Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Construction of novel Bi2S3@Zn-Co-cLDHs heterojunction for enhanced photocatalytic degradation of levofloxacin with persulfate activation under visible light: mechanism and degradation pathway
Nguyen Thi Mai Tho * and
Minh An Tran Nguyen
* and
Minh An Tran Nguyen
Faculty of Chemical Engineering, Industrial University of Ho Chi Minh City, Ho Chi Minh City, Vietnam. E-mail: nguyenthimaitho@iuh.edu.vn
First published on 21st July 2025
Abstract
This study effectively synthesized the novel Bi2S3@Zn-Co calcined layered double hydroxides heterojunction (Bi2S3@ZC-cLDHs) via co-precipitation and thermal methods. ZC-LDHs built with a Zn2+/Co2+ molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, after calcination at 600 °C, yielded a blend of ZnO and ZnCo2O4 oxide, uniformly distributed on Bi2S3 rods. Bi2S3@ZC-cLDHs heterostructures exhibited superior photocatalytic efficiency for levofloxacin (LF) degradation compared to Bi2S3 and ZC-cLDHs under same catalytic conditions. The enhanced photodegradation efficiency results from the increased surface area and the establishment of a heterojunction at the interface of Bi2S3 rods and ZC-cLDHs. In addition, the photocatalytic degradation efficiency of LF enhanced from 74.8% to 90.1% with the addition of persulfate (PS) as an activating under visible light, utilizing a catalyst loading of Bi2S3@ZC-cLDHs at 1.0 g L−1, initial concentration of 20 ppm, PS loading of 0.25 g L−1, and light exposure duration of 90 minutes. The Z-scheme established the photocatalytic mechanism for the degradation of LF using Bi2S3@ZC-cLDHs with PS activation. Radical trapping tests demonstrated that O2˙− and h+ were the significant active species. The combination of PS and catalyst had a synergistic effect, wherein S2O82− interacted with electrons to create SO4˙− during the photocatalytic process. The analysis using LC-MS provided a thorough understanding of possible photocatalytic breakdown path of LF; the photoproducts were small-sized molecules with little impact on the environment.
1, after calcination at 600 °C, yielded a blend of ZnO and ZnCo2O4 oxide, uniformly distributed on Bi2S3 rods. Bi2S3@ZC-cLDHs heterostructures exhibited superior photocatalytic efficiency for levofloxacin (LF) degradation compared to Bi2S3 and ZC-cLDHs under same catalytic conditions. The enhanced photodegradation efficiency results from the increased surface area and the establishment of a heterojunction at the interface of Bi2S3 rods and ZC-cLDHs. In addition, the photocatalytic degradation efficiency of LF enhanced from 74.8% to 90.1% with the addition of persulfate (PS) as an activating under visible light, utilizing a catalyst loading of Bi2S3@ZC-cLDHs at 1.0 g L−1, initial concentration of 20 ppm, PS loading of 0.25 g L−1, and light exposure duration of 90 minutes. The Z-scheme established the photocatalytic mechanism for the degradation of LF using Bi2S3@ZC-cLDHs with PS activation. Radical trapping tests demonstrated that O2˙− and h+ were the significant active species. The combination of PS and catalyst had a synergistic effect, wherein S2O82− interacted with electrons to create SO4˙− during the photocatalytic process. The analysis using LC-MS provided a thorough understanding of possible photocatalytic breakdown path of LF; the photoproducts were small-sized molecules with little impact on the environment.
1 Introduction
Antibiotics are pharmacological agents that may suppress bacterial proliferation, therefore used for the treatment of bacterial infections, inflammation, and several other diseases.1,2 Levofloxacin (LF) is a third-generation fluoroquinolone antibiotic that has a wide range of antibacterial action in both people and animals.3 LF has significant chemical stability, bio-refractory properties, demonstrates little metabolic clearance in humans, and is released into the environment post-usage.4,5 The discharged LF may ultimately enter water sources, leading to the emergence of drug-resistant bacteria and antibiotic resistance, posing a threat to aquatic creatures and human health.6 Consequently, finding reliable and practical ways to eliminate medicines like LF from the aquatic environment is thus urgent.Layered double hydroxides (LDHs) are anionic clay minerals characterized by a layered structure and the general formula [M2+1−aM3+a(OH)2]a+·[An−a/n]·mH2O, where M2+ represents a divalent metal cation and M3+ denotes a trivalent metal cation, has a positively charged octahedral structure.7,8 It equilibrates these positively charged layers by interposing negatively charged inorganic or organic anions between them. LDHs serve as precursors for cLDHs upon calcination at an appropriate temperature, resulting in the decomposition of LDHs into cLDHs, which include divalent metal oxides and spinel oxides. The capacity of cLDHs to restructure LDHs via the method of “memory effect” has led to prompted significant research in the domain of adsorption.9,10
Nowadays, advanced oxidation processes (AOPs) using semiconductor photocatalysis have become a crucial and highly endorsed method for the effective degradation of organic pollutants, especially antibiotics, due to their high efficiency, cost-effectiveness, simplicity, and energy conservation.1,11,12 Numerous semiconductor materials have been investigated potentially photocatalytic materials by absorbing visible light and producing reactive free radicals (ROS) ˙OH, O2˙−; photogenerated electrons, and holes (e−/h+) for the degradation of organic waste.13,14 The special interactive effects of the two oxide components of cLDHs with larger surface areas than LDHs have led to the recent widespread application of cLDHs in photocatalysis.
Certain research has shown considerable success in using cLDHs or cLDHs heterostructures as photocatalysts for the degradation of contaminants such as CoFe-cLDHs,7 NiCoFe-LDHs, Ti-MOF/NiFeLDHs8 nevertheless, there is a limitation of studies on ZnCo-cLDHs.
Wide light absorption range, effective charge separation, superior redox potential, and exceptional stability are all desirable properties of a photocatalyst.3 Upon evaluating the characteristics, pure semiconductors exhibit notable deficiencies, including insufficient band gap energy in Bi2S3, resulting in rapid recombination of e−/h+ pairs,15,16 and excessive band gap energy in ZnO,17 which hinders light absorption, thereby contributing to the low efficiency of organic matter decomposition. To increase the durability of materials and the efficiency of photoinduced pair separation, researchers have implemented several active strategies, which includes doping metals and decorating substrate on semiconductors, or coupling with other semiconductors to create heterojunction photocatalysts.3,14,18
In addition, to improve the catalytic efficacy of semiconductors, an enhanced oxidation technique using activated persulfate has been investigated for pollutant treatment.19,20 Usually, PS does not directly interact with pollutants; however, it is activated by solar-driven photocatalytic materials to generate reactive species, hence improving its capacity to decompose pollutants. At this time, persulfate and catalyst have a synergistic effect; S2O82− quickly traps photogenerated electrons to produce SO4˙−, decreasing the recombination of charge carriers, and SO4˙− may also interact with H2O to provide ˙OH radicals, therefore augmenting the capacity to destroy organic substances.7,21,22
From the researched strategies to improve the photocatalytic efficiency of semiconductor materials, specifically cLDHs. In this work, we addressed the following problems: (1) synthesis of cLDHs materials using LDHs precursors obtained from cobalt and zinc salts via the co-precipitation method, disperse on the surface of Bi2S3 rods (Bi2S3@ZC-cLDHs). (2) A comprehensive examination of the photocatalytic of Bi2S3@ZC-cLDHs for activating PS to degrade LF antibiotics in simulated light conditions. (3) Investigation of the photocatalytic mechanism of Bi2S3@ZC-cLDHs, activation mechanism of PS, and identification of possible degradation pathway of LF. (4) Evaluating the reusability and stability of Bi2S3@ZC-cLDHs heterostructures.
2 Experimental
2.1 Materials and chemicals
Bismuth(III) nitrate pentahydrate (Bi(NO3)3·5H2O, 98.0%), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O; 98%); zinc acetate dihydrate (Zn(CH3COO)2·6H2O, 98%), sodium hydroxide (NaOH, 99%) was purchased from Sigma-Aldrich, USA. Ethylene glycol ((CH2OH)2, 99%), levofloxacin ((C18H20FN3O4) 99%); ethanol (C2H5OH, 99.5%); tert-butanol (99.5%); p-benzoquinone; ethylenediamine tetra acetic acid disodium salt (Na2EDTA, 99%); cexadecyl trimethyl ammonium bromide (CTAB, 99%) and thiourea (H2NCSNH2, 99%), were purchased from Xilong Scientific Co., Ltd China.2.2 Synthesis of Bi2S3@ZC-cLDHs
2.3 Photocatalytic activity evaluation
The photocatalytic efficacy of the synthesized samples was evaluated for the degradation of LF using PS as an activator under visible light. The photocatalytic occurred in a catalytic system including 0.1 g of Bi2S3@ZC-cLDHs evenly spread in 100 mL of LF 20 ppm (Cin). The photocatalytic system has two glass layers; the inner layer is LF solution and catalyst to create a uniformly stirred suspension throughout the reaction, while the outer layer features a steady water circulation system kept at the room temperature. A 300 W halogen lamp (64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 HLX 150 W, 24 V, Osram, Germany) simulating visible light was emitted with a maximum intensity of approximately 100 mW cm−2, directed at the suspension. Before being exposed to lighting, the suspension is stirred in darkness for 30 minutes, for the suspension establishes equilibrium/desorption, following which the LF concentration (Co) is measured. The loading of PS activator is introduced into the photocatalytic system during the commencement of illumination, which lasts for 90 minutes. 5 mL of the suspension in the system are removed and filtered through a 0.22 mm filter to eliminate solids after fifteen minutes of illumination. With wavelength of 290 nm for LF, the UV-vis spectrophotometer was used to track the concentration of the degenerated solution (Ct) over time. To examine the variables impacting the photocatalytic process, the influencing parameters will be altered while the other parameters stay unchanged. Analysis of reactive oxygen species, suitable quantities of p-benzoquinone (p-BQ), methanol (MeOH), tert-butanol (TBA), and Na2-EDTA were added into the photocatalytic system before beginning light irradiation.
640 HLX 150 W, 24 V, Osram, Germany) simulating visible light was emitted with a maximum intensity of approximately 100 mW cm−2, directed at the suspension. Before being exposed to lighting, the suspension is stirred in darkness for 30 minutes, for the suspension establishes equilibrium/desorption, following which the LF concentration (Co) is measured. The loading of PS activator is introduced into the photocatalytic system during the commencement of illumination, which lasts for 90 minutes. 5 mL of the suspension in the system are removed and filtered through a 0.22 mm filter to eliminate solids after fifteen minutes of illumination. With wavelength of 290 nm for LF, the UV-vis spectrophotometer was used to track the concentration of the degenerated solution (Ct) over time. To examine the variables impacting the photocatalytic process, the influencing parameters will be altered while the other parameters stay unchanged. Analysis of reactive oxygen species, suitable quantities of p-benzoquinone (p-BQ), methanol (MeOH), tert-butanol (TBA), and Na2-EDTA were added into the photocatalytic system before beginning light irradiation.
3 Results and discussion
3.1 Characterization
The X-ray diffraction patterns (XRD) of the Bi2S3, ZC-cLDHs, and Bi2S3@ZC-cLDHs as synthesized samples are shown in Fig. 1A. The ZC-cLDHs sample exhibits peaks at 2θ positions 31.77°; 34.34°; 36.56°; 47.61°; 56,61°; 62.82°; 65.13°; 68.12° and 69.11° corresponding to the (100), (002), (101), (102), (110), (200), (112), and (004) planes, aligning with the peaks of ZnO23 (JCPDS, 36-1451), and the peaks at 2θ positions 31.5°; 36.76°; 44.70°; 55.52°; 59.11° and 65.01° correspond to the (220), (311), (400), (422), (511) and (440) planes, recognizing with the standard peaks of ZnCo2O4 (refs. 24 and 25) (JCPDS-23-1390). Fig. 1A at 2θ from 30 to 40° shows the overlap of the diffraction peaks at locations 31.77° and 36.56° of ZnO and 31.5° and 36.76° of ZnCo2O4. | ||
| Fig. 1 (A) XRD; (B) FT-IR; (C) UV-Vis-DRS ; (D and E) the Eg and (F) PL of Bi2S3, ZC-cLDHs, and Bi2S3@ZC-cLDHs samples. | ||
The peaks at 2θ positions 25.17°; 28.65°; 31.93°; 33.05°; 35.81°.45.55°; 46.67°; 52.79°; 59.33°. 65.02° and 71.98° of the Bi2S3 sample match with the typical peaks of Bi2S3 (ref. 26 and 27) (JCPDS No. 17-0320). The Bi2S3@ZC-cLDHs sample, with a mass ratio of Bi2S3/ZC-cLDHs at 15%, exhibited diffraction results indicating that, aside from the characteristic peaks of ZnO and ZnCo2O4 phase, the low-intensity peaks of Bi2S3 were either not distinctly visible or nearly covered up by the obvious peaks of ZnO and ZnCo2O4, with only the intensity of the strong peaks at the position of Bi2S3 being obvious. Furthermore, the peaks were distinct and acute, with no anomalous peaks present, indicating that the synthesized materials exhibited great purity and few contaminants.
Fig. 1B presents the Fourier transform infrared spectroscopy (FT-IR) of Bi2S3, ZC-cLDHs, and Bi2S3@ZC-cLDHs samples. The Bi2S3 sample exhibits two peaks at 1101–1069 cm−1 and 839 cm−1, corresponding to the vibrations of the Bi–S bond.28,29 The bands at 1620 cm−1 and 3347 cm−1 correspond to the bending and stretching vibrations of O–H in adsorbed H2O. The ZC-cLDHs exhibit two vibrational frequencies at 654 and 562 cm−1, corresponding to the Zn–O and Co–O bonds,21,30 respectively. Specifically for the Bi2S3@ZC-cLDHs sample, along the vibrations of Zn–O and Co–O bonds, Bi–S vibrations are also present, indicating an interaction between Bi2S3 and ZC-cLDHs that results in the establishment of Bi2S3@ZC-cLDHs heterostructures.
Diffuse reflectance spectroscopy (UV-Vis DRS) was used for determining the optical absorption of the synthesized samples.30 The findings demonstrate that the absorption spectrum of Bi2S3 extends from the visible light spectrum to the infrared region.15 ZC-cLDHs demonstrates two absorption wavelengths at 405 and 604 cm−1. The XRD findings indicate that ZC-cLDHs has two phases: ZnO and ZnCo2O4. Consequently, the absorption wavelength at 405 nm corresponds with ZnO,31 while the absorption wavelength at 604 nm belongs to ZnCo2O4.32 The combination of ZC-cLDHs with Bi2S3 results in Bi2S3@ZC-cLDHs exhibiting an absorption edge at 455 nm, which is blue-shifted relative to ZC-cLDHs. This shift may result from the interaction between Bi2S3 and ZC-cLDHs, which modifies the energy band, transitioning from visible light to infrared light. The results of determining the maximum absorption wavelength of the synthesized materials and calculating the band gap energy (Eg) using the Kubelka–Munk function33 are shown in Fig. 1C. ZC-cLDHs has an absorption wavelength of 405 nm, corresponding to an energy gap (Eg) of 2.01 eV for ZnCo2O4, and wavelength of 604 nm, corresponding to 2.97 eV for ZnO. Bi2S3@ZC-cLDHs is characterized by two Eg values of 1.79 eV and 2.68 eV, respectively (Fig. 1D). According to Fig. 1E the Bi2S3 sample is determined to have Eg = 1.5 eV.
The photoluminescence (PL) intensity correlates with the amplitude of the photogenerated electron–hole separation.34 The carrier separation increases as the PL intensity decreases, indicating enhanced photodegradation efficacy.35 Fig. 1F illustrates the PL spectra of ZC-cLDHs; Bi2S3 and Bi2S3@ZC-cLDHs, all measured at an excitation wavelength of 320 nm. The image clearly indicates that ZC-cLDHs and Bi2S3 exhibit robust fluorescence, indicating rapid recombination of electrons and holes.34 The fluorescence intensity of the synthesized sample is much lower than that of Bi2S3@ZC-cLDHs, suggesting that the combination of Bi2S3-ZC-cLDHs efficiently suppresses electron–hole recombination, hence enhancing the LF breakdown of the composites.36 Bi2S3@ZC-cLDHs are expected to function well as photocatalysts within the visible light spectrum.
3.2 XPS
X-ray photoelectron spectroscopy (XPS) was used for evaluating the surface chemical composition and elemental valence state of the Bi2S3@ZC-cLDHs composite,33 as seen in Fig. 2. The full scan spectrum of Bi2S3@ZC-cLDHs reveals the presence of Zn, Co, Bi, and S with no other elements detected, indicating that the synthesized sample exhibits great purity (Fig. 2A). The peak of C 1s is applied to a reference for charge correction. The atomic percentages of C, O, Co, Zn, Bi and S are 24.08%; 47.74%; 10.29%; 16.48%; 0.75%; 0.66% respectively. Zn 2p1/2 and Zn 2p3/2 have binding energy peaks at 1044.72 eV and 1021.73 eV in the high-resolution spectrum of Zn (Fig. 2B). The high-resolution XPS spectra of Bi 4f and S 2p exhibits two significant symmetric peaks at binding energy levels of 164.61 eV and 158.8 eV, typical of Bi 4f2/5 and Bi 4f7/2, confirming that Bi is in the Bi3+ oxidation state.15,37 The peaks of Bi 4d3/2 and Bi 4d5/2, respectively, at 470 and 495 eV are also visible in Fig. 2A. The two energy peaks at 159.68 eV and 163.54 eV are situated between Bi 4f5/2 and Bi 4f7/2, indicative of S 2p3/2 and S 2p1/2.16 | ||
| Fig. 2 XPS survey spectra (A); high resolution core level spectra of (B) Bi–S; (C) Zn 2p; (D) Co 2p; and (E) O 1s for Bi2S3@ZC-cLDHs. | ||
This result implies the establishment of Bi–S bonds in Bi2S3-ZC-cLDHs. Fig. 2C exhibits two binding energies at 795 eV and 780 eV, corresponding to Co 2p1/2 and Co 2p3/2, respectively.25 The deconvoluted results for these two binding energy peaks indicate that there are two energy peaks at 795.85 eV and 780.49 eV, typical of Co2+, and two energy peaks at 794.13 eV and 779.21 eV, corresponding to Co3+, in the spinel complex ZnCo2O4.33,38 Furthermore, two satellite peaks are seen at energy peaks of around 788 and 802 eV, indicating the existence of multivalent cobalt.38 The deconvolution at the 531 eV binding energy of O 1s shows three energy values: 529.6,530.84, and 532.44 eV (Fig. 2E). The binding energy of lattice oxygen (OL) at 529.6 eV corresponds to M–O bonds.39 The strong binding energy peak at 532.44 eV indicates a significant presence of oxygen vacancies (OV), and the energy position of 532.44 eV represents the adsorption of oxygen on the surface.40,41
 | ||
| Fig. 3 SEM of (A) Bi2S3, (B) ZC-cLDHs, (C) Bi2S3@ZC-cLDHs samples; TEM of (D) Bi2S3@ZC-cLDHs sample. | ||
The results of EDX analysis and EDX mapping for the Bi2S3@ZC-cLDHs sample are shown in Fig. 4. The EDX spectrum of the Bi2S3@ZC-cLDHs sample reveals the presence of elements Bi, S, Co, Zn, and O, with mass percentages of 13.79%, 2.11%, 12.73%, 46.55%, and 24.82%, and atomic percentages of 2.53%, 2.52%, 8.25%, 27.27%, and 59.41%, respectively. The EDX maping revealed the elemental composition of the Bi2S3@ZC-cLDHs, providing insight into the distribution of Bi, S, Co, Zn, and O within the composites.
3.3 Photocatalytic activity
The research assessed the photocatalytic efficacy of Bi2S3, ZC-cLDHs, and Bi2S3@ZC-cLDHs samples under identical catalyst loading conditions of 1.0 g L−1 and LF concentration of 20 ppm in two scenarios: (i) simulated sunshine (Bi2S3/Vis; ZC-cLDHs/Vis; Bi2S3@ZC-cLDHs/Vis). (ii) Simulated sunshine in conjunction with a dose of 0.25 g L−1 PS activator (Bi2S3/PS/Vis; ZC-cLDHs/PS/Vis; and Bi2S3@ZC-cLDHs/PS/Vis).
The experimental findings demonstrated that the combination of Vis and PS significantly enhanced the LF degradation efficiency of Bi2S3, ZC-cLDHs, and Bi2S3@ZC-cLDHs, suggesting that simulated sunlight activates PS to promote LF degradation. The studies indicated that Bi2S3/Vis; ZC-cLDHs/Vis and Bi2S3@ZC-cLDHs/Vis exhibited a photocatalytic efficiency of 35.8% (k = 0.02055 min−1) 59.8% (k = 0.0109 min−1) and 74.8% (k = 0.01686 min−1), whereas Bi2S3/PS/Vis; ZC-cLDHs/PS/Vis and Bi2S3@ZC-cLDHs/PS/Vis had a photocatalytic efficiency of 46.7% (k = 0.00818 min−1); 74.8% (k = 0.01872 min−1). and 90.1% (k = 0.02808 min−1). The photocatalytic efficiency of LF degradation of Bi2S3; ZC-cLDHs and Bi2S3@ZC-cLDHs with PS activator is higher than that without activator in the visible light region. In which, the LF decomposition rate of the Bi2S3@ZC-cLDHs/PS/Vis increased by over two-fold compared to the Bi2S3@ZC-cLDHs/Vis, as S2O82− carried out the electron reaction, leading to the formation of SO4˙−. This then interacted with H2O to produce ˙OH, enhancing the LF decomposition,45,46 according to eqn (i) and (ii).
| S2O82− + e− → SO4˙− + SO42− | (i) |
| SO4˙− + H2O → SO42− + ˙OH + H+ | (ii) |
The results indicate that the degrading efficiency of LF was not significantly diminished (6.3%) when the PS activator was paired with simulated irradiation light in the experiment. This suggests that the PS could only scarcely be activated to degrade LF in the absence of a catalyst. Furthermore, the findings of the experiment demonstrated that the photocatalytic efficacy of LF degradation of pure Bi2S3; ZC-cLDHs was rather low in both Vis and PS/Vis. A comparative investigation of the LF degradation efficiency of Bi2S3/PS/Vis and ZC-cLDHs/PS/Vis revealed that Bi2S3 and ZC-cLDHs had modest LF degradation efficiencies, ranging from around 46.8% to 70.8%. The findings indicated that the amalgamation of Bi2S3 and ZC-cLDHs significantly enhanced the photocatalytic efficiency for LF degradation to 90.1%. The photocatalyst performance was determined by the values of k, which were subsequently arranged in descending order of Bi2S3@ZC-cLDHs/PS/Vis (k = 0.02808 min−1) > ZC-cLDHs/PS/Vis (k = 0.01686 min−1) > Bi2S3/PS/Vis (0.0081 min−1). Similarly, the findings indicated that Bi2S3@ZC-cLDHs/Vis had a superior LF degradation efficiency of 74.5%, in contrast to Bi2S3/Vis (35.8%) and ZC-cLDHs/Vis (59.8%). The efficacy of the photocatalyst was assessed based on the k values, which were organised in decreasing order as follows: Bi2S3@ZC-cLDHs/Vis (k = 0.01871 min−1) > ZC-cLDHs/Vis (k = 0.0109 min−1) > Bi2S3/Vis (k = 0.0055 min−1).
The photocatalytic effects of Bi2S3@ZC-cLDHs heterojunction surpassed those of pure Bi2S3 and ZC-cLDHs, indicating that this heterojunction enhanced photocatalytic performance. The UV-Vis/DRS results show that Bi2S3 exhibits an absorption wavelength range from the ultraviolet to the visible the spectrum, with a low band gap energy. ZC-cLDHs comprises ZnCo2O4 with an Eg of 2.01 eV, and ZnO with an Eg of 2.97 eV. The Bi2S3@ZC-cLDHs sample exhibits a chemical interaction between ZC-cLDHs and Bi2S3 that alters the light absorption spectrum. The interaction between Bi2S3 and ZC-cLDHs form a heterojunction, leading to a synergistic impact between the two phases. This process, Bi2S3 establishes efficient intermediate energy levels that facilitate the transfer between electrons and holes, therefore decreasing the possibility of recombination between photogenerated e−/h+, enhancing the ability to decompose LF.15,30,31 Another characteristic is the material's morphology; the SEM maging results indicate that Bi2S3 has a rod-like structure, whereas ZC-cLDHs exhibits nanolayered structure. The precipitation of ZC-cLDHs on Bi2S3 enhances dispersion capability and surface area, reduces the aggregation tendency of Bi2S3-ZC-cLDHs, and facilitates light absorption, so expanding the catalytic efficacy of the composite.15
In addition, the photocatalytic activity of Bi2S3@ZC-cLDHs heterojunction for LF degradation was tested under natural light (Bi2S3@ZC-cLDHs/PS/sunlight); the experimental time in natural light was from 10 to 12 h local time. The degradation efficiency of Bi2S3@ZC-cLDHs/PS/sunlight was 92.1% greater than that of Bi2S3@ZC-cLDHs/PS/Vis, possibly due to the higher light intensity of sunlight compared to that provided by the Orsam lamp. Furthermore, Bi2S3@ZC-cLDHs have the capability to absorb both visible and ultraviolet spectra in natural light.47,48
The first-order kinetic equation,  was used to model the LF breakdown process of Bi2S3@ZC-cLDHs based on the experimental results. Co, Ct (mg L−1): initial concentration and the concentration of LF at any instant time t; and k: the rate constant (min−1).
was used to model the LF breakdown process of Bi2S3@ZC-cLDHs based on the experimental results. Co, Ct (mg L−1): initial concentration and the concentration of LF at any instant time t; and k: the rate constant (min−1).
Fig. 5B and C displays the linear equation, rate constant, and correlation coefficient r2 for the LF decomposition process of materials. The findings indicate that the r2 value varies from 0.9516–0.9918, demonstrating that the first-order apparent kinetic equation is entirely appropriate for assessing the LF conversion kinetics with the PS activator of the Bi2S3@ZC-cLDHs. The photocatalytic efficiency of the PS-activated Bi2S3@ZC-cLDHs sample is most effective, achieving 90.1%, with a rate constant of k = 0.02808 min−1. Table 1 displays comparisons between the Bi2S3@ZC-cLDHs synthesized in this study and other reported photcatalysts for the elimination LF with or without an activator PS. The Bi2S3@ZC-cLDHs compound catalyst with PS activator demonstrated a better LF degradation efficiency than the other catalysts.
| Photocatalysts | Catalyst dose (g L−1) | Levofloxacin concentration (mg L−1) | Activation | Degradation efficiency | Ref. |
|---|---|---|---|---|---|
| Ag3PO4/C3N4/ZnO | 0.5 | 100 mL; 10 | 89.2% | 49 | |
| AgFeO2/Ag3VO4 | 0.2 | 50 mL; 20 | 95.95% | 3 | |
| Co3O4/Bi2MoO6@g-C3N4 | 1.0 | 50 mL; 25 | 95.21% | 36 | |
| Co-Bi2Fe4O9 | 0.5 | 50 mL; 15 | PS 0.2 mM | 100% | 46 |
| Fe3O4/MoS2–O/biochar | 0.4 | 50 mL; 10 | 90.64% | 12 | |
| Bi2S3@ZC-cLDHs | 1.0 | 100 mL; 20 | PS 0.25 g L−1 | 90.1% | This paper |
3.4 Cycling test
The durability and reusability of photocatalytic material are essential considerations to put the material into practical applications. Fig. 6A presents the assessment outcomes of the durability and LF degradation efficacy of the Bi2S3@ZC-cLDHs sample after four reutilizations. The Bi2S3@ZC-cLDHs, after the first photocatalytic process, was filtered, rinsed with distilled water, dried, and then utilized in the subsequent photocatalytic process under identical catalytic conditions as the first instance. The experimental findings indicate that the photocatalytic effectiveness of Bi2S3@ZC-cLDHs in degrading LF after 4th reuses decreased in the following sequence: 90.1%, 87.2%, 84.16%, and 79.1%. The XRD and IR spectra (Fig. 6B and C) of the synthesised Bi2S3@ZC-cLDHs sample before and after the fourth reuse indicate a stable structure, with little changes in the main peaks and a slight decrease in peak strength. The buildup of by-products on the photocatalyst's surface may be the cause; the repetitive washing of Bi2S3@ZC-cLDHs diminishes the bonding characteristics of Bi2S3-ZC-cLDHs. SEM image (Fig. 6D)results indicate that upon reuse, the surface area of Bi2S3@ZC-cLDHs exhibits minor alterations; the Bi2S3 rods begin to deform, and ZC-cLDHs display agglomeration and uneven dispersion on the rod surface, resulting in modified surface area and consequently diminishing the capacity to interact with light photons. | ||
| Fig. 6 (A) The photocatalytic stability, (B) XRD, (C) FTIR and (D) SEM pattern before and after the 4th recycling of Bi2S3@ZC-cLDHs. | ||
3.5 Photocatalytic mechanism and degradation pathways
In the optimization experiment above, p-BQ, MeOH, TBA and Na2-EDTA were used as trapping agents to capture O2˙−; SO4˙− and ˙OH, ˙OH; h+ respectively, to identify the ROS that are major to the LF degradation process in the Bi2S3@ZC-cLDHs heterojunction.11,14 As shown in Fig. 7A and B trapping agents' incorporation reduced the degradation efficiency of LF. The LF decomposition efficiency dropped little to 62.4% (k = 0.01234 min−1) when SO4˙− and ˙OH were captured with MeOH, and by 79.8% (k = 0.02097 min−1) when ˙OH was captured with TBA. The degradation rate FL of Bi2S3@ZC-cLDHs in the presence of MeOH was inferior to that seen with TBA, suggesting that the presence of SO4˙− in ROS may augment the degradation FL for Bi2S3@ZC-cLDHs. However, with p-BQ and Na2-EDTA, the degradation efficiency decreased significantly to 32.8% (k = 0.00525 min−1) and 30.1% (k = 0.00437 min−1). The findings indicated that h+ and O2˙− were the primary agents in the LF breakdown process. The free radicals breakdown FL in the following order: h+ > O2˙− > ˙OH > SO4˙−.
 | ||
| Fig. 7 (A and B) Trapping experiments of ROS (h+, SO4˙− and ˙OH, ˙OH and O2˙− using p-BQ, MeOH, TBA and Na2-EDTA). | ||
XRD and UV-Vis diffraction analyses revealed that ZC-cLDHs had two phases, ZnO and ZnCo2O4, with band gap energies measured at 2.97 eV and 2.2 eV, respectively. The conduction band (CB) and valence band (VB) potentials of these two semiconductors were computed using the following eqn (iii) and (iv).16,55
| EVB = X − Ee + 0.5 Eg | (iii) |
| ECB = EVB − Eg | (iv) |
Upon exposure to sunlight, both Bi2S3 and ZC-cLDHs semiconductors within the Bi2S3@ZC-cLDHs heterojunction absorb light, resulting in the excitation of electrons in the VB, therefore generating (e−/h+). In the ZC-cLDHs semiconductor, the CB potential of ZnCo2O4 is lower than that of ZnO, and both potentials are more negative than the O2/O2˙− potential  .30 Consequently, this excited electrons in the CB of ZnCo2O4 transfer to the CB of ZnO, where they combine with (e−) from ZnO to react with surface-adsorbed O2, resulting in the formation of O2˙−, which subsequently oxidizes LF resulting in products. Furthermore, these charged electrons can also disintegrate straightaway into LF. Moreover, the LF decomposition rate is enhanced, diminishing the recombination of photogenerated electrons and holes, since the S2O82− activator can trap this electron produced on the catalyst's surface and produce SO4˙− and ˙OH to further decompose LF.
.30 Consequently, this excited electrons in the CB of ZnCo2O4 transfer to the CB of ZnO, where they combine with (e−) from ZnO to react with surface-adsorbed O2, resulting in the formation of O2˙−, which subsequently oxidizes LF resulting in products. Furthermore, these charged electrons can also disintegrate straightaway into LF. Moreover, the LF decomposition rate is enhanced, diminishing the recombination of photogenerated electrons and holes, since the S2O82− activator can trap this electron produced on the catalyst's surface and produce SO4˙− and ˙OH to further decompose LF.
For Bi2S3, Evb = 0.3 eV is more positive than the standard potential of O2/O2˙−, but Evb = 1.99 eV is lower than the standard potential of ˙OH/H2O (E = 1.99 eV vs. NHE). The (e−) are incapable of reacting with O2 to produce O2˙−, and the (h+) in the valence band cannot oxidize H2O to create. Consequently, the charges produced by light do not traverse the traditional type-II heterojunction charge transfer mechanism. Consequently, they stay trapped inside the Bi2S3 structure, causing decreased efficiency in the capture and use of solar energy for chemical processes. This is accomplished via the direct charge transfer mechanism in alignment with the Z-scheme (Fig. 8). The (e−) at the conduction band of Bi2S3 transition to the VB of ZC-cLDHs, where they combine with the holes of ZC-cLDHs. in addtion, the (e−) in the CB of Bi2S3 may react with S2O82− to generate SO4˙− and ˙OH radicals, facilitating the degradation of LF. For the ZC-cLDHs, ZnO's Ecb = 2.775 eV is greater than the standard oxidation potential of ˙OH/H2O, which means it react with H2O to produce ˙OH to decompose LF. In contrast, ZnCo2O4's Evb = 1.445 eV is smaller than the standard oxidation potential of ˙OH/H2O, meaning it cannot oxidize H2O to form ˙OH; instead, the (h+) can directly decompose LF to the product.
 | ||
| Fig. 9 The degradation of LF has performed based on times: (A). t = 0 min; (B) t = 30 min; (C) t = 45 min; (D) t = 60 min; (E) t = 75 min; and (F) t = 90 min. | ||
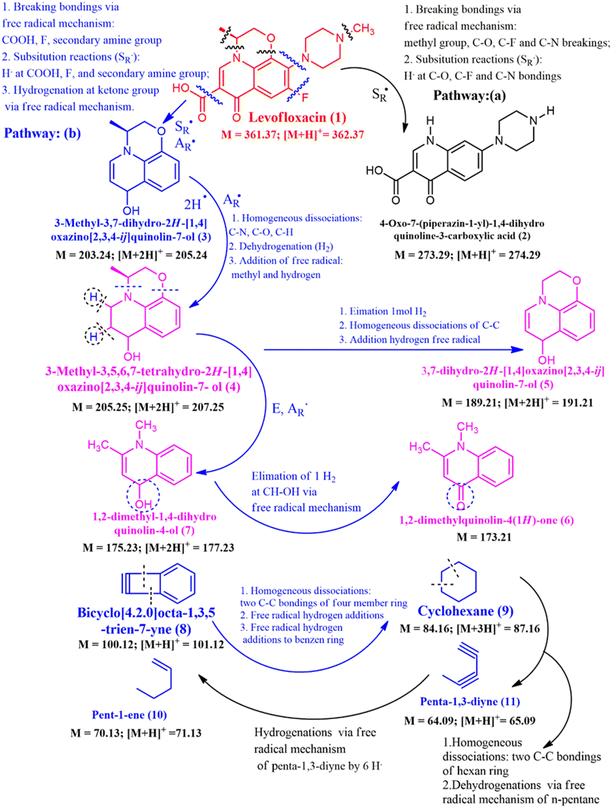
At time of 45 min, as seen in Fig. 9C, compound (1), the LF has reduced around value of 75% that is transform to ion fragments of 274.51, 205.20, 177.36, 173.50, 101.54, 87.39, 71.22, and 65.48 that belong to [M + H]+ = 274.29 (1), [M]+ = 205.25 (4), [M+2H]+ = 177.23 (7), [M]+ = 173.50 (6), [M + H]+ = 101.12 (8), [M + H]+ = 87.16 (9), [M + H]+ = 71.13 (10), and [M + H]+ = 65.09 (11), respectively, as seen in Scheme 1. As seen in Fig. 9D–F that are corresponded to times of 60, 75 and 90 min of the degradations and at time of 60 min, the LF almost has been degraded 100%. It has been transferred to small fragment ions such as 205.15, 191.25, 177.36, 173.53 101.59, 87.54, 71.27, and 65.59 that are detected [M + 2H]+ (3), c (5), [M + 2H]+ (7), [M]+ (6), [M + H]+ (8), [M + 3H]+, [M + H]+ (10), and [M + H]+ (11), respectively, as seen in Scheme 1. Molecule ions that have lower molecule weights have appeared in higher centrations such as [M + H]+ (10), and [M + H]+ (11) and has proved that degradations to low molecule weights such as compound (10) and compound (11) are simple alkene and diyne derivatives as seen in Fig. 9D. As seen in Scheme 1, the decompositions of LF or compound (1) to compounds (2–11) has been detected based on LC-MS results at time of 60 min that remained zero percent of LF, via reactions that are free radical substitution (SR˙), free radical addition (AR˙), elimination reaction, and hydrogenation via free radical reaction mechanism. The free radicals that have participated in reactions in Scheme 1 are explained via active mechanisms in Fig. 7A and B such as ROS.14,36,56 All reactions have conducted the Bi2S3@ZC-cLDHs heterojunction photocatalysts reactions. As exposed in Scheme 1, the reaction of LF (1) has yielded (3) via two typical types that have determined the free radical substitution (SR˙) and the free radical addition (AR˙) by breaking bonding via the free radical mechanism: COOH, F, and secondary amine group and substitution reactions (SR˙): H atom of COOH, F, and secondary amine group, finally, the hydrogenation at ketone group via free radical mechanism. Compound (3) is like compound (P9) or compound (L7) in articles.2,3 The transform of (1) to compound (2) has performed by substitution reaction (SR˙) that also explained as previous article via fragment (P28) or (P5)2,49 via the homogeneous dissociations at C–N, C–O, and C–H bonding, the dehydrogenation, and addition of free radial of methyl and hydrogen, compound (3) has transformed to (4) that is similar to degradation as compound (P13) in article.49 Compound (4) has yielded compound (7) via E and AR˙ reactions and it also transformed to (5) via elimination of 1 mol H2, homogenous dissociation of C–C bonding and additional hydrogen via free radical reaction. Fragment (7) to (6) has been detected by the elimination of 1 mol H2 at CH–OH bonding via free radical mechanism reaction.
The small compound (8) has yielded compound (9) via some mechanisms such as homogeneous dissociations of two C–C bonding of four-member rings, free radical hydrogen additions, and free radical addition of hydrogen atoms to benzene derivatives. Compound (9) has the transform to compound (11) by mechanism and compound (11) has transformed to compound (10) via some mechanisms in Scheme 1. Compounds (8), (9), (10), and (11) are small compounds and compounds (10) and (11) are expecting compounds of degradation. Our project expects these compounds will be changed to CO2 and H2O, but the final analysis result of GC-MS has not been detected. The degradation of LF (1) to small molecular weight such as compound (10) to (11) as GC-MS results and proposal mechanism reactions exposed advances in degradation mechanism of LF that compared to the former articles.1,3–5
4 Conclusion
The synthesis of the novel Bi2S3@ZC-cLDH heterojunction was accomplished effectively. ZC-cLDHs include two phases, ZnO and ZnCo2O4, uniformly distributed on the surface of Bi2S3 rods. The photoactivity of the Bi2S3@ZC-cLDHs heterojunction was greatly enhanced by the addition of PS; the combined action of Bi2S3@ZC-cLDHs and PS activated PS to function as an electron acceptor, facilitating the separation of photogenerated electron–hole pairs. As a result, the LF decomposition rate of Bi2S3@ZC-cLDHs/PS/Vis increased by 2.2 times the rate of Bi2S3@ZC-cLDHs/Vis. The combination of Bi2S3 and ZC-cLDHs significantly improved the degradation of LF under visible light, shown by the LF degradation rate of the Bi2S3@ZC-cLDHs/PS/Vis system being 1.6 times superior to that of the ZC-cLDHs/PS/Vis system. High charge separation and transfer efficiency have been predicted by the Z-scheme for the LF degrading process of Bi2S3@ZC-cLDHs/PS/Vis. The Bi2S3@ZC-cLDHs demonstrated significant stability after four reuses and exceptional reusability.The degradation products resulting from the LF photodegradation of Bi2S3@ZC-cLDHs were identified as uncomplicated and low-toxicity compounds. The Bi2S3@ZC-cLDHs heterojunction with PS activator under visible light is shown to be an effective and environmentally friendly method for removing LF from water in this study.Data availability
We send the DAS of the article “Manuscript ID: RA-ART-05-2025-003086 TITLE: construction of novel Bi2S3@Zn-Co-cLDHs heterojunction for enhanced photocatalytic degradation of levofloxacin with persulfate activation under visible light: mechanism, degradation pathway” https://doi.org/10.6084/m9.figshare.28916051.Author contributions
Nguyen Thi Mai Thoparticipated in methodology, validation, writing, reviewing, and project administration. Minh An Tran Nguyen participated in editing the draft manuscript.Conflicts of interest
The authors claim that they have no known competing interests that could have an impact on the research described in this publication.Acknowledgements
This work is supported by Faculty of Chemical Engineering (FCE), Industrial University of Ho Chi Minh City (IUH), Ho Chi Minh, Vietnam.References
- X. Lu, L. Wu, L. Liang, D. Liu, Y. Chen, Y. Zeng, M. Zhong and B. Jia, J. Water Process Eng., 2023, 56, 104427 CrossRef.
- M. Mahjoore, M. Honarmand and A. Aryafar, Environ. Sci. Pollut. Res., 2023, 30, 44439–44456 CrossRef CAS PubMed.
- Z. Chen, B. Ning, Y. Cai, M. Liu, P. Xu, P. Zhang, G. Xiao and Y. He, J. Taiwan Inst. Chem. Eng., 2023, 151, 105126 CrossRef CAS.
- L. Liu, R. Zhan, M. Zhang, J. Li, Z. Wang, H. Mi and Y. Zhang, J. Environ. Chem. Eng., 2022, 10(3), 107435 CrossRef CAS.
- Q. Jin, D. Ji, Y. Chen, Z. Tang and Y. Fu, Sep. Purif. Technol., 2022, 282, 120104 CrossRef CAS.
- M. Mahjoore, M. Honarmand and A. Aryafar, Environ. Sci. Pollut. Res., 2023, 30, 44439–44456 CrossRef CAS PubMed.
- S. Park, N. Choi, T. H. Kim, D. H. Lee, Y. Park and Y. Hwang, J. Mol. Liq., 2024, 408, 125285 CrossRef CAS.
- P. Bobde, A. K. Sharma, D. Panchal, A. Sharma, R. K. Patel, R. S. Dhodapkar and S. Pal, Int. J. Ionics, 2023, 20, 5733–5752 CAS.
- M. Xie, X. Luo, C. Liu, S. You, S. Rad, H. He, Y. Huang and Z. Tu, RSC Adv., 2022, 12, 25833–25843 RSC.
- T. V. M. Sreekanth, H. P. Dang, N. Q. Thang, N. Van Cuong and N. T. M. Tho, Environ. Sci.:Adv., 2025, 4, 663–675 CAS.
- S. W. Lv, J. M. Liu, N. Zhao, C. Y. Li, F. E. Yang, Z. H. Wang and S. Wang, Sep. Purif. Technol., 2020, 253, 117413 CrossRef CAS.
- R. Li, Z. Ji, Z. Hu, Z. Zhao, X. Wang, A. Song, X. Lu, Z. Zhang and A. Cai, Biomass Convers. Biorefin., 2025, 15, 6345–6363 CrossRef CAS.
- D. Renuka Devee, T. Sivanesan, R. M. Muthukrishnan, D. Pourkodee, P. Mohammed Yusuf Ansari, S. M. Abdul Kader and R. Ranjani, Chem. Phys. Impact, 2024, 8, 100605 CrossRef.
- Y. Hu, X. Wang, Y. Wang, S. Li, B. Cui and Y. Du, ACS Appl. Nano Mater., 2023, 6, 10768–10778 CrossRef CAS.
- J. Liao, Y. Zhong, Z. He, H. Ding, K. Chen and D. Chen, J. Mater. Sci.: Mater. Electron., 2021, 32, 1022–1032 CrossRef CAS.
- H. A. Kashmery and S. I. El-Hout, Opt. Mater., 2023, 135, 113231 CrossRef CAS.
- K. Goswami, R. Ananthakrishnan and S. Mandal, Mater. Chem. Phys., 2018, 206, 174–185 CrossRef CAS.
- M. A. Ahmed, B. M. Mahran, A. M. Abbas, M. A. Tarek and A. M. Saed, J. Dispersion Sci. Technol., 2022, 43, 349–363 CrossRef CAS.
- R. Su, Z. Li, F. Cheng, X. Dai, H. Wang, Y. Luo and L. Huang, Water, Air, Soil Pollut., 2023, 234, 754 CrossRef CAS.
- J. Zhang, S. Zhang, X. Bian, Y. Yin, W. Huang, C. Liu, X. Liang and F. Li, Molecules, 2024, 29, 4055 CrossRef CAS PubMed.
- M. Sabri, A. Habibi-Yangjeh and S. Ghosh, J. Photochem. Photobiol., A, 2020, 391, 112397 CrossRef CAS.
- G. Liu, Y. Lin, S. Li, C. Shi, D. Zhang and L. Chen, Environ. Sci. Pollut. Res., 2023, 30, 87830–87850 CrossRef CAS PubMed.
- T. T. T. Nguyen, Y. N. N. Nguyen, X. T. Tran, T. T. T. Nguyen and T. Van Tran, J. Environ. Chem. Eng., 2023, 11(5), 111003 CrossRef CAS.
- M. I. A. Abdel Maksoud, G. S. El-Sayyad, N. Mamdouh and W. M. A. El Rouby, J. Inorg. Organomet. Polym. Mater., 2022, 32, 3621–3639 CrossRef CAS.
- X. Wang, P. Wu, Z. Zhao, L. Sun, Q. Deng, Z. Yin and X. Chen, J. Mater. Sci.: Mater. Electron., 2020, 31, 4895–4904 CrossRef CAS.
- X. Yu, J. Zhou, Q. Li, W. N. Zhao, S. Zhao, H. Chen, K. Tao and L. Han, Dalton Trans., 2019, 48, 9057–9061 RSC.
- M. Wang, L. Yang, J. Yuan, L. He, Y. Song, H. Zhang, Z. Zhang and S. Fang, RSC Adv., 2018, 8, 12459–12470 RSC.
- D. Ayodhya and G. Veerabhadram, Environ. Technol., 2021, 42, 826–841 CrossRef CAS PubMed.
- S. V. P. Vattikuti, P. C. Nagajyothi and J. Shim, J. Mater. Sci.: Mater. Electron., 2019, 30, 5681–5690 CrossRef CAS.
- B. Tan, Y. Fang, Q. Chen, X. Ao and Y. Cao, Opt. Mater., 2020, 109, 110470 CrossRef CAS.
- N. Thi Mai Tho, B. The Huy, D. N. Nha Khanh, N. Quoc Thang, N. Thi Phuong Dieu, B. Dai Duong and N. Thi Kim Phuong, ChemistrySelect, 2018, 3, 9986–9994 CrossRef CAS.
- K. Yang, Y. Zhang, C. Meng, F. F. Cao, X. Chen, X. Fu, W. Dai and C. Yu, Appl. Surf. Sci., 2017, 391, 635–644 CrossRef CAS.
- N. Alomayrah, M. Ikram, S. Zulfiqar, S. Alomairy, M. S. Al-Buriahi, I. Shakir, M. F. Warsi and E. W. Cochran, RSC Adv., 2024, 14, 24874–24897 RSC.
- Y. Zhou, S. Feng, C. Ma, W. Wu, Z. Ye, X. Dai, Y. Wang and X. Cao, Ceram. Int., 2022, 48(21), 31334–31343 CrossRef CAS.
- X. Li, Y. Li, X. Guo and Z. Jin, Front. Chem. Sci. Eng., 2023, 17, 606–616 CrossRef CAS.
- H. Wei, F. Meng, W. Yu, J. Li and H. Zhang, Sep. Purif. Technol., 2023, 318, 123940 CrossRef CAS.
- Y. Cao, X. Yuan, H. Chen, H. Wang, Y. Chen, J. Chen, H. Huang, Y. Mou, Z. Shangguan and X. Li, Chem. Eng. J., 2023, 456, 140971 CrossRef CAS.
- R. Jahanshahi, H. H. Moghadam, S. Sobhani and J. M. Sansano, RSC Adv., 2024, 14, 26424–26436 RSC.
- N. H. Nam, N. Q. Hung, N. T. H. Anh, N. Q. Thang and N. T. M. Tho, RSC Adv., 2024, 14, 32436–32450 RSC.
- T. S. Rad, Z. Ansarian, R. D. C. Soltani, A. Khataee, Y. Orooji and F. Vafaei, J. Hazard. Mater., 2020, 399, 123062 CrossRef CAS PubMed.
- W. Zhang, C. Xu, E. Liu, J. Fan and X. Hu, Appl. Surf. Sci., 2020, 515, 146039 CrossRef CAS.
- J. Zhang, Y. Ma, W. Zhang, X. Huang, X. Wang, Y. Huang and P. Zhang, J. Cleaner Prod., 2022, 365, 132810 CrossRef CAS.
- J. Wang, B. Wang, Z. Wang, L. Chen, C. Gao, B. Xu, Z. Jia and G. Wu, J. Colloid Interface Sci., 2021, 586, 479–490 CrossRef CAS PubMed.
- H. Y. Zhu, R. Jiang, Y. Q. Fu, R. R. Li, J. Yao and S. T. Jiang, Appl. Surf. Sci., 2016, 369, 1–10 CrossRef CAS.
- J. Yang, M. Huang, S. Wang, X. Mao, Y. Hu and X. Chen, Water, 2020, 12, 3583 CrossRef CAS.
- X. Zhong, Z. S. Zou, H. L. Wang, W. Huang and B. X. Zhou, Materials, 2019, 12(23), 3952 CrossRef CAS PubMed.
- P. H. Lee, Y. C. Huang and T. W. Liang, Int. J. Environ. Sci. Technol., 2025, 22, 6945–6956 CrossRef CAS.
- A. E. Noua, D. Kaya, G. Sigircik, T. Tuken, F. Karadag and A. Ekicibil, J. Mater. Sci.: Mater. Electron., 2024, 35, 1220 CrossRef CAS.
- S. Luo, Z. Pang, N. Ding and H. Liu, Res. Chem. Intermed., 2023, 49, 5517–5539 CrossRef CAS.
- Q. Wang, B. Wang, Y. Ma and S. Xing, Chem. Eng. J., 2018, 354, 473–480 CrossRef CAS.
- X. Du, X. Bai, L. Xu, L. Yang and P. Jin, Chem. Eng. J., 2020, 384, 123245 CrossRef CAS.
- W. Liu, J. Zhou and J. Yao, Ecotoxicol. Environ. Saf., 2020, 190(110062) CAS.
- Y. Li, H. Zhang, D. Zhang, S. Yao, S. Dong, Q. Chen, F. Fan, H. Jia and M. Dong, Molecules, 2024, 29, 1169 CrossRef CAS PubMed.
- H. Zhang, L. chao Nengzi, Z. Wang, X. Zhang, B. Li and X. Cheng, J. Hazard. Mater., 2020, 383, 121236 CrossRef CAS PubMed.
- H. T. Nguyen, V. D. Doan, T. L. Huong Nguyen, A. T. Nguyen, Q. H. Tran, V. A. Tran and V. T. Le, RSC Adv., 2025, 15, 6241–6259 RSC.
- M. A. Meetani, A. Alzamly, N. Al-Dubaili, M. F. Malik, N. Elmerhi, N. I. Albadawi, S. Hisaindee, R. Selvaraj and M. A. Rauf, Desalin. Water Treat., 2020, 183, 325–334 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |