Open Access Article
Open Access ArticleSynthesis, structural characterization, and DFT investigation of a mixed-valence Co(III)/Co(II) complex stabilized by supramolecular interactions†
Susovan Beraa,
Sudip Bhuniaa,
Rosa M. Gomila b,
Antonio Frontera
b,
Antonio Frontera b and
Shouvik Chattopadhyay
b and
Shouvik Chattopadhyay *a
*a
aDepartment of Chemistry, Inorganic Section, Jadavpur University, Kolkata 700032, India. E-mail: shouvik.chattopadhyay@jadavpuriuniversity.in; Tel: +91-33-24572941
bDepartament de Químca, Universitt de les Illes Balears, Crta de Valldemossa km 7.5, 07122 Palma de Mallorca, Baleares, Spain. E-mail: toni.frontera@uib.es
First published on 12th May 2025
Abstract
A dinuclear mixed-valence cobalt(III/II) complex, [(DMSO)(SCN)CoIIILCoII(NCS)2(OH2)]·DMF, has been synthesized and structurally characterized by elemental analysis, spectroscopy, and single-crystal X-ray diffraction. The structure reveals a hexa-coordinated cobalt(III) center in an octahedral geometry and a penta-coordinated cobalt(II) center adopting a square pyramidal geometry. To support the oxidation state assignment, a spin density analysis was carried out, confirming spin localization on the cobalt(II) center. Additionally, a comprehensive DFT study was performed to evaluate key supramolecular interactions in the solid state. Theoretical analysis of selected assemblies using molecular electrostatic potential (MEP) mapping, QTAIM, and NCIplot methods reveals the energetic and directional features of dominant hydrogen bonds, including NH⋯S and OH⋯O interactions. The substantial interaction energies (up to −31.4 kcal mol−1) and topological descriptors underscore the structure-directing role of these noncovalent contacts in the formation of one-dimensional supramolecular chains.
Introduction
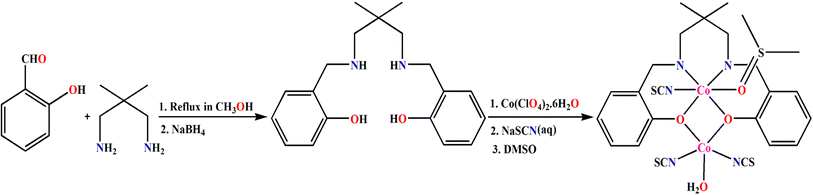
Polynuclear complexes of different transition and non-transition metals have attracted the interest of inorganic chemists and material scientists due to their amazing photo-catalytic and bio-mimicking catalytic activities, potential energy applications, ability to detect different trace elements and explosives, fascinating magnetic properties, applications in opto-electronics and medicinal chemistry, etc.1–15 Among them, complexes of cobalt represent a unique class due to their ability to show SMM (single molecule magnet) and field induced SMM behaviors.16–23 The use of cobalt complexes as catalysts for the oxidation of water to oxygen under sunlight thereby producing clean and sustainable energy is well established.24–28 Schiff bases and their reduced analogues have also been used by several research groups to produce mixed valence complexes (containing both +II and +III oxidation states) of cobalt.29–31 Most of the complexes are dinuclear or trinuclear, although tetranuclear complexes are also reported in literature.32–41 The SMM behavior or field induced SMM behaviour of many di and trinuclear mixed valence cobalt(III/II) complexes with Schiff base or reduced Schiff base ligands is also reported in literature.42,43 The complexes have also been used to fabricate different opto-electronic devices.44–46 Many cobalt complexes were shown to have different bio-mimicking catalytic abilities, e.g. phosphatase mimicking activity (to catalyze the hydrolysis of (4-nitrophenyl)phosphate ester), catechol mimicking activity (to catalyze the aerial oxidation of 3,5-DTBC to 3,5-DTBQ (3,5-di-tert-butylbenzoquinone)), phenoxazinone synthase mimicking activity (to catalyze the aerial oxidation of o-aminophenol to 2-aminophenoxazine-3-one) etc.47–52 Different non-covalent interactions, e.g. H-bonding, CH⋯π, π⋯π, cation⋯π, anion⋯π interactions etc. in the solid state structures of the complexes play an important role to grow different supramolecular architectures.53–61 Crystal engineers, theoretical chemists and supramolecular chemists are interested to analyze different supramolecular structures in their solid state structures and like to estimate the energy of different interactions.62–67In the present work, a reduced Schiff base ligand, (2,2-dimethyl-1,3-propanediyl)bis(iminomethylene)bis(phenol) (H2L), was synthesized and employed to prepare a dinuclear, mixed-valence cobalt complex, [(DMSO)(SCN)CoIIILCoII(NCS)2(OH2)]·DMF. The complex has been fully characterized, including single-crystal X-ray diffraction analysis, which reveals distinct coordination environments for the cobalt(III) and cobalt(II) centers. To gain deeper insight into the electronic structure and the supramolecular organization in the solid state, we carried out a detailed theoretical study. Spin density mapping was used to confirm the oxidation state distribution, while molecular electrostatic potential (MEP) analysis, QTAIM, and NCIplot methods were employed to elucidate the nature and strength of key intermolecular interactions.
In this context, our motivation was to synthesize and characterize a new dinuclear mixed-valence cobalt(III/II) complex stabilized by supramolecular interactions. Mixed-valence cobalt systems are of considerable interest due to their potential applications in catalysis, molecular magnetism, and materials science.29,37,38 Moreover, studying the role of hydrogen bonding and other weak intermolecular interactions provides important insights into the supramolecular assembly of such complexes, which can be exploited to tailor functional properties. Although the present work is focused on fundamental structural and bonding aspects, the complex synthesized could serve as a promising candidate for future studies aimed at developing catalysts or molecular magnetic materials.
Experimental
Materials
The chemicals used for the synthesis of the complex were purchased from Sigma-Aldrich, India. No further purification of the materials was required prior to their use.Caution! Although not encountered during experimental work, perchlorate salts of metal complexes with organic ligands are potentially explosive. Only a small amount of material should be prepared and it should be handled with care.
The methanolic solution of the dicondensed reduced Schiff base ligand H2L (approximately 2 mmol), was then added to a 5 mL methanolic solution of Co(ClO4)2·6H2O (∼2 mmol, 730 mg; purity: ≥98%) and the resulting reaction mixture was stirred at room temperature for 1 h to produce a brown colour solution. Then 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol/water solution (∼5 mL) of sodium thiocyanate (80 mg, ∼1 mmol; purity: ≥98%) was added to the resulting solution followed by the dropwise addition of DMF (purity: ≥99%) with constant stirring for another 1 h then the reaction mixture was allowed to stand overnight. Dark single crystals of the complex started to appear at the bottom of the beaker within a few days and was collected by filtration and then dried in open atmosphere at room temperature.
1 (v/v) methanol/water solution (∼5 mL) of sodium thiocyanate (80 mg, ∼1 mmol; purity: ≥98%) was added to the resulting solution followed by the dropwise addition of DMF (purity: ≥99%) with constant stirring for another 1 h then the reaction mixture was allowed to stand overnight. Dark single crystals of the complex started to appear at the bottom of the beaker within a few days and was collected by filtration and then dried in open atmosphere at room temperature.
The chemical composition was confirmed by elemental analyses (carbon, hydrogen and nitrogen) by using a PerkinElmer 240C elemental analyzer. IR spectrum in KBr (4500 to 500 cm−1) was recorded with a PerkinElmer RX-1 FTIR. The electronic absorption spectrum (800–200 nm) in acetonitrile solution was collected using a SHIMADZU UV1900i, UV-vis spectrophotometer.
Yield: 464 mg (∼60%) based on Cobalt. Anal. Calc. for C27H39Co2N6O5S4 (FW: 773.78): C, 41.9; H, 5.08; N, 10.86. Found: C, 42.0; H, 5.1; N, 10.9%, FT-IR (KBr, cm−1): 3411 (νOH), 3158 (νN–H), 2977–2932 (νC–H), 2071 (νNCS). UV-vis, λmax (nm), [εmax (L mol−1 cm−1)] (acetonitrile): 262 (1.6 × 104), 345 (7.1 × 103), 622 (0.82 × 103). Magnetic moment: 4.98 BM.
Crystal data: C24H32Co2N5O4S4, C3H7NO; FW = 773.78; monoclinic space group P21/n, a = 14.7901(13) Å; b = 15.1095(12) Å; c = 16.3477(14) Å, β = 105.661(3), V = 3517.6(5) Å3, Z = 4, DCalc = 1.461 gm cm−3, F(000) = 1604, mu = 1.224 mm−1. The data were collected at 273 K under the flow of liquid nitrogen using a ‘Bruker D8 QUEST area detector’ diffractometer equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Numbers of independent reflections = 7761. The molecular structure was solved by direct method and refined by full-matrix least squares on F2 using SHELXL-97 to R1 = 0.1303, wR2 = 0.2126 using 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 reflections for the complex.68 R(int) = 0.097, no. of parameters = 407. [R1 = Σ‖Fo| − |Fc‖/Σ|Fo|, wR2 = Σw(|Fo|2 − |Fc|2)2/Σw(|Fo|2)1/2] non-hydrogen atoms were refined with anisotropic thermal parameters. The hydrogen atoms attached to nitrogen were located by difference Fourier maps and were kept at fixed positions. All other hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms. Multi-scan empirical absorption corrections were applied to the data using the program SADABS.69
550 reflections for the complex.68 R(int) = 0.097, no. of parameters = 407. [R1 = Σ‖Fo| − |Fc‖/Σ|Fo|, wR2 = Σw(|Fo|2 − |Fc|2)2/Σw(|Fo|2)1/2] non-hydrogen atoms were refined with anisotropic thermal parameters. The hydrogen atoms attached to nitrogen were located by difference Fourier maps and were kept at fixed positions. All other hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms. Multi-scan empirical absorption corrections were applied to the data using the program SADABS.69
The PBE0 hybrid functional was selected for all calculations because of its well-established performance in accurately predicting electronic structures, spin distributions, and noncovalent interaction energies in transition-metal complexes.73 In particular, PBE0 is known to offer a balanced treatment of exchange and correlation effects, which is critical for systems involving both localized and delocalized electronic features. The addition of the D4 dispersion74 correction ensures a reliable description of noncovalent interactions, which are central to this study. Previous benchmarks have demonstrated the good accuracy of PBE0 for coordination compounds and supramolecular assemblies involving first-row transition metals.80
Results and discussion
Synthesis
The Schiff base and reduced Schiff base ligand {(2,2-dimethyl-1,3-propanediyl)bis(iminomethylene)bis(phenol) (H2L)}, was synthesized following the reported method.81–84 The reduced Schiff base ligand was then mixed with Co(ClO4)2·6H2O and sodium thiocyanate in methanol with constant stirring at room temperature to yield the complex. Synthetic route to the formation of the complex has been shown in Scheme 1.[[(DMSO)(NCS)CoIIILCoII(NCS)2(OH2)](DMF)]. The single crystal X-ray structural analysis indicates that the complex crystallizes in the monoclinic, P21/n space group. The structure of the complex is shown in Fig. 1 with the selective atomic numbering scheme. The oxidation states of Co(1) and Co(2) have been assigned as +III and +II respectively. Discrimination between cobalt(III) and cobalt(II) centers is based on bond length considerations.
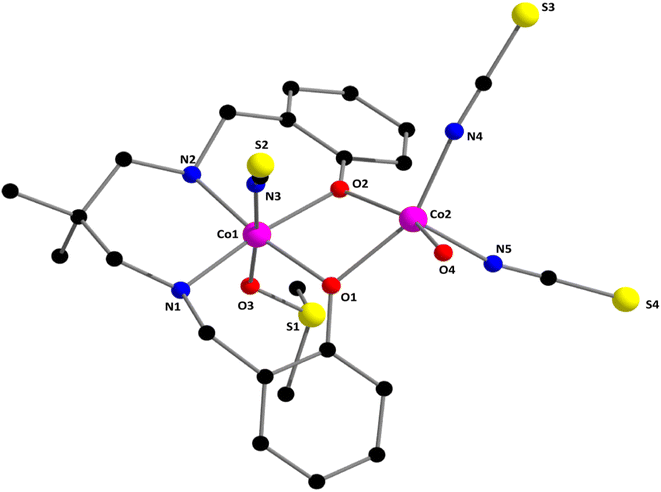 | ||
| Fig. 1 Perspective view of complex 1 with selective atom numbering scheme. Selected bond lengths (Å): Co(1)–O(1) 1.927(3), Co(1)–O(2) 1.925(4), Co(1)–O(3) 1.923(5), Co(1)–N(1) 1.986(5), Co(1)–N(2) 1.975(5), Co(1)–N(3) 1.898(6), Co(2)–O(1) 2.077(4), Co(2)–O(2) 2.041(3), Co(2)–O(4) 2.095(5), Co(2)–N(4) 2.011(6), Co(2)–N(5) 2.018(6). Important angles have been listed in Table S1 (ESI).† | ||
Co(1) is equatorially coordinated by the two amine nitrogen atoms [N(1), N(2)] and two phenolate oxygen atoms [O(1), O(2)], from the tetradentate reduced Schiff base ligand. The axial positions are occupied by the thiocyanate nitrogen atom, N(3) and sulphur (S1) atom of coordinated solvent DMSO. On the other hand, Co(2) is equatorially coordinated by two phenolate oxygen atoms, [O(1), O(2)], two nitrogen atoms, N(4) and N(5) of two terminal thiocyanate groups and an oxygen atom [(O(4))] of a coordinated water molecule to complete its penta-coordinated square pyramidal geometry. Relatively large Co(1)⋯Co(2) distances {3.068(1) Å} are not indicative of any cobalt–cobalt bonding.85–89 The thiocyanates are quasi-linear with the N–C–S angles being 178.9(6)°, 178.0(7)° and 177.6(6)°, as expected.90 The saturated six-membered chelate ring [Co(1)–N(1)–C(8)–C(9)–C(12)–N(2)] has envelope conformation with the puckering parameters, q = 0.548(6)Å; θ = 12.05(5)°; ϕ = 185(3)°.91,92
Hirshfeld surface analysis
The Hirshfeld surface is mapped over dnorm (range ∼0.1 Å to 1.5 Å), shape index and curvedness (Fig. S1†). Red spots on the Hirshfeld surfaces denote the dominant interactions. The intermolecular interactions appear as distinct spikes in the 2D fingerprint plot shows the different spikes with their corresponding interactions. The dominant interactions in the complex correspond to H⋯H/H⋯H (45%), C⋯H/H⋯C (16.8%), S⋯H/H⋯S (30.8%), O⋯H/H⋯O (3%) and N⋯H/H⋯N (2.6%) contacts. 2D fingerprint plot of the complex is shown in Fig. S2.†IR and electronic spectroscopy study
The absence of any major band at around 1600 cm−1 (corresponding to azomethine bond) in the IR spectrum of the complex confirms the complete reduction of the azomethine group. A strong band for the terminal N-bonded thiocyanate group in complex is observed at 2071 cm−1.90 Broad bands in the range of 2859–3072 cm−1 may be attributed to the C–H stretching vibrations.93–95 The band around 3158 cm−1 may be assigned to N–H stretching vibrations.96 A distinct band is observed in the IR spectrum of the complex at 3411 cm−1 due to O–H stretching vibration.97 The IR spectrum of this complex is given in Fig. S3.†The complex shows intense absorption bands around 243–275 nm, which may be assigned as to n–π* transitions.98 The band at ca. 345 nm may be attributed to a ligand-to-metal charge transfer transition (LMCT).98,99 A band at 620 nm may be assigned as d–d transition.95 The electronic spectrum of the complex in acetonitrile medium at room temperature in the range 200–900 nm is shown in Fig. S4.†
Electrochemical studies
Cyclic voltammetry of the complex (with 10−3 M solution in DMF) was performed a with glassy carbon as the working electrode, Ag/AgCl as the reference electrode, and platinum wire as the auxiliary electrode incorporating TBAB (tetrabutylammonium bromide) as supporting electrolytet 300 K in argon atmosphere. The potential range covered by the cyclic voltammetry was −2.0 V to +2.0 V. The scan rate was 150 mV s−1.The complex exhibits two one-electron responses, one of which is irreversible redox signal, and the other is quasi-reversible (Fig. 2). The quasi-reversible redox signal consists of an oxidation peak, Epa at +1.41 V and a reduction peak, Epc at +1.31 V, whereas the irreversible signal consists only a reduction peak, Epc at −0.66 V. The quasi-reversible redox signal may be attributed to the CoII → CoIII oxidation and the CoIII → CoII reduction, whereas the irreversible signal is attributed to the CoIII → CoII reduction.100,101 The detailed electrochemical data is given in Table 1.
| Scan rate (mV s−1) | Epa (V) (CoII → CoIII) | Epc (V) (CoIII → CoII) | E1/2 (V) (CoII → CoIII) | ΔEp (CoII → CoIII) | Epc (V) (CoIII → CoII) |
|---|---|---|---|---|---|
| a E1/2 denotes the half-wave potential. E1/2 = (Epa + Epc)/2 and ΔEp = (Epa − Epc). | |||||
| 150 | +1.41 | +1.31 | +1.36 | +0.10 | −0.66 |
DFT calculations
The theoretical investigation began with a spin density analysis to support the assignment of the paramagnetic cobalt(II) center to the five-coordinate site, and the diamagnetic cobalt(III) ion to the octahedral site. As shown in Fig. 3, the spin density is predominantly localized on the cobalt(II) center (2.83 e), with minor delocalization onto the directly bonded atoms (0.15 e), and only a negligible residual spin density on the cobalt(III) center (0.02 e).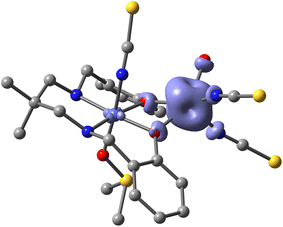 | ||
| Fig. 3 Spin density plot of the complex (isovalue 0.004 a.u.) at the PBE0-D4/def2-TZVP level of theory. | ||
Next, we analyzed selected assemblies extracted from the solid-state structure of the complex to investigate the energetic characteristics of key hydrogen bonds (see Fig. 4). In particular, we focused on a supramolecular assembly in which two complexes are bridged by two DMF molecules, forming an R44 (8) synthon (Fig. 4a). Additionally, two NH⋯S hydrogen bonds are established between the coordinated amino groups of one complex and the thiocyanate ligands of an adjacent complex, resulting in homodimeric assemblies (Fig. 4b). These dimers further propagate into one-dimensional supramolecular chains in the solid state. The DFT analysis aims to quantify these hydrogen bonds and is complemented by a combined QTAIM and NCIplot approach, as this combination is well suited to identifying real-space interactions and revealing their attractive nature.
 | ||
| Fig. 4 Partial view of the tetrameric (a) and homodimeric (b) assemblies observed in the solid state of the complex. Distances in Å. | ||
To gain further insight, we computed the molecular electrostatic potential (MEP) surface of the complex to identify the most electron-rich (nucleophilic) and electron-deficient (electrophilic) regions. As shown in Fig. 5, the highest positive MEP values are located on the hydrogen atoms of the coordinated amino groups (59.0 and 57.7 kcal mol−1), as well as in the region influenced by both methyl groups of the coordinated DMSO ligand (55.2 kcal mol−1). Significant positive potentials are also observed on the hydrogen atoms of the aliphatic linker between the amino groups (37.7 kcal mol−1) and on the hydrogen atoms of the coordinated water molecule (38.9 and 46.4 kcal mol−1). In contrast, the most negative MEP values are found on the thiocyanate ligands coordinated to the cobalt(II) center (−52.7 and −56.5 kcal mol−1), followed by the thiocyanate ligand bound to the cobalt(III) center. These findings are consistent with the supramolecular assemblies depicted in Fig. 4 and support the structure-directing role of NH⋯S and OH⋯O hydrogen bonding interactions.
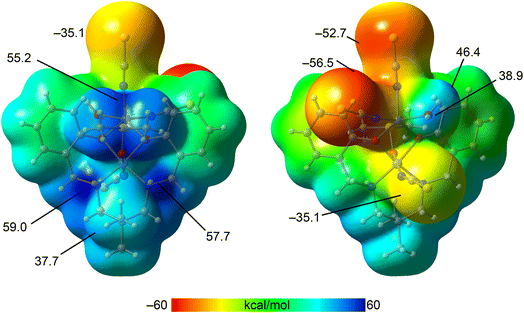 | ||
| Fig. 5 Two views of the MEP surface of the complex. Energies at selected points of the surface are indicated in kcal mol−1. Isovalue 0.001 a.u. | ||
The QTAIM/NCIplot analysis of the assemblies is presented in Fig. 6. The OH⋯O(DMF) hydrogen bonds forming the R44 (8) supramolecular ring are clearly identified by bond critical points (BCPs) and bond paths connecting the hydrogen and oxygen atoms. These hydrogen bonds are further visualized by blue reduced density gradient (RDG) isosurfaces, indicative of their strong and attractive nature. The calculated formation energy is substantial (−31.4 kcal mol−1), highlighting the significance of this synthon in the solid-state architecture of the complex. The individual strengths of the hydrogen bonds are noted adjacent to the BCPs in Fig. 6a, showing a difference of 1.7 kcal mol−1. This variation is consistent with the MEP analysis, which revealed distinct electrostatic potential values at the two hydrogen atoms of the coordinated water molecule.
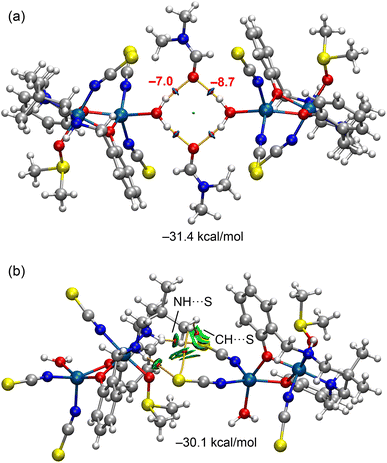 | ||
| Fig. 6 QTAIM/NCIplot analysis of the tetramer (a) and the homodimer (b) of the complexes. The formation energies of the assemblies are also indicated. | ||
The combined QTAIM/NCIplot analysis of the homodimer is presented in Fig. 6b. Four bond critical points (BCPs) and corresponding bond paths are observed, connecting the two monomeric units. Two of these correspond to NH⋯S hydrogen bonds, while the other two involve CH⋯S contacts between methyl hydrogen atoms and the sulfur atoms of the thiocyanate ligands. The NH⋯S interactions are characterized by small, disk-shaped RDG isosurfaces, typical of classical hydrogen bonds. In contrast, the CH⋯S contacts exhibit more extended RDG isosurfaces that encompass both the sulfur and carbon atoms of the SCN ligand, a shape more commonly associated with CH⋯π interactions. The directionality of these CH⋯S contacts suggests a significant contribution from the π-system of the thiocyanate acting as an electron donor. The interaction energy for the dimer is also notably large and negative (−30.1 kcal mol−1), supporting the formation of infinite one-dimensional supramolecular chains in the solid state, stabilized by the combined effect of NH⋯S and CH⋯S interactions.
The theoretical results obtained at the PBE0-D4/def2-TZVP level show good agreement with the experimental observations. The spin density analysis confirms that the unpaired electron density is predominantly localized on the five-coordinate Co(II) center, while the Co(III) center remains essentially diamagnetic, consistent with the bond length analysis derived from single-crystal X-ray diffraction. In addition, the DFT-calculated supramolecular assemblies reveal strong NH⋯S and OH⋯O hydrogen bonds, which match the key interactions identified in the crystal structure. The substantial calculated interaction energies (up to −31.4 kcal mol−1) corroborate the importance of these contacts in stabilizing the extended one-dimensional network observed experimentally. Therefore, the theoretical findings not only support but also enhance the interpretation of the experimental structural data.
Conclusion
In summary, we have synthesized and structurally characterized a novel dinuclear cobalt(III/II) complex derived from a reduced Schiff base ligand. The complex features distinct coordination geometries around the cobalt centers, as confirmed by single-crystal X-ray diffraction. A detailed DFT study, including spin density mapping, molecular electrostatic potential (MEP) surfaces, and topological analyses (QTAIM and NCIplot), has provided valuable insights into the electronic distribution and the nature of the noncovalent interactions that stabilize the supramolecular architecture. The strong NH⋯S and OH⋯O hydrogen bonds, as well as weaker CH⋯S contacts, contribute significantly to the formation of extended 1D networks in the solid state. These results underscore the importance of combining experimental and theoretical approaches to understand the role of noncovalent forces in the assembly of mixed-valence coordination complexes and may guide future design of supramolecular materials with tailored properties.Data availability
All data underlying the results are available as part of the article and no additional source data are required.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. Bera and S. Bhunia thank the UGC, India, for awarding Senior Research Fellowships. The authors are grateful to Projects PID2020-115637GB-I00 and PID2023-148453NB-I00 funded by the Ministerio de Ciencia, Innovación y Universidades of Spain MCIU/AEI/10.13039/501100011033 and FEDER, UE.Notes and references
- P. Koley, B. Ghosh, J. Bhattacharyya and A. Hazari, Mol. Catal., 2024, 569, 114523 CrossRef CAS.
- L. K. Das, A. Biswas, J. S. Kinyon, N. S. Dalal, H. Zhou and A. Ghosh, Inorg. Chem., 2013, 52, 11744–11757 CrossRef CAS PubMed.
- J. Mandal, A. Dey, S. Sarkar, M. Khatun, P. Ghorai, P. P. Ray, P. Mahata and A. Saha, Inorg. Chem., 2024, 63, 4527–4544 CrossRef CAS PubMed.
- J. Mandal, P. Brandão, S. Benmansour, C. J. Gómez-García and A. Saha, Cryst. Growth Des., 2022, 22, 7544–7554 CrossRef CAS.
- H.-H. Zhang, Y.-B. Ren, Z.-L. Yuan, N.-X. Kang, S. Mehdi, C.-C. Xing, X.-Y. Liu, Y.-P. Fan, B.-J. Li and B.-Z. Liu, Rare Met., 2023, 42, 1935–1945 CrossRef CAS.
- Y. J. Lee and S. –K. Park, Rare Met., 2024, 43, 522–532 CrossRef CAS.
- J. Wu, Y. Zhang, Q. Hong, H. Yang, L. Zhang, M. Zhang and L. Yu, Chin. Chem. Lett., 2025, 36, 110165 CrossRef CAS.
- A. K. Ghosh, D. Ghoshal, E. Zangrando, J. Ribas and N. R. Chaudhuri, Inorg. Chem., 2005, 44, 1786–1793 CrossRef CAS.
- B. Santra, P. Kalita, S. Chandra, D. Mandal, V. Kumar, R. S. Narayanan, A. Dey, N. Chrysochos, V. Huch, S. Biswas, D. Ghoshal, E. C. Sañudo, B. Sarkar, C. Schulzke, V. Chandrasekhar and A. Jana, Dalton Trans., 2020, 49, 2527–2536 RSC.
- M. Shit, M. Mahapatra, N. Sepay, C. Sinha, B. Dutta and M. H. Mir, Chem.–Eur. J., 2024, 30, e202402425 CrossRef CAS PubMed.
- S. Ahmed, D. Sahoo, P. Brandão, S. Bhunia, N. B. Manik and C. Sinha, Inorg. Chim. Acta, 2024, 572, 122277 CrossRef CAS.
- S. S. Bera and M. Szostak, ACS Catal., 2022, 12, 3111–3137 CrossRef CAS PubMed.
- R. Debnath, P. Ghosh and S. Koner, Appl. Organomet. Chem., 2025, 39, e7815 CrossRef CAS.
- K. Das, T. N. Mandal, S. Roy, A. Jana, S. Konar, C.-M. Liu, A. K. Barik and S. K. Kar, Polyhedron, 2011, 30, 715–724 CrossRef CAS.
- R. Sen, D. K. Hazra, S. Koner, M. Helliwell, M. Mukherjee and A. Bhattacharjee, Polyhedron, 2010, 29, 3183–3191 CrossRef CAS.
- E. Zahradníková, C. Pichon, C. Duhayon, J.-P. Sutter, P. Halaš and B. Drahoš, RSC Adv., 2024, 14, 28138–28147 RSC.
- I. Banerjee, A. Jana, S. Singh, J. Marek, E. d. Barco and M. Ali, Polyhedron, 2013, 66, 162–166 CrossRef CAS.
- A. A. Pavlov, Y. V. Nelyubina, S. V. Kats, L. V. Penkova, N. N. Efimov, A. O. Dmitrienko, A. V. Vologzhanina, A. S. Belov, Y. Z. Voloshin and V. V. Novikov, J. Phys. Chem. Lett., 2016, 7, 4111–4116 CrossRef CAS.
- A. S. Belov, S. A. Belova, N. N. Efimov, V. V. Zlobina, V. V. Novikov, Y. V. Nelyubina, Y. V. Zubavichus, Y. Z. Voloshin and A. A. Pavlov, Dalton Trans., 2023, 52, 2928–2932 RSC.
- S. A. Belova, A. S. Belov, A. A. Danshina, Y. V. Zubavichus, D. Y. Aleshin, A. A. Pavlov, N. N. Efimov and Y. Z. Voloshin, Dalton Trans., 2024, 53, 1482–1491 RSC.
- N. Plyuta, S. Petrusenko, V. N. Kokozay, T. Cauchy, F. Lloret, M. Julve, J. Cano and N. Avarvari, Dalton Trans., 2022, 51, 4760–4771 RSC.
- A. Zabala-Lekuona, A. Landart-Gereka, M. M. Quesada-Moreno, A. J. Mota, I. F. Díaz-Ortega, H. Nojiri, J. Krzystek, J. M. Seco and E. Colacio, Inorg. Chem., 2023, 62, 20030–20041 CrossRef CAS.
- S. S. Massoud, F. A. Mautner, H. Sakiyama, F. R. Louka, N. H. M. Salem, R. C. Fischer, A. Torvisco, T. Guizouarn and G. Velmurugan, Eur. J. Inorg. Chem., 2025, e202400777 CrossRef.
- J. G. McAlpin, T. A. Stich, W. H. Casey and R. D. Britt, Coord. Chem. Rev., 2012, 256, 2445–2452 CrossRef CAS.
- B. S. Brunschwig, M. H. Chou, Q. Creutz, P. Ghosh and N. Sutin, J. Am. Chem. Soc., 1983, 105, 4832–4833 CrossRef CAS.
- M. Schilling, G. R. Patzke, J. Hutter and S. Luber, J. Phys. Chem. C, 2016, 120, 7966–7975 CrossRef CAS.
- M. M. Najafpour and H. Feizi, Catal. Sci. Technol., 2018, 8, 1840–1848 RSC.
- D. S. Nesterov and O. V. Nesterova, Catalysts, 2018, 8(12), 602 CrossRef.
- A. Ray, G. M. Rosair, R. Kadam and S. Mitra, Polyhedron, 2009, 28, 796–806 CrossRef CAS.
- T. G. Dastidar and S. Chattopadhyay, Polyhedron, 2022, 211, 115511 CrossRef CAS.
- A. Hazari, L. K. Das, R. M. Kadam, A. Bauzá, A. Frontera and A. Ghosh, Dalton Trans., 2015, 44, 3862–3876 RSC.
- E. Evangelio, N. P. Rath and L. M. Mirica, Dalton Trans., 2012, 41, 8010–8021 RSC.
- Y.-C. Su, C.-Y. Tsai, L.-S. Huang, C.-H. Lin and B.-T. Ko, Dalton Trans., 2019, 48, 12239–12249 RSC.
- S. Dutta, P. Biswas, U. Florke and K. Nag, Inorg. Chem., 2010, 49, 7217–7219 CrossRef.
- Z.-L. You, Acta Crystallogr., 2005, C61, m295 CAS.
- J. Tang, F. Huang, Y. Wei, H. Bian, W. Zhang and H. Liang, Dalton Trans., 2016, 45, 8061–8072 RSC.
- Z.-L. You, D.-H. Shi, C. Xu, Q. Zhang and H.-L. Zhu, Eur. J. Med. Chem., 2008, 43, 862–871 CrossRef CAS PubMed.
- R. S. Sarkar, S. Banerjee and S. Chattopadhyay, Polyhedron, 2024, 254, 116916 CrossRef CAS.
- D.-H. Shi, Z.-L. You, C. Xu, Q. Zhang and H.-L. Zhu, Inorg. Chem. Commun., 2007, 10, 404–406 CrossRef CAS.
- X. He, C.-Z. Lu and C.-D. Wu, J. Coord. Chem., 2006, 59, 977–984 CrossRef CAS.
- C. Fukuhara, E. Asato, T. Shimoji and K. Katsura, J. Chem. Soc., Dalton Trans., 1987, 1305–1311 RSC.
- A. Banerjee, S. Banerjee, C. J. G. Garcia, S. Benmansour and S. Chattopadhyay, Dalton Trans., 2020, 49, 16778–16790 RSC.
- A. Banerjee, C. J. G. Garcia, S. Benmansour, R. M. Gomila, A. Frontera and S. Chattopadhyay, Polyhedron, 2022, 220, 115802 CrossRef CAS.
- A. Banerjee, D. Das, P. P. Ray, S. Banerjee and S. Chattopadhyay, Dalton Trans., 2021, 50, 1721–1732 RSC.
- S. Bhunia, M. Das, S. Banerjee, M. G. B. Drew, P. P. Ray and S. Chattopadhyay, RSC Adv., 2024, 14, 11185–11196 RSC.
- R. S. Sarkar, A. Biswas, P. P. Ray, R. M. Gomila, M. G. B. Drew, S. Banerjee, A. Frontera and S. Chattopadhyay, CrystEngComm, 2023, 25, 1006–1017 RSC.
- A. Hazari, A. Das, P. Mahapatra and A. Ghosh, Polyhedron, 2017, 134, 99–106 CrossRef CAS.
- A. Banerjee and S. Chattopadhyay, Polyhedreon, 2020, 177, 114290 CrossRef CAS.
- K. Ghosh, M. G. B. Drew and S. Chattopadhyay, Inorg. Chim. Acta, 2018, 482, 23–33 CrossRef CAS.
- K. Ghosh, S. Roy, A. Ghosh, A. Banerjee, A. Bauza, A. Frontera and S. Chattopadhyay, Polyhedron, 2016, 112, 6–17 CrossRef CAS.
- K. Ghosh, K. Harms and S. Chattopadhyay, ChemistrySelect, 2017, 2, 8207–8220 CrossRef CAS.
- K. Ghosh, K. Harms, A. Franconetti, A. Frontera and S. Chattopadhyay, J. Organomet. Chem., 2019, 883, 52–64 CrossRef CAS.
- S. Roy, M. G. B. Drew, A. Bauzá, A. Frontera and S. Chattopadhyay, Dalton Trans., 2017, 46, 5384–5397 RSC.
- S. Tsuzuki and A. Fujii, Phys. Chem. Chem. Phys., 2008, 10, 2584–2594 RSC.
- T.-H. Huang and M.-H. Zhang, Inorg. Chim. Acta, 2014, 416, 28–34 CrossRef CAS.
- B. Mirtamizdoust, A. Karamad, F. Mojtabazade, H. Hosein-Monfared, R. Bikas, Z. Zák, H. Erfani, S. Jadoun and A. K. Mishra, ACS Omega, 2024, 9, 5563–5575 CrossRef CAS.
- M. Nishio, CrystEngComm, 2004, 6, 130–158 RSC.
- M. Bazargan, M. Mirzaei, A. S. Hamid, Z. H. Kafshdar, H. Ziaekhodadadian, E. Momenzadeh, J. T. Mague, D. M. Gil, R. M. Gomila and A. Frontera, CrystEngComm, 2022, 24, 6677–6687 RSC.
- U. Mukhopadhyay, D. Choquesillo-Lazarte, J. Niclós-Gutiérrez and I. Bernal, CrystEngComm, 2004, 6, 627–632 RSC.
- A. M. Keys, D. W. Kastner, L. L. Kiessling and H. J. Kulik, Chem. Sci., 2025, 16, 1746–1761 RSC.
- R. Gaur, S. Roy, P. Kallem and F. Banat, J. Mol. Struct., 2022, 1265, 133400 CrossRef CAS.
- P. K. Bhaumik, A. Frontera and S. Chattopadhyay, Inorg. Chim. Acta, 2021, 515, 120023 CrossRef CAS.
- Y. V. Torubaev, D. K. Rai, I. V. Skabitsky, S. Pakhira and A. Dmitrienko, New J. Chem., 2019, 43, 7941–7949 RSC.
- F. Biedermann and H.-J. Schneider, Chem. Rev., 2016, 116, 5216–5300 CrossRef CAS PubMed.
- N. Bäumer, K. K. Kartha, S. Buss, I. Maisuls, J. P. Palakkal, C. A. Strassert and G. Fernández, Chem. Sci., 2021, 12, 5236–5245 RSC.
- A. N. Malik, M. N. Tahir, A. Ali, M. Ashfaq, M. Ibrahim, A. E. Kuznetsov, M. A. Assiri and M. Y. Sameeh, ACS Omega, 2023, 8, 25034–25047 CrossRef CAS PubMed.
- J. Antony, R. Sure and S. Grimme, Chem. Commun., 2015, 51, 1764–1774 RSC.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3 Search PubMed.
- G. M. Sheldrick, Sadabs, V2014/5, Software for Empirical Absorption Correction, University of Göttingen, Institute fur Anorganische Chemie der Universitat, Gottingen, Germany, 1999 Search PubMed.
- M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19–32 RSC.
- H. F. Clausen, M. S. Chevallier, M. A. Spackman and B. B. Iversen, New J. Chem., 2010, 34, 193–199 RSC.
- A. L. Rohl, M. Moret, W. Kaminsky, K. Claborn, J. J. McKinnon and B. Kahr, Cryst. Growth Des., 2008, 8, 4517–4525 CrossRef CAS.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- R. Ahlrichs, M. Bär, M. Hacer, H. Horn and C. Kömel, Chem. Phys. Lett., 1989, 162, 165–169 CrossRef CAS.
- R. F. W. Bader, Chem. Rev., 1991, 91, 893–928 CrossRef CAS.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS.
- D. Coskun, S. V. Jerome and R. A. Friesner, J. Chem. Theory Comput., 2016, 12, 1121–1128 CrossRef CAS.
- W.-J. Lian, X.-T. Wang, C.-Z. Xie, H. Tian, X.-Q. Song, H.-T. Pan, X. Qiao and J.-Y. Xu, Dalton Trans., 2016, 45, 9073–9087 RSC.
- P. Middya, S. D. Sarkar and S. Chattopadhyay, J. Mol. Struct., 2025, 1322, 140242 CrossRef CAS.
- S. Bhunia and S. Chattopadhyay, Inorg. Chim. Acta, 2025, 577, 122475 CrossRef CAS.
- R. S. Sarkar, C. J. G. García, S. Benmansour and S. Chattopadhyay, Polyhedron, 2025, 269, 117425 CrossRef CAS.
- J. Welby, L. N. Rusere, J. M. Tanski and L. A. Tyler, Inorg. Chim. Acta, 2009, 362, 1405–1411 CrossRef CAS.
- E. Baca-Solis, S. Bern‘es, H. Vazquez-Lima, M.-E. Boulon, R. E. P. Winpenny and Y. Reyes-Ortega, ChemistrySelect, 2016, 1, 6866–6871 CrossRef CAS.
- X. He, C.-Z. Lu and C.-D. Wu, J. Coord. Chem., 2006, 59, 977–984 CrossRef CAS.
- A. D. Khalaji and S. Triki, Russ. J. Coord. Chem., 2011, 37, 664–667 CrossRef CAS.
- S. Banerjee, M. Nandy, S. Sen, S. Mandal, G. M. Rosair, A. M. Z. Slawin, C. J. G. Garcia, J. M. Clemente-Juan, E. Zangrando, N. Guidolin and S. Mitra, Dalton Trans., 2011, 40, 1652–1661 RSC.
- S. Bera, S. Bhunia, R. M. Gomila, M. G. B. Drew, A. Frontera and S. Chattopadhyay, RSC Adv., 2023, 13, 29568–29583 RSC.
- D. Cremer, Acta Crystallogr., Sect. B: Struct. Sci., 1984, 40, 498–500 CrossRef.
- J. C. A. Boeyens, J. Cryst. Mol. Struct., 1978, 8, 317–320 CrossRef.
- T. Basak, S. Roy, S. Banerjee and S. Chattopadhyay, Inorg. Chim. Acta, 2022, 543, 121186 CrossRef CAS.
- S. Roy, B. Halder, R. M. Gomila, A. Frontera, M. G. B. Drew and S. Chattopadhyay, Inorg. Chim. Acta, 2024, 573, 122323 CrossRef CAS.
- S. Bera, A. Frontera and S. Chattopadhyay, Polyhedron, 2025, 14, 117422 CrossRef.
- S. Chattopadhyay, M. S. Ray, S. Chaudhuri, G. Mukhopadhyay, G. Bocelli, A. Cantoni and A. Ghosh, Inorg. Chim. Acta, 2006, 359, 1367–1375 CrossRef CAS.
- A. Bhattacharyya, M. Das, A. Bauza, S. Herrero, R. G. Prieto, A. Frontera and S. Chattopadhyay, New J. Chem., 2017, 41, 13585–13592 RSC.
- A. Ray, G. M. Rosair, G. Pilet, B. Dede, C. J. Gomez-García, S. Signorella, S. Bellú and S. Mitra, Inorg. Chim. Acta, 2011, 375, 20–30 CrossRef CAS.
- H. A. R. Pramanik, P. C. Paul, P. Mondal and C. R. Bhattacharjee, J. Mol. Struct., 2015, 1100, 496–505 CrossRef CAS.
- S. Bhunia, M. Das, S. Banerjee, M. G. B. Drew, P. P. Ray and S. Chattopadhyay, RSC Adv., 2024, 14, 11185–11196 RSC.
- M. Das and S. Chattopadhyay, Polyhedron, 2013, 50, 443–451 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S4 and Table S1. CCDC 2441974. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra02432c |
| This journal is © The Royal Society of Chemistry 2025 |