Open Access Article
Open Access ArticleSalt-free dyeing of cotton fabric via graft polymerization with chitosan using dimethyl itaconate as a renewable cross-linker†
Aqsa Majeed ,
Rosario Carmenini
,
Rosario Carmenini ,
Erica Locatelli
,
Erica Locatelli ,
Letizia Sambri
,
Letizia Sambri and
Mauro Comes Franchini
and
Mauro Comes Franchini *
*
Department of Industrial Chemistry “Toso Montanari”, University of Bologna, V. Piero Gobetti 85, 40129, Bologna, Italy. E-mail: mauro.comesfranchiniunibo.it
First published on 21st July 2025
Abstract
The dyeing of the cotton fabric with reactive dyes is the most commonly used process in textile industry. However, the extensive use of inorganic salts poses significant environment and health concerns. To address this, the recent studies has focused on modifying cotton fabric using bio-degradable resources. In this work, chitosan and dimethyl itaconate were grafted on cotton fabric through graft polymerization reaction using ammonium per sulphate as initiator. The modified cotton was characterized through FTIR, SEM and EDS techniques. Under the condition of no organic salt, the modified and non-modified cotton were dyed with C.I. reactive blue-4, C.I. reactive orange-16, and C.I. reactive black-5 under varying dye concentration, time and temperature. The colour strength properties, analysed through K/S values, revealed that the modified cotton showed the better K/S value as compared to non-modified cotton. Furthermore, a comparative analysis of colour fastness to washing and rubbing indicated that the modification did not adversely affect the colour depth.
Introduction
Cotton fabric is one of the most widely used cellulosic materials in the textile industry because of its excellent chemical properties, biodegradability, bio-compatible nature and final texture of the textiles.1 In the textile industry, reactive dyes enjoy widespread appreciation and in fact they are one of the most used dyes for the dyeing of cotton fabric, because of their wide range of excellent colour shades and brilliant colour fastness properties.2 Reactive dyes can create a covalent bond between the reactive group of the dye and the hydroxyl groups of the cellulosic backbone by nucleophilic substitution under alkaline conditions. However, when the cotton fabric is immersed in water, at neutral or basic pH, the surface becomes anionic due to the deprotonation of the hydroxyl groups of cellulose; the acquired negative charge generates a repulsion with the anionic reactive dyes resulting in high static repulsion between both molecules.3,4To minimize the repulsion between the dye and the cotton fabric, a huge amount of inorganic salts like sodium chloride (NaCl) and Glauber's salt (Na2SO4) are added (typically 30–100 g L−1) in the dye bath.5 In this scenario, these salts act as dying promoters because the cations of the inorganic salt interact with the slightly negative surface of the water-immersed cotton, resulting in an increased interaction with the anionic dye molecule6 thus enhancing the dye fixation and colour pervasiveness.7 However, the use of these inorganic salts in the dyeing process increases the risk of potential harm to the environment and to the human health. Indeed, according to a reported study almost 1.6 million litres of water are used to produce 8000 kg of dyed clothes and approximately 80% of the dye-containing wastewater is released untreated in the environment.8 This wastewater is considered the main cause of water pollution because of its high pH, loads of effluents, and the high amount of salts.9 This polluted water drained into the soil resulting in a salination problem, and a World Bank's report indicates that around 17–20% of water pollution comes from the textile industry.9
The main aim of this paper is to address the problem of using excessive amount of inorganic salts into the process of dyeing. To overcome this problem, different approaches have been proposed such as modification of the structure of reactive dyes, the use of biodegradable dyeing promoters,10 alternative dyeing methods,11 the use of natural mordants,12 and the chemical modification of cotton fabrics by introducing cationic groups.13 Among all of them, graft polymerization of cotton is gaining attention in the recent literature over other methods because this approach, using suitable compounds, can introduce a high number of cationic groups on the textile thus increasing the electrostatic affinity between the cotton and the dye molecules. Therefore, the chemical cationization of cellulose fabric modified the cotton surface, originally negative charged by introducing more cationic sites. This modification transforms the fabric, facilitation its interaction with the anionic reactive dyes, therefore lowering the amount of salts needed.14 For instance, different cationic agents such as 3-chloro-2-hydroxylpropyl trimethyl ammonium chloride (CHPTAC),15,16 (3-acrylamide propyl) trimethylammonium chloride (AAHTAPC),17 dendritic polymers,18 quaternary ammonium nanopartciles19 and biopolymers20 and a combination of epoxy polyemers and biopolymers21 are reported to in literature. However, these commercially available polymers have different limitations such as high dosage of cationic agents like CHPTAC leading to high cost and environmental concerns.22 Furthermore, quaternary ammonium cationic groups require high concentrations of alkali during the modification process23 and primary amino containing polymers (i.e. polyethyleneimine) have low adsorption efficiency problem.24
To overcome the above mentioned problems, this study is carried out using bio-degradable polymers such as chitosan and dimethyl itaconate. In particular chitosan, a biodegradable biomass, composed of (β-1-4) linked residues of glucosamine and N-acetyl-glucosamine is the only polycation available in nature and its cationization density depends on the pH of media25 As such, chitosan has received great attention for cotton cationization. Previous studies reported the chemical fixation of the chitosan on the cotton using different crosslinkers such as poly-carboxylic acids and metal adsorbents.26 However, these chemical derivatives produce several side effects on the mechanical properties of the fabric, its degradation, and the release of toxic and irritant compounds during its consumption.8
Industrial interest is clearly aimed at sustainable approaches for dyeing fabrics and in this sense the use of renewable and biodegradable biomass together with building blocks from renewable sources is an emerging method in the literature and with great application potential.
Itaconic acid, or 2-methylenesuccinic acid, is an unsaturated dicarboxylic acid traditionally obtained by the distillation of naturally occurring citric acid and nowadays produced by fermentation with engineered bacteria.27 This building block was recently included in the top 12 building block chemicals by the US Department of Energy thanks to its sustainable production, negligible toxicity and extensive applicability.28
In the current study, we describe good reactive dye fixation without any use of salts through graft polymerization of cotton carried out by grafting chitosan on cellulosic fabric with the addition of a bio-based molecule, the dimethyl itaconate as a novel functional crosslinker. The herein presented grafting approach is carried out through a free radical polymerization using ammonium persulfate as an initiator, which is safe to use, efficient, and soluble in water.1
The dye uptake was investigated using three commercial dyes C.I. reactive blue-4, C.I. reactive orange-16, and C.I. reactive black-5. The dyeing properties of the modified (MC) and non-modified cotton (NMC) fabrics were compared and analysed through K/S values. The modified fabric was characterized, and the application properties of this method were investigated.
Experimental
Materials
Chitosan, dimethyl itaconate, and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received. Mill-scoured and bleached cotton fabric was a generous loan from Zaitex srl (Dueville, Italy) company and used without modification. All the vinyl sulfone dyes used were reactive black-5 (Sigma-Aldrich), reactive orange-16 (BLD Pharma), and reactive blue-4 (BLD Pharma). All the dyes were of commercial grade and were used without any modification. All other reagents, namely sodium chloride (NaCl) and sodium sulfate (Na2SO4) were commonly used laboratory grade reagent.Graft polymerization of cotton fabric with chitosan and dimethyl itaconate
In a 500 mL round-bottomed flask equipped with a magnetic stirrer, 1.0 g of chitosan was dissolved in 2% acetic acid solution (100 mL). The mixture was kept under continuous stirring until a homogenous mixture was made. Subsequently, 3.0 g (0.01 mol) of dimethyl itaconate was added to the reaction mixture and stirred at room temperature for 10 min. After that, 1.0 g of cotton fabric was added into the reaction flask and allowed to stir for an additional 10 min at 40 °C. Once the mixture was homogenized, 1.0 g (0.0004 mol) of ammonium persulfate (APS) dissolved in 10 mL of water was added into the reaction system under a nitrogenous atmosphere and the temperature was raised to 70 °C for 4 h under continuous stirring and inert atmosphere during which the colour of the reaction mixture changed from pale yellow to white. The reaction was stopped by letting the air go into the reaction mixture, followed by its precipitation with cold methanol. Modified cotton (MC) fabric was washed several times with cold methanol to remove unreacted monomers and then air dried. Cotton fabrics without any treatment, herein called non-modified cotton (NMC) was used as comparison throughout this study.Characterization of modified cotton
The modification of the cotton was confirmed with Fourier Transform Infra-Red Spectroscopy (FT-IR) recorded on a NICOLET 6700 FTIR-ATR spectrometer from Thermo Fischer Scientific Co., Waltham, MA, USA. Scanning electron microscopy and energy dispersive spectroscopy (EDS) (ZEISS model EVO 50 EP equipped with an OXFORD INCA 350 EDS system) were used to analyse the surface morphology of modified and non-modified cotton fabrics on the surface vs. interior and to confirm the presence of elements used for the modification of cellulosic fabric. All the observations were made in EP (environmental pressure 10–20 kV and 80 PA of pressure in chamber). In order to analyze the color homogeneity and the penetration of the colorants inside the modified dyed cotton fabric, optical-microscopy (Digital Microscope KH-7700 equipped with the lens MX-5040RZ) was conducted from the surface and interior (yarn cross-sections) of the fabric.Dyeing of modified cotton and non-modified cotton
The dyeing of chitosan-itaconic dimethyl modified cotton (MC) and dyeing of the non-modified cotton (NMC) was carried out at a liquor-to-goods ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a 250 mL round-bottomed flask equipped with a magnetic stirrer fabrics for C.I. reactive blue-4, C.I. reactive orange-16, and C.I. reactive black-5 (Fig. 1). Before analysing the different dyeing parameters such as dye concentration, time and temperature, different samples for the MC and NMC were dyed at different pH (1, 2, 3, 4, 5, 6 and 7) to adjust the effect of pH media on the dyeing of the substrate. It was found that the MC showed best dyeability at pH: 6, and NMC at pH: 8 and was controlled for the further experiments by adjusting the pH of the dyebath before dyeing the cotton fabric (data available in ESI†).
1 in a 250 mL round-bottomed flask equipped with a magnetic stirrer fabrics for C.I. reactive blue-4, C.I. reactive orange-16, and C.I. reactive black-5 (Fig. 1). Before analysing the different dyeing parameters such as dye concentration, time and temperature, different samples for the MC and NMC were dyed at different pH (1, 2, 3, 4, 5, 6 and 7) to adjust the effect of pH media on the dyeing of the substrate. It was found that the MC showed best dyeability at pH: 6, and NMC at pH: 8 and was controlled for the further experiments by adjusting the pH of the dyebath before dyeing the cotton fabric (data available in ESI†).
 | ||
| Fig. 1 Chemical structure of reactive dyes (a) C.I. reactive blue-4; (b) C.I. reactive black-5; (c) C.I. reactive orange-16. | ||
After adjusting the pH, dyeing of the modified cotton was carried out using 1% w.o.f dye concentration added in a 100 mL volume of dye bath. The temperature of the dye bath was increased to 40 °C, followed by a gradual increase in temperature to 60 °C. After that, the modified cotton fabric was added into the dye bath, and the dyeing process continued for 60 min at 60 °C before the fabric was removed from the dye bath. The dyeing of the non-modified cotton was carried out by almost the same procedure, except the addition of sodium chloride (30 g L−1) and sodium carbonate (8 g L−1) into the dye bath to promote the bonding between the dye and cotton fabric. To rinse off un-attached dye from the fabric, washing of the fabric was carried out with cold water (approximately 200 mL for 0.3 g cotton) and then with warm water (40 °C for 10 min), followed by washing with non-ionic detergent at 90 °C for 15 min. Then the fabric was washed with cold water and dried in the air.
Optimization of dyeing conditions of modified and non-modified cotton
To optimize the dyeing conditions, dyeing of the modified (MC) and non-modified cotton (NMC) was carried out at 1% w.o.f to determine the optimize pH conditions keeping the same material-to-liquor ratio of the dye bath. Once the pH is adjusted, different dye concentrations (1%, 3%, 5%, and 7% w.o.f) were optimized. Once the dye concentration was optimized, another set of experiments was carried out to analyse the effect of different temperatures (50 °C, 60 °C, 70 °C, and 80 °C) on the dye uptake of the fabrics, followed by the optimization of the time of dyeing (30 min, 60 min, 90 min, and 120 min). Once all the experiments were conducted, optimum conditions were selected based on the colour strength values of dyed samples.Colour strength (K/S) and dye uptake (%) measurement
The colour strength of the dyed fabrics was recorded on a PerkinElmer UV/vis spectrophotometer Lambda 35, equipped with integrating sphere, in the form of reflectance (R) and K/S values were measured using the Kubelka–Munk equation.where S: scattering coefficient; R: reflectance at λmax; K: absorbance coefficient.
The reflectance was measured in the in the range of 400–800 nm and then the K/S values were calculated at λmax using Kubelka–Munk equation.
The percentage dye uptake also known as dye exhaustion of the modified cotton was measured with UV-vis spectrophotometry Carry 3500. The maximum absorption wavelength of dye bath was calculated before and after the dyeing of modified cotton with C.I. reactive black-5, C.I. reactive blue-4 and C.I. reactive orange-16 using the equation mentioned below.
Colour fastness measurement
Keeping in view the ISO standards of colour fastness, rubbing (ISO 105 X-12) and washing (ISO-105-C03) fastness of the modified and non-modified dyed fabrics was measured on a scale of 1–5 on the grey scale. The wash fastness of the fabric was observed with S-1002 two-dye bath dyeing and testing apparatus and rub fastness was obtained using a Y (B) 571-II crock-meter and the obtained values were recorded in the form of a table.Results and discussions
Mechanism of graft polymerization of chitosan and dimethyl itaconate on cellulosic fabric
Graft polymerization of cotton was carried out through the free radical polymerization mechanism which involves three stages: initiation, propagation, and termination. The process for the grafting of chitosan and dimethyl itaconate onto cotton is outlined in Fig. 2. In this process, ammonium persulfate (APS) was chosen as the initiator since it is a colourless, water-soluble compound that generates radicals under mild conditions. At the stage of initiation, APS produce free radicals on the reactants, which the drive the polymerization of dimethyl itaconate and propagetion of reaction resulting in the grafting of chitosan on cotton. Dimethyl itaconate acted as crosslinker taking advantage by its α,β-unsaturated activated system. | ||
| Fig. 2 Schematic presentation of graft polymerization of cotton with chitosan using dimethyl itaconate as a cross-linker. | ||
Control experiments to understand the importance of reactants and the reaction mechanism
Characterization of modified cotton
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of esters. This peak therefore confirmed the presence of dimethyl itaconate. On the other hand, as reported in the literature the small shift in the range of wavenumber and transmittance demonstrated the presence of chitosan in the modified cotton.31 Therefore, the IR spectrum of modified cotton confirms the graft polymerization of cotton by chitosan and dimethyl itaconate.
O group of esters. This peak therefore confirmed the presence of dimethyl itaconate. On the other hand, as reported in the literature the small shift in the range of wavenumber and transmittance demonstrated the presence of chitosan in the modified cotton.31 Therefore, the IR spectrum of modified cotton confirms the graft polymerization of cotton by chitosan and dimethyl itaconate.
From Fig. 3(a), the nitrogen content of the modified fabric increased by 2.98% which indicates the insertion of chitosan in MC. In addition, the carbon content of the modified cotton was also increased because of the addition of dimethyl itaconate and chitosan to the cotton fabric. This EDS test demonstrated that the chitosan and dimethyl itaconate had been homogenously grafted on cellulosic fabric.
Optimization and colorimetric properties of modified and non-modified cotton fabric dyed under different parameters
In the conventional methods of dyeing, salt plays a critical role in promoting dye absorption on the cotton fabric. The other key parameters that affect the dyeing process include dye concentration, time of dyeing, and temperature. The process curve used for dyeing modified (MC) and non-modified cotton (NMC) is shown in Fig. 5. Given that we modified the cotton to promote the salt-free dyeing method, MC was dyed without any salt, in contrast to the NMC dyeing, where 30 g L−1 of sodium chloride (NaCl) was added to enhance the dye attachment to the cotton fabric. Moreover, to find the best dying conditions, the dyeing experiments of modified cotton (MC) and non-modified cotton (NMC) with C.I. reactive blue-4, C.I. reactive orange-16, and C.I. reactive black-5 were carried out varying the dye concentration, the temperature and the dying time. | ||
| Fig. 5 Process curve for dyeing and washing of modified (MC) and non-modified cotton (NMC) with reactive dye. | ||
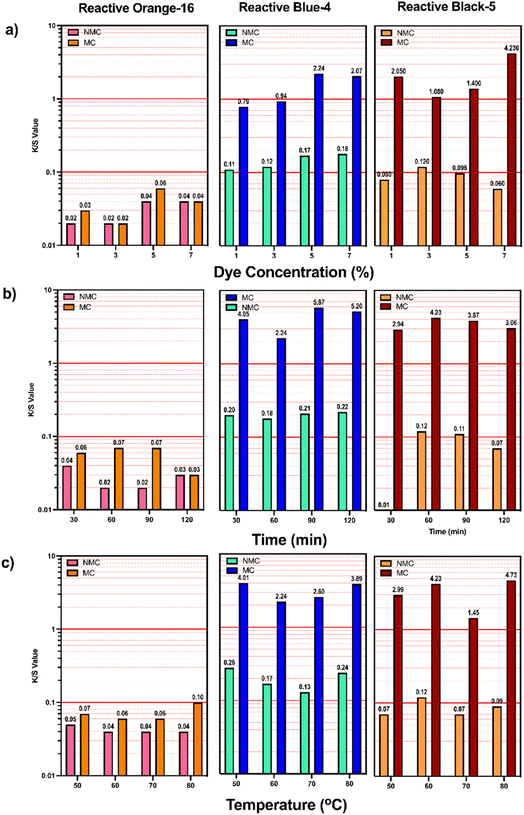
The specific values of these parameters and their effect on colour strength are reported in Table 1 and Fig. 6. For the optimization of the dye concentration, the process was carried out using the dye at 1%, 3%, 5%, and 7% w.o.f respectively. It was found that for reactive blue-4, the values of K/S increased using 5% w.o.f, and then a narrow decrease was observed at 7% w.o.f for the non-modified cotton. The same trend was observed for the modified cotton, even if a minor difference between 5% and 7% w.o.f was observed. This trend showed that the optimized dye concentration for reactive blue-4 dye is 5% w.o.f, and a higher concentration had no obvious effect, leading to an increase of unreacted dye molecules in the dye bath.
| Dye | Optimized conditions for dyeing | R%a | K/Sb | |||
|---|---|---|---|---|---|---|
| MCc | NMCd | MCc | NMCd | |||
| a R%: reflectance percentage.b K/S: colour strength.c MC: modified cotton.d NMC: non-modified cotton. | ||||||
| C.I. reactive blue-4 | Dyeing concentration | 15.75 | 55.93 | 2.24 | 0.17 | |
| 5% | ||||||
| Dyeing temperature | 10.69 | 47.61 | 4.01 | 0.28 | ||
| 50 °C | ||||||
| Dyeing time | 7.31 | 53.56 | 5.87 | 0.20 | ||
| 90 min | ||||||
| C.I. reactive black-5 | Dyeing concentration | 9.63 | 60.43 | 4.23 | 0.12 | |
| 3% NMC | 7% MC | |||||
| Dyeing temperature | 9.63 | 60.43 | 4.23 | 0.12 | ||
| 60 °C | ||||||
| Dyeing time | 9.63 | 60.43 | 4.23 | 0.12 | ||
| 60 min | ||||||
| C.I. reactive orange-16 | Dyeing concentration | 71.85 | 73.53 | 0.06 | 0.04 | |
| 5% | ||||||
| Dyeing temperature | 63.7 | 70.95 | 0.10 | 0.05 | ||
| 50 °C NMC | 80 °C MC | |||||
| Dyeing time | 69.4 | 73.73 | 0.07 | 0.04 | ||
| 30 min | ||||||
Similarly, the effect of temperature was optimized performing the dying at 50 °C, 60 °C, 70 °C, and 80 °C. It was observed that for the reactive blue-4, the MC and NMC at 50 °C showed the best K/S values, i.e., 4.01 and 0.28, respectively. Moreover, for the time of dyeing, it was found that the best colour strength, i.e., 5.87, was observed at 90 min for MC and K/S 0.22 at 120 min for NMC, but for non-modified cotton there is a slight difference between the K/S values for 90 min and 120 min, that are 0.21 and 0.22, respectively. Therefore, also for NMC, 90 min was considered the optimized dyeing time for reactive blue-4.
Similarly, the optimization of the dyeing conditions for reactive black-5 was carried out as shown in Fig. 6 for modified and non-modified cotton and it was found that for NMC, the value of K/S enhanced increasing the dye concentration from 1% to 3% but it gradually decreased at 5% and 7% of dye concentration. In contrast, for the MC, the best dye concentration was found to be 7%. Assessing the effect of temperature, the K/S value for modified cotton increased heating till 60 °C (K/S 4.23), but some fluctuations were observed with a further temperature increase. However, the difference between K/S values across range 60–80 °C was not significant, leading to the conclusion that 60 °C can be considered as the optimal temperature for the dyeing of the modified cotton.
Also, with NMC, the optimal K/S value was 60 °C. Furthermore, to optimize the effect of dyeing time on MC/NMC, it was observed that MC obtained optimal K/S value after 60 min of dyeing which then decreased after 90 min and 120 min. However, the effect of time was less pronounced for the non-modified cotton, with minimal change in K/S value from 30 min to 90 min (K/S 0.093, 0.12, 0.11 respectively) followed by a gradual decrease at 120 min (K/S 0.07). Therefore, based on these findings, 60 min was considered as the optimized time also for the dyeing of NMC.
Finally, reactive orange-16 was used to dye MC and NMC and, also in this case, the optimization of dyeing parameters was carried out (Fig. 6). Colour strength values for MC decreased from 1% to 3% and then the highest K/S value was observed at 5% w.o.f. In contrast, for NMC the dye concentration has no obvious effect for 1% and 3% as the K/S value remains constant at 0.02, which then increases at 5% (K/S 0.04) and remains constant at 7%. Hence, also for NMC, 5% w.o.f was considered as the optimal dye concentration. While observing the effect of temperature, it was found that for MC the best colour strength was exhibited when the dyeing temperature was at 80 °C (K/S 0.1). In the case of NMC 50 °C (K/S 0.05) was found to be the best temperature for dyeing. Moreover, MC exhibited the highest colour strength (K/S 0.06) after 30 min of dyeing, because at higher times i.e., 60 min, and 90 min there was no noticeable difference in K/S values. A similar trend was noticed for NMC, in which the highest K/S value was found at 30 min (K/S 0.04), and the lowest was observed at 90 min (K/S 0.01).
However, it was concluded that, as compared to the huge difference between the colour strength of MC/NMC of C.I. reactive blue-4 and C.I. reactive black-5, the colour strength values of reactive orange-16 are comparable between MC/NMC.
According to the Kubelka–Munk equation, the reflectance and the colour strength are inversely related to each other. If the value of reflectance increases, then the K/S value would decrease.32 As reported in Table 1, the values of reflectance and K/S significantly improved for the MC in comparison to NMC. The high value of reflectance for NMC as compared to MC demonstrates that the absorbance of the dye is higher for the MC. Moreover, the greater values of K/S for MC also show that the MC has more dye uptake and colour strength.
It was also observed that the percentage dye uptake of the modified cotton is exceptional. The percentage dye uptake was calculated according to the above mentioned (II) equation for each dye concentration. It was found that the highest dye upatek was at highest K/S values i.e. 84.61%, 84.09% and 85.43% for reactive orange-16, reactive black-5 and reactive blue-4 respectively. It explains that the only small percentage of the dyed solution was discarded as a effluents without any electrolytes for the process of MC dyeing. From the obtained results, we demonstrated that the introduction of chitosan and dimethyl itaconate promotes bond formation between the dye and the fabrics thanks to the presence of amino groups and electron withdrawing groups. In addition to that, grafting process slightly alter the surface morphology of the cotton, as evidenced by SEM analysis, facilitated the dye penetration into the fabric. Therefore, the grafting process enhanced the cotton fabric overall reactivity towards dyes, offering improved colour strength.
Comparison of colour fastness between modified and non-modified cotton
Colour fastness is the measurement of resistance of a dyed fabric to change in any of its colour characteristics against the pristine fabric in case of washing and rubbing. These colour changes are measured according to the grey scale that ranges from 1 to 5 (5 means unchanged to secondary material, while rating 1 expresses a major change in colour comparison to secondary material, that are the pristine fabric). Therefore, when the dyed fabric is washed or rubbed with the raw fabric, the transfer of colour from dyed to the raw fabric is measured on the grey scale.33 We evaluated the colour fastness to washing and rubbing (dry and wet) for MC and NMC concerning the colour change and colour bleeding to the multifiber (acetate, cotton, nylon, polyester, acrylic, and wool) fabric. Fastness ratings of fabric dyed at optimum conditions with C.I. reactive blue-4, C.I. reactive black-5 and C.I. reactive orange-16 are presented in Table 2.| Fabric sample | Rubbing fastness | Washing fastness and colour staining to multifiber | ||||||
|---|---|---|---|---|---|---|---|---|
| Dry | Wet | Acetate | Cotton | Nylon | Polyester | Acrylic | Wool | |
| Colourfastness for modified cotton | ||||||||
| Reactive blue-4 | 4/5 | 3/4 | 5 | 3 | 5 | 5 | 5 | 5 |
| Reactive black-5 | 4/5 | 4 | 5 | 3/4 | 5 | 5 | 5 | 5 |
| Reactive orange-16 | 4/5 | 4 | 5 | 4 | 5 | 5 | 4/5 | 5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Colourfastness for non-modified cotton | ||||||||
| Reactive blue-4 | 4/5 | 4 | 5 | 4/5 | 5 | 5 | 5 | 5 |
| Reactive black-5 | 4/5 | 4 | 5 | 4/5 | 5 | 5 | 4/5 | 5 |
| Reactive orange-16 | 4/5 | 4 | 5 | 4/5 | 5 | 5 | 4/5 | 5 |
Results reported in Table 2 showed that the fastness ratings of the modified cotton and non-modified cotton are comparable to each other in case of rubbing and washing. It was also noted that in the case of wet rubbing, the MC also showed excellent colour strength as NMC, which shows that the dye is covalently attached to the fabric. However, in the case of washing, modified cotton showed a lower rating (for MC: 3 and NMC: 4/5) for reactive blue-4 and (MC: 3/4 and 4/5 for NMC) in the case of cotton, but for other parts of multifiber, the values are almost similar in both cases. It can be concluded that, as reported in Fig. 6 for the reactive blue-4 and reactive black-5, MC has a much deeper shade as compared to NMC. Hence, generally, with the first washing, MC has stained more than untreated fabric. Therefore, it is concluded that the grafting of cotton with chitosan and dimethyl itaconate does not negatively affect the colour fastness properties of the textile. Additionally, despite the salt-free dyeing for MC, the colour fastness properties remains comparable to that of NMC.
Conclusion
In conclusion, cellulosic fibres were modified using an important biomass, the chitosan, and dimethyl itaconate, a novel and bio-based crosslinker, through a graft polymerization reaction and subsequently characterized by FTIR, EDS, and SEM analysis finding that the modification has no negative effect on the surface out without salt in contrast to the non-modified cotton dyeing in which 25–30 g L−1 of salt are routinely employed. Optimization of dyeing conditions was conducted across varying ranges of dye concentration, temperature, and time. Notably, K/S values for the dyeing of modified cotton (MC) were higher than those of non-modified cotton (NMC), even under salt-free dyeing conditions. Comparative analysis of colour fastness between MC and NMC also demonstrated that the modification has no negative impact on colour fastness and staining. Therefore, it is concluded that thanks to the combination of a graft polymerization approach on cotton with chitosan and dimethyl itaconate the use of salt can be avoided without compromising the colour strength and colour fastness properties.Data availability
The data supporting this article have been included in the ESI available online.†Author contributions
A. M. and R. C. prepared the original draft and provided all the experimental procedures. M. C. F. planned and supervised the project. L. S. and E. L. review the manuscript and provided structural characterization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks are due to the Made in Italy – Circular and Sustainable (MICS) Extended Partnership, funded by the European Union Next-Generation EU (Piano Nazionale di Ripresa e Resilienza (PNRR) – Missione 4, Componente 2, Investimento 1.3 – D. D. 1551.11-10-2022, PE00000004). We thank the company Zaitex Spa (Dueville, Italy), for the generous loan of the cotton material and for the precious help in some characterizations.Notes and references
- K. Li, X. Li, Y. Li and C. Wu, J. Nat. Fibers, 2023, 20, 1–14 Search PubMed.
- M. N. Amin and R. S. Blackburn, ACS Sustain. Chem. Eng., 2015, 3, 725–732 CrossRef CAS.
- A. M. Grancarić, A. Tarbuk and I. Ristić, Article in Fibres and Textiles in Eastern Europe, 2013 Search PubMed.
- T. S. Aysha, N. S. Ahmed, M. S. El-Sedik, Y. A. Youssef and R. M. El-Shishtawy, Sci. Rep., 2022, 12, 22339 CrossRef CAS PubMed.
- M. A. R. Bhuiyan, A. Shaid and M. A. Khan, Chem. Mater. Eng., 2014, 2, 96–100 Search PubMed.
- A. Dessie, Adv. Res. Text. Eng., 2022, 7, 1077 Search PubMed.
- W. Ma, S. Zhang, B. Tang and J. Yang, Color. Technol., 2005, 121, 193–197 CAS.
- D. Alonso-Segura, L. Hernández-García, J. Menchaca-Arredondo, M. Sánchez, B. Chamorro-Garza and R. Garza-Hernández, Polymers, 2021, 13, 2–16 CrossRef PubMed.
- J. Sharma, S. Sharma and V. Soni, Reg. Stud. Mar. Sci., 2021, 45, 101802 Search PubMed.
- J. Ru, X. Qian and Y. Wang, Sci. Rep., 2018, 8, 13045 CrossRef PubMed.
- Z. Khatri, M. H. Memon, A. Khatri and A. Tanwari, Ultrason. Sonochem., 2011, 18, 1301–1307 CrossRef CAS PubMed.
- K. Iqbal, H. Afzal, M. O. R. Siddiqui, U. Bashir, K. Jan, A. Abbas and H. A. Abid, Sustainable Chem. Pharm., 2023, 33, 2352–5541 Search PubMed.
- N. Arivithamani and V. R. Giri Dev, J. Cleaner Prod., 2017, 149, 1188–1199 CrossRef CAS.
- J. Correia, K. T. Rainert and F. R. Oliveira, et al., Cellulose, 2020, 27, 8527–8550 CrossRef CAS.
- N. Arivithamani and V. R. G. Dev, Clean Technol. Environ. Policy, 2017, 19, 2317–2326 CrossRef CAS.
- S. Zhai, Y. Li, W. Dong, H. Zhao, K. Ma, H. Zhang, H. Wang, Y. Zhao, X. Li and Z. Cai, Cellulose, 2022, 29, 633–646 CrossRef CAS.
- A. Patiño, C. Canal, C. Rodríguez, G. Caballero, A. Navarro and J. M. Canal, Cellulose, 2011, 18, 1073–1083 CrossRef.
- M. Sadeghi-Kiakhani and S. Safapour, Fibers Polym., 2015, 16, 1075–1081 CrossRef CAS.
- Z. Wei, D. Xinlan and Z. Jinjie, Fibres Text. East. Eur., 2018, 26, 116–121 CrossRef CAS.
- N. Arivithamani, S. Agnes Mary, M. Senthil Kumar and V. R. Giri Dev, Clean Technol. Environ. Policy, 2014, 16, 1207–1215 CrossRef CAS.
- K. Singha, S. Maity and M. Singha, Int. J. Textil. Sci., 2013, 1, 69–77 CrossRef.
- J. Setthayanond, F. Netzer, K. Seemork, P. Suwanruji, T. Bechtold, T. Pham and A. P. Manian, Cellulose, 2023, 30, 4697–4711 CrossRef CAS.
- X. Liu, F. Zhang, S. Liu, Q. Zhao, J. He, J. Wei and X. Dong, J. Cleaner Prod., 2024, 442, 141154 CrossRef CAS.
- J. Xia, C. Zhang, X. Liu, J. He and X. Dong, Green Chem., 2022, 24, 9180–9190 RSC.
- S. (Gabriel) Kou, L. M. Peters and M. R. Mucalo, Int. J. Biol. Macromol., 2021, 169, 85–94 CrossRef PubMed.
- G. Zhang, R. Qu, C. Sun, C. Ji, H. Chen, C. Wang and Y. Niu, J. Appl. Polym. Sci., 2008, 110, 2321–2327 CrossRef CAS.
- J. Becker, A. Lange, J. Fabarius and C. Wittmann, Curr. Opin. Biotechnol., 2015, 36, 168–175 CrossRef CAS PubMed.
- T. Klement and J. Büchs, Bioresour. Technol., 2013, 135, 422–431 CrossRef CAS PubMed.
- E. H. Portella, D. Romanzini, C. C. Angrizani, S. C. Amico and A. J. Zattera, Mater. Res., 2016, 19, 542–547 CrossRef CAS.
- C. Chung, M. Lee and E. K. Choe, Carbohydr. Polym., 2004, 58, 417–420 CrossRef CAS.
- B. Arik, K. Titandioksit, V. E. Kitosan, S. Hibrit, S.-J. Kaplamalar, İ. Kaplanmiş, P. Kumaşin, A. Ve and Y. Ö. Değerlendirilmesi, Evaluation of Antibacterial and Structural Properties of Cotton Fabric Coated by Chitosan/Titania and Chitosan/Silica Hybrid Sol-Gel Coatings Search PubMed.
- R. B. Love, S. Oglesby and I. Gailey, J. Soc. Dyers Colour., 1965, 81, 609–614 CrossRef.
- W. D. Schindler and P. J. Hauser, in Chemical Finishing of Textiles, Elsevier, 2004, pp. 144–156 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02510a |
| This journal is © The Royal Society of Chemistry 2025 |