O8, Na2S2O8 and (NH4)2S2O8 (Entry 14, Table 1) could also produce 6a in 58% and 65% yields, respectively (Entries 13 and 14, Table 1).
| Entry | Solvent/H2O | (v/v) | PC | Oxidant | Yield(%)a |
|---|---|---|---|---|---|
| a Isolated yield.b The reaction was performed in green light.c The reaction was performed in blue light.d The reaction was performed in purple light.e The reaction was performed in an air atmosphere.f The reaction was performed in the absence of photocatalyst.g The reaction was performed in the dark. | |||||
| 1 | CH3OH | Neat | PC-1 | K2S2O8 | 40 |
| 2 | DMF | Neat | PC-1 | K2S2O8 | 38 |
| 3 | CH3CN | Neat | PC-1 | K2S2O8 | 31 |
| 4 | CH3CN/H2O | 4/1 | PC-1 | K2S2O8 | 65 |
| 5 | Dioxane/H2O | 4/1 | PC-1 | K2S2O8 | 50 |
| 6 | CH3OH/H2O | 4/1 | PC-1 | K2S2O8 | 27 |
| 7 | DMF/H2O | 4/1 | PC-1 | K2S2O8 | 0 |
| 8 | DMSO/H2O | 4/1 | PC-1 | K2S2O8 | 0 |
| 9 | CH3CN/H2O | 4/1 | PC-2 | K2S2O8 | 63 |
| 10 | CH3CN/H2O | 4/1 | PC-3 | K2S2O8 | 66 |
| 11 | CH3CN/H2O | 4/1 | PC-4 | K2S2O8 | 84 |
| 12 | CH3CN/H2O | 4/1 | PC-5 | K2S2O8 | 65 |
| 13 | CH3CN/H2O | 4/1 | PC-4 | Na2S2O8 | 58 |
| 14 | CH3CN/H2O | 4/1 | PC-4 | (NH4)2S2O8 | 65 |
| 15b | CH3CN/H2O | 4/1 | PC-4 | K2S2O8 | 72 |
| 16c | CH3CN/H2O | 4/1 | PC-4 | K2S2O8 | 95 |
| 17d | CH3CN/H2O | 4/1 | PC-4 | K2S2O8 | 62 |
| 18 | CH3CN/H2O | 4/1 | PC-4 | None | 0 |
| 19e | CH3CN/H2O | 4/1 | PC-4 | K2S2O8 | 0 |
| 20f | CH3CN/H2O | 4/1 | None | K2S2O8 | 85 |
| 21g | CH3CN/H2O | 4/1 | PC-4 | K2S2O8 | 90 |
Further optimization of various light sources indicated that blue light was the best choice, whereas green and purple lights were relatively less effective (Entries 15–17, Table 1). Control experiments showed that oxidative (Entry 18, Table 1) and nitrogen atmospheres (Entry 19, Table 1) were necessary for the conversion. Notably, spiro[4.5]trienone 6a could still be generated in 85 and 90% yields in the absence of the photosensitizer (Entry 20, Table 1) or in the dark (Entry 21, Table 1), respectively. In this case, several substrates were selected to verify that photo-irradiation was crucial to the reaction. The yields of spiro[4.5]trienones decreased dramatically when the reactions were carried out under oxidative conditions with only K2S2O8 at room temperature. Even at 90 °C, 6d, 6h and 6i could only produce much lower product yields compared to the standard reaction conditions. When AgNO3 was introduced as the catalyst at room temperature, 6b, 6d, 6f and 6h could be isolated in only 24–43% product yields. By increasing the reaction temperature to 90 °C, the yield of 6d increased to 64% (Table 2). Except for the selected template substrate 5a, all of the other substrates in Table 2 could only produce much lower yields of the corresponding products at room temperature. These results explicitly indicated that visible light was very important for this transition-metal-free cyclization transformation to obtain elevated reaction yields at room temperature in a nitrogen atmosphere. Additionally, substrate 5a′ did not produce any product in CH3CN/H2O, CH3OH/H2O or dioxane/H2O under the standard reaction conditions, demonstrating that a methoxy (–OMe) group at the para-position of phenyl was essential for the conversion (Table 3). Therefore, the optimal reaction conditions were established as a combination of 5a (0.1 mmol), PhSO2Na (0.2 mmol), and 9-thioxanthone (0.02 mmol) in the presence of K2S2O8 (0.2 mmol) under the irradiation of blue light and a nitrogen atmosphere. As shown in Table 4, with the optimized conditions in hand, we then investigated the scope and generality of this metal-free visible-light-promoted dearomative ipso-spirocyclization reaction with respect to various phenylacrylamides. Substrates bearing electron-donating groups, such as –Me (6b and 6c) and the stronger electron-donating group –OMe (6d and 6e), produced azaspiro[4.5]trienones in excellent yields. Substrates bearing electron-withdrawing groups, for example, –F (6f), –Cl (6g and 6h), –Br (6i and 6j), –I (6k), and –CF3 (6l), also provided good product yields. To our delight, 5m and 5n afforded azaspiro[4.5]trienones 6m and 6n in 66% and 30% yields, respectively. Next, several similarly structured sulfonyl radicals were introduced in the reaction, which resulted in the formation of 6o–6s in 64–98% yields. When sodium trifluoromethanesulfinate (Langlois reagent) was used as the sulfonyl donor, 6t′ was isolated in 41% yield instead of 6t. The amide-protected free substrate 5u only afforded 6u in 19% yield. By contrast, N-acetyl substrate 5v did not form product 6v.
| Entry | Sub | K2S2O8b | K2S2O8c | AgNO3, K2S2O8d | AgNO3, K2S2O8e |
|---|---|---|---|---|---|
| a Isolated yield.b The reaction was performed in the presence of K2S2O8 (2.0 equiv.) at room temperature.c The reaction was performed in the presence of K2S2O8 (2.0 equiv.) at 90 °C.d The reaction was performed in the presence of AgNO3 (0.2 equiv.) and K2S2O8 (2.0 equiv.) at room temperature.e The reaction was performed in the presence of AgNO3 (0.2 equiv.) and K2S2O8 (2.0 equiv.) at 90 °C. | |||||
| 1 | 6b | 28% | 71% | 43% | — |
| 2 | 6d | 7% | 16% | 24% | 64% |
| 3 | 6f | 6% | 69% | 43% | — |
| 4 | 6h | 5% | 38% | 27% | — |
| 5 | 6i | 16% | 30% | 77% | — |
a Reaction conditions: 5a (0.1 mmol), PhSO2Na (0.2 mmol), PC-4 (0.02 mmol, 0.2 equiv.), and K2S2O8 (0.2 mmol, 2.0 equiv.) in CH3CN/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 2.0 mL) under the irradiation of blue LEDs in a nitrogen atmosphere; isolated yield. 1, v/v, 2.0 mL) under the irradiation of blue LEDs in a nitrogen atmosphere; isolated yield. |
|---|
 |
Coumarin is an important pharmaceutical structural motif and shows a broad spectrum of medicinal properties and biological activities.28 The increasing importance and widespread usage of coumarin derivatives have drawn attention to their synthetic methods,29 among which metal-catalyzed and organocatalytic methods have proven to be the most effective. Several metal-catalyzed and/or organocatalytic synthetic strategies30 for coumarin have been investigated and reported in recent years. Therefore, after successfully introducing the sulfonyl radical onto spiro[4.5]trienones, we continued trying to expand the application scope of the proposed method to access more versatile sulfonyl-substituted coumarin31 scaffolds using aryl propiolates. Solvent screening results showed that in CH3CN/H2O (7/1, v/v, 2 mL, Entry 5, Table 5), 7a could offer coumarin 8a in 77% yield; other proportions (Entries 1–4 and 6, Table 5) produced lower product yields, and other solvent systems gave similar results (Entries 7–12, Table 5). Alteration of PCs (Entries 13 and 14, Table 5) or oxidants (Entries 15 and 17, Table 5) resulted in lower yields. The yield of 8a decreased dramatically when the reaction was performed in the absence of 9-thioxanthone (Entry 18, Table 5) or visible light (Entry 20, Table 5). No product could be detected in the absence of K2S2O8 (Entry 19, Table 5). Therefore, the optimal reaction conditions were quickly established as a combination of substrate 7a (0.1 mmol, 1.0 equiv.), PhSO2Na (0.2 mmol, 2.0 equiv.), 9-thioxanthone (0.02 mmol, 0.2 equiv.) and K2S2O8 (0.2 mmol, 2.0 equiv.) in CH3CN/H2O (7/1, v/v, 2.0 mL) under irradiation with a 23 W white LED in a nitrogen atmosphere (for details, see ESI Table S4 and Page S20†). As shown in Table 6, aryl propiolates with strong electron-donating substituents were efficient under the reaction conditions to afford coumarins in 56–77% yields (8a–8d). Notably, an intermediate product of 8b and 8b′ was also isolated in 17% yield (for details, see the ESI, Pages S22 and S23†). Substrates with electron-donating and -withdrawing groups substituted simultaneously afforded the corresponding coumarin products (8e and 8f) in moderate yields, respectively. The annulation of substrates with the electron-poor group produced the desired coumarins (8g–8j) in 46–60% yields.
| Entry | Solvent/H2O | (v/v) | PC | Oxidant | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield.b The reaction was performed in the dark. | |||||
| 1 | CH3CN | Neat | 9-Thioxanthone | K2S2O8 | Trace |
| 2 | CH3CN/H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 50 |
| 3 | CH3CN/H2O | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 63 |
| 4 | CH3CN/H2O | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 60 |
| 5 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 77 |
| 6 | CH3CN/H2O | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 49 |
| 7 | CH3OH/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 28 |
| 8 | Dioxane/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 59 |
| 9 | DCE/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 0 |
| 10 | PhCF3/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | Trace |
| 11 | DMF/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 0 |
| 12 | DMSO/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 29 |
| 13 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Eosin disodium | K2S2O8 | 28 |
| 14 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Solvent red 72 | K2S2O8 | 29 |
| 15 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | Na2S2O8 | 31 |
| 16 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | Ce(NH4)2(NO3)6 | 22 |
| 17 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | (NH4)2S2O8 | Trace |
| 18 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
None | K2S2O8 | 28 |
| 19 | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | None | 0 |
| 20b | CH3CN/H2O | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9-Thioxanthone | K2S2O8 | 26 |
a Reaction conditions: 5a (0.1 mmol), PhSO2Na (0.2 mmol), PC-4 (0.02 mmol, 0.2 equiv.), and K2S2O8 (0.2 mmol, 2.0 equiv.) in CH3CN/H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 2.0 mL) under the irradiation of white LEDs in a nitrogen atmosphere; isolated yield. 1, v/v, 2.0 mL) under the irradiation of white LEDs in a nitrogen atmosphere; isolated yield. |
|---|
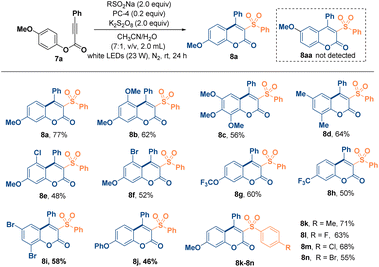 |
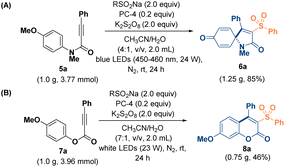
On the other hand, different sodium sulfonate sources, such as halogens, –CF3, –OCF3 and –Oph substituents, were used to synthesize coumarin products (8k–8n) with yields ranging from 55% to 71%. Similar to spiro[4.5]trienones, spiro[5.5]trienone skeletons are also widely found in natural products and pharmaceuticals. Consequently, the development of efficient methods for the construction of these privileged structures has also been an important task in organic synthesis. In a previous report, biaryl ynones32 were utilized to synthesize spiro[5.5]trienones. Therefore, we also hoped to extend the scope of this sulfonylated spiro-cyclization to spiro[5.5]trienones using this reaction method (Table 7). To our delight, various propargyl esters 9 afforded the corresponding spiro[5.5]trienone products 10a and 10b (62–58% yield) under the standard conditions. Several sulfonyl radicals introduced in the reaction could also produce spiro[5.5]trienones 10c–10f in 49–60% yields, affording the desired spiro[5.5]trienones. Similarly, when 4-methoxybenzyl-3-phenylpropiolate 11 was subjected to the same conditions, 7-methoxy-5-phenyl-4-(phenylsulfonyl)benzo[c]oxepin-3(1H)-ones (12a and 12b) were successfully isolated in 46–56% yields. Even when the reactions were performed on the gram scale, cyclization involving the sulfonyl radical proceeded excellently to afford spiro[4.5]trienone product 6a in 85% yield (Fig. 2A). Aryl propiolates formed coumarin 8a with PhSO2Na in 46% yield (Fig. 2B). The introduction of the radical scavenger reagent 2,2,6,6-tetramethylpiperidinyloxy (TEMPO; 2.0 equivalents) in the reaction mixture under the standard reaction conditions completely suppressed the conversions. Spiro[4.5]trienone product 6a (Fig. 3A) and coumarin 10a (Fig. 3B) were not detected, and the starting materials were recovered in 96% and 95% yields, respectively.
a Reaction conditions: 9 or 11 (0.1 mmol), PhSO2Na (0.2 mmol), PC-4 (0.02 mmol, 0.2 equiv.), and K2S2O8 (0.2 mmol, 2.0 equiv.) in CH3CN/H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 7 1 or 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 2.0 mL) under the irradiation of blue or white LEDs in a nitrogen atmosphere; isolated yield. 1, v/v, 2.0 mL) under the irradiation of blue or white LEDs in a nitrogen atmosphere; isolated yield. |
|---|
 |
To gain a deep insight into the formation process of spiro[4.5]trienones, H2O18 was introduced in the reaction mixture instead of H2O (Fig. 4A). High-resolution mass spectrometry (HRMS) analysis showed that the oxygen atom of the product ketone carbonyl group was a mixture of O18 and O16, indicating that the oxygen atom of the ketone originated from the original substrate –16OMe group and reaction solvent (H2O18) (also see ESI Fig. S5 and S6, Pages S8 and S9†). Therefore, a plausible mechanism for the reactions (Fig. 4B) was proposed based on the experimental results presented above. First, the benzenesulfonyl radical was formed via the oxidation of the excited-state photosensitizer 9-thioxanthone (PC-4). Then, a radical addition reaction with the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond occurred to afford intermediate Int-1, which subsequently produced intermediate Int-2 via intramolecular radical addition. The demethylation24,26 of Int-3 afforded normal spiro[4.5]trienone 6h. Meanwhile, a H2O18 nucleophilic attack, followed by deprotonation and MeOH elimination25a reactions of intermediate Int-3 afforded the spiro[4.5]trienone O18 product 6h′. As shown in Fig. 4C, similar to the formation of 6a, the benzenesulfonyl radical was first formed via the oxidation of the excited-state photosensitizer 9-thioxanthone (PC-4). Then, the benzenesulfonyl radical addition reaction with 7a afforded intermediate Int-4, which subsequently afforded intermediate Int-5 via intramolecular radical addition. The oxidation of Int-5 afforded Int-6, followed by 1,2-ester migration33 to afford Int-6, and the coumarin product 8a finally formed via dehydrogenation aromatization.
C bond occurred to afford intermediate Int-1, which subsequently produced intermediate Int-2 via intramolecular radical addition. The demethylation24,26 of Int-3 afforded normal spiro[4.5]trienone 6h. Meanwhile, a H2O18 nucleophilic attack, followed by deprotonation and MeOH elimination25a reactions of intermediate Int-3 afforded the spiro[4.5]trienone O18 product 6h′. As shown in Fig. 4C, similar to the formation of 6a, the benzenesulfonyl radical was first formed via the oxidation of the excited-state photosensitizer 9-thioxanthone (PC-4). Then, the benzenesulfonyl radical addition reaction with 7a afforded intermediate Int-4, which subsequently afforded intermediate Int-5 via intramolecular radical addition. The oxidation of Int-5 afforded Int-6, followed by 1,2-ester migration33 to afford Int-6, and the coumarin product 8a finally formed via dehydrogenation aromatization.
 | ||
| Fig. 4 Isotope experiment of 5h (A). Proposed reaction mechanism for 5h to 6h + 6h′ (B) and proposed mechanism of the preparation of coumarin 8a (C). | ||
To confirm the proposed reaction process, density functional theory (DFT) calculations were also performed to gain additional insights into the reaction mechanism (Fig. 5). Two sequences (Paths 1 and 2) were compared using the Gaussian 16C 01 program with M06-2X/6-311G(d,p)). By taking the change in oxidation and dehydrogenation processes into consideration, the energy of intermediate 2Int4 is set as the zero point of the total electronic energy (unit in kcal mol−1). The zero point of the total electronic energy in brackets after double backslash is the ground state of corresponding intermediate (for example, 1Int6 and 18a, black line, Path 1). The higher spin multiplicity of the system are shown in the blue line (Path 2), and the corresponding excited states are shown in the pink line (Path 3). The change in the energy of isomer product 8aa is shown in the red line (Path 1–i). The vinyl radical intermediate 2Int4 is generated via the addition of the benzenesulfinate radical to substrate 7a. Then, the C atom radical of the vinyl attacks and bonds with the C1 atom in the bending structure of 2Int4-c to afford 2Int5. Path 1 (Path 1: 2Int4 → 2Int4-c → 2Int5 → 1Int6 → 1Int7 → 18a, dark line) is more favorable than Path 1–i (Path 1–i: 2Int4 → 2Int4-c → 2Int5-I → 1Int6-I → 18aa, red line) because the total electronic energy of 2Int5 is 7.2 kcal mol−1 lower than that of 2Int5-i. The final product 18a of the aromatic structure undergoes intramolecular 1,2-migration, whose energy is 3.3 kcal mol−1 lower than that of isomer 18aa. This result indicated that the formation process of product 8a was much more favorable than the formation process of isomer 8aa.
Conclusions
In conclusion, a metal-free visible-light-promoted radical cascade cyclization reaction approach to access diverse heterocyclic spiro-trienones, coumarins and their derivatives under mild irradiation conditions was reported. The results of radical scavenger and isotope experiments showed that the reaction involved radical addition, cyclization and deprotonation to afford the desired products. The mechanistic study of the synthesis of coumarins was also validated using the results of DFT calculations. Further study of the application of phenylsulfinyl radicals in organic synthesis is in progress in our laboratory.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are grateful to the Research Foundation for Doctoral of Huanghuai University (no. 12011942), National Scientific Research Project Cultivation Fund of Huanghuai University (No. 110719421001), Programs for Science and Technology Development of Henan Province (No. 232102310360, 212102310329, 242102310440 and 252102311232), the National Natural Science Foundation of China (No. U2004191), Graduate Education Reform Project of Henan Province (No. 2023SJGLX336Y and 2023SJGLX093Y) and the Key Scientific Research Projects of Universities in Henan Province (No. 23B150007 and 25B150032) for the support of this research.Notes and references
- Y. L. Yang, F. R. Chang and Y. C. Wu, Annosqualine: A Novel Alkaloid from the Stems of Annona Squamosa, Helv. Chim. Acta, 2004, 87, 1392–1399 CrossRef CAS.
- (a) A. J. S. Alves, N. G. Alves, M. I. L. Soares and T. M. V. D. Pinho e Melo, Strategies and Methodologies for the Construction of Spiro-γ-lactams: An Update, Org. Chem. Front., 2021, 8, 3543–3593 Search PubMed; (b) W. C. Yang, M.-M. Zhang and J.-G. Feng, Recent Advances in the Construction of Spiro Compounds via Radical Dearomatization, Adv. Synth. Catal., 2020, 362, 4446–4461 CrossRef CAS; (c) R. J. Song and Y. X. Xie, Recent Advances in Oxidative Ipso-Annulation of N-Arylpropiolamides, Chin. J. Chem., 2017, 35, 280–288 CrossRef CAS; (d) C. R. Reddy, S. K. Prajapti, K. Warudikar, R. Ranjan and B. B. Rao, Ipso-Cyclization: An Emerging Tool for Multifunctional Spirocyclohexadienones, Org. Biomol. Chem., 2017, 15, 3130–3151 RSC.
- (a) T. Dohi, A. Maruyama, M. Yoshimura, K. Morimoto, H. Tohma and Y. Kita, Versatile Hypervalent-iodine (III)-Catalyzed Oxidations with m-Chloroperbenzoic Acid as a Cooxidant, Angew. Chem., Int. Ed., 2005, 44, 6193–6196 CrossRef CAS; (b) D. Magdziak, S. J. Meek and T. R. R. Pettus, Cyclohexadienone Ketals and Quinols: Four Building Blocks Potentially Useful for Enantioselective Synthesis, Chem. Rev., 2004, 104, 1383–1429 CrossRef CAS.
- (a) B. X. Tang, D. J. Tang, S. Tang, Q. F. Yu, Y. H. Zhang, Y. Liang, P. Zhong and J. H. Li, Selective Synthesis of Spiro[4,5]trienyl Acetates via an Intramolecular Electrophilic Ipso-Iodocyclization Process, Org. Lett., 2008, 10, 1063–1066 CrossRef CAS; (b) Q. Yin and S. L. You, Intramolecular Alkene Electrophilic Bromination Initiated Ipso-Bromocyclization for the Synthesis of Functionalized Azaspirocyclohexadienones, Org. Lett., 2012, 14, 3526–3529 CrossRef CAS PubMed; (c) D. Yugandhar, S. Kuriakose, J. B. Nanubolu and A. K. Srivastava, Synthesis of Alkaloid-Mimicking Tricyclic Skeletons by Diastereo- and Regioselective Ugi/Ipso-Cyclization/Aza-Michael Cascade Reaction in One-Pot, Org. Lett., 2016, 18, 1040–1043 CrossRef CAS PubMed.
- (a) A. H. Bansode, S. R. Shaikh, R. G. Gonnade and N. T. Patil, Intramolecular Ipso-Arylative Cyclization of Aryl-alkynoates and N-Arylpropiolamides with Aryldiazonium Salts through Merged Gold/Visible Light Photoredox Catalysis, Chem. Commun., 2017, 53, 9081–9084 RSC; (b) S. Chiba, L. Zhang and J. Y. Lee, Copper-Catalyzed Synthesis of Azaspirocyclohexadienones from α-Azido-N-arylamides under an Oxygen Atmosphere, J. Am. Chem. Soc., 2010, 132, 7266–7267 CrossRef CAS; (c) T. Nemoto, Z. Zhao, T. Yokosaka, Y. Suzuki, R. Wu and Y. Hamada, Palladium-Catalyzed Intramolecular Ipso-Friedel–Crafts Alkylation of Phenols and Indoles: Rearomatization-Assisted Oxidative Addition, Angew. Chem., Int. Ed., 2013, 52, 2217–2220 CrossRef CAS PubMed; (d) S. Rousseaux, J. García-Fortanet, M. A. Del Aguila Sanchez and S. L. Buchwald, Palladium (0)-Catalyzed Arylative Dearomatization of Phenols, J. Am. Chem. Soc., 2011, 133, 9282–9285 CrossRef CAS PubMed; (e) Q. F. Wu, W. B. Liu, C. X. Zhuo, Z. Q. Rong, K. Y. Ye and S. L. You, Iridium-Catalyzed Intramolecular Asymmetric Allylic Dearomatization of Phenols, Angew. Chem., Int. Ed., 2011, 50, 4455–4458 CrossRef CAS.
- (a) D. P. Jin, P. Gao, D. Q. Chen, S. Chen, J. Wang, X. Y. Liu and Y. M. Liang, AgSCF3-Mediated Oxidative Trifluoromethythiolation of Alkynes with Dearomatization to Synthesize SCF3-Substituted Spiro[4,5]trienones, Org. Lett., 2016, 18, 3486–3489 CrossRef CAS PubMed; (b) D. Zheng, J. Y. Yu and J. Wu, Generation of Sulfonyl Radicals from Aryldiazonium Tetrafluoroborates and Sulfur Dioxide: The Synthesis of 3-Sulfonated Coumarins, Angew. Chem., Int. Ed., 2016, 55, 11925–11929 CrossRef CAS.
- (a) J.-W. Yuan, C.-X. Mou, Y. Zhang, W.-Y. Hu, L.-R. Yang, Y.-M. Xiao, P. Mao, S.-R. Zhang and L.-B. Qu, Transition-metal Catalyzed Oxidative Spirocyclization of N-aryl Alkynamides with Methylarenes under Microwave Irradiation, Org. Biomol. Chem., 2021, 19, 10348–10358 RSC; (b) M. Li, R.-J. Song and J.-H. Li, Nickel-Promoted Oxidative Ipso-Annulation of N-(p-Methoxyaryl)propiolamides with α-CarbonylAlkyl Bromides, Chin. J. Chem., 2017, 35, 299–302 CrossRef CAS; (c) S. Manna, P. K. S. Ashwathappa and K. R. Prabhu, Visible Light-Mediated-Ipso-Annulation of Activated Alkynes:Access to 3-Alkylated Spiro[4,5]-trienones, Thiaspiro[4,5]-trienones and Azaspiro[4,5]-trienones, Chem. Commun., 2020, 56, 13165–13168 RSC; (d) P. P. Zeng, X. X. Huang, W. Tang and Z. W. Chen, Copper-Catalyzed Cascade Radical Cyclization of Alkynoates: Construction of Aryldifluoromethylated Coumarins, Org. Biomol. Chem., 2021, 19, 10223–10227 RSC; (e) W.-T. Wei, R.-J. Song, X.-H. Ouyang, Y. Li, H.-B. Li and J.-H. Li, Copper-Catalyzed Oxidative Ipso-Carboalkylation of Activated Alkynes with Ethers Leading to 3-Etherified Azaspiro[4.5]trienones, Org. Chem. Front., 2014, 1, 484–489 RSC; (f) P. Chen, J.-H. Fan, W.-Q. Yu, B.-Q. Xiong, Y. Liu, K.-W. Tang and J. Xie, Alkylation/Ipso-Cyclization of Active Alkynes Leading to 3-Alkylated Aza- and Oxa-spiro[4,5]-trienones, J. Org. Chem., 2022, 87, 5643–5659 CrossRef CAS; (g) S. Manna and K. R. Prabhu, Visible-Light-Mediated Vicinal Difunctionalization of Activated Alkynes with Boronic Acids: Substrate-Controlled Rapid Access to 3-Alkylated Coumarins and Unsaturated Spirocycles, Org. Lett., 2023, 25, 810–815 CrossRef CAS.
- C. R. Reddy, D. H. Kolgave, U. Ajaykumar and R. Ramesha, Copper (II)-Catalyzed Oxidative Ipso-Annulation of N-Arylpropiolamides and Biaryl Ynones with 1,3-Diketones: Construction of Diketoalkyl Spirotrienones, Org. Biomol. Chem., 2022, 20, 6879–6889 RSC.
- A. Mathuri, B. Pal, M. Pramanik and P. Mal, Chemodivergent Chalcogenation of Aryl Alkynoates or N-Arylpropynamides Using 9-Mesityl-10-Methylacridinium Perchlorate Photocatalyst, J. Org. Chem., 2023, 88, 10096–10110 CrossRef CAS.
- (a) Y.-C. Wang, J.-B. Liu, H. W. Zhou, P. Rojsitthisak, G. Y. S. Qiu and W. L. Xie, Ortho-Hydroxylative Ipso-Cyclization of N-arylpropiolamide, J. Org. Chem., 2020, 85, 1906–1914 CrossRef CAS PubMed; (b) Y. Chen, F.-Y. Lu, R.-X. Li, Z. Guan and Y.-H. He, Visible-light-mediated Synthesis of Bromo-containing Azaspirotrienediones from N-phenylpropynamides, Asian J. Org. Chem., 2021, 10, 668–673 CrossRef CAS; (c) X. X. Li, B. B. Zhang, B. Y. Zhao, X. F. Wang, L. Z. Xu and Y. F. Du, Synthesis of 3-Halogenated Quinolin-2-Ones from N-Arylpropynamides via Hypervalent Iodine (III) Mediated. Umpolung Process, Adv. Synth. Catal., 2022, 364, 1427–1433 CrossRef CAS; (d) T. Liu, Y. M. Li, L. L. Jiang, J. A. Wang, K. Jin, R. Zhang and C. Y. Duan, Photo-Mediated Synthesis of Halogenated Spiro[4,5]trienones of N-Aryl Alkynamides with PhI(OCOCF3)2 and KBr/KCl, Org. Biomol. Chem., 2020, 18, 1933–1939 RSC; (e) B. Pal, A. Mathuri, A. Manna and P. Mal, CsPbBr3 Perovskite Photocatalyst in Chemodivergent Functionalization of N-Methylalkanamides Using CBr4, Org. Lett., 2023, 25, 4075–4079 CrossRef CAS; (f) B.-X. Tang, Y.-H. Zhang, R.-J. Song, D.-J. Tang, G.-B. Deng, Z.-Q. Wang, Y.-X. Xie, Y.-Z. Xia and J.-H. Li, Intramolecular Ipso-Halocyclization of 4-(p-Unsubstituted-aryl)-1-alkynes Leading to Spiro[4,5]trienones: Scope, Application, and Mechanistic Investigations, J. Org. Chem., 2012, 77, 2837–2849 CrossRef CAS; (g) K. Yu, X. Q. Kong, J. J. Yang, G. D. Li, B. Xu and Q. J. Chen, Electrochemical Oxidative Halogenation of N-Aryl Alkynamides for the Synthesis of Spiro[4.5]trienones, J. Org. Chem., 2021, 86, 917–928 CrossRef CAS PubMed; (h) D. Yugandhar and A. K. Srivastava, Efficient Construction of Azaspiro[4.5]trienone Libraries via Tandem Ugi 4CC/Electrophilic Ipso-Iodocyclization in One-Pot, ACS Comb. Sci., 2015, 17, 474–481 CrossRef CAS.
- (a) F. Chen, Y. Zheng, H. Yang, Q.-Y. Yang, L.-Y. Wu and N. N. Zhou, Iron-Catalyzed Silylation and Spirocyclization of Biaryl-Ynones: A Radical Cascade Process toward Silylated Spiro[5.5]trienones, Adv. Synth. Catal., 2022, 364, 1537–1542 CrossRef CAS; (b) P. Gao, W. W. Zhang and Z. C. Zhang, Copper-Catalyzed Oxidative Ipso-Annulation of Activated Alkynes with Silanes: An Approach to 3-Silyl Azaspiro[4,5]trienones, Org. Lett., 2016, 18, 5820–5823 CrossRef CAS.
- (a) F. Zeng, X. Chen, K. Sun, H. Zhu, X. Yuan, Y. Liu, L. Qu, Y. Zhao and B. Yu, Visible-Light-Induced Metal-Free Cascade Cyclization of N-arylpropiolamides to 3-Phosphorylated, Trifluoromethylated and Thiocyanated azaspiro[4.5]trienones, Org. Chem. Front., 2021, 8, 760–766 RSC; (b) L.-J. Wang, A.-Q. Wang, Y. Xia, X.-X. Wu, X.-Y. Liu and Y.-M. Liang, Silver-Catalyzed Carbon-Phosphorus Functionalization of N-(p-Methoxyaryl) Propiolamides Coupled with Dearomatization: Access to Phosphorylated Aza-Decenones, Chem. Commun., 2014, 50, 13998–14001 RSC.
- D. Xia, L.-Y. Shen, Y. C. Zhang and W.-C. Yang, Radical Spirocyclization of Biaryl Ynones for the Construction of NO2-Containing Spiro[5.5]trienones, New J. Chem., 2022, 46, 20061–20064 RSC.
- (a) X.-H. Ouyang, R.-J. Song, Y. Li, B. Liu and J.-H. Li, J. Metal-Free Oxidative Ipso-Carboacylation of Alkynes: Synthesis of 3-Acylspiro[4,5]trienones from N-Arylpropiolamides and Aldehydes, J. Org. Chem., 2014, 79, 4582–4589 CrossRef CAS PubMed; (b) C. R. Reddy, U. A. Kumar, A. D. Patil and R. Ramesh, Ipso-Cyclization of Unactivated Biaryl Ynones Leading to Thio-Functionalized Spirocyclic Enones, Org. Biomol. Chem., 2023, 21, 6379–6388 RSC; (c) C. M. Volla, A. M. Nair, A. H. Shinde and S. Kumar, Metal-free Spirocyclization of N-Arylpropiolamides with Glyoxylic Acids: Access to Complex Azaspiro-fused Tricycles, Chem. Commun., 2020, 56, 12367–12370 RSC; (d) C. R. Reddy, D. H. Kolgave, M. Subbarao, M. Aila and S. K. Prajapti, Ag-Catalyzed Oxidative Ipso-Cyclization via Decarboxylative Acylation/Alkylation: Access to 3-Acyl/Alkyl-spiro[4.5]trienones, Org. Lett., 2020, 22, 5342–5346 CrossRef CAS PubMed; (e) V. J. Roy, N. Dagar, S. Choudhury and S. R. Roy, Unified Approach to Diverse Heterocyclic Synthesis: Organo-Photocatalyzed Carboacylation of Alkenes and Alkynes from Feedstock Aldehydes and Alcohols, J. Org. Chem., 2023, 88, 15374–15388 CrossRef CAS; (f) P. Chen, J. Xie, Z. Chen, B.-Q. Xiong, Y. Liu, C.-A. Yang and K.-W. Tang, Visible-Light-Mediated Nitrogen-Centered Radical Strategy: Preparation of 3-Acylated Spiro[4,5]trienones, Adv. Synth. Catal., 2021, 363, 1–8 CrossRef; (g) Y. Liu, Q.-L. Wang, C.-S. Zhou, B.-Q. Xiong, P.-L. Zhang, C.- A Yang and K.-W. Tang, Visible-Light-Mediated Ipso-Carboacylation of Alkynes: Synthesis of 3-Acylspiro[4,5]trienones from N-(p-methoxyaryl)propiolamides and Acyl Chlorides, J. Org. Chem., 2018, 83, 2210–2218 CrossRef CAS PubMed.
- (a) X. X. Li, Y. X. Wang, Y. X. Ouyang, Z. Y. Yu, B. B. Zhang, J. R. Zhang, H. F. Shi, H. Zuilhof and Y. F. Du, Unexpected Substituent Effects in Spiro-Compound Formation: Steering N-Aryl Propynamides and DMSO toward Site-Specific Sulfination in Quinolin-2-ones or Spiro[4,5]trienones, J. Org. Chem., 2021, 86, 9490–9502 CrossRef CAS PubMed; (b) W.-C. Gao, T. Liu, Y.-F. Cheng, H.-H. Chang, X. Li, R. Zhou, A.-L. Wei and Y. Qiao, AlCl3-Catalyzed Intramolecular Cyclization of N-Arylpropynamides with N-Sulfanylsuccinimides: Divergent Synthesis of 3-Sulfenyl Quinolin-2-ones and Azaspiro[4,5]trienones, J. Org. Chem., 2017, 82, 13459–13467 CrossRef CAS PubMed; (c) L. Z. Xu, F. X. Sun, H. Zhao, H. F. Shi, Y. H. Wu, X. X. Li and Y. F. Du, Synthesis of Trifluoromethylthiolated Quinolinones via Trifluoromethanesulfanamide-Induced Electrophilic Intramolecular Cyclization of N-Arylpropynamides, Adv. Synth. Catal., 2023, 365, 3837–3842 CrossRef CAS; (d) C. R. Reddy, U. Ajaykumar and D. H. Kolgave, Expeditious Access to Spiro-Fused 2,5-Cyclohexadienones via Thio(seleno)cyanative Ipso-Cyclization, J. Org. Chem., 2020, 85, 15521–15531 CrossRef.
- (a) A. Recchi, P. Rosa, D. F. Back and G. Zeni, Selenium-Promoted Electrophilic Cyclization of Arylpropiolamides: Synthesis of 3-Organoselenyl Spiro[4,5]trienones, Org. Biomol. Chem., 2020, 18, 3544–3551 RSC; (b) H. Sahoo, G. S. Grandhi, I. Ramakrishna and M. Baidya, Metal-free Switchable ortho/Ipso-Cyclization of N-aryl Alkynamides: Divergent Synthesis of 3-Selenyl Quinolin-2-ones and Azaspiro[4,5]trienones, Org. Biomol. Chem., 2019, 17, 10163–10166 RSC; (c) J. W. Hua, Z. Fang, M. Bian, T. Ma, M. Yang, J. Xu, C. K. Liu, W. He, N. Zhu, Z. Yang and K. Guo, Electrochemical Synthesis of Spiro[4.5]trienones through Radical-Initiated Dearomative Spirocyclization, ChemSusChem, 2020, 13, 2053–2059 CrossRef CAS PubMed; (d) H. Sahoo, A. Mandal, S. Dana and M. Baidya, Visible Light-Induced Synthetic Approach for Selenylative Spirocyclization of N-Aryl Alkynamides with Molecular Oxygen as Oxidant, Adv. Synth. Catal., 2018, 360, 1099–1103 CrossRef CAS; (e) C. R. Reddy, M. Subbarao, D. H. Kolgave, U. Ajaykumar and P. P. Vinaya, Access to Diverse Seleno-Spirocyclohexadienones via Ag (II)-Catalyzed Selenylative Ipso-Annulation with Se and Boronic Acids, ACS Omega, 2022, 7, 38045–38052 CrossRef PubMed; (f) J.-W. Yuan, Q. Chen, W.-T. Wu, J.-J. Zhao, L.-R. Yang, Y.-M. Xiao, P. Mao and L.-B. Qu, Selectfluor-mediated Construction of 3-Arylselenenyl and 3,4-Bisarylselenenyl spiro[4.5]trienones via Cascade Annulation of N-Phenylpropiolamides with Diselenides, New J. Chem., 2022, 46, 9451–9460 RSC.
- G. Zeni, B. Godoi, C. K. Jurinic, A. L. Belladona and R. F. Schumacher, Transition Metal-Free Synthesis of Carbo- and Heterocycles via Reaction of Alkynes with Organylchalcogenides, Chem. Rec., 2021, 21, 1–17 CrossRef.
- Y. Y. Luo, L. Y. Lv and Z. P. Li, Light-Promoted Germylation of Aryl Propiolamides/Alkynoates: Synthesis of Ge-Containing Spiro[4.5]trienones and Vinylgermanes, ChemCatChem, 2023, e202300467 CrossRef CAS.
- (a) M. Tohnishi, H. Nakao, T. Furuya, A. Seo, H. Kodama, K. Tsubata, S. Fujioka, H. Kodama, T. Hirooka and T. N. Flubendiamide, a Novel Insecticide Highly Active against Lepidopterous Insect Pests, J. Pestic. Sci., 2005, 30, 354–360 CrossRef CAS; (b) Y. Shen, C. A. Zificsak, J. E. Shea, X. G. Lao, O. Bollt, X. F. Li, J. G. Lisko, J. P. Theroff, C. L. Scaife, M. A. Ator, B. A. Ruggeri, B. D. Dorsey and S. K. K. Design, Synthesis and Biological Evaluation of Sulfonyl Acrylonitriles as Novel Inhibitors of Cancer Metastasis and Spread, J. Med. Chem., 2015, 58, 1140–1158 CrossRef CAS PubMed.
- (a) C. Jia, D. Piao, T. Kitamura and Y. Fujiwara, New Method for Preparation of Coumarins and Quinolinones via Pd-Catalyzed Intramolecular Hydroarylation of C-C Triple Bonds, J. Org. Chem., 2000, 65, 7516–7522 CrossRef CAS; (b) Z. Zhang, P. He, H. G. Du, J. X. Xu and P. F. Li, Sulfur-Mediated Electrophilic Cyclization of Aryl-Substituted Internal Alkynes, J. Org. Chem., 2019, 84, 4517–4524 CrossRef CAS PubMed; (c) S. Mondal, D. Manna and G. Mugesh, Selenium-Mediated Dehalogenation of Halogenated Nucleosides and Its Relevance to the DNA Repair Pathway, Angew. Chem., Int. Ed., 2015, 54, 9298–9302 CrossRef CAS PubMed; (d) C.-F. Lee, Y.-C. Liu and S. S. Badsara, Transition-Metal-Catalyzed C-S Bond Coupling Reaction, Chem.–Asian J., 2014, 9, 706–722 CrossRef CAS PubMed; (e) Y. Li, M. Wang and X. Jiang, Controllable Sulfoxidation and Sulfenylation with Organic Thiosulfate Salts via Dual Electron- and Energy-Transfer Photocatalysis, ACS Catal., 2017, 7, 7587–7592 CrossRef CAS; (f) M. Wang, Q. Fan and X. Jiang, Metal-Free Construction of Primary Sulfonamides through Three Diverse salts, Green Chem., 2018, 20, 5469–5473 RSC; (g) M. S iauciulis, S. Sapmaz, A. P. Pulis and D. J. Procter, Dual Vicinal Functionalisation of Heterocycles via An Interrupted Pummerer Coupling/[3,3]-Sigmatropic Rearrangement Cascade, Chem. Sci., 2018, 9, 754–759 RSC; (h) L. Smith, S. Coote, H. Sneddon and D. Procter, Beyond the Pummerer Reaction: Recent Developments in Thionium Ion Chemistry, Angew. Chem., Int. Ed., 2010, 49, 5832–5844 CrossRef CAS; (i) N.-W. Liu, S. Liang and G. Manolikakes, Recent Advances in the Synthesis of Sulfones, Synthesis, 2016, 48, 1939–1973 CrossRef CAS; (j) K. M. Borys and Z. Ochal, Synthetic Approaches to Aryl Halomethyl Sulfones, Curr. Org. Chem., 2016, 20, 963–970 CrossRef CAS; (k) G. B. Huang, X. Y. Li, J. R. Luo, Z. H. Luo and M. X. Tan, Recent Progress on Synthesis of Sulfur Compounds by Sodium Sulfinates, Chin. J. Org. Chem., 2019, 39, 617–624 CrossRef CAS.
- (a) P.-J. Zhu, Z.-Z. Yu, Y.-F. Lv, J.-L. Zhao, Y.-Y. Tong, Q.-D. You and Z.-Y. Jiang, Discovery of 3,5-Dimethyl-4-Sulfonyl-1H-Pyrrole-Based Myeloid Cell Leukemia 1 Inhibitors with High Affinity, Selectivity, and Oral Bioavailability, J. Med. Chem., 2021, 64, 11330–11353 CrossRef CAS PubMed; (b) K. A. Scott1 and J. T. Njardarson, Analysis of US FDA-Approved Drugs Containing Sulfur Atoms, Top. Curr. Chem., 2018, 376, 1–34 CrossRef; (c) T. Nagase, T. Takahashi, T. Sasaki, A. Nagumo, K. Shimamura, Y. Miyamoto, H. Kitazawa, M. Kanesaka, R. Yoshimoto, K. Aragane, S. Tokita and N. Sato, Synthesis and Biological Evaluation of a Novel 3-Sulfonyl-8-azabicyclo[3.2.1]octane Class of Long Chain Fatty Acid Elongase 6 (ELOVL6) Inhibitors, J. Med. Chem., 2009, 52, 4111–4114 CrossRef CAS PubMed; (d) R. Artschwager, D. J. Ward, S. Gannon, A. J. Brouwer, H. Langemheen, H. Kowalski and R. M. J. Liskamp, Potent and Highly Selective Inhibitors of the Proteasome Trypsin-like Site by Incorporation of Basic Side Chain Containing Amino Acid Derived Sulfonyl Fluorides, J. Med. Chem., 2018, 61, 5395–5411 CrossRef CAS.
- Y. Liu, Q.-L. Wang, B.-Q. Xiong, P.-L. Zhang, C.-A. Yang, Y.-X. Gong, J. Liao and Q. Zhou, Visible-light-mediated Ipso-Carbosulfonylation of Alkynes: Synthesis of 3-Sulfonylspiro[4,5]trienones from Propiolamides and Sulfonyl Chlorides under Transition-metal-free Conditions, Synlett, 2018, 29, 2396–2403 CrossRef CAS.
- J. Wen, W. Wei, S. Xue, D. Yang, Y. Lou, C. Gao and H. Wang, Metal-free Oxidative Spirocyclization of Alkyneswith Sulfonylhydrazides Leading to 3-Sulfonated Azaspiro[4,5]trienones, J. Org. Chem., 2015, 80, 4966–4972 CrossRef CAS.
- W. Wei, H. H. Cui, D. S. Yang, H. L. Yue, C. L. He, Y. L. Zhang and H. Wang, Visible-light-enabled Spirocyclization of Alkynes Leading to 3-Sulfonyl and 3-Sulfenyl Azaspiro[4,5]trienones, Green Chem., 2017, 19, 5608–5613 RSC.
- (a) Y. Liu, Q.-L. Wang, Z. Chen, Q. Zhou, B.-Q. Xiong, P.-L. Zhang and K.-W. Tang, Visible-light Promoted One-pot Synthesis of Sulfonated Spiro[4,5]trienones from Propiolamides, Anilines and Sulfur Dioxide under Transition Metal-free Conditions, Chem. Commun., 2019, 55, 12212–12215 RSC; (b) A. M. Nair, I. Halder, S. Khan and C. M. R. Volla, Metal Free Sulfonylative Spirocyclization of Alkenyl and Alkynyl Amides via Insertion of Sulfur Dioxide, Adv. Synth. Catal., 2020, 362, 224 CrossRef CAS.
- X. W. Shan, Y. Gao, Y. R. Lu, R. Huang, T. T. Sun, K. Lu and X. Zhao, Visible-light Promoted Cascade Cyanoalkylsulfonylation/Ipso-Cyclization of N-arylpropiolamides toward Sulfonated Spiro[4,5]trienones via SO2 Insertion, Org. Biomol. Chem., 2023, 21, 4823–4832 RSC.
- X. Sun, J.-P. Zhu, Q.-C. Qiu, Y.-L. He, D.-R. Hu, X.-L. Li, G.-P. Lu, Y.-H. Yuan, X.-F. Zhang, X. B. Xu, M. Yu and B. Wu, Metal-Free Visible-Light-Driven Cascade Cyclization Reaction to Synthesize 2-Oxindoles via Benzoyl and Phenylsulfinyl Radicals with Acrylamide Derivatives, Org. Biomol. Chem., 2022, 20, 8042–8048 RSC.
- (a) F. G. Medina, J. G. Marrero, M. Macías-Alonso, M. C. González, I. Córdova-Guerrero, A. G. Teissier García and S. Osegueda-Robles, Coumarin Heterocyclic Derivatives: Chemical Synthesis and Biological Activity, Nat. Prod. Rep., 2015, 32, 1472–1507 RSC; (b) N. S. Reddy, M. R. Mallireddigari, S. Cosenza, K. Gumireddy, S. C. Bell, E. P. Reddy and M. V. R. Reddy, Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity, Bioorg. Med. Chem. Lett., 2004, 14, 4093–4097 CrossRef CAS PubMed.
- E. Calcio Gaudino, S. Tagliapietra, K. Martina, G. Palmisano and G. Cravotto, Recent Advances and Perspectives in the Synthesis of Bioactive Coumarins, RSC Adv., 2016, 6, 46394–46405 RSC.
- S. M. Farid, B. Seifinoferest, M. Gholamhosseyni, B. Larijania and M. Mahdavi, Modern Metal-Catalyzed and Organocatalytic Methods for Synthesis of Coumarin Derivatives: a review, Org. Biomol. Chem., 2022, 20, 4846–4883 RSC.
- (a) W. Wei, J. W. Wen, D. S. Yang, M. Y. Guo, Y. Y. Wang, J. M. You and H. Wang, Direct and metal-free arylsulfonylation of alkynes with sulfonylhydrazides for the construction of 3-sulfonated coumarins, Chem. Commun., 2015, 51, 768–771 RSC; (b) D. Q. Zheng, J. Y. Yu and J. Wu, Generation of Sulfonyl Radicals from Aryldiazonium Tetrafluoroborates and Sulfur Dioxide: The Synthesis of 3-Sulfonated Coumarins, Angew. Chem., Int. Ed., 2016, 55, 11925–11929 CrossRef CAS; (c) X. F. Wang, T. Liu, D. Q. Zheng, Q. Zhong and J. Wu, Synthesis of 3-(((2,3-dihydrobenzofuran-3-yl)methyl)sulfonyl) coumarins through the reaction of 2-(allyloxy)anilines, sulfur dioxide, and aryl propiolates, Org. Chem. Front., 2017, 4, 2455–2458 RSC; (d) W. Q. Wu, Y. N. An, J. X. Li, S. R. Yang, Z. Z. Zhu and H. F. Jiang, Iodine-catalyzed cascade annulation of alkynes with sodium arylsulfinates: assembly of 3-sulfenylcoumarin and 3-sulfenylquinolinone derivatives, Org. Chem. Front., 2017, 4, 1751–1756 RSC; (e) Z. K. Chen, N.-W. Liu, M. Bolte, H. J. Ren and G. Manolikakes, Visible-light mediated 3-component synthesis of sulfonylated coumarins from sulfur dioxide, Green Chem., 2018, 20, 3059–3070 RSC.
- (a) Y. Li, L. J. Li, C. Y. Guo, Q. Q. Yan, H. X. Zhou, Y. Wang, Z.-Q. Liu and Z. J. Li, Nitro-Spirocyclization of Biaryl Ynones with tert-Butyl Nitrite: Access to NO2-Substituted Spiro[5.5]trienones, J. Org. Chem., 2023, 88, 4854–4862 CrossRef CAS; (b) D. Xia and X.-F. Duan, Iron-Catalyzed Dearomatization of Biaryl Ynones with Aldehydes via Double C-H Functionalization in Eco-Benign Solvents: Highly Atom-Economical Synthesis of Acylated Spiro[5.5]trienones, J. Org. Chem., 2021, 86, 15263–15275 CrossRef CAS PubMed; (c) Y. Zhang, C. C. Ma, J. Struwe, J. Feng, G. F. Zhu and L. Ackermann, Electrooxidative Dearomatization of Biaryls: Synthesis of Tri- and Difluoromethylated Spiro[5.5]trienones, Chem. Sci., 2021, 12, 10092–10096 RSC; (d) J. Wang, X.-X. Lu, R.-P. Yang, Z.-H. Xiang, B.-B. Zhang, S. J. Chao, L. X. Liu, Y. H. Yan and X. F. Shang, Synthesis of Spiro[5.5]trienones- and Spiro[4.5]trienones-Fused Selenocyanates via Electrophilic Selenocyanogen Cyclization and Dearomative Spirocyclization, J. Org. Chem., 2022, 87, 13089–13101 CrossRef CAS PubMed; (e) C. R. Reddy and D. H. Kolgave, Electrochemical Selenylative Carbannulation of Biaryl Ynones to Seleno-Dibenzocycloheptenones/Spiro[5.5]Trienones, J. Org. Chem., 2021, 86, 17071–17081 CrossRef; (f) J.-N. Li, Z.-J. Li, L.-Y. Shen, P. H. Li, Y. C. Zhang and W.-C. Yang, Synthesis of Polychloromethylated and Halogenated Spiro[5.5]trienones via Dearomative Spirocyclization of Biaryl Ynones, Org. Biomol. Chem., 2022, 20, 6659–6666 RSC; (g) W.-C. Yang, M.-M. Zhang, Y. Sun, C.-Y. Chen and L. Wang, Electrochemical Trifluoromethylthiolation and Spirocyclization of Alkynes with AgSCF3: Access to SCF3-Containing Spiro[5.5]trienones, Org. Lett., 2021, 23, 6691–6696 CrossRef CAS PubMed; (h) F.-S. He, L. J. Su, F. Y. Yu, Z. M. Tang and J. Wu, Construction of Sulfonated Spiro[5,5]trienones from Sulfur Dioxide via Iron-Catalyzed Dearomative Spirocyclization of Biaryls, Org. Chem. Front., 2022, 9, 1937–1942 RSC.
- S. Sau and P. Mal, 3-Nitro-Coumarin Synthesis via Nitrative Cyclization of Aryl Alkynoates Using tert-Butyl Nitrite, Chem. Commun., 2021, 57, 9228–9231 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra03553h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |