Open Access Article
Open Access ArticleSafflower plant extract as a sustainable corrosion inhibitor for carbon steel in acidic media: a combined electrochemical and computational study
Qahtan A. Yousif *a,
Sumit Sahil Malhotrab,
Mahmoud A. Bedair
*a,
Sumit Sahil Malhotrab,
Mahmoud A. Bedair *c,
Azaj Ansari
*c,
Azaj Ansari *b and
Ali K. Hadid
*b and
Ali K. Hadid
aDepartment of Materials Engineering, College of Engineering, University of Al-Qadisiyah, Iraq. E-mail: qahtan.adnan@qu.edu.iq
bDepartment of Chemistry, School of Basic Sciences, Central University of Haryana, Mahendergarh, 123031, Haryana, India. E-mail: ajaz.alam2@gmail.com
cDepartment of Chemistry, College of Science, University of Bisha, P.O. Box 511, Bisha, 61922, Saudi Arabia. E-mail: mbedair@ub.edu.sa; m_bedier@yahoo.com
dCollege of Health and Medical Technologies, University of Al-Kafeel, Iraq
First published on 23rd June 2025
Abstract
This study investigates the corrosion inhibition efficiency of safflower plant (SP) extract on carbon steel in hydrochloric acid (HCl) solutions. The SP extract, obtained through Soxhlet extraction, was tested for its ability to reduce corrosion using electrochemical techniques, including potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), and electrochemical frequency modulation (EFM). The study revealed that the SP extract functions as an effective mixed-type inhibitor, significantly reducing the corrosion current density and enhancing inhibition efficiency at concentrations up to 2.5 g L−1. It achieved an inhibition efficiency of 89.56% at 2.5 g L−1. The adsorption mechanism is described in terms of both physical and chemical adsorption processes, with the Langmuir isotherm fitting the adsorption data. Computational modeling using density functional theory (DFT) further supported the experimental findings, identifying key active compounds in SP extract that contribute to its inhibitory performance. The study demonstrates the potential of SP extract as a sustainable and eco-friendly corrosion inhibitor for industrial applications.
1. Introduction
The corrosion of metals is widely recognized as a significant economic phenomenon. Corrosion significantly reduces the longevity of metals, especially steel, which is widely utilized.1,2 Using acid solutions can significantly damage enterprises due to the substantial costs associated with corrosion-related maintenance and production losses.3,4 Halting corrosive reactions lowers the economy's growing need for natural resources and the amount of hazardous materials created and released into the environment.5,6 The chemical composition of these compounds will impact the corrosion process.7,8 Reactive and unstable metals engage in electrochemical processes in their surroundings, creating more stable elements and reducing energy.9,10 Carbon steel is crucial in numerous industries, emphasizing its significance in the oil and gas sector.11,12 Carbon steel is the most extensively utilized engineering material globally, contributing to an estimated 85 percent of annual steel production.13,14 Carbon steel corrosion poses a significant challenge for numerous industries, especially in the oil and gas sector.15,16 Its detrimental effects extend beyond production stability and can have a detrimental impact on project economics.5,17 Studies of carbon steel corrosion in acidic media, particularly hydrochloric acid, have become more critical since acid solutions are widely used in industry.18,19 The corrosion of metallic objects in an acidic solution is quite expensive.20,21 In the past, industrial corrosion inhibitors included inorganic salts and organic substances. However, these inhibitors were ineffective at delaying corrosion because they were expensive and dangerous and posed health and environmental dangers in the event of a severe loss in output.22–24 Thus, most conventional organic and inorganic corrosion inhibitors are hazardous and can cause environmental problems when disposed of.25 Regulations related to the environment further limit their applicability.26 For example, environmental pollution occurs when oilfield inhibitors are released from offshore production platforms into the ocean, endangering marine life.27 Green or ecologically friendly corrosion inhibitors prepared from natural materials have emerged. Since 1960, plant extracts have been used to prevent corrosion in an environmentally sustainable manner.17,28 The first plant extract used for this purpose was tannin.29 There are many different plant parts and components from which extracts can be prepared.30 Many chemical structures, such as aromatic rings, carbonyl, carboxylic, amine, and hydroxyl, can be found in these natural sources.31,32 These substances work as efficient inhibitors to retard the corrosion of metals and alloys, particularly in acidic environments.33 Because so many plant resources are available, these plant extracts are also widely accessible.34 However, alternatives are more eco-friendly and derived from plants, which can be used instead of synthetic corrosion inhibitors.35,36 Several researchers have come up with hypotheses to try to explain this phenomenon since the way green corrosion inhibitors work depends on the structure of the active ingredient.37–41 Thus, plant extracts' inhibitory mechanism is often based on their physical or chemical adsorption on the metal surface, which creates a barrier that prevents the corrosive species from accessing the metal and slows down metal breakdown.17,30 An examination of research on developing plant inhibitors for carbon steel indicates that these studies have predominantly concentrated on carbon steel in HCl environments.42,43 This is because carbon steel is extensively used in various applications, such as chemical and electrochemical processes like acid pickling, cleaning, and etching, which require using acidic solutions, particularly HCl.44,45 Numerous organic compounds, including flavonoids, alkaloids, phenols, and tannins, found in plant extracts have demonstrated corrosion-inhibitive properties.46 The plant extracts contain phenolic compounds that effectively adsorb on the metal surface and impede corrosion.47 Various extraction methods, such as Soxhlet extraction and ultrasound-assisted extraction, can obtain extracts from different plant parts, such as seeds, stems, pulp, and leaves, altering the significant constituents and their anticorrosive activity.48 Many studies have used plant extracts as corrosion inhibitors for various metals and alloys under different corrosive conditions.17,49,50 Only Fouda and his group, as well as Nasibi and his group, have conducted any recent studies on using the safflower plant (SP) as a corrosion inhibitor.51,52 Both studies used an infusion of the plant's leaves as an inhibitor to investigate the corrosion effect on commercial 60/40-brass samples and mild steel, respectively. This research used the Soxhlet method to investigate how SP extract, a cheap and naturally occurring substance, inhibits carbon steel corrosion in HCl acid solutions using electrochemical and surface analysis methods. Density functional theory (DFT) helps us understand the electrical properties of molecules that stick to surfaces at the molecular and atomic levels. This lets us study how well plant extracts stop corrosion and how they work, as well as learn more about how chemicals react and work to prevent corrosion.2. The experimental methods
2.1. SP extract preparation
Since antiquity, safflower flowers (Fig. 1) have been cultivated for their use in fabric dyeing, culinary coloring, and seasoning applications. The terminology “Safflower” is derived from its substantial contribution to the dyeing sector. The seeds' oil produces illumination, paint, and varnish. | ||
| Fig. 1 Leaves of safflower flowers and the chemical structure of some important molecules present in it. | ||
The desiccated safflower flower samples were procured from a local vendor, pulverized into a powder using a blender and processed through pure water extraction. The powder (2.0 g) of crushed leaves was extracted twice at 95 °C using Soxhlet extraction in deionized water (1000 mL). The acquired solution was subsequently concentrated at −0.05 MPa and desiccated at 80 °C. SP has been used at concentrations between 0.5 and 2.5 g L−1. Because it is hygroscopic, the extract must be stored dry and cold in a small, tightly sealed container. The largest and most significant constituents of the SP extract were identified by GS/mass (Shimadzu Instruments), and the chemical results showed that the safflower concentrate contains 2-piperidinone, N-[4-bromo-n-butyl], 2-hexyl-1-decanol, 2-piperidone, 9-octadecenoic acid, undec-10-yonic acid, and tetradecyl ester.
2.2. Material preparation
Commercially available materials were used without any additional purification. The acid solutions were prepared by diluting 37% hydrochloric acid (Sigma-Aldrich) with distilled water. Each experiment utilized 150 mL of electrolyte. The working electrode consisted of carbon steel specimens with a nominal composition of 2.19% C, 1.36% Mn, 3.10% Si, 0.10% Cr, 0.12% Mo, 0.21% Ni, and 0.59% Cu, and the remaining balance was Fe. The coupons were trimmed to dimensions of 1.5 × 1.5 cm and then coated with polyester resin, resulting in a final surface area of 1 cm2. The carbon steel substrates were manually abraded using several grades of emery paper, ranging from rough to smooth, to achieve an appropriate soft primary surface. Subsequently, the substrates were degreased using acetone and benzene solvents and rinsed with distilled water before each electrochemical experiment. A 0.5 N hydrochloric acid (HCl) solution was prepared by diluting 37% HCl with distilled water. Each experiment utilized 150 mL of the electrolyte.2.3. Electrochemical measurement and surface inspection
A typical three-electrode cell configuration was used for the electrochemical testing, with carbon steel as the working electrode, platinum as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. Before measurements, the working electrode (carbon steel) was immersed in the test solution (0.5 M HCl ± SP extract) for 30 min to achieve a stable Eocp. The potentiostat device was employed to study polarization at a 3 mV s−1 scan rate. Eqn (1) was used to calculate the inhibitory efficiency53 (ηP).
 | (1) |
 | (2) |
 | (3) |
To investigate the alterations in surface morphology, the electrodes were exposed to an SP inhibitor; the specimens were immersed in a solution of 0.5 N hydrochloric acid containing 2.5 g L−1 safflower extract at 25 °C. After 72 hours of waiting, the electrodes were removed from the solution, washed with distilled water, and dried. Then, images were taken using a Sigma 300 machine set to energy-dispersive X-ray analysis (EDX) for field emission scanning electron microscopy (FE-SEM). The FE-SEM profile details are recorded as resolution: 1 kV (2.2 nm), maximum scan speed (ions/pixel), accelerating voltage: 0.02–30 kV.
2.4. Computational analysis techniques
We modelled the compounds 1 to 5 (compound 1: 2-hexyldecan-1-ol, compound 2: octadec-9-enoic acid, compound 3: 1-(4-bromobutyl)piperidin-2-one, compound 4: piperidin-2-one and compound 5: tetradecyl undec-10-ynoate) with the help of Gauss view 6.1 (ref. 56) software and performed geometry optimizations by using Gaussian 16.57 In this current research work, B3LYP58,59 functional and 6-311G (d,p)60 basis set is employed for all the optimizations. The density of states (DOS) is calculated by using GaussSum software.61 Hydrogens are omitted for the better clarification of pictures.3. Results and discussion
3.1. Electrochemical assessments
 | ||
| Fig. 2 OCP vs. time curves for steel in 0.5 N HCl solution without and with different concentrations of SP at 25 °C. | ||
Similar behavior is shown in the literature.63,64 Positive shifts in OCP values suggest the formation of a protective coating on the surface of carbon steel, which reduces its dissolution. This phenomenon can be attributed to the adsorption of SP extracts onto the surface of the carbon steel. Fig. 2 demonstrates that the OCP value of the blank solution differs only slightly (maximum of 0.012 V) from the OCP values of the different concentrations of SP in a 0.5 N HCl solution. This shows that SP can be categorized as a mixed-type corrosion inhibitor. In a highly acidic environment, the anodic process involves the transfer of metal ions into the solution. In contrast, the cathodic process reduces hydrogen ions to generate hydrogen gas.65 SP is classified as a mixed-type corrosion inhibitor, which can influence both the anodic and cathodic processes.
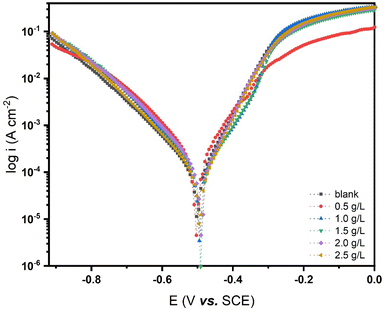 | ||
| Fig. 3 Potetiodynamic polarization curves for steel in 0.5 N HCl solution without and with different concentrations of SP at 25 °C. | ||
As indicated in Table 1, the following electro-chemical and kinetics parameters can be calculated by extrapolating Tafel polarization curves: corrosion potential (Ecorr), corrosion current density (icorr), anodic Tafel slope (βa), cathodic Tafel slope (βc), coverage area (θ), and inhibition efficiency (ηP). The Tafel plots reveal that adding the inhibitor shifts the polarization curves towards less negative potentials, aligning with the OCP method's results. Similarly, we found that the corrosion current density decreased as the concentration of the SP inhibitor in the 0.5 N HCl acid solution increased, leading to a rise in the effectiveness of inhibition. The addition of various concentrations of SP inhibitor in this investigation moderately influences the values of βa and βc. This demonstrates that the SP inhibitor alters the corrosion reaction and controls both the cathodic and anodic reactions. The lowest icorr value (213.6 μA for Blank, 22.28 μA for SP extract 2.5 g L−1) was recorded at the highest inhibitor concentration. The inhibitory efficiency increases at 2.5 g L−1 concentration (ηP % = 89.56%). Previous studies23,24,66 have elucidated these findings, demonstrating that the decrease in icorr results from the adsorption of SP extract molecules onto the metal surface and the creation of films on the active sites. This film formation reduces the medium's corrosive nature at the carbon steel and acid solution interface. Table 1 establishes a direct correlation between the corrosion current density and the corrosion rate. The carbon steel's surface coverage increases as SP concentration increases. A protective layer formed by more inhibitor molecules adsorbing to the surface lowers the corrosive current. Thus, the Ecorr values change slightly when an SP inhibitor is present, as shown in Table 1. As stated in the OCP evaluation, a maximum deviation of 09 mV was observed between the blank solution's Ecorr value and the Ecorr values of the different SP concentrations in a 0.5 N HCl solution (this value does not exceed 85 mV). Because of this, we say that this inhibitor is mixed.67 The mixed-type behavior of SP extract can be attributed to its ability to adsorb onto both the anodic and cathodic sites of the carbon steel surface through nitrogen- and oxygen-containing functional groups present in the extract. These functional groups, such as –OH, –NH, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (as confirmed by ATR-FTIR analysis), interact with the metal surface via donor–acceptor mechanisms, thereby reducing both the dissolution of iron and the evolution of hydrogen gas.68 The SP extract adsorbs onto the active sites of carbon steel, thereby inhibiting both anodic oxidation and cathodic reduction reactions,69 which explains SP's inhibitory characteristics.
O (as confirmed by ATR-FTIR analysis), interact with the metal surface via donor–acceptor mechanisms, thereby reducing both the dissolution of iron and the evolution of hydrogen gas.68 The SP extract adsorbs onto the active sites of carbon steel, thereby inhibiting both anodic oxidation and cathodic reduction reactions,69 which explains SP's inhibitory characteristics.
| Conc. (g L−1) | Ecorr vs. SCE (mV) | icorr (μA cm−2) | βa (mV dec−1) | βc (mV dec−1) | k (mpy) | θ | ηp % |
|---|---|---|---|---|---|---|---|
| — | −500 | 213.6 | 83.60 | −127.7 | 196.2 | — | — |
| 0.5 | −503 | 87.64 | 73.10 | −86.20 | 107.4 | 0.5897 | 58.97 |
| 1.0 | −496 | 76.55 | 87.50 | −101.0 | 97.15 | 0.6416 | 64.16 |
| 1.5 | −492 | 54.23 | 80.80 | −96.30 | 85.46 | 0.7461 | 74.61 |
| 2.0 | −496 | 36.45 | 77.80 | −108.7 | 78.35 | 0.8293 | 82.93 |
| 2.5 | −491 | 22.28 | 66.40 | −92.50 | 46.27 | 0.8956 | 89.56 |
 | ||
| Fig. 4 Electrochemical frequency modulation curves for steel in 0.5 N HCl solution without and with different concentrations of SP at 25 °C. | ||
| Conc. (g L−1) | icorr (μA cm−2) | βa (mV dec−1) | βc (mV dec−1) | CF-2 | CF-3 | k (mpy) | θ | ηE % |
|---|---|---|---|---|---|---|---|---|
| — | 398.8 | 33 | −38 | 1.499 | 2.225 | 234.0 | — | — |
| 0.5 | 193.2 | 84 | −102 | 1.899 | 3.023 | 89.57 | 0.5155 | 51.55 |
| 1.0 | 148.7 | 89 | −112 | 1.930 | 2.998 | 69.56 | 0.6271 | 62.71 |
| 1.5 | 121.9 | 90 | −103 | 1.831 | 3.192 | 52.01 | 0.6943 | 69.43 |
| 2.0 | 96.11 | 91 | −105 | 2.059 | 3.006 | 30.14 | 0.7590 | 75.90 |
| 2.5 | 63.50 | 86 | −113 | 2.003 | 3.190 | 23.88 | 0.8407 | 84.07 |
Increasing the SP inhibitor's concentration decreases icorr while simultaneously expanding the inhibitor's effectiveness. The SP extract inhibitor was successful since the rate of corrosion dropped from 234.0 mpy to 46.88 mpy. Given that the usual values for CF-2 and CF-3 are 2.0 and 3.0, respectively,71 any discrepancies in the causality factor values from the theoretical benchmarks may be attributed to a little perturbation amplitude, insufficient spectral frequency resolution, or a malfunctioning inhibitor. If the causative factors closely align with the expected values, a causal link exists between the perturbation signal and the response signal. The statistics are thereafter regarded as reliable. Table 2 shows casualty factor values that are becoming close to the standard values, which means the data are promising and support both the Tafel slopes and the icorr. As mentioned earlier, applying eqn (3) yielded the inhibitory efficiency (ηF %). SP has the highest inhibition efficiency, at 84.07%, based on the obtained percentage of efficiencies.
 | ||
| Fig. 5 Nyquist plots (a), Bode, phase angle plots (b), Nyquist plots at different immersion time (c) for steel in 0.5 N HCl solution without and with different concentrations of SP at 25 °C. | ||
The Bode plots indicate that with an increase in SP concentration, the peak magnitude also elevates, implying a decrease in the capacitive behavior of steel attributed to SP adsorption on the steel surface.73
An equivalent circuit model (Fig. 5a) was used to analyze the EIS data, with the findings described in Table 3. Rs, Rct, and Rf denote the resistances linked to the solution at the interface of the working/reference electrodes, the charge transfer mechanism, and the surface layer, respectively. In this context, the total resistance is represented by polarization resistance (Rp = Rct + Rf). Constant phase elements (CPE) denote the capacitance of the double layer (CPEdl) and the surface film (CPEf), with their values indicating the system's non-ideal characteristics.74,75 The value ‘n' measures the divergence from optimal capacitance, affected by the porous configuration of the metal surface. The chi-squared value (χ2), which is less than 10−2, indicates a robust concordance between the experimental data and the model.76 The Rp values showed a distinct correlation with differing SP concentrations, indicating improved protective efficacy with greater dose. EIS measurements were also conducted by immersing a carbon steel electrode in a 0.5 M HCl solution containing 2.5 g per L SP extract for varying durations (2–12 hours). The results are presented in Fig. 5(c). The Nyquist plot displays a capacitive reactance semicircle that increases in radius with longer immersion while maintaining its shape. This suggests that SP's inhibition efficiency strengthens over time without altering its inhibition mechanism. The slight variation in arc radius across immersion periods further supports the long-term protective effect of SP.
| Inhibitor | Conc. (g L−1) | Rs (Ω cm2) | Rf (Ω cm2) | CPEf | Cf (μF cm−2) | Rct (Ω cm2) | CPEdl | Cdl (μF cm−2) | Chi squared (χ2) | α° | Rp (Rf + Rct) | θ | ηz % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yo1 (m Ω−1 sn cm−2) | n1 | Yo2 (μ Ω−1 sn cm−2) | n2 | ||||||||||||
| Blank | 0.00 | 0.9527 | — | — | — | — | 24.09 | 318.60 | 0.8434 | 128.99 | 3.11 × 10−3 | −55.92 | 24.09 | — | — |
| SP | 0.50 | 1.067 | 4.983 | 71.78 | 0.99975 | 71.64 | 81.31 | 316.00 | 0.7007 | 66.14 | 3.40 × 10−3 | −64.46 | 86.29 | 0.7208 | 72.08 |
| 1.00 | 0.9098 | 7.987 | 57.11 | 0.99896 | 56.65 | 89.02 | 292.10 | 0.7457 | 84.14 | 5.08 × 10−3 | −64.73 | 97.01 | 0.7517 | 75.17 | |
| 1.50 | 0.967 | 13.819 | 53.39 | 0.99988 | 53.34 | 117.9 | 287.70 | 0.7462 | 91.01 | 3.93 × 10−3 | −63.54 | 131.72 | 0.8171 | 81.71 | |
| 2.00 | 1.008 | 16.403 | 45.86 | 0.99987 | 45.82 | 119.5 | 261.80 | 0.7395 | 77.26 | 5.92 × 10−3 | −68.52 | 135.90 | 0.8227 | 82.27 | |
| 2.50 | 0.8207 | 19.134 | 41.83 | 0.99887 | 41.49 | 137.8 | 230.60 | 0.7985 | 96.58 | 8.10 × 10−3 | −66.03 | 156.93 | 0.8465 | 84.65 | |
The inhibition effectiveness increased with greater concentrations of SP, reaching a maximum of 84.65% at 2.5 g L−1, demonstrating its effective corrosion inhibition capability. Moreover, the CPEdl value decreased with the increasing SP concentration, attributable to the substitution of water molecules at the metal/solution interface by SP molecules.77,78 This resulted in the effective protection of the steel surface from corrosion in 0.5 N HCl solution via the development of a protective extract layer. Table 3 illustrates a clear association between elevated SP concentrations and augmented charge transfer resistance (Rct), resulting in enhanced inhibitory efficiency.
3.2. Effects of temperature and thermodynamic considerations
Inhibitor applications in corrosive systems are significantly restricted by their diminished efficacy as temperatures fluctuate, which validates the effectiveness of the SP extract inhibitor as temperatures rise. To achieve this, it is crucial to recognize that the impact of temperature on the inhibited acid–metal reaction is intricate.79 This is because the metal surface undergoes numerous modifications, including the rapid etching and desorption of the inhibitor, as well as the inhibitor's decomposition and rearrangement in certain instances. Nevertheless, it provides the capacity to calculate numerous thermodynamic functions and kinetic considerations for the adsorption and inhibition processes, which are instrumental in determining the type of adsorption inhibitor. An experiment was conducted to investigate the effect of temperature on the dissolution of steel in a controlled environment. Experiments were carried out at different temperatures (298–318 K) to study the behavior of a carbon steel electrode in a 0.5 N HCl acidic solution. Additionally, specific concentrations of SP extract inhibitor were added to determine its effectiveness as a corrosion inhibitor at elevated temperatures and to understand the nature of its adsorption. Based on the analysis of these data, it is possible to infer the occurrence of either adsorption (chemisorption) or physisorption.80 As the temperature increases, the strength of chemisorption is enhanced, while physical adsorption becomes more prominent at lower temperatures.The polarization curves for carbon steel in a 0.5 N HCl acidic solution and an SP extract inhibitor at different temperatures can be seen in Fig. 6. Based on the data provided, the cathodic and anodic branches show increased current density with rising temperature, indicating enhanced electrochemical activity. Variations in temperature significantly affect the Tafel slopes obtained from polarization measurements. As the temperature rises, the values of corrosion potential tend to increase but eventually reach a point where they stabilize and no longer change.
 | ||
| Fig. 6 Potentiodynamic polarization curves for carbon steel in a 0.5 N HCl acidic solution and an SP extract inhibitor at different temperatures. | ||
Fig. 7 illustrates the relationship between the absolute solution temperature (K), the corrosive current density, and corrosion efficiency in the corrosion medium. From Fig. 7, it was clear that as the temperature rises, so does the corrosive current density of the carbon steel electrode in a solution that does not have the SP extract inhibitor. The reason for this is that as the temperature of the solution rises, reactive species move more quickly to the electrode surface and along all other corrosion pathways, including electrochemical and chemical reactions. Compared to the uninhibited system, the corrosion current densities of the inhibited systems are consistently lower. In the present study, these results suggest that the temperature range under investigation is appropriate for the SP inhibitor. Fig. 7 shows how the investigated SP inhibitor changed the ηp % of carbon steel in a 0.5 N HCl acidic solution when the temperature was changed. According to this graph, the ability to provide protection decreases as the temperature of the solution increases. The performance is characterized by chemical adsorption. The reason for this is that increasing the reaction temperature reduces the strength of the bond between the inhibitor species and the metal interface. The formation and alteration of a coordinate bond result from the transfer of the π-density of molecular electrons from inhibitor molecules to open d-orbitals81 at the electrode surface. This process forms the foundation of the chemisorption mechanism. Fig. 8 shows the Arrhenius plot for carbon steel deterioration in the absence and presence of SP extract. The following equation, based on the Arrhenius model,82 connects the apparent activation energy (Ea) and the (icorr) SP inhibitor:
 | ||
| Fig. 7 The relationship between the absolute solution temperature (K), the corrosive current density (A), and corrosion efficiency in the corrosion medium (B). | ||
The slope of the straight line in Fig. 8 is utilized to calculate Ea values. In comparing the Ea values of the uninhibited acidic solution (15.03 kJ mol−1) and the inhibited one (8.916 kJ mol−1), it is evident that the chemical adsorption process is more pronounced in the former. This could be attributed to a change in the overall degradation pattern, shifting from exposed surfaces to areas that have undergone adsorption. This results in a significant decrease in activation energy at an enhanced level of the protective process. This is done by overlapping the electron density of the SP extract molecules with unfilled d-orbitals of iron to control the anodic process of a corrosion reaction and validate the chemical adsorption mechanistic pathway via the formation of a coordination bond. The Langmuir isotherm was used to analyze the experimental data of the adsorption process of the SP extract inhibitor on carbon steel in the 0.5 N HCl corrosive solutions under investigation. Fig. 9 presents the Langmuir adsorption model80 shown as Cinh/θ vs. Cinh.
Thermodynamic parameters are necessary for comprehending the mitigation process's mechanistic pathway. The variableΔG0ads, representing the degree of standard free energy, can be acquired via the formula recorded in previous publications.83 The ΔG0ads results are typically utilized to confirm the adsorption classification84 between the carbon steel surface and SP extract inhibitor. The computed values for the adsorption constant (Kads) and the standard Gibbs free energy change (ΔG0ads) are 1.991 L g−1 and −35.94 kJ mol−1, respectively. The decent value of Kads indicates that the SP inhibitor molecules bind more efficiently with the carbon steel surface, providing improved protection. These findings were consistent with previous studies in the literature.85,86 The Kads value showed that the adsorption of SP inhibitor molecules on the surface of carbon steel was a complicated process that involved both chemical and physical adsorption. This means that between −20 and −40 kJ mol−1, physisorption and chemisorption occur together.87
3.3. FE-SEM investigation
The surface morphology of carbon steel was analyzed to explore the inhibitory properties of SP extract. Fig. 10 shows FE-SEM images of the carbon steel surface after being in 0.5 N HCl for 72 hours with and without 2.5 g L−1 of SP extract inhibitor. There were deep ravines and large pits on the carbon steel surface without SP, which was significantly different from the polished surface (Fig. 10a). As shown in Fig. 10b, this indicated severe corrosion of carbon steel in a 0.5 N HCl solution.88 However, it was clear that the carbon steel surface had a smoother shape when 2.5 g L−1 of SP was added.89 This differs from the polished surface morphology; in the presence of SP extract, the inhibitor forms a dispersed aggregative layer across the entire steel surface, indicating effective adsorption. These events also showed that the inhibitor molecules adsorbed to the carbon steel's surface and formed a protective film. As seen in Fig. 10c, this blocked the corrosion that the acidic solution was causing.3.4. Adsorption of SP analysis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H–) stretching vibration due to asymmetric stretching of alkene. Also, the peak intensity at 2962.3 cm−1 corresponded to (–C–H) stretching vibration due to alkanes. It was also strong at 2962.3 cm−1 for a stretching C–H sp3 of hydrocarbon chains. So, it was seen to be weak at 2424.4 cm−1 for stretching of methylene (–CH2–) groups symmetrically. In addition, a peak at 2054.4 cm−1 was the corresponding aldehyde peak due to C
C–H–) stretching vibration due to asymmetric stretching of alkene. Also, the peak intensity at 2962.3 cm−1 corresponded to (–C–H) stretching vibration due to alkanes. It was also strong at 2962.3 cm−1 for a stretching C–H sp3 of hydrocarbon chains. So, it was seen to be weak at 2424.4 cm−1 for stretching of methylene (–CH2–) groups symmetrically. In addition, a peak at 2054.4 cm−1 was the corresponding aldehyde peak due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) stretching vibration. Moreover, the attained peak at 1672.6 cm−1 is behind carbonyl (C
stretching vibration. Moreover, the attained peak at 1672.6 cm−1 is behind carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibrations. Also, the strengths seen at 1596.3 cm−1 and 1485.5 cm−1 were due to amines' N–H bending vibrations and amides' –C–N stretching vibrations. However, the peak at 1272.5 cm−1 was associated with (–CH2) out-of-plane deformation vibration modes. Meanwhile, the peak at 1110.4 cm−1 corresponds to a symmetric stretch of the –(C–O–C) bond. Another critical point is that the (C
O) stretching vibrations. Also, the strengths seen at 1596.3 cm−1 and 1485.5 cm−1 were due to amines' N–H bending vibrations and amides' –C–N stretching vibrations. However, the peak at 1272.5 cm−1 was associated with (–CH2) out-of-plane deformation vibration modes. Meanwhile, the peak at 1110.4 cm−1 corresponds to a symmetric stretch of the –(C–O–C) bond. Another critical point is that the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H) bending of trans-disubstituted olefin groups causes the peak at 801.3 cm−1. So, the peak at 604.8 cm−1 is due to carbon–hydrogen bonds, which can be put into two groups: symmetric in-plane and out-of-plane deformation bending modes. It can be seen in Fig. 11 that the ATR-FTIR spectra of the protective film formed on the carbon steel surface showed all the characteristic peaks of the SP pure inhibitor, indicating that the inhibitor adsorbed on the carbon steel surface. From the obvious peaks, function groups such as C
C–H) bending of trans-disubstituted olefin groups causes the peak at 801.3 cm−1. So, the peak at 604.8 cm−1 is due to carbon–hydrogen bonds, which can be put into two groups: symmetric in-plane and out-of-plane deformation bending modes. It can be seen in Fig. 11 that the ATR-FTIR spectra of the protective film formed on the carbon steel surface showed all the characteristic peaks of the SP pure inhibitor, indicating that the inhibitor adsorbed on the carbon steel surface. From the obvious peaks, function groups such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH appear on the carbon steel surface. Besides, there were small changes, and some frequencies of weak function groups were shifted significantly in the case of SP-carbon steel, as shown in Fig. 11. The above findings clearly illustrate that the molecules of the SP extract enhanced the inhibition of corrosion by the adsorption process and hence retarded the corrosion.
O and NH appear on the carbon steel surface. Besides, there were small changes, and some frequencies of weak function groups were shifted significantly in the case of SP-carbon steel, as shown in Fig. 11. The above findings clearly illustrate that the molecules of the SP extract enhanced the inhibition of corrosion by the adsorption process and hence retarded the corrosion.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and n–π* transition from C
N and n–π* transition from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups. After metal exposure, the spectrum exhibits the same absorption peaks with small modifications. The absorption peaks showed a minor hypsochromic shift. Changes in the absorption band confirm the combination of SP and Fe2+ ions formed during the corrosion reaction.90
O functional groups. After metal exposure, the spectrum exhibits the same absorption peaks with small modifications. The absorption peaks showed a minor hypsochromic shift. Changes in the absorption band confirm the combination of SP and Fe2+ ions formed during the corrosion reaction.90
3.5. Computational results
The FMOs research is further substantiated by the density of states (DOS) analysis shown in Fig. 15, which quantifies the number of states within a unit energy interval of the system.100,101 The density of states (DOS) quantifies the number of states within a unit energy interval and is crucial for analyzing fragment interactions and their contributions to molecular orbitals (MOs).
The analysis of FMOs, their percentage composition, and the density of states around HOMOs and LUMOs demonstrated that differing electron-withdrawing strengths of acceptor components may substantially modify the electron density distribution on molecular orbitals. Positive and negative energy values on the density of states graph indicate bonding and antibonding orbitals, respectively. A value of 0 indicates the absence of contact between bonding orbitals. DOS computations validate the FMO results. The distribution patterns of HOMO and LUMO, as well as the energy gap between them, fluctuate based on the acceptor groups. Thus, DOS spectra can quantitatively evaluate the existence of contacts.
Additionally, these frontier molecular orbitals (FMOs) are employed to examine global reactivity descriptors and analyze the properties of the investigated compounds 1–5.102 This information can be used to understand the relationship between energy, structure and reactivity characteristics of complexes such as IP (Ionization Potential), EA (Electron Affinity), electronegativity (χ), hardness (η), softness (s), and dipole moment (μ). The global reactivity descriptors utilizing Koopman's theorem103,104 can be described as follows,
| IP (ionization potential) = −EHOMO | (4) |
| EA (electron affinity) = −ELUMO | (5) |
 | (6) |
 | (7) |
 | (8) |
 | (9) |
A low electronegativity (χ) value often signifies the inhibitors' tendency to donate electrons to the metal surface. In contrast, inhibitor molecules with elevated electronegativity have an increased propensity to receive an electron from the steel contact, a process known as “back-donation”, thereby forming a stronger connection with the metal surface.105 Table 4 indicates that all species have elevated electronegativity, indicating their tendency to participate in back-donation and establish stronger connections with the metal contact. Moreover, softness (s) and hardness (η) function as metrics for the stability and reactivity of inhibitor compounds. Soft molecules have enhanced protective properties relative to hard molecules due to their efficient electron transfer to the contacting metal surface during adsorption, rendering them more effective corrosion inhibitors.106
| Compounds | HOMO (eV) | LUMO (eV) | Eg (eV) | η (eV) | μ (debye) | S (eV) | χ (eV) | ΔEback-donation (eV) |
|---|---|---|---|---|---|---|---|---|
| 1 | −7.34 | −0.89 | 6.44 | 3.22 | 1.43 | 0.31 | −4.11 | −0.80 |
| 2 | −6.65 | −0.07 | 6.58 | 3.29 | 1.86 | 0.30 | −3.36 | −0.82 |
| 3 | −6.15 | −1.40 | 4.75 | 2.37 | 4.61 | 0.42 | −3.77 | −0.59 |
| 4 | −6.63 | −0.63 | 6.00 | 3.00 | 4.05 | 0.33 | −3.63 | −0.75 |
| 5 | −7.35 | −0.04 | 7.31 | 3.65 | 1.38 | 0.27 | −3.69 | −0.91 |
As shown in Table 4, compound 3 demonstrates superior inhibitory properties attributed to its higher σ value and lower η value compared to the other species. Additionally, the inhibitor's ability to donate or accept electrons is evaluated by the fraction of ΔEback-donation. Moreover, when the ΔEback-donation value is negative, it signifies that the molecule withdraws an electron from the metal surface atoms. In response, the metal atoms, now electron-deficient, tend to reclaim an electron from the molecule through back-donation. This dynamic exchange is energetically favourable and leads to a stronger interaction between the molecule and the metal surface.107 As illustrated in Table 4, the ΔEback-donation values for all compounds 1–5 are negative, suggesting that back-donation is a preferred mechanism for these species to establish a robust bond with the metal interface. The dipole moment serves as another critical parameter within the corrosion protection prognosis approach. An increase in dipole moment leads to enhanced adsorption of the molecule onto the metallic surface and a corresponding increase in distortion energy.108 Consequently, a higher dipole moment generally correlates with improved corrosion inhibition. As shown in Table 4, compound 3 exhibits a significantly higher dipole moment (4.61 debye) compared to the other compounds (compound 1: 1.43 debye, compound 2: 1.86 debye, compound 4: 4.05 debye, compound 5: 1.38 debye). The superior inhibition efficiency of the SP extract at 2.5 g L−1 (89.56%) correlates well with the presence of compound 3 as the dominant constituent. Its favorable electronic structure including the presence of electronegative atoms (N and O) supports coordination with Fe atoms, forming a protective layer that reduces corrosion, the lowest ΔE value (indicating enhanced reactivity and adsorption tendency) that facilitates electron transfer between the inhibitor and the metal surface and the highest dipole moment (suggesting stronger interaction with the metal surface) that enhances adsorption by promoting orientation toward the charged metal surface, provides a molecular-level explanation for the observed electrochemical behavior.109 This suggests that compound 3 has a greater affinity for adsorption on the metal surface, potentially resulting in enhanced corrosion protection.
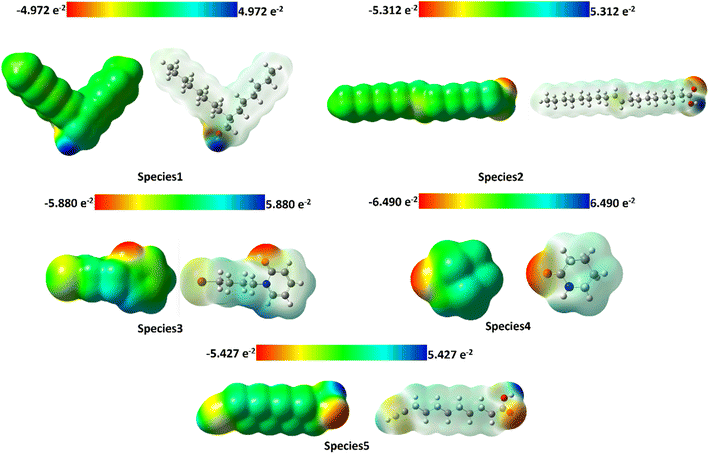
Compound 1 exhibits a red colour surrounding the oxygen atom, a blue colour over the hydrogen of the hydroxyl group, and a green colour enveloping the entire decane carbon chain. Compound 2 displays a red color surrounding the oxygen atom, a blue color over the hydrogen of the hydroxyl group, and a green color encompassing the entire octadec-9-ene carbon chain. Compound 3 exhibits a red color surrounding the oxygen atom, a blue color over the hydrogen of the piperdone ring, and a green color enveloping the entire bromobutyl chain.
Compound 4 exhibits a red color surrounding the oxygen atom, a blue color over the “NH” group that is azane of the piperdone ring, and a green color enveloping the entire piperidine ring. Compound 5 displays a red color surrounding the oxygen atom, a blue color over the hydrogen of the hydroxyl group, and a green color encompassing the entire tetradecane chain see Fig. 16. The electronegative potential values for compound 1, compound 2, compound 3, compound 4 and compound 5 are −4.972 × 10−2, −5.312 × 10−2, −5.880 × 10−2, −6.490 × 10−2 and −5.427 × 10−2 respectively. MEP maps indicate that compound 4 possesses the highest electronegative potential compared to the other species.
3.6. Inhibition mechanism of SP extract
Based on experimental observations (EIS, ATR-FTIR, FE-SEM/EDX) and theoretical calculations (DFT), we propose the following inhibition mechanism for SP extract on carbon steel in 0.5 M HCl solution:In the absence of the inhibitor, corrosive species such as H+, Cl−, and H2O can easily reach the steel surface, leading to severe corrosion.120 When Sp extract is introduced into the medium, it contains several electron-rich compounds, including 2-piperidinone, N-[4-bromo-n-butyl], 9-octadecenoic acid and tetradecyl undec-10-ynoate. These compounds possess heteroatoms (N, O) and conjugated systems, enabling them to donate electron pairs to vacant d-orbitals of Fe atoms on the carbon steel surface. This leads to the formation of: Fe–N and Fe–O coordination bonds, confirmed by ATR-FTIR analysis showing characteristic peaks corresponding to these bonds after immersion in SP-containing solution. A protective adsorbed layer that physically blocks aggressive ions (H+, Cl−) from reaching the metal surface.121 From PDP results: The Ecorr shift was less than ±85 mV, indicating mixed-type inhibition, where both anodic dissolution and cathodic hydrogen evolution are suppressed. DFT calculations further support this behavior by showing that key compounds (especially compound 3) exhibit strong reactivity toward both oxidation and reduction reactions. The variation in Rct and CPEdl with SP concentration fits well with the Langmuir isotherm, suggesting: monolayer adsorption of inhibitor molecules on the steel surface and no lateral interactions between adsorbed molecules. Maximum surface coverage at 2.5 g L−1, consistent with the highest inhibition efficiency observed. FE-SEM/EDX analysis showed a smooth, uniform layer formed on the steel surface in the presence of SP extract. EDX spectra confirmed the presence of N and O from the extract on the inhibited surface, reinforcing the adsorption mechanism. DFT results highlighted compound 3 (1-(4-bromobutyl)piperidin-2-one) as the most active component due to: highest HOMO energy → strong electron donating capability, lowest LUMO energy → high electron accepting tendency, smallest energy gap (ΔE) → enhanced reactivity and High dipole moment → better orientation towards the metal surface. These properties align with the observed strong adsorption and high inhibition efficiency.
Table 5 presents data on plant extracts, as reported in the literature, along with their inhibition efficiencies.122–125 The inhibition efficiency of SP extract against carbon steel alloy corrosion was comparable and exceeded that of several extracts in literature. The inclusion of ‘method of extraction’ and ‘plant part used’ highlights the importance of preparation techniques and source material in determining the efficacy of plant-based corrosion inhibitors. For instance, the Soxhlet extraction method used in this study ensures a higher yield of active compounds compared to simple maceration or soaking methods, potentially contributing to the superior inhibition efficiency observed for SP extract. In this study, SP extract achieved higher efficiency (PDP = 89.56%) than previously reported extracts (76.5%, 77.84%, 82.0%, and 87.89%). These results indicate that this research builds logically on prior studies of plant extracts as green inhibitors.
| Extract name | Extraction method | Plant part used | Medium | Substrate | Concentration | IE % | Ref. |
|---|---|---|---|---|---|---|---|
| Dactyloctenium aegyptium extract | — | Whole plant | 0.5 M HCl | Carbon steel | 800 ppm | PDP: 76.5 | 122 |
| EIS: 70.6 | |||||||
| Kopsia teoi extracts (KTDAE) | Maceration | Aerial parts | 0.5 M HCl | Mild steel | 500 ppm | PDP: 77.84 | 123 |
| EIS: 87.04 | |||||||
| Heat-assisted extracted Melilotus officinalis (HAE) | Heat-assisted extraction | Leaves | 0.5 M HCl | Carbon steel | 800 ppm | PDP: 82.0 | 124 |
| EIS: 82.3 | |||||||
| Cleome arabica L. extract | Soaking | Whole plant | 0.5 M HCl | AISI 1045 carbon steel | 1.0 g L−1 | PDP: 87.89 | 125 |
| EIS: 77.8 | |||||||
| Safflower extract (SP) | Soxhlet extraction | Whole plant (flower/leaves) | 0.5 M HCl | Carbon steel | 2.5 g L−1 | PDP: 89.56 | This study |
| EFM: 84.07 | |||||||
| EIS: 84.65 |
4. Conclusion
The findings of this study demonstrate that safflower plant (SP) extract is a highly effective green corrosion inhibitor for carbon steel in acidic environments. The extract significantly reduces the corrosion rate and enhances inhibition efficiency, particularly at higher concentrations (up to 2.5 g L−1). The mechanism of inhibition is attributed to both physical and chemical adsorption, as supported by experimental and computational data. The Langmuir adsorption model successfully described the inhibitor's adsorption behavior. Theoretical studies using density functional theory (DFT) further elucidated the interaction between key active compounds in the SP extract and the metal surface, confirming their role in inhibiting corrosion. Computational modeling and analysis of the five studied complexes (compounds 1–5) reveal significant insights into their corrosion inhibition potential. Among them, compound 3 stands out as the most effective inhibitor, demonstrated by its highest HOMO energy, lowest LUMO energy, and smallest energy gap (ΔE), which indicate strong electron-donating capability and superior adsorption onto metal surfaces. Density of States (DOS) analysis and global reactivity descriptors, including ionization potential, electron affinity, and dipole moment, further confirm compound 3's superior inhibitory properties. While Molecular Electrostatic Potential (MEP) mapping highlights compound 4's highest electronegative potential, compound 3's optimal electronic structure and reactivity make it the most promising candidate for corrosion protection. The SP extract provides a promising, eco-friendly alternative to conventional synthetic inhibitors, making it suitable for applications in industries where acidic solutions are used.Data availability
The datasets generated during and/or analyzed during the current study are available from the author on reasonable request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are thankful to the Deanship of Graduate Studies and Scientific Research at University of Bisha for supporting this work through the Fast-Track Research Support Program.References
- Q. A. Yousif, Z. Fadel, A. M. Abuelela, E. H. Alosaimi, S. Melhi and M. A. Bedair, RSC Adv., 2023, 13, 13094–13119 RSC.
- K. Sayin, H. Jafari and F. Mohsenifar, J. Taiwan Inst. Chem. Eng., 2016, 68, 431–439 CrossRef CAS.
- A. A. Bahraq, I. B. Obot, M. A. Al-Osta and M. Ibrahim, Constr. Build. Mater., 2024, 412, 134808 CrossRef CAS.
- M. Baladi, Q. A. Yousif, M. Valian and M. Salavati-Niasari, J. Alloys Compd., 2022, 896, 163032 CrossRef CAS.
- S. Abd El Wanees, M. G. A. Saleh, M. I. Alahmdi, N. H. Elsayed, M. M. Aljohani, M. Abdelfattah, K. A. Soliman, M. Lotfy Alalati and S. S. Elyan, Mater. Chem. Phys., 2024, 311, 128484 CrossRef.
- H. Jafari, F. Mohsenifar and K. Sayin, J. Taiwan Inst. Chem. Eng., 2016, 64, 314–324 CrossRef CAS.
- Z. Fadel and Q. A. Yousif, J. Phys.:Conf. Ser., 2020, 1664, 012093 CrossRef CAS.
- M. Gab-Allah, M. Bedair, A. Elged, S. Soliman, E. Badr and M. Bakr, Egypt. J. Chem., 2023, 66, 2013–2031 Search PubMed.
- B. Yan, J. Lv, S. Zhou, Z. Wu, X. Liu, B. Li, Q. Gao, W. Wu and G. Chen, Biomass Bioenergy, 2024, 184, 107198 CrossRef CAS.
- H. Jafari, K. Akbarzade and I. Danaee, Arabian J. Chem., 2019, 12, 1387–1394 CrossRef CAS.
- M. N. Majeed and Q. A. Yousif, J. Nanostruct., 2022, 12, 616–624 CAS.
- A. Fahmy, M. El Sabbagh, M. Bedair, A. Gangan, M. El-Sabbah, S. M. El-Bahy and J. F. Friedrich, J. Adhes. Sci. Technol., 2021, 35, 1734–1751 CrossRef CAS.
- Z. Song, L. Liu, M. Z. Guo, H. Cai, Q. Liu, S. Donkor and H. Zhao, Case Stud. Constr. Mater., 2024, 20, e02992 Search PubMed.
- H. Jafari, E. Ameri, M. Rezaeivala, A. Berisha and J. Halili, J. Phys. Chem. Solids, 2022, 164, 110645 CrossRef CAS.
- P. D. Desai, C. B. Pawar, M. S. Avhad and A. P. More, Vietnam J. Chem., 2023, 61, 15–42 CrossRef CAS.
- S. Melhi, M. A. Bedair, E. H. Alosaimi, A. A. O. Younes, W. H. El-Shwiniy and A. M. Abuelela, RSC Adv., 2022, 12, 32488–32507 RSC.
- A. Royani, M. Hanafi, R. Haldhar and A. Manaf, J. Engine Res., 2024, 12(3), 321–327 CrossRef.
- Q. A. Yousif, A. Abdel Nazeer, Z. Fadel, L. A. Al-Hajji and K. Shalabi, ACS Omega, 2024, 9, 14153–14173 CrossRef CAS PubMed.
- E. S. Gad, M. A. Abbas, M. A. Bedair, O. E. El-Azabawy and S. M. Mukhtar, Sci. Rep., 2023, 13, 1–27 CrossRef PubMed.
- B. Benzidia, M. Barbouchi, M. Rehioui, H. Hammouch, H. Erramli and N. Hajjaji, J. King Saud Univ., Sci., 2023, 35, 102986 CrossRef.
- H. Jafari, I. Danaee, H. Eskandari and M. RashvandAvei, J. Mater. Sci. Technol., 2014, 30, 239–252 CrossRef CAS.
- T. N. Tran, T. T. Tran, D. S. Jo, P. S. N. Dong, V. K. Nguyen, T. L. Huynh and N. Nguyen Dang, J. Ind. Eng. Chem., 2024, 135, 175–187 CrossRef CAS.
- Q. A. Yousif, IOP Conf. Ser.: Earth Environ. Sci., 2021, 790, 012072 CrossRef.
- Q. A. Yousif, M. A. Bedair, Z. Fadel, F. Al-Odail and A. M. Abuelela, Inorg. Chem. Commun., 2024, 164, 112454 CrossRef CAS.
- H. Ferkous, A. Sedik, A. Delimi, R. Redjemia, K. Abdesalem, C. Boulechfar, A. Abdennouri, A. Madaci, M. Berredjem, A. Boublia, M. Sajid Ali, B. H. Jeon, K. Kumar Yadav and Y. Benguerba, J. Mol. Liq., 2024, 394, 123781 CrossRef CAS.
- Z. Fadel and Q. A. Yousif, IOP Conf. Ser.: Earth Environ. Sci., 2021, 790, 012074 CrossRef.
- B. Weng, M. Y. Qi, C. Han, Z. R. Tang and Y. J. Xu, ACS Catal., 2019, 9, 4642–4687 CrossRef CAS.
- R. Lachhab, M. Galai, A. Ech-chebab, R. A. Belakhmima, M. E. Touhami and I. Mansouri, Ceram. Int., 2024, 50, 4282–4295 CrossRef CAS.
- B. Benzidia, M. Barbouchi, R. Hsissou, M. Zouarhi, H. Erramli and N. Hajjaji, Curr. Res. Green Sustainable Chem., 2022, 5, 100299 CrossRef CAS.
- N. Alipanah, A. Dehghani, A. Hossein Jafari Mofidabadi, M. Salamati and B. Ramezanzadeh, J. Mol. Liq., 2024, 393, 123554 CrossRef CAS.
- Y. Di, X. Li, Z. Chen, X. Yin, Y. Chen, Y. Liu and W. Yang, J. Mol. Struct., 2022, 1268, 133737 CrossRef CAS.
- H. Mahmoudi-Moghaddam, M. Amiri, H. Akbari Javar, Q. A. Yousif and M. Salavati-Niasari, Arabian J. Chem., 2022, 15, 103988 CrossRef CAS.
- Y. Meng, S. Li and Z. Zhang, Heliyon, 2024, 10, e24688 CrossRef CAS PubMed.
- J. xin Song, H. jie Zhang, M. hong Niu, Y. zhu Guo and H. ming Li, Ind. Crops Prod., 2024, 214, 118443 CrossRef.
- M. Valian, F. Soofivand, A. Khoobi, Q. A. Yousif and M. Salavati-Niasari, Arabian J. Chem., 2022, 16, 104401 CrossRef.
- J. Wang, Y. Cao, J. Xue, X. Zhang, Y. Liang, K. Chen, C. Huang and D. Zheng, Corros. Sci., 2024, 231, 111994 CrossRef CAS.
- M. H. Shahini, M. Ramezanzadeh and B. Ramezanzadeh, Colloids Surf., A, 2022, 634, 127990 CrossRef CAS.
- A. Kumar, J. Kumar and Nishtha, Mater. Today: Proc., 2022, 64, 141–146 CAS.
- D. S. Chauhan, M. A. Quraishi, A. A. Sorour and C. Verma, J. Pet. Sci. Eng., 2022, 215, 110695 CrossRef CAS.
- A. M. Abuelela, J. Kaur, A. Saxena, M. A. Bedair, D. K. Verma and E. Berdimurodov, Sci. Rep., 2023, 13, 19367 CrossRef CAS PubMed.
- K. Zakaria, M. A. Abbas and M. A. Bedair, J. Mol. Liq., 2022, 352, 118689 CrossRef CAS.
- B. Liao, Z. Luo, S. Wan and L. Chen, J. Ind. Eng. Chem., 2023, 117, 238–246 CrossRef CAS.
- Q. A. Yousif, Z. Fadel, A. M. Abuelela, E. H. Alosaimi, S. Melhi and M. A. Bedair, RSC Adv., 2023, 13, 13094–13119 RSC.
- M. Galai, K. Dahmani, O. Kharbouch, M. Rbaa, N. Alzeqri, L. Guo, A. A. AlObaid, A. Hmada, N. Dkhireche, E. Ech-chihbi, M. Ouakki, M. E. Touhami and I. Warad, J. Phys. Chem. Solids, 2024, 184, 111681 CrossRef CAS.
- H. Jafari and E. Ameri, Anti-Corros. Methods Mater., 2024, 71, 632–639 CrossRef CAS.
- R. Aslam, G. Serdaroglu, S. Zehra, D. Kumar Verma, J. Aslam, L. Guo, C. Verma, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2022, 348, 118373 CrossRef CAS.
- H. Ahchouch, M. El house, A. H. Al-Moubaraki, E. A. Noor, A. Hadfi, A. Driouiche, L. Bammou, M. Belkhaouda, R. Salghi, M. Chafiq, A. Chaouiki and Y. G. Ko, Arabian J. Chem., 2024, 17, 105593 CrossRef CAS.
- O. Ninich, A. Et-tahir, K. Kettani, M. Ghanmi, J. Aoujdad, S. El Antry, M. Ouajdi and B. Satrani, J. Ethnopharmacol., 2022, 285, 114889 CrossRef.
- M. Bedair, M. Metwally, S. Soliman, A. Al-Sabagh, A. Salem and T. Mohamed, Al-Azhar Bull. Sci., 2015, 26, 1–14 CrossRef.
- R. Haldhar, S.-C. Kim, D. Prasad, M. A. A. Bedair, I. Bahadur, S. Kaya, O. Dagdag and L. Guo, J. Mol. Struct., 2021, 1242, 130822 CrossRef CAS.
- A. E. A. S. Fouda, A. A. Nazeer and A. Saber, J. Korean Chem. Soc., 2014, 58, 160–168 CrossRef CAS.
- M. Nasibi, D. Zaarei, G. Rashed and E. Ghasemi, Chem. Eng. Commun., 2013, 2003, 367–378 CrossRef.
- M. N. Majeed, Q. A. Yousif and M. A. Bedair, ACS Omega, 2022, 7, 29850–29857 CrossRef CAS.
- A. Gangan, M. ElSabbagh, M. A. Bedair, H. M. Ahmed, M. El-Sabbah, S. M. El-Bahy and A. Fahmy, Arabian J. Chem., 2021, 14, 103391 CrossRef CAS.
- M. A. Bedair, A. M. Abuelela, S. Melhi, Q. A. Yousif, V. V. Chaban and E. H. Alosaimi, Mater. Chem. Phys., 2024, 312, 128644 CrossRef CAS.
- Semichem Inc., Semichem Inc:GaussView 6, 2016 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, 2016.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- M. J. Frisch, J. A. Pople and J. S. Binkley, J. Chem. Phys., 1984, 80, 3265–3269 CrossRef CAS.
- A.-R. A. Allouche, J. Comput. Chem., 2011, 32, 174–182 CrossRef CAS PubMed.
- M. A. Bedair, A. M. Abuelela, W. M. Zoghaib and T. A. Mohamed, J. Mol. Struct., 2021, 1244, 130927 CrossRef CAS.
- F. Bertin, G. R. Joshi, J. Kittel, C. Sagnard, F. Ropital, M. Martinez, C. Bosch and K. Wolski, Corros. Sci., 2024, 227, 111778 CrossRef CAS.
- M. A. Al-Masoud, M. M. Khalaf, I. M. A. Mohamed, K. Shalabi and H. M. Abd El-Lateef, J. Ind. Eng. Chem., 2022, 112, 398–422 CrossRef CAS.
- T. K. Sarkar, M. Yadav and I. B. Obot, Chem. Eng. J., 2022, 431, 133481 CrossRef CAS.
- H. Yan, S. Dai, P. Liu and Y. Zhu, Corros. Sci., 2024, 227, 111750 CrossRef CAS.
- M. A. Bedair, Q. A. Yousif, Z. Fadel, S. Melhi, F. A. Al-Odail and A. M. Abuelela, J. Mol. Struct., 2025, 1328, 141282 CrossRef CAS.
- M. A. Bedair, Results Surf. Interfaces, 2025, 19, 100511 CrossRef.
- A. M. Ashmawy, R. Said, I. A. Naguib, B. Yao and M. A. Bedair, ACS Omega, 2022, 7, 17849–17860 CrossRef CAS.
- K. Rasheeda, N. P. Swathi, V. D. P. Alva, S. Samshuddin, T. A. Aljohani, I. Baig, F. Y. Alomari and A. H. Alamri, S. Afr. J. Chem. Eng., 2024, 48, 80–94 Search PubMed.
- N. A. Odewunmi, M. A. J. Mazumder and S. A. Ali, J. Mol. Liq., 2022, 360, 119431 CrossRef CAS.
- M. A. Bedair, H. M. Elaryian, A. H. Bedair, R. M. Aboushahba, A. El-Aziz and S. Fouda, Inorg. Chem. Commun., 2023, 148, 110304 CrossRef CAS.
- M. A. Abbas, E. I. Arafa, M. A. Bedair, A. S. Ismail, O. E. El-Azabawy, S. A. Baker and H. I. Al-Shafey, Arabian J. Sci. Eng., 2023, 48, 7463–7484 CrossRef CAS.
- M. A. Bedair, Inorg. Chem. Commun., 2024, 167, 112693 CrossRef CAS.
- H. M. Elaryian, M. A. Bedair, A. H. Bedair, R. M. Aboushahba and A. E.-A. S. Fouda, RSC Adv., 2022, 12, 29350–29374 RSC.
- M. A. Bedair, E. H. Alosaimi and S. Melhi, J. Adhes. Sci. Technol., 2021, 37, 1–31 Search PubMed.
- H. Zhu, Y. Huo, W. Wang, X. He, S. Fang and Y. Zhang, Process Saf. Environ. Prot., 2021, 148, 624–635 CrossRef CAS.
- M. A. Bedair, S. A. Soliman and M. S. Metwally, J. Ind. Eng. Chem., 2016, 41, 10–22 CrossRef CAS.
- R. Kellal, D. Benmessaoud Left, Z. S. Safi, A. Thoume, N. A. Wazzan, O. S. AL-Qurashi and M. Zertoubi, Mater. Chem. Phys., 2024, 314, 128846 CrossRef CAS.
- R. Lei, S. Deng, Y. Qiang, D. Xu, G. Du, D. Shao and X. Li, Colloids Surf., A, 2024, 687, 133358 CrossRef CAS.
- B. lan Lin, X. xin Zhou, T. hu Duan, C. Zhao, J. hao Zhu and Y. ye Xu, Arabian J. Chem., 2024, 17(1), 105410 CrossRef.
- L. Cui, W. Kang, H. You, J. Cheng and Z. Li, J. Bio-Tribo. Corros., 2021, 7, 1–14 CrossRef.
- T. Nesane, N. E. Madala, M. M. Kabanda, L. C. Murulana and I. Bahadur, Colloids Surf., A, 2023, 667, 131405 CrossRef CAS.
- S. Lamghafri, W. Daoudi, A. El Aatiaoui, O. Dagdag, H. Kim, A. Berisha, W. B. W. Nik, A. J. Obaidullah, K. K. Yadav, A. Zarrouk and A. Lamhamdi, J. Mol. Struct., 2024, 1306, 137924 CrossRef CAS.
- B. Tamilselvi, D. S. Bhuvaneshwari, S. Padmavathy and P. B. Raja, J. Mol. Liq., 2022, 359, 119359 CrossRef CAS.
- M. A. Abbas and M. A. Bedair, Z. Phys. Chem., 2019, 233, 225–254 CrossRef CAS.
- S. S. Alarfaji, I. H. Ali, M. Z. Bani-Fwaz and M. A. Bedair, Molecules, 2021, 26, 3183 CrossRef CAS PubMed.
- M. A. Abbas, E. I. Arafa, E. S. Gad, M. A. Bedair, O. E. El-Azabawy and H. I. Al-Shafey, Inorg. Chem. Commun., 2022, 143, 109758 CrossRef CAS.
- M. A. Abbas, M. A. Bedair, O. E. El-Azabawy, E. S. Gad, M. A. Abbas, M. A. Bedair, O. E. El-Azabawy and E. S. Gad, ACS Omega, 2021, 6, 15089–15102 CrossRef CAS PubMed.
- H. M. Elaryian, M. A. Bedair, A. H. Bedair, R. M. Aboushahba and A. E.-A. S. Fouda, J. Mol. Liq., 2022, 346, 118310 CrossRef CAS.
- M. A. Gebril, M. A. Bedair, S. A. Soliman, M. F. Bakr and M. B. I. Mohamed, J. Mol. Liq., 2022, 349, 118445 CrossRef CAS.
- A. Kokalj, Corros. Sci., 2021, 180, 109016 CrossRef CAS.
- M. A. Deyab, Q. Mohsen and O. A. A. El-Shamy, Colloids Surf., A, 2024, 702, 135024 CrossRef.
- I. Lukovits, E. Kálmán and F. Zucchi, Corrosion, 2001, 57, 3–8 CrossRef CAS.
- M. A. Bedair, H. M. Elaryian, E. S. Gad, M. Alshareef, A. H. Bedair, R. M. Aboushahba and A. S. Fouda, RSC Adv., 2023, 13, 478–498 RSC.
- H. M. Abd El-Lateef, K. Shalabi and A. H. Tantawy, New J. Chem., 2020, 44, 17791–17814 RSC.
- M. Goyal, H. Vashist, S. Kumar, I. Bahadur, F. Benhiba and A. Zarrouk, J. Mol. Liq., 2020, 315, 113705 CrossRef CAS.
- A. Dehghani, A. H. Mostafatabar, G. Bahlakeh and B. Ramezanzadeh, J. Mol. Liq., 2020, 316, 113914 CrossRef CAS.
- M. A. Bedair, A. M. Abuelela, M. Alshareef, M. Owda and E. M. Eliwa, RSC Adv., 2023, 13, 186–211 RSC.
- S. S. Malhotra, M. Kumar, M. K. Gupta and A. Ansari, Mater. Today Commun., 2024, 39, 109208 CrossRef CAS.
- S. S. Malhotra, M. Ahmed, M. Kumar, M. A. Amin, S. M. El-Bahy, Z. M. El-Bahy, R. K. Mohapatra and A. Ansari, Mater. Today Commun., 2024, 41, 110182 CrossRef CAS.
- M. A. Bedair, S. A. Soliman, M. A. Hegazy, I. B. Obot and A. S. Ahmed, J. Adhes. Sci. Technol., 2019, 33, 1139–1168 CrossRef CAS.
- M. Govindarajan, M. Karabacak, S. Periandy and D. Tanuja, Spectrochim. Acta, Part A, 2012, 97, 231–245 CrossRef CAS PubMed.
- A. M. Abuelela, M. A. Bedair, E. S. Gad, Y. F. El-Aryan, W. A. A. Arafa, A. K. Mourad, H. Nady and S. Eid, Sci. Rep., 2024, 14, 13310 CrossRef CAS PubMed.
- E. A. Badr, M. A. Bedair and S. M. Shaban, Mater. Chem. Phys., 2018, 219, 444–460 CrossRef CAS.
- M. K. Awad, M. S. Metwally, S. A. Soliman, A. A. El-Zomrawy and M. A. bedair, J. Ind. Eng. Chem., 2014, 20, 796–808 CrossRef CAS.
- M. A. Bedair, M. M. B. El-Sabbah, A. S. Fouda and H. M. Elaryian, Corros. Sci., 2017, 128, 54–72 CrossRef CAS.
- A. M. Abuelela, M. A. Bedair, W. M. Zoghaib, L. D. Wilson and T. A. Mohamed, J. Mol. Struct., 2021, 1230, 129647 CrossRef CAS.
- M. A. Bedair, J. Mol. Liq., 2016, 219, 128–141 CrossRef CAS.
- M. A. A. Bedair, S. A. A. Soliman, M. F. Bakr, E. S. S. Gad, H. Lgaz, I.-M. M. Chung, M. Salama and F. Z. Alqahtany, J. Mol. Liq., 2020, 317, 114015 CrossRef CAS.
- M. Kumar, A. K. Talakkal, R. K. Mohapatra and A. Ansari, J. Mol. Model., 2023, 29, 336 CrossRef CAS.
- M. Ahmed, M. K. Gupta and A. Ansari, J. Mol. Model., 2023, 29, 358 CrossRef CAS.
- O. Yadav, M. Ansari and A. Ansari, Spectrochim. Acta, Part A, 2022, 278, 121331 CrossRef CAS PubMed.
- M. Kumar, M. Ansari and A. Ansari, Spectrochim. Acta, Part A, 2023, 284, 121774 CrossRef CAS PubMed.
- R. K. Mohapatra, M. Azam, P. K. Mohapatra, A. K. Sarangi, M. Abdalla, L. Perekhoda, O. Yadav, S. I. Al-Resayes, K. Jong-Doo, K. Dhama, A. Ansari, V. Seidel, S. Verma and M. K. Raval, J. King Saud Univ., Sci., 2022, 34, 102086 CrossRef.
- Monika and A. Ansari, Struct. Chem., 2023, 34, 825–835 CrossRef CAS.
- O. Yadav, M. Kumar, H. Mittal, K. Yadav, V. Seidel and A. Ansari, Front. Pharmacol., 2022, 13, 982484 CrossRef CAS.
- S. Raheem, T. Jan, A. Qayum, O. Yadav, M. Mustafa, A. Ansari, G. Mustafa Peerzada, S. K. Singh and M. Ahmad Rizvi, Polyhedron, 2023, 244, 116597 CrossRef CAS.
- M. Kumar, M. K. Gupta, M. Ansari and A. Ansari, Phys. Chem. Chem. Phys., 2024, 26, 4349–4362 RSC.
- H. Jafari, F. Mohsenifar and K. Sayin, Iran. J. Chem. Chem. Eng., 2018, 37, 85–103 CAS.
- H. Jafari and K. Sayin, J. Taiwan Inst. Chem. Eng., 2015, 56, 181–190 CrossRef CAS.
- Raghvi, A. Saxena, J. Kaur, E. Berdimurodov and D. K. Verma, J. Indian Chem. Soc., 2024, 101(10), 101317 CrossRef CAS.
- M. T. Muhammad, M. H. Hussin, M. H. Abu Bakar, T. S. Hamidon, S. S. Azahar, K. Awang, M. Litaudon and M. N. Azmi, Mater. Chem. Phys., 2024, 322, 129584 CrossRef CAS.
- M. Iranpour, A. Babaei and M. Bagherzadeh, Clean Chem. Eng., 2025, 100149 CrossRef.
- I. Ait Bouabdallah, F. Adjal, A. Zaabar, A. Benchikh, D. Guerniche, C. Ait Ramdane-Terbouche, A. P. Piedade, M. Z. Ibrahim, N. Nasrallah and A. Abdi, RSC Adv., 2024, 14, 36423–36436 RSC.
| This journal is © The Royal Society of Chemistry 2025 |