Open Access Article
Open Access ArticleSustainable antioxidant and antibacterial activities of zinc ion and caffeic acid-coupled carboxymethyl chitosan
Yuhui Zhanga,
Wenna Zhaia,
Mengdi Xiaa,
Yangzi Raoa,
Yuxin Yangb,
Xiao Wang*b and
Jianfeng Zhang *ac
*ac
aSchool of Material Science and Chemical Engineering, Ningbo University, Ningbo, Zhejiang 315211, China. E-mail: zjf@nbu.edu.cn
bHealth Science Center, Ningbo University, Ningbo, Zhejiang 315211, China. E-mail: wangxiao@nbu.edu.cn
cNingbo Fondxy New Materials Limited Corporation, Ningbo, Zhejiang 315210, China
First published on 25th June 2025
Abstract
Carboxymethyl chitosan (CMCS), a water-soluble derivative of chitosan, exhibits good biocompatibility. However, the limited bioactivities impede its potential applications. This work presents a novel CMCS derivative, termed CMCS–Zn–CA, which was prepared by complexing CMCS with Zn2+ and caffeic acid (CA) under microwave irradiation. Structural characterization was conducted using FT-IR, UV, XRD, TGA, and XPS analyses. Results showed that the scavenging rates of CMCS–Zn–CA on DPPH and hydroxyl radical were 95.45% and 92.45%, respectively, while those of CMCS were only 12.62% and 9.21%, respectively. The antibacterial assay on S. aureus and E. coli revealed that CMCS–Zn–CA demonstrated much lower minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) than CMCS, indicating the excellent inhibition ability of CMCS–Zn–CA on both strains. The time-kill curves and plate count confirmed the antibacterial activity of CMCS–Zn–CA, and SEM results validated its efficacious ability in disrupting the cell barrier. Furthermore, CMCS–Zn–CA demonstrated sustainable antioxidant activity by retaining 93.9% of the CA content and 95.4% of the DPPH radical scavenging rate and prolonged the milk storage time by maintaining a higher pH, lower acidity and lower bacterial count. In conclusion, the CMCS complex displayed sustainable antioxidant and antibacterial activities, suggesting its great potential in practical applications.
1. Introduction
Free radicals, especially reactive oxygen species, are by-products of human metabolism, and an excess of reactive oxygen species can react with various macromolecules in organisms, leading to cell damage and various diseases, such as heart disease, respiratory diseases, and tumors.1,2 S. aureus and E. coli are typical foodborne pathogens that are spread through contaminated food and drinking water, and they can inflict serious harm to human health by causing symptoms of meningitis and septicemia.3 As a result, extensive studies have attempted to develop effective antioxidant and antibacterial reagents for theoretical research or practical application.4–7 However, very few studies have been reported on the successful and effective incorporation of both bioactivities in one reagent. Therefore, it is imperative to develop new reagents that simultaneously possess high antioxidant and antibacterial properties.Chitosan (CS), a natural cationic polysaccharide derived from the deacetylation of chitin, has been widely used in food packaging, fruit preservation, and pharmaceutical development.8–10 Nevertheless, the limitation of CS's physical and biological properties, such as its poor water solubility and low antioxidant and antibacterial activities, has obstructed the practical and potential applications of CS, and numerous studies have tried to develop novel CS derivatives for broader application.11–13 Carboxymethyl chitosan (CMCS) is a category of popular water-soluble macromolecule derived from the carboxymethylation of chitosan14–16 and is extensively used in many fields for its optimized properties.17,18 However, it should be noted that the inferior bioactivities of CMCS have limited its practical application, and various methods have been developed to improve the antioxidant and antibacterial properties of CMCS; for instance, metal ion complexation and natural antioxidant grafting.19
Zinc, an essential trace element for the human body, plays an important role in human growth and development, and it is considered a promising material owing to its particular antibacterial activity; furthermore, zinc can enhance the antioxidant capacity of complexes by reducing their oxidation potential.20–22 Considering the advantages of chemical and biological properties of zinc, researchers have incorporated zinc into polysaccharides to form complexes and enhance their antioxidant and antibacterial abilities.23 Tang et al. found that CMCS can interact with zinc ions through chelation, and the chelation process was significantly influenced by the reaction conditions.24 R. L. Patale et al. obtained CMCS–Zn complexes that exhibited impressive antibacterial properties, with an inhibition zone of 20 mm against S. aureus.25 Nevertheless, it should be noted that CMCS–Zn cannot meet the practical requirement of the antioxidant and antibacterial activities. Furthermore, to the best of our knowledge, there is currently a lack of literature on simultaneously testing the two bioactivities of CMCS–Zn.
Polyphenols are a class of plant-derived compounds with noteworthy antioxidant properties, and exhibit enhanced antioxidant ability when combined with CMCS.26,27 So far, numerous polyphenols, such as caffeic acid, ferulic acid, and gallic acid, have been reported to bind to CMCS at amino and hydroxyl sites for improved bioactivities.28,29 Among the above-mentioned polyphenols, caffeic acid (CA) has attracted considerable attention owing to its natural antioxidant properties. Furthermore, CA can form complexes with enhanced water solubility and antioxidant activity when grafted onto chitosan or CMCS, exhibiting DPPH and hydroxyl radical scavenging rates that are several times greater than those of CMCS alone.30–32 Despite the impressive progress made thus far in antioxidant research, scientists continue to seek new approaches to improve the antioxidant activity of polyphenols.
In summary, CMCS, Zn, and CA individually exhibit excellent biological properties as reported in the literature. However, the preparation and bioactivity of the complexes formed from the three components remain unexplored. Herein, we developed a novel ternary complex of CMCS with zinc and CA using a free radical-mediated grafting method under microwave irradiation. The structures of the complexes were characterized, and the antioxidant activity was determined by measuring their ability to scavenge DPPH and hydroxyl radicals. The stability of the samples was evaluated by measuring the retained DPPH radical scavenging rate and CA content. Meanwhile, the antibacterial capacity of the complexes was evaluated by their inhibiting effect against E. coli and S. aureus, and their potential application was ultimately assessed in milk preservation.
2. Materials and methods
2.1 Materials
CMCS, potassium persulfate, ferrous sulfate and zinc acetate were all purchased from Shanghai Macklin Biochemical Co., Ltd. CA was purchased from Shanghai Meryer Biochemical Technology Co., Ltd. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was obtained from Tokyo Chemical Industry. The dialysis tubes (MWCO 8000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) were supplied by Shanghai Yuanye Biochemical Technology Co., Ltd. All reagents were used without further purification. E. coli (ATCC 25922) and S. aureus (ATCC 43300) were provided by the Microbiology Laboratory of Ningbo University and cryopreserved at −20 °C.
000 Da) were supplied by Shanghai Yuanye Biochemical Technology Co., Ltd. All reagents were used without further purification. E. coli (ATCC 25922) and S. aureus (ATCC 43300) were provided by the Microbiology Laboratory of Ningbo University and cryopreserved at −20 °C.
2.2 Preparation of CMCS–Zn complex
The CMCS–Zn complexes was prepared at room temperature (approximately 25 °C) under nitrogen protection, as previously described in the literature with minor modifications.33 Briefly, CMCS (0.5 g) was dissolved in distilled water (50 mL, 1% w/v), followed by the dropwise addition of zinc acetate solution (10 mL, 0.1 mol L−1) under continuous stirring. The precipitate was gradually generated by adjusting the pH of the mixed solution with 0.1 mol L−1 of NaHCO3. The precipitate was centrifuged, and CMCS–Zn was obtained through freeze-drying.2.3 Preparation of CMCS–Zn–CA complex
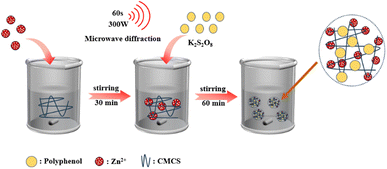
CMCS–Zn–CA was synthesized using a microwave-assisted method with a mass ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (CMCS–Zn–CA) (Fig. 1). In detail, 10 mL of 0.1 mol per L zinc acetate was slowly added into a solution of CMCS (0.5 g/50 mL water) under stirring, and 10 mL of 0.02 mol per L potassium persulfate was added dropwise. Subsequently, 0.2 g of CA was added into the solution, followed by stirring for 60 min. The reaction was initiated in a 300 W microwave oven for 1 minute, put up for cooling, and the operation was repeatedly processed for three times. Subsequently, the mixture was poured into the dialysis bag (MWCO 8000–14
2 (CMCS–Zn–CA) (Fig. 1). In detail, 10 mL of 0.1 mol per L zinc acetate was slowly added into a solution of CMCS (0.5 g/50 mL water) under stirring, and 10 mL of 0.02 mol per L potassium persulfate was added dropwise. Subsequently, 0.2 g of CA was added into the solution, followed by stirring for 60 min. The reaction was initiated in a 300 W microwave oven for 1 minute, put up for cooling, and the operation was repeatedly processed for three times. Subsequently, the mixture was poured into the dialysis bag (MWCO 8000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da), dialyzed against distilled water for 72 h, and CMCS–Zn–CA was obtained after freeze-drying, yielding 0.6128 g as a yellowish-brown powder.
000 Da), dialyzed against distilled water for 72 h, and CMCS–Zn–CA was obtained after freeze-drying, yielding 0.6128 g as a yellowish-brown powder.
2.4 Determination of grafting rate
The grafting rate of CMCS–Zn–CA was determined using the Folin–Ciocalteu method with slight modifications.34 In brief, 1 mL of 1 mg per mL CMCS–Zn–CA solution was mixed with 2.5 mL of Folin–Ciocalteu reagent (after a ten-fold dilution) and left to react in the dark for 5 min. Then, 4 mL of Na2CO3 (5%, w/v) was added to the mixture. The absorbance of the solution was measured at 760 nm after being fully shaken and reacted at 30 °C for 2 h. The standard curve was constructed using the CA concentration and absorbance data, and the grafting rate of the samples was expressed as mg CA equivalents per gram of CMCS–Zn–CA (mg CAE per g).2.5 Structural characterization
The structures were characterized by FTIR, UV-vis, XRD, TGA and XPS. The FTIR spectra were measured in the range of 4000–400 wavenumbers by a spectrometer (Thermo Nicolet 6700). The UV-vis spectra were measured from 200 nm to 400 nm with a spectrophotometer (LAMBDA 850+). The XRD spectra were determined in the 2θ range of 5–70° by an X-ray diffractometer (Bruker D8). The thermal stability of the complexes was evaluated on a thermal gravimetric analyzer (STA 2500) under nitrogen protection in the temperature range of 25–550 °C and heating rate of 10 °C min−1. XPS data were measured on an X-ray photoelectron spectrometer (Thermo Scientific) using Al Kα X-ray with 12 kV of working voltage.2.6 Antioxidant activity assessment
where A1 is the absorbance of the sample, and A0 is the absorbance of the control (anhydrous ethanol instead of the sample).
where As is the absorbance of the sample, A0 is the absorbance of the blank (water instead of the sample), and Ac is the absorbance of the control (water instead of H2O2).
2.7 Sustainability of antioxidant activity
The sustainability of CMCS–Zn–CA was determined according to the literature.35 Specifically, 1 mg mL−1 of the sample solution was initially prepared, stored at room temperature, and the CA content and DPPH radical scavenging capacity were measured in the upcoming 6 days. The percentages of the remaining CA content and DPPH radical scavenging activity were calculated to evaluate the sustainability of CMCS–Zn–CA.2.8 Determination of antibacterial activities
2.9 Application assessment in milk storage
3. Results and discussion
3.1 Preparation of CMCS–Zn–CA
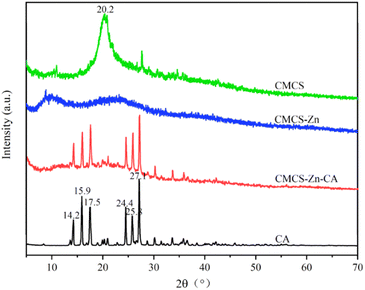
CMCS–Zn–CA was prepared by a free radical-mediated grafting method under microwave irradiation (Fig. 1). The process can be divided into three key steps: (i) the chelation of zinc ions with the hydroxyl of CMCS to produce CMCS–Zn complexes, facilitating the breaking of the O–H and N–H bonds; (ii) the formation of the oxygen radical and nitrogen radical from CMCS–Zn by the dissociation of O–H and N–H in the presence of sulfate radicals generated by the decomposition of persulfate; and (iii) the complexing of O–H and N–H in the CA molecules with the oxygen radical and nitrogen radical in CMCS–Zn to form the ternary complex CMCS–Zn–CA. The grafting rate of CMCS–Zn–CA was evaluated by measuring the total CA content in the complexes, and an optimized grafting rate of 84.21 mg CAE per g could be achieved. The subsequent bioactivity tests demonstrate that the bioactivity of CMCS–Zn–CA is linearly dependent on the grafting rate, i.e., a higher grafting rate results in greater bioactivity. Furthermore, the preparation of CMCS–Zn–CA under microwave condition should use potassium persulfate as the initiator, instead of the traditional initiator VC and H2O2.40 This is because H2O2 can be easily decomposed. The hydroxyl radical produced is less stable than the sulfate radical, resulting in disappointing reaction consequences.3.2 Characterization of CMCS–Zn and CMCS–Zn–CA
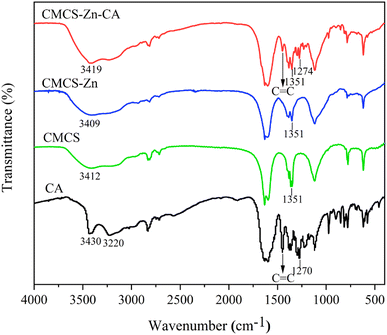
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching at 1450 cm−1. CMCS exhibited a broad peak at 3412 cm−1 (N–H and O–H stretching) and a characteristic C–N stretching band at 1351 cm−1. Upon zinc complexation, CMCS–Zn showed a slight shift in the N–H/O–H stretching band to 3409 cm−1, indicating successful metal chelation. The CMCS–Zn–CA spectrum retained the C–N stretching signature (∼1351 cm−1), while incorporating aromatic C
C stretching at 1450 cm−1. CMCS exhibited a broad peak at 3412 cm−1 (N–H and O–H stretching) and a characteristic C–N stretching band at 1351 cm−1. Upon zinc complexation, CMCS–Zn showed a slight shift in the N–H/O–H stretching band to 3409 cm−1, indicating successful metal chelation. The CMCS–Zn–CA spectrum retained the C–N stretching signature (∼1351 cm−1), while incorporating aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching features (1450–1650 cm−1). Changes in the hydroxyl peak position and intensity further confirmed the successful incorporation of both zinc ions and CA into the CMCS structure.
C stretching features (1450–1650 cm−1). Changes in the hydroxyl peak position and intensity further confirmed the successful incorporation of both zinc ions and CA into the CMCS structure.
| Sample | C (%) | N (%) | O (%) | Zn (%) |
|---|---|---|---|---|
| CA | 69.3 | 0 | 30.7 | 0 |
| CMCS | 58.88 | 5.4 | 35.72 | 0 |
| CMCS–Zn | 58.02 | 5.76 | 31.82 | 4.4 |
| CMCS–Zn–CA | 58.66 | 4.6 | 35.06 | 1.68 |
3.3 Antioxidative activity assessment
3.4 Sustainability of antioxidant activity
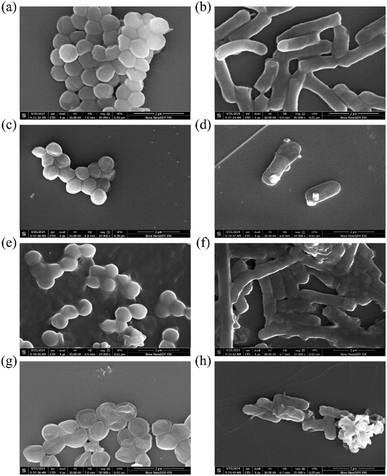
Polyphenols have been well-known for their excellent antioxidant properties. However, their instability in aqueous solution and susceptibility to external environment result in a serious loss of their antioxidant capacity. The main purpose of this work was to explore potential antioxidants with sustained capacity, so we tried to make a comparison between the sustainability of CMCS–Zn–CA and CA in a natural storage condition, as shown in Fig. 9. A gradual decline can be seen in the CA content and DPPH radical scavenging rate of CA and CMCS–Zn–CA over time. However, after six days of storage, CMCS–Zn–CA maintained 93.9% of its initial CA content and 95.4% of its DPPH radical scavenging activity, significantly outperforming free CA (81.7% and 87.9%, respectively). These results demonstrate that incorporation into the CMCS–Zn complex substantially enhances the CA stability.3.5 Assessment of antibacterial activity
| Strains | Concentration (μg mL−1) | |||||
|---|---|---|---|---|---|---|
| CMCS | Zn | CA | CMCS–Zn | CMCS–Zn–CA | ||
| a MIC: minimal inhibitory concentration, MBC: minimal bactericide concentration. | ||||||
| MIC | S. aureus | 2000 | 500 | 2000 | 250 | 125 |
| E. coli | >2000 | 2000 | >2000 | 1000 | 500 | |
| MBC | S. aureus | >2000 | 2000 | >2000 | 1000 | 500 |
| E. coli | >2000 | >2000 | >2000 | >2000 | 1000 | |
| Bacteria | Sample | CK | 1× | 2× | 4× |
|---|---|---|---|---|---|
| S. aureus | CMCS | 12.93 | 8.41 | 7.62 | 7.54 |
| CMCS–Zn | 13.57 | 6.00 | 4.24 | 2.99 | |
| CMCS–Zn–CA | 12.42 | 3.38 | 0 | 0 | |
| E. coli | CMCS | 8.81 | 8.31 | 8.04 | 7.95 |
| CMCS–Zn | 8.66 | 7.23 | 6.34 | 4.85 | |
| CMCS–Zn–CA | 8.55 | 1.15 | 0 | 0 |
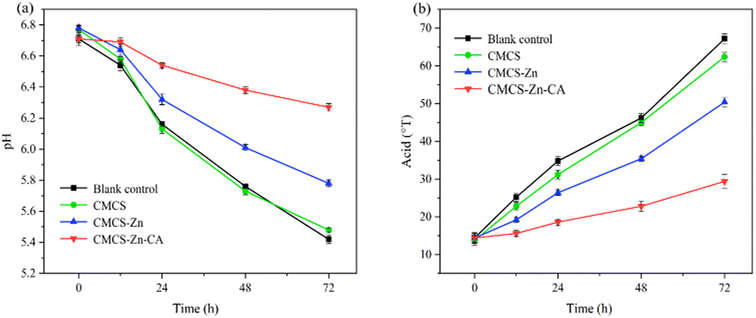
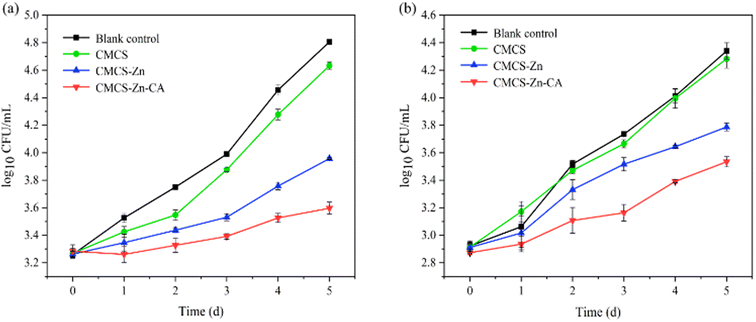
3.6 Application assessment in milk storage
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 and E. coli reaching 4.34
log10 CFU mL−1 and E. coli reaching 4.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1. Although no significant inhibition of bacterial growth was observed in the CMCS-treated milk, bacterial counts of S. aureus and E. coli after treatment with CMCS–Zn–CA decreased to 3.59
log10 CFU mL−1. Although no significant inhibition of bacterial growth was observed in the CMCS-treated milk, bacterial counts of S. aureus and E. coli after treatment with CMCS–Zn–CA decreased to 3.59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1 and 3.53
log10 CFU mL−1 and 3.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU mL−1, respectively, indicating that CMCS–Zn–CA can effectively inhibit the growth of both bacteria. Furthermore, it can be seen that the inhibition of S. aureus was more pronounced than that of E. coli, suggesting a stronger effect on Gram-positive bacteria.
log10 CFU mL−1, respectively, indicating that CMCS–Zn–CA can effectively inhibit the growth of both bacteria. Furthermore, it can be seen that the inhibition of S. aureus was more pronounced than that of E. coli, suggesting a stronger effect on Gram-positive bacteria.
4. Conclusions
A ternary complex, CMCS–Zn–CA, was successfully prepared by employing the free radical mediated grafting method under microwave irradiation. The structure was characterized by FTIR, UV-vis, XRD, TGA and XPS, and the grafting rate of CMCS–Zn–CA was found to be 84.21 mg CAE per g. The antioxidant activity assays revealed that CMCS–Zn–CA possesses an exceptional capacity to scavenge both DPPH and hydroxyl radicals, with a scavenging rate exceeding 90%. The sustainability test showed that CMCS–Zn–CA demonstrated a CA content of 93.9% and a DPPH radical scavenging rate of 95.4% after six days, while only 81.7% and 87.9% for CA, respectively. Furthermore, MIC and MBC measurements showed that the MICs of CMCS–Zn–CA against S. aureus and E. coli were 125 μg mL−1 and 500 μg mL−1, respectively. Meanwhile, the MBCs of CMCS–Zn–CA against the two strains were 500 μg mL−1 and 1000 μg mL−1, respectively, which were significantly lower than that of CMCS. The time-kill curves and plate counts demonstrated that CMCS–Zn–CA has an excellent inhibitory effect on both bacteria. The mechanism exploration indicated that the bacterial damage caused by CMCS–Zn–CA was significantly greater than that by CMCS and CMCS–Zn. It could be inferred that the antibacterial action might be achieved by destroying the cell barrier of bacteria. The results of the application assessment showed that CMCS–Zn–CA can result in a higher pH value, lower acidity and lower bacteria count, indicating superior antioxidative and antibacterial ability to prolong the milk storage time. In summary, the present study proposes novel Zn2+ and caffeic acid coupled complexes with excellent simultaneous antioxidant and antibacterial activities, and the sustainability of its high bioactivities forecasts the brilliant prospect in the application of health care and food preservation.Data availability
Data will be made available upon request.Author contributions
Yuhui Zhang: conceptualization, data curation, formal analysis, investigation, writing – original draft. Wenna Zhai: formal analysis, investigation. Mengdi Xia: formal analysis, validation. Yangzi Rao: data curation, investigation, validation. Yuxin Yang: formal analysis, methodology. Xiao Wang: visualization, project administration, supervision. Jianfeng Zhang: writing – review & editing, visualization, project administration, funding acquisition, supervision.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Open Research Fund from Zhejiang Collaborative Innovation Center for High Value Utilization of byproducts from Ethylene Project (Ningbo Polytechnic College) in 2021–2022 (No. NZXT202103) and the Natural Science Foundation of Ningbo (No. 2024J407).References
- Z. Tao, J. Wang and H. Wu, et al., ACS Appl. Mater. Interfaces, 2023, 15, 11474–11484 CrossRef CAS PubMed.
- J. Xie, N. Wang and X. Dong, et al., ACS Appl. Mater. Interfaces, 2018, 11, 2579–2590 CrossRef PubMed.
- H. Zhang, M. Liu and Y. Liu, et al., Spectrochim. Acta, Part A, 2025, 325, 125119 CrossRef CAS PubMed.
- G. Huang, X. Mei and J. Hu, Curr. Drug Targets, 2017, 18, 1296–1300 CAS.
- X. Guo, J. Food Process. Eng., 2024, 47, 14690 CrossRef.
- P. Li, L. Sun and S. Xue, et al., SmartMat, 2022, 3, 226–248 CrossRef CAS.
- H. Z. Zhang, L. L. Gan and H. Wang, et al., Mini-Rev. Med. Chem., 2017, 17, 122–166 CrossRef CAS PubMed.
- P. K. Dutta, J. Dutta and V. S. Tripathi, J. Sci. Ind. Res., 2004, 63, 20–31 CAS.
- S. Manna, A. Seth and P. Gupta, et al., ACS Biomater. Sci. Eng., 2023, 9, 2181–2202 CrossRef CAS PubMed.
- J. Jin, B. Luo and S. Xuan, et al., Int. J. Biol. Macromol., 2024, 266, 131253 CrossRef CAS PubMed.
- T. Yan, C. Li and Q. Ouyang, et al., React. Funct. Polym., 2019, 137, 38–45 CrossRef CAS.
- Y. Yu, Z. Su and Y. Peng, et al., Int. J. Biol. Macromol., 2025, 289, 138772 CrossRef CAS PubMed.
- Q. Hu and Y. Luo, Carbohydr. Polym., 2016, 151, 624–639 CrossRef CAS PubMed.
- L. Wang, Z. Luo and J. Yan, et al., Ultrason. Sonochem., 2020, 68, 105184 CrossRef CAS PubMed.
- M. I. H. Mondal and F. Ahmed, J. Text. Inst., 2020, 111, 49–59 CrossRef CAS.
- E. Cohen and E. Poverenov, Chem.–Eur. J., 2022, 28, 67 Search PubMed.
- L. Upadhyaya, J. Singh and V. Agarwal, et al., Carbohydr. Polym., 2013, 91, 452–466 CrossRef CAS PubMed.
- M. Kurniasih, Purwati and T. Cahyati, et al., Int. J. Biol. Macromol., 2018, 119, 166–171 CrossRef CAS PubMed.
- J. Liu, J. F. Lu and J. Kan, et al., Int. J. Biol. Macromol., 2013, 62, 85–93 CrossRef CAS PubMed.
- M. E. Bodini, M. A. del Valle and R. Tapia, et al., Polyhedron, 2001, 20, 1005–1009 CrossRef CAS.
- A. Vijayakumar, R. Mohan and P. Jayaprakash, J. Indian Chem. Soc., 2024, 101, 101375 CrossRef CAS.
- X. Lin, K. Jin and L. He, et al., ChemistrySelect, 2023, 8, 1–10 Search PubMed.
- J. Zhao, D. Qian and L. Zhang, et al., RSC Adv., 2024, 14, 10410–10415 RSC.
- D. N. S. Hon and L. G. Tang, J. Appl. Polym. Sci., 2000, 77, 2246–2253 CrossRef CAS.
- R. L. Patale and V. B. Patravale, Carbohydr. Polym., 2011, 85, 105–110 CrossRef CAS.
- V.-L. Truong and W.-S. Jeong, Int. J. Mol. Sci., 2021, 22, 9109 CrossRef CAS PubMed.
- M. Ma, M. Gu and S. Zhang, et al., Int. J. Biol. Macromol., 2024, 259, 129267 CrossRef CAS PubMed.
- R. Bai, H. Yong and X. Zhang, et al., Int. J. Biol. Macromol., 2020, 143, 49–59 CrossRef CAS PubMed.
- L. Bian, H. Sun and Y. Zhou, et al., Molecules, 2022, 27, 8496 CrossRef CAS PubMed.
- R. J. Robbins, J. Agric. Food Chem., 2003, 51, 2866–2887 CrossRef CAS PubMed.
- J. Liu, X.-y. Wen and J.-f. Lu, et al., Int. J. Biol. Macromol., 2014, 65, 97–106 CrossRef CAS PubMed.
- S.-H. Yu, H.-Y. Hsieh and J.-C. Pang, et al., Food Hydrocoll., 2013, 32, 9–19 CrossRef CAS.
- X. Wang, Y. Du and H. Liu, Carbohydr. Polym., 2004, 56, 21–26 CrossRef CAS.
- M. Curcio, F. Puoci and F. Iemma, et al., J. Agric. Food Chem., 2009, 57, 5933–5938 CrossRef CAS PubMed.
- J. Wang, H. Li and Z. Chen, et al., Ind. Crops Prod., 2016, 89, 152–156 CrossRef CAS.
- M. J. Moreno-Vásquez, M. Plascencia-Jatomea and S. Sánchez-Valdes, et al., Polymers, 2021, 13, 1375 CrossRef PubMed.
- M. Matejczyk, R. Świsłocka and A. Golonko, et al., Adv. Med. Sci., 2018, 63, 14–21 CrossRef PubMed.
- S. Mao, Y. Zeng and Y. Ren, et al., Food Hydrocoll., 2025, 160, 110722 CrossRef CAS.
- Y. Shi, Y. Li and K. Yang, et al., Food Control, 2023, 144, 109374 CrossRef CAS.
- C. H. Zhang, X. Q. Yu and Y. J. Diao, et al., Iran. Polym. J., 2021, 30, 81–91 CrossRef CAS.
- E. O. Olanipekun, O. Ayodele and O. C. Olatunde, et al., Int. J. Biol. Macromol., 2021, 183, 1971–1977 CrossRef CAS PubMed.
- F. Li, Y. Yan and C. Gu, et al., Foods, 2022, 11, 3548 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |