Open Access Article
Open Access ArticleHierarchical porous adsorbent from asphaltenes fibers and its application for methyl orange removal†
Hiyam Khalil a,
Amgad Salama
a,
Amgad Salama *bc,
TriDung Ngod,
Thomas Kaminskid and
Maen M. Husein
*bc,
TriDung Ngod,
Thomas Kaminskid and
Maen M. Husein *a
*a
aDepartment of Chemical & Petroleum Engineering, University of Calgary, Calgary, AB T2N 1N4, Canada. E-mail: mhusein@ucalgary.ca
bDepartment of Mechanical Engineering, University of Saskatchewan, Saskatoon, SK S7N 5A9, Canada. E-mail: amgad.salama@uregina.ca
cDepartment of Mechanical and Aerospace Engineering, Nazarbayev University, Astana 010000, Kazakhstan
dEnergy Services, InnoTech Alberta, Edmonton, AB, Canada
First published on 15th July 2025
Abstract
This study explores the preparation of hierarchical porous adsorbent starting from asphaltenes fibers. Solid or aqueous KOH, Fe(NO3)3, and Al(NO3)3 activation agents were mixed with the fibers followed by treatment at 573 K for 24 h under air atmosphere. The resulting structures were characterized and assessed as adsorbents for methyl orange from aqueous solutions. Asphaltenes fibers modified with solid Al(NO3)3 exhibited the highest adsorption capacity (6.32 mg g−1) and removal efficiency (79%) at 298 K and pH = 3. The intraparticle diffusion kinetic model fitted the experimental data across two time zones corresponding to initial diffusion into the mesopores followed by diffusion into micropores. The second zone could equally be modeled by a pseudo-second order model corresponding to chemisorption onto active sites. The equilibrium uptake was best described by Langmuir isotherm, indicating monolayer chemisorption of endothermic nature (ΔH0 = 8.41 kJ mol−1). The modified fibers retained significant adsorption capacity with 76.22% of initial adsorption capacity over five cycles, demonstrating their stability and reusability. This study highlights the potential of chemically activated asphaltenes fibers as effective adsorbents for wastewater treatment.
1. Introduction
The quality of natural waters is increasingly compromised by calcitrant pollutants from industrial, agricultural, and domestic sources. Contaminants such as heavy metals and organic compounds that pose serious health and environmental risks1 continue to find their way into the different water bodies. Methyl orange (MO) is a synthetic organic azo dye widely used in textiles, printing, and as a pH indicator.2 MO wide use, resistance to biodegradation as well as its potential toxicity to aquatic ecosystems make it a significant water contamination.3Among the different water treatment methods, adsorption has proven highly effective due to its adaptability and affordability. The performance of the adsorption process depends largely on the adsorbent material, which must effectively capture pollutants and contribute to no or little back contamination.4,5 Carbon-based adsorbents, including graphene oxide (GO), carbon nanotubes (CNTs), and activated carbon, are widely used due to their high surface area, stability, and reusability. However, materials such as GO and CNTs are relatively costly and can complicate post-treatment due to their nanoscale size and mechanical stability, raising additional environmental concerns.6 As a result, there is a growing need to develop cost-effective, reusable, and eco-friendly adsorbents for sustainable water treatment.
Asphaltenes particles, a by-product of the petroleum industry, present a promising alternative. Asphaltenes are abundant, inexpensive, and can be chemically modified. Asphaltenes pose challenges during oil production and processing, leading to significant losses.7 On the other hand, the use of asphaltenes particles as adsorbents has been successfully demonstrated, especially when chemically activated with KOH. KOH-activated asphaltenes particles exhibited remarkable adsorption for CO2 (7.15 mmol g−1) and H2S (12.86 mmol g−1) under atmospheric pressure at 298 K, alongside a stable cyclic adsorption–desorption performance.8 Moreover, asphaltenes particles mixed with KOH in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mass ratio and heated to 873 K displayed high surface area (970 m2 g−1) and significant adsorption for methylene blue (218.15 mg g−1).9 Nevertheless, KOH activation has some drawbacks, including the corrosiveness of KOH, which requires careful handling and disposal.10 The alkaline by-products from KOH activation must be neutralized before disposal.10 In addition, excessive KOH reactivity may damage the carbon structure.11 Alternative activation agents such as Fe(NO3)3 and Al(NO3)3 address these shortcomings and proved effective at creating porous structures. For example, iron-containing carbon foam (Fe-CF) with hierarchical porous structure was synthesized by first carbonizing a mixture of epoxy resin and nano-magnesium oxide, followed by Fe(NO3)3 activation.12 Hierarchical porous carbon was also created using glucose and Fe(NO3)3·9H2O in ZnCl2–KCl molten salt.13 The impact of different amounts of Fe(NO3)3·9H2O on the surface area and adsorption capabilities for methylene blue and methyl orange was examined.13 Lastly, Al(NO3)3 activation contributed to stable hierarchical micropores and mesopores structure on zeolite.14
2 mass ratio and heated to 873 K displayed high surface area (970 m2 g−1) and significant adsorption for methylene blue (218.15 mg g−1).9 Nevertheless, KOH activation has some drawbacks, including the corrosiveness of KOH, which requires careful handling and disposal.10 The alkaline by-products from KOH activation must be neutralized before disposal.10 In addition, excessive KOH reactivity may damage the carbon structure.11 Alternative activation agents such as Fe(NO3)3 and Al(NO3)3 address these shortcomings and proved effective at creating porous structures. For example, iron-containing carbon foam (Fe-CF) with hierarchical porous structure was synthesized by first carbonizing a mixture of epoxy resin and nano-magnesium oxide, followed by Fe(NO3)3 activation.12 Hierarchical porous carbon was also created using glucose and Fe(NO3)3·9H2O in ZnCl2–KCl molten salt.13 The impact of different amounts of Fe(NO3)3·9H2O on the surface area and adsorption capabilities for methylene blue and methyl orange was examined.13 Lastly, Al(NO3)3 activation contributed to stable hierarchical micropores and mesopores structure on zeolite.14
Asphaltenes fibers offer significant handling advantages over asphaltenes particles due to their larger size and fibrous structure, which make them easier to separate from treated water, hence reducing back contamination. Asphaltenes fibers robustness also allows for multiple reuse cycles, contributing to cost-effectiveness.15 While KOH activation of asphaltenes-based carbon fibers has been studied,16 Fe(NO3)3 and Al(NO3)3 activation has not been explored. This study explores the use of Fe(NO3)3 and Al(NO3)3 as activation agents and aims at establishing hierarchical porous carbons (HPCs) starting from asphaltenes fibers. KOH activation is compared, together with the solid and wet impregnation techniques. MO removal from aqueous solution was used to assess the performance of the hierarchical adsorbent, and adsorption isotherms and kinetics were established.
2. Experimental methods
2.1. Asphaltenes fibers modification
The as-received asphaltenes fibers (AsphF) used in this study was provided by InnoTech Alberta (Edmonton, AB, Canada). MO has a chemical formula of C14H14N3NaO3S and molar mass of 327.33 g mol−1 (85% pure, ACS, Canada). The chemical structure of MO is shown in Fig. 1. Chemical reagents such as KOH (≥85% pure), Fe(NO3)3·9H2O (≥98% pure), and Al(NO3)3·9H2O (≥98% pure) were all obtained from Sigma-Aldrich (Canada) and used as received. Double-distilled water was used for all experiments.Solid or aqueous solution of KOH, Fe(NO3)3·9H2O, or Al(NO3)3·9H2O is used to synthesize chemically activated AsphF following Scheme 1. The AsphF are mixed with the different reagents at 1 g fiber![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g reagent using solid mixing or impregnation. Impregnation involves dissolving 0.6 g of the chemical reagent in 10 mL of water before mixing with AsphF. The mixture is then placed in an oven at 573 K for 24 h under air atmosphere. The activated AsphF were collected and left to cool to room temperature naturally. The fibers are then washed with distilled water to ensure a neutral pH and left to dry in the oven at 343 K for 3 h. Control samples exposed only to the heat treatment step were collected for comparison.
2 g reagent using solid mixing or impregnation. Impregnation involves dissolving 0.6 g of the chemical reagent in 10 mL of water before mixing with AsphF. The mixture is then placed in an oven at 573 K for 24 h under air atmosphere. The activated AsphF were collected and left to cool to room temperature naturally. The fibers are then washed with distilled water to ensure a neutral pH and left to dry in the oven at 343 K for 3 h. Control samples exposed only to the heat treatment step were collected for comparison.
The modified samples were given short names as follows: AsphF-T for the heat-treated sample without chemicals; AsphF/KOHs-T and AsphF/KOHaq-T for samples treated with solid and aqueous KOH; AsphF/Fe(NO3)3s-T and AsphF/Fe(NO3)3aq-T for samples treated with solid and aqueous Fe(NO3)3; and AsphF/Al(NO3)3s-T and AsphF/Al(NO3)3aq-T for samples treated with solid and aqueous Al(NO3)3. These names are also explained in the Abbreviations section.
The preparation of chemically activated AsphF potentially offers a cost-effective strategy for developing functional adsorbents suitable for water treatment applications. Asphaltenes are an abundant and low-cost byproduct of the petroleum industry.7 The activating agents used in this work, including KOH, Fe(NO3)3, and Al(NO3)3, are widely available and reasonably priced.17–19 The thermal activation process relies on heating in air, which helps reduce operational complexity and energy requirements. We note that the preparation of asphaltenes fibers through electrospinning has also been reported as cost-effective, with the raw asphaltenes valued at approximately $0.04 USD per kg.20 A recent techno-economic analysis further indicates that the production cost of asphaltenes-based carbon fibers can be kept below $9 USD per kg.21 Asphaltenes fibers could be less expensive in comparison with some conventional carbon fiber precursors such as polyacrylonitrile (PAN), which typically cost ∼$25 USD per kg.21 These economic and material advantages support the feasibility of using asphaltene-based adsorbents in large-scale water treatment systems, especially where affordability and material availability are key considerations.
2.2. Modified AsphF characterization tests
The morphology of the modified AsphF was examined using scanning electron microscopy (SEM) (Phenom, Thermo Fisher Scientific, USA) operating at 15 kV accelerating voltage under low vacuum of 50 Pa. Elemental composition of carbon and oxygen for the untreated and chemically treated AsphF was studied using energy-dispersive X-ray spectroscopy (EDX) analysis was performed with Phenom, Thermo Fisher Scientific (USA). Nitrogen adsorption/desorption isotherms were established using a Gemini VII 2390 surface area analyzer (Micromeritics, USA) at 77 K after degassing the samples in N2 environment at 393 K for 24 h. The specific surface area and pore size distribution of the asphaltenes samples were calculated using the adsorption/desorption isotherms of N2 at 77 K using the multi-point Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) equations. Additionally, Fourier-transform infrared spectroscopy (FTIR) was performed on an Agilent Cary 630 spectrometer (Agilent, USA) over a wavelength range of 650–4000 cm−1. Each sample was scanned 64 times to identify the functional groups.2.3. Batch adsorption experiments
Batch adsorption experiments were carried out in an incubator shaker (Orbital Shaker – Incubator ES-20, Grant-bio, Canada) to assess the effectiveness of the AsphF in adsorbing MO before and after modification. The effect of solution pH (3 to 11) and temperature (298 K, 313 K, and 333 K) on the adsorption was evaluated. Detailed conditions for the batch adsorption experiments are summarized in Table 1.| Study | Adsorbent | Ci (mg L−1) | t (h) | T (K) | pH |
|---|---|---|---|---|---|
| Adsorption performance | Modified/unmodified AsphF included in this study | 20 | 24 | 298 | 7 |
| Kinetics | AsphF/Al(NO3)3s-T | 20–30 | 0–72 | 298 | 7 |
| Isotherms | AsphF/Al(NO3)3s-T | 5–30 | 24 | 298–333 | 7 |
| Thermodynamics | AsphF/Al(NO3)3s-T | 20 | 24 | 298–333 | 7 |
| Impact of pH | AsphF/Al(NO3)3s-T | 20 | 24 | 298 | 3–11 |
To investigate the performance of the different adsorbents, a specified amount of the modified/unmodified AsphF was mixed with 10 mL of 20 mg per L MO solution to achieve 2.5 g L−1 adsorbent dosage at pH ∼ 7.0, 200 rpm and 298 K. Preliminary tests from the kinetics study showed that MO adsorption after 24 h is sufficiently close to equilibrium, while still preserving the chemistry of the mixture from external variable, e.g. CO2 dissolution. The modified AsphF with the optimal adsorption performance; namely AsphF/Al(NO3)3s-T, was selected to carry out further studies. Detailed kinetics study was conducted by varying contact time 0–72 h at 298 K, 2.5 g L−1 adsorbent, and pH ∼ 7.0 for MO initial concentrations, Ci, of 20, 25, and 30 mg L−1. The adsorption isotherms were established by varying Ci between 5–30 mg L−1 and the thermodynamic parameters were obtained for 298 K, 313 K, and 333 K at pH ∼ 7.0. The effect of solution pH on MO removal was examined within a pH range of 3–11 at 298 K, 2.5 g L−1 adsorbent, and Ci = 20 mg L−1. The pH was adjusted as needed with 0.1 M HCl or 0.1 M NaOH solution and was measured by pH meter (Mettler Toledo Biotechnology S210, Canada). MO concentration was determined by measuring its maximum absorbance at λmax = 464 nm using UV-vis spectrophotometer (Shimadzu, model UV-2600, Japan). A calibration curve correlating absorbance at 464 nm with MO concentration (1.25–35 mg L−1) was establish based on Beer–Lambert law.21 Doubled distilled water was used as a blank. MO uptake by modified/unmodified AsphF at a given time, qt (mg g−1), and the percent removal (%) of MO were calculated per eqn (1) and (2), respectively.
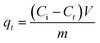 | (1) |
 | (2) |
| Kinetic model (rate equation) | Integral equation | Equation number | Reference |
|---|---|---|---|
| Pseudo-first order | qt = qe(1 − e−k1t) | (3) | 22 |
 |
(4) | ||
| Pseudo-second order |  |
(5) | |
 |
(6) | ||
| Elovich |  |
(7) | |
| Intraparticle diffusion |  |
(8) | |
| Bangham | 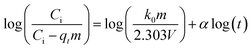 |
(9) | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isotherm models | |||
| Langmuir | 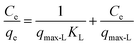 |
(10) | 23 |
 |
(11) | ||
| Freundlich | 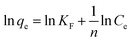 |
(12) | |
| Temkin | qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A + B A + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce |
(13) | |
 |
(14) | ||
| Dubinin–Radushkevich (D–R) | ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qmax-D–R − βε2 qmax-D–R − βε2 |
(15) | |
 |
(16) | ||
 |
(17) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Thermodynamic relation | |||
 |
(18) | 24 | |
| K = KL × 1000 × M × C0 | (19) | ||
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
(20) | ||
2.4. Adsorbent regeneration
To evaluate the reusability of the adsorbents, regeneration tests were conducted. The tests involved mixing 2.5 g per L AsphF/Al(NO3)3s-T with 10 mL of 20 mg per L MO solution at pH = 7 at room temperature. Following equilibrium, the adsorbent was recovered using Whatman filter paper (no. 1, 11 μm), mixed into 20 mL ethanol for 1 h at 298 K, then washed repeatedly with distilled water to remove residual MO or ethanol. After washing, the regenerated adsorbent was dried at 343 K for 3 h and reused.3. Results and discussion
3.1. Characterization of the modified AsphF
The SEM images in Fig. 2 and S1† capture the structural changes in AsphF under various treatment conditions. The control samples (AsphF and AsphF/W) in Fig. 2a and S1a† depict a destroyed structure likely due to thermal stress/hydrolysis during heat treatment at 573 K under air atmosphere. Fig. 2b shows the samples treated with KOH, where solid mixing resulted in the formation of small pores, whereas the impregnation method caused significant destruction of the fiber structure. Fig. 2c and S1c† reveal that both solid Al(NO3)3·9H2O mixing and impregnation produced pores; nevertheless, solid mixing generated larger and more abundant pores. Similarly, the samples treated with solid and aqueous Fe(NO3)3·9H2O in Fig. 2d and S1d,† respectively, depict the formation of pores and ash in both treatments. Again, solid mixing exhibits a higher extent of pores and a more preserved structure which qualifies as hierarchical structure. Furthermore, the intact nature of the product and the ease of separating the fibers after treatment highlight the structural integrity achieved through solid mixing. These observations underscore the effectiveness of activating AsphF using solid mixing in preserving fiber structure and increasing porosity, rendering it a superior method compared to impregnation. Therefore, the rest of the characterization tests were performed on samples modified by solid mixing. The oxidizing agents Al(NO3)3·9H2O and Fe(NO3)3·9H2O help in conserving the structure of AsphF during heat treatment by virtue of NO3− (nitrate ions). Nitrate ions act as mild oxidizing agents, facilitating controlled oxidation away from excessive degradation or fragmentation of the fiber structure, while incorporating oxygen-containing functional groups. This explanation draws from previous research on acid oxidizing agents, e.g. HNO3, to stabilize asphaltenes-derived carbon fibers.25 | ||
| Fig. 2 SEM images of AsphF with and without solid activation under air atmosphere at 573 K for 24 h: (a) AsphF and AsphF-T (b) AsphF/KOHs-T (c) AsphF/Al(NO3)3s-T (d) AsphF/Fe(NO3)3s-T. | ||
Activation time is also one of the factors that affect the morphology of AsphF.26 As the activation time increased, the surface of the AsphF became rougher, as evident in the SEM images of Fig. S2.†
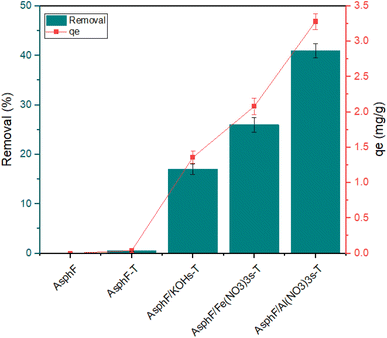
The hierarchical porous structures in Fig. 2 dictated the nitrogen adsorption–desorption isotherms in Fig. 3. The untreated AsphF exhibit minimal nitrogen adsorption, suggesting very low porosity, while chemically modified fibers, especially AsphF/KOHs-T, AsphF/Fe(NO3)3s-T, and AsphF/Al(NO3)3s-T, displayed significant adsorption and distinct hysteresis loops, confirming the presence of mesopores.27 The pore size distribution for the chemically modified fibers in Table 3 showed substantial mesopores (30–50 Å) volume (Vmeso) of range 0.075–0.101 cm3 g−1 with a small contribution from micropores (<20 Å) volume (Vmicro) of range 0.0004–0.00009 cm3 g−1. The untreated AsphF show the lowest specific surface area (SBET) of 1.27 m2 g−1 and minimal total pore volume (Vtotal). AsphF-T exhibit a slight increase in SBET (2.12 m2 g−1) and pore volumes. Significantly enhanced properties are observed in AsphF/KOHs-T showing an SBET of 66.57 m2 g−1 and a notable increase in mesopore volume (0.075 cm3 g−1). The highest surface area is achieved with AsphF/Al(NO3)3s-T and AsphF/Fe(NO3)3s-T, with SBET values of 110.2 m2 g−1 and 91.80 m2 g−1, respectively, and corresponding increase in Vtotal and Vmeso. These results suggest that Fe(NO3)3 and Al(NO3)3 are superior chemical activation agents to the more widely used KOH.
 | ||
| Fig. 3 (a) Nitrogen adsorption–desorption isotherms of the modified and unmodified AsphF (dashed lines indicate desorption); (b) pore size distribution. | ||
| Type of isotherm | SBETa (m2 g−1) | Vtotalb,e (cm3 g−1) | Vmicroc (cm3 g−1) | Vmesod (cm3 g−1) | |
|---|---|---|---|---|---|
| a SBET = specific surface area based on BET.b Vtotal = total pore volume at P/Po ∼ 0.99.c Vmicro = T-plot micropore volume.d Vmeso = Vtotal − Vmicro.e Cumulative volume of pores using BJH method.f N/D = not determined. | |||||
| AsphF | I | 1.27 | 0.002 | 0.002 | N/Df |
| AsphF-T | I | 1.94 | 0.003 | 0.003 | N/Df |
| AsphF/KOHs-T | IV | 66.57 | 0.075 | 0.0004 | 0.075 |
| AsphF/Al(NO3)3s-T | IV | 110.2 | 0.081 | 0.0053 | 0.076 |
| AsphF/Fe(NO3)3s-T | IV | 91.80 | 0.101 | 0.00009 | 0.101 |
The FTIR spectra in Fig. 4 display the structural changes in untreated and chemically modified asphaltenes fibers. For AsphF, peaks at 2922 cm−1 and 2847 cm−1 correspond to the asymmetric and symmetric stretching vibrations of aliphatic C–H bonds, indicating the presence of long aliphatic chains.28,29 The peak at 2105 cm−1 suggests the presence of weak C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C stretching vibrations or carbonaceous materials, which are common in asphaltenes structures.30 The band at 1565 cm−1 is attributed to the stretching vibrations of aromatic C
C stretching vibrations or carbonaceous materials, which are common in asphaltenes structures.30 The band at 1565 cm−1 is attributed to the stretching vibrations of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, confirming the aromatic nature of asphaltenes.29,31 The peak at 1438 cm−1 corresponds to aliphatic C–H bending vibrations, further validating the presence of aliphatic hydrocarbons.29 The peak at 1032 cm−1 can be attributed to the ester linkages found in the asphaltenes molecule.32 For the modified AsphF, distinct changes are observed. In AsphF-T and AsphF/KOHs-T, the peak at 2922 cm−1 and 2847 cm−1 disappeared, likely due to thermal oxidation. AsphF/Fe(NO3)3s-T display additional peak at 835 cm−1 attributed to Fe–O stretching and nitrate group vibrations, confirming the incorporation of iron species.33,34 These modifications also show a decrease in the intensity of the aliphatic C–H peaks, suggesting partial surface oxidation. Similarly, AsphF/Al(NO3)3s-T exhibit enhanced reduction in the intensity of the aromatic functional groups. These results confirm that chemical treatment introduces oxygen-containing and metal–oxygen functional groups while preserving the aromatic backbone of AsphF, which aligns with the gentle oxidizing nature of nitrate ions (NO3−) discussed earlier. Incorporating functional groups into the fibers while maintaining their structural integrity, ultimately increases the number of active binding sites.
C bonds, confirming the aromatic nature of asphaltenes.29,31 The peak at 1438 cm−1 corresponds to aliphatic C–H bending vibrations, further validating the presence of aliphatic hydrocarbons.29 The peak at 1032 cm−1 can be attributed to the ester linkages found in the asphaltenes molecule.32 For the modified AsphF, distinct changes are observed. In AsphF-T and AsphF/KOHs-T, the peak at 2922 cm−1 and 2847 cm−1 disappeared, likely due to thermal oxidation. AsphF/Fe(NO3)3s-T display additional peak at 835 cm−1 attributed to Fe–O stretching and nitrate group vibrations, confirming the incorporation of iron species.33,34 These modifications also show a decrease in the intensity of the aliphatic C–H peaks, suggesting partial surface oxidation. Similarly, AsphF/Al(NO3)3s-T exhibit enhanced reduction in the intensity of the aromatic functional groups. These results confirm that chemical treatment introduces oxygen-containing and metal–oxygen functional groups while preserving the aromatic backbone of AsphF, which aligns with the gentle oxidizing nature of nitrate ions (NO3−) discussed earlier. Incorporating functional groups into the fibers while maintaining their structural integrity, ultimately increases the number of active binding sites.
The oxygen versus carbon composition of the untreated and chemically treated AsphF is given in Fig. 5. AsphF-T show no significant change in oxygen content, indicating a limited oxygenated functional group. In contrast, treatments with KOH, Fe(NO3)3s, and Al(NO3)3s result in a substantial reduction in carbon content relative to oxygen, suggesting successful surface functionalization through oxidation and/or formation of oxygenated species and metal complexes. This observation aligns with literature interpretation, where also the slight decrease in carbon content was attributed to partial oxidation.25 This transformation is critical for the stabilization process as it facilitates the cross-linking of asphaltenes structures, pore creation, enhancing the thermal stability and structural integrity of the resulting carbon fibers.
 | ||
| Fig. 5 Elemental composition of carbon (C) and oxygen (O) for the untreated and chemically treated AsphF. | ||
3.2. Adsorption study
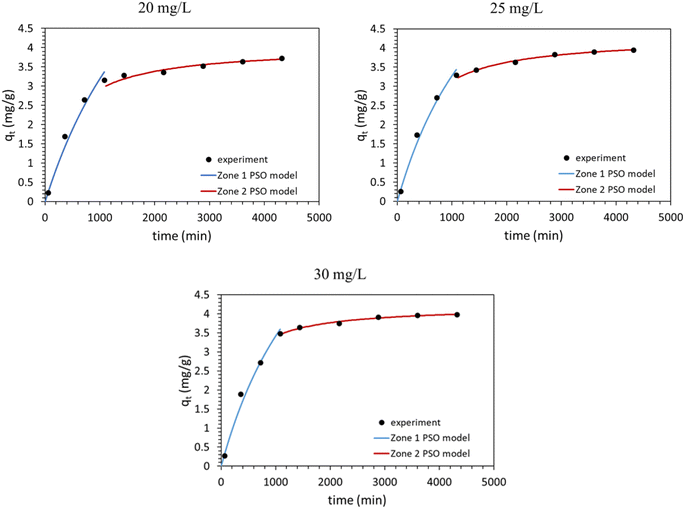
Generally, PSO displayed a higher correlation coefficient (R2 ∼ 0.9830) across all concentrations (Table S1 and Fig. S6†) compared to the other models. PSO assumes chemical interactions, via electron transfer or valency forces, govern the rate of adsorption. It is noted that the adsorption of MO on different adsorbents has been previously described using PSO kinetics.3,23,35 Nevertheless, a closer look at MO adsorption data for the different initial concentrations reveals two distinct adsorption zones; t ≤ 18 h (zone 1) and t ≥ 18 h (zone 2). Fig. 7 and Table 4 show R2 based on the linearized form of PSO for the two zones for different MO initial concentrations. For example, for 20 mg L−1 initial concentration of MO, the first zone exhibits low R2 value (∼0.5970), whereas the second zone displays high R2 value (∼0.9987). To thoroughly investigate these zones, Weber and Morris intraparticle diffusion model was applied.36 The intraparticle diffusion model (Fig. 8) was fitted to the two adsorption zones. The fit appears excellent for the two zones (R2 ≥ 0.9488). Physically, zone 1 describes diffusion into mesopores. As evidenced by the higher ki,1 value and negative intercept, I, the initial adsorption into mesopores represents rapid molecular diffusion into the larger pores. Conversely, zone 2 describes diffusion into micropores, as reflected by lower ki,2 value and positive I. It is noted that zone 2 could be equally described by the pore diffusion model and the chemisorption model, which suggests diffusion into micropore or chemisorption mechanism.2,22,36–38
| Zone 1 | Zone 2 | |||||
|---|---|---|---|---|---|---|
| 20 mg L−1 | 25 mg L−1 | 30 mg L−1 | 20 mg L−1 | 25 mg L−1 | 30 mg L−1 | |
| PSO | ||||||
| R2 | 0.5970 | 0.8035 | 0.7554 | 0.9987 | 0.9997 | 0.9997 |
| qe-c (mg g−1) | 10.67 | 9.24 | 9.75 | 4.02 | 4.29 | 4.20 |
| k2 (g mg−1 min−1) | 0.00005 | 0.00075 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Intraparticle diffusion | ||||||
| R2 | 0.9940 | 0.9967 | 0.9959 | 0.9831 | 0.9492 | 0.9488 |
| I (mg g−1) | −0.621 | −0.630 | −0.645 | 2.623 | 2.731 | 3.166 |
| ki (g mg−1 min−0.5) | 0.122 | 0.016 | ||||
As the temperature increased from 298 K to 333 K, the adsorption capacity of the maximum uptake (qmax-L) also increased from 3.95 mg g−1 to 5.14 mg g−1. The thermodynamic analysis discussed in the next section shows a positive ΔH0 value, indicating an endothermic reaction that further supports a shift of the equilibrium toward more adsorption at higher temperatures. The separation factor (RL) values extracted from Langmuir isotherm (Table 5), decreased from 0.09 to 0.07 with increasing temperature while 0 < RL < 1, which imply favorable adsorption of MO onto the AsphF/Al(NO3)3s-T adsorbent surface.23
| 298 K | 313 K | 333 K | |
|---|---|---|---|
| R2 | 0.9917 | 0.9893 | 0.9782 |
| qmax-L (mg g−1) | 3.95 | 4.31 | 5.14 |
| KL (L mg−1) | 0.48 | 0.57 | 0.69 |
| RL | 0.09 | 0.08 | 0.07 |
The calculated values of ΔH0, ΔS0 and ΔG0 in Table 6 provide valuable insight into the adsorption of MO onto AsphF/Al(NO3)3s-T. As reported in the literature,39 ΔG0 values for physical adsorption typically range from −20 to 0 kJ mol−1, while those for chemical adsorption are generally between −400 and −80 kJ mol−1. Based on Table 6, ΔG0 values ranged from −29.65 to −34.13 kJ mol−1, suggesting that the adsorption process was not a single physical or chemical adsorption but features both. This finding aligns with the results from the kinetic model, which point to multiple mechanisms influencing the adsorption process. It suggests that the adsorption of MO on AsphF/Al(NO3)3s-T is more complex than the PSO model suggests, highlighting that chemisorption is not the only factor affecting the adsorption rate. The negative values of ΔG0 indicate that the adsorption process is spontaneous and becomes more favorable at higher temperatures. While reflecting an endothermic adsorption process, the positive ΔH0 value of 8.41 kJ mol−1, in principle, contributes to more positive ΔG0. The spontaneity of the adsorption process can be explained by the steps involved during adsorption. Specifically, water molecules initially adsorbed onto the surface must be desorbed before any MO dye molecules are adsorbed. Since desorption of water molecules is an endothermic reaction, and adsorption is typically exothermic, it appears that the heat absorbed during water desorption exceeds the heat released during dye adsorption. This results in an overall endothermic process. Additionally, the molar volume of water molecules is much smaller than that of MO dyes, meaning numerous water molecules must be displaced to accommodate a single dye molecule.40 The positive entropy change indicates an increase in the overall randomness at the solid–liquid interface during adsorption. While adsorption decreases randomness by organizing dye molecules onto the adsorbent surface, desorption of water molecules increases the system disorder, which in turn dominates the overall change in entropy contributing positive ΔS0. This results agree with results obtained by ref. 40.
| T (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (kJ K−1 mol−1) | R2 |
|---|---|---|---|---|
| 298 | −29.65 | 8.41 | 0.13 | 0.9995 |
| 313 | −31.59 | |||
| 333 | −34.13 |
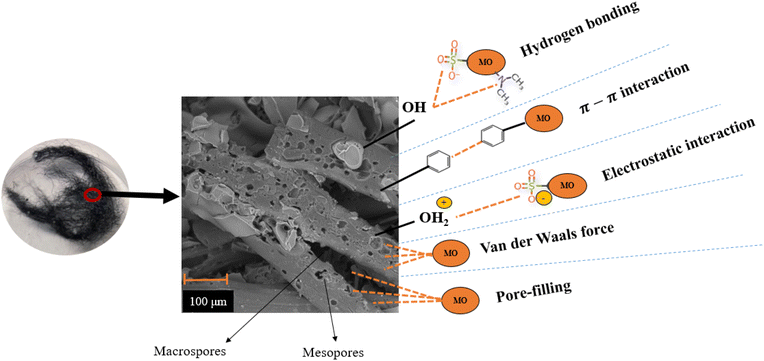 | ||
| Fig. 11 Adsorption mechanisms of MO into AsphF/Al(NO3)3s-T. Note: micropores are not visible in this magnification. | ||
Although this study used pure methyl orange, the main adsorption mechanisms are expected to apply to other similar anionic dyes. Nevertheless, real wastewater may contain a combination of contaminants that may compete on adsorption sites, and/or alter the chemistry of a given contaminant, and/or adsorbent. Wastewaters belonging to certain industries may be considered in future work.
 | ||
| Fig. 12 Regeneration performance of AsphF/Al(NO3)3s-T toward MO adsorption experiments (298 K, 24 h, 200 rpm, Ci = 20 mg L−1, 2.5 g adsorbent per L). | ||
The comparison of various adsorbents used for MO removal is shown in Table 7, highlighting their maximum uptake (qmax), pH conditions, and contact times. Among the adsorbents, AsphF/Al(NO3)3s-T from this study demonstrate a good adsorption capacity of 6.32 mg g−1 at pH = 3 with a contact time of 24 h.
| Adsorbent | qmax (mg g−1) | pH | Contact time | Reference |
|---|---|---|---|---|
| Chitosan beads | 5.60 | 8 | 24 h | 44 |
| Functionalized multiwalled carbon nanotubes | 52.86 | 2.3 | 2 h | 45 |
| Rice husk | 1.30 | 2 | 35 min | 46 |
| Graphene oxide | 16.83 | 3 | 100 min | 47 |
| Bottom ash | 3.62 | 3 | 4 h | 48 |
| Modified asphaltenes particles | 7.80 | 3 | 120 h | 23 |
| Modified asphaltenes fibers (AsphF/Al(NO3)3s-T) | 6.32 | 3 | 24 h | This study |
Although the results are promising, there are some limitations to consider. The adsorption capacity of the modified fibers, while improved, is still lower than some advanced materials such as nanostructured adsorbents. In addition, the use of Fe(NO3)3, and Al(NO3)3 may lead to small amounts of metal leaching, which should be evaluated before applying the adsorbent on a larger scale. The experiments in this study were performed using a single dye under controlled conditions, but real wastewater usually contains a variety of contaminants that could affect adsorption. Moreover, while batch adsorption experiments do not capture the adsorbent performance in an industrial setting, column adsorption may be considered in a future study.
4. Conclusions
This study successfully developed hierarchical porous asphaltenes fibers chemically activated using KOH, Fe(NO3)3, and Al(NO3)3 via solid and impregnation mixing methods. Among the synthesized adsorbents, Al(NO3)3-modified asphaltenes fibers prepared through solid mixing exhibited the highest adsorption performance for MO dye, with a maximum uptake of 6.32 mg g−1 and 79% removal efficiency at pH = 3 and 298 K. The adsorption process followed the pseudo second order kinetic model and/or intraparticle diffusion. Initially, adsorption is driven by diffusion into mesopores, while in the second zone, it proceeds through either diffusion into micropores or chemisorption at active sites. Isotherm studies revealed that the Langmuir model best described the equilibrium uptake, confirming monolayer adsorption on a homogenous surface. Thermodynamic parameters demonstrated the endothermic and spontaneous nature of the adsorption, with positive entropy changes (ΔS0 = 0.13 kJ K−1 mol−1) suggesting increased randomness at the solid–liquid interface. Regeneration experiments highlighted the adsorbent stability and reusability, with a 23.78% decline in adsorption capacity over five cycles. These findings underline the durability and cost-effectiveness of modified asphaltenes fibers.Abbreviations
Symbol
| AsphF | As-received asphaltenes fibers |
| AsphF-T | Heat treated asphaltenes fibers |
| AsphF/W-T | Heat treated impregnated asphaltenes fibers |
| AsphF/KOHs-T | Heat treated asphaltenes fibers solid mixed with KOH |
| AsphF/Fe(NO3)3s-T | Heat treated asphaltenes fibers solid mixed with Fe(NO3)3 |
| AsphF/Al(NO3)3s-T | Heat treated asphaltenes fibers solid mixed with Al(NO3)3 |
| AsphF/KOHaq-T | Heat treated asphaltenes fibers impregnated with KOH |
| AsphF/Fe(NO3)3aq-T | Heat treated asphaltenes fibers impregnated with Fe(NO3)3 |
| AsphF/Al(NO3)3aq-T | Heat treated asphaltenes fibers impregnated with Al(NO3)3 |
| Ci | Initial concentration of MO (mg L−1) |
| Ct | Concentration of MO after a specific time (mg L−1) |
| Ce | Equilibrium MO concentration (mg L−1) |
| qt | Adsorption uptake at a specific time (mg g−1) |
| qe | Equilibrium adsorption capacity (mg g−1) |
| V | Volume of the solution containing the adsorbate (L) |
| m | Mass of the adsorbent used in the adsorption process (g) |
| k1 | Rate constant of the pseudo-first-order adsorption process (min−1) |
| k2 | Rate constant of the pseudo-second-order adsorption process (g mg−1 min−1) |
| ki | Intraparticle diffusion rate constant (g mg−1 min−0.5) |
| I | Intercept of the intraparticle diffusion model related to the boundary layer thickness (mg g−1) |
| k0 | Bangham constant (mL g−1 L−1) |
| qmax-L | Langmuir maximum adsorption uptake (mg g−1) |
| KL | Langmuir isotherm constant (L mg−1) |
| RL | Separation factor constant (unitless) |
| KF | Freundlich isotherm constant (mg g−1 (L mg−1)1/n) |
| 1/n | Adsorption intensity factor (unitless) |
| B | Amount of adsorption heat (J mol−1) |
| A | Temkin equilibrium binding constant (L mg−1) |
| R | Universal gas constant 8.314 (J mol−1 K−1) |
| T | Absolute temperature (K) |
| 1/bT | Adsorption potential of adsorbent |
| qmax-D–R | Dubinin–Radushkevich maximum adsorption uptake (mg g−1) |
| E | Mean adsorption energy (J mol−1) |
| K | Thermodynamic equilibrium constant (unitless) |
| ΔS0 | Standard entropy change of the adsorption (J mol−1 K−1) |
| ΔH0 | Standard enthalpy change of the adsorption (J mol−1) |
| MW | Molar mass (g mol−1) |
| C0 | Standard concentration of the adsorbate (mol L−1) |
| ΔG0 | Standard Gibbs free energy change (J mol−1) |
Greek symbol
| αElovich | Initial adsorption rate constant (mg g−1 min−1) |
| αBangham | Constant in Bangham model (unitless) |
| βElovich | Desorption constant (g mg−1) |
| βD–R | Activity coefficient related to adsorption mean free energy (mol2 J−2) |
| ε | Constant related to Polanyi potential (J2 mol−2) |
Data availability
All data generated or analyzed during this study are either included in this published article or are available from the corresponding author upon reasonable request. The core datasets supporting the adsorption experiments, material characterization (including FTIR spectra, BET surface area data, and SEM images), and kinetic and isotherm modeling results are available for academic and non-commercial use. Due to the file size and formatting of certain raw data outputs, they have not been uploaded publicly but can be shared in a suitable format upon request. Researchers interested in reproducing or further developing the study findings are encouraged to contact the corresponding author to obtain access to the original experimental datasets and supporting documentation.Conflicts of interest
There are no conflicts to declare.References
- WHO, WHO Global Water, Sanitation and Hygiene Annual Report 2019, 2020 Search PubMed.
- K. Jedynak, M. Repelewicz, K. Kurdziel and D. Wideł, Mesoporous carbons as adsorbents to removal of methyl orange (Anionic dye) and methylene blue (cationic dye) from aqueous solutions, Desalin. Water Treat., 2021, 220, 363–379 CrossRef CAS.
- H. Alyasi, H. Mackey and G. McKay, Adsorption of Methyl Orange from Water Using Chitosan Bead-like Materials, Molecules, 2023, 28, 6561 CrossRef CAS PubMed.
- W. Chen, X. Zhang, M. Mamadiev and Z. Wang, Sorption of perfluorooctane sulfonate and perfluorooctanoate on polyacrylonitrile fiber-derived activated carbon fibers: In comparison with activated carbon, RSC Adv., 2017, 7, 927–938 RSC.
- S. Satyam and S. Patra, Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review, Heliyon, 2024, 10(9), e29573 CrossRef CAS PubMed.
- D. A. Gkika, A. C. Mitropoulos and G. Z. Kyzas, Why reuse spent adsorbents? The latest challenges and limitations, Sci. Total Environ., 2022, 822, 153612 CrossRef CAS PubMed.
- S. Fakher, M. Ahdaya, M. Elturki and A. Imqam, Critical review of asphaltene properties and factors impacting its stability in crude oil, J. Pet. Explor. Prod. Technol., 2020, 10, 1183–1200 CrossRef CAS.
- N. H. M. H. Tehrani, M. S. Alivand, D. M. Maklavany, A. Rashidi, M. Samipoorgiri, A. Seif and Z. Yousefian, Novel asphaltene-derived nanoporous carbon with N-S-rich micro-mesoporous structure for superior gas adsorption: Experimental and DFT study, Chem. Eng. J., 2019, 358, 1126–1138 CrossRef CAS.
- M. A. Rabeea, T. A. Zaidan, A. H. Ayfan and A. A. Younis, High porosity activated carbon synthesis using asphaltene particles, Carbon Lett., 2020, 30, 199–205 CrossRef.
- O. Ioannidou and A. Zabaniotou, Agricultural residues as precursors for activated carbon production-A review, Renewable Sustainable Energy Rev., 2007, 11(9), 1966–2005 CrossRef CAS.
- K. Y. Foo and B. H. Hameed, Preparation and characterization of activated carbon from pistachio nut shells via microwave-induced chemical activation, Biomass Bioenergy, 2011, 35(7), 3257–3261 CrossRef CAS.
- X. Zhang, L. Lin, W. Gao, Y. Zhou and Q. Lin, A novel Fe-containing carbon foam with hierarchical porous structure for efficient removal of organic dyes, Diam. Relat. Mater., 2023, 140, 110492 CrossRef CAS.
- S. Li, H. Zhang, S. Hu, J. Liu, Q. Zhu and S. Zhang, Synthesis of hierarchical porous carbon in molten salt and its application for dye adsorption, Nanomaterials, 2019, 9(8), 1098 CrossRef CAS PubMed.
- A. K. Ishkildina and O. S. Travkina, Effects of the Method for the Preparation of Synthetic Aluminosilicate on the Properties of ZSM-5, Pet. Chem., 2024, 64, 181–185 CrossRef CAS.
- H. L. Girard, P. Bourrianne, D. Chen, A. Jaishankar, J. L. Vreeland, R. E. Cohen, K. K. Varanasi and G. H. McKinley, Asphaltene Adsorption on Functionalized Solids, Langmuir, 2020, 36(14), 3894–3902 CrossRef CAS PubMed.
- S. Abedi, High performance electrode and catalyst nano-materials for energy storage devices: Supercapacitors, pseudocapacitors and zinc-air batteries made from asphaltene based carbon fibers, PhD thesis, University of Alberta, 2023.
- Sigma-Aldrich, Potassium hydroxide, ACS reagent, ≥85%, pellets, https://www.sigmaaldrich.com/CA/en/product/sigald/221473 Search PubMed.
- Sigma-Aldrich, Iron(III) nitrate nonahydrate, ACS reagent, ≥98%, https://www.sigmaaldrich.com/US/en/product/sigald/216828 Search PubMed.
- Sigma-Aldrich, Aluminum nitrate nonahydrate, ACS reagent, ≥98%, https://www.sigmaaldrich.com/US/en/product/sigald/237973 Search PubMed.
- A. G. De Crisci, R. Gieleciak, M. H. Mobarok, M. Ali, T. D. Ngo, K. Goswami, A. Khan and J. Chen, Producing asphaltene fibres from bitumen-derived asphaltenes for carbon fibre development: Part one–Electrospinning, Can. J. Chem. Eng., 2023, 101(5), 2633–2645 CrossRef CAS.
- M. A. Behnajady, N. Modirshahla and H. Fathi, Kinetics of decolorization of an azo dye in UV alone and UV/H2O2 processes, J. Hazard. Mater., 2006, 136(3), 816–821 CrossRef CAS PubMed.
- I. D. Mall, V. C. Srivastava and N. K. Agarwal, Removal of Orange-G and Methyl Violet dyes by adsorption onto bagasse fly ashdkinetic study and equilibrium isotherm analyses, Dyes Pigm., 2006, 69(3), 210–223 CrossRef CAS.
- A. Eshraghian, L. Yu, G. Achari and U. Sundararaj, Development of an effective asphaltene-derived adsorbent for wastewater treatment: Characterization and methyl orange removal study, J. Environ. Chem. Eng., 2023, 11(1), 109221 CrossRef CAS.
- X. Zhou, X. Yu, J. Hao and H. Liu, Comments on the calculation of the standard equilibrium constant using the Langmuir model in Journal of Hazardous Materials 422 (2022) 126863, J. Hazard. Mater., 2022, 429, 1–3 Search PubMed.
- D. Leistenschneider, P. Zuo, Y. Kim, Z. Abedi, D. G. Ivey, A. de Klerk, X. Zhang and W. Chen, A mechanism study of acid-assisted oxidative stabilization of asphaltene-derived carbon fibers, Carbon Trends, 2021, 5, 100090 CrossRef CAS.
- E. De Rose, S. Bartucci, C. Poselle Bonaventura, G. Conte, R. G. Agostino and A. Policicchio, Effects of activation temperature and time on porosity features of activated carbons derived from lemon peel and preliminary hydrogen adsorption tests, Colloids Surf., A, 2023, 672, 131727 CrossRef CAS.
- K. Phothong, C. Tangsathitkulchai and P. Lawtae, The analysis of pore development and formation of surface functional groups in bamboo-based activated carbon during co2 activation, Molecules, 2021, 26(18), 5641 CrossRef CAS PubMed.
- I. Zojaji, A. Esfandiarian and J. Taheri-Shakib, Toward molecular characterization of asphaltene from different origins under different conditions by means of FT-IR spectroscopy, Adv. Colloid Interface Sci., 2021, 289, 102314 CrossRef CAS PubMed.
- X. Qiyong, K. Wyclif, P. Jingjun, R. Xiong, W. Deng, S. Zhang, J. Guo and Y. Yang, Analysis of Xinjiang asphaltenes using high precision spectroscopy, RSC Adv., 2020, 10(65), 39425–39433 RSC.
- M. Alhreez and D. Wen, Molecular structure characterization of asphaltene in the presence of inhibitors with nanoemulsions, RSC Adv., 2019, 9(34), 19560–19570 RSC.
- A. S. Shalygin, E. S. Milovanov, E. P. Kovalev, S. S. Yakushkin, S. G. Kazarian and O. N. Martyanov, In Situ FTIR Spectroscopic Imaging of Asphaltene Deposition from Crude Oil under n-Heptane and Acetone Flows, Pet. Chem., 2022, 62, 1087–1095 CrossRef CAS.
- W. T. Welch, R. R. Bledsoe, B. K. Wilt and M. B. Sumner, Process for analysis of asphaltene content in hydrocarbon mixtures by middle infrared spectroscopy, US Pat., 6087662, filed May 22, 1998, and issued July 11, 2000 Search PubMed.
- S. F. Aval, A. Akbarzadeh, M. R. Yamchi, F. Zarghami, K. Nejati-Koshki and N. Zarghami, Gene silencing effect of SiRNA-magnetic modified with biodegradable copolymer nanoparticles on hTERT gene expression in lung cancer cell line, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 188–193 CrossRef PubMed.
- M. K. Trivedi and A. B. Dahryn Trivedi, Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment, J. Chromatogr. Sep. Tech., 2015, 6(5), 1000282 Search PubMed.
- A. Abollé, K. Y. Urbain, K. Ollo, K. Y. Tchonrontcha and K. A. Rodrigue, Adsorption of Methyl Orange on Corncob Activated Carbon: Kinetic, Equilibrium, and Thermodynamic Studies, Earthline J. Chem. Sci., 2022, 8, 205–224 Search PubMed.
- C. Sutherland, A diffusion-chemisorption kinetic model for simulating biosorption using forest macro-fungus, fomes fasciatus, International Research Journal of Plant Science, 2010, 1(4), 107–117 Search PubMed.
- N. K. Lazaridis and D. D. Asouhidou, Kinetics of sorptive removal of chromium(VI) from aqueous solutions by calcined Mg-Al-CO3 hydrotalcite, Water Res., 2003, 37(12), 2875–2882 CrossRef CAS PubMed.
- A. Pholosi, E. B. Naidoo and A. E. Ofomaja, Intraparticle diffusion of Cr(VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study, S. Afr. J. Chem. Eng., 2020, 32, 39–55 Search PubMed.
- C. Li, Z. Xiong, J. Zhang and C. Wu, The Strengthening Role of the Amino Group in Metal-Organic Framework MIL-53 (Al) for Methylene Blue and Malachite Green Dye Adsorption, J. Chem. Eng. Data, 2015, 60(11), 3414–3422 CrossRef CAS.
- S. Hussain, M. Kamran, S. A. Khan, K. Shaheen, Z. Shah, H. Suo, Q. Khan, A. B. Shah, W. U. Rehman, Y. O. Al-Ghamdi and U. Ghani, Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films, Int. J. Biol. Macromol., 2021, 168, 383–394 CrossRef CAS PubMed.
- Y. O. Khaniabadi, R. Heydari, H. Nourmoradi, H. Basiri and H. Basiri, Low-cost sorbent for the removal of aniline and methyl orange from liquid-phase: Aloe Vera leaves wastes, J. Taiwan Inst. Chem. Eng., 2016, 68, 90–98 CrossRef CAS.
- R. Lafi and A. Hafiane, Removal of methyl orange (MO) from aqueous solution using cationic surfactants modified coffee waste (MCWs), J. Taiwan Inst. Chem. Eng., 2016, 58(4), 424–433 CrossRef CAS.
- Y. S. Reddy, T. J. Jose, B. Dinesh, R. N. Kumar, P. S. Kumar and K. Kaviyarasu, Equilibrium, kinetic, and thermodynamic study of Direct Yellow 12 dye adsorption by biomass-derived porous graphitic activated carbon, Biomass Convers. Biorefin., 2024, 6817–6833 Search PubMed.
- W. A. Morais, A. L. P. de Almeida, M. R. Pereira and J. L. C. Fonseca, Equilibrium and kinetic analysis of methyl orange sorption on chitosan spheres, Carbohydr. Res., 2008, 343(14), 2489–2493 CrossRef CAS PubMed.
- Y. Yao, H. Bing, X. Feifei and C. Xiaofeng, Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes, Chem. Eng. J., 2011, 170(1), 82–89 CrossRef CAS.
- D. R. Tchuifon, S. G. Anagho, E. Njanja, J. N. Ghogomu, N. G. Ndifor-Angwafor and T. Kamgaing, Equilibrium and kinetic modelling of methyl orange adsorption from aqueous solution using rice husk and egussi peeling, Int. J. Chem. Sci., 2014, 12(3), 741–761 CAS.
- D. Robati, B. Mirza, M. Rajabi, O. Moradi, I. Tyagi, S. Agarwal and V. K. Gupta, Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase, Chem. Eng. J., 2016, 284, 687–697 CrossRef CAS.
- A. Mittal, A. Malviya, D. Kaur, J. Mittal and L. Kurup, Studies on the adsorption kinetics and isotherms for the removal and recovery of Methyl Orange from wastewaters using waste materials, J. Hazard. Mater., 2007, 148(1–2), 229–240 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra03061g |
| This journal is © The Royal Society of Chemistry 2025 |