Open Access Article
Open Access ArticleA new azo dye for colorimetric determination of lead(II) ions†
Sefiu Olalekan Olaleye *ab and
Uzma Bibi
*ab and
Uzma Bibi c
c
aDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Ibadan, Orita UI, Ibadan, 200284, Nigeria. E-mail: solaleye5@gmail.com
bDepartment of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmacy, Federal University Oye-Ekiti, Ekiti, Nigeria
cThird World Center for Science and Technology, H. E. J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi, 75270, Pakistan
First published on 19th June 2025
Abstract
A new azo dye, S9b (1-amino-4-[(E)-2-(8-hydroxyquinolin-5-yl)diazen-1-yl]-9,10-dihydroanthracene-9,10-dione), was developed and evaluated as a colorimetric chemosensor for lead(II) ions. Upon Pb2+ addition, the S9b solution color changed from rosy-brown to sandy-brown. The UV-vis spectrum of the S9b–Pb2+ complex exhibited a hyperchromic shift compared to free S9b and a bathochromic shift relative to S9b complexes with other metal ions. Optimized conditions comprised an ethanol solvent system, pH 6.0, a reaction time of 2 min, and a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of S9b to Pb2+.The response of S9b was linear at Pb2+ concentrations of 3.90–9.36 μg mL−1. The calculated detection limit, quantitation limit and binding constant were 1.55 μg mL−1, 4.71 μg mL−1 and 3.07 × 104 L2 g−2, respectively. Determination of Pb2+ was not significantly affected by other interfering cations (Ag+, Co2+, Cu2+, Fe2+, Fe3+, Na+, K+, Ni2+, Hg2+, Ca2+, Zn2+, Mg2+, and Al3+). The S9b-based method demonstrated recoveries of 100.03–103.11% and relative standard deviations (RSDs) of 0.06–2.07% for Pb2+ in spiked water samples, with accuracy and precision comparable to atomic absorption spectroscopy (AAS). FTIR studies and DFT calculations indicated that Pb2+ binding to S9b occurs via the heterocyclic nitrogen and phenolic hydroxyl groups of the azo dye. The sensor demonstrated reusability following regeneration with EDTA, which dissociated the Pb2+ complex. This study highlights the potential of the anthracene-9,10-dione-based azo dye as a simple, eco-friendly, and rapid chemosensor for Pb2+ detection in aqueous systems.
1 molar ratio of S9b to Pb2+.The response of S9b was linear at Pb2+ concentrations of 3.90–9.36 μg mL−1. The calculated detection limit, quantitation limit and binding constant were 1.55 μg mL−1, 4.71 μg mL−1 and 3.07 × 104 L2 g−2, respectively. Determination of Pb2+ was not significantly affected by other interfering cations (Ag+, Co2+, Cu2+, Fe2+, Fe3+, Na+, K+, Ni2+, Hg2+, Ca2+, Zn2+, Mg2+, and Al3+). The S9b-based method demonstrated recoveries of 100.03–103.11% and relative standard deviations (RSDs) of 0.06–2.07% for Pb2+ in spiked water samples, with accuracy and precision comparable to atomic absorption spectroscopy (AAS). FTIR studies and DFT calculations indicated that Pb2+ binding to S9b occurs via the heterocyclic nitrogen and phenolic hydroxyl groups of the azo dye. The sensor demonstrated reusability following regeneration with EDTA, which dissociated the Pb2+ complex. This study highlights the potential of the anthracene-9,10-dione-based azo dye as a simple, eco-friendly, and rapid chemosensor for Pb2+ detection in aqueous systems.
1. Introduction
Water contamination by heavy metals poses significant risks to human health and ecosystems. Among these metals, lead (Pb) is particularly hazardous, exerting toxic effects even at trace concentrations. Lead(II) ions are extensively utilized in industries such as battery manufacturing, automotive production, paints, ceramics, and mining.1–4 Environmental Pb2+ contamination primarily occurs through rainwater-mediated leaching of soil particles contaminated by anthropogenic activities. The World Health Organization (WHO) stipulates a guideline value of 10 μg L−1 for lead in drinking water, underscoring its acute toxicity.5 Chronic exposure to Pb2+ induces severe renal and neurological damage,4 disrupts reproductive systems in both sexes (reducing sperm count, motility, and altering morphology),6,7 and is linked to adverse pregnancy outcomes, including miscarriage, preterm birth, and developmental deficits.8 At the cellular level, Pb2+ interferes with DNA transcription and compromises membrane integrity,9 necessitating robust environmental monitoring strategies.Conventional analytical techniques for Pb2+ detection, such as atomic absorption/emission spectrometry (AAS/AES), inductively coupled plasma mass spectrometry (ICP-MS), and anodic stripping voltammetry (ASV), offer high sensitivity but are hindered by operational complexity, high cost, and huge technicality.10–16 Consequently, chemosensors—particularly colorimetric probes—have emerged as promising alternatives due to their simplicity, cost-effectiveness, and potential for real-time field applications.
Azo dyes, widely employed in the textile industry,17 are gaining attention as colorimetric chemosensors owing to their distinct chromogenic properties. Despite numerous optical sensors reported for Pb2+ ion detection,18–24 a comprehensive literature review highlights that azo-functionalized systems, in particular, remain significantly underexplored for Pb2+ detection. For instance, Ghorbanian et al.25 developed a benzothiazole azo dye for Pb2+ sensing in DMSO-water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v, pH 7), exhibiting a 72 nm hypsochromic shift (blue to pink) with a detection limit of 0.67 μM. Similarly, Wang et al.26 reported an azobenzene-based sensor in acetonitrile-water (1
4 v/v, pH 7), exhibiting a 72 nm hypsochromic shift (blue to pink) with a detection limit of 0.67 μM. Similarly, Wang et al.26 reported an azobenzene-based sensor in acetonitrile-water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 7.21), achieving a limit of detection (LOD) of 5.44 μM via a yellow-to-magenta transition. While these systems demonstrate feasibility, dimethylsulfoxide and acetonitrile utilized as sensing milieus are not eco-friendly.
1 v/v, pH 7.21), achieving a limit of detection (LOD) of 5.44 μM via a yellow-to-magenta transition. While these systems demonstrate feasibility, dimethylsulfoxide and acetonitrile utilized as sensing milieus are not eco-friendly.
Anthracene-9,10-dione derivatives, small organic molecules with high molar absorptivity and visible-region absorbance/emission maxima,27,28 have shown promise as chemosensors for metal ions.29–32 However, to our knowledge, no azo-functionalized anthracene-9,10-dione derivatives have been reported for Pb2+ detection. In continuation of our efforts in sensor design,33 we herein present a new, fast and simple azo derivative of 1,4-diaminoanthracene-9,10-dione as a selective colorimetric probe for Pb2+ in ethanol.
2. Experimental
2.1 Materials and instrument
All the reagents and chemicals were obtained from BDH, Poole, England. Thin-layer chromatography (TLC) was developed on pre-coated silica gel aluminum plates (Kieselgel 60F254, E. Merck, Germany). 1H NMR and 13C NMR spectra were recorded on Bruker BioSpin GmbH Avance NEO 600 MHz, UK. UV-vis spectral data were acquired on the Spectroquant Pharo 300, Merck, Germany. FTIR spectra data were obtained on FT-IR Spectrometer Spectrum Two PerkinElmer, USA. ESI-MS measurements were recorded on AmaZon speed ESI-Ion trap mass spectrometer (Bruker, UK). The pH of the solution was obtained on pH meter (Hanna, US).2.2 Methodology
The purified compound was analyzed by Attenuated total reflectance Fourier-transform infrared spectroscopy (ATR-FTIR), 1H/13C nuclear magnetic resonance (NMR; acetone-d6, 600 MHz), and electrospray ionization mass spectrometry (ESI-MS).
2.3 Chemosensor measurement
A stock solution of S9b (2.3428 mM) was prepared by mixing 1.5 mL of S9b (1 mg mL−1) with 125 μL of buffer solution. 100 μL of S9b solution was added to each vial containing five equivalents of solution of nitrate salts of Ag+, Co2+, Cu2+, Fe2+, Fe3+, Na+, K+, Ni2+, Pb2+, Hg2+, Ca2+, Zn2+, Mg2+, and Al3+. The mixture was swirled for 60 seconds and made up to 5 mL with ethanol. The color changes were observed, and UV-visible scans of the mixture were recorded between 300 and 700 nm on the UV-visible spectrophotometer. Triplicate measurements were performed to ensure reproducibility.2.4 Optimization studies
2.5 Analytical method development
 | (1) |
 | (2) |
Accuracy was assessed as percentage recovery, while precision was evaluated via relative standard deviation (RSD). Statistical comparisons between the S9b and AAS methods were conducted using Student's t-test (for means) and the F-test (for variances).
2.6 Determination of binding mechanism
A solution of 8.401 mg Pb(NO3)2 in 2 mL deionized water was added dropwise to 1 mg mL−1 N,N-dimethylformamide (DMF) solution of S9b. The mixture was magnetically stirred for 30 min at ambient temperature. The resulting precipitate was isolated via vacuum filtration, washed thoroughly with deionized water, and air-dried. Fourier-transform infrared (FT-IR) spectroscopy was employed to compare the spectra of free S9b and its Pb2+ complex, enabling identification of functional groups involved in Pb2+ coordination.2.7 Reversibility experiment
To investigate the reversibility of Pb2+ binding to S9b, 134 μL of S9b (2.3428 mM) and 66 μL of Pb2+ solution (2.3428 mM) were combined in a vial. The mixture was vortex-mixed for 10 s and incubated for 2 min at ambient temperature. Subsequently, 134 μL of disodium ethylenediaminetetraacetic acid (Na2EDTA; 2.3428 mM) was introduced, followed by vortex-mixing and incubation for 2 min. The final solution was diluted to 5 mL with ethanol, and its UV-vis absorption spectrum was recorded across 200–700 nm to assess spectral changes induced by competitive EDTA chelation.2.8 Selectivity assessment of S9b toward Pb2+ in the presence of competing metal ions
To evaluate the selectivity of S9b for Pb2+, 134 μL of S9b stock solution (2.3428 mM) and 5 molar equivalents (eq.) of Pb2+ were added to each of 13 vials. The mixtures were vortex-mixed for 10 s, followed by the addition of 5 eq of competing metal ions: Ag+, Co2+, Cu2+, Fe2+, Fe3+, Na+, K+, Ni2+, Hg2+, Ca2+, Zn2+, Mg2+, and Al3+, to individual vials. After vortex-mixing and incubation for 2 min at ambient temperature, each solution was diluted to 5 mL with ethanol. Absorbance measurements were recorded at 500 nm (λmax) using a UV-vis spectrophotometer. Triplicate determinations were performed, and the mean absorbance values (±standard deviation) were plotted on a bar chart to compare the response of S9b–Pb2+ complexes in the presence of interfering ions.2.9 Density functional theory
Density functional theory (DFT) calculations were conducted using the Gaussian 09 program, Revision C.01. The gas-phase geometry of S9b, initially constructed using GaussView software, was optimized using the Coulomb-attenuating hybrid exchange–correlation functional (CAM-B3LYP) with the 6-311++G (d,p) basis set, assuming a singlet spin state and neutral charge. For the S9b–Pb2+ complex, geometry optimization was performed using the LanL2DZ effective core potential and basis set (with 5D and 7F angular momentum functions) under a +2 charge state.3. Results and discussion
3.1 Synthesis of S9b (1-amino-4-[(E)-2-(8-hydroxyquinolin-5-yl)diazen-1-yl]-9,10-dihydroanthracene-9,10-dione)
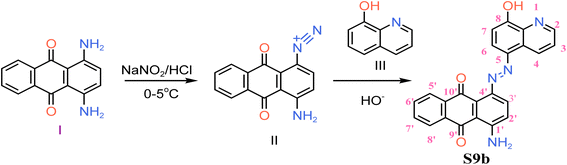
S9b was synthesized by first diazotizing of 1,4-diaminoanthracene-9,10-dione (I) at one of the aromatic amines with moderate acid concentration of 1 M hydrochloric acid and subsequent diazocoupling of the diazonium product (II) with 8-hydroxyquinoline (III) as shown in Scheme 1. Diazocoupling took place at para position to the phenolic group of (III). The chemical structure of S9b was confirmed by spectroscopic techniques.3.2 Characterization of S9b
Violet; yield: 51.55%; FTIR (ATR, ν, cm−1): C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1630), N
O (1630), N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1500), N–H (3400, 3290), C–N (1281), C
N (1500), N–H (3400, 3290), C–N (1281), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1580); 1H NMR (600 MHz, acetone): δ 7.74 (dd, J = 5.7, 3.3 Hz, 1H, H-2), 7.69 (d, J = 8.3 Hz, 1H, H-4), 7.63–7.66 (m, 1H, H-3), 7.56 (d, J = 8.6 Hz, 1H, H-3′), 7.46 (d, J = 2.4 Hz, 1H, H-6), 7.28–7.25 (m, 2H, H-5′, 8′), 7.24 (d, J = 2.6 Hz, 1H, H-2′), 7.19 (d, J = 7.1 Hz, 1H, H-7), 7.16 (dd, J = 7.3, 2.7 Hz, 2H, H-6′, 7′), 3.60 (s, 2H, H-11); 13C NMR (600 MHz, acetone): δ 139.815, 133.433, 131.981, 130.525, 130.280, 129.597, 129.066, 128.993, 128.478, 127.704, 127.433, 126.910, 126.570, 125.401, 124.858, 119.824, 114.604; MS (ESI, m/z): 395.1 [M+ + H]. Anal. calcd. C23H14O13N4: C, 70.04; H, 3.57; O, 12.18; N, 14.20; found: C, 70.10; H, 3.55; O, 12.14; N, 14.26. The spectra are shown in the ESI† (Fig. S1–S5).
N (1580); 1H NMR (600 MHz, acetone): δ 7.74 (dd, J = 5.7, 3.3 Hz, 1H, H-2), 7.69 (d, J = 8.3 Hz, 1H, H-4), 7.63–7.66 (m, 1H, H-3), 7.56 (d, J = 8.6 Hz, 1H, H-3′), 7.46 (d, J = 2.4 Hz, 1H, H-6), 7.28–7.25 (m, 2H, H-5′, 8′), 7.24 (d, J = 2.6 Hz, 1H, H-2′), 7.19 (d, J = 7.1 Hz, 1H, H-7), 7.16 (dd, J = 7.3, 2.7 Hz, 2H, H-6′, 7′), 3.60 (s, 2H, H-11); 13C NMR (600 MHz, acetone): δ 139.815, 133.433, 131.981, 130.525, 130.280, 129.597, 129.066, 128.993, 128.478, 127.704, 127.433, 126.910, 126.570, 125.401, 124.858, 119.824, 114.604; MS (ESI, m/z): 395.1 [M+ + H]. Anal. calcd. C23H14O13N4: C, 70.04; H, 3.57; O, 12.18; N, 14.20; found: C, 70.10; H, 3.55; O, 12.14; N, 14.26. The spectra are shown in the ESI† (Fig. S1–S5).
3.3 Spectroscopic response of S9b to cations
The interaction of S9b with nitrate salts of Ag+, Co2+, Cu2+, Fe2+, Fe3+, Na+, K+, Ni2+, Pb2+, Hg2+, Ca2+, Zn2+, Mg2+, and Al3+ in ethanol was investigated using UV-vis absorption spectroscopy. Free S9b exhibited a rosy-brown coloration in ethanol, which changed to a distinct sandy-brown exclusively upon addition of Pb2+ (Fig. 1a). No significant color changes were observed with other cations, even at five equivalents.UV-vis spectral analysis (Fig. 1b) further corroborated this color behavior. While the absorption profiles of S9b remained largely unperturbed in the presence of Ag+, Co2+, Cu2+, Fe2+, Fe3+, Na+, K+, Ni2+, Hg2+, Ca2+, Zn2+, Mg2+, and Al3+, Pb2+ induced a pronounced red shift accompanied by a hyperchromic effect at 500 nm. This spectral pattern is attributed to ligand-centered electronic transitions (n → π*) and potential d–d interactions facilitated by the coordination geometry of the S9b–Pb2+ complex. The wavelength of maximum absorptivity difference (500 nm) was subsequently utilized for quantitative Pb2+ determination.
The unique behavior of Pb2+ likely arises from its larger ionic radius, higher polarizability, and soft Lewis acid character, which enhance its complementarity with the portal of electron-rich phenolic hydroxyl and quinoline nitrogen moieties of S9b. Additionally, the ability of Pb2+ to adopt distorted coordination geometries due to the inert pair effect (retention of its 6s2 electrons) may stabilize distinct electronic transitions that other cations can't.
3.4 Job's plot experiment
To elucidate the binding stoichiometry between S9band Pb2+, a Job's plot of continuous variations was constructed by recording the absorbance at 500 nm across a series of solutions with varying mole ratios of S9b to Pb2+ (Fig. 2a). The plot exhibited a distinct maximum at a mole fraction of S9bof 0.67, beyond which the absorbance plateaued, indicating saturation of the interaction. This inflection point corresponds to a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry (S9b
1 binding stoichiometry (S9b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb2+), consistent with the coordination of two ligand molecules (S9b) to a single Pb2+ ion.
Pb2+), consistent with the coordination of two ligand molecules (S9b) to a single Pb2+ ion.
3.5 Response time and selection of diluting solvents
For colorimetric sensors, rapid response kinetics are critical for real-time applications. To evaluate the behavior of S9b toward Pb2+, the absorbance of the S9b–Pb2+ complex was monitored at 500 nm over 30 minutes in ethanol. As shown in Fig. 2b, the absorbance increased sharply within the first 2 minutes, reaching a maximum intensity, followed by a gradual decline. This suggests that the binding equilibrium between S9b and Pb2+ is established rapidly, with optimal signal stability achieved at 2 minutes. The subsequent decrease in absorbance may arise from slow ligand dissociation under prolonged ambient conditions. Therefore, the reaction between S9b and Pb2+ was allowed to stay for 2 minutes.The performance of S9b as a Pb2+ sensor was also evaluated across solvents of varying polarity and donor properties, including ethanol, methanol, acetone, DMSO, and DMF. Notably, the S9b–Pb2+ complex exhibited no significant variation in coloration or absorbance intensity at 500 nm across these solvents. Hence, ethanol was selected as the reaction medium due to its low toxicity, biodegradability, and cost-effectiveness, aligning with green chemistry principles.
3.6 Effects of pH on reaction between S9b and lead(II) ions
Azo-based chemosensors are inherently sensitive to pH variations due to the protonation equilibria of their functional groups, which modulate electronic transitions and metal-binding affinity.33 To evaluate the pH response of S9b as a Pb2+ sensor, the absorbance response was investigated across a pH range of 4–10 (Fig. 2c). The sensor exhibited stable absorbance values between pH 4 and 6, followed by a sharp increase at pH 8, with maximal signal intensity observed under basic conditions.This pH-dependent behavior is attributed to structural and electronic changes in S9b. In alkaline media (pH > 7), deprotonation of the hydroxyl group on the 8-hydroxyquinoline moiety generates a phenoxide ion, enhancing electron delocalization across the conjugated azo-chromophore. The resulting electron-rich phenoxide strengthens coordination to Pb2+ via lone pair donation, likely forming a stable chelate complex that amplifies ligand-to-metal charge transfer (LMCT) transitions. Conversely, under acidic conditions (pH < 6), protonation of the azo bridge (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) disrupts conjugation, diminishing the chromophore's π-electron system and reducing its ability to engage in electronic transitions, thereby lowering absorbance.
N–) disrupts conjugation, diminishing the chromophore's π-electron system and reducing its ability to engage in electronic transitions, thereby lowering absorbance.
Notably, while the highest absorbance occurred at pH 8, practical considerations necessitated optimum pH 6 for Pb2+ determination. Basic pH levels risk hydroxide precipitation of Pb2+ (as Pb(OH)2), complicating quantification, whereas overly acidic conditions impair sensor performance. The choice of pH 6 balances optimal chromophore activity with minimized interference from lead hydrolysis, ensuring reliable detection in environmentally relevant aqueous systems.
3.7 Analytical performance of S9b for Pb2+ detection
The quantitative relationship between the absorption intensity of S9b and Pb2+ concentration was established via a linear regression analysis (Fig. 2d). The sensor exhibited a linear response (R2 = 0.9967) within the Pb2+ concentration range of 3.90–9.36 μg mL−1, as described by the equation: y = 0.0017x + 0.3993 (n = 3). Where y represents absorbance and x is Pb2+ concentration (μg mL−1). The high correlation coefficient adheres to the ICH Q2(R1) guideline requirement for linearity (R2 ≥ 0.98),36 validating the method's suitability for quantitative analysis.The calculated binding constant (k), 3.07 × 104 L2 g−2, was obtained from modified Benesi–Hildebrand's eqn (3) based on 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry:37 The plot of
1 stoichiometry:37 The plot of  against
against 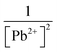 is shown in the ESI (Fig S6†).
is shown in the ESI (Fig S6†).
 | (3) |
Further modification of eqn (3) gives eqn (4)
 | (4) |
 | (5) |
The constant reflects strong affinity between S9b and Pb2+, consistent with reported values for Pb2+ sensors.38,39 Sandell's sensitivity, a measure of method detectability, was calculated as 58.823 ng cm−2, indicating that a low concentration of Pb2+ can induce a measurable absorbance change.
The limit of detection (LOD) and limit of quantification (LOQ) were determined using the formulae 3.3 σ/s and 10σ/s, respectively, where σ is the standard deviation of the intercept of the calibration equation and s is the slope of the calibration curve. The LOD and LOQ values of 1.55 μg mL−1 and 4.71 μg mL−1, respectively, demonstrate S9b's capability to detect Pb2+ at the part-per-million (ppm) level. While this sensitivity aligns with conventional colorimetric sensors, future modifications to the S9b structure by introducing electron-withdrawing groups or auxiliary binding sites could enhance detection to sub-ppm levels for trace environmental or biological applications.
3.8 Binding mechanism of S9b with lead(II)ions
To probe the binding mechanism between S9b and Pb2+, Fourier-transform infrared (FTIR) spectroscopy was conducted. Comparative analysis of the FTIR spectra of free S9b and its Pb2+ complex (Fig. 3) revealed distinct shifts in key vibrational modes. The C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the azo-quinoline moiety, originally observed at 1281 cm−1 and 1580 cm−1 in free S9b, shifted to 1270 cm−1 and 1560 cm−1, respectively, in the Pb2+ complex. These shifts indicate electron donation from the nitrogen and –OH groups of quinoline to Pb2+, weakening the bond strengths and reducing their vibrational frequencies.
N stretching vibrations of the azo-quinoline moiety, originally observed at 1281 cm−1 and 1580 cm−1 in free S9b, shifted to 1270 cm−1 and 1560 cm−1, respectively, in the Pb2+ complex. These shifts indicate electron donation from the nitrogen and –OH groups of quinoline to Pb2+, weakening the bond strengths and reducing their vibrational frequencies.
Notably, the O–H bending vibration at 1371 cm−1, associated with the phenolic hydroxyl group of 8-hydroxyquinoline in free S9b, diminished significantly in the complex, suggesting deprotonation of the hydroxyl group upon coordination to Pb2+. This is further corroborated by the emergence of new vibrational bands at 567 cm−1 and 440 cm−1, assigned to Pb–O and Pb–N stretching modes, respectively. These findings confirm direct coordination of Pb2+ to both the deprotonated phenolic oxygen and the quinoline nitrogen of S9b.
In contrast, the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration of the anthraquinone core remained unaltered in both free S9b and its Pb2+ complex, indicating no involvement of the ketonic oxygen in metal binding. This observation aligns with prior studies on anthraquinone-based sensors, where the carbonyl group retains its electronic independence during complexation.39,40 The FTIR data support a binding mechanism wherein Pb2+ interacts selectively with the nitrogen and the deprotonated phenolic oxygen of the 8-hydroxyquinoline moiety. This bidentate coordination mode enhances the stability of the S9b–Pb2+ complex, consistent with the 2
O) stretching vibration of the anthraquinone core remained unaltered in both free S9b and its Pb2+ complex, indicating no involvement of the ketonic oxygen in metal binding. This observation aligns with prior studies on anthraquinone-based sensors, where the carbonyl group retains its electronic independence during complexation.39,40 The FTIR data support a binding mechanism wherein Pb2+ interacts selectively with the nitrogen and the deprotonated phenolic oxygen of the 8-hydroxyquinoline moiety. This bidentate coordination mode enhances the stability of the S9b–Pb2+ complex, consistent with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry inferred from Job's plot analysis (Fig. 2a) and the UV-vis spectral changes.
1 stoichiometry inferred from Job's plot analysis (Fig. 2a) and the UV-vis spectral changes.
3.9 Reversibility and reusability of S9b
To assess the reversibility of the S9b–Pb2+ interaction, the complex was treated with disodium ethylenediaminetetraacetate (Na2EDTA), a strong chelating agent with high affinity for Pb2+. Upon addition of Na2EDTA, the hyperchromic absorbance band of the S9b–Pb2+ complex at 500 nm diminished completely, and the resulting spectrum became superimposable with that of free S9b (Fig. 4). This spectral restoration confirms the displacement of Pb2+ from the complex by EDTA, regenerating unbound S9b through competitive chelation.The reversibility of the binding interaction underscores the dynamic equilibrium between S9b and Pb2+, while the restoration of the ligand's original spectral profile demonstrates its structural integrity post-metal release. These findings suggest that S9b can be reused in sensing applications, as the ligand retains its functionality after Pb2+ removal. Furthermore, the ability to regenerate S9b via EDTA treatment highlights its potential for recyclability in cost-effective or continuous monitoring systems.
3.10 Analytical application of S9b in environmental samples
The azo-based chemosensor S9b was successfully validated for the determination of Pb2+ in three environmental water samples (well, river, and tap water). Initial analysis confirmed the absence of detectable Pb2+ in unspiked samples. To evaluate accuracy and precision, known concentrations of Pb2+ were spiked into the samples, and recoveries were calculated using both S9b and atomic absorption spectroscopy (AAS) (Table 1).| Water source | Spiked (mg L−1) | S9b method | AAS | t test | F test | ||
|---|---|---|---|---|---|---|---|
| % Recoverya | RSD | % Recoverya | RSD | ||||
| a Quadruplicate determinations. | |||||||
| Well | 4.68 | 103.11 | 0.50 | 99.11 | 0.10 | ||
| 7.02 | 103.28 | 0.46 | 101.37 | 0.15 | 0.88 | 0.93 | |
| 8.58 | 100.19 | 0.64 | 99.63 | 0.06 | |||
| River | 4.68 | 103.63 | 0.31 | 100.35 | 0.05 | ||
| 7.02 | 102.12 | 0.12 | 101.18 | 0.12 | 0.99 | 0.79 | |
| 8.58 | 100.89 | 1.16 | 103.84 | 0.15 | |||
| Tap | 4.68 | 102.57 | 2.07 | 101.25 | 1.25 | ||
| 7.02 | 100.74 | 0.54 | 102.07 | 0.98 | 0.79 | 0.27 | |
| 8.58 | 100.03 | 0.06 | 103.18 | 0.37 | |||
The S9b method demonstrated excellent accuracy, with recovery percentages ranging from 100.03% to 103.11%, and high precision, reflected by relative standard deviation (RSD) values of 0.06% to 2.07%. These results affirm the practicality of S9b for Pb2+ quantification in aqueous matrices. Statistical validation via F- and t-tests further confirmed the method's reliability: p-values exceeding 0.05 for both tests indicated no significant difference between the means and variances of the S9b and AAS datasets. Furthermore, this statistical equivalence underscores S9b as a viable alternative to AAS for Pb2+ detection in environmental monitoring.
3.11 Competition experiments
To evaluate the selectivity of S9b for Pb2+ in complex matrices, competition experiments were conducted with potential interfering cations (Ag+, Co2+, Cu2+, Fe2+, Fe3+, Na+, K+, Ni2+, Hg2+, Ca2+, Zn2+, Mg2+, and Al3+). The absorbance intensity of the S9b–Pb2+ complex at 500 nm was monitored in the presence of these cations at 5 eq concentrations to Pb2+ (Fig. 5). Notably, the absorbance signal for Pb2+ remained largely unaffected by the coexistence of competing ions, even at elevated concentrations. This insensitivity to interference underscores the selectivity of S9b for Pb2+. These findings confirm that S9b retains its analytical performance in multicomponent systems, making it suitable for Pb2+ detection in environmentally water samples where competing ions are ubiquitous. | ||
| Fig. 5 Absorption changes of S9b (24.74 μg mL−1) with Pb2+ (5 equivalents) and metal ions (5 equivalents) in ethanol. | ||
3.12 DFT studies
To corroborate the proposed 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry (S9b
1 binding stoichiometry (S9b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pb2+) inferred from Job's plot analysis, density functional theory (DFT) calculations were performed on S9b and its Pb2+ complex. The optimized geometries of free S9b and the S9b–Pb2+ complex (Fig. 6a and 7a) revealed significant structural reorganization upon metal coordination. Free S9b adopts a twisted conformation, evidenced by a dihedral angle (N39–C25–C30–O37) of 0.2396°, which transitions to a fully planar configuration in the Pb2+ complex. This planarization facilitates chelation of Pb2+ via the nitrogen of the quinoline heterocycle and the deprotonated phenolic oxygen, forming a stable six-membered coordination ring.
Pb2+) inferred from Job's plot analysis, density functional theory (DFT) calculations were performed on S9b and its Pb2+ complex. The optimized geometries of free S9b and the S9b–Pb2+ complex (Fig. 6a and 7a) revealed significant structural reorganization upon metal coordination. Free S9b adopts a twisted conformation, evidenced by a dihedral angle (N39–C25–C30–O37) of 0.2396°, which transitions to a fully planar configuration in the Pb2+ complex. This planarization facilitates chelation of Pb2+ via the nitrogen of the quinoline heterocycle and the deprotonated phenolic oxygen, forming a stable six-membered coordination ring.
 | ||
| Fig. 6 (a). Optimized geometry of S9b; molecular orbitals of energy levels of (b). HOMO (c). HOMO+1 (d). HOMO+2 (e). LUMO (f). LUMO+1 (g). LUMO+2. | ||
 | ||
| Fig. 7 (a). Optimized geometry of S9b–Pb2+; molecular orbitals of energy levels of (b). HOMO (c). HOMO+1 (d). HOMO+2 (e). LUMO (f). LUMO+1 (g). LUMO+2. | ||
Electronic structure analysis further elucidated the spectral behavior of the complex. The energy gaps (ΔE) for the three lowest-energy transitions (HOMO → LUMO, HOMO+1 → LUMO+1, HOMO+2 → LUMO+2) exhibited an ascending trend (Fig. 6 and 7), with ΔE values for S9b–Pb2+ consistently lower than those of free S9b. This reduction in ΔE aligns with the observed bathochromic shift in UV-vis spectra, as smaller energy gaps correlate with longer absorption wavelengths.41 Additionally, the enhanced oscillator strength of these transitions explains the hyperchromic effect in the complex, attributed to intensified ligand-to-metal charge transfer (LMCT) upon Pb2+ binding. The DFT results rationalize the experimental UV-vis and FTIR data, affirming the proposed 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and binding mode (Fig. 2, inset). The planarization of S9b, coupled with electronic delocalization in the Pb2+ complex, underscores the ligand's electronic complementarity for selective Pb2+ sensing.
1 stoichiometry and binding mode (Fig. 2, inset). The planarization of S9b, coupled with electronic delocalization in the Pb2+ complex, underscores the ligand's electronic complementarity for selective Pb2+ sensing.
3.13 Comparison of S9b method with prior approaches
Table 2 summarizes the response time, limit of detection (LOD), chemosensing millieu, and applications of the anthracene-9,10-dione azo dye sensor (S9b) alongside prior Pb(II) detection methods. Similar to existing approaches, the performance of S9b was pH-dependent; however, its design integrated practicality and environmental relevance, distinguishing it from conventional systems. The synthesis of S9b employed a straightforward diazotization and diazo-coupling protocol, contrasting with the laborious, nanoscience-intensive procedures reported by Saputri et al.,42 Ratnarathorn et al.,44 and Wang et al.47| Sensor | Solvent | Reaction time | LOD (μg mL−1) | Analytical range (μg mL−1) | Real samples |
|---|---|---|---|---|---|
| a Unit in μM. | |||||
| Gold nanoparticle42 | Glycine–NaOH buffer | 10 min | 9.5 | 0–10 | Lake |
| Probe-infused polymer monolithic43 | Solid state | 40 s | 2.8 × 10−4 | 0–0.3 | Cigarette samples and water |
| Azobenzene26 | Acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
30 min | 5.44a | NA | Natural water, live water flea |
| Functionalized GNP44 | Aqueous | 15 min | 5 × 10−4 | 0–0.01 | Breast milk, drinking water |
| Schiff base45 | MeOH/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 s | 6.78 × 10−3 | 6 × 10−3 to 41.2a | Water |
| Anthracene derived chalcone39 | MeOH/H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) 1 (v/v) |
5 min | 0.94a | NR | Ground and sewage water |
| Rhodamine 6G46 | Acetonitrile–water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) 1 (v/v) |
<1 min | 2.7 × 10−3 a | 0.01–10a | Sea shell foods |
| Fluophors-gold nanoparticle47 | Aqueous | 40 min | 1.1 × 10−4 | 0.0016–0.021 | Waste and tap water |
| Curcumin cyclohexanone37 | Aqueous | 5 min | 0.5a | 2–20a | Tap and river |
| Gold nano clusters48 | Aqueous | 1 min | 50a | NR | Deionized water |
| Anthracene-9,10-dione azo dye (this study) | Ethanol | 2 min | 1.55 (7.49a) | 3.90–9.36 | River, tap and well |
The S9b sensor exhibited a rapid response time of 2 min for Pb(II) detection in aqueous samples, significantly saving analytical time than the methods of Wang et al. (30 min),26 Ratnarathorn et al. (15 min),44 and Wang et al. (40 min).47 Nevertheless, solid-state composite systems, such as the polymer monolithic probe by Sivaraman et al.43 and the Rhodamine 6G-based strategy by Wan et al.,46 achieved sub-minute response times owing to enhanced structural templating that facilitates faster Pb(II) diffusion to binding sites.
Environmental compatibility is a key advantage of S9b, as ethanol—a green, water-miscible solvent—serves as the sensing medium. In contrast, methods by Wang et al.,26 Wan et al.,46 and Prabhu et al.39 relied on acetonitrile or methanol, which pose ecological and operational challenges for on-site applications. The LOD of S9b (1.5 μg mL−1) rendered it suitable for monitoring moderately contaminated water systems (ppm-level detection), bridging a critical gap between sensitivity and practicality. While this LOD surpassed those of Saputri et al. (9.5 μg mL−1)42 and Bian et al. (50 μM),48 some colorimetric methods exhibited superior sensitivity.43,44,46
4. Conclusion
We present S9b, a new anthracene-9,10-dione-based azo dye chemosensor for selective and sensitive Pb2+ detection in environmental waters. Operating optimally in ethanol (pH 6, 2 min response), S9b exhibited a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry with Pb2+, validated by Job's plot. The sensor demonstrated selectivity for Pb2+ against common interfering cations, reusability via EDTA displacement, and statistical equivalence to AAS in accuracy and precision. FTIR and DFT studies revealed Pb2+ coordination through the quinoline nitrogen and deprotonated phenolic oxygen, with spectral shifts rationalized by reduced HOMO–LUMO energy gaps.
1 binding stoichiometry with Pb2+, validated by Job's plot. The sensor demonstrated selectivity for Pb2+ against common interfering cations, reusability via EDTA displacement, and statistical equivalence to AAS in accuracy and precision. FTIR and DFT studies revealed Pb2+ coordination through the quinoline nitrogen and deprotonated phenolic oxygen, with spectral shifts rationalized by reduced HOMO–LUMO energy gaps.
While S9b shows promise, its detection limit (1.55 ppm) and ethanol dependency limit trace-level analysis in aqueous systems. Future efforts should prioritize structural modifications with electron-withdrawing groups to enhance sub-ppm sensitivity and aqueous compatibility. This study advances anthracene-9,10-dione-based chemosensor design and underscores molecular engineering's role in addressing environmental health challenges through sustainable sensing technologies.
Data availability
The data used to support the findings of the study are available in the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the RSC Research Fund grant (R22-2366730013) awarded to S. O. O. to carry out the study. The provision of an enabling environment to conduct this research by Dr Babatunde Akeem Saka, the director of the Centre for Biomedical Research Initiatives, and the assistance of Dr Maria Aqeel Khan of the Third World Center for Science and Technology, H. E. J. Research Institute of Chemistry, and International Center for Chemical and Biological Sciences are well acknowledged.References
- M. S. Collin, S. K. Venkatraman, N. Vijayakumar, V. Kanimozhi, S. M. Arbaaz, R. G. S. Stacey, J. Anusha, R. Choudhary, V. Lvov, G. I. Tovar, F. Senatov, S. Koppala and S. Swamiappan, J. Hazard. Mater. Adv., 2022, 7, 100094 CAS.
- J. E. Gall, R. S. Boyd and N. Rajakaruna, Environ. Monit. Assess., 2015, 187, 1–21 CrossRef CAS PubMed.
- A. Violante, V. Cozzolino, L. Perelomov, A. G. Caporale and M. Pigna, J. Soil Sci. Plant Nutr., 2010, 10, 268–292 Search PubMed.
- N. Gupta, D. K. Khan and S. C. Santra, Bull. Environ. Contam. Toxicol., 2008, 80, 115–118 CrossRef CAS PubMed.
- F. Edition, WHO Chron., 2011, 38, 104–108 Search PubMed.
- S. J. S. Flora, G. Flora and G. Saxena, in Lead, ed. J. S. Casas and J. Sordo, Elsevier Science BV, 2006, vol. 4, pp. 158–228 Search PubMed.
- H. M. Wu, D.-T. Lin-Tan, M.-L. Wang, H.-Y. Huang, C.-L. Lee, H.-S. Wang, Y.-K. Soong and J. L. Lin, Reprod. Biol. Endocrinol., 2012, 10, 9 CrossRef PubMed.
- D. C. Bellinger, Birth Defects Res., Part A, 2005, 73, 409–420 CrossRef CAS PubMed.
- C. G. Yedjou, C. K. Tchounwou, S. Haile, F. Edwards and P. B. Tchounwou, Ethn. Dis., 2010, 20, 101 Search PubMed.
- S. L. Zhao, F. S. Chen, J. Zhang, S. B. Ren, H. D. Liang and S. S. Li, J. Ind. Eng. Chem., 2015, 27, 362–367 CrossRef CAS.
- G. Billon and C. M. G. Van Den Berg, Electroanalysis, 2004, 16, 1583–1591 CrossRef CAS.
- M. Sha, Y. Xiaomei and C. Xiumin, Chem. J. Internet, 2007, 9(7), 31 Search PubMed.
- N. C. Munksgaar, G. J. Batterham and D. L. Parry, Mar. Pollut. Bull., 1998, 36, 527–534 CrossRef.
- M. R. Cave, O. Butler, J. M. Cook, M. S. Cresser, L. M. Garden and D. L. Miles, J. Anal. At. Spectrom., 2000, 15, 181–235 RSC.
- M. R. Cave, O. Butler, S. R. N. Chenery, J. M. Cook, M. S. Cresser and D. L. Miles, J. Anal. At. Spectrom., 2001, 16, 194–235 RSC.
- S. C. Wilschefski and M. R. Baxter, Clin. Biochem. Rev., 2019, 40, 115–133 Search PubMed.
- S. Sarkar, A. Banerjee, H. Urmi, B. Raju and B. Rajib, Water Conserv. Sci. Eng., 2017, 2, 121–131 CrossRef.
- K. M. Lee, X. Chen, W. Fang, J. M. Kim and J. Yoon, Macromol. Rapid Commun., 2011, 32, 497–500 CrossRef CAS PubMed.
- H. Zheng, X. Q. Zhan, Q. N. Bian and X.-J. Zhang, Chem. Commun., 2013, 49, 429–447 RSC.
- H. Son, G. Kang and J. H. Jung, Analyst, 2012, 137, 163–169 RSC.
- P. Chen, B. Greenberg, S. Taghavi, C. Romano, D. van der Lelie and C. He, Angew. Chem., Int. Ed., 2005, 44, 2715–2719 CrossRef CAS PubMed.
- Z. Hu, C. Lin, X. Wang, L. Ding, C. Cui, S. Liu and H. Y. Lu, Chem. Commun., 2010, 46, 3765–3767 RSC.
- M. Liu, X. Lou, J. Du, M. Guan, J. Wang, X. Ding and J. Zhao, Analyst, 2012, 137, 70–72 RSC.
- Y. Chen and J. Jiang, Org. Biomol. Chem., 2012, 10, 4782–4787 RSC.
- M. Ghorbanian, S. Asghari and M. Tajbakhsh, Spectrochim. Acta, Part A, 2023, 296, 122652 CrossRef CAS PubMed.
- Y. Wang, S. Hu, Y. Zhang, H. Gong, R. Sun, W. Mao, D. Wang and Y. Chen, J. Photochem. Photobiol., A, 2018, 355, 101–108 CrossRef CAS.
- A. K. K. Bhasin, P. Chauhan and S. Chaudhary, Sens. Actuators, B, 2019, 294, 116–122 CrossRef CAS.
- P. Kumar, A. Ghosh and D. A. Jose, Analyst, 2019, 144, 594–601 RSC.
- M. Barzegar, M. F. Mousavi, H. Khajehsharifi, M. Shamsipur and H. Sharghi, IEEE Sens. J., 2005, 5, 392–397 CAS.
- A. Rahmani, M. Barzegar, M. Shamsipur, H. Sharghi and M. F. Mousavi, Anal. Lett., 2000, 33, 2611–2629 CrossRef CAS.
- S. Riahi, M. F. Mousavi, M. Shamsipur and H. A. Sharghi, Electroanalysis, 2003, 15, 1561–1565 CrossRef CAS.
- X. Li, X. Ma and M. Huang, Talanta, 2009, 78, 498–505 CrossRef CAS PubMed.
- S. O. Olaleye, S. O. Idowu, A. Shakil and M. A. Khan, RSC Adv., 2025, 15, 3497–3514 RSC.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Angew. Chem., 2013, 52, 11998–12013 CrossRef CAS PubMed.
- A. Shrivastava and V. B. Gupta, Chron. Young Sci., 2011, 2, 21–25 CrossRef.
- European Medicines Agency, ICH guideline Q2(R2) on validation of analytical procedures, https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf, accessed 20 May 2025.
- M. Sadia, J. Khan, R. Khan, S. W. A. Shah, A. Zada, M. Zahoor, R. Ullah and E. A. Ali, Heliyon, 2025, 11, e41125 CrossRef CAS PubMed.
- T. Anand, G. Sivaraman, A. Mahesh and D. Chellappa, Anal. Chim. Acta, 2015, 853, 596–601 CrossRef CAS PubMed.
- J. Prabhu, K. Velmurugan and R. Nandhakumar, Spectrochim. Acta, Part A, 2015, 144, 23–28 CrossRef CAS PubMed.
- S. Kim, S. Gwon and J. Bae, J. Fiber Soc., 2014, 70, 254–257 CrossRef CAS.
- L. Hou, X. Kong, Y. Wang, J. Chao, C. Li, C. Dong, Y. Wang and S. Shuang, J. Mater. Chem. B, 2017, 5, 8957–8966 RSC.
- F. A. Saputri, T. S. Aulia, C. Jatmika, R. Iswandana, S. Megantara and V. A. Dhumale, RSC Adv., 2025, 15, 6931–6937 RSC.
- S. P. Sivaraman, P. Srinivasan, D. K. Madhu, P. Deivasigamani and A. M. Mohan, J. Hazard. Mater., 2025, 487, 137247 CrossRef PubMed.
- N. Ratnarathorn, O. Chailapakul and W. Dungchai, Talanta, 2015, 132, 613–618 CrossRef CAS PubMed.
- N. Karachi, O. Azadi, R. Razavi, A. Tahvili and Z. Parsaee, J. Photochem. Photobiol., A, 2018, 360, 152–165 CrossRef CAS.
- J. Wan, K. Zhang, C. Li, Y. Li and S. Niu, Sens. Actuators, B, 2017, 246, 696–702 CrossRef CAS.
- S. Wang, J. Sun and F. Gao, Analyst, 2015, 140, 4001–4006 RSC.
- R. Bian, X. Wu, F. Chai, L. Li, L. Zhang, T. Wang, C. Wang and Z. Su, Sens. Actuators, B, 2017, 241, 592–600 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02486b |
| This journal is © The Royal Society of Chemistry 2025 |