 Open Access Article
Open Access ArticleAdvances in Co3O4 nanomaterial-based photocatalysts for water purification: mechanisms, green synthesis, activation of oxidants, waste-derived sources, and computational insights
Van Dien Dang
a,
Nguyen Thi Hong Nhung
b,
Iqra Rabani
c,
Nguyen Tien Tran
de,
Bui Thi Phuong Thuy
 f and
Hai Bang Truong
f and
Hai Bang Truong
 *gh
*gh
aFaculty of Biology and Environment, Ho Chi Minh City University of Industry and Trade, 140 Le Trong Tan, Tan Phu District, Ho Chi Minh 700000, Vietnam
bFaculty of Applied Science and Technology (FAST), Nguyen Tat Thanh University, 331 National Highway 1A, An Phu Dong Ward, District 12, Ho Chi Minh City 700000, Vietnam
cAntwerp Engineering, Photoelectrochemistry and Sensing (A-PECS), University of Antwerp, Groenenborgerlaan 171, 2020 Antwerp, Belgium
dCenter for Advanced Chemistry, Institute of Research and Development, Duy Tan University, Da Nang 550000, Vietnam
eFaculty of Natural Sciences, Duy Tan University, Da Nang 550000, Vietnam
fFaculty of Fundamental Sciences, Van Lang University, Ho Chi Minh City, Vietnam
gOptical Materials Research Group, Science and Technology Advanced Institute, Van Lang University, Ho Chi Minh City, Vietnam
hFaculty of Applied Technology, School of Technology, Van Lang University, Ho Chi Minh City, Vietnam. E-mail: truonghaibang@vlu.edu.vn
First published on 5th June 2025
Abstract
Water scarcity remains a critical global challenge, affecting billions of people and significantly impacting ecosystems, economies, and public health. Among various water treatment technologies, photocatalysis has emerged as a highly effective method for degrading a wide range of contaminants. Cobalt oxide (Co3O4) has gained considerable attention as a photocatalyst due to its unique structural, electronic, and optical properties. Despite extensive research on the synthesis and application of Co3O4-based photocatalysts, a comprehensive review summarizing recent advancements and modifications in Co3O4 nanomaterials over the past five years is notably lacking. This review critically examines the fundamental photocatalytic mechanisms of Co3O4 nanomaterial-based systems, systematically discussing their advantages and inherent limitations. Additionally, it explores emerging research trends, including biosynthesis, facile recovery, synthesis from waste-derived sources, and computational techniques, alongside the prevailing challenges shaping this field. Furthermore, the review identifies key research directions for the future development and optimization of Co3O4-based nanostructures, emphasizing their potential to enhance photocatalytic efficiency for water purification. By addressing these aspects, this work aims to bridge existing knowledge gaps and provide a foundation for future innovations in sustainable water treatment technologies.
1. Introduction
Water scarcity is a severe global issue affecting billions of people and posing significant challenges to ecosystems, economies, and public health. According to UNESCO, the number of urban residents facing water scarcity is expected to reach 2.5 billion by 2050.1 Thus, potential solutions for effective water treatment and reusability are urgently required to promote more sustainable and livable futures. Among the water treatment methods, photocatalysis offers distinct advantages in efficiently degrading a wide spectrum of pollutants.2,3 This eco-friendly process minimizes byproducts, utilizes renewable solar energy, and reduces chemical and energy demands.4 Cost-effective and operable under ambient conditions, photocatalysis is scalable and versatile, suitable for applications from small systems to large-scale plants.5 Compared to chlorination or reverse osmosis, it is more sustainable, less dependent on chemicals, and particularly effective against emerging contaminants.6 Photocatalysis involves a series of interconnected processes.7 It begins with light absorption, followed by the separation of electrons and holes. These charge carriers then migrate to the photocatalyst's surface, where active species are formed and conduct the reduction and oxidation reactions. At its core, photocatalysis utilizes optical energy to activate catalysts, producing reactive oxygen species with strong oxidative properties capable of degrading water contaminants.8Cobalt oxide (Co3O4) is a promising photocatalytic material due to its distinctive structural, electronic, and optical features.9 As a p-type semiconductor with a spinel configuration, Co3O4 exhibits excellent redox activity, facilitated by its mixed oxidation states (Co2+ and Co3+). Its narrow bandgap (1.5–2.4 eV) enables visible light absorption, crucial for solar energy-driven photocatalysis.10 Co3O4 is cost-effective, easy to synthesize, environmentally compatible, and offers excellent chemical stability and reversible redox performance.11 However, its performance is limited by charge recombination, photocorrosion under prolonged light exposure, and structural degradation in harsh environments.10 The bulk form of Co3O4 often exhibits limited surface area, restricting the quantity of active sites and further diminishing photocatalytic activity.12 Despite cobalt's relative abundance, the scalability and economic viability of large-scale Co3O4 production remain challenges for broader application in photocatalysis.
The preparation of materials at the nanoscale has emerged as a prominent strategy in recent years to significantly enhance the efficiency and versatility of photocatalysis.13 Nanostructures, including nanoparticles, nanorods, nanosheets, and nanotubes, exhibit a high surface-to-volume ratio, thereby increasing the quantity of active sites available for pollutant adsorption and catalytic reactions, which in turn improves overall photocatalytic performance.6,14 Additionally, the dimension, shape, and composition of nanomaterials can be precisely tailored to modulate their electronic properties, such as the bandgap, thereby enabling the absorption of a broader spectrum of solar radiation.15 Doping these nanomaterials with metals, non-metals, or engineering heterojunctions with other semiconductors further enhances charge carrier separation, mitigating electron–hole recombination and optimizing photocatalytic efficiency.16
Despite extensive research on the synthesis and application of Co3O4-based photocatalysts, there remains a notable paucity of review articles addressing the advancements and modifications in Co3O4-based nanomaterial systems in the recent five years for the efficient decomposition of pollutants in water. This review provides a critical assessment of the underlying photocatalytic mechanisms, delineating the advantages and limitations inherent to these systems. In addition, it offers an in-depth analysis of the prevailing research trends, including biosynthesis, facile recovery, synthesis from waste-derived sources, photocatalytic activation of oxidants, and computational techniques, alongside the prevailing challenges shaping this field. Finally, the review outlines prospective research trajectories for the development and optimization of Co3O4 nanostructures in the photocatalytic remediation of contaminated water, emphasizing key areas of significant potential for future exploration.
2. Physical and chemical characteristics of Co3O4
Co3O4 is a mixed-valence transition metal oxide that crystallizes in a spinel structure, where Co2+ ions occupy tetrahedral sites and Co3+ ions are located in octahedral positions within a close-packed oxygen lattice.17 This distinctive arrangement confers Co3O4 with remarkable redox flexibility and facilitates efficient electron transfer and oxygen mobility, which are essential for photocatalytic activity. As a p-type semiconductor, Co3O4 exhibits a relatively narrow band gap in the range of 1.4 to 2.2 eV,18,19 enabling strong absorption in the visible region of the electromagnetic spectrum. The valence band is composed of O 2p orbitals, while Co 3d orbitals form the conduction band, allowing effective excitation of electron–hole pairs under solar illumination.20 This optical behavior makes Co3O4 highly attractive for sustainable photocatalytic water treatment, particularly in solar-driven pollutant degradation processes. One of the standout advantages of Co3O4 is its robust thermal and chemical stability. The material retains its crystalline structure at temperatures exceeding 800 °C and remains stable across a wide pH spectrum.21,22 This durability ensures reliable long-term performance, particularly in harsh or variable water treatment environments. Although cobalt exists naturally in various minerals such as cobaltite, erythrite, and heterogenite, Co3O4 used in photocatalysis is typically produced through synthetic processes to ensure purity, controlled morphology, and reproducible performance.23 These laboratory-based preparations are necessary to generate nanoscale Co3O4 with tailored surface area, crystal facets, and defect densities, which are not easily obtained from naturally occurring sources.24 Compared to many conventional materials, this level of tunability greatly enhances Co3O4's catalytic performance and practical utility. The photocatalytic performance of Co3O4 is heavily influenced by its physicochemical features, which are governed by the synthesis method. A wide range of fabrication techniques have been developed to engineer Co3O4 nanostructures with desirable morphology and crystallinity.25 Tailored nanostructures not only increase the surface-to-volume ratio but also introduce oxygen vacancies and unsaturated cobalt sites that act as active centers for photocatalytic reactions. The surface defects enhance visible-light absorption and promote the formation of reactive oxygen species, such as hydroxyl and superoxide radicals, which are pivotal in decomposing organic contaminants. Building upon the crucial role of surface structure and defects, the following section reveals deeper into how different synthesis routes shape the morphology of Co3O4 nanomaterials and influence their photocatalytic behavior.3. Synthesis and morphology of Co3O4 nanomaterial-based photocatalysts
The morphology of Co3O4 nanomaterials plays a pivotal role in governing their photocatalytic efficacy, as it influences key factors such as light absorption, charge separation, surface reactivity, and the availability of active sites. Among the various Co3O4 nanostructures, nanospheres are the most prevalent due to their versatile synthesis methods, including hydrothermal, solvothermal, co-precipitation, calcination, and biosynthesis.26–31 The diameters and band gap energies of Co3O4 nanospheres exhibit a wide range, spanning from 6 to 100 nm and 1.77 to 3.86 eV, respectively, which significantly impacts their photocatalytic performance. Different from most recent studies that synthesize Co3O4 from synthetic chemical precursors, Dhiman and Gupta successfully synthesized Co3O4 nanoparticles from discarded batteries for photocatalytic decomposition of methylene blue dye32 (Table 1). In their approach, various solutions, including Cyphos IL 102, HCl, and oxalic acid, were employed to extract cobalt from a mixture of metals. Co3O4 nanoparticles were subsequently formed via a calcination process, demonstrating the ability to completely degrade 50 mg L−1 of MB within 3 hours of solar light exposure. While this study presents a promising approach to converting hazardous waste into a material for environmental remediation, further investigations are necessary to fully elucidate the electronic, optical, and photocatalytic features of the as-synthesized Co3O4 nanomaterials.| System (dose) | Synthesis method & light source | Pollutant (concentration) | Removal efficiency (irradiation time) | Ref. |
|---|---|---|---|---|
| a Light intensity is not reported in the referenced literature. | ||||
| Nanoparticle Co3O4 (0.25 g L−1) | Extraction of cobalt from waste batteries using Cyphos IL 102 and calcination (600 °C) | Methylene blue (50 mg L−1) | UV-VIS: 100% (180 min) | 32 |
| Solar lighta | ||||
| Nanosheet Co3O4 (0.6 g L−1) | Calcination with rose petal as template (550 °C) | Methylene blue (10 mg L−1) | UV-VIS: 92% (60 min) | 33 |
| Xenon lampa | ||||
| Mesoporous nanoparticles Co3O4 (1 g L−1) | Sol–gel with soft template and calcination (650 °C) | Tetracycline (20 mg L−1) | UV-VIS: 66% (105 min) | 34 |
| Xenon lamp (300 W) | ||||
| Quantum dot Co3O4 (0.3 g L−1) | Solvothermal (80 °C) & mixing | Tetracycline (20 mg L−1) | UV-VIS: 33% (80 min) | 35 |
| Xenon lamp (150 W) | ||||
| Nanocage cube Co3O4 (0.8 g L−1) | Calcination from ZIF-67 (500 °C) | Tetracycline (30 mg L−1) | UV-VIS: 14% (240 min) | 36 |
| LED lamp (1 W) | ||||
| Polyhedral nanoparticle Co3O4 | Calcination from ZIF-67 (300 °C) | Norfloxacin (20 mg L−1) | UV-VIS: 38% (90 min) | 37 |
| Xenon lamp (150 W) | ||||
| Spherical Co3O4 (0.2 g L−1) | Green synthesis using Piper betle extract and calcination (600 °C) | Eriochrome T (60 mg L−1) | UV-VIS: 94% (80 min) | 26 |
| 6 UV-lamp (6 W) | ||||
| Nanoparticle Co3O4 (1 g L−1) | Green synthesis using Aloe barbadensis and calcination (900 °C) | Rhodamine B (10 mg L−1) | UV-VIS: 97% (255 min) | 27 |
| Sunlighta | ||||
| Nanoparticle SF-Co3O4 (100 g L−1) | Green synthesis using sunflower seeds (500 °C) | Methylene blue | UV-VIS: 90% (120 min) | 38 |
| Nanoparticle GC-Co3O4 (100 g L−1) | Green synthesis using green chilli (500 °C) | UV-VIS: 70% (120 min) | ||
| Xenon lamp (150 W) | ||||
For alternative morphologies, J. Yang et al. employed rose petal as a template to fabricate Co3O4 nanosheets with an ultrathin structure (∼0.1 μm) through calcination method33 (Fig. 1a). The Co3O4 nanosheets exhibited a porous architecture with an increased surface area (52 m2 g−1), which facilitated enhanced charge transport and increased methylene blue dye adsorption, thereby promoting effective photocatalytic degradation. However, the photocatalytic mechanism underlying methylene blue degradation was not thoroughly elucidated, as the study did not measure the energy band structure of Co3O4 nor the generation of active species during the process. Co3O4 mesoporous have garnered significant notice in recent research due to their inherent advantages, including large surface areas, highly accessible active sites, enhanced diffusion of pollutants into the material and degradation products out of it, and improved stability of nanoparticles by preventing sintering during photocatalytic reactions. For instance, mesoporous Co3O4 nanoparticles were synthesized via sol–gel technique, utilising the soft template F-127, for the photocatalytic degradation of the tetracycline antibiotics34 (Fig. 1b). The Co3O4 exhibited a large surface area (SBET = 178 m2 g−1) with an average pore diameter of 8 nm, which facilitated the efficient transport and penetration of antibiotic molecules to the active sites. Under optimized conditions, at a dosage of 1 g L−1, the Co3O4 photocatalyst achieved 66% degradation of tetracycline (20 mg L−1) within 1.5 hours of light irradiation.
 | ||
| Fig. 1 Co3O4 nanomaterial-based photocatalysts with varied morphologies in the application for water treatment in the recent five years: nanosheet Co3O4 (ref. 33) (a), mesoporous Co3O4 (ref. 34) (b), quantum dot Co3O4 (ref. 35) (c), polyhedral Co3O4 (ref. 38) (d), and cubic Co3O4 (ref. 37) (e). Reprinted with permission from ref. 33 Copyright (2019), ref. 34 Copyright (2023), ref. 35 Copyright (2022), ref. 38 Copyright (2022), and ref. 37 Copyright (2024), with permission from Elsevier. | ||
Nanosized Co3O4 quantum dots exhibit several advantageous properties, including a short charge transfer path, enhanced photostability, broad light absorbance, and a quantum confinement effect, all of which contribute to improved electron–hole pair generation and separation, thereby enhancing catalytic efficiency. Additionally, Co3O4 quantum dots can be uniformly dispersed in solvents such as water or ethanol, facilitating their easy incorporation onto the surface of photocatalysts via a temperature-dependent deposition process. In a representative study, Zhang et al. prepared Co3O4 quantum dots by solvothermal methods for the photocatalytic treatment of tetracycline35 (Fig. 1c). The individual Co3O4 quantum dots demonstrated limited photocatalytic activity under visible light illumination (33% of tetracycline degradation in 80 min of light exposure). Although quantum dots can absorb a wide range of light, their small size (3–5 nm) led to rapid charge carrier recombination and poor stability under prolonged exposure to pollutants. Moreover, the high surface-to-volume ratio of the Co3O4 quantum dots resulted in the creation of surface trap states. These traps can capture charge carriers and prevent them from contributing to photocatalytic reactions, which reduces the overall photocatalytic efficiency. Consequently, it is often recommended to combine Co3O4 in quantum dot structure with another semiconductor to form composite materials, thereby creating additional charge transfer pathways and facilitating the effective generation of active species.
The synthesis of Co3O4 nanomaterials from cobalt-based metal–organic framework (MOF) precursors, particularly ZIF-67, has emerged as a prominent research focus in recent years. Co3O4-based MOFs integrate the distinctive physicochemical properties of both MOFs and Co3O4 contributing to enhanced photocatalytic effectiveness. Specifically, MOF structures contain hollow cavities that facilitate multiple reflections and refractions of absorbed light, thereby prolonging its residence time and increasing the generation of photoexcited electron–hole pairs. Imanuella et al. successfully synthesized Co3O4 nanocages with a cubic morphology by calcining ZIF-67 at 500 °C.36 However, the resulting Co3O4 exhibited relatively low photocatalytic performance, achieving only 14% degradation of tetracycline (30 mg L−1) after six hours of simulated solar irradiation. Nevertheless, when incorporated into a composite system with CdS, the Co3O4 nanocage facilitated charge separation through the formation of an F-scheme heterojunction, significantly enhancing tetracycline removal to 83%. In another study, Adhikari et al. synthesized polyhedral Co3O4 nanoparticles by calcining ZIF-67 at 300 °C38 (Fig. 1d). The material demonstrated moderate photocatalytic activity, decomposing 38% of norfloxacin under 90 minutes of visible-light exposure. Furthermore, the Co3O4 nanoparticles were reported to form a p–n heterojunction with MoS2, exhibiting a type-II band alignment that significantly improved the migration rates of photoexcited charge carriers, thereby enhancing overall photocatalytic performance.
It was noticed from the studies above that the morphology of Co3O4 nanomaterial-based photocatalysts significantly influences their light-harvesting efficiency, charge dynamics, and catalytic reactivity. Controlled morphological engineering, including the development of hierarchical, porous, and composite structures, is crucial for optimizing performance in environmental and energy applications. Porous and hollow Co3O4 nanostructures improve photocatalytic efficiency by increasing surface area and active site availability, while mesoporous and macroporous frameworks improve reactant diffusion and reaction kinetics. Morphologies designed to trap light, such as hierarchical and porous configurations, enhance photon absorption, and nanostructures with tunable dimensions, like quantum dots, enable bandgap optimization for broader solar spectrum utilization. Additionally, core–shell and layered morphologies of Co3O4 nanomaterials improve durability by mitigating photocorrosion and structural degradation under operational conditions.
4. Biosynthesis of Co3O4 nanomaterial-based photocatalysts
The biosynthesis of nanomaterials has attracted lots of attention in recent research due to its sustainability and efficiency. This biologically driven approach employs microorganisms and plants as natural nanofactories, obviating the need for hazardous chemicals. Moreover, biosynthesis occurs under ambient temperature and pressure conditions while utilizing renewable or waste-derived biomaterials, thereby enhancing its economic viability and energy efficiency.The biosynthesis of nanomaterials generally follows two main mechanistic pathways. The first is the biochemical reduction mechanism, in which naturally occurring reducing agents convert metal ions into their zero-valent or oxide forms, initiating the nucleation and growth of nanocrystals. Several research groups have recently adopted this biochemical reduction mechanism in the green synthesis of Co3O4 nanomaterials for water purification applications (Table 1). Most Co3O4 nanomaterials synthesized via the biochemical reduction pathway exhibit spherical or quasi-spherical morphologies. In 2022, S. Kumar et al. successfully synthesized spherical Co3O4 nanoparticles using Piper betle extract, achieving a yield ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Co3O4
1 (Co3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) extract)26 (Fig. 2). The photocatalytic activity of the Co3O4 was comprehensively assessed through quantum yield, space-time yield, and figure-of-merit calculations. At an optimal concentration of 0.2 g L−1, the material demonstrated 94% breakdown of Eriochrome T dye (60 mg L−1) within 80 minutes. Although Co3O4 exhibits a high band gap energy (3.86 eV), defect states such as oxygen and cobalt vacancies or interstitials enable charge carrier excitation under solar or visible light. The study did not verify the photocatalytic generation and mechanistic roles of reactive oxygen species. Moreover, future research should aim to identify the key components in P. betle leaf extract in order to optimize the extraction process, selectively enrich active compounds, and maximize the yield and quality of Co3O4 nanoparticles. Sarvalkar et al. synthesized Co3O4 nanospheres with diameters ranging from 6 to 20 nm using an Aloe barbadensis extract-mediated green approach.27 The biogenically derived Co3O4 was evaluated for the decomposition of rhodamine B under sunlight exposure. The system required an extended period of 24 hours in darkness to attain adsorption equilibrium between Co3O4 and the target dye. Additionally, the photocatalytic efficiency was relatively low, as a high catalyst loading of 1 g L−1 was necessary to achieve 97% degradation of 10 mg L−1 rhodamine B over 255 minutes. Furthermore, the study did not elucidate the photocatalytic mechanism, as it did not explore the electronic band structure or identify the reactive species responsible for the degradation process. The composition of A. barbadensis extract was not clarified to identify the key bioactive compounds involved in the reduction of metal ions to nanoparticles. In another study, Khalid et al. prepared two distinct Co3O4 nanomaterials using sunflower seed and chili extract for the photocatalytic removal of methylene blue dye.38 Despite identical synthesis conditions, Co3O4 nanomaterials derived from different bio-extracts exhibited varied properties and photocatalytic performance. The Co3O4 nanoparticles derived from sunflower seed exhibited smaller particle size, a lower bandgap, and higher electrical conduction compared to those prepared using chili extract, resulting in superior photocatalytic performance for methylene blue degradation. These differences likely arise from the distinct bioactive compound compositions of each extract, which were not analyzed in the study. Noticeably, the high catalyst concentration required for the photocatalytic experiments (100 g L−1) presents a significant cost limitation for practical applications. Additionally, the synthesized materials demonstrated bifunctional properties, exhibiting potential for both photocatalytic water treatment and electrochemical energy storage.
extract)26 (Fig. 2). The photocatalytic activity of the Co3O4 was comprehensively assessed through quantum yield, space-time yield, and figure-of-merit calculations. At an optimal concentration of 0.2 g L−1, the material demonstrated 94% breakdown of Eriochrome T dye (60 mg L−1) within 80 minutes. Although Co3O4 exhibits a high band gap energy (3.86 eV), defect states such as oxygen and cobalt vacancies or interstitials enable charge carrier excitation under solar or visible light. The study did not verify the photocatalytic generation and mechanistic roles of reactive oxygen species. Moreover, future research should aim to identify the key components in P. betle leaf extract in order to optimize the extraction process, selectively enrich active compounds, and maximize the yield and quality of Co3O4 nanoparticles. Sarvalkar et al. synthesized Co3O4 nanospheres with diameters ranging from 6 to 20 nm using an Aloe barbadensis extract-mediated green approach.27 The biogenically derived Co3O4 was evaluated for the decomposition of rhodamine B under sunlight exposure. The system required an extended period of 24 hours in darkness to attain adsorption equilibrium between Co3O4 and the target dye. Additionally, the photocatalytic efficiency was relatively low, as a high catalyst loading of 1 g L−1 was necessary to achieve 97% degradation of 10 mg L−1 rhodamine B over 255 minutes. Furthermore, the study did not elucidate the photocatalytic mechanism, as it did not explore the electronic band structure or identify the reactive species responsible for the degradation process. The composition of A. barbadensis extract was not clarified to identify the key bioactive compounds involved in the reduction of metal ions to nanoparticles. In another study, Khalid et al. prepared two distinct Co3O4 nanomaterials using sunflower seed and chili extract for the photocatalytic removal of methylene blue dye.38 Despite identical synthesis conditions, Co3O4 nanomaterials derived from different bio-extracts exhibited varied properties and photocatalytic performance. The Co3O4 nanoparticles derived from sunflower seed exhibited smaller particle size, a lower bandgap, and higher electrical conduction compared to those prepared using chili extract, resulting in superior photocatalytic performance for methylene blue degradation. These differences likely arise from the distinct bioactive compound compositions of each extract, which were not analyzed in the study. Noticeably, the high catalyst concentration required for the photocatalytic experiments (100 g L−1) presents a significant cost limitation for practical applications. Additionally, the synthesized materials demonstrated bifunctional properties, exhibiting potential for both photocatalytic water treatment and electrochemical energy storage.
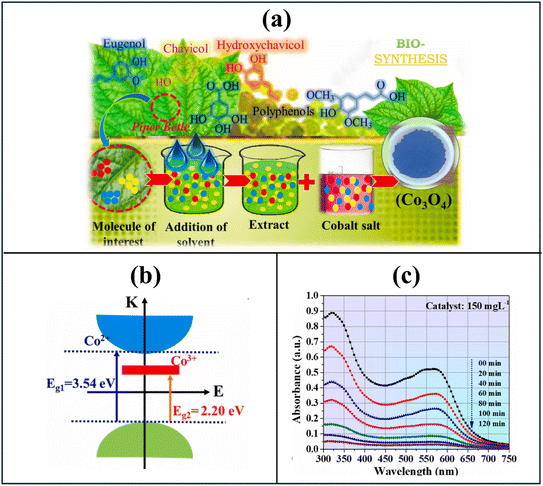 | ||
| Fig. 2 Biosynthesis of Co3O4 nanoparticles from Piper betle extract26 for photocatalytic degradation of Eriochrome Black T: synthesis process (a), electronic structure (b), and photocatalytic performance in degradation of Eriochrome Black T (c). Reprinted with permission from ref. 26, Copyright (2022), with permission from Elsevier. | ||
The second biosynthesis pathway involves capping or stabilization, in which biomolecules bind to the surface of forming nanomaterials to prevent agglomeration and regulate growth, thereby maintaining monodispersity and controlling their shape. In the study of Yang et al. (2019),33 Co3O4 nanosheets were fabricated using a biotemplate derived from rose flower petals. The petal extract, rich in polysaccharides and proteins containing amine and carboxyl groups, formed complexes with Co2+ ions and inhibited their aggregation. As a result, cobalt ions were uniformly distributed on the petal surface and subsequently converted into Co3O4 nanosheets after calcination. These nanosheets exhibited significantly enhanced photocatalytic activity, degrading methylene blue three times faster than commercial Co3O4. The authors attributed this performance to the 2D porous structure, which facilitated electron migration from the bulk to the catalyst surface. However, the study did not investigate the generation of reactive species or elucidate the photocatalytic degradation mechanism.
The bandgap, structural morphology, and optical characteristics of Co3O4 nanomaterials synthesized via biological approaches are significantly influenced by the choice of biological precursors and the specific parameters governing the biosynthesis process. A critical analysis of existing studies indicates that a comprehensive understanding of the biomolecular interactions governing the synthesis of Co3O4 nanoparticles remains largely unexplored. To address this gap, advanced spectroscopic and microscopic techniques should be employed to elucidate the precise roles of enzymes, proteins, and phytochemicals in the nucleation and growth processes of Co3O4 nanoparticles, which are fundamental for controlling their morphological and electronic properties.
Further research is required to evaluate the feasibility of diverse biological sources for Co3O4 synthesis to enhance the material's structural integrity and functional performance. Additionally, optimizing key synthesis parameters, such as annealing temperature, reaction pH, and the ratio of plant extract to Co3O4 precursor, is essential for achieving well-defined nanostructures with tailored physicochemical characteristics. Moreover, in-depth investigations into the reaction mechanisms, phase purity, and surface chemistry of biogenically synthesized Co3O4 are imperative to advance its potential applications, particularly in the photocatalytic remediation of contaminated water.
5. Photocatalytic systems with Co3O4 nanomaterials incorporated into other materials
5.1. Metal doping
Both metal doping and photocatalytic composite formation are extensively utilized approaches for improving the photocatalytic efficiency of materials. However, in recent years, research on the modification of Co3O4 through metal doping has been relatively limited, likely due to inherent challenges such as structural instability induced by lattice distortions and the tendency of doped nanomaterials to agglomerate or undergo uncontrolled growth, ultimately compromising their nanoscale features. In a study conducted by Keerthana et al. (2021), Sn was doped onto Co3O4 via the sol–gel and calcination method39 for the treatment of methylene blue dye (Table 2). The 2% Sn-doped Co3O4 exhibited a methylene blue degradation efficiency of 75% within 180 minutes, representing only a 7% improvement over pristine Co3O4. This enhancement was attributed to the role of Sn doping in facilitating the growth of Co3O4 nanoparticles with reduced agglomeration and smaller particle size. The optimal Sn/Co3O4 sample maintained stable performance over three photocatalytic cycles. However, this limited testing, combined with the lack of post-reaction characterizations, prevents a thorough assessment of its long-term stability. Further investigations are necessary to elucidate the precise role of Sn doping and to comprehensively explore the photocatalytic mechanism governing dye degradation in Sn-doped Co3O4.| Photocatalytic systems (dose) | Synthesis method/Light source | Pollutant (concentration) | Removal efficiency | Ref. |
|---|---|---|---|---|
| a Light intensity is not reported in the referenced literature. | ||||
| Metal-doped system | ||||
| Sn/Co3O4 (0.2 g L−1) | Sol–gel & calcination (400 °C) | Methylene blue | UV-VIS: 75% (180 min) | 39 |
| Light source: N/A | ||||
| PtO/Co3O4 (1 g L−1) | Sol–gel & impregnation | Foron blue (100 mg L−1) | UV-VIS: 100% (30 min) | 40 |
| Xenon lamp (500 W) | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Straddling type-I heterojunction composite | ||||
| Bi6Cr2O15/Co3O4 (0.15 g L−1) | Sonochemical precipiation | Methylene blue (25 mg L−1) | UV-VIS: 91% (160 min) | 41 |
| Halogen lamp (500 W) | ||||
| Co3O4/TiO2 (2 g L−1) | Impregnation using soft template | Ciprofloxacin (10 mg L−1) | UV-VIS: 100% (60 min) | 42 |
| Xenon lamp (300 W) | ||||
| rGO-Co3O4/ZnO (dose: N/A) | Two-step hydrothermal | Parathion (25 mg L−1) | UV-VIS: 99.6% (140 min) | 28 |
| Light source: N/A | ||||
| Bi2O3/BN/Co3O4 (1 g L−1) | Calcination & impregnation | Norfloxacin (5 mg L−1) | HPLC: 98% (180 min) | 29 |
| Xenon lamp (500 W) | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Type-II heterojunction composite | ||||
| Co3O4(QDs)/Bi2WO6 (0.3 g L−1) | Solvothermal & mixing | Tetracycline (20 mg L−1) | UV-VIS: 80% (20 min) | 35 |
| Xenon lamp (300 W) | ||||
| Red P/Co3O4 (0.4 g L−1) | Hydrothermal and physical mixing | Malachite green (20 mg L−1) | UV-VIS: 94% (20 min) | 43 |
| Xenon lamp (300 W) | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| S-scheme composite | ||||
| Li2MnO3/Co3O4 (1 g L−1) | Sol–gel with soft template | Tetracycline (25 mg L−1) | UV-VIS: 100% (120 min) | 44 |
| Xenon lamp (300 W) | ||||
| CuCo2O4/Co3O4 (1 g L−1) | Sol–gel with soft template | Tetracycline (20 mg L−1) | UV-VIS: 100% (90 min) | 34 |
| Xenon lamp (300 W) | ||||
| MoSSe/Co3O4 (0.2 g L−1) | Solvothermal | Tetracycline (10 mg L−1) | UV-VIS: 94% (90 min) | 30 |
| Xenon lamp (300 W) | ||||
| Co3O4/MoS2/SrTiO3 | Wet impregnation | Methylene blue (100 mg L−1) | UV-VIS: 95% (100 min) | 45 |
| Halogen lamp (500 W) | ||||
| Co3O4/CuBi2O4/SmVO4 (0.3 g L−1) | Mixing & calcination | Carbamazepine (10 mg L−1) | UV-VIS: 76% (300 min) | 46 |
| Xenon lamp (300 W) | ||||
| Fe3O4/Co3O4/TiO2 (1 g L−1) | Sol–gel | Tetracycline (10 mg L−1) | UV-VIS: 100% (150 min) | 47 |
| Natural sunlighta | TOC: 100% (240 min) | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Straddling and S-scheme composite | ||||
| CeO2/Co3O4/Ag/Ag3PO4 (1 g L−1) | Electrospining & impregnation | Methylene blue (10 mg L−1) | UV-VIS: 93% (80 min) | 48 |
| LED lighta | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Magnetic composite | ||||
| ZnCo2O4/Co3O4 (0.52 g L−1) | Green synthesis using Stevia extract & calcination | Acid violet 7 (13 mg L−1) | UV-VIS: 93.5% (70 min) | 49 |
| Osram lamp (125 W) | ||||
| Ag/Co3O4/NiFe2O4 (0.05 g L−1) | Multi-step precipitation | Tetracycline (10 mg L−1) | UV-VIS: 100% (250 min) | 50 |
| LED light (1070 W m−2) | ||||
| Co3O4/MK-30 | Green synthesis using Carissa edulis extract & calcination | Methylene blue (10 mg L−1) | UV-VIS: 98% (45 min) | 51 |
| Light source: N/A | ||||
In another study, Mohamed et al. successfully modified mesoporous Co3O4 nanocrystals with PtO using the impregnation method.40 The optimized 0.9% PtO-doped Co3O4 at a concentration of 1 g L−1, achieved complete decomposition of 100 mg L−1 foron blue dye within one hour of visible-light irradiation, whereas unmodified Co3O4 nanoparticles degraded only 55% of the dye under the same conditions. The increased photocatalytic activity of PtO-doped Co3O4 was attributed to the ability of PtO to withdraw excited electrons from the conduction band of Co3O4, thereby facilitating the reduction of adsorbed O2 into active radicals. Additionally, PtO was reported to establish a heterojunction electric field at the PtO/Co3O4 interface, effectively suppressing electron–hole recombination and significantly enhancing photocatalytic efficiency. However, no energy band determination of the synthesized materials was performed, rendering the inferences regarding the underlying photocatalytic mechanism inconclusive. The lack of post-reaction characterizations hinders a full assessment of PtO/Co3O4's long-term stability, and its practical applicability remains unclear due to the absence of testing under realistic water conditions.
5.2. Co3O4-based photocatalytic nanocomposites
Preparing photocatalytic composites offers several advantages over metal doping, making it a more effective and sustainable approach. Composite materials provide greater flexibility in design, allowing precise control over interfacial interactions, band alignment, and morphology. Different types of Co3O4-based photocatalytic composites were prepared in recent years for polluted water treatment.Janani et al. prepared Bi6Cr2O15/Co3O4 composite via a sonochemical precipitation method for the photocatalytic treatment of methylene blue41 (Table 2). Similarly, Alhaddad et al. fabricated a Co3O4/TiO2 composite using the impregnation method with a soft template for the treatment of the antibiotic ciprofloxacin.42 Mohammadi et al. developed rGO-Co3O4/ZnO nanocomposite by through a two-step hydrothermal process for the degradation of parathion28 (Fig. 3a). In a different investigation, Wang et al. synthesized Bi2O3/BN/Co3O4 photocatalyst via calcination and impregnation methods for the removal of norfloxacin. A common characteristic among these Co3O4-based photocatalytic systems is the formation of a straddling type-I heterojunction, which is generally considered the least effective due to its inefficient charge separation and high electron–hole recombination rates. In a straddling type-I heterojunction, the conduction and valence bands of one semiconductor are fully enclosed within the band structure of the second component, causing the accumulation of both photogenerated electrons and holes in the same material. This band alignment significantly increases the probability of electron–hole recombination, thereby limiting photocatalytic efficiency. Although comparing pollutant removal efficiencies across different systems is complicated by varying experimental conditions and target contaminants, a representative example is the Bi2O3/BN/Co3O4 composite.29 As a straddling type-I heterojunction, it required 3 hours under high-intensity xenon lamp irradiation (500 W) and a large catalyst dosage (1 g L−1) to degrade just 5 mg L−1 of antibiotics, illustrating the inherent limitations of this heterojunction design. Moreover, several critical limitations persist in these studies regarding the mechanistic understanding of photocatalysis. In the cases of Bi6Cr2O15/Co3O4 and Bi2O3/BN/Co3O4, the electronic band structure and the primary active species involved in the photocatalysis were not determined. For the Co3O4/TiO2 composite, no experimental evidence was provided to confirm the establishment of a p–n heterojunction or to elucidate the charge transmission pathways between the composite's constituents. Additionally, for rGO-Co3O4/ZnO system, no optimization studies were conducted to refine the synthesis process and identify the most effective composite configuration. Addressing these gaps through systematic experimental investigations will be vital for advancing the design and functionality of Co3O4 nanomaterial-based photocatalysts.
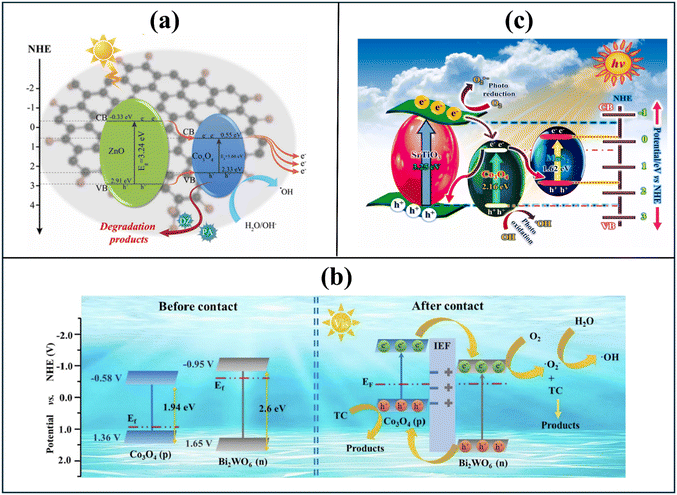 | ||
| Fig. 3 Photocatalytic mechanism of the Co3O4 nanomaterial-based composites: Straddling type-I heterojunction system of rGO-Co3O4/ZnO composite28 (a), type-II and p–n heterojunction system of Co3O4(QDs)/Bi2WO6 composite35 (b), and dual Z-scheme system of Co3O4/MoS2/SrTiO3 composite45 (c). Reprinted with permission from ref. 28 Copyright (2023), ref. 35 Copyright (2022), ref. 45 Copyright (2024) with permission from Elsevier. | ||
Compared to type-I heterojunctions, type-II and S-scheme heterojunctions enable more efficient utilization of visible light due to their favorable band alignment and charge transfer mechanisms. Zhang et al. prepared a Co3O4/Bi2WO6 composite by solvothermal and mixing techniques for the decomposition of tetracycline.35 The optimized 10% Co3O4/Bi2WO6 composite demonstrated an 80% degradation efficiency for tetracycline (20 mg L−1) within 20 minutes of visible light irradiation. The high photocatalytic activity was attributed to the construction of both p–n and type-II heterojunctions, which effectively suppressed charge carrier recombination (Fig. 3b). Especially, the degrading pathway of tetracycline and the generation of intermediates during photocatalysis were systematically investigated using advanced techniques, including three-dimensional excitation-emission matrix fluorescence and liquid chromatography/quadrupole time-of-flight mass spectrometry. Similarly, Tao et al. synthesized a heterogenous type-II red P/Co3O4 composite via hydrothermal and physical mixing methods for the degradation of malachite green.43 The red P/Co3O4 photocatalytic system, at a dosage of 0.4 g L−1, achieved 94% degradation of malachite green (20 mg L−1) within just 20 minutes. However, a prolonged adsorption time of 30 minutes was required before the photocatalytic reaction commenced. A notable limitation of such type-II photocatalytic composites is that electrons and holes migrate to the conduction and valence bands with lower energy levels, thereby restricting their reactivity and ability to generate highly reactive radical species. In both the Co3O4/Bi2WO6 and red P/Co3O4 systems, photogenerated holes were transferred from the valence band of the secondary component to the less positive valence band of Co3O4. This hindered their capacity to oxidize water molecules and produce strong hydroxyl radicals, thereby limiting the overall oxidative potential of the system.
S-scheme heterojunctions facilitate enhanced redox capabilities by preserving highly energetic electrons and holes in spatially distinct locations, thereby improving charge carrier separation and promoting superior photocatalytic activity.52 Several recent studies have explored Co3O4-based photocatalytic composites incorporating S-scheme charge transfer pathways. For instance, the research group of Armenia synthesized two S-scheme photocatalytic systems, Li2MnO3/Co3O4 and CuCo2O4/Co3O4, using a sol–gel approach with the F-127 surfactant as a soft template for the decomposition of tetracycline in water.34,44 These nanocomposites were designed to integrate both S-scheme charge transfer and p–n junction formation between the p-type semiconductor Co3O4 and complementary n-type semiconductors, thereby elevating the separation of charge carriers and facilitating the generation of reactive radicals, such as superoxide radicals ˙O2− and hydroxyl radicals ˙OH. However, further refinement in defining the energy band positions and band gaps of these systems is required to establish a more accurate understanding of photoinduced electron–hole mobility within the nanocomposites.
Ternary composites featuring a dual S-scheme charge transfer mechanism have demonstrated superior photocatalytic efficiency among Co3O4-based materials. In a representative study, Venkatesh G et al. prepared a Co3O4/MoS2/SrTiO3 composite via the wet impregnation method for the photocatalytic degradation of methylene blue dye.45 The implementation of the double S-scheme mechanism facilitated the accumulation of electrons at the conduction bands of SrTiO3 and MoS2, while photogenerated holes remained in the valence band of Co3O4 (Fig. 3c). This efficient charge separation and transfer process enabled the generation of multiple reactive species, ultimately leading to the effective degradation of 95% of methylene blue (100 mg L−1) within 100 min of light irradiation. Especially, the density functional theory method was employed to calculate the energy band structure and electronic density of states of the materials, thereby providing a robust theoretical foundation for elucidating the charge transfer process. The development of materials for environmental remediation should prioritize simplification, cost-effectiveness, and enhanced practical applicability. The synthesis of Co3O4-based quaternary composites through complex preparation routes should be avoided to facilitate scalable production. Most existing research has concentrated on the synthesis of Co3O4-based catalysts in powder form, which, despite offering high surface area, presents challenges in post-treatment separation and reuse. However, strategies to improve catalyst recovery, such as immobilizing the photocatalyst on floating carriers, polymeric membranes, or porous sponge-like substrates, remain underexplored. These approaches not only facilitate easy separation but also enhance the potential for continuous-flow or in situ water treatment systems, which are essential for practical and large-scale applications.
5.3. Magnetic Co3O4-based photocatalytic nanocomposites
Photocatalysts with facile recovery mechanisms are essential to ensuring sustainability, economic viability, and large-scale implementation. Heidari-Asil et al. synthesize nanoparticles ZnCo2O4/Co3O4 via a green synthesis approach using Carissa edulis extract,49 enabling convenient recovery through an external magnetic field. By adjusting the concentration of Stevia extract, the glycoside content was effectively modified, significantly influencing the morphology and dimensions of the ZnCo2O4/Co3O4 nanostructures. The optimized nanocomposite exhibited high structural uniformity and achieved a degradation efficiency of 93.5% for acid violet 7 within 70 minutes of light exposure. The nanomaterial exhibits weak ferromagnetism at room temperature, with a remanence of 0.004 emu per g and coercivity of 48 Oe. This magnetic behavior enables rapid separation using an external magnet, facilitating catalyst recovery and contributing to a high photocatalytic efficiency after ten reuse cycles (93.5% in the first run and 81.7% after ten cycles). However, a notable limitation of this study is the absence of band gap measurements for the semiconductor components, which would provide greater insight into the photocatalytic mechanism of the composite.Kumar et al. synthesized a magnetically recyclable composite by integrating polyhedral Co3O4 nanoparticles with the magnetic semiconductor NiFe2O4 for photocatalytic H2O2 production in tetracycline degradation50 (Fig. 4a). Molecular dynamics simulations revealed that the adsorption capacity of O2 on the Co3O4 surface was more than twice that observed on NiFe2O4, further contributing to the system's enhanced photocatalytic efficiency (Fig. 4b). Beyond the formation of an S-scheme heterojunction between Co3O4 and NiFe2O4, Ag doping played a pivotal role in enhancing H2O2 production. The presence of Ag nanoparticles significantly improved charge carrier dynamics by efficiently extracting photoexcited electrons from Co3O4 and NiFe2O4, thereby facilitating the direct reduction of O2 molecules into H2O2 (Fig. 4c). The nanocomposite showed superparamagnetic behavior with a high saturation magnetization of approximately 14.2 emu per g, highlighting its potential for magnetically recoverable photocatalytic applications. The composite successfully achieved complete degradation of tetracycline (10 mg L−1) within 250 minutes. However, its optimal performance at pH 3 presents a significant limitation, restricting its applicability for the rapid treatment of conventionally polluted water.
 | ||
| Fig. 4 Magnetization curve (a), molecular dynamics simulation for the adsorption of O2 on two components of the composite (b), and photocatalytic mechanism of the Ag/Co3O4/NiFe2O4 photocatalytic system (c). Reprinted with permission from the ref. 50 Copyright (2022) with permission from Elsevier. | ||
In a separate study, Co3O4 nanoparticles were dispersed onto magnetic montmorillonite K30 (MK-30) nanosheets via an ultrasonication-assisted method, demonstrating effective photocatalytic degradation of tetracycline.51 In addition to imparting magnetic properties for rapid recovery, MK-30, with its negatively charged surface, was reported to facilitate the withdrawal of photogenerated positive holes, thereby enhancing charge carrier separation within the Co3O4/MK-30 composite and improving photocatalytic performance. Although the magnetic properties of Co3O4/MK-30 were not directly analyzed, the catalyst was easily recovered using an external magnetic field after each cycle, indicating magnetic separability. This enabled stable photocatalytic performance over five reuse cycles, highlighting its potential for efficient and reusable water treatment applications.
6. Co3O4 nanomaterial-based photocatalysts for oxidant activation in water treatment
Photocatalytic activation of oxidants is an advanced process that utilizes a photocatalyst to absorb light energy, initiating redox reactions that activate oxidants such as hydrogen peroxide (H2O2), persulfates (S2O82−) and peroxymonosulfates (HSO5−). This activation leads to the generation of reactive oxygen species, which facilitate the decomposition of organic pollutants. Co3O4, a mixed-valence spinel oxide containing both Co2+ and Co3+, serves as an efficient catalyst for oxidant activation through cyclic redox reactions, enabling effective electron transfer in oxidation–reduction processes even in the absence of light. However, photocatalytic activation of oxidants by Co3O4 has been reported to significantly enhance oxidation reactions due to the additional interaction of oxidants with photoexcited charge carriers, thereby improving overall catalytic efficiency.In a study by Drummer et al. (2024), Co3O4 was synthesized via a green synthesis approach using spent coffee extract and employed for the activation of HSO5− in the treatment of tartrazine dye in water.53 Under visible light, photoexcited electrons in Co3O4's conduction band reduced HSO5−, initiating a series of reactions that generate various reactive species, including ˙O2−, ˙OH, ˙SO4−, 1O2, and ˙SO5−. Among these, sulfate and hydroxyl radicals identified as the primary active species responsible for the degradation of tartrazine (Fig. 5a). This photocatalytic activation enhanced the degradation rate constant by 37.6% compared to the reaction without light. The study further highlighted that the performance of the Co3O4/HSO5− system was highly dependent upon the type of light source used, including LED light, simulated sunlight, and natural sunlight. Minimal cobalt leaching (∼0.0001%) suggests that Co3O4 activates HSO5− via a stable, heterogeneous mechanism for sulfate radical generation. Despite its promising photocatalytic performance, the study identified challenges related to the recovery and reuse of the nanomaterial, limiting its recyclability to only two cycles. To address this limitation, it is suggested that a growth template be employed during synthesis. To enhance structural stability and facilitate efficient retrieval of the nanocatalyst for repeated applications.
 | ||
| Fig. 5 Photocatalytic activation mechanism of oxidants by Co3O4 (ref. 53) (a), Co3O4/g-C3N4 (ref. 31) (b), and Co3O4@P–C3N4/α-Fe2O3 (ref. 54) (c). Reprinted with permission from the ref. 53 Copyright (2024), ref. 31 Copyright (2021), and ref. 54 Copyright (2024) with permission from Elsevier. | ||
Similarly, nanospherical Co3O4 was integrated on laminated g-C3N4 to construct a heterostructured photocatalyst for the activation of HSO5− in the degradation of atrazine.31 Density functional theory calculations revealed that the enhanced photocatalytic activity of Co3O4/g-C3N4 composite is primarily attributed to modifications in its electronic band structure, which promote efficient charge carrier separation and transfer. Upon the incorporation of Co3O4, the uniform distribution of Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) within g-C3N4 was disrupted, thereby enhancing electron delocalization and mobility. Notably, for g-C3N4 to function effectively as a photocatalyst, its HOMO was required to be predominantly composed of the Co 3d state orbitals, serving as active sites for redox reactions. The activation of HSO5− in this system was predominantly driven by photoexcited electrons at conduction bands of g-C3N4 and g-C3N4 and by Co(II) (Fig. 5b). Scavenging experiments revealed that ˙OH, ˙SO4−, and e− are the dominant reactive species contributing the atrazine decomposition, whereas h+ and ˙O2− play relatively minor roles. The system achieved 79% degradation of atrazine (10 mg L−1) within 35 minutes of light irradiation. The Co3O4/g-C3N4 catalyst demonstrated high stability, as confirmed by recycling tests for atrazine degradation, cobalt ion leaching assessments, and structural analyses via XRD and XPS.
In a different work, Rangaraj et al. fabricated a ternary composite by incorporating Co3O4 with Fe2O3 and P-doped g-C3N4 for the activation of persulfate in the treatment of bisphenol A.54 The composite exhibited a low charge carrier recombination rate, ascribed to the formation of a double Z-scheme heterojunction, which effectively enhanced charge separation and transfer. Additionally, the composite demonstrated magnetic properties, facilitating its retrieval and reuse after catalytic reactions. Persulfate has been reported to react with Co(II), Fe(II), and photogenerated electrons in the conduction bands of the composite components, resulting in the generation of sulfate radicals (Fig. 5c). In this study, the synergistic action of reactive species, including h+, ˙OH, ˙SO4−, facilitated the efficient degradation of bisphenol A (20 mg L−1), achieving a removal efficiency of 90% within 90 minutes. However, the study did not explore the optimal composition ratios of the individual components during the synthesis process. Furthermore, it did not include an analysis of the energy band structure or investigate the potential shifts in band alignment resulting from the combination of p- and n-type semiconductors within the composite system.
From the reviewed studies, persulfate and peroxymonosulfate have been commonly selected as oxidants activated by Co3O4-based photocatalysts for advanced water treatment. This preference is likely due to their ability to generate various reactive species, particularly strong radicals such as ˙SO4− and ˙OH, which exert synergistic effects in the degradation of recalcitrant pollutants. Among the two, persulfate has been recommended as the more effective oxidant. This is because PS is primarily reduced by photoinduced electrons from the catalyst to produce sulfate radicals, whereas PMS can also be oxidized by photogenerated holes to form HSO5−, a weaker oxidant with lower redox potential. Moreover, prolonged exposure to strong oxidants and reactive species may degrade Co3O4 structure and limit reusability. Therefore, comprehensive stability assessments, including recycling tests, leaching analysis, and post-reaction characterizations, are essential to ensure practical applicability and sustained performance under real-world conditions.
7. Computational applications
Computational approaches, particularly Density Functional Theory (DFT), play a pivotal role in photocatalysis research by providing atomic-scale insights into the electronic, structural, and energetic properties of photocatalytic materials. However, studies focusing on Co3O4-based nanomaterials for photocatalytic water treatment have rarely integrated computational methods with experimental techniques to optimize photocatalyst design, explore band structure and density of states (DOS), or elucidate photocatalytic reaction pathways.In the study conducted by Mohammadi et al.,55 GO/Co3O4/ZnO photocatalytic nanocomposite was employed for the degradation of the insecticide parathion. The response surface methodology employing a quadratic polynomial model was applied to design the experiments and optimize the photocatalytic performance by evaluating key operational parameters. Through statistical analysis, the optimal conditions were identified as a pH of 12, parathion concentration of 5 mg L−1, photocatalyst dosage of 0.12 g, reaction duration of 2.3 hours, and light intensity of 14 W cm−2. In a separate study, Chen et al. applied DFT to model the crystal structure, simulate the energy band structure, and analyze the density of states of Au/Co3O4/Bi2MoO6 composite used for photocatalytic decomposition of methyl orange dye.56 The computational simulations revealed that incorporating Co3O4 into the composite significantly reduced the band gap (1.84 eV for Co3O4/Bi2MoO6 compared to 2.72 eV for Bi2MoO6) and enhanced the O 2p and Co 3d electron density near the Fermi level, thereby promoting charge carrier transfer and improving photocatalytic efficiency. Furthermore, Guo et al. synthesized a visible light-activated Co3O4/Bi2MoO6 nanocomposite for peroxymonosulfate-assisted degradation of the antibiotic norfloxacin.57 DFT calculations were successfully employed to elucidate the photocatalytic mechanism, revealing the energy band structure and confirming the formation of a p–n heterojunction between Co3O4 and Bi2MoO6, which enhanced charge separation and transfer. Similarly, Y. Wang et al. incorporated Co3O4 nanodots onto TiO2 nanosheets to fabricate Co3O4/TiO2 nanocomposite for the photocatalytic degradation of antibiotic enrofloxacin.58 DFT simulations were utilized to predict the energy band structure and electron density distribution. TiO2 exhibited a lower Fermi level and work function compared to Co3O4, indicating a directional electron flow from TiO2 to Co3O4 at the interface (Fig. 6). Additionally, the density of states analysis revealed that interfacial oxygen atoms contributed to heterojunction formation, thereby facilitating charge transfer across the interface. This charge redistribution led to the alignment of Fermi levels between the two components, resulting in the formation of a built-in electric field that enhanced electron–hole separation and migration, ultimately improving the photocatalytic performance.
 | ||
| Fig. 6 The calculated density of states for (a) TiO2 and (c) Co3O4, alongside the electrostatic potential for (b) TiO2 and (d) Co3O4. Panel (e) depicts the built-in electric field formed at the TiO2/Co3O4 interface. Reprinted with permission from the ref. 58, Copyright (2020) with permission from Elsevier. | ||
8. Future perspectives and challenges
The research on Co3O4 nanomaterials for water treatment continues to be a highly promising field, offering substantial opportunities for further exploration. To achieve breakthroughs in the synthesis of Co3O4 nanostructures with novel morphologies and advanced electronic and optical properties, state-of-the-art synthesis strategies, such as template-assisted pyrolysis, atomic layer deposition, and nanoconfinement techniques, should be prioritized to precisely tailor the structural and physicochemical characteristics of Co3O4.MOF-derived Co3O4 nanomaterials present a distinctive platform for designing hierarchical nanostructures with high surface area, tunable porosity, and controllable morphology. Despite their promising photocatalytic activity, challenges related to inefficient charge carrier separation persist due to intrinsic band structure limitations. Therefore, future research should focus on band gap engineering through cation doping, defect modulation, and oxygen vacancy engineering to enhance light absorption, facilitate charge movement, and prolong charge carrier lifetimes.
The capacity of Co3O4 to activate oxidants should be further explored to maximize its application for water purification. Key research directions should include mechanistic elucidation of oxidation pathways, optimization of oxidant-to-photocatalyst ratios, and development of light-assisted Fenton-like and sulfate radical-based oxidation systems. Prolonged exposure to oxidants may degrade Co3O4 structure and limit reusability, highlighting the need for thorough stability assessments to ensure long-term applicability.
Prolonged photocatalytic reactions can lead to aggregation, cobalt ion leaching, structural degradation, and challenges in catalyst recovery, all of which hinder the practical application of Co3O4 nanomaterials. Core-shell coating is a solution to enhance the structural integrity and chemical stability of Co3O4. Immobilization of Co3O4 nanomaterials on substrates such as membranes, glass, ceramics, or polymer films allows convenient recovery without compromising photocatalytic efficiency. Additionally, integrating Co3O4 with other magnetic semiconductors to develop S-scheme heterojunction nanocomposites should be a priority, as the S-scheme charge transfer mechanism has demonstrated superior photocatalytic performance while maintaining facile recovery via magnetic separation.
To align with environmental sustainability goals, research efforts should focus on eco-friendly synthesis methodologies, such as bio-derived precursors, solvent-free synthesis, and low-temperature calcination processes. Utilizing biomass-derived templates or waste-derived metal sources for precursors could reduce the environmental impact and improve the economic feasibility of Co3O4 photocatalysts.
To enable commercial application of Co3O4-based photocatalysts, future studies must aim for high degradation efficiencies within short irradiation times, even under visible or solar light. In addition, consistent performance over multiple reuse cycles and in complex real wastewater matrices must be demonstrated. Standardization of photocatalytic metrics such as apparent quantum yield and total organic carbon removal will allow meaningful comparison between systems. Furthermore, low-cost, scalable synthesis, combined with magnetic recovery and low catalyst dosage, will be essential for reducing operational costs and enabling large-scale implementation.
A comprehensive understanding of the photocatalytic mechanisms in Co3O4-based systems necessitates the integration of cutting-edge experimental and computational techniques. For example, Operando X-ray photoelectron spectroscopy is useful to monitor band edge shifts under real–time reaction conditions. Hard X-ray photoelectron spectroscopy is effective for probing buried interfaces in heterojunctions. Moreover, frequency-resolved Mott–Schottky analysis is very helpful for precise determination of band edge positions and charge carrier dynamics. Beside the experimental methods, computational techniques like density functional theory calculations and molecular dynamics simulations should be conducted simultaneously to predict electronic structures, charge transport mechanisms, and band alignment and verify the experimental results.
9. Conclusions
Despite extensive research on Co3O4 nanomaterial-based photocatalysts, a critical gap remains in comprehensive reviews that consolidate recent advancements in their application for water treatment. This review provided an in-depth analysis of key photocatalytic mechanisms, inherent advantages, existing limitations, and prospective research directions of Co3O4 nanomaterial-based photocatalysts for water treatment in the recent five years. Various Co3O4 nanostructures have been synthesized through diverse methodologies for their implementation as photocatalysts in wastewater treatment. Furthermore, extensive efforts have been dedicated to modifying Co3O4 and integrating it with other functional materials to enhance charge carrier separation, improve degradation efficiency, and facilitate catalyst recovery for practical applications. However, a significant number of studies have yet to elucidate the precise catalytic mechanisms and the roles of reactive oxygen species in degradation processes.Future perspectives and challenges on the Co3O4 nanomaterial-based photocatalysts for water treatment were proposed. To enhance Co3O4 nanomaterial's performance, advanced synthesis techniques should be applied for precise control over its structure and properties. MOF-derived Co3O4 offers high surface area and tunable porosity, but improved band gap engineering via doping, defect modulation, and oxygen vacancy control is needed for better charge separation. Further studies should optimize Co3O4 nanomaterial's role in oxidant activation, including light-assisted Fenton-like and sulfate radical-based systems. Challenges like aggregation, leaching, and recovery must be addressed through core–shell coatings, immobilization, and magnetic S-scheme heterojunctions. Sustainable synthesis approaches should improve environmental and economic viability. Finally, integrating advanced spectroscopic techniques with computational modeling is essential for understanding charge transfer mechanisms. Addressing these challenges will optimize Co3O4 nanomaterial-based photocatalysts for scalable and sustainable water treatment solutions.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Van Dien Dang: writing (original draft), investigation, visualization. Nguyen Thi Hong Nhung: writing (original draft), Iqra Rabani: conceptualization, writing (original draft), Nguyen Tien Tran: writing (original draft), Bui Thi Phuong Thuy: writing (original draft), Hai Bang Truong: writing (review& editing), project administration, supervision, validation.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- A. Boretti and L. Rosa, npj Clean Water, 2019, 2, 1–6 Search PubMed.
- I. Rabani, M. S. Tahir, F. Afzal, H. B. Truong, M. Kim and Y.-S. Seo, J. Environ. Chem. Eng., 2023, 11, 109235 CrossRef CAS.
- S. K. Ray, H. B. Truong, Z. Arshad, H. S. Shin and J. Hur, Membr. Water Treat., 2020, 11, 257–274 Search PubMed.
- H. Bang Truong, X. Cuong Nguyen and J. Hur, J. Environ. Manage., 2023, 345, 118895 CrossRef CAS PubMed.
- Y. Yang, X. Li, C. Zhou, W. Xiong, G. Zeng, D. Huang, C. Zhang, W. Wang, B. Song, X. Tang, X. Li and H. Guo, Water Res., 2020, 184, 116200 CrossRef CAS PubMed.
- M. Ismael, J. Alloys Compd., 2020, 846, 156446 CrossRef CAS.
- C. Prasad, H. Tang and I. Bahadur, J. Mol. Liq., 2019, 281, 634–654 CrossRef CAS.
- Z. Yu, F. Li and Q. Xiang, J. Mater. Sci. Technol., 2024, 175, 244–257 CrossRef CAS.
- H. H. Do, Q. Van Le and H. B. Truong, Korean J. Chem. Eng., 2024, 41, 2227–2237 CrossRef CAS.
- R. Subagyo, A. Yudhowijoyo, N. A. Sholeha, S. S. Hutagalung, D. Prasetyoko, M. D. Birowosuto, A. Arramel, J. Jiang and Y. Kusumawati, J. Colloid Interface Sci., 2023, 650, 1550–1590 CrossRef CAS PubMed.
- H. H. Do and H. B. Truong, Beilstein J. Nanotechnol., 2023, 14, 904–911 CrossRef CAS PubMed.
- J. Ma, H. Wei, Y. Liu, X. Ren, Y. Li, F. Wang, X. Han, E. Xu, X. Cao, G. Wang, F. Ren and S. Wei, Int. J. Hydrogen Energy, 2020, 45, 21205–21220 Search PubMed.
- H. B. Truong, B. T. Huy, S. K. Ray, Y.-I. Lee, J. Cho and J. Hur, Chem. Eng. J., 2020, 399, 125733 CrossRef CAS.
- A. M. Nasir, J. Jaafar, F. Aziz, N. Yusof, W. N. W. Salleh, A. F. Ismail and M. Aziz, J. Water Process Eng., 2020, 36, 101300 CrossRef.
- H. B. Truong, T. T. L. Doan, N. T. Hoang, N. Van Tam, M. K. Nguyen, L. G. Trung, J. S. Gwag and N. T. Tran, J. Environ. Sci., 2024, 139, 569–588 CrossRef CAS PubMed.
- F. Lu and D. Astruc, Coord. Chem. Rev., 2018, 356, 147–164 Search PubMed.
- D. Zhou, J. Yu, J. Tang, X. Y. Li and P. Ou, Adv. Energy Mater., 2025, 15, 2404007 Search PubMed.
- A. Matsuda, R. Yamauchi, D. Shiojiri, G. Tan, S. Kaneko and M. Yoshimoto, Appl. Surf. Sci., 2015, 349, 78–82 CrossRef CAS.
- S. Vladimirova, V. Krivetskiy, M. Rumyantseva, A. Gaskov, N. Mordvinova, O. Lebedev, M. Martyshov and P. Forsh, Sensors, 2017, 17, 2216 CrossRef PubMed.
- N. Bayati-Komitaki, S. H. Ganduh, A. H. Alzaidy and M. Salavati-Niasari, Biomed. Pharmacother., 2024, 180, 117457 Search PubMed.
- O. Brummel and J. Libuda, Catal. Letters, 2020, 150, 1546–1560 Search PubMed.
- V. Mandić, S. Kurajica, M. Plodinec and I. Panžić, Catalysts, 2022, 12, 1162 CrossRef.
- G. Ziwa, R. Crane and K. A. Hudson-Edwards, Minerals, 2020, 11, 22 CrossRef.
- N. R. Khalid, A. Gull, F. Ali, M. B. Tahir, T. Iqbal, M. Rafique, M. A. Assiri, M. Imran and M. Alzaid, Ceram. Int., 2022, 48, 32009–32021 CrossRef CAS.
- L. Zhang, H. Li, K. Li, L. Li, J. Wei, L. Feng and Q. Fu, J. Alloys Compd., 2016, 680, 146–154 CrossRef CAS.
- S. Kumar, G. Kaur, M. Rawat, Y. F. Tsang, K. Y. Lin and K. H. Kim, J. Clean. Prod., 2022, 361, 132242 CrossRef CAS.
- P. D. Sarvalkar, A. S. Jamadar, S. S. kakade, A. B. Magdum, P. K. Pawar, J. B. Yadav, M. S. Nimbalkar, N. R. Prasad, A. A. Ramteke and K. K. K. Sharma, Results Eng, 2024, 22, 102094 CrossRef CAS.
- A. Mohammadi, H. Mirhosseini, A. Hekmatiyan, L. Abdolahi, F. Mehrabi and M. Shahmirzaei, J. Environ. Chem. Eng., 2023, 11, 110912 CrossRef CAS.
- N. Wang, W. Wang, D. Qi, G. Kang, B. Wang, H. Zhang, J. Ruan, R. Lei, Z. Zhang, S. Zhang and H. Zhou, Chemosphere, 2024, 352, 141481 CrossRef CAS PubMed.
- X. Xiong, J. Zhang, C. Chen, S. Yang, J. Lin, J. Xi and Z. Kong, J. Alloys Compd., 2022, 926, 166863 CrossRef CAS.
- Q. Yang, J. An, Z. Xu, S. Liang and H. Wang, Colloids Surf., A, 2021, 614, 126161 CrossRef CAS.
- S. Dhiman and B. Gupta, Environ. Technol. Innov., 2021, 23, 101765 CrossRef CAS.
- J. Yang, M. Wang, S. Zhao, Y. Liu, W. Zhang, B. Wu and Q. Liu, Int. J. Hydrogen Energy, 2019, 44, 870–879 CrossRef CAS.
- S. H. Almenia, A. A. Ismail, K. A. Alzahrani and M. Aljahdali, J. Photochem. Photobiol. A Chem., 2023, 438, 114507 CrossRef CAS.
- X. Zhang, H. Zhang, J. Yu, Z. Wu and Q. Zhou, Appl. Surf. Sci., 2022, 585, 152547 CrossRef CAS.
- N. Imanuella, K. H. Ng, L. A. Tuyen and N. Q. Hung, Appl. Catal. B: Environ, 2024, 358, 124401 CrossRef CAS.
- S. Adhikari, S. Mandal and D. H. Kim, Appl. Surf. Sci., 2024, 653, 159374 CrossRef CAS.
- N. R. Khalid, A. Gull, F. Ali, M. B. Tahir, T. Iqbal, M. Rafique, M. A. Assiri, M. Imran and M. Alzaid, Ceram. Int., 2022, 48, 32009–32021 CrossRef CAS.
- S. P. Keerthana, R. Yuvakkumar, P. S. Kumar, G. Ravi, D. V. N. Vo and D. Velauthapillai, Chemosphere, 2021, 277, 130325 CrossRef CAS PubMed.
- R. M. Mohamed, A. A. Ismail, A. S. Basaleh and H. A. Bawazir, J. Photochem. Photobiol. A Chem., 2022, 428, 113859 CrossRef CAS.
- B. Janani, A. Syed, H. A. AL-Shwaiman, M. M. Alkhulaifi, A. M. Elgorban and S. S. Khan, Colloids Surf., A, 2021, 622, 126671 CrossRef CAS.
- M. Alhaddad, A. A. Ismail, Y. G. Alghamdi, N. D. Al-Khathami and R. M. Mohamed, Opt. Mater., 2022, 131, 112643 CrossRef CAS.
- J. Tao, M. Zhang, X. Gao, H. Zhao, Z. Ren, D. Li, J. Li, R. Zhang, Y. Liu and Y. Zhai, Mater. Chem. Phys., 2020, 240, 122185 CrossRef CAS.
- S. H. Almenia, A. A. Ismail, K. A. Alzahrani and M. Aljahdali, J. Alloys Compd., 2023, 953, 170127 CrossRef CAS.
- G. Venkatesh, P. D. Shobana, N. Elavarasan, A. Rajesh, S. Senthilnathan and V. Vighesh, Mater. Sci. Semicond. Process., 2024, 169, 107909 CrossRef.
- P. J. Mafa, M. E. Malefane, A. O. Idris, B. B. Mamba, D. Liu, J. Gui and A. T. Kuvarega, J. Colloid Interface Sci., 2021, 603, 666–684 CrossRef CAS PubMed.
- M. M. Abutalib, H. M. Alghamdi, A. Rajeh, O. Nur, A. M. Hezma and M. A. Mannaa, J. Mater. Res. Technol., 2022, 20, 1043–1056 CrossRef CAS.
- M. M. Sabzehmeidani, H. Karimi and M. Ghaedi, Mater. Res. Bull., 2022, 147, 111629 CrossRef CAS.
- S. A. Heidari-Asil, S. Zinatloo-Ajabshir, H. A. Alshamsi, A. Al-Nayili, Q. A. Yousif and M. Salavati-Niasari, Int. J. Hydrogen Energy, 2022, 47, 16852–16861 CrossRef CAS.
- U. Kumar, J. Kuntail, A. Kumar, R. Prakash, M. R. Pai and I. Sinha, Appl. Surf. Sci., 2022, 589, 153013 CrossRef CAS.
- T. Chellapandi, S. M. Roopan and G. Madhumitha, Environ. Res., 2023, 219, 115052 CrossRef CAS PubMed.
- F. Li, Z. Fang, Z. Xu and Q. Xiang, Energy Environ. Sci., 2024, 17, 497–509 RSC.
- S. Drummer, O. Mkhari and M. Chowdhury, Next Nanotechnol., 2024, 6, 100069 CrossRef.
- V. M. Rangaraj, S. Devaraju, T. G. Reddy, H. Zafar, D. H. Anjum and V. Mittal, Mater. Res. Bull., 2024, 179, 112925 CrossRef CAS.
- A. Mohammadi, H. Mirhosseini, A. Hekmatiyan, L. Abdolahi, F. Mehrabi and M. Shahmirzaei, J. Environ. Chem. Eng., 2023, 11, 110912 CrossRef CAS.
- Q. Chen, B. Miao, Y. Hao, H. Wang and Q. Chen, J. Alloys Compd., 2022, 902, 163804 CrossRef CAS.
- J. Guo, C. H. Shen, J. Sun, X. J. Xu, X. Y. Li, Z. H. Fei, Z. T. Liu and X. J. Wen, Sep. Purif. Technol., 2021, 259, 118109 CrossRef CAS.
- Y. Wang, C. Zhu, G. Zuo, Y. Guo, W. Xiao, Y. Dai, J. Kong, X. Xu, Y. Zhou, A. Xie, C. Sun and Q. Xian, Appl. Catal. B: Environ, 2020, 278, 119298 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |






