 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of recent developments in rare earth-doped nanophosphors for emerging technological applications
R. Kirana,
Nagaraj Kamathb,
M. I. Sayyedc,
Aljawhara H. Almuqrind and
Sudha D. Kamath *a
*a
aDepartment of Physics, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka, India. E-mail: sudha.kamath@manipal.edu
bDepartment of Humanities and Management, Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, Karnataka, India
cRenewable Energy and Environmental Technology Center, University of Tabuk, Tabuk, 47913, Saudi Arabia
dDepartment of Physics, College of Science, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh, 11671, Saudi Arabia
First published on 12th June 2025
Abstract
This article explores the synthesis of rare-earth-doped nanophosphors (RENPs), focusing on selecting optimal host materials like tungstates, vanadates, and aluminates to enhance luminescent properties. The incorporation of rare-earth elements, such as Eu3+, Tb3+, Dy3+, Sm3+, etc., significantly improves emission spectra and efficiency by utilizing their unique electronic configurations and interactions with the host lattice. Nanophosphors were synthesized through sol–gel, combustion, and microwave-assisted methods, resulting in materials with tunable photoluminescence, high luminous efficiency, and appropriate chromaticity for wide-bandgap lighting applications. The study further investigates upconversion nanophosphors (UCNPs), demonstrating exceptional attributes like long luminescence lifetimes, high sensitivity, low energy loss, and tunable emission spectra. These characteristics make UCNPs ideal for advanced sensing applications. Additionally, RENPs exhibit great potential in thermoluminescence dosimetry, providing accurate radiation dose measurements with minimal fading and energy-dependent responses, thus advancing both medical and environmental dosimetry.
1. Introduction
The domain of materials science and engineering is progressing at an accelerated pace, driven by the growing demand for materials that can meet the evolving needs of modern technology across diverse industrial, scientific, and commercial sectors.1–4 In material design, a key consideration is not only the material's versatility across various applications but also its ability to overcome the challenges encountered in real-world implementation.5–7 As a result, extensive global research is focused on developing novel materials that satisfy the rigorous demands of both everyday and cutting-edge technological applications.8 Fig. 1 displays the various applications of advanced materials in modern science.9Rare earth-doped phosphors are one such unique class of materials that have attracted considerable attention in materials science due to their exceptional ability to emit controlled light, alongside remarkable thermal and chemical stability.10 Over time, a wide variety of host materials for phosphors have been explored in the literature, each contributing distinct properties that enhance the performance and applicability of phosphor-based systems.11 The continued exploration of novel host matrices remains critical for advancing the efficiency and functionality of phosphors, particularly for their application in an expanding range of technological fields. In recent years, the integration of nanotechnology with phosphors has led to the synthesis of rare earth-doped nanophosphors (RENPs), which have quickly emerged as a potential candidate to enhance the performance of light-emitting diodes (LEDs).12 A nanophosphor (NP) is a luminescent material with a particle size typically ranging from 1 to 100 nanometers. Fig. 2 illustrates the volume of research articles published over the past 24 years within the domain of NPs.
 | ||
| Fig. 2 Publications per year with the keyword “nano phosphor” (Google Scholar, accessed April 30, 2025). | ||
It is important to note that the unique spectroscopic properties of RENPs arise not from the quantum size effects but from a combination of factors, including particle size, doping composition, site symmetry, dopant-ligand distance, crystalline phase, and the strength of coordination. Surface effects also play a significant role in determining their overall luminescent behaviour.13 Furthermore, the reduction of phosphor particle size to the nanoscale confers significant advantages, including superior optical, thermal, and mechanical properties. These enhanced characteristics make NPs central to the development of next-generation, energy-efficient lighting systems. For example, the quantum efficiency (QE) of LaSr2AlO5:1 mol% Sm3+ nanophosphor was measured at 94.49%, making it comparable to commercially available phosphors for LED applications.14 Additionally, the luminescent lifetime of the NPs spans a wide range, from a few microseconds and milliseconds to several seconds and even minutes, highlighting their potential for diverse lighting applications.15–18
Additionally, NPs have shown great promise in nanomedicine and biomedical imaging. RENPs exhibit unique properties, such as up-conversion, anti-Stokes shifts, high quantum efficiency, long luminescence lifetime, narrow emission spectra, and excellent colour purity, which make them ideal candidates for a range of medical applications.19,20 They are also employed as luminescent markers for diagnostics, therapeutic imaging, and biological labeling.21 Additionally, their ability to reduce UV-induced photo damage to biological samples and their resistance to photo-bleaching and photo-blinking offer distinct advantages for long-term imaging and monitoring of biological processes.22–24
In light of the growing importance of RENPs, this paper presents an extensive review of the literature, critically examining the various types of NPs, their synthesis, and applications across various fields. Through this review, we aim to provide a deeper understanding of the current state of research, explore the potential of these materials in diverse applications, and identify promising areas for future advancements in the development of RENP materials.
2. Synthesis of nanophosphors
In this section, we have explored various facets of NP synthesis, all of which are essential for optimizing the performance of the phosphor material.2.1 Selection of phosphor host
A critical factor in the fabrication of phosphors is the selection of an appropriate host material. An ideal host should create an environment conducive to the efficient incorporation of dopant ions, thereby enhancing the material's performance for its intended application. Depending on the specific requirements of the application, the host material must exhibit excellent thermal, optical, and structural stability. Below, we present a summary of some of the most widely used phosphor hosts. A list of some of the prominent phosphor hosts is mentioned in Fig. 3.2.2 Rare earth elements as the dopant
Rare earth (RE) elements constitute a group of 17 chemical elements in the periodic table, comprising the 15 lanthanides along with yttrium and scandium, which are included due to their analogous chemical behaviour.60 These elements predominantly exist in either the +2 or +3 oxidation states. RE ions exhibiting 4fN → 4fN transitions are characterized by superior coherence properties, highly resolved absorption lines, and long lifetimes. On the other hand, ions undergoing 4fN → 4fN−15d transitions demonstrate shorter lifetimes, broad absorption spectra spanning the ultraviolet to visible regions, and enhanced oscillator strengths.61 From the RE ions' spectroscopic properties, most RE elements are filled with one or more electrons in their outermost 4f orbital. Thus, different RE (trivalent/divalent ions) elements have abundant energy levels due to differences in their arrangement of 4f electrons. The spectroscopic characteristics of RE ions reveal that the majority of these elements possess one or more electrons occupying their outermost 4f orbitals. Consequently, both trivalent and divalent RE ions exhibit abundant energy levels, arising from the distinct configurations of their 4f electron arrangements.62,63 Table 1 depicts the list of RE elements along with basic information, including atomic number, electronic configuration, and various oxidation states.| Rare earth element | Atomic number | Symbol | Electronic configuration | Oxidation states |
|---|---|---|---|---|
| Scandium | 21 | Sc | [Ar]3d14s2 | +3 |
| Yttrium | 39 | Y | [Kr]4d15s2 | +3 |
| Lanthanum | 57 | La | [Xe]4f05d16s2 | +3 |
| Cerium | 58 | Ce | [Xe]4f15d16s2 | +3, +4 |
| Praseodymium | 59 | Pm | [Xe]4f36s2 | +3, +4 |
| Neodymium | 60 | Nd | [Xe]4f46s2 | +3, +4 |
| Promethium | 61 | Pr | [Xe]4f56s2 | +3 |
| Samarium | 62 | Sm | [Xe]4f66s2 | +2, +3 |
| Europium | 63 | Eu | [Xe]4f76s2 | +2, +3 |
| Gadolinium | 64 | Gd | [Xe]4f75d16s2 | +3 |
| Terbium | 65 | Tb | [Xe]4f96s2 | +3, +4 |
| Dysprosium | 66 | Dy | [Xe]4f106s2 | +3, +4 |
| Holmium | 67 | Ho | [Xe]4f116s2 | +3 |
| Erbium | 68 | Er | [Xe]4f126s2 | +3 |
| Thulium | 69 | Tm | [Xe]4f136s2 | +2, +3 |
| Ytterbium | 70 | Yb | [Xe]4f146s2 | +2, +3 |
| Lutetium | 71 | Lu | [Xe]4f145d16s2 | +3 |
Furthermore, it was noted that the emission spectra of RE-doped phosphors exhibit minimal sensitivity to their chemical environment.66 This unique feature enables the fine-tuning of emission colors by introducing suitable dopants into the host lattice without necessitating alterations to the host material itself. The remarkably sharp line spectra characteristic of RE-doped phosphors arise from the low-lying, partially filled 4f subshell. These 4f electrons are effectively shielded by the outer 5p and 5s orbitals, resulting in a well-defined set of distinct energy levels.67,68
When RE ions are introduced into a host lattice, the perturbations arising from the host induce the mixing of the host's even-parity p or d orbitals with the odd-parity f orbitals of the dopant ions. This interaction leads to a relaxation of the selection rules governing electronic transitions, thereby enhancing both the absorption efficiency and the emission yield of the doped system.69,70 Furthermore, the extent of relaxation is also contingent upon the symmetry of the environment surrounding the RE ions, with higher symmetry facilitating a greater relaxation effect.71 The excitation of RE-doped phosphors can occur either directly through the activator ion or indirectly via a sensitizer (A), which may itself be an RE ion, or through the incorporation of a dopant, termed a sensitizer (S). The sensitizer absorbs incident energy and subsequently transfers it to the RE activator, facilitating luminescence.72,73 Fig. 4(a) and (b) represents the schematic representation of the luminescence mechanism within a phosphor host, highlighting the roles of both activator and sensitizer in the energy transfer process.
 | ||
| Fig. 4 Luminescence mechanism in phosphors with (a) activator alone and (b) activator along with sensitizer. | ||
2.3 Synthesis methods
The investigation of advanced synthesis techniques continues to be a highly active research domain, facilitating the development of more efficient organic and inorganic phosphors. Below, we outline some of the most prominent methods employed for NP synthesis.3. Applications of nanophosphors
3.1 Lighting applications
Solid-state lighting (SSL) technology, encompassing LEDs and organic LEDs (OLEDs), has revolutionized the lighting industry. Its advancement is driven by the increasing demand for energy-efficient solutions and severe environmental requirements. SSL has widely utilized phosphors doped with RE ions. Fig. 5 presents the milestones in phosphor material development along with the defining criteria for each generation.87 However, there is still significant scope for further research in this field, with a primary focus on reducing electrical energy consumption.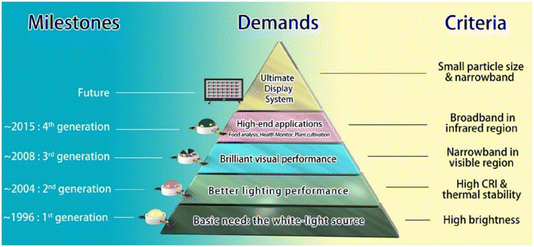 | ||
| Fig. 5 Milestones in phosphor development reflecting demands from basic white-light lighting to advanced applications. Reprinted with permission from ref. 87 copyright 2022, American Chemical Society. | ||
To achieve efficient white light emission and cover the visible spectrum, the following key factors must be considered to enhance performance and effectiveness.
(a) Using a single host material with multiple RE activators reduces WLED costs and simplifies synthesis.
(b) The colour rendering index (CRI) evaluates a light source's ability to accurately reproduce the colours of illuminated objects. CRI values range from 55 to 65, considered to be fair, and 65–75 are categorized as good, and values above 75 represent an excellent light source.
(c) Correlated color temperature (CCT) is a key parameter that defines the colour appearance of a light source and is measured in kelvin. Lower CCT values, around 2000 K to 3000 K, produce a warm light used in residential lighting, while higher values, ranging from 4000 K to 6500 K, create a cooler, daylight-like illumination suitable for commercial and industrial applications.
(d) Phosphor materials should exhibit thermal stability to withstand temperature variations. Additionally, they must possess chemical stability to enhance synthesis efficiency and ensure the formation of desired compounds.
(e) Finally, the phosphor should exhibit a high quantum yield (QY), defined as the ratio of emitted photons to absorbed photons, ensuring efficient light conversion.
Some of the notable works in the field of lighting applications using NPs are discussed in this section. In certain instances, the host matrix was held constant while exploring the luminescent behaviour under varying conditions, specifically, through single doping, co-doping, and the incorporation of charge compensators. Conversely, in other studies, the dopant ions were kept fixed while different host lattices were investigated to enable a comparative analysis of their influence on key optical and structural properties.
To start with, Saha et al. studied the luminescence behaviour of Li+ and Eu3+ co-doped nano magnesium aluminate (MgAl2O4) phosphors prepared via the sol–gel method.88 When doped solely with Eu3+, the substitution of Mg2+ by Eu3+ led to a net positive charge due to their differing valence states, creating vacancies that act as luminescence quenchers.89 To address this issue, they used Li+ as a charge compensator and co-doped it into optimized MgAl2O4: Eu3+. The ideal Eu3+ concentration was 2 mol%, while Li+ content varied from 0.5 to 2.5 mol%, keeping Eu3+ constant. Spectroscopic analysis revealed that the incorporation of Li+ ions resulted in a twofold enhancement of PL intensity, with the peak intensity achieved at a Li+ concentration of 2 mol%. Moreover, the integration of Li+ was found to enhance the QE by increasing the probability of radiative transitions within the system. The study does not examine thermal quenching behaviour, which is vital for evaluating phosphor performance under high-temperature LED operation. Also, the stability under prolonged UV exposure was not tested, which is critical for real-world lighting and display systems. Whereas Manjula et al. used a low-temperature solution combustion method with oxalyl dihydrazide (ODH) as fuel to prepare MgAl2O4:Eu3+ nanophosphors.90 They found that the optimum Eu3+ concentration was 5 mol% and concluded that the dipole–dipole interaction was the dominant mechanism for concentration quenching. The obtained band gap of the pure sample was 5.1 eV using DFT calculations, whereas using DRS measurements, it was found to vary between 4.6 eV to 4.9 eV for the doped samples.
Additionally, they carried out photocatalytic measurements, which were missing from the works of Saha et al. They demonstrated effective photocatalytic performance in degrading FOR dye under UV light, achieving an absorbance of 96% after 135 minutes of illumination. The MgAl2O4 photocatalyst maintained consistent activity over five consecutive cycles, indicating good recycling stability. To determine the active species involved in the photocatalytic decolouration, they used AgNO3, alcohol, and ethylenediaminetetraacetic acid (EDTA) as the different scavengers. The outcomes of the rate of photodegradation of dye along with various concentrations, recycling stability, and scavenging examinations are presented in Fig. 6(a)–(d). Therefore, it can be concluded that the significant reduction in efficiency upon EDTA addition confirms that holes (h+) play the dominant role in the photocatalytic decolouration mechanism. Recently, Halefoglu et al. and Lamonova et al. have explored the luminescent characteristics of MgAl2O4 phosphors doped with Sm3+ and Tb3+ ions, respectively. However, their investigations did not consider the influence of charge compensating agents, nor did they provide a comprehensive analysis of the thermal behaviour of the synthesized materials, leaving important aspects of phosphor performance underexplored.91,92 Next, Panigrahi et al. reported the co-doped MgAl2O4:Eu3+,Dy3+ nanophosphors, prepared via a modified Pechini-type sol–gel method.93 Owing to the proximity in energy levels between the 4F9/2 state of Dy3+ and the 5D0 state of Eu3+, resonant energy transfer (ET) occurs through a multipolar interaction mechanism.94 To explore tunable emission properties, two series of samples were synthesized: Mg(1−x−0.02)Al2O4:0.02Dy3+,xEu3+ (x = 0.1, 0.2, 0.3, 0.4, and 0.5 mol%) and Mg(1−y−0.02)Al2O4:0.02Eu3+,yDy3+ (y = 0.2, 0.3, 0.4, 0.5, and 1 mol%). The PL emission spectra of the samples are recorded under 351 nm excitation, revealing an intriguing trend: while maintaining Dy3+ concentration at 2 mol%, the intensities of Dy3+ emissions at 488 nm and 575 nm diminished progressively with an increase in Eu3+ concentration (0.1–0.5%), accompanied by a simultaneous intensification of Eu3+ emission peaks. Conversely, fixing Eu3+ concentration while incrementally increasing Dy3+ concentration unexpectedly led to a substantial enhancement of Eu3+ emission intensity. Among the synthesized phosphors, NPs co-doped with 2 mol% Dy3+ and 0.2 mol% Eu3+ exhibited superior white light emission under UV excitation, with chromaticity coordinates of (0.31, 0.33) aligning closely with white light.95 Additionally, the NPs demonstrated a remarkably high absolute quantum yield of nearly 67% and a CCT value of 6494 K. These findings establish MgAl2O4:2% Dy3+,0.2% Eu3+ as a highly promising candidate for WLED applications. Despite extensive studies on RE-doped MgAl2O4 nanophosphors, a significant gap remains in understanding their PL behavior at elevated temperatures. In particular, the thermal quenching characteristics have not been thoroughly explored. To substantiate the potential of these materials for high-performance LED applications, it is imperative to conduct temperature-dependent PL analyses to accurately determine the quenching temperature (TQ). Such insights are essential for assessing their thermal stability and operational reliability under real-world device conditions.
 | ||
| Fig. 6 (a) Photodegradation rate of the dye (b) effect of dye concentration on degradation rate (c) recyclability and stability of MgAl2O4 (d) scavenging examinations. The figures were reproduced from ref. 93 with copyright permission from John Wiley and Sons. | ||
Shivram et al. reported the synthesis of CaTiO3:Eu3+ nanophosphors using a low-temperature solution combustion method, achieving a crystallite size of approximately 40–45 nm.96 Under 398 nm excitation, a strong red emission centered at 615 nm was observed, attributed to the hypersensitive 5D0 → 7F2 transition of Eu3+. The optimal dopant concentration was identified as 3 mol%, and the corresponding CIE chromaticity coordinates closely matched the NTSC red standards, making the phosphor suitable for LED applications. In a separate study, Mazzo et al. synthesized CaTiO3:Eu3+ nanophosphors via a polymeric precursor route, yielding smaller particle sizes in the range of 24–35 nm.97 They determined the optimal Eu3+ concentration to be 1.5 mol%, beyond which concentration quenching set in. Furthermore, annealing was found to enhance the optical band gap due to lattice reorganization at elevated temperatures. Despite these promising findings, both studies lacked evaluation of temperature-dependent PL and thermal quenching, which are critical for real-world LED reliability. Additionally, neither study included an analysis of colour purity, decay dynamics, or lifetime measurements. Some of these limitations were addressed by Singh et al., who employed a chemical co-precipitation method to prepare CaTiO3:Eu3+ nanophosphors.98 Their study identified an optimal Eu3+ concentration of 5 mol%, with quenching attributed to dipole–quadrupole interactions. The optimized phosphor exhibited a quantum efficiency of 17.46%, correlated color temperatures (CCT) between 1740 and 2522 K, and CIE coordinates of (0.63, 0.37), confirming its potential as a red-emitting phosphor for domestic lighting. Further advancements were made by Sasidharan et al., who investigated Y3+ co-doping in CaTiO3:Eu3+ systems. By fixing the Eu3+ concentration at 7 mol%, they found that co-doping with 5 mol% Y3+ enhanced the red emission intensity at 614 nm by a factor of 10 compared to Eu-only samples.99 This enhancement was accompanied by a notable increase in colour purity (from 82.9% to 91.4%), a maximum luminescence lifetime of 1.314 ms, and a peak internal quantum efficiency of 81.6%, as confirmed via Judd–Ofelt analysis. In 2017, Singh and Manam synthesized CaTiO3:Dy3+ nanophosphors using a solid-state reaction method and demonstrated efficient blue and yellow emissions under 386 nm UV excitation, with optimal luminescence at 4 mol% of dopant.100 This composition yielded near-white CIE coordinates (0.28, 0.32) and a highly correlated color temperature of 9222 K, indicating promise for cold white light applications. The study also highlighted the role of dipole–dipole interactions in concentration quenching and reported moderate thermal stability. Building on this, the 2021 work by Shanbhag et al. introduced Li+ co-doping and SiO2 core–shell coating via solution combustion synthesis, achieving significantly enhanced luminescence and quantum efficiency (∼93%) with retained emission characteristics.101 The incorporation of Li+ (0.5 mol%) and the SiO2 shell effectively reduced non-radiative surface defects and improved crystallinity, leading to higher color purity (∼92%) and optimal CCT (6532 K). Despite the advancements, critical aspects of the material system remain insufficiently addressed. Specifically, the role and mechanism of Li+ ions in facilitating charge compensation and lattice incorporation lack comprehensive elucidation through advanced spectroscopic techniques such as X-ray photoelectron spectroscopy (XPS). Additionally, the atomic-level interactions between the SiO2 shell and the CaTiO3 host matrix have not been thoroughly investigated, limiting our understanding of the interface chemistry and its influence on luminescence efficiency. Moreover, long-term stability assessments, including photostability, thermal endurance under continuous excitation, and integration into functional LED prototypes, are absent from the current literature. While the contributions of Singh et al. and Sasidharan et al. have addressed foundational aspects such as structural characterization and luminescence optimization, essential parameters, including the effects of charge compensators, material reusability, and operational reliability under high-power excitation, remain unexplored. These factors are critical for the practical deployment of CaTiO3-based nanophosphors in robust, high-performance solid-state lighting applications.
Next, Kumar et al. explored the structural and photoluminescent characteristics of RE-doped GdSr2AlO5 nanophosphors, synthesized via a low-cost, urea-assisted gel combustion method.102–104 The materials doped individually with Dy3+, Eu3+, and Sm3+ ions are tailored for WLED applications, aiming to overcome the limitations of conventional lighting materials such as poor colour rendering and the absence of full-spectrum emission. All synthesized phosphors retained a well-defined tetragonal crystal structure, verified through XRD and Rietveld refinement. SEM and transmission electron TEM confirmed homogeneous, nanometer-sized particles (typically 31–35 nm). Each dopant imparted distinct emission properties: Dy3+ (optimal at 3 mol%) exhibited characteristic blue (488 nm), yellow (582 nm), and red (669 nm) emission bands arising from intra-4f transitions, producing a balanced white light when excited at 351 or 272 nm. Eu3+ (also optimal at 3 mol%) showed dominant red emission at 612 nm due to the hypersensitive 5D0 → 7F2 transition under 268 nm excitation, making it suitable for generating warm white light. Sm3+ (optimal at 4 mol%) emitted strong orange-red light centered at 603 nm under 273 nm excitation, primarily from the 4G5/2 → 6H7/2 transition. All systems demonstrated effective energy transfer from the Gd3+ host to the respective RE ions, a key factor in enhancing emission intensity. Notably, concentration quenching beyond the optimal dopant level was observed, attributed to non-radiative energy losses via dipole–dipole or quadrupole–quadrupole interactions among neighbouring activator ions. Judd–Ofelt analysis and lifetime measurements were conducted in detail for the Eu3+ system, yielding a quantum efficiency of 67.62% and confirming a favourable asymmetric environment around Eu3+ ions. Thermal stability for Eu3+ was also assessed, with a thermal quenching activation energy of 0.216 eV, suggesting strong potential for practical LED integration. In contrast, the Dy3+ and Sm3+ doped systems lacked equivalent thermal and quantum efficiency analyses, presenting an opportunity for further study. Despite the promising outcomes, thermal quenching behaviour and long-term photostability were only partially addressed, especially lacking in Dy3+ and Sm3+ doped systems. Critical parameters for commercial viability, such as Colour Rendering Index (CRI), CCT, and luminous efficacy, were not quantitatively evaluated. Additionally, while the excitation was primarily conducted in the UV region, behavior under standard blue LED excitation (around 450 nm), which is common in current WLED technology, remains unexplored. There is also untapped potential in exploring various RE doping and co-doping strategies (e.g., Dy3+/Sm3+ or Eu3+/Sm3+) that could enable more flexible colour tuning and mitigate concentration quenching. Hooda et al. focused on BaYZn3AlO7 phosphors doped with Eu3+ and Er3+ ions, employing solution combustion synthesis to achieve red and yellowish-green emissions, respectively.105,106 In a parallel study, Kaushik et al. synthesized Tb3+ doped BaYZn3AlO7 nanophosphors using the same combustion-based technique, targeting efficient green emission.107 Each dopant induces distinct emission colours. Eu3+ yields strong red emission at 611 nm, Er3+ produces yellowish-green emission at 527 and 566 nm, and Tb3+ exhibits green emission centered at 543 nm, highlighting the tunability of the host lattice for solid-state lighting applications, especially in warm and white LEDs. Morphologically, the nanoparticles ranged from 36 nm to 86 nm and showed characteristic porous, agglomerated features typical of combustion-synthesized materials. While the Eu3+ doped sample was thoroughly analyzed using Judd–Ofelt theory to explain its luminescence mechanism and quenching behavior, such detailed energy transfer studies were absent in the Er3+ and Tb3+ studies. Additionally, quantum efficiency was reported only for the Tb3+ system (78%), with no corresponding data for the Eu3+ and Er3+ systems. A significant gap across all studies is the lack of long-term photostability data and comparative synthesis methods to evaluate performance in practical scenarios. Future work should explore co-doping strategies, thermal degradation studies, and integration into functional devices to fully realize the potential of these nanophosphors in commercial lighting technologies. Next, the sequence of research by Phogat et al. (2021), Devi et al. (2023), and Solanki et al. (2025) presented a coherent and progressive development of Eu3+ doped vanadate nanophosphors, focusing on enhancing red emission for solid-state lighting applications.108–110 In the foundational work by Phogat et al. Ca9Bi(VO4)7:Eu3+ nanophosphors were synthesized via a solution combustion method, demonstrating an intense red emission at 614 nm, and a high quantum efficiency at 70 mol% Eu3+, making it a strong candidate for near-UV WLEDs.108 This research emphasized structural refinement using Rietveld analysis and photoluminescent behaviour characterized through Judd–Ofelt theory. Building on these findings, Devi et al. replaced Bi3+ with Y3+ in the host matrix, resulting in Ca9Y(VO4)7:Eu3+ nanophosphors with an even higher quantum efficiency (65.7%) and maintained strong emission at 619 nm.109 Their study also incorporated Dexter's theory and the Inokuti–Hirayama model to detail dipole–dipole interactions responsible for concentration quenching, thus offering deeper insight into energy transfer mechanisms. Extending this trajectory, Solanki et al. replaced Y3+ with La3+, synthesizing Ca9La(VO4)7:Eu3+ nanophosphors that not only sustained high photoluminescent performance (emission at 615 nm, QE ∼ 58.67%) but also pioneered their application in latent fingerprint detection (LFP), showcasing dual-use functionality for both lighting and forensic technologies.110 Furthermore, Phogat et al. also extended their analysis to other dopants also.111,112 They prepared Ca9Bi(VO4)7:Sm3+ nanophosphors synthesized via solution combustion, and they observed intense orange-red emission centered around 600–610 nm due to the 4G5/2 → 6H7/2 transition.111 This composition achieved high quantum efficiency (∼81%) and revealed efficient energy transfer from the VO43− group to Sm3+ ions. Dalal et al. substituted Bi3+ with Gd3+, fabricating Ca9Gd(VO4)7:Sm3+ phosphors.113 This study achieved improved thermal stability, quantum efficiency (82.84%), and extended emission in the reddish-orange region (606 nm), facilitated by the substitution of slightly smaller Gd3+ ions with Sm3+, resulting in minimal structural distortion and a narrow bandgap (3.66 eV), enhancing radiative transitions. Furthermore, they also explored the Ca9La(VO4)7:Sm3+ system, reporting intense orange-red luminescence with 10 mol% Sm3+ as the optimal doping level.114 The substitution of La3+ with ionic properties similar to Sm3+ was structurally favourable and resulted in a robust trigonal framework. The optimized sample displayed a slightly reduced bandgap (3.42 eV), increased emission intensity, and favourable chromaticity coordinates (0.5868, 0.4027), confirming its application potential in modern lighting technologies. Across all three works, the solution combustion method remained a consistent, low-cost, and energy-efficient synthesis route. Additionally, an extensive overview of optical studies of various RENPs has been systematically compiled in Table 2.
| Nanophosphor | Synthesis method | λex (nm) | λemax (nm) | CCT (K) | CP (%) | Ref. |
|---|---|---|---|---|---|---|
| a λex – excitation wavelength, λemax – maximum emission wavelength, CP – colour purity. | ||||||
| Y4Al2O9:Sm3+ | Gel combustion | 406 | 602 | 1664–1764 | 87.4–92.1 | 115 |
| Y4Al2O9:Dy3+ | Combustion | 348 | 483 | 5037.88–6609.22 | — | 116 |
| SrGdAlO4:Sm3+ | Solution combustion | 407 | 600 | — | — | 117 |
| Ba3Y4O9:Tb3+ | Solution combustion | 292 | 544 | — | — | 118 |
| SrLaAlO4:Dy3+ | Solution combustion | 352 | 484 | 7.4 × 103 to 11.1 × 103 | 23.02–39.27 | 119 |
| BaSrGd4O8:Dy3+ | Solution combustion | 352 | 570 | 1714–2700 | — | 120 |
| GdAlO3:Er3+ | Gel combustion | 377 | 546 | 5749–6005 | 76.66–90.44 | 121 |
| Ca9La(PO4)7:Dy3+ | Solution combustion | 352 | 485 | 6973–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 425 |
11.30–36.5 | 122 |
| GdAl2O9:Er3+ | Solution combustion | 382 | 558 | 5983–7169 | — | 123 |
| NaCaVO4:Dy3+ | Combustion | 310 | 579 | 3306–3369 | 50.80–52.30 | 124 |
| KSrVO4:Sm3+ | Combustion | 402 | 600 | 1645 | — | 125 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours is critical for competing with commercial standards such as YAG:Ce3+. The growing demand for high-power pc-WLEDs presents additional challenges, such as efficiency in LED chips, luminescence saturation of phosphors, and thermal degradation at elevated temperatures (∼200 °C). While YAG:Ce3+ remains effective as a yellow-emitting phosphor, alternatives like the red-emitting KSF:Mn4+ show performance limitations, particularly saturation at input intensities exceeding 0.1 W mm−2. Thus, identifying robust red phosphors for high-power applications remains a key research goal.126 Furthermore, the reliance on blue InGaN chips in current pc-WLEDs raises health concerns due to potential overexposure to blue light, prompting interest in ultraviolet (UV) or violet chip-based systems. These systems typically incorporate either a broadband white-light-emitting phosphor or a combination of red, green, and blue phosphors. However, limitations such as lower chip efficiency and large Stokes shifts reduce the overall efficacy of UV/violet-excited pc-WLEDs. Nevertheless, these systems are promising for applications requiring warm, low-intensity lighting, such as nighttime illumination. Integrating machine learning approaches is proving effective in predicting materials with optimal combinations of structural and optical properties.127,128
000 hours is critical for competing with commercial standards such as YAG:Ce3+. The growing demand for high-power pc-WLEDs presents additional challenges, such as efficiency in LED chips, luminescence saturation of phosphors, and thermal degradation at elevated temperatures (∼200 °C). While YAG:Ce3+ remains effective as a yellow-emitting phosphor, alternatives like the red-emitting KSF:Mn4+ show performance limitations, particularly saturation at input intensities exceeding 0.1 W mm−2. Thus, identifying robust red phosphors for high-power applications remains a key research goal.126 Furthermore, the reliance on blue InGaN chips in current pc-WLEDs raises health concerns due to potential overexposure to blue light, prompting interest in ultraviolet (UV) or violet chip-based systems. These systems typically incorporate either a broadband white-light-emitting phosphor or a combination of red, green, and blue phosphors. However, limitations such as lower chip efficiency and large Stokes shifts reduce the overall efficacy of UV/violet-excited pc-WLEDs. Nevertheless, these systems are promising for applications requiring warm, low-intensity lighting, such as nighttime illumination. Integrating machine learning approaches is proving effective in predicting materials with optimal combinations of structural and optical properties.127,1283.2 Sensing applications
RENPs have gathered significant attention for sensing applications due to their unique optical properties. These phosphors, doped with RE ions, exhibit tunable emission spectra under specific excitation conditions, enabling their use in temperature, pressure, and gas sensors. Furthermore, UCNPs provide enhanced sensitivity and signal reliability, particularly for remote and non-contact sensing applications. Additionally, the long luminescence lifetime and low energy loss of these phosphors make them ideal candidates for real-time monitoring and detection in various environments. Fig. 7(a) and (b) illustrate the distinctive optical properties of UCNPs and their corresponding sensing applications, respectively.129 Some of the most prominent advancements within this domain are presented below.To assess the suitability of the prepared NPs for sensing applications, it is essential to consider several key parameters that influence their performance. The following section outlines the critical factors that determine the effectiveness of NPs in metal ion and temperature sensing applications.
Here, Fo and F represent the fluorescence intensities of phosphor before and after the presence of metal ions, respectively. [Q] denotes the concentration of the metal ion, while KSV is the Stern–Volmer constant. Some of the important parameters in the case of metal ion sensing include.
(a) The detection limit (LOD). LOD refers to the lowest concentration of a metal ion that can be reliably detected, and it is given by131
Here, s represents the standard deviation, while b denotes the slope of the calibration curve.
(b) Quantification detection limits (LOQ). It is defined as the lowest concentration of a metal ion that can be accurately quantified with a reasonable degree of precision and accuracy. It is given by132
(c) Half-quenching concentration (C0.5). Half-quenching concentration is defined as the concentration of metal ions at which the fluorescence intensity reduces to half of its initial value (Fo/2). On substituting this condition in the Stern–Volmer equation and simplifying, we get
Below we have listed some of the important works in the field of phosphor-based metal ion sensors, including the detection of Cd2+, Pb2+, Fe3+, As3+, and Cr3+ ions using various phosphor systems. The two studies by Tashi et al. and Saif et al. present distinct approaches to the development of lanthanide-doped nanophosphors for heavy metal ion sensing. Tashi et al. hydrothermally synthesized NaGdF4 nanophosphor co-doped with Eu3+ and Ce3+ for chemical sensing of heavy metal ions (Cd2+, Pb2+, and Cr3+) in wastewater.133 These NPs exhibit an energy transfer from Ce3+ to Gd3+ to Eu3+, allowing them to act as a sensitive probe under UV excitation. Although the study includes structural and spectroscopic characterizations and indicates successful detection of Cr3+, Pb2+, and Cd2+, it lacks specific analytical performance metrics such as detection limits, response time, or interference from other ions. Furthermore, its application is limited to controlled laboratory settings, without validation using actual environmental samples. In contrast, the study by Saif et al. synthesized BaZrO3:Eu3+ nanophosphor for the detection of chromium ions from tannery leather and wastewater.134 They presented a more targeted and application-focused approach. The BaZrO3:Eu3+ nanophosphor, synthesized using a sonochemical sol–gel method, exhibits strong and stable red fluorescence that is selectively quenched in the presence of Cr3+ ions. Unlike the broader scope of Tashi et al., Saif et al. focus solely on Cr3+ detection but provide a comprehensive evaluation, including LOD = 3.8 × 10−9 mol L−1, a high KSV value of 7.05327 × 107 mol L−1, and excellent performance in real tannery leather and wastewater samples. The study confirms that quenching occurs via a static coulombic interaction, substantiated by lifetime and absorption-emission overlap studies. Tashi et al. exploit a multi-ion sensitization mechanism for enhanced luminescence and multi-ion sensing, and Saif et al. demonstrate the practical utility of their sensor with rigorous analytical validation. The work by Tashi et al. lacks real-world testing, quantitative sensitivity data, and interference analysis. Therefore, the NaGdF4:Eu3+/Ce3+ system could be further investigated for its potential selectivity and comparative performance against the BaZrO3:Eu3+ system when sensing Cr3+. Next, we transition into a comparison of two distinct approaches to Fe3+ ion sensing using lanthanide-doped nanophosphors. In the first study, Mahmoud et al. developed a multifunctional Bi12SiO20:Pr3+ nanophosphor via a hydrothermal method.135 This material exhibits a strong pinkish-red emission under UVA light and demonstrates versatility across three key applications: latent fingerprint visualization, anti-counterfeiting ink formulation, and Fe3+ ion detection in drinking water. The study offers a thorough performance analysis, with a Stern–Volmer quenching response over the concentration range of 0 to 17 × 10−5 mol L−1 and a detection limit of 1.56 × 10−5 mol L−1. The nanophosphor also displays high selectivity toward Fe3+ ions, as evidenced by pronounced fluorescence quenching under UV exposure. Notably, real sample testing in drinking water revealed high recovery rates and excellent correlation with standard atomic absorption spectroscopy, further establishing the material's practical applicability. They also examined the PL lifetimes and morphology in detail, contributing valuable insight into the sensor's stability and efficiency under varying environmental conditions. A more recent study by Singhaal et al. introduced a polyethylenimine (PEI)-functionalized NaCeF4:Tb3+/Eu3+ nanophosphor, also synthesized hydrothermally.136 This system functions as a dual-mode sensor for Fe3+ ions and picric acid (PA). The PEI layer enhances water dispersibility and promotes complexation with analytes, enabling selective quenching of the Eu3+ emission, especially at the 594 nm peak. The material demonstrated a low detection limit of 1.39 ppm for Fe3+ and a high KSV value of 3.8 × 104 M−1, indicating both sensitivity and selectivity. A distinctive feature of this study is the integration of reduced graphene oxide (RGO), used to explore energy transfer processes and further fine-tune PL properties. Together, these studies exemplify the diversity in material design and sensing strategy within Fe3+ ion detection. Mahmoud et al. emphasize practical applicability and multifunctional use, while Singhaal et al. offer an advanced, tunable platform with potential for future expansion. Furthermore, Kumar and Roy presented a highly sensitive and selective luminescence-based method for detecting As3+ in water using Eu3+ doped GdVO4 nanophosphors synthesized via a hydrothermal method.137 These nanophosphors exhibit strong red emission due to energy transfer from VO43− groups to Eu3+ ions, and their fluorescence is quenched in the presence of As3+ due to covalent bonding with surface Eu–OH groups, causing nanoparticle aggregation. The system showed an excellent LOD of 39 nM, well below the WHO permissible limit of 130 nM, and was tested successfully in real tap and river water samples with high accuracy and minimal interference from other ions. This robust selectivity across varying pH levels and fast response time of 30 seconds underscores its potential for practical field use in environmental monitoring.
where Iij is the fluorescence intensity for the transitions from the upper (i = 2) and lower (i = 1) thermalizing energy levels to a terminal level j, and T is the absolute temperature. Some of the recently reported temperature sensing properties of NPs include the work by Singh et al., who focused on Ca0.79−xBixEr0.01Yb0.2MoO4 nano phosphors synthesized via gel-combustion methods.140 Here, co-doping with Bi3+ ions introduced local symmetry distortions around the Er3+ sites without altering the overall tetragonal CaMoO4 structure. This local asymmetry was found to significantly enhance the upconversion (UC) emission intensity by approximately 25 times compared to the undoped samples, primarily by suppressing non-radiative decay pathways. This enhancement resulted in an SR value of 0.0068 K−1 at 300 K. However, this study was limited to room temperature measurements, and no absolute sensitivity value was presented, restricting its practical application scope for broader temperature ranges. Building upon this foundation, Gouraha et al. employed a microwave-assisted synthesis route to prepare ErxYbyCa1−x−yMoO4 nano phosphors.141 Microwave synthesis enabled finer control over particle size (∼15 nm), improved crystallinity, and rapid phase formation at lower energy inputs compared to conventional methods. The UC emission under 980 nm excitation was significantly improved, and temperature sensing capabilities were extended up to 398 K. The maximum SR value reported was approximately 0.0170 K−1 at 398 K, indicating a better thermal response than that achieved by Singh et al. Moreover, Gouraha et al. expanded the functionality of the material by demonstrating catalytic efficiency in the selective oxidation of benzoin and benzyl alcohols, positioning these phosphors not only as optical sensors but also as potential catalysts. In a further progression, they also synthesized CaMoO4:Er3+/Yb3+ nanophosphors with enhanced upconversion and down conversion properties, again using a microwave-assisted approach but with optimized doping levels.142 The material exhibited robust temperature sensing capabilities, achieving a maximum SA value of 10.74 × 10−3 K−1 at 500 K, thus significantly outperforming the previous two studies in terms of both sensitivity and operational temperature range. Despite these advancements, notable research gaps persist across all three studies. None of the studies addressed low-temperature sensing capabilities, leaving the behaviour of these materials below 300 K unexplored. Similarly, thermal cycling durability, which is essential for practical deployment in fluctuating temperature environments, remains unexplored. Next, we have presented the collective research on vanadate-based nanomaterials doped with various lanthanide ions (Tm3+, Er3+, Sm3+, Eu3+, Nd3+, Yb3+, and Dy3+), primarily focusing on significant advancements in morphology control, luminescent tuning, and temperature sensing applications. The study led Wang et al. synthesized LuVO4:Ln3+ (Ln = Tm, Er, Sm, Eu) nano/micro-structures via solvothermal synthesis using tartaric acid as a morphology-directing agent.143 They achieved the formation of diverse structures and demonstrated tunable multicolour emissions ranging from blue to white light by adjusting lanthanide dopant types and concentrations. However, while the study significantly advanced morphology-controlled photoluminescent materials, it lacked exploration into functional applications like thermal sensing, limiting its practical impact. Building upon structural achievements, the studies by Kolesnikov et al. introduced a crucial improvement by focusing on optical thermometry with LuVO4:Nd3+/Yb3+ nanophosphors.144 They exploited phonon-assisted UC mechanisms to achieve highly sensitive, non-contact temperature measurements. Improvements included achieving relative thermal sensitivities up to 2.6% K−1 and temperature resolutions as fine as 0.2 K across a wide temperature range of 323 to 873 K.145 These studies also analyzed the energy transfer efficiency between Nd3+ and Yb3+ centers and the influence of Yb3+ concentrations on thermometric performance aspects that were not addressed in Wang's earlier work. The 2023 paper, again by Kolesnikov et al. extended these advances to LaVO4 systems doped with Dy3+ and Sm3+. This study proposed a multimode thermometric approach, not just relying on luminescence intensity ratios, but also incorporating the temperature-induced red shift of the charge transfer band, a novel sensing mechanism enhancing sensitivity and reliability.146 The highest sensitivity achieved (Sr = 2.07% K−1 for LaVO4:Sm3+) for the temperature range 173–573 K. Nevertheless, this work was restricted to a moderate temperature range and focused on monoclinic LaVO4 rather than tetragonal LuVO4, hinting at a structural dependency that might limit generalizability. However, though Kolesnikov's works were functionally advanced, they still faced challenges related to long-term operational stability, potential agglomeration of NPs, and performance inconsistencies. Furthermore, scalability and repeatability under real-world conditions remain unexplored across all studies. Furthermore, the comparative analysis of YVO4-based luminescent nanomaterials doped with various lanthanide ions, across the studies conducted by Kolesnikov et al. (2017, 2018, 2021) and Hong Zhou (2020), shows a systematic evolution in optical nano thermometry applications. In 2017, Kolesnikov et al. synthesized Nd3+ doped YVO4 nanophosphors to function as non-contact thermal sensors based on near-infrared emission, specifically utilizing three independent thermometric parameters: FIR, spectral redshift (Stark level transitions), and full width at half maximum (FWHM) broadening.147 The study demonstrated that each parameter responded uniquely over a wide thermal window from 123 to 873 K, but the limited sensitivity of individual parameters and high thermal quenching at elevated temperatures exposed the need for more selective luminescent probes. Advancing this field, the 2018 study by Kalinichev under Kolesnikov's supervision introduced a ratiometric thermal sensing strategy utilizing the thermally coupled transitions between the 4F5/2 and 4F3/2 excited states of Nd3+ ions.148 By selecting a broader energy gap (several hundred cm−1) between these levels, the study achieved an increased Boltzmann population difference, leading to a markedly enhanced relative thermal sensitivity of up to 1.3% K−1, and allowed temperature detection with sub-degree resolution around 313 K and 673 K. Nevertheless, issues such as inhomogeneous particle sizes, aggregation tendencies in aqueous dispersions, and decreasing emission intensity at high dopant concentrations remained critical challenges. In 2020, Zhou et al. studied the temperature sensing properties of Pr3+ doped YVO4 phosphors by exploring both thermally coupled (3P1 → 3P0) and non-thermally coupled (1D2 → 3P0) level transitions.149 They demonstrated that the non-thermally coupled FIR technique, making use of transitions separated by a larger energy gap (∼1000 cm−1), could yield a higher SR value of 1.137% K−1 at 313 K, outperforming conventional thermally coupled FIR methods. This improvement was attributed to reduced cross-relaxation pathways and minimized thermal back-transfer processes. However, the study noted slight distortions in the YVO4 crystal lattice after high-temperature sintering, potentially due to stress-induced anisotropic shrinkage, thus affecting luminescence stability under cyclic thermal stress. Finally, in the 2021 work led again Kolesnikov et al. systematically compared co-doped Eu3+/Nd3+ and singly doped Eu3+ and Nd3+ NPs.150 Co-doped systems exhibited moderate sensitivity (∼0.8% K−1) but suffered from non-radiative energy transfer inefficiencies, whereas the singly doped systems maintained discrete energy channels for each ion, resulting in enhanced thermal resolution down to 0.4 K. The use of ratiometric luminescence between the 5D0 → 7F2 transition of Eu3+ and the 4F3/2 → 4I11/2 transition of Nd3+, coupled with Boltzmann analysis, enabled precise and reproducible thermal readings, although slight inhomogeneities in nanoparticle dispersion introduced minor uncertainties in FIR calibration. But, in the case of Nd3+ based systems, the limited absorption cross-section at biological windows (∼800 nm) restricts application under low-power excitation; for Pr3+ and Eu3+ doped materials, concentration quenching and incomplete suppression of cross-relaxation mechanisms limit maximum achievable sensitivities.
 | ||
| Fig. 8 Various strategies for temperature readout in luminescence thermometry include (a) intensity-based (b) band-shift-based (c) bandwidth-based (d) polarization-based (e) ratiometric (f) kinetics-based and (g) single-band ratiometric. Reproduced with permission under Creative Commons CC BY 4.0 license from ref. 138 copyright@2022 The Authors. | ||
| Nano phosphor | Synthesis method | Temperature range (K) | Maximum absolute sensitivity | Ref. |
|---|---|---|---|---|
| BaTiO3:Er3+,Yb3+ | Co-precipitation | 300–505 | 0.00192 K−1 at 410 K | 151 |
| YMoO4:Er3+,Yb3+,Zn2+ | Co-precipitation | 300–523 | 0.0785 K−1 at 300 K | 152 |
| Lu2Ti2O7:Yb3+,Er3+ | Hydrothermal | 298–573 | 0.00313 K−1 at 536 K | 153 |
| CaSrSiO4:Tb3+ | Sintering | 10–290 | 17.6 × 10−4 K−1 at 70 K | 154 |
| NaZnPO4:Er3+,Eu3+,Yb3+ | Co-precipitation | 300–503 | 7.3 × 10−3 K−1 at 503 K | 155 |
| Gd2(MoO4)3:Er3+,Yb3+,Li+,Zn2+ | Co-precipitation | 300–473 | 38.7 × 10−3 K−1 at 473 K | 156 |
| Gd2O3:Er3+,Yb3+,Zn2+ | Combustion | 297–577 | 0.0116 K−1 at 297 K | 157 |
| NaGdF4:Yb3+,Ho3+,Ce3+ | Hydrothermal | 300–500 | 0.1446 K−1 at 500 K | 158 |
| NaGdF4: Yb3+,Ho3+ | Hydrothermal | 303–523 | 0.17 K−1 at 523 K | 159 |
| ZnGa2O4: Cr3+,Bi3+ | Hydrothermal | 303–503 | 0.017 K−1 at 493 K | 160 |
| LaOF:0.05Yb3+,Tm3+ | Hydrothermal | 303–573 | 0.0046 K−1 at 303 K | 161 |
3.3 Thermoluminescence dosimetry applications
Humans are continuously exposed to different forms of radiation originating from various sources. As shown in Fig. 9, these sources can be broadly categorized into natural and artificial radiation sources.162,163 Among natural sources, the Sun is a major contributor of electromagnetic radiation. Other natural sources include terrestrial radionuclides, which are radioactive elements naturally present in the Earth's crust, and cosmogenic radiation, which results from interactions between cosmic rays and atmospheric particles.164 Environmental radiation is pervasive and is present in the soil, the food we consume, the water we drink, and the air we breathe. One of the most significant contributors to natural background radiation is radon gas, a radioactive decay product of uranium, which can be inhaled from the air or ingested through the food and water supply.165 On the other hand, artificial sources include technological devices such as mobile phones, as well as medical diagnostic procedures and industrial activities, which add to the overall radiation exposure, although typically at lower levels compared to natural sources. | ||
| Fig. 9 Various forms of artificial and natural radiation sources. Reprinted with permission from ref. 163, page no. 277, copyright (2022), Elsevier. | ||
The process of measuring and calculating the amount of radiation absorbed by a material is known as dosimetry. This is typically carried out using a small instrument known as a dosimeter.166 Among the various dosimetric methods available, the present study focused on the thermally stimulated luminescence (TSL) technique.167 The fundamental principle of this method is that the intensity of thermoluminescence (TL) emitted by a phosphor material is directly related to the radiation dose it has absorbed, thus allowing for the estimation of unknown radiation exposures. Thermoluminescent dosimeters (TLDs), which are widely used for this purpose, are manufactured in multiple forms, including powders, chips, rods, and ribbons.168 Their design also makes them convenient for personal use, commonly incorporated into wearable items such as badges and rings. The glow curve obtained during the TLD readout process provides critical information about the energy depth of the traps within the material that are responsible for the observed luminescence. Additionally, the linear response to varying radiation doses and the relatively low fading over time are key features that contribute to the widespread application of TLDs in radiation dosimetry.169,170
RENPs are integral to the study of TL, a process wherein light is emitted by wide-bandgap materials upon heating to sub-incandescent temperatures. This luminescent phenomenon arises not from the direct influence of heat but as a consequence of prior energy absorption from an external stimulus, with thermal energy functioning merely as a catalyst to release the stored energy. The usage of TL is particularly useful in dosimetry, as the intensity of emitted light correlates linearly with the absorbed energy. The intricate mechanisms underlying TL are illustrated in Fig. 10, delineated through sequential processes labeled as (a) to (e).171 The interaction of atoms with ionizing radiation generates free charge carriers (a), which travel through the crystalline lattice and become trapped at the trapping centers (b and c). Heating the TL material releases these carriers, leading to recombination (e and f), during which light is emitted if radiative recombination occurs.
Some of the released carriers may be retrapped (d) or recombined non-radiatively. A TL model includes traps and recombination centers, described by rate equations. The plot of thermoluminescence intensity against the heating rate is known as the TL glow curve. This curve is instrumental in determining key parameters, including activation energy (E), frequency factor (s), and order of kinetics (b), which characterize the TL glow peak. In the following section, we have compiled recent advancements in the field of TL dosimetry using RENPs.171,172 The field of radiation dosimetry has witnessed significant progress with the development of RENPs, particularly those activated with Eu3+ ions. From 2017 to 2024, a series of studies have explored various host matrices such as Y2O3, LiF, YVO4, BaSO4, ZnGa2O4, and SrMgAl10O17, incorporating Eu3+ and sometimes co-doped with other lanthanide ions like Sm3+ and Dy3+. These materials have been examined for their TL response, structural stability, and suitability for γ, β, X-ray, and proton dosimetry. One of the earliest foundational works in this timeline was conducted by Shivaramu et al., who synthesized Eu3+ doped Y2O3 nanophosphors via the solution combustion method.173 Their study revealed that these materials demonstrated strong TL emission when exposed to γ-radiation, primarily due to the presence of deep electron trap centers. The TL glow curve was well defined, and the emission intensity correlated positively with dose, indicating suitability for dosimetric applications. The material's chemical stability and wide bandgap of 5.8 eV contributed to better glow curve reproducibility and minimal fading. Expanding on this, Kakade et al. synthesized Eu3+ doped Y2O3 using the sol–gel method.174 The study demonstrated that the structural and luminescent properties could be fine-tuned by adjusting Eu3+ concentration and annealing temperature. The TL studies revealed four distinct glow peaks at 100 °C, 117 °C, 139 °C, and 301 °C. While the first low-temperature peak faded rapidly, the others remained stable, offering a balance between sensitivity and signal retention. These characteristics established Y2O3:Eu3+ as a reliable material for high-dose TL applications, particularly in environmental and industrial monitoring. Kumar et al. focused on co-doped LiF nanophosphors containing Sm3+, Dy3+, and varying concentrations of Eu3+ ions.175 Synthesized via a chemical co-precipitation method, the LiF:Sm3+, Dy3+, and Eu3+ samples demonstrated excellent TL performance over an extended dose range of 0.1 to 30 kGy. The TL glow curve exhibited a strong peak between 398 K and 412 K. Using Chen's peak shape method and the TLAnal program, activation energies ranging from 0.71 to 2.24 eV were calculated along with frequency factors and kinetic orders. The phosphors showed linear TL behavior across the tested dose range and maintained a low fading of 10% over 27 days, making them highly effective for long-term radiation monitoring. This study also established the importance of deliberate defect engineering to enhance electron trapping and dosimetric response. Another study from the same group explored LiF co-doped with Sm3+ and Eu3+, revealing similar TL characteristics with a defined glow peak and consistent trap behaviour.176 These results suggested that co-doping not only improves TL intensity but also modifies trap depth, enabling a more stable dosimetric material. Next, Osorio et al. explored YVO4 doped with Eu3+ and co-doped with Dy3+ synthesized via the co-precipitation method.177 The inclusion of Dy3+ enhanced trap density and TL response by introducing deeper trap levels. The PL intensity and TL glow curves were influenced by crystallite size and preparation conditions. Importantly, the study found that these phosphors responded consistently to both β and γ irradiation, indicating their versatility in dosimetry. The ability of Dy3+ to act as a charge carrier trap significantly improved radiative recombination efficiency, leading to a higher and more stable TL output. Nattudurai et al. introduced BaSO4:Eu3+ as a novel phosphor for TL dosimetry under orthovoltage X-rays and low-energy proton irradiation.178 Using Co-60 γ-rays, the study demonstrated a linear TL response to doses ranging from 0.01 to 2 Gy. The maximum glow peak was recorded at 212 °C, with a TL signal that scaled linearly with absorbed dose across all radiation types. However, the material showed strong energy dependence, with TL calibration slopes varying significantly between X-ray and proton beams. Despite this, the material's reusability, small size, and high sensitivity made it a valuable dosimeter if properly calibrated in the user beam. A more recent investigation by Barad et al. involved ZnGa2O4:Eu3+ synthesized using gel combustion.179 The TL glow curve analysis identified five distinct peaks, with the main peak around 190 °C. The dose–response curve showed excellent linearity up to 100 Gy with R2 = 0.989 and a sublinear response beyond that range. Deconvolution techniques were used to determine trap depth and frequency factors. The optimal dopant concentration was found to be 2% Eu3+. The high chemical and thermal stability of the ZnGa2O4 host lattice, along with strong persistent luminescence and a high density of trap centers, makes it a suitable candidate for medium-dose range dosimetry, especially in industrial or medical contexts. While each system showed specific advantages, some recurring limitations were observed. In materials like BaSO4, energy dependence under different radiation sources can limit universal application unless specific calibrations are performed. Low-temperature glow peaks, especially in Y2O3 and LiF systems, were prone to fading due to shallow trap levels, affecting long-term signal reliability. Additionally, synthesis techniques such as gel combustion and sol–gel methods, while offering fine control over morphology and dopant distribution, may pose challenges in scalability and reproducibility across larger production batches. Nonetheless, significant improvements have been made in the field. The evolution from singly doped to co-doped systems enhanced both TL intensity and trap complexity. Co-dopants like Dy3+ and Sm3+ proved effective in introducing deeper and more stable traps, broadening the dose–response range, and reducing signal fading. In conclusion, recently a considerable advancement in the design and application of Eu3+ activated nanophosphors for TL dosimetry. In the next few papers, we have provided the evaluation of five similar host systems doped with Dy3+. We start with the study by Bahl et al., who synthesized a novel co-doped phosphor CaSO4:Dy,Mn using a modified recrystallization method.180 Their primary goal was to enhance the sensitivity of the well-established CaSO4:Dy phosphor, which, despite its wide usage in medical and environmental dosimetry, required improved performance for lower dose detection. The introduction of Mn as a co-dopant at an optimized ratio of Dy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 0.025
Mn = 0.025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.075 within a fixed total concentration of 0.1 mol% significantly increased the TL intensity, almost doubling that of the conventional CaSO4:Dy and surpassing that of LiF:Mg,Cu,P. Importantly, the TL glow peak remained at ∼240 °C, a desirable temperature for practical dosimetry applications, and the phosphor displayed a stable fading profile, with only ∼11% signal loss over three months. The glow curve deconvolution revealed a complex trapping mechanism, yet it retained reproducibility and linearity in the low-dose range, making it especially suitable for applications such as diagnostic radiology and nuclear medicine, where precise detection of microgray level exposures is crucial. Complementing this development, Mandlik et al. focused on extending the dosimetric range of CaSO4:Dy by reducing its particle size to the nanometer regime through a chemical co-precipitation technique.181 The resulting nanorod-shaped CaSO4:Dy, with dimensions around 20 nm in diameter and 200 nm in length, exhibited a major TL glow peak at approximately 283 °C after annealing at 700 °C. Structural analyses via XRD and TEM confirmed phase changes and morphological evolution during annealing, which directly influenced the TL behaviour. This nanophosphor demonstrated a wide and unsaturated dose response up to 10 kGy, unlike its microcrystalline counterpart, which is saturated at ∼100 Gy. Moreover, fading studies showed only minor signal degradation, reinforcing its potential for high-dose dosimetry applications such as sterilization, industrial irradiation, and radiation therapy monitoring. In a related study, Mandlik et al. investigated the CaSO4:Eu nanophosphors, emphasizing the impact of particle size on both TL and PL properties.182 The microcrystalline phosphor was first prepared via the acid recrystallization method and subsequently subjected to ball milling to yield various particle sizes, ranging from 5 μm to as small as 50 nm. Additionally, nanocrystalline CaSO4:Eu was synthesized using the chemical co-precipitation method for comparison. It was observed that the TL intensity of the phosphor decreased significantly with particle size reduction, particularly for samples under 200 nm. This trend was attributed to the increased presence of surface defects and non-radiative recombination centers, which quench the luminescence. Despite this, the 50 nm sample maintained a linear TL response over a wide dose range of 1–10 kGy, making it suitable for both low and high radiation dose applications. The study also confirmed that Eu ions were present in both Eu2+ and Eu3+ states, contributing to the dual-mode emission observed in PL spectra. While NPs offer enhanced surface area and tunable properties, it is critical to balance grain size and structural integrity to preserve luminescence performance. Similarly, the TL behaviour of sodium sulfate-based phosphors was explored by Vidya et al. through the synthesis of Na2SO4:Dy and LiNaSO4:Dy via the slow evaporation technique, followed by calcination at 400 °C.183 Their work revealed that Dy3+ incorporation into Na2SO4 led to the appearance of glow peaks around 61 °C and 175 °C, indicating the formation of shallow and deep traps, respectively. Meanwhile, the co-doping with Li+ ions in LiNaSO4 resulted in a significant shift in glow peak positions and a general reduction in TL intensity. The authors interpreted this behaviour as the result of lithium-induced structural changes and possible recombination suppression due to charge compensation mechanisms. Despite these effects, both phosphors exhibited linear dose responses up to 5 kGy and good reproducibility, suggesting their utility for mid-dose dosimetry applications. Additionally, the activation energies and frequency factors were derived using peak shape analysis, offering deeper insight into the trap dynamics of these systems. However, the relatively low glow peak temperatures may limit their use in thermally unstable environments or field deployments with fluctuating ambient conditions. The most recent and advanced material in this comparative review is the Dy3+ doped Zn0.66Mg0.3Al2O4 phosphor, synthesized by Pathak et al. employing a solution combustion synthesis route, The researchers prepared a spinel-phase nanophosphor optimized with 4 mol% Dy3+ ions.184 Structural characterization via XRD, FTIR, and SEM confirmed the successful incorporation of Dy3+ into the host lattice and the presence of nanostructured particles (∼10–25 nm). TL measurements revealed glow peaks at 246–250 °C across doses ranging from 600 to 1000 Gy, with nearly linear dose–response behavior and minimal fading of nearly 4.25% over 20 days. The high activation energy, ranging from 0.93 to 0.97 eV, and deep trap characteristics suggest excellent stability and low thermal noise, rendering the material highly suitable for high-dose applications such as nuclear reactor surveillance, space mission dosimetry, and industrial accelerator operations. Moreover, a substantial body of literature has been dedicated to exploring the dosimetric applications of NPs, with numerous pioneering studies summarized in Table 4.
0.075 within a fixed total concentration of 0.1 mol% significantly increased the TL intensity, almost doubling that of the conventional CaSO4:Dy and surpassing that of LiF:Mg,Cu,P. Importantly, the TL glow peak remained at ∼240 °C, a desirable temperature for practical dosimetry applications, and the phosphor displayed a stable fading profile, with only ∼11% signal loss over three months. The glow curve deconvolution revealed a complex trapping mechanism, yet it retained reproducibility and linearity in the low-dose range, making it especially suitable for applications such as diagnostic radiology and nuclear medicine, where precise detection of microgray level exposures is crucial. Complementing this development, Mandlik et al. focused on extending the dosimetric range of CaSO4:Dy by reducing its particle size to the nanometer regime through a chemical co-precipitation technique.181 The resulting nanorod-shaped CaSO4:Dy, with dimensions around 20 nm in diameter and 200 nm in length, exhibited a major TL glow peak at approximately 283 °C after annealing at 700 °C. Structural analyses via XRD and TEM confirmed phase changes and morphological evolution during annealing, which directly influenced the TL behaviour. This nanophosphor demonstrated a wide and unsaturated dose response up to 10 kGy, unlike its microcrystalline counterpart, which is saturated at ∼100 Gy. Moreover, fading studies showed only minor signal degradation, reinforcing its potential for high-dose dosimetry applications such as sterilization, industrial irradiation, and radiation therapy monitoring. In a related study, Mandlik et al. investigated the CaSO4:Eu nanophosphors, emphasizing the impact of particle size on both TL and PL properties.182 The microcrystalline phosphor was first prepared via the acid recrystallization method and subsequently subjected to ball milling to yield various particle sizes, ranging from 5 μm to as small as 50 nm. Additionally, nanocrystalline CaSO4:Eu was synthesized using the chemical co-precipitation method for comparison. It was observed that the TL intensity of the phosphor decreased significantly with particle size reduction, particularly for samples under 200 nm. This trend was attributed to the increased presence of surface defects and non-radiative recombination centers, which quench the luminescence. Despite this, the 50 nm sample maintained a linear TL response over a wide dose range of 1–10 kGy, making it suitable for both low and high radiation dose applications. The study also confirmed that Eu ions were present in both Eu2+ and Eu3+ states, contributing to the dual-mode emission observed in PL spectra. While NPs offer enhanced surface area and tunable properties, it is critical to balance grain size and structural integrity to preserve luminescence performance. Similarly, the TL behaviour of sodium sulfate-based phosphors was explored by Vidya et al. through the synthesis of Na2SO4:Dy and LiNaSO4:Dy via the slow evaporation technique, followed by calcination at 400 °C.183 Their work revealed that Dy3+ incorporation into Na2SO4 led to the appearance of glow peaks around 61 °C and 175 °C, indicating the formation of shallow and deep traps, respectively. Meanwhile, the co-doping with Li+ ions in LiNaSO4 resulted in a significant shift in glow peak positions and a general reduction in TL intensity. The authors interpreted this behaviour as the result of lithium-induced structural changes and possible recombination suppression due to charge compensation mechanisms. Despite these effects, both phosphors exhibited linear dose responses up to 5 kGy and good reproducibility, suggesting their utility for mid-dose dosimetry applications. Additionally, the activation energies and frequency factors were derived using peak shape analysis, offering deeper insight into the trap dynamics of these systems. However, the relatively low glow peak temperatures may limit their use in thermally unstable environments or field deployments with fluctuating ambient conditions. The most recent and advanced material in this comparative review is the Dy3+ doped Zn0.66Mg0.3Al2O4 phosphor, synthesized by Pathak et al. employing a solution combustion synthesis route, The researchers prepared a spinel-phase nanophosphor optimized with 4 mol% Dy3+ ions.184 Structural characterization via XRD, FTIR, and SEM confirmed the successful incorporation of Dy3+ into the host lattice and the presence of nanostructured particles (∼10–25 nm). TL measurements revealed glow peaks at 246–250 °C across doses ranging from 600 to 1000 Gy, with nearly linear dose–response behavior and minimal fading of nearly 4.25% over 20 days. The high activation energy, ranging from 0.93 to 0.97 eV, and deep trap characteristics suggest excellent stability and low thermal noise, rendering the material highly suitable for high-dose applications such as nuclear reactor surveillance, space mission dosimetry, and industrial accelerator operations. Moreover, a substantial body of literature has been dedicated to exploring the dosimetric applications of NPs, with numerous pioneering studies summarized in Table 4.
| Nano phosphor | Radiation type | Dose | Deconvoluted TL peaks (K) | Order of kinetics | E (eV) | Frequency factor (s−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Lu3Al5GaO12:Ce3+ | β rays | 50 Gy | 323.3 | 1 | 0.65 | 2.51 × 1011 | 187 |
| 343.1 | 1 | 0.68 | 2.37 × 1011 | ||||
| 361 | 1 | 0.72 | 2.25 × 1011 | ||||
| 388.4 | 1 | 0.78 | 2.09 × 1011 | ||||
| ZnAl2O4:Dy3+ | γ rays | 10 Gy | 446 | 1 | 0.17 | 1.9 × 105 | 188 |
| 469 | 1 | 0.11 | 1.7 × 105 | ||||
| 598 | 1 | 0.37 | 1.0 × 105 | ||||
| 648 | 1 | 0.59 | 2.7 × 105 | ||||
| SrZr4(PO4)6:Pr3+ | UV | 30 min | 486 | 2 | 0.69 | 1.64 × 106 | 189 |
| MgO:Li,Sm | γ rays | 15 Gy | 442 | 1.9 | 1.37 | 1.8 × 1015 | 190 |
| CdSiO3:Gd3+ | UV | 10 min | 346.25 | 2 | 0.68 | 2.62 × 108 | 191 |
| 392.05 | 2 | 0.67 | 7.61 × 106 | ||||
| 436.76 | 2 | 0.95 | 7.59 × 108 | ||||
| KCl:Sm3+ | Proton ion | 250 keV | 535 | 1 | 1.51 | 2.6 × 1013 | 192 |
| 583 | 2 | 1.65 | 1.9 × 1014 | ||||
| 512 | 1 | 1.31 | 8.5 × 1011 | ||||
| Carbon ion | 250 keV | 550 | 1 | 1.10 | 7.2 × 1010 | ||
| 606 | 2 | 1.45 | 6.2 × 1012 | ||||
| 608 | 1 | 0.99 | 5.9 × 109 | ||||
| K2Ca2(SO4)3:Eu3+ | Electrons | 6 MeV | 423 | 1 | 0.97 | 1.29 × 1011 | 193 |
| 439 | 1 | 1.08 | 8.50 × 1011 | ||||
| 477 | 1 | 1.23 | 3.21 × 1012 | ||||
| 532 | 1 | 0.74 | 1.81 × 106 | ||||
| γ rays | 10 kGy | 422 | 1 | 0.99 | 2.66 × 1011 | ||
| 435 | 1 | 1.03 | 2.73 × 1011 | ||||
| 479 | 1 | 1.24 | 3.88 × 1012 | ||||
| 532 | 1 | 0.75 | 1.72 × 106 | ||||
| LiMgBO3:Dy3+ | Ag9+ | 120 MeV | 355 | 2 | 0.92 | 4.22 × 1012 | 194 |
| 387 | 2 | 0.72 | 6.28 × 108 | ||||
| 410 | 2 | 0.65 | 2.45 × 107 | ||||
| 567 | 2 | 0.88 | 1.11 × 107 | ||||
| γ rays | 5 Gy | 376 | 2 | 1.26 | 3.65 × 1016 | ||
| 397 | 2 | 1.07 | 1.79 × 1013 | ||||
| SnO2:Eu3+ | γ rays | 15 kGy | 366.5 | 1.40 | 0.65 | 2.36 × 108 | 195 |
| 440 | 1.56 | 0.98 | 4.73 × 1010 | ||||
| 529 | 1.42 | 1.10 | 6.46 × 109 | ||||
| 624 | 2.00 | 1.17 | 2.58 × 1010 | ||||
| CaF2:Ce3+ | γ rays | 5 Gy | 394 | 2.02 | 1.73 | 3.2 × 1021 | 196 |
| 411 | 1.63 | 1.24 | 2.8 × 1014 | ||||
| 425 | 1.66 | 1.65 | 9.4 × 1018 | ||||
| 445 | 1.73 | 1.17 | 2.1 × 1012 | ||||
| 556 | 1.30 | 1.97 | 1.1 × 1017 | ||||
| 594 | 1.57 | 1.84 | 4.9 × 1015 | ||||
| 632 | 1.14 | 1.49 | 6.4 × 1010 |
4. Conclusions
In conclusion, this paper highlights the significant advancements in the synthesis and optimization of rare-earth-doped nano phosphors (RENPs), with a focus on the strategic selection of host materials such as tungstates, vanadates, and aluminates. The incorporation of rare-earth elements has proven to significantly enhance the PL properties and efficiency of these materials, leading to the development of phosphors with tunable emission spectra, high luminous efficiency, and desirable chromaticity for advanced lighting applications. The careful modulation of dopant concentrations and energy transfer mechanisms plays a critical role in optimizing these materials for specific applications, particularly for lighting technologies. Furthermore, the UCNPs have been demonstrated for various advanced sensing applications, alongside electrochemical and photocatalytic applications. Also, the use of synthesis methods such as sol–gel, combustion, and microwave-assisted techniques further improves their performance for environmental and biomedical monitoring. Lastly, RENPs exhibit exceptional promise in TL dosimetry, showcasing their ability to provide precise radiation dose measurements with minimal fading and reliable dose-dependent responses, which underscores their suitability for medical and environmental dosimetry applications. These findings open the door for the further development and implementation of RENPs in a broad range of scientific and industrial fields.Data availability
This review does not include any primary research findings, software, or code, nor does it present new data generation or analysis.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R2), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors acknowledge the financial support from the Manipal Academy of Higher Education, Manipal, India.References
- S. A. M. Tofail, E. P. Koumoulos, A. Bandyopadhyay, S. Bose, L. O'Donoghue and C. Charitidis, Mater. Today, 2018, 21, 22–37 CrossRef.
- J. O. Gidiagba, C. Daraojimba, K. A. Ofonagoro, N. L. Eyo-Udo, B. A. Egbokhaebho, O. A. Ogunjobi and A. A. Banso, Engineering Science & Technology Journal, 2023, 4, 84–100 Search PubMed.
- N. Ninduwezuor-Ehiobu, O. A. Tula, C. Daraojimba, K. A. Ofonagoro, O. A. Ogunjobi, J. O. Gidiagba, B. A. Egbokhaebho and A. A. Banso, Engineering Science & Technology Journal, 2023, 4, 140–168 Search PubMed.
- K. A. Olu-lawal, O. K. Olajiga, A. K. Adeleke, E. C. Ani and D. J. P. Montero, Int. J. Appl. Res. Soc. Sci., 2024, 6, 279–291 CrossRef.
- M. W. Tibbitt, C. B. Rodell, J. A. Burdick and K. S. Anseth, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 14444–14451 CrossRef CAS PubMed.
- C. C. Ahia and E. L. Meyer, Phys. Status Solidi A, 2023, 221(3), 2300293 CrossRef.
- S. Forest, D. McDowell, S. Müller and E. Werner, Oberwolfach Rep., 2024, 21, 657–730 Search PubMed.
- Z. Q. S. Nwokediegwu, K. I. Ibekwe, V. I. Ilojianya, E. A. Etukudoh, O. B. Ayorinde and C. Author, Engineering Science & Technology Journal, 2024, 5, 367–384 Search PubMed.
- V. K. Verma and S. Verma, Mater. Today Proc., 2024 DOI:10.1016/j.matpr.2024.05.004.
- R. S. Yadav, Monika, S. B. Rai and S. J. Dhoble, Prog. Solid State Chem., 2020, 57, 100267 CrossRef CAS.
- B. Moine and G. Bizarri, Mater. Sci. Eng., B, 2003, 105, 2–7 CrossRef.
- M. Cesaria and B. Di Bartolo, Nanomaterials, 2019, 9, 1048 CrossRef CAS PubMed.
- M. Cesaria and B. Di Bartolo, in Quantum Nano-Photonics, Springer, Dordrecht, 2018, pp. 27–77 Search PubMed.
- P. Kumar, D. Singh and H. Kumar, Mater. Chem. Phys., 2024, 320, 129418 CrossRef CAS.
- M. Rathaiah, P. Haritha, A. D. Lozano-Gorrín, P. Babu, C. K. Jayasankar, U. R. Rodríguez-Mendoza, V. Lavín and V. Venkatramu, Phys. Chem. Chem. Phys., 2016, 18, 14720–14729 RSC.
- D. Bidwai, N. K. Sahu, S. J. Dhoble, A. Mahajan, D. Haranath and G. Swati, Methods Appl. Fluoresc., 2022, 10, 032001 CrossRef CAS PubMed.
- L.-X. Yan, Z.-Y. Yan, X. Zhao, L.-J. Chen, T.-X. Liu and X.-P. Yan, J. Colloid Interface Sci., 2024, 662, 11–18 CrossRef CAS PubMed.
- P. Sehrawat, R. K. Malik, R. Punia, S. P. Khatkar and V. B. Taxak, J. Alloys Compd., 2021, 879, 160371 CrossRef CAS.
- W. Feng, C. Han and F. Li, Adv. Mater., 2013, 25(37), 5287–5303 CrossRef CAS PubMed.
- Y. I. Park, J. H. Kim, K. T. Lee, K. S. Jeon, H. B. Na, J. H. Yu, H. M. Kim, N. Lee, S. H. Choi, S. I. Baik, H. Kim, S. P. Park, B. J. Park, Y. W. Kim, S. H. Lee, S. Y. Yoon, I. C. Song, W. K. Moon, Y. D. Suh and T. Hyeon, Adv. Mater., 2009, 21, 4467–4471 CrossRef CAS.
- S. Ghosh and K. Ghosh, in Handbook of II–VI Semiconductor-Based Sensors and Radiation Detectors, Springer International Publishing, Cham, 2023, pp. 615–632 Search PubMed.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 5808–5829 CrossRef CAS PubMed.
- C. Yang, X. Zhang, J. Kang, C. Wei, P. Sang, S. Lin, B. Sun, J. Fan, B. Jiang, Y. Li, X. Chen, J. Xu, H. Chen and L. Zhang, J. Mater. Sci. Technol., 2023, 166, 1–20 CrossRef CAS.
- Z. Huang, B. Chen, B. Ren, D. Tu, Z. Wang, C. Wang, Y. Zheng, X. Li, D. Wang, Z. Ren, S. Qu, Z. Chen, C. Xu, Y. Fu and D. Peng, Adv. Sci., 2023, 10(3), 2204925 CrossRef CAS PubMed.
- Y. Xu, Y. Wang, L. Shi, L. Xing and X. Tan, Opt. Laser Technol., 2013, 54, 50–52 CrossRef CAS.
- A. M. Kaczmarek, K. Van Hecke and R. Van Deun, Inorg. Chem., 2014, 53, 9498–9508 CrossRef CAS PubMed.
- Z. Liu, Y. Dong, M. Fu and C. Wang, Dalton Trans., 2023, 52, 16819–16828 RSC.
- M. I. Khan, Y.-D. Chang, Q. Chen, J. Salta, Y.-S. Lee, C. J. O'Connor and J. Zubieta, Inorg. Chem., 1994, 33, 6340–6350 CrossRef CAS.
- E. J. Baran, Coord. Chem. Rev., 2024, 502, 215549 CrossRef CAS.
- M. Suzuki, T. Yamaguchi, N. Fukushima and M. Koyama, J. Appl. Phys., 2008, 103(3) DOI:10.1063/1.2838470.
- B. Chen, J. Yu and X. Liang, Langmuir, 2011, 27, 11654–11659 CrossRef CAS PubMed.
- A. Muley, S. B. Dhoble, P. Ramesh, R. S. Yadav and S. J. Dhoble, Prog. Solid State Chem., 2022, 66, 100347 CrossRef CAS.
- L. Xi, Y. Wang, L. Yin and P. D. Townsend, J. Alloys Compd., 2020, 820, 153094 CrossRef CAS.
- T. Gotoh, M. Jeem, L. Zhang, N. Okinaka and S. Watanabe, J. Phys. Chem. Solids, 2020, 142, 109436 CrossRef CAS.
- J. Niu, B. K. Rao and P. Jena, J. Chem. Phys., 1997, 107, 132–140 CrossRef CAS.
- A. M. Efa, K. A. Matori, M. H. M. Zaid, C. A. C. Abdullah, N. Zainuddin, M. Z. H. Mayzan and S. L. Kamis, Silicon, 2024, 16, 5327–5336 CrossRef CAS.
- A. Ruckman, G. Beckler, W. Guthrie, M. Jesuit, M. Boyd, I. Slagle, R. Wilson, N. Barrow, N. S. Tagiara, E. I. Kamitsos, S. Feller and C. B. Bragatto, Solid State Ionics, 2021, 359, 115530 CrossRef CAS.
- N. I. Leonyuk, V. V. Maltsev and E. A. Volkova, Molecules, 2020, 25, 2450 CrossRef CAS PubMed.
- S. Wang, Y. Xu, T. Chen, W. Jiang, J. Liu, X. Zhang, W. Jiang and L. Wang, Chem. Eng. J., 2021, 404, 125912 CrossRef CAS.
- K. Kirdsiri, R. Rajaramakrishna, B. Damdee, H. J. Kim, N. Nuntawong, M. Horphathum and J. Kaewkhao, Solid State Sci., 2019, 89, 57–66 CrossRef CAS.
- M. Mutailipu, K. R. Poeppelmeier and S. Pan, Chem. Rev., 2021, 121, 1130–1202 CrossRef CAS PubMed.
- M. Takesue, H. Hayashi and R. L. Smith, Prog. Cryst. Growth Charact. Mater., 2009, 55(3–4), 98–124 CrossRef CAS.
- K. Sharma and S. V. Moharil, Cryst. Res. Technol., 2023, 58(6), 2200272 CrossRef CAS.
- N. Ugemuge, Y. R. Parauha and S. J. Dhoble, in Energy Materials: Fundamentals to Applications, Elsevier, 2021, pp. 445–480 Search PubMed.
- L. Qin, J. Chen, X. Chen, H. Shao and Z. Wang, J. Lumin., 2021, 238, 118228 CrossRef CAS.
- L. Zhao, F. Fan, X. Chen, Y. Wang, Y. Li and B. Deng, J. Mater. Sci.: Mater. Electron., 2018, 29, 5975–5981 CrossRef CAS.
- R. A. Barve, N. Suriyamurthy, B. S. Panigrahi and B. Venkatraman, Radiat. Prot. Dosim., 2015, 163, 430–438 CrossRef CAS PubMed.
- D. A. Bradley, R. P. Hugtenburg, A. Nisbet, A. T. A. Rahman, F. Issa, N. M. Noor and A. Alalawi, Appl. Radiat. Isot., 2012, 71, 2–11 CrossRef CAS PubMed.
- L. Kövér, A. Némethy, I. Cserny, A. Nisawa, Y. Ito and H. Adachi, Surf. Interface Anal., 1994, 22, 45–50 CrossRef.
- H. T. B. Thao and T. Yamakawa, Soil Sci. Plant Nutr., 2009, 55, 228–234 CrossRef CAS.
- A. Ducousso-Détrez, J. Fontaine, A. Lounès-Hadj Sahraoui and M. Hijri, Microorganisms, 2022, 10, 609 CrossRef PubMed.
- R. Murugavel, A. Choudhury, M. G. Walawalkar, R. Pothiraja and C. N. R. Rao, Chem. Rev., 2008, 108, 3549–3655 CrossRef CAS PubMed.
- H. Khiar, N. Barka and A. Puga, Coord. Chem. Rev., 2024, 510, 215814 CrossRef CAS.
- Y. Zhao, C. C. Lin, Y. Wei, T.-S. Chan and G. Li, Opt. Express, 2016, 24, 4316 CrossRef CAS PubMed.
- P. Sengupta, J. Hazard. Mater., 2012, 235–236, 17–28 CrossRef CAS PubMed.
- Q. Li, G. Wu, D. A. Cullen, K. L. More, N. H. Mack, H. T. Chung and P. Zelenay, ACS Catal., 2014, 4, 3193–3200 CrossRef CAS.
- S. M. Abo-Naf, M. S. El-Amiry and A. A. Abdel-Khalek, Opt. Mater., 2008, 30, 900–909 CrossRef CAS.
- Y. Xu, Y. Li, R. Lei, Y. Hua, F. Huang and S. Xu, J. Lumin., 2023, 263, 120110 CrossRef CAS.
- J. Dang, D. Mei, Y. Wu and Z. Lin, Coord. Chem. Rev., 2021, 431, 213692 CrossRef CAS.
- J.-C. G. Bünzli, Acc. Chem. Res., 2006, 39, 53–61 CrossRef PubMed.
- P. Dorenbos, J. Lumin., 2000, 91, 91–106 CrossRef CAS.
- L. Smentek, Phys. Rep., 1998, 297, 155–237 CrossRef CAS.
- R. C. Ropp, J. Electrochem. Soc., 1964, 111, 311 CrossRef CAS.
- P. Kim, A. Anderko, A. Navrotsky and R. Riman, Minerals, 2018, 8, 106 CrossRef.
- I. Gupta, S. Singh, S. Bhagwan and D. Singh, Ceram. Int., 2021, 47, 19282–19303 CrossRef CAS.
- S. K. Gupta, K. Sudarshan and R. M. Kadam, Mater. Today Commun., 2021, 27, 102277 CrossRef CAS.
- R. Rajeswari, N. Islavath, M. Raghavender and L. Giribabu, Chem. Rec., 2020, 20(2), 65–88 CrossRef CAS PubMed.
- X. Wang, J. Xu, J. Yu, Y. Bu, J. Marques-Hueso and X. Yan, Phys. Chem. Chem. Phys., 2020, 22(27), 15120–15162 RSC.
- A. N. Belsky and J. C. Krupa, Displays, 1999, 19, 185–196 CrossRef CAS.
- L. A. Riseberg and M. J. Weber, in Progress in Optics, ed. E. Wolf, 1977, vol. 14, pp. 89–159 Search PubMed.
- S. K. Gupta, K. S. Prasad, N. Pathak and R. M. Kadam, J. Mol. Struct., 2020, 1221, 128776 CrossRef CAS.
- Deepali and M. Jayasimhadri, J. Mater. Sci.: Mater. Electron., 2023, 34, 1999 CrossRef CAS.
- Z. Zhang, J. Yan, Q. Zhang, G. Tian, W. Jiang, J. Huo, H. Ni, L. Li and J. Li, Inorg. Chem., 2022, 61, 16484–16492 CrossRef CAS PubMed.
- R. K. Tamrakar, D. P. Bisen and N. Brahme, J. Radiat. Res. Appl. Sci., 2014, 7, 550–559 Search PubMed.
- J.-H. Lee, H.-J. Park, K. Yoo, B.-W. Kim, J. C. Lee and S. Park, J. Eur. Ceram. Soc., 2007, 27, 965–968 CrossRef CAS.
- C.-C. Lin, K.-M. Lin and Y.-Y. Li, J. Lumin., 2007, 126, 795–799 CrossRef CAS.
- P. M. Kakade, A. R. Kachere, P. D. Sahare, A. V. Deshmukh, S. D. Dhole, S. R. Jadkar and N. T. Mandlik, J. Alloys Compd., 2022, 928, 167106 CrossRef CAS.
- L. G. Jacobsohn, M. W. Blair, S. C. Tornga, L. O. Brown, B. L. Bennett and R. E. Muenchausen, J. Appl. Phys., 2008, 104(12), 124303 CrossRef.
- Z. Chen and Y. Yan, J. Mater. Sci., 2006, 41, 5793–5796 CrossRef CAS.
- L. Wang, S. Liu, K. Huang, X. Ye, Y. Yang and Z. Zhou, J. Rare Earths, 2011, 29, 1049–1052 CrossRef CAS.
- T. Kim and S. Kang, Mater. Res. Bull., 2005, 40, 1945–1954 CrossRef CAS.
- M. Vasile, P. Vlazan and N. M. Avram, J. Alloys Compd., 2010, 500, 185–189 CrossRef CAS.
- A. V. Murugan, A. K. Viswanath, V. Ravi, B. A. Kakade and V. Saaminathan, Appl. Phys. Lett., 2006, 89(12), 123120 CrossRef.
- M. K. Mahata, K. Kumar and V. K. Rai, Spectrochim. Acta, Part A, 2014, 124, 285–291 CrossRef CAS PubMed.
- W. Pan, G. Ning, X. Zhang, J. Wang, Y. Lin and J. Ye, J. Lumin., 2008, 128, 1975–1979 CrossRef CAS.
- C. E. Rivera-Enríquez, A. Fernández-Osorio and J. Chávez-Fernández, J. Alloys Compd., 2016, 688, 775–782 CrossRef.
- M.-H. Fang, Z. Bao, W.-T. Huang and R.-S. Liu, Chem. Rev., 2022, 122, 11474–11513 CrossRef CAS PubMed.
- S. Saha, S. Das, U. K. Ghorai, N. Mazumder, B. K. Gupta and K. K. Chattopadhyay, Dalton Trans., 2013, 42, 12965 RSC.
- Z. Guo, H. Jiang, H. Li, H. Zhang, C. Liu, R. Zhao, Z. Yang, H. Tang, J. Li, J. Zhang and J. Zhu, Appl. Mater. Today, 2024, 37, 102095 CrossRef.
- M. S. N., C. M., R. Naik, R. V., N. H., R. C. R., S. B. S. and N. k. A., Luminescence, 2023, 38, 1149–1166 CrossRef CAS PubMed.
- K. Lamonova, I. Danilenko, G. Volkova, S. Orel, Y. Pashkevich, A. Prokhorov, O. Viagin, P. Maksimchuk, V. Seminko, Y. Malyukin and V. Kurnosov, J. Lumin., 2022, 241, 118493 CrossRef CAS.
- Y. Z. Halefoglu, G. Souadi, M. Ayvacikli, K. Bulcar, M. Topaksu, A. Canimoglu, O. Madkhali, R. Karmouch and N. Can, Appl. Radiat. Isot., 2024, 203, 111101 CrossRef CAS PubMed.
- K. Panigrahi, S. Saha, S. Sain, R. Chatterjee, A. Das, U. K. Ghorai, N. S. Das and K. K. Chattopadhyay, Dalton Trans., 2018, 47, 12228–12242 RSC.
- R. Cao, Z. Lai, Y. Cao, F. Cheng, C. Liao, S. Nie, X. Yi and J. Wang, New J. Chem., 2023, 47, 10025–10035 RSC.
- M. Liu, H. Hao, G. Liao, C. Li, K. Liu, N. Wang, Q. Niu and X. Yin, Adv. Opt. Mater., 2023, 11(15), 2202918 CrossRef CAS.
- M. Shivram, S. C. Prashantha, H. Nagabhushana, S. C. Sharma, K. Thyagarajan, R. Harikrishna and B. M. Nagabhushana, Spectrochim. Acta, Part A, 2014, 120, 395–400 CrossRef CAS PubMed.
- T. M. Mazzo, L. M. da Rocha Oliveira, L. R. Macario, W. Avansi, R. da Silveira André, I. L. V. Rosa, J. A. Varela and E. Longo, Mater. Chem. Phys., 2014, 145, 141–150 CrossRef CAS.
- D. K. Singh and J. Manam, J. Mater. Sci.: Mater. Electron., 2016, 27, 10371–10381 CrossRef CAS.
- S. Sasidharan, G. Jyothi and K. G. Gopchandran, J. Lumin., 2021, 235, 118048 CrossRef CAS.
- D. K. Singh and J. Manam, Electron. Mater. Lett., 2017, 13, 292–301 CrossRef CAS.
- V. V. Shanbhag, S. C. Prashantha, T. R. S. Shekhar, H. Nagabhushana, R. Naik, K. M. Girish, S. Ashwini, D. Rangappa and D. S. Prasanna, Ceram. Int., 2021, 47, 10346–10354 CrossRef CAS.
- P. Kumar, D. Singh, I. Gupta and H. Kumar, Ceram. Int., 2023, 49, 29010–29024 CrossRef CAS.
- P. Kumar, D. Singh and I. Gupta, J. Alloys Compd., 2023, 966, 171410 CrossRef CAS.
- P. Kumar, D. Singh and I. Gupta, RSC Adv., 2023, 13, 7703–7718 RSC.
- A. Hooda, A. Khatkar, M. Kumar, R. K. Malik, S. Devi, S. P. Khatkar and V. B. Taxak, J. Alloys Compd., 2020, 823, 153641 CrossRef CAS.
- A. Hooda, S. P. Khatkar, S. Devi and V. B. Taxak, Optik, 2022, 257, 168774 CrossRef CAS.
- S. Kaushik, S. Devi, M. Kumar, H. Dalal, N. Sehrawat, R. K. Malik, A. Srivastava and M. Srivastava, Appl. Phys. A: Mater. Sci. Process., 2022, 128, 198 CrossRef CAS.
- P. Phogat, S. P. Khatkar, R. K. Malik, J. Dalal, A. Hooda and V. B. Taxak, J. Lumin., 2021, 234, 117984 CrossRef CAS.
- P. Devi, P. Sehrawat, M. Sheoran, H. Dalal, N. Sehrawat and R. K. Malik, J. Mater. Sci.: Mater. Electron., 2023, 34, 867 CrossRef CAS.
- D. Solanki, P. Devi, H. Dalal, N. Sehrawat, M. Kumar, O. Garg and R. K. Malik, Indian J. Phys., 2025, 99, 1867–1879 CrossRef CAS.
- P. Phogat, S. P. Khatkar, R. K. Malik, J. Dalal, M. Punia and V. B. Taxak, J. Mater. Sci.: Mater. Electron., 2021, 32, 8615–8627 CrossRef CAS.
- P. Phogat, S. P. Khatkar, R. K. Malik, Sushma, J. Dalal, P. Hooda and V. B. Taxak, Mater. Chem. Phys., 2021, 270, 124828 CrossRef CAS.
- H. Dalal, M. Kumar, S. Kaushik, P. Sehrawat, M. Sheoran, N. Sehrawat and R. K. Malik, J. Electron. Mater., 2023, 52, 2780–2793 CrossRef CAS.
- H. Dalal, M. Kumar, P. Devi, N. Sehrawat, D. Solanki, S. Kumar and R. K. Malik, J. Opt., 2024, 1–11 Search PubMed.
- P. Kumar, D. Singh, I. Gupta, S. Singh, S. Nehra and R. Kumar, J. Lumin., 2023, 257, 119703 CrossRef CAS.
- P. Kumar, D. Singh, I. Gupta, S. Singh, S. Nehra and R. Kumar, Opt. Mater., 2023, 138, 113677 CrossRef CAS.
- A. Hooda, S. P. Khatkar, A. Khatkar, R. K. Malik, J. Dalal, S. Devi and V. B. Taxak, Mater. Chem. Phys., 2019, 232, 39–48 CrossRef CAS.
- S. Devi, A. Khatkar, A. Hooda, V. B. Taxak, P. Boora, P. Dhankhar and S. P. Khatkar, J. Solid State Chem., 2020, 288, 121333 CrossRef CAS.
- P. Sehrawat, A. Khatkar, P. Boora, M. Kumar, R. K. Malik, S. P. Khatkar and V. B. Taxak, Ceram. Int., 2020, 46, 16274–16284 CrossRef CAS.
- H. Dalal, M. Kumar, P. Sehrawat, M. Sheoran, N. Sehrawat, S. Kumar and R. K. Malik, J. Mater. Sci.: Mater. Electron., 2022, 33, 13743–13756 CrossRef CAS.
- P. Kumar, D. Singh, I. Gupta, S. Singh and V. Kumar, Luminescence, 2022, 37, 2028–2040 CrossRef CAS PubMed.
- P. Chhillar and P. B. Doon, Inorg. Chem. Commun., 2024, 159, 111844 CrossRef CAS.
- I. Gupta, D. Singh, S. Singh, P. Kumar, S. Bhagwan and V. Kumar, Chem. Phys. Lett., 2023, 814, 140350 CrossRef CAS.
- P. Jamwal, N. Lalotra, P. Sharma and K. Pathania, Bull. Mater. Sci., 2024, 47, 186 CrossRef CAS.
- P. Biswas, V. Kumar, O. M. Ntwaeaborwa and H. C. Swart, Mater. Res. Express, 2015, 2(2), 025010 CrossRef CAS.
- K. Panigrahi and A. Nag, J. Phys. Chem. C, 2022, 126, 8553–8564 CrossRef CAS.
- T. Takeda, Y. Koyama, H. Ikeno, S. Matsuishi and N. Hirosaki, Sci. Technol. Adv. Mater., 2024, 25(1), 2421761 CrossRef PubMed.
- M. Novita, A. S. Chauhan, R. M. D. Ujianti, D. Marlina, H. Kusumo, M. T. Anwar, M. Piasecki and M. G. Brik, J. Lumin., 2024, 269, 120476 CrossRef CAS.
- J. Kumar and I. Roy, Talanta Open, 2024, 9, 100302 CrossRef.
- L. Tashi, M. Kumar, Z. ul Nisa, N. Nelofar and H. N. Sheikh, New J. Chem., 2020, 44, 1009–1020 RSC.
- G. M. Khairy, A. S. Amin, S. M. N. Moalla, A. Medhat and N. Hassan, RSC Adv., 2022, 12, 27679–27686 RSC.
- M. Pajewska-Szmyt, B. Buszewski and R. Gadzała-Kopciuch, Mater. Chem. Phys., 2020, 242, 122484 CrossRef CAS.
- L. Tashi, M. Kumar, Z. ul Nisa, N. Nelofar and H. N. Sheikh, New J. Chem., 2020, 44, 1009–1020 RSC.
- M. Saif, R. Kamal and H. S. Hafez, J. Alloys Compd., 2019, 803, 658–663 CrossRef CAS.
- H. R. Mahmoud, M. Saif and R. Fouad, J. Alloys Compd., 2019, 805, 887–895 CrossRef CAS.
- R. Singhaal, L. Tashi, Z. ul Nisa, N. A. Ashashi, C. Sen, S. Devi and H. N. Sheikh, RSC Adv., 2021, 11, 19333–19350 RSC.
- J. Kumar and I. Roy, Environ. Sci.: Nano, 2025, 12(5), 2699–2709 RSC.
- L. Marciniak, K. Kniec, K. Elżbieciak-Piecka, K. Trejgis, J. Stefanska and M. Dramićanin, Coord. Chem. Rev., 2022, 469, 214671 CrossRef CAS.
- D. Sevic, M. S. Rabasovic, J. Krizan, S. Savic-Sevic, M. G. Nikolic, B. P. Marinkovic and M. D. Rabasovic, J. Phys. D Appl. Phys., 2019, 53, 015106 CrossRef.
- K. R, P. A, M. M. Kennedy S, V. Mishra, M. I. Sayyed, M. Rashad and S. D. Kamath, Phys. B, 2025, 697, 416712 CrossRef CAS.
- S. Gouraha, N. Jain, K. Anchal, G. Patel, S. Banerjee and J. Singh, Sci. Rep., 2024, 14, 27385 CrossRef CAS PubMed.
- S. Gouraha, S. Sinha, A. Srivastava and J. Singh, J. Photochem. Photobiol., A, 2025, 458, 115967 CrossRef CAS.
- Y. Wang, Y. Song, Y. Li, T. Cui, X. Zhou, Y. Sheng, K. Zheng, H. You and H. Zou, New J. Chem., 2017, 41, 709–716 RSC.
- I. E. Kolesnikov, E. V. Afanaseva, M. A. Kurochkin, E. I. Vaishlia, A. A. Kalinichev, E. Y. Kolesnikov and E. Lähderanta, ACS Appl. Mater. Interfaces, 2022, 14, 1757–1764 CrossRef CAS PubMed.
- I. E. Kolesnikov, E. V Afanaseva, M. A. Kurochkin, E. I. Vaishlia, E. Y. Kolesnikov and E. Lähderanta, Nanotechnology, 2022, 33, 165504 CrossRef PubMed.
- I. E. Kolesnikov, D. V. Mamonova, M. A. Kurochkin, V. A. Medvedev, G. Bai and E. Y. Kolesnikov, J. Alloys Compd., 2023, 965, 171388 CrossRef CAS.
- A. A. Kalinichev, M. A. Kurochkin, E. V. Golyeva, A. V. Kurochkin, E. Lähderanta, M. D. Mikhailov and I. E. Kolesnikov, J. Lumin., 2018, 195, 61–66 CrossRef CAS.
- I. E. Kolesnikov, A. A. Kalinichev, M. A. Kurochkin, E. V. Golyeva, E. Y. Kolesnikov, A. V. Kurochkin, E. Lähderanta and M. D. Mikhailov, Sci. Rep., 2017, 7, 18002 CrossRef CAS PubMed.
- H. Zhou, W. Gao, P. Cai, B. Zhang and S. Li, Solid State Sci., 2020, 104, 106283 CrossRef CAS.
- I. E. Kolesnikov, D. V. Mamonova, M. A. Kurochkin, E. Y. Kolesnikov, E. Lähderanta and A. A. Manshina, ACS Appl. Nano Mater., 2021, 4, 12481–12489 CrossRef CAS.
- M. K. Mahata, T. Koppe, T. Mondal, C. Brüsewitz, K. Kumar, V. K. Rai, H. Hofsäss and U. Vetter, Phys. Chem. Chem. Phys., 2015, 17, 20741–20753 RSC.
- M. Mondal and V. K. Rai, J. Ind. Eng. Chem., 2018, 60, 125–132 CrossRef CAS.
- J. Liao, Q. Wang, L. Lan, J. Guo, L. Nie, S. Liu and H. R. Wen, Curr. Appl. Phys., 2017, 17, 427–432 CrossRef.
- Q. Wu, S. Li, C. Li, Q. Xu, J. Sun, Z. Kang and C. Song, Appl. Phys. B, 2018, 124, 199 CrossRef.
- L. Mukhopadhyay and V. K. Rai, New J. Chem., 2018, 42, 13122–13134 RSC.
- A. Kumari, L. Mukhopadhyay and V. K. Rai, J. Rare Earths, 2019, 37, 242–247 CrossRef CAS.
- S. K. Ranjan, M. Mondal and V. K. Rai, Mater. Res. Bull., 2018, 106, 66–73 CrossRef CAS.
- T. Pang and J. Wang, Mater. Res. Express, 2018, 5(1), 2018 Search PubMed.
- Q. Qiang and Y. Wang, New J. Chem., 2019, 43, 5011–5019 RSC.
- E. Glais, M. Pellerin, V. Castaing, D. Alloyeau, N. Touati, B. Viana and C. Chanéac, RSC Adv., 2018, 8, 41767–41774 RSC.
- S. Tamboli, G. B. Nair, A. K. Sharma, S. J. Dhoble and H. C. Swart, Mater. Res. Bull., 2024, 169, 112503 CrossRef CAS.
- J. Sabol and P. Weng, Introduction to Radiation Protection Dosimetry, World Scientific, 1995 Search PubMed.
- A. Deshpande and S. J. Dhoble, in Radiation Dosimetry Phosphors, Elsevier, 2022, pp. 277–299 Search PubMed.
- S. Salminen-Paatero and J. Paatero, Int. J. Environ. Res. Publ. Health, 2021, 18, 10577 CrossRef CAS PubMed.
- D. Shahbazi-Gahrouei, M. Gholami and S. Setayandeh, Adv. Biomed. Res., 2013, 2, 65 CrossRef PubMed.
- W. L. Mclaughlin and M. F. Desrosiers, Radiat. Phys. Chem., 1995, 46, 1163–1174 CrossRef CAS.
- M. Martini and F. Meinardi, Riv. Nuovo Cimento, 1997, 20, 1–71 CrossRef CAS.
- T. Katsumata, R. Sakai, S. Komuro and T. Morikawa, J. Electrochem. Soc., 2003, 150, H111 CrossRef CAS.
- T. Kron, Radiat. Prot. Dosim., 1999, 85, 333–340 CrossRef CAS.
- S. K. Omanwar, K. A. Koparkar and H. S. Virk, Defect Diffusion Forum, 2013, 347, 75–110 Search PubMed.
- Y. R. Parauha and S. J. Dhoble, in Rare-Earth-Activated Phosphors: Chemistry and Applications, Elsevier, 2022, pp. 241–272 Search PubMed.
- A. R. Kadam, Y. R. Parauha, M. Michalska-Domanska, N. S. Dhoble and S. J. Dhoble, in Radiation Dosimetry Phosphors: Synthesis, Mechanisms, Properties and Analysis, Elsevier, 2022, pp. 251–277 Search PubMed.
- N. J. Shivaramu, B. N. Lakshminarasappa, K. R. Nagabhushana, F. Singh and H. C. Swart, Mater. Res. Express, 2017, 4, 115033 CrossRef.
- P. M. Kakade, A. R. Kachere, P. D. Sahare, A. V. Deshmukh, S. D. Dhole, S. R. Jadkar and N. T. Mandlik, J. Alloys Compd., 2022, 928, 167106 CrossRef CAS.
- A. Kumar, A. Kumar, R. Dogra, M. Manhas, S. Sharma, N. Singh and R. Kumar, Optik, 2020, 216, 164965 CrossRef CAS.
- A. Kumar, A. Kumar, R. Dogra, M. Manhas, S. Sharma and R. Kumar, Ceram. Int., 2018, 44, 15535–15541 CrossRef CAS.
- A. Fernández-Osorio, R. Redón, J. Medina-Pérez, M. Pedroza-Montero and M. Acosta, J. Clust. Sci., 2022, 33, 653–664 CrossRef.
- R. Nattudurai, D. Arous, N. F. J. Edin, A. Pandey and E. Malinen, Radiat. Phys. Chem., 2023, 208, 110928 CrossRef CAS.
- A. Barad, M. Topaksu, J. Hakami and N. Can, Ceram. Int., 2024, 50, 11458–11468 CrossRef CAS.
- S. Bahl, S. P. Lochab and P. Kumar, Radiat. Phys. Chem., 2016, 119, 136–141 CrossRef CAS.
- N. Mandlik, S. D. Dhole, P. D. Sahare, J. S. Bakare, A. Balraj and B. C. Bhatt, Appl. Radiat. Isot., 2019, 148, 253–261 CrossRef CAS PubMed.
- N. T. Mandlik, P. D. Sahare and S. D. Dhole, Appl. Radiat. Isot., 2020, 159, 109080 CrossRef CAS PubMed.
- Y. S. Vidya and B. N. Lakshminarasappa, Appl. Phys. A: Mater. Sci. Process., 2015, 118, 249–260 CrossRef CAS.
- P. Pathak, M. Singh, P. K. Mishra, S. Jani, R. Gupta, R. Brajpuriya and I. P. Sahu, Next Mater., 2025, 6, 100391 CrossRef.
- H. Nanto and G. Okada, Jpn. J. Appl. Phys., 2023, 62, 010505 CrossRef CAS.
- A. R. Kadam, Y. R. Parauha, M. Michalska-Domanska, N. S. Dhoble and S. J. Dhoble, in Radiation Dosimetry Phosphors, Elsevier, 2022, pp. 251–277 Search PubMed.
- S. Saadi, D. E. Kdib, A. Boukerika, R. Berreksi, A. Bentabet, S. Mahtout and Z. L. Mokrani, Radiat. Meas., 2024, 178, 107296 CrossRef CAS.
- A. D. Vartha, P. R. Patankar, P. D. Sahare, L. Sharma, A. R. Kachere, P. M. Kakade, B. Bai, S. D. Dhole and N. T. Mandlik, J. Radioanal. Nucl. Chem., 2024, 333, 4503–4522 CrossRef CAS.
- T. G. V. Kumar, K. Pavani, A. J. Neves, K. Munirathnam, S. N. Kumar, G. V. A. Reddy and B. Sreenivasulu, Appl. Phys. A: Mater. Sci. Process., 2024, 130, 49 CrossRef CAS.
- P. Seth, A. Jain, L. Goswami, G. Gupta and S. Aggarwal, J. Alloys Compd., 2023, 965, 171329 CrossRef CAS.
- A. Lingappa and B. M. Manohara, J. Lumin., 2022, 241, 118476 CrossRef CAS.
- M. Agarwal, S. K. Garg, K. Asokan and P. Kumar, J. Alloys Compd., 2020, 823, 153740 CrossRef CAS.
- N. T. Mandlik, P. D. Sahare, S. R. Rondiya, N. Y. Dzade, A. V. Deore, S. S. Dahiwale and S. D. Dhole, Opt. Mater., 2020, 109, 110272 CrossRef CAS.
- A. K. Bedyal, V. Kumar, O. M. Ntwaeaborwa and H. C. Swart, Ceram. Int., 2016, 42, 18529–18535 CrossRef CAS.
- M. S. Bhadane, S. S. Dahiwale, K. R. Sature, B. J. Patil, N. T. Mandlik, V. N. Bhoraskar and S. D. Dhole, J. Alloys Compd., 2017, 695, 1918–1923 CrossRef CAS.
- M. Zahedifar, E. Sadeghi, M. R. Mozdianfard and E. Habibi, Appl. Radiat. Isot., 2013, 78, 125–131 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |











